- 1Department of Neuroscience, Headache Center, Bambino Gesù Children Hospital, Rome, Italy
- 2Child Neurology Unit, Systems Medicine Department, Tor Vergata University Hospital of Rome, Rome, Italy
- 3Center for Sensory-Motor Interaction, Aalborg University, Aalborg, Denmark
Migraine is a frequent and very disabling disease, especially at pediatric age. Despite this, there are few controlled data on the prophylactic treatment of primary headaches in this category of age. Given that the recently introduced calcitonin gene-related peptide (CGRP) inhibitors (CGRP-r) are still limited to adulthood, there is no drug with exclusive indication for migraine treatment in pediatric age. This raises several limitations in terms of adherence and effectiveness of the therapy. Moreover, the scenario is complicated by placebo response, which is larger in children and adolescents than in adults and often leads to an improvement in the attack frequency even in absence of any active pharmacological treatment. Our aim was to investigate the real evidence concerning the prophylactic therapy of pediatric migraine by reviewing the clinical studies published between 2010 and 2019.
Introduction
According to epidemiological studies, the prevalence of headache in children varies from 5.9 to 82% (1). Migraine, the most common type of primary headache in children, is highly disabling even in childhood and adolescence. The average prevalence of pediatric migraine varies according to age, going from 3% in younger children to ~20% in adolescents (2). A noticeable social problem is represented by chronic migraine (more than 15 days with headache a month) that afflicts from 0.6 to 1.8% of children and adolescents (3).
The main reference for the diagnosis of primary headaches are the criteria of the International Headache Society (IHS) (4). These criteria have shown limitations when applied in the pediatric age (1, 5), although the last version (ICHD 3) considers some peculiarities of migraine in pediatric age, such as the shorted duration of pain and the unilateral/bilateral location of pain (1, 5).
Regarding therapies of pediatric migraine, there is a significant lack of clinical studies on acute and prophylactic therapy. This is partly due to differences between countries, where therapeutic approaches are based on cultural and political factors. Few clinical trials are available in pediatric patients and they often show conflicting findings. The paucity of data on the effectiveness of treatments in young migraineurs is also due to the power of placebo effect, in terms of reduction of both frequency and intensity of migraine attacks (6). Though representing a precious resource, the placebo effect can paradoxically represent an obstacle in controlled trials comparing the efficacy of pharmacological and non-pharmacological treatments with placebo.
Migraine prophylaxis aims at reducing the impact of migraine by improving the frequency and intensity of attacks. In children and adolescents, it should be considered when the frequency of attacks is higher than 4 attacks per month or the response to the symptomatic treatment is not satisfactory. In a previous retrospective review, Papetti et al. (7) emphasized the lack of definitive data on the possible drugs to be used.
Here, our aim is to investigate the actual evidence concerning prophylactic therapy of pediatric migraine by reviewing clinical studies published between 2010 and 2019.
Methods
Literature Search Strategy
We considered studies published from January 2010 to January 2019. Medline and Cochrane library were used for the research. Search words were: “migraine and treatment or therapy,” “migraine and prophylaxis,” and “migraine and guidelines.” The filters included clinical trials (CT), randomized control trials (RCTs), open label studies (OL), retrospective studies (RS), meta-analysis, multicenter studies, reviews and articles that were either published in the last 10 years. Our search was focused on the age group ranging from 0 to 18 years, although any article that included adult population but contained patients under the age of 18 years was also considered. Two authors (F.U. and L.P.) independently checked the studies identified by the literature search. All potentially relevant studies were reviewed by the two authors.
Search Results
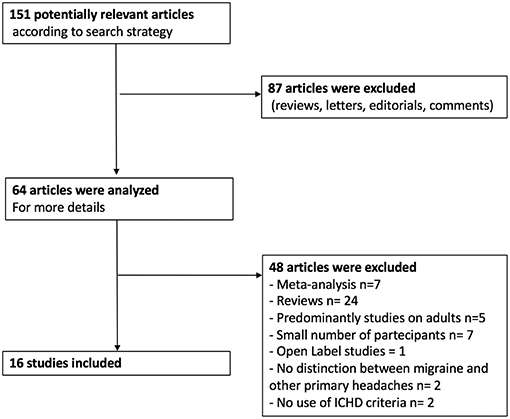
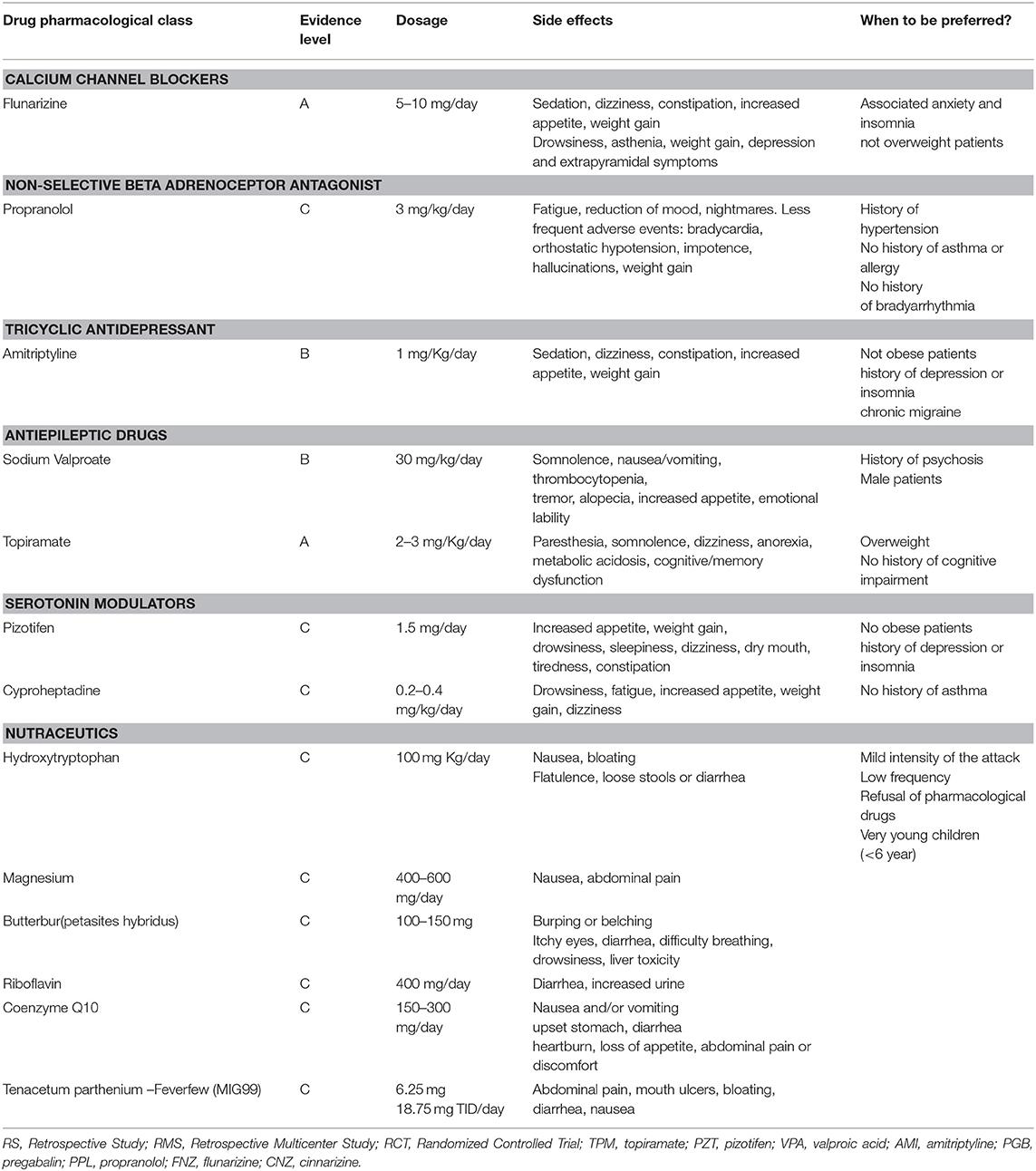
Using the above described strategy, 64 articles concerning preventive treatment of migraine in children were included in our study. Among them, there were 40 systematic reviews or meta-analysis of the literature concerning the prophylactic treatment of pediatric migraine, 21 clinical trials (CTs), and 3 retrospective studies (RSs). As for the CTs, 15 were randomized control trials (RCTs) and one was an open label study (OL) (Figure 1). All the included studies were published from 2010 to the present. Results of current evidence are resumed in Table 1.
Pharmacological Treatment
Calcium Channel Blockers
Flunarizine is a calcium channel blocker with properties on the cerebrovascular circulation. How flunarizine acts in preventing migraine is not yet established but it probably has both vascular and neuronal effects (8).
In an RS (2012), Basheer Peer et al. demonstrated that flunarizine (2.5–10 mg/day) shows good efficacy in children and adolescents (median age 13 years), leading to at least a 50% reduction in attack frequency in 57% of patients (41/72). Interestingly, the response rate was particularly high in patients with hemiplegic migraine (85%). The study also showed that flunarizine was well-tolerated with a reasonable safety profile. Side effects were observed in 21% of children and adolescents and included depression, weight gain and sedation (9). In a retrospective study of 475 patients, Kim et al. (10) showed that the efficacy and tolerability of flunarizine 5 mg/day were comparable to those of topiramate. The responder rate (50% reduction in headache days/month) was 80% (89/111 patients) for flunarizine (5 or 10 mg /day) and 81% (122/150 patients) for topiramate (from 25 to 100 mg/day). The frequency of adverse effects was higher in topiramate (10%) than flunarizine (6%) (10). In 2014, Topcu et al. used the PedMIDAS (the score of disability assessment in pediatric migraine) to evaluate the efficacy of different prophylactic therapies in 53 patients, recruited from a series of 88 patients suffering from migraines with an age ranging from 6 to 17 years. They found that topiramate (1–2 mg/Kg/day), propranolol (20–40 mg/day), and flunarizine (5–10 mg/day) significantly decreased PedMIDAS score. The number of days with analgesic treatment significantly decreased in the patients treated with topiramate and propranolol (p < 0.05), while it remained unchanged in the flunarizine (p > 0.05) (11). More recently, Toldo et al. (12) conducted a retrospective multicenter study among 706 patients with primary headaches. Preventive drugs were used in 19% of migraineurs and in 3% of patients with tension-type headache (12). In patients with migraine, the most used drug was flunarizine (18%), followed by antiepileptic drugs (7%) and pizotifen (6%). Flunarizine and pizotifen were the most effective drugs (72 and 82%, respectively) (12).
Flunarizine is licensed in Italy for patients over 18 years (7) and widely prescribed in Europe, while it is not licensed in the UK or the USA given the lack of published data in the development age. Placebo-controlled clinical trials in pediatric age are needed to confirm its effectiveness in pediatric migraine (13).
Beta-Blockers
Propranolol is a non-selective beta (b) adrenoceptor antagonist that blocks the b1,2 receptors. Propranolol started to be used in the prophylaxis of migraine for more than 50 years (14). Propranolol showed efficacy and high profile of tolerability in several clinical trials on adult migraine (4). On the contrary, there are only a few studies supporting the efficacy of propranolol in pediatric age (15–17).
In 2010, Bidabadi et al. compared the efficacy and safety of propranolol (started at a dosage of 3 mg/kg/day) and valproate (30 mg/Kg/day) for migraine prophylaxis in childhood. In this study, 60 patients were enrolled (30 in the group A that received propranolol 3 mg/kg/day and 30 in the group B treated by sodium valproate 30 mg/kg/day). The mean age of the patients was 9.85 ± 2.63 years. Headache frequency was significantly reduced by more than 50% in 83% of patients treated with propranolol and in 63% of patients treated with sodium valproate without significant differences between the drugs. Furthermore, no significant difference in side effects between the two groups was found (18). Eidlitz-Markus et al. (19) compared the efficacy of a low dose of propranolol (the initial dose was 0.47 ± 0.17 mg/kg/day) with a low dose of amitriptyline (mean initial dose, 0.26 ± 0.1 mg/kg/day) in children and adolescent suffering from severe migraine. Although the study was not blinded and placebo controlled, it included a large number of patients (118 with a mean age of 12.54 ± 3.14 years). Both propranolol and amitriptyline, when combined with non-pharmacologic treatments, showed efficacy in reducing the frequency of migraine attacks in children (reduction of attack frequency >50% per month in 80% of patients). Propranolol group showed less frequent side effects (19). In 2012, Fallah et al. compared efficacy and safety of propranolol (1 mg/kg/day) and topiramate (3 mg/kg/day) in a parallel single-blinded randomized clinical trial. Authors enrolled 100 patients that were divided in two groups (50 patients treated with propranolol and 50 patients treated with topiramate). After 3 months of treatment, 62% of patients treated with propranolol and 82% of patients treated with topiramate showed more than a 50% reduction in monthly headache frequency (p < 0.05). No serious adverse events were seen in both groups and, in particular, the main side effects after treatment with propranolol were mild hypotension and drowsiness (20). In a RCT (2013), Bakhshandeh Bali et al. compared effectiveness, safety and tolerability of propranolol (10 to 20 mg/day divided in two doses; group b) and pregabalin (50 to 75 mg/day; group a). After 4 and 8 weeks of pregabalin administration, headache frequency was reduced by 81.8 and 85.45%, respectively. Using the same treatment intervals, propranolol reduced monthly headache frequency by 64.54 and 68.25%, respectively. The difference between drugs was statistically significant (p = 0.04) (21).
Recent data showed that beta-blockers are rarely used in Italy, probably because their tolerability profile is not excellent and they are licensed over 18 years (4).
Tricyclic Antidepressant
Amitriptyline is one of the most used drugs for preventive treatment of pediatric migraine (22). It is also recommended in cases of tension-type headache associated with anxiety, insomnia and depression (22). Efficacy of amitriptyline prophylaxis is achieved with much lower doses than those required for anti-depressive therapy (10–20 mg/day up to 25–75 mg/day) (7). It is advisable to use increasing doses before reaching the maintenance dose in order to reduce the side effects and improve tolerability. Contraindications are cardiac, hepatic, renal, prostatic and thyroid diseases; glaucoma, hypotension, epilepsy, use of anti-MAO. Amitriptyline also should be used with caution for its anticholinergic effects. The most frequent adverse events are dry mouth, constipation, sedation, and increase in appetite, increased weight, occasionally orthostatic hypotension and cardiotoxicity (22).
As reported above, it was shown that low-dose propranolol and low-dose amitriptyline, if combined with non-pharmacological measures, were both effective in reducing migraine attacks frequency (19). Between July 2012 and November 2014, Hershey et al. conducted a double-blinded, placebo-controlled study with the aim to determine the most effective prophylactic treatment in children and adolescents (CHAMP study). Authors compared the efficacy of amitriptyline, topiramate, and placebo in 361 subjects (from 8 to 17 years of age). In a period of 6 months, 52% of patients receiving amitriptyline (dose 1 mg/kg per day), 55% of patients receiving topiramate (dose 2 mg/kg per day), and 61% of patients receiving placebo had a reduction in headache days of at least 50%, without any significant difference between groups. Furthermore, the patients treated with amitriptyline or topiramate presented higher rates of adverse events compared to placebo control group (23). In conclusion, considering the negative outcome of this study in terms of efficacy and the increased risk of undesirable effects from amitriptyline or topiramate in this sensitive category of patients, the benefit / risk ratio of these drugs is considered unfavorable. In an Iranian parallel, single-blinded randomized clinical trial, the efficacy of amitriptyline (1 mg/kg/day) was compared to melatonin (0.3 mg/kg/day) in a population of migraineurs ranging from 5 to 15 years. A reduction of more than 50% in monthly headache frequency was seen in 82.5 and 62%.5 of patients treated with amitriptyline and melatonin, respectively. Amitriptyline was significantly more effective (P = 0.04) (24). Amitriptyline showed a good efficacy for treatment of chronic headaches in association with cognitive behavioral therapy (25–28).
Antiepileptic Drugs
Sodium Valproate (500–1,500 mg/day) and topiramate (50–100 mg/day) were evaluated for prophylactic therapy of pediatric migraine in some controlled studies (7).
In the last 8 years, one RCT compared the efficacy of valproate and propranolol for the preventive treatment of migraine in the pediatric age. Sixty children (aged 5–15 years) with migraine without aura were included. Patients received propranolol (3 mg/kg/day) or sodium valproate (30 mg/kg/day) for at least 6 months. The main endpoint (reduction of more than 50% in monthly headache frequency) was observed in 83% of the propranolol group and in 63% of sodium valproate group without statistical significance. The global reduction of baseline headache frequency was better in the group of propranolol (p < 0.05) (18).
Topiramate is a first-line strategy for the treatment of migraine in adults. In 2014, the U.S. Food and Drug Administration (FDA) approved topiramate for migraine treatment in the pediatric patients aged 12 to 17 years (29). In adults, topiramate proved efficacious in the preventive treatment of migraine with and without aura in episodic and chronic form, and excessive use of symptomatic drugs (24, 30). In a parallel single-blinded randomized clinical pediatric trial, the efficacy and safety of topiramate (3 mg/Kg/day) and propranolol (1 mg/Kg/day) were compared, and the results showed that topiramate was more effective in reducing the monthly frequency, severity, duration and disability of the headache. Topiramate was superior to propranolol in reducing the frequency of the attacks by at least 50% (respectively 82 vs. 62% of patients) (31). In another study by the same authors, recruiting a population of 100 pediatric patients (mean age of 10.46 ± 2.11 years) treated with topiramate (3 mg/kg/day), the frequency and duration of headache attacks reduced from 15.34 ± 7.28 to 6.07 ± 3.16 attacks and from 2.28 ± 1.55 to 0.94 ± 0.35 h, respectively. The pediatric migraine disability assessment score was reduced from 32.4 ± 9.3 to 15.5 ± 6. Side-effects were seen in 21% of the patients, including hyperthermia, anorexia and weight loss, and drowsiness (32). Authors concluded that topiramate could be considered a safe and effective drug for migraine therapy in pediatric patients (32). As reported above, Kim et al. showed that the response rate, retention rate and the rate of side effects were not significantly different between flunarizine and topiramate (10). In a randomized, double-blind clinical study of 44 migraineurs (aged 4–15 years), Ashrafi et al. compared the efficacy and safety of cinnarizine and topiramate in the prevention of pediatric migraine. The primary endpoint was the monthly frequency of migraine. Measures of secondary efficacy were the intensity of monthly migraine and a response rate higher than 50%. During the double-blind phase of the study (week 8), both patients treated with cinnarizine and topiramate showed a statistically significant 50% responder rate (cinnarizine: 55%, p = 0.004; topiramate: 50%, p = 0.001). Also monthly migraine intensity reduced in both groups (p < 0.001) (33). After 12 weeks of treatment, a significant reduction of monthly migraine frequency was observed for both cinnarizine and topiramate (p < 0.05) with no significant differences between groups (33).
The CHAMP study failed in showing any superiority of treatment with amitriptyline or topiramate, as compared to placebo (23).
Verapamil, levetiracetam and zonisamide have also been studied for treatment of migraine, but there is a lack of evidence supporting their use in the pediatric population (34).
Serotonin Modulators
Pizotifen was studied in a placebo controlled trial conducted on 37 subjects (6–15 years), at a dosage of 1.5 mg/day, with a significant reduction in attack frequency and mild side effects (35). In a subsequent controlled study, the dose of 1–1.5 mg, administered for 6 months in 47 migraine subjects (7–14 years), was not more effective than placebo. Side effects consisted of sedation, increase in appetite and weight (36). In the last decade, no trials have been conducted on pizotifen from which definitive efficacy data can be drawn.
Cyproheptadine was first evaluated in an open study at a dosage of 0.2–0.4 mg/kg/day for 3–6 months, achieving a good improvement (68%) and a remission (21%) of the headache (37). This substance, usually used in younger patients, can have the same side effects as pizotifen, that is drowsiness, weight gain and tenderness. Contraindications consist of asthma, glaucoma and peptic ulcer.
Despite the lack of definitive data, Pizotifen is the only licensed drug in Italy for prophylaxis in migraineurs children (7, 38).
A recent survey on treatments for primary headaches, in 13 specialized juvenile Italian headache centers, reported that pizotifen (1 mg/kg/day) was one of the most efficacious (82% perceived by patients) and tolerated treatments for migraineurs children (12).
Non-pharmacological Approach
Nutraceutics and Herbals
The term Nutraceutical refers to all those compounds that derive from “nutrition” and “pharmaceutical.” It refers to the study of active ingredients of food origin that are supposed to have a beneficial function on human health. More active ingredients can be combined with each other to enhance their effects. The term “herbal” refers to all those compounds, such as plants or derivatives of medicinal plants. In general, nutraceutics are chosen to have fewer side effects and a more “natural” approach to the treatment of the disease. These products are generally marked in the absence of validative studies (efficacy and safety) (39).
Data on the use of nutraceuticals and herbals are available for the following molecules: magnesium, riboflavin, coenzyme Q10, butterbur, feverfew and hydroxytryptophan (40).
The rationale of the use of nutraceutics in the treatment of migraine is based on the involvement of these substances in anti-inflammatory or antioxidant molecular pathways or in the mitochondrial energy activity (39).
Despite the widespread use in clinical practice, there are few RCTs available for these substances. Thus, the level of evidence remains low (level b or c), as well as the recommendation (class III).
The few RCTs on magnesium, riboflavin, feverfew, and hydroxytryptophan are prior to 2010 and have not shown conclusive results (41–43).
A more recent RCT investigated the effect of coenzyme Q10 (100 mg/day) in the prophylaxis of pediatric migraine (44). A significant reduction in migraine frequency (p < 0.001), severity (p < 0.05), and duration (p < 0.05) was equally found in the placebo and CoQ10 groups (44).
Ginkolide B, in combination with other nutraceutics, was studied in pediatric open label studies. It is a platelet-activating factor (PAF) receptor antagonist, and would modulate pro-inflammatory mechanisms (42). One open-label trial verified the efficacy of a complex of ginkgolide B, coenzyme Q10, riboflavin and magnesium (doses not specified) in pediatric patients with migraine. After 3 months of treatment, the number of attacks in a month was significantly lower (45). Another open label study compared the efficacy of a combination of ginkgolide B (80 mg/day), coenzyme Q10 (20 mg/day), riboflavin (1.6 mg/day), and magnesium (300 mg/day) with a complex of L-tryptophan (250 mg/day), 5-hydroxytryptophan (50 mg/day), vitamin PP (9 mg/day), and vitamin B6 (1 mg/day) for a treatment period of 6 months. Both combinations were associated with a significant reduction of frequency of headache attacks with a major effect for the complex including ginkgolide B (39, 40).
Onabotulinumtoxin A
The use of botulinum toxin proved promising in adult patients with migraine, and in particular, its efficacy has been recognized in adults with chronic migraine. However, there are few retrospective data regarding the pediatric experience. This treatment is particularly useful in patients that present side effects of oral drugs or in drug resistant migraine (46). In a retrospective case series study, Ahmed et al evaluated tolerability and efficacy of botulinum toxin type A in the treatment of pediatric chronic headache (47). The study included 10 patients with age ranging from 11 to 17 years who received a standard 100-unit dose of onabotulinumtoxin A. The patients had attempted an average of 8.0 ± 2.40 SD therapies prior to botulinum toxin. A decrease in headache intensity was observed in 40% of patients and 20% noted a decrease in headache frequency with global improvement in quality of life (47). In 2012, Kabbouche et al. reviewed the data of pediatric patients who had received Onabotulinumtoxin A (average dose of 188.5 units±32 with a minimum dose of 75 units and maximum of 200) for chronic migraine in a pediatric headache center from 2004 to 2010. A significant reduction in the frequency of the headache attacks was observed (from 27.4 headache per month±5.2 to 21.3 ± 10.3; p < 0.05), while there was no significant change in the severity of pain (48).
Complementary Therapies
Non-pharmacological treatment for pediatric migraine includes cognitive behavioral therapy, acupuncture, and biofeedback.
As stated above, cognitive behavioral therapy (CBT) proved effective in treating chronic forms of migraine, although the best results were observed when this therapy was combined with pharmacological therapy, in particular amitriptyline (25–28).
A randomized study conducted on 135 patients (mean age 14.4 ± 2) with chronic migraine evaluated the efficacy at 20 weeks of the combined treatment with CBT plus amitriptyline vs. headache education plus amitriptyline. The authors found that 47% of patients in the CBT plus amitriptyline group had less than four headache days per month compared to 20% in the headache education plus amitriptyline group (p < 0.005). At 12 months post treatment, 72% of patients in the CBT plus amitriptyline group had less than four headache days per month compared to 52% in the headache education plus amitriptyline group (p < 0.05) (27).
In a recent RCT, two different training programs [multimodal cognitive-behavioral training (CBT) and applied relaxation (AR)] were compared with an educational intervention (EDU). Sixty-five children and adolescents with at least 2 attacks of headache per month were assigned to one of the three group. The main outcome endpoints included changes in headache frequency, intensity and duration, responder rate (50% reduction of headache frequency), and number of the attacks needed to treat (NNT). All three groups presented a significant reduction in headache frequency and duration, while no significant differences were observed in the intensity of pain. The group of CBT showed the highest responder rates (50% reduction of headache frequency) after 4 weeks of treatment (63 vs. 32% of AR and 19% of EDU). However, at follow-up after six months, no significant differences were found in the NNTs (CBT: 63%, AR: 56%, EDU: 55%). At follow-up assessment, the effects of the headache frequency remained stable in all groups (49).
There is only limited data on the use of acupuncture for the treatment of pediatric migraine. While efficacy of acupuncture in reducing the frequency of the attacks of migraine was shown in earlier studies (50, 51), no further result has been published in the last 10 years.
Although there are no studies in the last decade on the efficacy of biofeedback for the treatment of pediatric migraine, a recent meta-analysis resumes the main findings on this topic (52). It concludes that biofeedback showed efficacy in reducing attack frequency (p < 0.001) and duration (p < 0.001), and intensity of pain (p < 0.001). However, biofeedback demonstrated no adjuvant effect when combined with other behavioral and no more benefits than pharmacological treatment (52). It is worth to be underlined that data on biofeedback comes only from retrospective studies or pilot studies (53–55).
Overall, non-pharmacological treatment for migraine can be a valid alternative for selected patients.
The choice of a non-pharmacological therapy should be reserved for patients who have failed drug therapies or, as a first line treatment, in patients who cannot tolerate the side effects of drugs. However, most published studies on non-pharmacological treatments have been carried out in adults, while definite results in children and adolescents are still lacking. Therefore, further confirmation with rigorous randomized controlled trials is mandatory for the majority of these approaches (56).
Further Considerations and Future Prospectives
The main novelty of the last decade in the prophylaxis of pediatric migraine comes from the results of the CHAMP study. This study showed that pharmacological treatments, such as amitriptyline and topiramate, do not differ from placebo. Three main issues are raised by this study:
- First, placebo effect proves very powerful in pediatric age (about 60% of patients), thus it should be considered as a fundamental therapeutic resource. Placebo response rate is known to be high in pediatric migraine studies (25). The high therapeutic efficacy of placebo should not be considered only as a threat to the success of clinical studies, but it represents a therapeutic possibility in the treatment of pediatric migraine. Research should be addressed to further investigate the exact mechanisms connected with high placebo response rate in children with migraine. A higher knowledge in this field could allow us to use placebo as a non-harmful and effective treatment.
- The CHAMP study get us to wonder whether the use of pharmacological treatment is still allowed. Although the CHAMP results must be taken into account, we cannot forget the results of other RCTs reviewed in the present study and supporting the efficacy of some pharmacological treatments. Moreover, CHAMP trial did not consider some dynamics that may influence the course of migraine independently of drug therapy, such as psychological factors mostly linked to school attendance. It is known that untreated young migraineurs have a lower frequency of attacks in summer months, while they suffer more after the start of the school (57, 58). This means that whether the efficacy of placebo is measured in a favorable (e.g., from February to August), or unfavorable (e.g., from August to February) period can influence the response to therapy. In conclusion, we believe that CHAMP study should induce us to be even more rigorous in the treatment selection, considering the evidence-based data of efficacy and safety as being crucial for the therapeutic choice.
- Lastly, we must underline that there is no drug available in pediatric age with exclusive indication for migraine treatment (59). From this point of view, there is high expectation for the use of calcitonin gene-related peptide (CGRP) inhibitors (CGRP-r). The large trials conducted in the adult population (60, 61) have led Food and Drug Administration (FDA) to give the green light to commercialization of these drugs (Erenumab; Galcanezumab; Fremanezumab) in USA. The same drugs have been recently approved from European Medicines Agency (Erenumab; Galcanezumab). Although results from trials in children and adolescents are not available yet, the Pediatric and Adolescent Headache special interest group of the American Headache Society proposed recommendations on the use of these agents for pediatric headache disorders (62). The authors suggested that the use of CGRP receptor antagonists could be considered in postpubertal adolescent patients with frequent migraine attacks (≥8 headache days/month), who have moderate to severe disability associated with migraine (PedMIDAS score ≥30) and have failed ≥2 preventive therapies. For younger patients, who are refractory to multiple preventive therapies, CGRP receptor antagonists may also be considered with proper monitoring (e.g., bone health, linear growth, weight/BMI, infections) (62).
Author Contributions
LP and FU took care of the selection of the articles and wrote the manuscript. RM and ST contributed to the selection of articles. MF and GS contributed to the methodology. FV supervised the final manuscript. MV designed and supervised the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Torriero R, Capuano A, Mariani R, Frusciante R, Tarantino S, Papetti L, et al. Diagnosis of primary headache in children younger than 6 years: A clinical challenge. Cephalalgia. (2017) 37:947–54. doi: 10.1177/0333102416660533
3. Arruda MA, Chevis CF, Bigal ME. Recent advances in the management of chronic migraine in children. Expert Rev Neurother. (2018) 18:231–9. doi: 10.1080/14737175.2018.1438191
4. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
5. Balestri M, Papetti L, Maiorani D, Capuano A, Tarantino S, Battan B, et al. Features of aura in paediatric migraine diagnosed using the ICHD 3 beta criteria. Cephalalgia. (2018) 38:1742–7. doi: 10.1177/0333102417748571
6. Kacperski J, Bazarsky A. New developments in the prophylactic drug treatment of pediatric migraine: what is new in 2017 and where does it leave us? Curr Pain Headache Rep. (2017) 21:38. doi: 10.1007/s11916-017-0638-4
7. Papetti L, Spalice A, Nicita F, Paolino MC, Castaldo R, Iannetti P, et al. Migraine treatment in developmental age: guidelines update. J Headache Pain. (2010) 11:267e76. doi: 10.1007/s10194-010-0205-4
8. Abu-Arafeh I. Flunarizine for the prevention of migraine - a new look at an old drug. Dev Med Child Neurol. (2012) 54:204–5. doi: 10.1111/j.1469-8749.2011.04152.x
9. Peer Mohamed B, Goadsby PJ, Prabhakar P. Safety and efficacy of flunarizine in childhood migraine: 11 years' experience, with emphasis on its effect in hemiplegic migraine. Dev Med Child Neurol. (2012) 54:274–7. doi: 10.1111/j.1469-8749.2011.04154.x
10. Kim H, Byun SH, Kim JS, Lim BC, Chae JH, Choi J, et al. Comparison of flunarizine and topiramate for the prophylaxis of pediatric migraines. Eur J Paediatr Neurol. (2013) 17:45–9. doi: 10.1016/j.ejpn.2012.10.001
11. Topcu Y, Hiz Kurul S, Bayram E, Sozmen K, Yis U. The Paediatric migraine disability assessment score is a useful tool for evaluating prophylactic migraine treatment. Acta Paediatr. (2014) 103:e484–9. doi: 10.1111/apa.12752
12. Toldo I, Rattin M, Perissinotto E, De Carlo D, Bolzonella B, Nosadini M, et al. Survey on treatments for primary headaches in 13 specialized juvenile Headache Centers: the first multicenter Italian study. Eur J Paediatr Neurol. (2017) 21:507–21. doi: 10.1016/j.ejpn.2016.12.009
14. Rabkin R, Stables DP, Levin NW, Suzman MM. The prophylactic value of propranolol in angina pectoris. Am J Cardiol. (1966) 18:370–83. doi: 10.1016/0002-9149(66)90056-7
15. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand. (1974) 50:109–15. doi: 10.1111/j.1600-0404.1974.tb01350.x
16. Forsythe WI, Gillies D, Sills MA. Propanolol ('Inderal') in the treatment of childhood migraine. Dev Med Child Neurol. (1984) 26:737–41. doi: 10.1111/j.1469-8749.1984.tb08166.x
17. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics. (1987) 79:593–7.
18. Bidabadi E, Mashouf M. A randomized trial of propanolol versus sodium valproate for the prophylaxis of migraine in pediatric patients. Paediatr Drugs. (2010) 12:269–75. doi: 10.2165/11316270-000000000-00000
19. Eidlitz-Markus T, Dlugatch Y, Haimi-Cohen Y, Goldberg-Stern H, Zeharia A. Nonpharmacologic treatment of migraine with low-dose propranolol or amitriptyline. Pediatr Neurol. (2012) 46:345–9. doi: 10.1016/j.pediatrneurol.2012.03.017
20. Fallah R, Akhavan Karbasi S, Shajari A, Fromandi M. The efficacy and safety of topiramate for prophylaxis of migraine in children. Iran J Child Neurol. (2013) 7:7–11. doi: 10.1016/j.juro.2017.02.3337
21. Bakhshandeh Bali M, Rahbarimanesh AA, Sadeghi M, Sedighi M, Karimzadeh P, Ghofrani M. Comparison of propranolol and pregabalin for prophylaxis of childhood migraine: a randomised controlled trial. Acta Med Iran. (2015) 53:276–80.
22. Hershey AD, Powers SW, Bentti AL, Degrauw TJ. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache. (2000) 40:539–49. doi: 10.1046/j.1526-4610.2000.00085.x
23. Hershey AD, Powers SW, Coffey CS, Eklund DD, Chamberlin LA, Korbee LL, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. (2013) 53:799–816. doi: 10.1111/head.12105
24. Fallah R, Fazelishoroki F, Sekhavat L. A randomized clinical trial comparing the efficacy of melatonin and amitriptyline in migraine prophylaxis of children. Iran J Child Neurol. (2018) 12:47–54.
25. Kroon Van Diest AM, Ramsey R, Aylward B, Kroner JW, Sullivan SM, Nause K, et al. Treatment adherence to biobehavioral recommendations in pediatric migraine as measured by electronic monitoring: the Adherence in Migraine (AIM) study. Headache. (2016) 56:1137–46. doi: 10.1111/head.12836
26. Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. (2013) 310:2622–30. doi: 10.1001/jama.2013.282533
27. Kroner JW, Hershey AD, Kashikar-Zuck SM, LeCates SL, Allen JR, Slater SK, et al. Cognitive behavioral therapy plus amitriptyline for children and adolescents with chronic migraine reduces headache days to < 4 per month. Headache. (2016) 56:711–6. doi: 10.1111/head.12795
28. Kroner JW, Peugh J, Kashikar-Zuck SM, LeCates SL, Allen JR, Slater SK, et al. Trajectory of improvement in children and adolescents with chronic migraine: results from the cognitive-behavioral therapy and amitriptyline trial. J Pain. (2017) 18:637–44. doi: 10.1016/j.jpain.2017.01.002
29. FDA approves Topamax for migraine prevention in adolescents. J Pain Palliat Care Pharmacother. (2014) 28:191.
31. Fallah R, Divanizadeh MS, Karimi M, Mirouliaei M, Shamszadeh A. Topiramate and propranolol for prophylaxis of migraine. Indian J Pediatr. (2013) 80:920–4. doi: 10.1007/s12098-013-0976-0
32. Fallah R, Akhavan Karbasi S, Shajari A, Fromandi M. The efficacy and safety of topiramate for prophylaxis of migraine in children. Iran J Child Neurol. (2013) 7:7–11.
33. Ashrafi MR, Najafi Z, Shafiei M, Heidari K, Togha M. Cinnarizine versus topiramate in prophylaxis of migraines among children and adolescents: a randomized, double-blind clinical trial. Iran J Child Neurol. (2014) 8:18–27.
34. Loder E, Rizzoli P. Pharmacologic Prevention of migraine: a narrative review of the state of the art in 2018. Headache. (2018) 58(Suppl. 3):218–29. doi: 10.1111/head.13375
35. Lawrence ER, Hossain M, Littlestone W. Sanomigran for migraine prophylaxis; controlled multicenter trial in general practice. Headache. (1997) 17:109–111. doi: 10.1111/j.1526-4610.1977.hed1703109.x
36. Gillies D, Sills M, Forsythe I. Pizotifen (Sanomigran) in childhood migraine. A double-blind controlled trial. Eur Neurol. (1986) 25:32–5. doi: 10.1159/000115983
37. Billie B, Ludvigsson J, Sanner G. Prophylaxis of migraine in children. Headache. (1977) 17:61–3. doi: 10.1111/j.1526-4610.1977.hed1702061.x
38. Toldo I, De Carlo D, Bolzonella B, Sartori S, Battistella PA. The pharmacological treatment of migraine in children and adolescents: an overview. Expert Rev Neurother. (2012) 12:1133e42. doi: 10.1586/ern.12.104
39. Orr SL, Venkateswaran S. Nutraceuticals in the prophylaxis of pediatric migraine: Evidence-based review and recommendations. Cephalalgia. (2014) 34:568–83. doi: 10.1177/0333102413519512
40. Esposito M, Ruberto M, Pascotto A, Carotenuto M. Nutraceutical preparations in childhood migraine prophylaxis. Neurol Sci. (2012) 33:1365–8. doi: 10.1007/s10072-012-1019-8
41. Wang F, Van Den Eeden SK, Ackerson LM, Salk SE, Reince RH, Elin RJ. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. (2003) 43:601–10. doi: 10.1046/j.1526-4610.2003.03102.x
42. MacLennan KM, Darlington CL, Smith PL. The CNS effects of ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. (2002) 67:235–57. doi: 10.1016/S0301-0082(02)00015-1
43. Bruijn J, Duivenvoorden H, Passchier J, Locher H, Dijkstra N, Arts WF. Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia. (2010) 30:1426–34. doi: 10.1177/0333102410365106
44. Slater SK, Nelson TD, Kabbouche MA, LeCates SL, Horn P, Segers A, et al. A randomized, double-blinded, placebo-controlled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia. (2011) 31:897–905. doi: 10.1177/0333102411406755
45. Esposito M, Carotenuto M. Ginkgolide B complex efficacy for brief prophylaxis of migraine in schoolaged children: an open-label study. Neurol Sci. (2011) 32:79–81. doi: 10.1007/s10072-010-0411-5
46. Mack KJ. Management of chronic daily headache in children. Expert Rev Neurother. (2010) 10:1479–86. doi: 10.1586/ern.10.124
47. Ahmed K, Oas KH, Mack KJ, Garza I. Experience with botulinum toxin type A in medically intractable pediatric chronic daily headache. Pediatr Neurol. (2010) 43:316–9. doi: 10.1016/j.pediatrneurol.2010.06.001
48. Kabbouche M, O'Brien H, Hershey AD. Onabotulinumtoxin A in pediatric chronic daily headache. Curr Neurol Neurosci Rep. (2012) 12:114–7. doi: 10.1007/s11910-012-0251-1
49. Trautmann E1, Kröner-Herwig B. A randomized controlled trial of Internet-based self-help training for recurrent headache in childhood and adolescence. Behav Res Ther. (2010) 48:28–37. doi: 10.1016/j.brat.2009.09.004
50. Pintov S, Lahat E, Alstein M, Vogel Z, Barg J. Acupuncture and the opioid system: implications in management of migraine. Pediatr Neurol. (1997) 17:129–33. doi: 10.1016/S0887-8994(97)00086-6
51. Gottschling S, Meyer S, Gribova I, Distler L, Berrang J, Gortner L, et al. Laser acupuncture in children with headache: a double-blind, randomized, bicenter, placebo-controlled trial. Pain. (2008) 137:405–12. doi: 10.1016/j.pain.2007.10.004
52. Stubberud A, Varkey E, McCrory DC, Pedersen SA, Linde M. Biofeedback as prophylaxis for pediatric migraine: a meta-analysis. Pediatrics. (2016) 138:e20160675. doi: 10.1542/peds.2016-0675
53. Blume HK, Brockman LN, Breuner CC. Biofeedback therapy for pediatric headache: factors associated with response. Headache. (2012) 52:1377–86. doi: 10.1111/j.1526-4610.2012.02215.x
54. Shiri S, Feintuch U, Weiss N, Pustilnik A, Geffen T, Kay B, et al. A virtual reality system combined with biofeedback for treating pediatric chronic headache–a pilot study. Pain Med. (2013) 14:621–7. doi: 10.1111/pme.12083
55. Hesse T, Holmes LG, Kennedy-Overfelt V, Kerr LM, Giles LL. Mindfulness based intervention for adolescents with recurrent headaches: a pilot feasibility study. Evid Based Compl Alt Med. 2015:508958. doi: 10.1155/2015/508958
56. Dalla Libera D, Colombo B, Pavan G, Comi G. Complementary and alternative medicine (CAM) use in an Italian cohort of pediatric headache patients: the tip of the iceberg. Neurol Sci. (2014) 35(Suppl. 1):145–8. doi: 10.1007/s10072-014-1756-y
57. Pakalnis A, Heyer GL. Seasonal variation in emergency department visits among pediatric headache patients. Headache. (2016) 56:1344–7. doi: 10.1111/head.12888
58. Soriani S, Fiumana E, Manfredini R, Boari B, Battistella PA, Canetta E, et al. Circadian and seasonal variation of migraine attacks in children. Headache. (2006) 46:1571–4. doi: 10.1111/j.1526-4610.2006.00613.x
59. Papetti L, Salfa I, Battan B, Moavero R, Termine C, Bartoli B, et al. Features of primary chronic headache in children and adolescents and validity of Ichd 3 criteria. Front Neurol. (2019) 10:92. doi: 10.3389/fneur.2019.00092
60. Goadsby PJ, Paemeleire K, Broessner G, Brandes J, Klatt J, Zhang F, et al. Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. (2019) 39:817–26. doi: 10.1177/0333102419835459
61. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. (2018) 38:1442–54. doi: 10.1177/0333102418779543
Keywords: migraine, pediatric migraine, prophylactic drugs, therapy, treatment, guidelines, preventive
Citation: Papetti L, Ursitti F, Moavero R, Ferilli MAN, Sforza G, Tarantino S, Vigevano F and Valeriani M (2019) Prophylactic Treatment of Pediatric Migraine: Is There Anything New in the Last Decade? Front. Neurol. 10:771. doi: 10.3389/fneur.2019.00771
Received: 16 May 2019; Accepted: 02 July 2019;
Published: 16 July 2019.
Edited by:
Vincenzo Guidetti, Sapienza University of Rome, ItalyReviewed by:
Marco Carotenuto, University of Campania, Luigi Vanvitelli Caserta, ItalyAynur Özge, Mersin University, Turkey
Copyright © 2019 Papetti, Ursitti, Moavero, Ferilli, Sforza, Tarantino, Vigevano and Valeriani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Valeriani, bWFzc2ltaWxpYW5vLnZhbGVyaWFuaUBvcGJnLm5ldA==
†These authors have contributed equally to this work
 Laura Papetti
Laura Papetti Fabiana Ursitti
Fabiana Ursitti Romina Moavero
Romina Moavero Michela Ada Noris Ferilli1
Michela Ada Noris Ferilli1 Federico Vigevano
Federico Vigevano Massimiliano Valeriani
Massimiliano Valeriani