
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 30 July 2019
Sec. Movement Disorders
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00794
This article is part of the Research Topic Patient Empowerment and Person-centered Care in Movement Disorders View all 7 articles
 Floris Pieter Vlaanderen1*
Floris Pieter Vlaanderen1* Yvonne de Man1
Yvonne de Man1 Jesse H. Krijthe2
Jesse H. Krijthe2 Marit A. C. Tanke1
Marit A. C. Tanke1 A. S. Groenewoud1
A. S. Groenewoud1 Patrick P. T. Jeurissen1
Patrick P. T. Jeurissen1 Sabine Oertelt-Prigione3
Sabine Oertelt-Prigione3 Marten Munneke4
Marten Munneke4 Bastiaan R. Bloem4
Bastiaan R. Bloem4 Marjan J. Meinders1
Marjan J. Meinders1Objective: To reconstruct a sex-specific patient journey for Dutch persons with Parkinson's disease (PD) during the first 5 years after diagnosis.
Method: We analyzed a national administrative medical claims database containing data of all patients newly diagnosed with PD between 2012 and 2016 in the Netherlands. We performed time-to-event analysis to identify the moments when patients received care from neurologists, allied healthcare therapists or general practitioners. We also extracted relevant clinical milestones: unexpected hospitalization for PD, pneumonia, orthopedic injuries, nursing home admission, and death. Using these data, we constructed the patient journey stratified for sex.
Results: We included claims data of 13,518 men and 8,775 women with newly diagnosed PD in the Netherlands. While we found little difference in neurologist consultations, women visited general practitioners and physiotherapists significantly earlier and more often (all p-values < 0.001). After 5 years, 37.9% (n = 3,326) of women had visited an occupational therapist and 18.5% (n = 1,623) a speech and language therapist at least once. This was 33.1% (n = 4,474) and 23.7% (n = 3,204) for men. Approximately 2 years after diagnosis, PD-related complications (pneumonia, orthopedic injuries, and PD-related hospitalization) occurred for the first time (women: 1.8 years; men: 2.3 years), and after 5 years, 72.9% (n = 6,397) of women, and 68.7% (n = 9,287) of men had experienced at least one.
Discussion: Considering the strengths and limitations of our methods, our findings suggest that women experience complications and access most healthcare services sooner after diagnosis and more frequently than men. The identified sex differences extend the debate about phenotypical differences in PD between men and women.
During the course of the disease, a patient with Parkinson's disease (PD) visits many different healthcare providers from different disciplines (1). This “journey through the healthcare system” varies per individual because of heterogeneity of symptoms, differences in disease progression rate, and the occurrence of PD-related complications. One important source of this variation might be sex differences in the presentation of PD (2). For example, numerous studies confirm that the incidence, and prevalence of PD is higher in men (2–6), that the disease starts at an earlier age in men (2, 7) and that the disease progresses faster in men (7, 8). In women, PD tends to be more often tremor-dominant (2, 7, 9), while in men it is more often the akinetic-rigid type (2, 10, 11).
We do not know if these sex differences translate to different patient journeys between men and women with PD. But when striving for optimal patient-centered and integrated care, it is vital to understand what the patient journeys look like. As shown for other diseases (12–15) such insights can be used to improve access and optimize coordination of care. In this paper, we use medical claims data to reconstruct the sex-specific journey for Dutch PD patients during the first 5 years after diagnosis.
In the Netherlands, the patient's journey starts when a general practitioner makes a referral to a neurologist when symptoms of Parkinson's appear. Neurologists, all located in hospitals, make the diagnosis. Thereafter, a PD patient visits the hospital every 3 months, to see their neurologist, who is supported by nurses or nurse specialists. The nurse and neurologist work in close collaboration with allied healthcare professionals in the community, including, e.g., physiotherapists, occupational therapists and speech and language therapists. Hospital care is covered by the compulsory health insurance, whereas allied healthcare services are covered by additional insurance package, which is not compulsory but taken up by over 80% of the Dutch people. In addition, the Netherlands stands out with comparatively low out-of-pocket payments (16). This probably reduces any possible selection bias due to differences in price responsiveness among PD patients. In the analysis, we therefore focus on the most frequently involved healthcare disciplines (neurologists, allied healthcare therapists, and general practitioners) and on recognized clinical milestones (PD-related complications, nursing home admission, and death).
To reconstruct the PD patient journey, we used medical claims data of all PD patients diagnosed between 2012 and 2016 in the Netherlands. The dataset was made available through Vektis, a not-for-profit organization that combines claims data of all Dutch healthcare insurance companies (17). Since all Dutch citizens are obliged by law to have a healthcare insurance, the Vektis database contains the claims data of ~99.8% of the Dutch population (18) [17.3 million people (19)]. The claims database contains data on primary care, emergency care and hospital care, plus nursing home residency. The dataset was anonymized by Vektis, making available only the sex, year of birth, and a unique random identifier for each individual. The key to the identifiers was not available to the researchers.
Similar to a recent paper using similar Dutch claims data in PD (20), we included only patients who had at least one diagnosis-related group code (DRG code) for PD. In the Netherlands, PD can only be diagnosed by a neurologist. We therefore regarded the first PD-related neurology DRG as the moment of diagnosis and, as such, as the starting point of the journey.
To reconstruct the patient journey, we included the professionals most frequently involved in the treatment of PD. These are neurologists (together with specialized PD nurses, since both claim their activities under the DRG code of the hospital), physiotherapists, occupational therapists, speech and language therapists, and general practitioners. For every included claim, we calculated how many days after the first diagnosis the activity had occurred. Next, we selected the 1st, 10, 20, and 30th visit to the general practitioner and the allied healthcare therapists. Unlike these disciplines, claims related to hospital care are defined in a DRG model, rather than by a pay-per-visit model. The first PD-related DRG includes at least one visit to a neurologist, but the actual number of visits may be higher. A subsequent PD-DRG can only start 90 days after the initial PD-DRG, and contains at least one visit to a neurologist or specialized PD nurse. Third and subsequent PD-DRGs can only start 365 days after the previous one. Consequently, the maximum number of PD-DRGs within the first 5 years after diagnosis is six. We therefore selected the first six PD-DRGs to assess utilization of neurologist. In a similar way, we identified the time after diagnosis until five clinical milestones in the patient's journey had been reached, using a methodology previously used in a comparable analysis: (21) nursing home admission, hospitalization for three PD-related complications (unexpected hospitalization for PD, pneumonia, orthopedic injuries) (20, 22) and, finally, death.
We used event history analysis with Kaplan-Meyer estimators to determine after how many days the average patient received specific care or reached a clinical milestone. This method deals with differences in length of follow-up between patients. The follow-up length was calculated for every patient as the number of days from diagnosis till death or till December 31st 2016, i.e., the last data point in the dataset. The median values of the time-to-event analysis were plotted on a timeline, representing the journey of the average PD patient. We constructed one timeline for men, and one for women. We chose median values over mean values because of the considerable differences in length of follow-up in our sample. Sex differences were statistically analyzed using log rank tests.
This study was approved by the institutional review board of the Radboud University Medical Center with a waiver of consent for participants in the study.
All data, published or not published within the article, is accessible through Vektis. Analyses were performed in SAS.
We included medical claims data of all 22,293 newly diagnosed patients in the analyses. As shown in Table 1, the population consists mainly of elderly individuals, a minority of whom lives in a nursing home before diagnosis.
Figure 1 shows two timelines representing the sex-specific patient journey of men and women with PD. Table 2 shows the inter quartile ranges (IQRs).
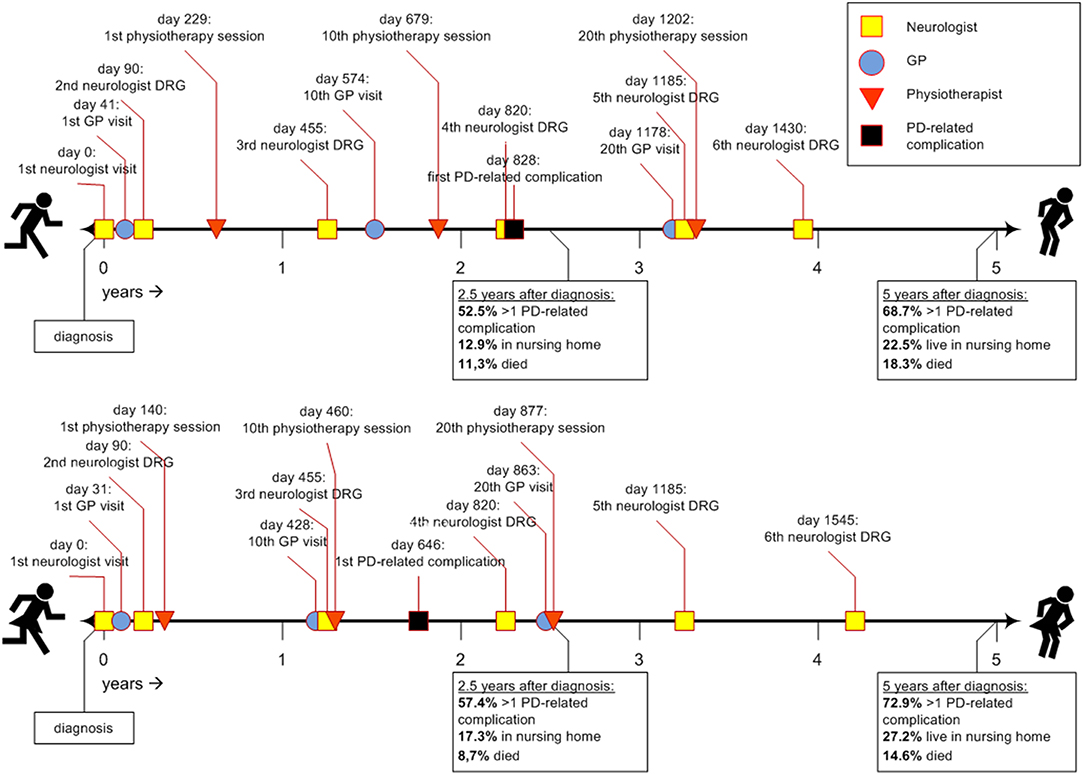
Figure 1. Timeline of the average Parkinson's patient journey during the first 5 years after diagnosis. DRG, diagnosis related group; GP, general practitioner; PD, Parkinson's disease.
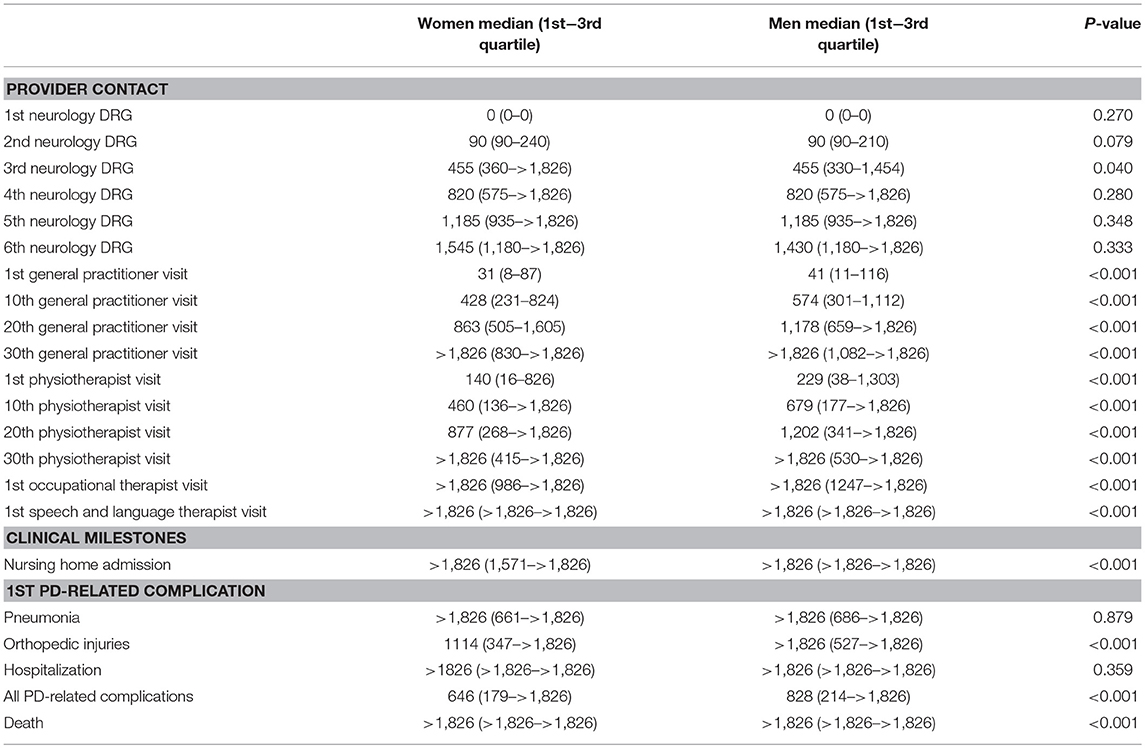
Table 2. Time (in days, since diagnosis) till relevant provider contacts and clinical milestones during the patient journey, by sex.
Approximately 1 month after diagnosis, patients first visited their general practitioner (women after 31 days; men after 41). Thereafter, women saw their general practitioner approximately once every 6 weeks (43 days). Men saw their general practitioner less often: approximately once every 8 to 9 weeks (59 days). For both sexes the frequency declined after the 20th visit (median >5 years for 30th visit). In all analyses, women visited their general practitioner significantly earlier than men (p-values < 0.001).
Three months after diagnosis, many patients saw their neurologist or specialized PD nurse again (both for men and women median = 90 days). However, a substantial part of the population also visited their neurologist or specialized PD nurse much later for the second time, i.e., not before 8 to 9 months after diagnosis [75th quartile value = 210 days (men) and 240 days (women)]. After the second visit, the frequency of claimed neurology DRGs was about once a year for both sexes, i.e., they visited a neurologist or specialized PD nurse at least once a year. Except for the third visit, where men used neurologist services slightly earlier than women, no significant sex differences were found.
Women with PD started their physiotherapy treatment ~5 months after diagnosis (median = 140 days). Their first 20 physiotherapy sessions took place about once every 5 to 6 weeks. Men started to visit a physiotherapist later after the diagnosis then women: 8 months after diagnosis (median = 229 days), and with a lower frequency: once every seven to 8 weeks. For both sexes the frequency declined after the 20th session (median >5 years for 30th physiotherapy sessions). In all analyses, women used physiotherapist services significantly earlier and within a shorter timespan than men (p-values < 0.001).
For occupational therapists and speech and language therapists, the median values for the first visits were not reached within the follow-up time of 5 years. Therefore, they are not displayed in Figure 1. After 5 years, 37.9% of the women (n = 3,326) and 33.1% of the men (n = 4,474) had visited an occupational therapist at least once. This was 18.5% for women (n = 1,623) and 23.7% for men (n = 3,204) for the first visit to speech and language therapist. These differences were statistically significant (p-values < 0.001).
Approximately 2 years after diagnosis, the first PD-related complication occurred. For women the median value was 1.8 years (IQR = 0.5–>5 years); for men this was 2.3 years (IQR = 0.6–>5 years; p-value < 0.001). We added the Kaplan-Meyer curve of this analysis in Figure 2. As shown in Table 2, orthopedic injuries were the most common complication, and occurred earlier in the course of the disease in women (p-value < 0.001). Five years after diagnosis, the percentage of patients that had experienced at least one PD-related complication was 72.9% in women (n = 6,397) and 68.7% in men (n = 9,287). The percentage of women admitted to a nursing home rose from 8.3% (n = 728) before diagnosis to 27.5% (n = 2,413) after 5 years of PD. In men this increase is from 5.0% (n = 676) to 22.5% (n = 3,042; p < 0.001). During the first 5 years after diagnosis, significantly fewer women died (14.6%) than men (18.3%, p-value = < 0.001).
The reconstruction of the Parkinson patient's journey through the Dutch healthcare sector during the first 5 years after diagnosis, reveals quantitative information about healthcare utilization and the occurrence of clinical milestones over time. It also reveals sex differences: in the Netherlands, women visit most of the included healthcare professionals sooner after diagnosis and more frequently. In addition, PD-related complications occur earlier in women than in men. A sizeable subgroup of patients is admitted to nursing homes within 5 years after diagnosis. Again, this happens more frequently in women. Finally, 14.6% of the women and 18.3% of the men died within 5 years after the diagnosis.
Our findings confirm and extend earlier work, from both inside and outside the Netherlands. The characteristics of our population are comparable to earlier work when it comes to general incidence (23) and the male predomination of the disease (2–6). Comparable values for age at diagnosis and percentage of early-onset PD were also found before (24). However, while most studies found a faster disease progression in men (7, 8), our findings suggest a more rapid disease progression in women, since they visited their healthcare professionals sooner and experienced orthopedic injuries earlier and more often after diagnosis. However, there might be other explanations for this observation. First, the included women were living relatively more often in a nursing home before diagnosis, indicating that they were probably in a worse physical condition at the outset. This might make them more prone to develop complications and also explain the more intense healthcare utilization. Patients living in nursing homes might also have easier access to the in-house allied health therapists. Second, the findings can indicate a doctor or patient delay in the diagnostic process in women, meaning that women receive the diagnosis relatively late in the course of the disease. This has been found earlier (25). Alternatively, women might find their way to healthcare professionals more effectively (or faster). This has been observed for other diseases (26, 27) but not previously for PD.
What surprised us was the high mortality rate and that patients experienced their first PD-related complication already 2 years after the diagnosis. No PD-specific literature is available to compare these results with. The average age at onset of 72 years is in line with other population-based cohort studies (28–30). The finding that 5.08.3% of the patients are living in a nursing home at diagnosis, which are relatively high, can be understood when considering that the Netherlands has relatively one of the largest long term care sectors in the world (31). Given that patients living in a nursing home are likely more vulnerable, this might explain the mortality and complication rates.
Our methods have strengths and limitations. An important strength is that our dataset contained all newly diagnosed patients in the country over a period of 5 consecutive years. This reduces a potential selection bias. However, some selection may have resulted from our inclusion criteria. Since we included all patients with a first PD DRG, there might be some cases where the initial diagnosis of PD was wrong. PD is hard to diagnose, with reported diagnostic error rates of >10% (32). Therefore, we cannot exclude that some patients had other conditions, in particular one of the forms of atypical parkinsonism (23). Our sample most likely included people who were incorrectly diagnosed. A review of the literature, including 11 studies, concluded that the validity of clinical diagnosis of PD is not satisfying, which was the case for both non-experts and movement disorder specialists (33). We were not able to correct for this error in our analysis. However, since our population characteristics matched well with previous reports, we do not think that all these factors affected our data on a large scale. Moreover, it is unlikely that this diagnostic error affected men and women differentially. Another strength is that our study is based on highly standardized claims data. And we only used items that, although self-reported by healthcare professionals, are known to be reliably completed (34).
As claims data don't include detailed information about the clinical status of the patients, we were not able to correct for factors that confound and/or modify the relationship between sex and complications (35). This holds particularly true for co-morbidity and PD-related complications that are more frequently associated to female sex (e.g., dyskinesia, motor, and non-motor complications) (11). Also, the sex-difference in the occurrence of orthopedic injuries might be explained by the female predominance in osteoporosis. Therefore, our findings require confirmation in other, independent datasets.
Finally, our findings might be difficult to extrapolate to other countries with another organization of the healthcare system. For example, duration of visits and treatment intensity can differ between countries. Moreover, the presence of ParkinsonNet in the Netherlands contributed to the quality and role of allied health care professionals, and stimulated access to specialized and multidisciplinary Parkinson care (36).
Comparable work on other diseases suggests that our reconstruction of the patient journey may lead to better patient-centered care delivery. Specifically, it provides healthcare professionals an overview of where and when particular physicians get involved, which might reveal errors in providers' perspectives (37). It might act as a useful tool to gain insight in patient experiences (12, 37), to reveal barriers to access (13, 38), to detect gaps in care delivery (39, 40), and to improve coordination and quality of care (38, 41). The identified sex differences might contribute to the debate about differences in PD between men and women, extending earlier work on different phenotypes to now include contrasts in healthcare utilization as well. We hope these insights can lead to better and more personalized care for PD patients of both sexes.
The datasets generated for this study are available on request to the corresponding author.
All authors contributed to the development of research questions and the selection and adaptation of the methods. FV, YM, and JK performed the analyses at Vektis. MT, AG, PJ, SO-P, MMu, BB, and MJM contributed in giving feedback during the writing process.
The Netherlands Organization for Health Research and Development (ZonMw, project number 91215076) and the Celsus Academy for sustainable Healthcare provided financial support for this study.
BB currently serves as Associate Editor for the Journal of Parkinson's disease, serves on the editorial of Practical Neurology and Digital Biomarkers, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen, UCB and Walk with Path, has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Abbvie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson's Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, and the Parkinson Vereniging.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Radder DL, de Vries NM, Riksen NP, Dimond SJ, Gros D, Gold DR, et al. Multidisciplinary care for people with Parkinson's disease: the new kids on the block! Expert Rev Neurotherapeutics. (2018) 19:145–57. doi: 10.1080/14737175.2019.1561285
2. Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM. Gender differences in Parkinson's disease: a clinical perspective. Acta Neurol Scand. (2017) 136:570–84. doi: 10.1111/ane.12796
3. Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. Parkinson's disease and Parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA working group. Italian Longitud Study Aging Neurol. (2000) 55:1358–63. doi: 10.1212/WNL.55.9.1358
4. Alves G, Müller B, Herlofson K, HogenEsch I, Telstad W, Aarsland D, et al. Incidence of Parkinson's disease in Norway: the norwegian parkwest study. J Neurol Neurosurg Psychiatry. (2009) 80:851–7. doi: 10.1136/jnnp.2008.168211
5. Kovács M, Makkos A, Aschermann Z, Janszky J, Komoly S, Weintraut R, et al. Impact of sex on the nonmotor symptoms and the health-related quality of life in Parkinson's disease. Parkinsons Dis. (2016) 2016:7951840. doi: 10.1155/2016/7951840
6. Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis. (2018) 4:21. doi: 10.1038/s41531-018-0058-0
7. Haaxma CA, Bloem BR, Borm GF, Oyen WJG, Lender KL, Eshius S, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2007) 78:819–24. doi: 10.1136/jnnp.2006.103788
8. Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand. (2000) 102:37–43. doi: 10.1034/j.1600-0404.2000.102001037.x
9. Solla P, Cannas A, Ibba FC, Loi F, Corona M, Orofino G, et al. Gender differences in motor and non-motor symptoms among sardinian patients with Parkinson's disease. J Neurol Sci. (2012) 323:33–9. doi: 10.1016/j.jns.2012.07.026
10. Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol. (2005) 252:1201–5. doi: 10.1007/s00415-005-0835-7
11. Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: a review. J Neurol. (2017) 264:1583–607. doi: 10.1007/s00415-016-8384-9
12. Kuo S, Huang KE, Davis SA, Feldman SR. The rosacea patient journey: a novel approach to conceptualizing patient experiences. Cutis. (2015) 95:37–43.
13. Mehta A, Belmatoug N, Bembi B, Deegan P, Elstein D, Göker-Alpan Ö, et al. Exploring the patient journey to diagnosis of Gaucher disease from the perspective of 212 patients with Gaucher disease and 16 Gaucher expert physicians. Mol Genet Metab. (2017) 122:122–9. doi: 10.1016/j.ymgme.2017.08.002
14. Hibbard JH, Mahoney E, Sonet E. Does patient activation level affect the cancer patient journey? Patient Edu Counsel. (2017) 100:1276–9. doi: 10.1016/j.pec.2017.03.019
15. Laveau F, Hammoudi N, Berthelot E, Belmin J, Assayag P, Cohen A, et al. Patient journey in decompensated heart failure: an analysis in departments of cardiology and geriatrics in the greater Paris university hospitals. Arch Cardiovas Dis. (2017) 110:42–50. doi: 10.1016/j.acvd.2016.05.009
16. Maarse H, Jeurissen P, Ruwaard D. Results of the market-oriented reform in the Netherlands: a review. Health Econ Policy Law. (2016) 11:161–78. doi: 10.1017/S1744133115000353
17. Vektis. Over Vektis. (2019). Available online at: https://www.vektis.nl/over-vektis (accessed January 10, 2019).
18. Saigusa R, Asano Y, Sumida H, Sato S. Zorgwijzer. Cijfers en Feiten over de Zorgverzekering. Barendrecht: Zorgwijzer (2019).
19. CBS. Bevolkingsteller. (2019). Available online at: https://www.cbs.nl/nl-nl/visualisaties/bevolkingsteller (accessed January 10, 2019).
20. Ypinga JHL, de Vries NM, Boonen LHHM, Koolman X, Munneke M, Zwinderman AH, et al. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. (2018) 17:153–61. doi: 10.1016/S1474-4422(17)30406-4
21. Bjornestad A, Pedersen KF, Tysnes OB, Alves G. Clinical milestones in Parkinson's disease: a 7-year population-based incident cohort study. Parkinsonism Relat Disord. (2017) 42:28–33. doi: 10.1016/j.parkreldis.2017.05.025
22. Muzerengi S, Herd C, Rick C, Clarke CE. A systematic review of interventions to reduce hospitalisation in Parkinson's disease. Parkinsonism Relat Disord. (2016) 24:3–7. doi: 10.1016/j.parkreldis.2016.01.011
24. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. (2003) 157:1015–22. doi: 10.1093/aje/kwg068
25. Saunders-Pullman R, Wang C, Stanley K, Bressman SB. Diagnosis and referral delay in women with Parkinson's disease. Gender Med. (2011) 8:209–17. doi: 10.1016/j.genm.2011.05.002
26. Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. (2003) 58:5–14. doi: 10.1037/0003-066X.58.1.5
27. Cohen D. Men need primary care at work, debate hears. BMJ. (2009) 338:b2471. doi: 10.1136/bmj.b2471
28. Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson's disease. Brain J Neurol. (2017) 140:429–41. doi: 10.1093/brain/aww291
29. Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time trends in the incidence of parkinson disease. JAMA Neurol. (2016) 73:981–9. doi: 10.1001/jamaneurol.2016.0947
30. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. (2006) 5:525–35. doi: 10.1016/S1474-4422(06)70471-9
31. Mosca I, van der Wees PJ, Mot ES, Wammes JJG, Jeurissen PPT. Sustainability of long-term care: puzzling tasks ahead for policy-makers. Int J Health Policy Manage. (2016) 6:195–205. doi: 10.15171/ijhpm.2016.109
32. Skinner TR, Scott IA, Martin JH. Diagnostic errors in older patients: a systematic review of incidence and potential causes in seven prevalent diseases. Int J General Med. (2016) 9:137–46. doi: 10.2147/IJGM.S96741
33. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. (2016) 86:566–76. doi: 10.1212/WNL.0000000000002350
35. Bloem BR, Ypinga JHL, Willis A, Canning CG, Barker RA, Munneke M, et al. Using medical claims analyses to understand interventions for parkinson patients. J Parkinson's Dis. (2018) 8:45–58. doi: 10.3233/JPD-171277
36. Bloem BR, Rompen L, Vries NM, Klink A, Munneke M, Jeurissen P. ParkinsonNet: a low-cost health care innovation with a systems approach from The Netherlands. Health Affairs. (2017) 36:1987–96. doi: 10.1377/hlthaff.2017.0832
37. Tan Q, Hildon ZJ, Singh S, Jing J, Thein TL, Coker R, et al. Comparing patient and healthcare worker experiences during a dengue outbreak in Singapore: understanding the patient journey and the introduction of a point-of-care test (POCT) toward better care delivery. BMC Infect Dis. (2017) 17:503. doi: 10.1186/s12879-017-2580-9
38. Kelly J, Dwyer J, Mackean T, O Donnell K, Willis E. Coproducing aboriginal patient journey mapping tools for improved quality and coordination of care. Aust J Primary Health. (2017) 23:536–42. doi: 10.1071/PY16069
39. Thrift-Perry M, Cabanes A, Cardoso F, Hunt KM, Cruz TA, Faircloth K. Global analysis of metastatic breast cancer policy gaps and advocacy efforts across the patient journey. Breast. (2018) 41:93–106. doi: 10.1016/j.breast.2018.06.005
40. Roughead EE, Semple SJ, Rosenfeld E. The extent of medication errors and adverse drug reactions throughout the patient journey in acute care in Australia. Int J Evid Based Healthcare. (2016) 14:113–22. doi: 10.1097/XEB.0000000000000075
Keywords: patient journey, Parkinson's disease, sex difference, personalized care, healthcare usage, early Parkinson's disease
Citation: Vlaanderen FP, de Man Y, Krijthe JH, Tanke MAC, Groenewoud AS, Jeurissen PPT, Oertelt-Prigione S, Munneke M, Bloem BR and Meinders MJ (2019) Sex-Specific Patient Journeys in Early Parkinson's Disease in the Netherlands. Front. Neurol. 10:794. doi: 10.3389/fneur.2019.00794
Received: 12 May 2019; Accepted: 10 July 2019;
Published: 30 July 2019.
Edited by:
Mayela Rodríguez-Violante, National Institute of Neurology and Neurosurgery (INNN), MexicoReviewed by:
Marina Picillo, University of Salerno, ItalyCopyright © 2019 Vlaanderen, de Man, Krijthe, Tanke, Groenewoud, Jeurissen, Oertelt-Prigione, Munneke, Bloem and Meinders. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Floris Pieter Vlaanderen, ZmxvcmlzLnZsYWFuZGVyZW5AcmFkYm91ZHVtYy5ubA==; Zi5wLnZsYWFuZGVyZW5AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.