- 1Neurosciences, Mental Health, and Sensory Organs (NESMOS) Department, Center for Experimental Neurological Therapies, S. Andrea Hospital-site, “Sapienza” University of Rome, Rome, Italy
- 2Department of Neurology and Psychiatry, “Sapienza” University of Rome, Rome, Italy
- 3Neuroimmunology Division, Department of Neurology, Neuroimaging Unit, Vanderbilt University Medical Centre, Nashville, TN, United States
- 4Neurologia – Centro Riferimento Regionale Sclerosi Multipla and Neuroscience Institute Cavalieri Ottolenghi, San Luigi Hospital, Turin, Italy
- 5D.A.I. Neurosciences and Mental Health, “Sapienza” University of Rome, Rome, Italy
- 6Università Cattolica, Fondazione Policlinico Universitario “A. Gemelli,” Rome, Italy
- 7Don Carlo Gnocchi Foundation Onlus, Milan, Italy
- 8National Centre of Epidemiology, National Institute of Health, Rome, Italy
- 9IRCCS Istituto Neurologico Mediterraneo (INM) Neuromed, Pozzilli, Italy
Introduction: To compare a schedule with cyclic withdrawal (CW) of interferon beta (IFN-b) 1b, respect to the full regimen (FR), in relapsing-remitting MS (RR-MS).
Methods: Participants were randomly assigned to CW or FR schedule and monthly monitored with brain MRI scans for 12 months (three of run-in and 9 of treatment). CW schedule included drug withdrawal for 1 month after two of treatment for a total of three quarters over the 9-month treatment period. The assessing neurologist and the expert neuroradiologists were blind. After the blind phase of the study all participants took their indicated disease modifying therapies in a prospectively planned, open-label extension phase (up to 120 months).
Results: Of 60 randomized subjects 56 (29 in FR and 27 in CW group) completed the single-blind phase: the two groups were comparable, except for a non-significant difference in the number of contrast-enhanced lesions (CEL) at the end of run-in. The two-sided 90% CI for the ratio between median number of cumulative CEL was 0.29–1.07, allowing to significantly reject the null hypothesis of a ratio ≥1.2 and to meet the primary end-point of non-inferiority (the threshold and the ratio between median were chosen according to the non-normal distribution of the data). The differences (CW vs. FR) were also non-significant for secondary end points: mean cumulative number of T2-weighted new and enlarging lesions (3.48 ± 5.34 vs. 3.86 ± 6.76); mean number and volume (cm3) of black holes (1.24 ± 1.61 vs. 2.71 ± 4.56; 489.11 ± 1488.12 vs. 204.48 ± 396.98); number of patients with at least an active scan (21 vs. 22); mean relapse rate (0.52 ± 0.89 vs. 0.34 ± 0.66), relapse risk ratio adjusted for baseline variables (2.15 [0.64–7.18]), EDSS score (1.0 [1–1.56] vs. 1.5 [1–1.78]), proportion of patients with antibodies anti-IFN (5 [21%] vs. 8 [36%]). Fifty-four patients (27 for each study arm) completed the open-label phase. The annualized RR, EDSS, proportion of patients shifting to progressive disease and hazard ratio of shifting, adjusting for baseline covariates, were comparable between the two study groups.
Conclusions: A calendar with CW was non-inferior than FR at the beginning of IFN-b therapy, and may not affect the long-term outcome.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT00270816
Introduction
Relapsing remitting multiple sclerosis (RR-MS) has become manageable thanks to a wide range of new treatments (1). However, these therapies last for decades and raise issues of safety, reduction in life quality, treatment adherence, and healthcare expenditure (2).
Furthermore, inflammation, neurodegeneration, and metabolic modifications co-exist and all need therapeutic intervention in MS; however, none of the approved disease modifying therapies (DMT) has been proven to tackle them all. The increasingly shared view is that combining therapies with different mechanisms of action may target the complex pathophysiology of the disease (3–5).
Hence, given the characteristics of available treatments, and within the perspective of facilitating the development of combination therapies, new regimens that reduce the treatment burden represent an unmet need in MS.
Work in our group using serial transcriptome analysis in peripheral blood mononuclear cells (PBMC) of patients treated with interferon beta (IFN-b) showed that gene expression changes are more pronounced during the 1st weeks of treatment, with a clear tendency to return to baseline levels 2–3 months after treatment initiation (6). This result provided the rationale for the design of a treatment regimen that includes cyclic withdrawals (CW) of treatment with IFN-b 1b. This schedule may in principle maintain the biological impact that is gradually lost due to the homeostatic response, be less cumbersome for patients and less expensive for the healthcare system. We therefore tested the hypothesis of non-inferiority of a schedule based on CW of IFN-b 1b compared to the canonical, full regimen (FR) in persons with RR-MS.
Patients and Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The present single-blind, phase-IV, randomized clinical study was performed at the Center for Experimental Neurological Therapies, Ospedale S. Andrea-site, “Sapienza” University of Rome, and Università Cattolica, Fondazione Policlinico Universitario “A. Gemelli,” Rome, Italy. The study was registered at the ClinicalTrial.gov (NCT00270816). The trial was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Ospedale S. Andrea, “Sapienza” University of Rome and each patient provided written informed consent.
Patients
Patients with RR-MS (7) were consecutively screened and enrolled between November 2006 and November 2008. The following were considered as inclusion criteria: age between 18 and 50 years (inclusive); no steroid treatment in the 2 months prior to the study and no previous exposure to any DMT. Exclusion criteria were: systemic diseases; pregnancy, inability to agree on the use of contraception for women of childbearing potential and breast-feeding, inability to undergo magnetic resonance imaging (MRI).
Protocol
The study design included a 3-month run-in period (months 1–3) and a 9-month treatment period (months 4–12). At the end of the run-in, patients were 1:1 randomized to receive either IFN-b 1b every other day consecutively (FR arm) or to CW arm (drug withdrawal for 1 month after two of treatment for a total of three quarters over the 9-month treatment period). Patients of the CW arm stopped IFN beta-1b 3 times, at months 6, 9, and 12. A list of randomization numbers and corresponding treatment numbers was computer-generated before the start of the study. A 2-physician-treating and assessing-model was used: the treating physician supervised drug administration, evaluated adverse events, and safety; the assessing physician evaluated outcome measures and was blind.
A prospectively planned, extension of the trial was conducted up to 120 months, during which the patients remained under the FR standard schedule of IFN-b 1b, or were shifted to the DMT that their neurologist in charge considered indicated.
Procedures
At first visit each patient underwent a clinical examination to rate disability with the Expanded Disability Status Score (EDSS) scale (8), performed routine blood tests, any required test to rule out MS mimickers, electrocardiogram, and brain MRI scan. Thereafter, each patient underwent monthly clinic visit and MRIs for the first 12 months, and every 6-month clinical with or without imaging assessment till month 120.
Clinical relapses were defined as the appearance of new symptoms or worsening of previous symptoms/signs associated with changes in the neurological examination lasting longer than 24 h in the absence of fever, infections or any other acute process that could be responsible for neurological worsening. Adverse events were defined as any untoward medical occurrence regardless of its causal relationship to the study treatment. The severity of the adverse events was graded as: mild (minimal or no required treatment and no interference with the patient's daily activities); moderate (low level of inconvenience or concern; may require therapeutic measures and cause some interference with functioning); severe (interruption of a patient's usual daily activities and requirement of systemic drug therapy or other treatment; usually incapacitating); life-threatening (immediate risk of death).
At the above specified time points all participants were imaged with gadolinium (Gd)-enhanced MRI of brain to calculate the number of contrast enhancing lesions (CELs) and the other outcome measures (see below). MRI was performed using a 1.5-T magnet (Philips Gyroscan NT 1.5). The magnet underwent several upgrades during the study period and care was taken that none of these upgrades affected the sequences in use. T2-weighted (T2W) spin-echo (SE) (TR = 2,000 ms; TE = 20/90 ms), fast fluid-attenuated inversion-recovery (FLAIR) (TR = 6,000 ms; TE = 150 ms) and T1-weighted (T1W) SE (TR = 550 ms; TE = 12 ms) sequences were acquired in the axial plane with 5-mm contiguous slices, and a field of view (FOV) = 240 mm, matrix = 256 X256. The hardcopy was analyzed by two experienced neuroradiologists working in pairs (when there was a disagreement a third senior neuroradiologist reviewed the images and a final consensus was reached). They were blind and detected the number of Gd-enhancing lesions, T2-hyperintense lesions, and T1-hypointense lesions. The number of chronic black holes (BH) on T1W MRI was assessed according to Bagnato et al. (9).
Neutralizing antibodies (NAB) status was evaluated with a bioassay based on the cytopathic effect (CPE) of encephalomyocarditis virus (EMC) on human lung carcinoma cells (A549) as previously described (10, 11). The assay was performed prior to study entry and at the end of the 9-month treatment phase. The neutralization titer of serum samples was calculated according to Kawade's formula (12), and expressed in 10-fold reduction unit (TRU) (13). A level of >20 TRU was considered as the threshold for positivity.
Outcomes Measures
Group (CW vs. FR) differences in the cumulative number of CELs over the first 9-month period was defined as the primary end point. Secondary end points included: group differences in cumulative number of new and enlarging T2-weighted lesions, number of patients with at least an active scan, relapse rate, number, and volume of BH, EDSS score, and proportion of NAB + patients. The outcome measures of the open label phase were the following: annualized relapses rate (ARR), EDSS, proportion of patients shifting to progressive MS [defined according to Lublin et al. (14) and Lublin et al. (15)].
Statistical Analysis
Group differences at study entry were assessed using a t-test for continuous variables and a chi-square test for categorical variables. For continuous variables, median and Interquartile Range (IQR) were also calculated. When the variable distribution was non-normal according to Shapiro-Wilk test, the two groups were compared performing a non-parametric K-sample test on the equality of medians.
At the end of the single-blind phase of the trial, mean, standard deviation, median, IQR, and the range values were calculated for the primary and secondary endpoints, separately for CW and FR patients. For the primary outcome, we established to compare the number of CELs between the two regimens thorough the mean difference or the ratio of medians according to the normal or non-normal distribution of the data. In case of normality distribution of the data, we considered 8.1 as the mean number of CELs observed in the FR regimen in a previous work of our group (observed by Pozzilli et al. in a period of 6 months) (16) and operatively set a difference of 1.5 cumulative CELs for non-inferiority of the CW vs. the FR schedule, and 2 cumulative CELs of standard deviation. In this scenario, we rejected the null hypothesis with a significance level of 0.05 if the upper limit of the two-sided 90% confidence interval (CI) of the difference between the means of the 2 regimens was < 1.5 CEL (17, 18). To compute a one-sided testing procedure for the difference of the means, with an alpha error = 5% and a study power = 0.80, a sample size of 46 patients was required. Thirty patients per group were recruited to account for a drop-out rate of 20%. Power analyses were performed using StudySize software (version 2.0).
In case of non-normal distribution of the data, we operationally considered a non-inferiority threshold for the ratio of median of 1.2, assuming a standard deviation on elog Scale of 0.3. In this case, recruiting 30 patients per group allow to get a power of 0.90 for a one sided 95% CI. The null hypothesis was that the ratio of the median of the CW group on the median of the FR group was ≥ 1.2 and therefore the non-inferiority was demonstrated if the upper limit of the two sided 90% CI for the ratio of medians was lower than 1.2. Normality was tested using the Shapiro-Wilk test.
For the secondary endpoints, we calculated the relapse risk ratio (RR) using a Poisson model adjusted for the baseline variables (gender, age, disease duration, relapse number before study entry, EDSS, and MRI metrics), and performed a non-parametric K-sample test on the equality of medians for the EDSS score and the MRI metrics, that showed a non-normal distribution. During the single-blind phase of the trial an intention to treat analysis was performed adopting a LOCF (last observation carried forward) method.
During the open-label extension of the trial, we compared the annual relapse rate and the EDSS score between CW and FR groups performing a non-parametric K-sample test on the equality of medians, because of the non-normal distribution of the above variables. The proportion of patients who shifted to a progressive disease was compared using a chi-square test. Individual follow-up started with the beginning of the open label phase and finished at the end of the follow-up period (120 months), death, loss at follow-up, or shifting to a progressive disease.
Results
Patients Disposition and Characteristics
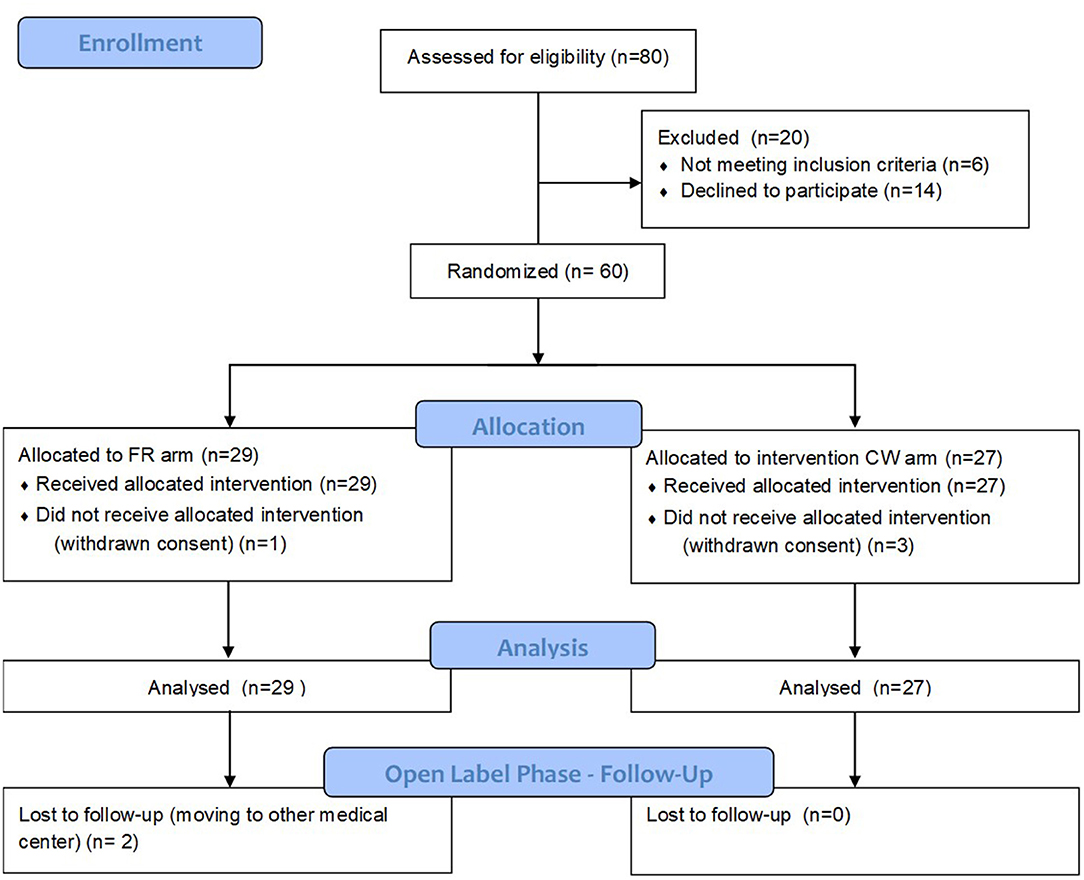
Figure 1 depicts eligibility assessment, patients screening, and inclusion. Table 1 reports baseline demographic and clinical characteristics of patients, as well as MRI metrics after the run in period. All the variables, including those showing a non-normal distribution, were balanced between the two groups.
Outcome Measures
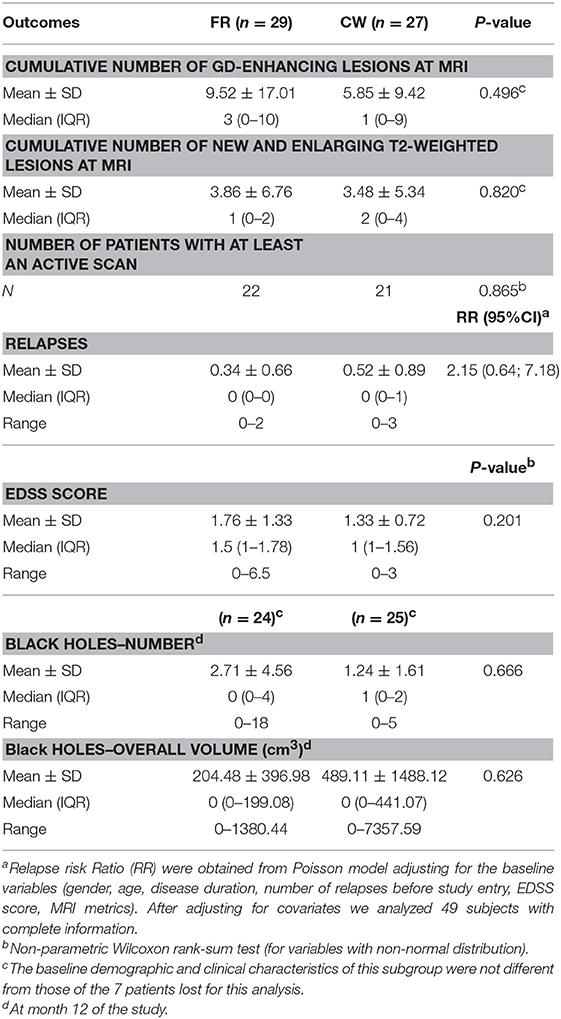
At the end of the trial, we verified that the normality was not satisfied for the primary outcome. Therefore, we tested the non-inferiority comparing the medians of the two groups. The median number of cumulative Gd-enhancing lesions at MRI was comparable between the two study arms, meeting the primary end-point: median 3 and IQR (0–10) for FR vs. median 1 (0–9) for CW; p = 0.496. Specifically, there was a 95% chance that the median number of cumulative Gd-enhancing lesions with the CW regimen was at most 1.2 times the median number of cumulative Gd-enhancing lesions with the FR schedule (Figure 2). The ratio of the median of the CW group on the median of the FR group and its relative two sided 90% CI was 1 (0.29; 1.07), allowing to significantly reject the null hypothesis of a ratio ≥1.2, and to confirm the non-inferiority of the CW regimen. Data related to the secondary endpoints of the first 9-month treatment period are summarized in Table 2. All secondary endpoints were met, although the study was not powered for any of them. The relapse RR, adjusted for baseline covariates, was comparable between CW and FR group (RR = 2.15, 95% CI: 0.64–7.18). Likewise, the mean number of cumulative new and enlarging T2-weighted lesions, the number of patients with at least an active scans, the number and volume of black holes and the median values of EDSS score did not show significant differences between the two study arms (respectively, p = 0.820; p = 0.865; p = 0.666; p = 0.626; and p = 0.201). The proportion of NAB + patients was similar between the two study arms: 8 (36%) in FR group and 5 (20%) in CW; p = 0.33 (data not shown).
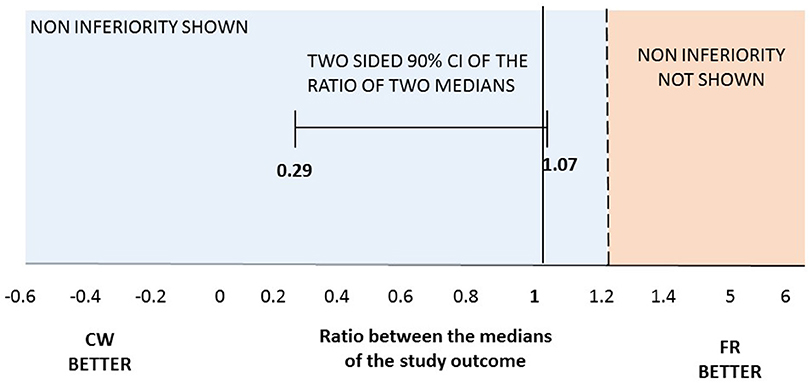
Figure 2. Two-sided 90%CI for the ratio between median number of cumulative Gd-enhancing lesions at MRI observed in the two regimens. Maximum ratio of 1.2 lesions was set for the one-tailed test with a significance level of 0.05. At month 12 median of the cumulative number of Gd-enhancing lesions at MRI was 1 (0–9) in CW vs. 3 (0–10) in FR patients.
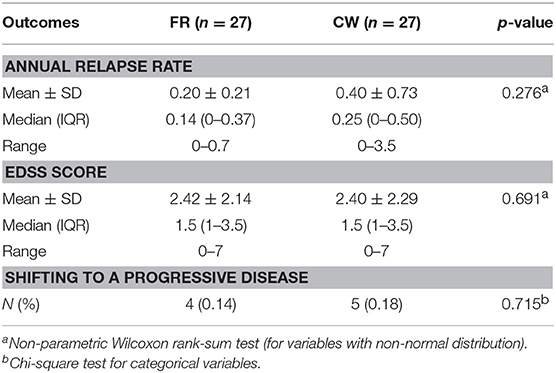
The open-label extension phase lasted between 8 and 10 years, depending upon individual patient recruitment date. In this phase, all patient who remained on IFN-b 1b took the FR, while others shifted to the DMT that their neurologist deemed indicated. The shifting to a second-line treatment occurred in a minority of cases (7/27 in both groups). Results of the open-label phase, summarized in Table 3, showed no difference between CW and FR study arms in the median of annual relapses rate (p = 0.276), the median of EDSS score (p = 0.691), and the proportion of patients shifting to a progressive phase of disease (p = 0.715).
No major adverse event was recorded throughout the trial. During the single-blind phase and the open-label extension of the trial the frequency and the nature of adverse events were within the established profile of IFN-b 1b, or the DMT that the patients shifted to, without differences between CW and FR study arms.
Discussion
Our pilot work compared two IFN-b 1b regimens in RR-MS and showed non-inferiority of a calendar with therapeutic holiday with respect to the approved schedule. The outcome measures proved to be comparable between the two study arms for both the clinical-MRI end-points, and the development of NAB to IFN-b during the blind phase of the trial.
The clinical metrics gathered over the open-label phase lasting up to 10 years (during which most of patients remained under continuous IFN-b therapy, or were shifted to the DMT that their neurologist in charge considered indicated) were also comparable between the two groups, suggesting that beginning IFN therapy with a cyclic dose variation should not affect the long-term clinical progression of MS. Our finding points to the possibility of an IFN-b schedule with better therapeutic index and cost-effectiveness ratio, at least in cases with relatively benign disease, taking into account that there are ~30% of patients stable on IFN-b long-term treatment. The estimated saving for a year of the therapy with 1-month holiday every quarter (as in our trial design) is about 5,000 euros/patient (or 20,000 dollar/patient in US). These improvements also open new perspectives for the design of combination therapies, a much-needed step in a disease with multiple mechanisms of damage, and a timely advancement now that treatments directed against the neurodegenerative component of the disease are emerging (19). This perspective contributes to the current relevance of our study, although the present landscape of the first-line DMT, that include oral drugs, has actually restricted the indications of injectable DMT. In fact, interferons remain a relevant option within available treatments, as witnessed also by the development of new IFN-b formulations aimed at reducing the frequency of the injections (20). In this context, if therapeutic holidays should prove feasible also with this class of IFNs, administered once every 2 weeks, the convenience of these injectable therapies would be further increased.
Previous work suggested an increased efficacy of IFN-b at higher dose in contrasting disease activity (21–23). Moreover, in a daily clinical setting, a switch to higher dose of IFN-b may be in principle prescribed to obtain a better control of disease course, though with uncertain outcomes on the risk of further relapses or increased disability (24). In accord with a substantial amount of pre-clinical and clinical research in conditions other than MS (25), we now suggest that the therapeutic holidays sustain the biological impact of IFN-b over time (in spite of the lower overall dose) by periodically interrupting feedback mechanisms that are anticipated in chronic treatment regimens (the homeostatic response during chronic treatments is well-documented also at the clinical level by the reduction of the flu-like syndrome after the beginning of IFN-b therapies, of gastrointestinal symptoms and flushing during dimethyl fumarate treatment and of other side-effects with various other drugs). A study on the continuous vs. intermittent therapy for chronic hepatitis C with interferon alfa-2a supports this interpretation (26). Notably, the block of the homeostatic response was not as strong as to imply a clinically significant resurgence of the flu-like syndrome at the beginning of each new cycle of treatment. In fact, though we did not plan formal questionnaires to compare the quality of life between the two study arms, and we had no adherence problems in FR group, virtually all participants experiencing the CW regimen reported satisfaction for the regular withdrawal. Overall, these results are in line with expectations based on our transcriptomics study in PBMC from IFN-treated patients, that prompted this pilot trial. It showed both homeostatic responses to IFN-b administration (that we used to design the dynamics of drug holiday), and sustained and lasting effects on up- or down-regulated genes, that antagonize some pathogenic loops of MS (6). Previous work on drug holiday for other DMT in MS are rare, with contrasting or disappointing results (27–29); however, this trial suggests to deepen temporal dynamics underpinning their biological effects in MS course, with the aim of reducing the treatment burden or favoring combination therapies.
Concerning the potential limitations of this trial, we chose to leave patients under CW regimen on a “true” therapeutic holiday: they were not given placebo during the month of IFN withdrawal, thus remaining un-blinded on their treatment schedule. In fact, blindness of neuroradiologists and the assessing neurologist was deemed sufficient considering the study end points. On the other hand, a sort of nocebo effect due to expectation of lower treatment response to the CW regimen did not produce measurable changes, being met the end points of non-inferiority. Another point of caution is the small sample size of the trial; it allowed us to obtain statistical significance, but warns against definitive conclusions and may require confirmative studies. This is in line with the pilot nature of our study, that was designed to minimize the possibility that loss of efficacy might occur in a larger population under CW regimen. The open-label phase of the study has the expected limitations of a trial long-term extension: non-prospective design, possibly selective dropout rate, un-blinded assessment and poor monitoring, underpowered sample size. The loss of only 2 patients in FR group at long-term follow-up, and the fact that the shifting to a second-line treatment occurred in a minority of cases in both study arms indicate the validity of the long follow-up we chose. Also, the analysis of the categorical results of the open-label part of the study, that were adjusted for the baseline characteristics of participants, might help mitigate, at least in part, the above potential shortcomings. However, the data coming from the unblinded trial phase should be interpreted with caution.
Randomized, non-inferiority trials are rare in MS likely because of the challenges that these studies raise, as recently elucidated (30). Though exploratory, this trial may be relevant in the MS therapeutic field, providing information that might improve the schedule of IFN-beta administration in persons with RR-MS. This information is relevant for clinical practice (31) and for the design of poly-therapies [an approach that is beginning to be tested: reference (32), and ClinicalTrials.gov- NCT02907177] aimed at combating the various mechanisms of damage that compose the complex pathophysiology of MS.
Data Availability
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Ethics Statement
The study was registered at the ClinicalTrials.gov (NCT00270816). The trial was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the local ethics committees and each patient provided written informed consent.
Author Contributions
GR and MS were the study principal investigators. MS, GR, FB, CP, and SR conceived and designed the study. SR, MFe, MB, AF, VN, MM, and MFr were assessing neurologists. FB provided image analyses. RM, MC, and AB acquired and analyzed data on antibodies anti-IFN. All the authors were involved in the assessment and interpretation of the data. MFe, FM, and NV performed the statistical analyses. GR, SR, MFe, FB, FM, NV, CP, and MS contributed to write the manuscript.
Funding
The study was supported by the Italian Ministry of Health (RF-2010-2321254) and the intramural research funds of the Centre for Experimental Neurological Therapies (CENTERS), Rome, Italy. CENTERS is supported by a special project of the Fondazione Italiana Sclerosi Multipla–FISM), Ospedale S. Andrea-site, NESMOS. Schering provided an unrestricted research grant to partially cover administrative cost of the study. No funding source had a role in the trial design, data collection, analysis and interpretation, and manuscript preparation.
Conflict of Interest Statement
SR receives research support from Italian Multiple Sclerosis Foundation. FB serves in the Scientific Advisory Board of Merck-Serono. RM receives research support from Italian Multiple Sclerosis Foundation. MC received speaker honoraria from Biogen Idec, Merck, and Teva. MB received onorary fees from Teva, Sanofy, Biogen Idec, Merck, Novartis. AF received onorary fees from Teva and Novartis. VN received speaker honoraria from Teva, Biogen Idec, Bayer Schering, Merck, Almirall, Genzyme, Novartis. MM received honoraria for speaking, advisory board/consulting from Teva, Almirall, Biogen, Bayer Schering, Merck, Novartis Pharmaceuticals, Ultragenix, and financial support for research activities from Merck, Almirall, Teva and Novartis Pharmaceuticals. AB served on the scientific advisory boards of Almirall, Bayer, Biogen Idec, and Genzyme; received speaker honoraria from Biogen Idec, Genzyme, Novartis, Sanofi-Aventis, and Teva; his institution has received grant support from Bayer, Biogen Idec, Merck, Novartis, Teva, the Italian Multiple Sclerosis Society, Fondazione Ricerca Biomedica ONLUS, and San Luigi ONLUS; is on the editorial board of Multiple Sclerosis International, Progress in Neuroscience, Dataset Papers in Neuroscience, Journal of Multiple Sclerosis, Neurology, and Therapy, and Multiple Sclerosis and Demyelinating Disorders; and received research support from Regione Piemonte, Italian Multiple Sclerosis Society, Associazione Ricerca Biomedica ONLUS, and San Luigi ONLUS. CP has received consulting and/or lecture fees and/or research funding and travel grant from Almirall, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva. MS receives research support from Italian Multiple Sclerosis Foundation (FISM) and research support with honorary fees from Teva, Bayer Schering, Genzyme, Biogen Idec, Merck and Novartis. GR receives research support from Italian Multiple Sclerosis Foundation (FISM).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the contribution of the late Prof. Anna Paola Batocchi to this paper.
Abbreviations
ARR, annualized relapses rate; BH, black holes; cBHs, chronic black holes; CDMS, clinically definite multiple sclerosis; CELs, contrast enhancing lesions; CI, Confidence Interval; CPE, cytopathic effect; CW, cyclic withdrawal; D-MEM, Dulbecco's modified eagle's medium DMT, disease modifying therapies; EDSS, Expanded Disability Status Score. CW, cyclic withdrawal; EMC, encephalomyocarditis virus; FCS, fetal calf serum. FLAIR, fast fluid- attenuated inversion-recovery FR, full regimen; HR, Hazard Ratio; Gd, gadolinium; IFN-b, interferon beta; IQR, Interquartile Range; LOCF, last observation carried forward; MRI, magnetic resonance imaging; MS, Multiple sclerosis NAB, Neutralizing antibodies; PBMC, peripheral blood mononuclear cells; RR, risk ratio; RR-MS, relapsing remitting multiple sclerosis; SE, spin-echo; T1W, T1-weighted; T2W, T2-weighted; TRU, 10-fold reduction unit.
References
1. Thompson AJ. A much-needed focus on progression in multiple sclerosis. Lancet Neurol. (2015) 14:133–5. doi: 10.1016/S1474-4422(14)70330-8
2. Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, et al. Minocycline in MS study team. trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med. (2017) 376:2122–33. doi: 10.1056/NEJMoa1608889
3. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. (2015) 14:183–93. doi: 10.1016/S1474-4422(14)70256-X
4. Salvetti M, Landsman D, Schwarz-Lam P, Comi G, Thompson AJ, Fox RJ. Progressive MS: from pathophysiology to drug discovery. Mult Scler. (2015) 21:1376–84. doi: 10.1177/1352458515603802
5. Faissner S, Mahjoub Y, Mishra M, Haupeltshofer S, Hahn JN, Gold R, et al. Unexpected additive effects of minocycline and hydroxychloroquine in models of multiple sclerosis:prospective combination treatment for progressive disease? Mult Scler. (2018) 24:1543–56. doi: 10.1177/1352458517728811
6. Annibali V, Di Giovanni S, Cannoni S, Giugni E, Bomprezzi R, Mattei C, et al. Gene expression profiles reveal homeostatic dynamics during interferon-beta therapy in multiple sclerosis. Autoimmunity. (2007) 40:16–22. doi: 10.1080/08916930601135241
7. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. (2001) 50:121–7. doi: 10.1002/ana.1032
8. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/WNL.33.11.1444
9. Bagnato F, Jeffries N, Richert ND, Stone RD, Ohayon JM, McFarland HF, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. (2003) 126:1782–9. doi: 10.1093/brain/awg182
10. Bertolotto A, Malucchi S, Milano E, Castello A, Capobianco M, Mutani R. Interferon b neutralizing antibodies in multiple sclerosis: neutralizing activity and cross-reactivity with three different preparations. Immunopharmacology. (2000) 48:95–100. doi: 10.1016/S0162-3109(00)00182-X
11. Antonelli G, Bagnato F, Pozzilli C, Simeoni E, Bastianelli S, Currenti M, et al. Development of neutralizing antibodies in patients with relapsing-remitting multiple sclerosis treated with IFN-b1a. J Interferon Cytokine Res. (1998) 18:345–50. doi: 10.1089/jir.1998.18.345
12. Kawade Y. Quantitation of neutralization of interferon by antibodies. Methods Enzymol. (1986) 119:558–73. doi: 10.1016/0076-6879(86)19076-8
13. Grossberg SE, Kawade Y, Kohase M, Klein JP. The neutralization of interferons by antibody. II. neutralizing antibody unitage and its relationship to bioassay sensitivity: the tenfold reduction unit, J Interferon Cytokine Res. (2001) 21:743–55. doi: 10.1089/107999001753124471
14. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. (1996) 46:907–11. doi: 10.1212/WNL.46.4.907
15. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2014) 83:278–86. doi: 10.1212/WNL.0000000000000560
16. Pozzilli C, Bastianello S, Koudriavtseva T, Gasperini C, Bozzao A, Millefiorini E, et al. Magnetic resonance imaging changes with recombinant human interferon-beta-1a: a short term study in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. (1996) 61:251–8. doi: 10.1136/jnnp.61.3.251
17. Committee for Proprietary Medicinal Products. Points to consider on switching between superiority and non-inferiority. Br J Clin Pharmacol. (2001) 52:223–8. doi: 10.1046/j.1365-2125.2001.01397-3.x
18. Pocock SJ. The pros and cons of noninferiority trials. Fundam Clin Pharmacol. (2003) 17:483–90. doi: 10.1046/j.1472-8206.2003.00162.x
19. Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. (2017) 390:2481–9. doi: 10.1016/S0140-6736(17)32346-2
20. Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Advance study investigators. Pegylated interferon β-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomized, phase 3, double-blind study. Lancet Neurol. (2014) 13:657–65. doi: 10.1016/S1474-4422(14)70068-7
21. Schwid SR, Panitch HS. Full results of the evidence of interferon dose-response-european North American comparative efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther. (2007) 29:2031–48. doi: 10.1016/j.clinthera.2007.09.025
22. Di Rezze S, Gupta S, Durastanti V, Millefiorini E, Pozzilli C, Bagnato F. An exploratory study on interferon beta dose effect in reducing size of enhancing lesions in multiple sclerosis. Mult Scler. (2007) 13:343–7. doi: 10.1177/1352458506071172
23. Hartung HP. High-dose, high-frequency recombinant interferon beta-1a in the treatment of multiple sclerosis. Expert Opin Pharmather. (2009) 10:291–309. doi: 10.1517/14656560802677882
24. Prosperini L, Borriello G, De Giglio L, Leonardi L, Barletta V, Pozzilli C. Management of breakthrough disease in patients with multiple sclerosis: when an increasing of Interferon beta dose should be effective? BMC Neurol. (2011) 11:26. doi: 10.1186/1471-2377-11-26
25. Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. (2017) 38:542–57. doi: 10.1016/j.it.2017.05.005
26. Negro F, Baldi M, Mondardini A, Leandro G, Chaneac M, Manzini P, et al. Continuous versus intermittent therapy for chronic hepatitis C with recombinant interferon alfa-2a. Gastroenterology. (1994) 107:479–85. doi: 10.1016/0016-5085(94)90174-0
27. Tanaka M, Kinoshita M, Tanaka K. Intermittent drug holidays in fingolimod therapyfor multiple sclerosis. Mult Scler. (2018) 24:236–7. doi: 10.1177/1352458517722647
28. Takahashi K. Effect of dosage reduction on peripheral blood lymphocyte count in patients with multiple sclerosis receiving long-term fingolimod therapy. J Clin Neurosci. (2019) 63:91–4. doi: 10.1016/j.jocn.2019.01.034
29. Killestein J, Vennegoor A, Strijbis EM, Seewann A, van Oosten BW, Uitdehaag BM, et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol. (2010) 68:392–5. doi: 10.1002/ana.22074
30. Mauri L, D'Agostino RB. Challenges in the design and interpretation of noninferiority trials. N Engl J Med. (2017) 377:1357–67. doi: 10.1056/NEJMra1510063
31. Trojano M, Pellegrini F, Fuiani A, Paolicelli D, Zipoli V, Zimatore GB, et al. New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol. (2007) 61:300–6. doi: 10.1002/ana.21102
32. Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide multiple sclerosis trial group and the MRI analysis center. teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized phase ii trial. Neurology. (2012) 78:1877–85. doi: 10.1212/WNL.0b013e318258f7d4
Keywords: relapsing-remitting multiple sclerosis, interferon beta 1b, non-inferiority, cyclic withdrawal, contrast-enhanced lesions, black holes
Citation: Romano S, Ferraldeschi M, Bagnato F, Mechelli R, Morena E, Caldano M, Buscarinu MC, Fornasiero A, Frontoni M, Nociti V, Mirabella M, Mayer F, Bertolotto A, Pozzilli C, Vanacore N, Salvetti M and Ristori G (2019) Drug Holiday of Interferon Beta 1b in Multiple Sclerosis: A Pilot, Randomized, Single Blind Study of Non-inferiority. Front. Neurol. 10:695. doi: 10.3389/fneur.2019.00695
Received: 13 February 2019; Accepted: 13 June 2019;
Published: 16 July 2019.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Maria José Sá, Centro Hospitalar São João, PortugalEva Havrdova, Charles University in Prague, Czechia
Copyright © 2019 Romano, Ferraldeschi, Bagnato, Mechelli, Morena, Caldano, Buscarinu, Fornasiero, Frontoni, Nociti, Mirabella, Mayer, Bertolotto, Pozzilli, Vanacore, Salvetti and Ristori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Salvetti, bWFyY28uc2FsdmV0dGlAdW5pcm9tYTEuaXQ=; Giovanni Ristori, Z2lvdmFubmkucmlzdG9yaUB1bmlyb21hMS5pdA==
†These authors have contributed equally to this work
 Silvia Romano
Silvia Romano Michela Ferraldeschi
Michela Ferraldeschi Francesca Bagnato3
Francesca Bagnato3 Rosella Mechelli
Rosella Mechelli Marzia Caldano
Marzia Caldano Arianna Fornasiero
Arianna Fornasiero Marco Frontoni
Marco Frontoni Massimiliano Mirabella
Massimiliano Mirabella Flavia Mayer
Flavia Mayer Carlo Pozzilli
Carlo Pozzilli Nicola Vanacore
Nicola Vanacore Marco Salvetti
Marco Salvetti Giovanni Ristori
Giovanni Ristori