
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 16 August 2019
Sec. Neurotrauma
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00875
This article is part of the Research Topic Developing Successful Neuroprotective Treatments for TBI: Translational Approaches, Novel Directions, Opportunities and Challenges View all 24 articles
Current efficacious treatments for traumatic brain injury (TBI) are lacking. Establishment of a protective gut microbiota population offers a compelling therapeutic avenue, as brain injury induces disruptions in the composition of the gut microbiota, i.e., gut dysbiosis, which has been shown to contribute to TBI-related neuropathology and impaired behavioral outcomes. The gut microbiome is involved in the modulation of a multitude of cellular and molecular processes fundamental to the progression of TBI-induced pathologies including neuroinflammation, blood brain barrier permeability, immune system response, microglial activation, and mitochondrial dysfunction, as well as intestinal motility and permeability. Additionally, gut dysbiosis further aggravates behavioral impairments in animal models of TBI and spinal cord injury, as well as negatively affects health outcomes in murine stroke models. Recent studies indicate that microbiota transplants and probiotics ameliorate neuroanatomical damage and functional impairments in animal models of stroke and spinal cord injury. In addition, probiotics have been shown to reduce the rate of infection and time spent in intensive care of hospitalized patients suffering from brain trauma. Perturbations in the composition of the gut microbiota and its metabolite profile may also serve as potential diagnostic and theragnostic biomarkers for injury severity and progression. This review aims to address the etiological role of the gut microbiome in the biochemical, neuroanatomical, and behavioral/cognitive consequences of TBI, as well as explore the potential of gut microbiome manipulation in the form of probiotics as an effective therapeutic to ameliorate TBI-induced pathology and symptoms.
Traumatic brain injury (TBI) is a major cause of death and disability in the United States and represents one of the most prevalent injury types sustained by the worldwide population (1). Reports spanning the last two decades underscore the human and financial burden of TBI in the United States, with an annual incidence of ~1.4 million cases (2), prevalence of ~3.17 million with a long-term TBI-induced disability (3), and an annual economic burden of billions of dollars (4). Importantly, these disabilities are a result of not only the mechanical damage sustained due to the initial injury (primary), but also the subsequent cellular and molecular damage that exacerbates in the following hours, days, weeks, and years post-injury (secondary) (5, 6). The etiology of secondary injury is multifaceted and may constitute altered cerebral blood flow, excitotoxicity, inflammation, microglial activation, metabolic anomalies, mitochondrial dysfunction, and oxidative stress resulting in transient or lifelong behavioral and cognitive deficits (5–9). TBI severity is categorized based on the Glasgow Coma Scale (GCS), in which patients are scored on the basis of clinical symptoms, and the resulting overall score classifies their injury as mild (score: 13–15), moderate (score: 9–12), or severe (score: <9) (10, 11). Overall, TBI complexity occurs on a spectrum ranging from mild to severe, diffuse to focal, and single to repeated exposures in brain vs. multi-organs, which leads to injury-specific heterogeneous pathobiological responses that cannot be regarded as a single condition (12).
Despite decades of rigorous preclinical research in which much insight into the heterogeneous nature of brain injury has been gained, efficacious therapeutics for TBI-induced neuropathologies and behavioral/cognitive impairments are lacking (13–15). Given the prevalence of TBI-related disabilities, it is imperative to consider novel treatment strategies. Restoration of the gut microbiome by gut eubiotic therapeutics is one such compelling avenue, which is capable of modulating the bi-directional relationship between TBI-induced disruptions of the gut microbiome and the influence of this gut dysbiosis on the pathophysiology of TBI-induced secondary injury progression (16, 17).
Gut microbiota refer to the bacteria, archaea, viruses, and eukaryotic microbes that reside primarily within the colon, but also within the stomach and small intestine (18). This commensal bacterial community accounts for 0.2–1 kg of an adult's bodyweight (18, 19), outnumbering mammalian cells by as much as 10:1, though more recent estimates indicate a ratio of ~1:1 (18), and contains ~100 fold more unique genes than the human genome (20). Bacteroidetes and Firmicutes phyla compose the majority of the gut microbiota, with Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia being present in fewer numbers. However, gut microbiota composition differs among individuals as diet, age, gender, environment, and genetics all influence bacterial strains/populations (21–23). The activity and composition of this microbial population is involved in a surprising number of biological processes, including homeostasis of the central nervous system (CNS) (24–26). This relationship is referred to as the microbiota-gut-brain axis (MGBA) (27), with communication between the gut microbiota and the CNS occurring through a neuro-endocrino-immunological network (28).
Perhaps the most direct route of communication within the MGBA is among the gut microbiota, enteric nervous system (ENS), and vagus nerve. Neuroactive compounds produced by gut bacteria influence the activity of sensory neurons of the ENS, which in turn modulates the afferent activity of the vagus nerve (29). These compounds consist of bacterial metabolites, neurotransmitters, neurotrophic factors, cytokines, and endotoxins (30–32). Nervous system signaling originating from the gastrointestinal tract is then integrated by the nucleus of the solitary tract (33) and relayed to other brain nuclei (34). Gut microbiota also play a fundamental role in the development and functioning of the host immune system (35). Homeostasis of host immune system function is predicated upon proper gastrointestinal neuromuscular control, maintenance of intestinal wall integrity, and intact ENS/vagus nerve signaling (36, 37), aspects of gastrointestinal health that are, in part, regulated by the gut microbiome. Perturbations in the composition of the gut microbiota are known to lead to a weakening of the intestinal-host barrier (38), allowing gastrointestinal content to be released into the blood stream and other parts of the body, a condition referred to as “leaky gut” (39), which can lead to neuroinflammation. For example, peripheral administration of the bacterial endotoxin lipopolysaccharide induces cytokine expression within the hypothalamus-pituitary-axis, resulting in regional neurotoxicity and systemic inflammation (40, 41). Notably, the cross-talk among the gut bacteria, ENS, and vagus nerve cohesively regulates the host immune and inflammatory responses to modulate CNS function (42, 43). Finally, cognitive and behavioral changes (e.g., stress) have repeatedly been shown to alter the composition of the gut microbiota, demonstrating both feed-forward and feedback mechanisms within the MGBA (44).
Gut microbiome composition has been linked to a variety of illness and disease states (45, 46), with research dating back over seven decades establishing a relationship between the metabolic products of gut bacteria and hepatic encephalopathy (47, 48). More recent research has linked the gut microbiota to inflammatory diseases (49) and several CNS-related disorders, including autism (50, 51), depression (28, 52), and anxiety (53, 54), as well as Alzheimer's disease (55) and Parkinson's disorder (55, 56). However, it is difficult to prove causation and directionality when discussing gut microbiome changes observed in human neuropsychiatric and neurodegenerative conditions (57). For these reasons, rodents are commonly used when investigating the MGBA as they (1) possess similar, but not identical, core intestinal bacterial populations to humans (58, 59) and (2) can be maintained “germ free” (devoid of gut microbiota) or gnotobiotic (gut microbiota of known composition).
Eubiotic therapeutics that alter the gut microbiome through diet, microbiota transplants, antibiotics, and pre-/probiotics influence both systemic and CNS-related processes. Microbiota transplants have been shown to influence obesity levels in rodents (60) and humans (61), as well as effectively treat recurrent Clostridium difficile infection (62). Meanwhile, probiotics have shown promise in the treatment of patients with ulcerative colitis (63) and antibiotics are now commonly used to eliminate the bacterial populations involved in hepatic encephalopathy (64). Probiotics have also been shown to reduce anxiety- and depressive-like symptoms in animals, with limited evidence indicating similar results in humans (53). Furthermore, gut microbiome alterations have been shown to ameliorate autism-like behaviors in mice (65), with probiotics having been suggested as a therapeutic strategy for individuals with post-traumatic stress disorder (66). Among other research findings [as reviewed by (67)], this has led some researchers to suggest “psychobiotics” as a new therapeutic approach for neurological and neuropsychiatric illnesses (68, 69).
Pertinent for TBI research is the bi-directional relationship that exists between brain injury and the gut microbiome (Figure 1). Research in brain and spinal cord injury (SCI) animal models has demonstrated that CNS injury disrupts the motility and permeability of the intestinal wall (70, 71) and perturbs the composition of the gut microbiome (17, 72), leading to a host-maladaptive state referred to as gut dysbiosis (73). Conversely, gut dysbiosis influences the pathophysiology of traumatic CNS injury (74, 75). For example, following SCI, significant changes in the composition of the gut microbiota were observed, namely a decrease in Bacteroidetes and increase in Firmicutes, with post-injury changes in the gut microbiome persisting out to 1 month and predicting the degree of locomotor impairment (76). A similar relationship was observed in a controlled cortical impact (CCI) rodent model of moderate TBI, with bacterial changes occurring as early as 2 h following injury, persisting out to 7 days post-injury, and correlating with lesion volume. However, the opposite alteration in gut microbiota was observed with a decrease in Firmicutes and increase in bacterial families within the Bacteroidetes and Proteobacteria phyla (77). Furthermore, a recent study by Treangen et al. (78) reported gut dysbiosis with significant decreases in Lactobacillus gasseri, Ruminococcus flavefaciens, and Eubacterium ventriosum and significant increases in Eubacterium sulci and Marvinbryantia formatexigens at 24 h post-CCI in mice. L. gasseri displayed the most drastic change with a 4-fold log decrease in abundance as compared to baseline, though it should be noted that a less pronounced decease was also observed following sham procedures. As L. gasseri is a member of the phylum Firmicutes, this work complements the findings of Nicholson et al., and provides for a potential eubiotic target as L. gasseri inhabits the human gut microbiome (79). Investigations into TBI-induced gut dysbiosis in humans is limited, though a recent study in severely injured patients with polytrauma reported a decrease in Bacteroidales, Fusobacteriales, and Verrucomicrobiales, as well as an increase in Clostridiales and Enterococcus within 72 h of injury (80).
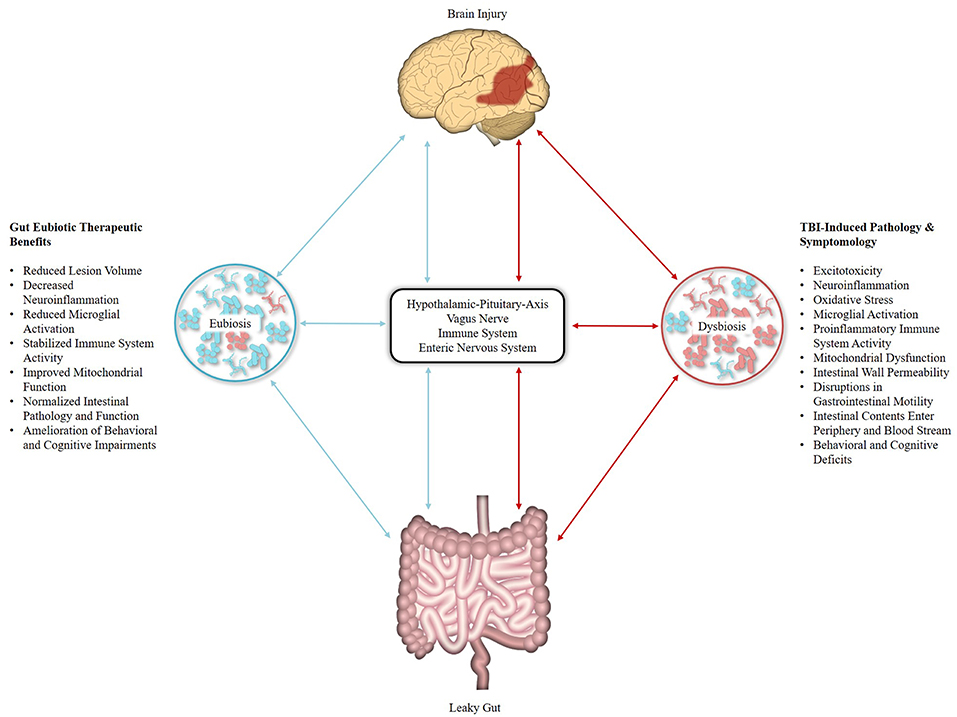
Figure 1. Effects of traumatic brain injury (TBI) and eubiotic therapies within the microbiota-gut-brain axis (MGBA). Brain injury induces disruptions within the MGBA through multiple pathways [Represented in Red]. Resulting perturbations complete a bi-directional positive feedback mechanism that contributes to the secondary injury characteristics of TBI. Resolution of gut dysbiosis by eubiotic therapeutics may act to break this cycle [Represented in Blue], thus reducing the impact of secondary injury pathology and improving TBI biochemical, pathological, and behavioral outcomes.
Gut dysbiosis also affects the integrity and permeability of the blood brain barrier (BBB) (81). Coupled with TBI-induced physical disruptions to the BBB (82), intestinal contents and the associated upregulation of the pro-inflammatory immune response more easily permeate the CNS, resulting in increased microglial activity, neuroinflammation, and neuropathology (83, 84). Microglial maturation and function within the CNS have been shown to be influenced by the gut microbiome in BBB-intact animals (85, 86), a relationship expected to be enhanced by increased BBB permeability. Therefore, it is likely that TBI-induced gut dysbiosis is a contributing factor in increased microglial activation following CNS injury (86). Post-injury mitochondrial dysfunction in terms of energy production (i.e., ATP synthesis) observed in TBI (87, 88) may also be impacted by gut dysbiosis, as studies have revealed a link between gut bacterial metabolites and mitochondrial function (26, 89).
Importantly, experimenter-induced alterations in the composition of the gut microbiota community regulate immune system activity, neuropathology, and behavior following CNS injury. In a gnotobiotic mouse model of ischemic stroke, an expansion of Proteobacteria accompanied by a contraction in Firmicutes and Bacteroidetes altered immune system homeostasis by increasing peripheral neuroprotective anti-inflammatory Treg cells and decreasing pro-inflammatory γδ T cells, resulting in a reduction in ischemic brain injury (90). However, the large-scale depletion of cultivatable gut microbiota by a broad-spectrum antibiotic in a mouse model of focal cerebral ischemia prior to injury resulted in decreased rates of survival and an increase in the development of severe acute colitis (74). Furthermore, if gut dysbiosis was experimentally induced by a broad-spectrum antibiotic prior to SCI, both neurological impairment and spinal cord pathology were exacerbated, likely due to changes in immune system activity (76). These studies demonstrate the complex relationships within the MGBA, revealing that the bacterial populations present at the time of injury influence the degree of neuropathology and functional impairment following TBI. Such knowledge establishes the basis for both the monitoring and manipulation of the gut microbiota as a means to diagnose and ameliorate the pathophysiology and symptomology of brain injuries.
Monitoring the extent of gut dysbiosis may provide a diagnostic tool for the identification of TBI severity, providing information for treatment guidance. Fecal metabolomes have already been used as biomarkers for several ailments including Crohn's disease and colorectal cancer (91, 92), and a recent study by Houlden et al. (72) demonstrated a positive correlation between the degree of gut dysbiosis and the severity of a closed-head-impact rodent model. Importantly, the profile of gut microbiota changes observed following TBI differed from those following ischemic brain injury by 72 h post-injury, indicating that different forms of brain injury uniquely impact the gut microbiome (72).
Beyond monitoring, manipulation of the gut microbiome via eubiotic therapies (e.g., microbiota transplants and pre/probiotics) presents an exciting treatment target for TBI (Figure 1). Several of the ailments associated with TBI-induced pathology that affect the microbiota are improved by the intake of probiotics, such as intestinal motility and permeability, health of the intestinal cellular lining, intestinal inflammation, and systemic immune response (93–95). Furthermore, perturbations in bacterial composition initially appear 24–72 h following trauma (72, 80); a time period corresponding to the pathophysiology of TBI-induced secondary injury, representing an ideal treatment window. As substantial alterations in the gut microbiome can occur 24–48 h following dramatic changes in diet (96, 97), eubiotic therapies could fundamentally shift the gut microbiome to a beneficial state in time to mitigate aspects of TBI-associated secondary injury. Preclinical studies support this concept as microbiota transplants have been shown to reduce brain lesion size and improve health outcomes in mouse models of ischemic stroke (98) and restore microglial function (85). Probiotic derived bacterial metabolites may also serve to modulate mitochondrial homeostasis (99) as gut microbiota generate short-chain fatty acid products such as butyrate, propionate, and acetate (100). Together with dietary ketones, these gut microbiome products serve as alternative energy sources for the injured brain and may improve bioenergetics function following TBI and SCI (101, 102). Additionally, gut microbiota-generated butyrate serves as a histone deacetylation (HDAC) inhibitor, offering additional benefits as HDACs play an important role in neuroprotection following CNS injuries (103) and enhance cognitive function in neuropsychiatric disorders (104). Furthermore, the butyric acid-producing probiotic Clostridium butyricum improved neurological deficits, reduced brain edema, attenuated neurodegeneration, and ameliorated BBB impairment (105), as well as improved spatial memory in mouse models of weight-drop impact head injury and cerebral ischemia, respectively (83). Probiotic supplements rich in lactobacilli and bifidobacteria have also been shown to improve spatial memory in a cognitively impaired mouse model (106) and one explanation for these observed improvements is evidenced by VSL#3 (a commercial, medical-grade probiotic rich in lactic acid bacteria) rescuing hippocampal neurogenesis via Ly6Chi monocytes in mice with antibiotic-induced gut dysbiosis (107). Treatment with VSL#3 also decreases circulating levels of TNFα, lessens cerebral monocyte infiltration, and reduces microglial activation (108). In mice that received SCI, VSL#3 provided the day of injury and extending for 35 days post-injury reduced neuropathology, improved locomotor recovery, and triggered a protective immune response through an increase in the number of Treg cells (109).
Importantly, human preclinical trials in brain injury patients with GCSs of 5–12 (i.e., moderate to severe TBI) indicate that manipulation of the gut microbiome through lactobacilli-rich probiotic supplementation within the first 48 h of admission with continued treatment for between 5 and 21 days can reduce nosocomial infection rate (110), decrease gastrointestinal dysfunction (111), lessen the incidence of ventilator-associated pneumonia (111), and shorten the time spent in intensive care (112). These observed benefits are commonly attributed to probiotic-induced reductions in systemic and central inflammation (113, 114). No studies exist examining the behavioral/cognitive outcomes of probiotic supplementation on TBI patients; however, probiotics have been shown to improve behavior and cognition in individuals with Alzheimer's disease (115) and depression (116), as well as healthy individuals (117). Probiotic supplementation for patients with penetrating TBI may be additionally useful as the long-term use of antibiotics is recommended for the reduction of infection, morbidity, and mortality rates (118, 119). As discussed, antibiotic-induced disruptions of the gut microbiome can lead to worsened TBI-related outcomes, potentially guiding medical practices toward adjunctive probiotic treatments to mitigate or minimize complex downstream pathobiological responses following TBI.
Provided the bi-directional relationship between the gut microbiome and TBI-associated pathology, resolution of gut dysbiosis represents a compelling therapeutic target. Probiotics consisting of lactobacilli, bifidobacteria, and other butyrate-producing gut bacteria appear most beneficial, providing a eubiotic therapy that enhances MGBA function through their anti-inflammatory and positive mitochondrial energetic properties. However, recent work revealed that antibiotic-induced microbiome perturbations and probiotic colonization display strong inter-species and inter-individual differences that may not have been apparent in previous investigations (120, 121). Additionally, differing courses/compositions of eubiotic treatments may need to be considered based on the type and severity of CNS injury, as these parameters produce dissimilar gut dysbiosis profiles (72). Therefore, resolution of gut dysbiosis as a therapeutic option requires investigations that yield information on the specific changes that occur to the gut microbiota following different types and severities of TBI, as well as optimal doses, treatment window, duration of treatment, and efficacy of experimentally-induced gut microbiome alterations across age and gender. Data that are sorely lacking (17, 93). Ultimately, this information could be used to develop a powerful diagnostic tool or eubiotic therapy to alleviate trauma brought on by brain injury.
MR wrote the manuscript. JP contributed to manuscript revision. DS read and approved the submitted version.
This effort was funded by the U.S. Army Medical Research and Development Command's Combat Casualty Care Research Program (H_001_2018_WRAIR).
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. (2007) 22:341–53.
2. Summers CR, Ivins B, Schwab KA. Traumatic brain injury in the United States: an epidemiologic overview. Mt Sinai J Med. (2009) 76:105–10. doi: 10.1002/msj.20100
3. Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. (2008) 23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac
4. Humphreys I, Wood RL, Phillips CJ, Macey S. The costs of traumatic brain injury: a literature review. Clinicoecon Outcomes Res. (2013) 5:281–7. doi: 10.2147/CEOR.S44625
5. Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. (2009) 76:97–104. doi: 10.1002/msj.20104
6. Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. (2011) 70:374–83. doi: 10.1002/ana.22455
7. Leung LY, Wei G, Shear DA, Tortella FC. The acute effects of hemorrhagic shock on cerebral blood flow, brain tissue oxygen tension, and spreading depolarization following penetrating ballistic-like brain injury. J Neurotrauma. (2013) 30:1288–98. doi: 10.1089/neu.2012.2715
8. Grandhi R, Tavakoli S, Ortega C, Simmonds MJ. A review of chronic pain and cognitive, mood, and motor dysfunction following mild traumatic brain injury: complex, comorbid, and/or overlapping conditions? Brain Sci. (2017) 7:E160. doi: 10.3390/brainsci7120160
9. Simon DW, Mcgeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. (2017) 13:171–91. doi: 10.1038/nrneurol.2017.13
10. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. (1974) 2:81–4. doi: 10.1016/S0140-6736(74)91639-0
11. Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir. (1976) 34:45–55. doi: 10.1007/BF01405862
12. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. (2017) 26:1118–30. doi: 10.1177/0963689717714102
13. Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: is more better? Prog Neurobiol. (2016) 142:45–67. doi: 10.1016/j.pneurobio.2016.05.002
14. Kochanek PM, Bramlett HM, Shear DA, Dixon CE, Mondello S, Dietrich WD, et al. Synthesis of findings, current investigations, and future directions: operation brain trauma therapy. J Neurotrauma. (2016) 33:606–14. doi: 10.1089/neu.2015.4133
15. Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, et al. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol. (2017) 37:571–85. doi: 10.1007/s10571-016-0400-1
16. Kharrazian D. Traumatic brain injury and the effect on the brain-gut axis. Altern Ther Health Med. (2015) 21(Suppl. 3):28–32.
17. Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. (2017) 66:31–44. doi: 10.1016/j.bbi.2017.05.009
18. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
19. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. (2017) 179:204–22. doi: 10.1016/j.trsl.2016.08.002
20. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
21. Yang AL, Kashyap PC. A clinical primer of the role of gut microbiome in health and disease. Trop Gastroenterol. (2015) 36:1–13. doi: 10.7869/tg.238
22. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. (2016) 7:313–22. doi: 10.1080/19490976.2016.1203502
23. Takagi T, Naito Y, Inoue R, Kashiwagi S, Uchiyama K, Mizushima K, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol. (2018) 54:53–63. doi: 10.1007/s00535-018-1488-5
24. Galland L. The gut microbiome and the brain. J Med Food. (2014) 17:1261–72. doi: 10.1089/jmf.2014.7000
25. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. (2017) 174:651–60. doi: 10.1002/ajmg.b.32567
26. Bajpai P, Darra A, Agrawal A. Microbe-mitochondrion crosstalk and health: an emerging paradigm. Mitochondrion. (2017) 39:20–25. doi: 10.1016/j.mito.2017.08.008
27. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. (2017) 46:77–89. doi: 10.1016/j.gtc.2016.09.007
28. Lima-Ojeda JM, Rupprecht R, Baghai TC. “I Am I and My Bacterial Circumstances”: linking gut microbiome, neurodevelopment, and depression. Front Psychiatry. (2017) 8:153. doi: 10.3389/fpsyt.2017.00153
29. Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. (2017) 3:3. doi: 10.1038/s41531-016-0002-0
30. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. (2012) 10:735–42. doi: 10.1038/nrmicro2876
31. Hsiao EY, Mcbride SW, Hsien S, Sharon G, Hyde ER, Mccue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
32. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
33. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. (2000) 85:1–17. doi: 10.1016/S1566-0702(00)00215-0
34. Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci. (2016) 73:737–55. doi: 10.1007/s00018-015-2083-z
35. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
36. Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. (2017) 17:94. doi: 10.1007/s11910-017-0802-6
37. Yoo BB, Mazmanian SK. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. (2017) 46:910–26. doi: 10.1016/j.immuni.2017.05.011
38. Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. (2017) 11:9. doi: 10.3389/fnbeh.2017.00009
39. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
40. Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. (1994) 27:157–62. doi: 10.1016/0169-328X(94)90197-X
41. Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. (2000) 20:6309–16. doi: 10.1523/JNEUROSCI.20-16-06309.2000
42. Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. (1998) 840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x
43. Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. (2015) 63:38–57. doi: 10.1007/s12026-015-8718-1
44. Patterson TT, Nicholson S, Wallace D, Hawryluk GWJ, Grandhi R. Complex feed-forward and feed-back mechanisms underlie the relationship between traumatic brain injury and the gut-microbiota-brain axis. Shock. (2018) 52:318–25. doi: 10.1097/SHK.0000000000001278
45. Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. (2015) 31:69–75. doi: 10.1097/MOG.0000000000000139
46. Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. (2017) 81:369–82. doi: 10.1002/ana.24901
47. Phear EA, Ruebner B, Sherlock S, Summerskill WH. Methionine toxicity in liver disease and its prevention by chlortetracycline. Clin Sci. (1956) 15:93–117.
48. Martini GA, Phear EA, Ruebner B, Sherlock S. The bacterial content of the small intestine in normal and cirrhotic subjects: relation to methionine toxicity. Clin Sci. (1957) 16:35–51.
49. Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. (2018) 360:j5145. doi: 10.1136/bmj.j5145
50. Cenit MC, Sanz Y, Codoner-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. (2017) 23:5486–98. doi: 10.3748/wjg.v23.i30.5486
51. Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci. (2017) 11:120. doi: 10.3389/fncel.2017.00120
52. Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. (2015) 28:1–6. doi: 10.1097/YCO.0000000000000117
53. Foster JA, Mcvey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
54. Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci USA. (2016) 113:E3130–9. doi: 10.1073/pnas.1600324113
55. Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. (2017) 8:90–102. doi: 10.1007/s13238-016-0338-6
56. Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. (2016) 158:52–62. doi: 10.1016/j.pharmthera.2015.11.012
57. Mu C, Yang Y, Zhu W. Gut microbiota: the brain peacekeeper. Front Microbiol. (2016) 7:345. doi: 10.3389/fmicb.2016.00345
58. Krych L, Hansen CH, Hansen AK, Van Den Berg FW, Nielsen DS. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE. (2013) 8:e62578. doi: 10.1371/journal.pone.0062578
59. Flemer B, Gaci N, Borrel G, Sanderson IR, Chaudhary PP, Tottey W, et al. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes. (2017) 8:428–39. doi: 10.1080/19490976.2017.1334033
60. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
61. John GK, Mullin GE. The gut microbiome and obesity. Curr Oncol Rep. (2016) 18:45. doi: 10.1007/s11912-016-0528-7
62. Brown WR. Fecal microbiota transplantation in treating Clostridium difficile infection. J Dig Dis. (2014) 15:405–8. doi: 10.1111/1751-2980.12160
63. Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. (2017) 46:389–400. doi: 10.1111/apt.14203
64. Patidar KR, Bajaj JS. Antibiotics for the treatment of hepatic encephalopathy. Metab Brain Dis. (2013) 28:307–12. doi: 10.1007/s11011-013-9383-5
65. Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. (2016) 165:1762–75. doi: 10.1016/j.cell.2016.06.001
66. Leclercq S, Forsythe P, Bienenstock J. Posttraumatic stress disorder: does the gut microbiome hold the key? Can J Psychiatry. (2016) 61:204–13. doi: 10.1177/0706743716635535
67. Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. (2016) 22:589–605. doi: 10.5056/jnm16018
68. Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. (2016) 39:763–81. doi: 10.1016/j.tins.2016.09.002
69. Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. (2017) 179:223–44. doi: 10.1016/j.trsl.2016.10.002
70. Hang CH, Shi JX, Li JS, Wu W, Yin HX. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. (2003) 9:2776–81. doi: 10.3748/wjg.v9.i12.2776
71. Bansal V, Costantini T, Kroll L, Peterson C, Loomis W, Eliceiri B, et al. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. (2009) 26:1353–9. doi: 10.1089/neu.2008.0858
72. Houlden A, Goldrick M, Brough D, Vizi ES, Lenart N, Martinecz B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. (2016) 57:10–20. doi: 10.1016/j.bbi.2016.04.003
73. Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, et al. Microbiota and neurological disorders: a gut feeling. Biores Open Access. (2016) 5:137–45. doi: 10.1089/biores.2016.0010
74. Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. (2016) 47:1354–63. doi: 10.1161/STROKEAHA.115.011800
75. Winek K, Meisel A, Dirnagl U. Gut microbiota impact on stroke outcome: fad or fact? J Cereb Blood Flow Metab. (2016) 36:891–8. doi: 10.1177/0271678X16636890
76. Kigerl KA, Mostacada K, Popovich PG. Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics. (2017) 15:60–7. doi: 10.1007/s13311-017-0583-2
77. Nicholson SE, Watts LT, Burmeister DM, Merrill D, Scroggins S, Zou Y, et al. Moderate traumatic brain injury alters the gastrointestinal microbiome in a time-dependent manner. Shock. (2018) 52:240. doi: 10.1097/SHK.0000000000001211
78. Treangen TJ, Wagner J, Burns MP, Villapol S. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. (2018) 9:2757. doi: 10.3389/fimmu.2018.02757
79. Lepage P, Leclerc MC, Joossens M, Mondot S, Blottiere HM, Raes J, et al. A metagenomic insight into our gut's microbiome. Gut. (2013) 62:146–58. doi: 10.1136/gutjnl-2011-301805
80. Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, et al. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surgery andamp; Acute Care Open. (2017) 2:e000108. doi: 10.1136/tsaco-2017-000108
81. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
82. Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. (2011) 2:492–516. doi: 10.1007/s12975-011-0125-x
83. Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. (2016) 1642:180–8. doi: 10.1016/j.brainres.2016.03.042
84. Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. (2017) 8:15062. doi: 10.1038/ncomms15062
85. Erny D, Hrabe De Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
86. Erny D, Hrabe De Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. (2017) 150:7–15. doi: 10.1111/imm.12645
87. Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol. (2009) 218:381–9. doi: 10.1016/j.expneurol.2009.05.023
88. Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, et al. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp Neurol. (2014) 257:106–13. doi: 10.1016/j.expneurol.2014.04.020
89. Frye RE, Rose S, Chacko J, Wynne R, Bennuri SC, Slattery JC, et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl Psychiatry. (2016) 6:e927. doi: 10.1038/tp.2016.189
90. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. (2016) 22:516–23. doi: 10.1038/nm.4068
91. Bennike T, Birkelund S, Stensballe A, Andersen V. Biomarkers in inflammatory bowel diseases: current status and proteomics identification strategies. World J Gastroenterol. (2014) 20:3231–44. doi: 10.3748/wjg.v20.i12.3231
92. Zackular JP, Rogers MA, Ruffin MTT, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res. (2014) 7:1112–21. doi: 10.1158/1940-6207.CAPR-14-0129
93. Verna EC, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol. (2010) 3:307–19. doi: 10.1177/1756283X10373814
94. Van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. (2013) 34:208–15. doi: 10.1016/j.it.2013.01.005
95. Sun B, Hu C, Fang H, Zhu L, Gao N, Zhu J. The effects of lactobacillus acidophilus on the intestinal smooth muscle contraction through PKC/MLCK/MLC signaling pathway in TBI mouse model. PLoS ONE. (2015) 10:e0128214. doi: 10.1371/journal.pone.0128214
96. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. (2009) 1:6ra14. doi: 10.1126/scitranslmed.3000322
97. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
98. Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. (2016) 36:7428–40. doi: 10.1523/JNEUROSCI.1114-16.2016
99. Han B, Sivaramakrishnan P, Lin CJ, Neve IAA, He J, Tay LWR, et al. Microbial genetic composition tunes host longevity. Cell. (2017) 169:1249–62 e1213. doi: 10.1016/j.cell.2017.05.036
100. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. (2015) 7:2839–49. doi: 10.3390/nu7042839
101. Stilling RM, Van De Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
102. Cavaleri F, Bashar E. Potential synergies of beta-hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab. (2018) 2018:7195760. doi: 10.1155/2018/7195760
103. Lu J, Frerich JM, Turtzo LC, Li S, Chiang J, Yang C, et al. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci USA. (2013) 110:10747–52. doi: 10.1073/pnas.1308950110
104. Volmar C-H, Wahlestedt C. Histone deacetylases (HDACs) and brain function. Neuroepigenetics. (2015) 1:20–7. doi: 10.1016/j.nepig.2014.10.002
105. Li H, Sun J, Du J, Wang F, Fang R, Yu C, et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. (2018) 30:e13260. doi: 10.1111/nmo.13260
106. Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. (2013) 240:287–96. doi: 10.1016/j.neuroscience.2013.02.055
107. Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, et al. Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. (2016) 15:1945–56. doi: 10.1016/j.celrep.2016.04.074
108. D'mello C, Ronaghan N, Zaheer R, Dicay M, Le T, Macnaughton WK, et al. Probiotics Improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci. (2015) 35:10821–30. doi: 10.1523/JNEUROSCI.0575-15.2015
109. Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. (2016) 213:2603–20. doi: 10.1084/jem.20151345
110. Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. (2011) 15:R290. doi: 10.1186/cc10579
111. Pavelescu D, Mirea L, Grintescu I. Could selected probiotics have beneficial effects on clinical outcome of severe traumatic brain injury patients? Critical Care. (2014) 18:P472. doi: 10.1186/cc13662
112. Falcao De Arruda IS, De Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci. (2004) 106:287–92. doi: 10.1042/CS20030251
113. Wang J, Liu KX, Ariani F, Tao LL, Zhang J, Qu JM. Probiotics for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of high-quality randomized controlled trials. PLoS ONE. (2013) 8:e83934. doi: 10.1371/journal.pone.0083934
114. Brenner LA, Stearns-Yoder KA, Hoffberg AS, Penzenik ME, Starosta AJ, Hernandez TD, et al. Growing literature but limited evidence: a systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav Immun. (2017) 65:57–67. doi: 10.1016/j.bbi.2017.06.003
115. Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. (2016) 8:256. doi: 10.3389/fnagi.2016.00256
116. Steenbergen L, Sellaro R, Van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. (2015) 48:258–64. doi: 10.1016/j.bbi.2015.04.003
117. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. (2007) 61:355–61. doi: 10.1038/sj.ejcn.1602546
118. Kazim SF, Shamim MS, Tahir MZ, Enam SA, Waheed S. Management of penetrating brain injury. J Emerg Trauma Shock. (2011) 4:395–402. doi: 10.4103/0974-2700.83871
119. Carney N, Totten AM, O'reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
120. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-Antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. (2018) 174:1406–23 e1416. doi: 10.1016/j.cell.2018.08.047
Keywords: traumatic brain injury, therapy, gut microbiome, microbiota-gut-brain axis, gut dysbiosis
Citation: Rice MW, Pandya JD and Shear DA (2019) Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Front. Neurol. 10:875. doi: 10.3389/fneur.2019.00875
Received: 05 July 2018; Accepted: 29 July 2019;
Published: 16 August 2019.
Edited by:
Firas H. Kobeissy, University of Florida, United StatesReviewed by:
Sonia Villapol, Houston Methodist Research Institute, United StatesCopyright © 2019 Rice, Pandya and Shear. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew W. Rice, bWF0dC53LnJpY2VAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.