- 1Department of Emergency Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 2Department of Psychiatry, Beitou Branch, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 3Department of Neurology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 4School of Public Health, National Defense Medical Center, Taipei, Taiwan
Background: Perceived stress has been related to migraine. The relationship between sex, migraine frequency, and severity of perceived stress remains unclear. We investigated perceived stress among migraineurs.
Methods: This cross-sectional case-control study involved 577 clinical outpatients at a tertiary hospital in Taiwan. Demographic and clinical data, including migraine characteristics, were collected. Migraineurs were stratified by episode frequency, aura and sex, and analyses were controlled for confounding variables. Multivariable linear regressions were used to inspect whether migraine frequency (1–4, 5–8, 9–14, or ≥15 headache days per month) was associated with perceived stress as assessed by the Perceived Stress Scale (PSS).
Results: Perceived stress was significantly higher in high frequency migraineurs (mean ± standard deviation (SD), 23.3 ± 8.7) than in low frequency migraineurs (mean ± SD, 21.9 ± 9.2; P < 0.05). After stratifying the analysis by sex, this result was observed in male subjects, but was insignificant in female subjects. In addition, the relationship between migraine frequency and perceived stress was not prominent in aura-present or -absent subgroups.
Conclusions: Higher perceived stress was associated with higher migraine frequency, but not in chronic migraine and female subgroups. Adaptation to migraine and various psychiatric comorbidities may contribute to these associations.
Background
Migraine is an important public health issue due to its high prevalence, affecting more than one billion people worldwide (1–4). As ranked by the World Health Organization, headache disorders are among the top 10 disabling conditions for both sexes and among the top five most disabling disorders for women (5). Since childhood, headache affects about 60% of children and adolescents worldwide, thus affecting school, physical activities, peer and family relationships. (6) Several triggers may increase migraine frequency, such as (1) variations in hormones; (2) changes in weather, meals, caffeine, medication, obesity, sleep quality, and (3) stressful events (7). Mounting evidence indicates that several comorbidities, such as anxiety, depression, sleep disturbances, vascular accidents, epilepsy, restless legs syndrome, and stress, are associated with migraine (4, 8, 9). In addition, a high prevalence of psychiatric comorbidities in patients with episodic and chronic headache, especially for anxiety and affective disorders has been reported (10). On the other hand, severe migraine attacks also may worsen these conditions (11).
Stress is an adaptive response to the perceived demands created by physical or psychological stimuli (12). Cohen et al. (13) created the perceived stress scale (PSS) to measure the levels to which situations in one's life are considered stressful. In subsequent decades, several studies have indicated that perceived stress is associated with multi-morbidity, mental illness, lower quality of life, and even increased mortality (14–16). Additionally, Guidetti et al. indicated that headache develops when humans face strong sensory stimuli, and physical or emotional stress might be the result of an evolved defense mechanism as a mediator of the environmental stressors and psychological factors (17). Previous studies have demonstrated that migraineurs have higher levels of perceived stress than controls (18). Perceived stress is considered to precipitate, exacerbate and perpetuate migraine (18, 19). Higher perceived stress levels are considered to occur more frequently in female than male migraineurs (12). Collectively, stress is commonly associated with migraine.
Although the relationship between migraine and stress is reliable, to our knowledge, currently no studies have definitively shown a positive trend between migraine frequency and perceived stress intensity. Furthermore, a previous study found no difference in mean PSS scores between migraineurs and controls after adjusting for depression/anxiety (19), suggesting that perceived stress in migraineurs could be affected by other factors, including depression/anxiety, that remain to be investigated. In addition, the difference in perceived stress between migraineurs with or without aura remains unclear.
Therefore, we tested the hypothesis that perceived stress intensity may predict migraine frequency, regardless of the presence of auras. In particular, we investigated the relationship between perceived stress intensity and migraine frequency while controlling for potentially confounding factors, such as sleep quality, employment status, education, smoking status, alcohol intake, coffee consumption, and depression/anxiety.
Methods
Patients
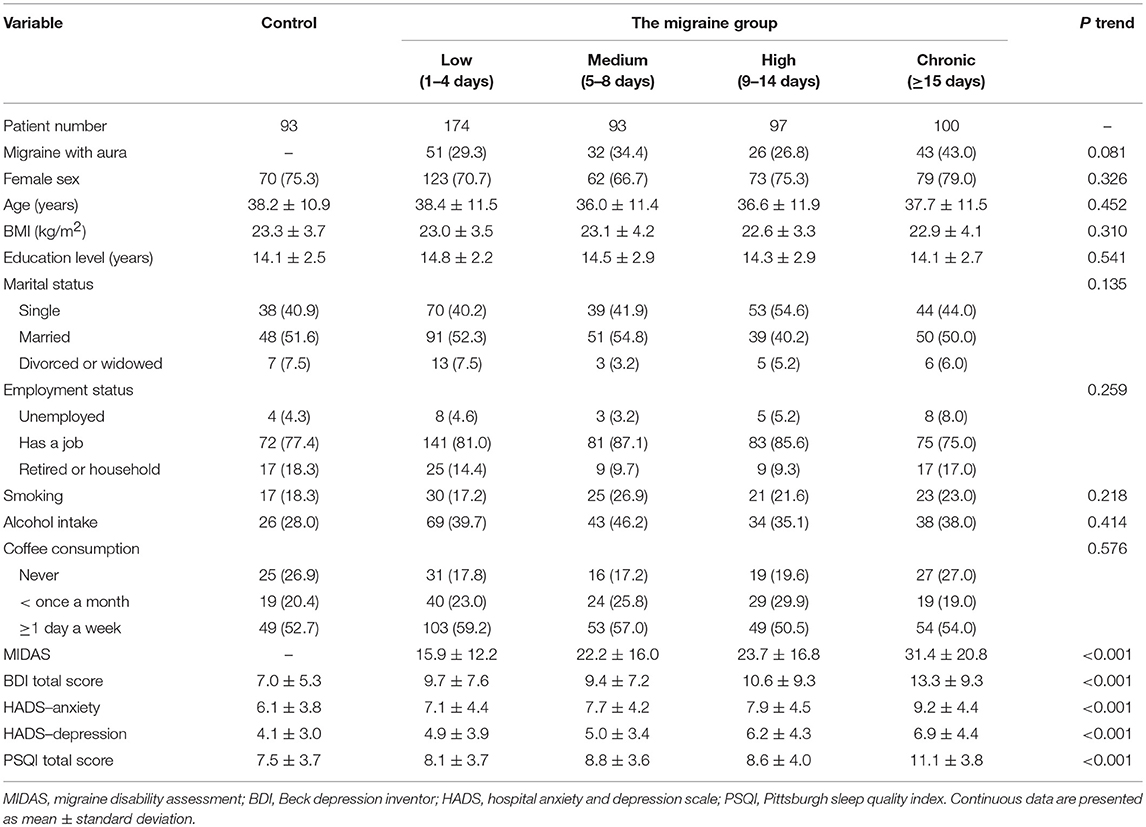
Our cross-sectional study enrolled 557 subjects visiting an outpatient headache clinic in the Department of Neurology at Tri-Service General Hospital between January 2016 and August 2018. All participants signed a written informed consent form after a full written and verbal explanation of the study. The study protocol was approved by the Institutional Review Board of the TSGH (No. 2-106-05-163). Patients with migraine, of both sexes, with and without auras, were enrolled after giving informed consent according to International Headache Society criteria (20). Among the study subjects, 100 experienced chronic, 97 high, 93 medium, and 174 low frequency migraine (>15, 9–14, 5–8, and 1–4 headache days/month, respectively). In addition, 93 volunteers without migraine, who had no family history of migraine and no previous diagnosis of other primary or secondary headache disorders, except for episodic tension-type headaches (<6 days/year), were selected.
Each participant who completed the screening questionnaire was interviewed by a board-certified neurologist and headache specialist (F-CY) to confirm the migraine diagnosis and exclude emotion-related headaches, as determined according to the International Classification of Headache Disorders, 3rd edition (beta version) (21, 22).
Psychometrics Analysis
Patients were interviewed with a structured questionnaire packet containing the PSS (13), Beck Depression Inventory (BDI) (23), Hospital Anxiety and Depression Subscales (HADS) (24), restless legs syndrome (RLS) screening questionnaire from the RLS Foundation (25), Pittsburgh Sleep Quality Index (PSQI) (26), and Migraine Disability Assessment questionnaire (MIDAS) (27). The PSS, a 14-item scale with seven positively stated items that are scored in reverse direction, was used to measure perceived stress. Each item is rated on a five-point scale (0, never; 1, almost never; 2, sometimes; 3, fairly often; 4, very often). Patients who scored ≥18 on the BDI (maximum score = 63) were classified as depressed. The HADS is a 14-item scale, with seven items related to anxiety and seven related to depression, each rated on a four-point scale (0, not at all; 1, sometimes; 2, often and 3, all the time), giving a maximum subscale score of 21. The PSQI estimates sleep quality over the prior month, with a final score of 6 indicating poor sleep. Participants who answered “yes” to at least 6/11 RLS symptom screening questionnaire items were considered to have a high probability of RLS. The MIDAS, a 5-item questionnaire, evaluated disability over the previous 3 months.
Statistical Analysis
Continuous and categorical data were expressed as mean ± standard deviation (SD) and frequency and proportion, respectively. Patients with migraines were classified into four ordinal migraine frequency groups. Linear trends in variable distributions were assessed across the control and ordinal migraine frequency groups using the Cochran-Armitage χ2 test for categorical variables or a linear contrast of general linear model for continuous variables. The PSS total score was pairwise compared among the four migraine frequency groups using a multivariable linear regression analysis adjusted for all the variables listed in Table 1. The multivariable linear regression analysis was stratified further by aura and sex. Finally, we conducted a multivariable linear regression analysis to explore factors associated with PSS total score in the patients with migraine. Differences with a 2-sided P < 0.05 were considered statistically significant. No adjustment for multiple testing (multiplicity) was made in this study. Statistical analyses were conducted using SPSS 22 (IBM SPSS, Inc., Armonk, NY, USA).
Results
Characteristics of the Study Subjects
Table 1 lists the characteristics of subjects in the control (93, 16.7%) and migraine frequency (464, 83.3%) groups. There was no significant difference in terms of aura, sex, age, body mass index (BMI), education level, marital status, employment status, smoking, alcohol drinking, or coffee consumption between the groups. The scores for the migraine disability, anxiety, depression, and sleep quality assessments worsened as migraine frequency increased (P < 0.001).
PSS Total Score
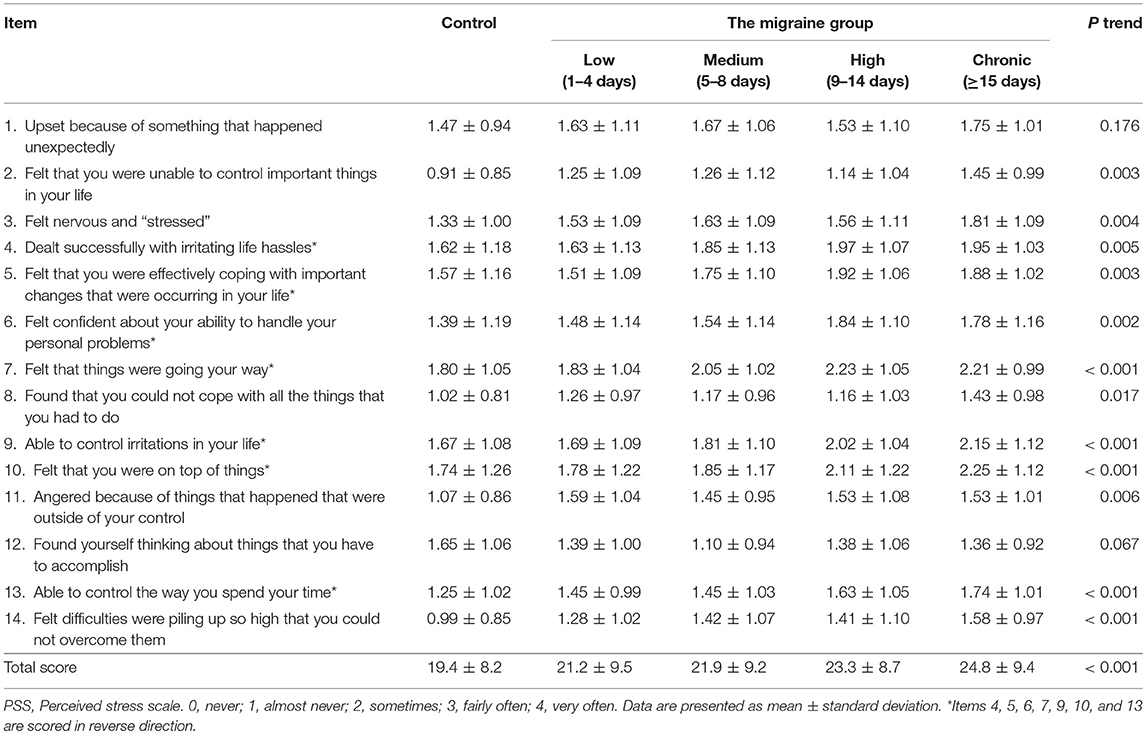
Table 2 lists the descriptive statistics for items on the PSS scale in the control and migraine frequency groups. Except for items 1 and 12, the scores of the 12 items on the PSS scale increased as migraine frequency increased (P < 0.05). With regard to total score, perceived stress was correlated positively with a higher frequency of migraine (P < 0.001). Noticeably, there was no significant difference in total PSS score between the two groups (P > 0.99, Bonferroni multiple comparison, data not shown).
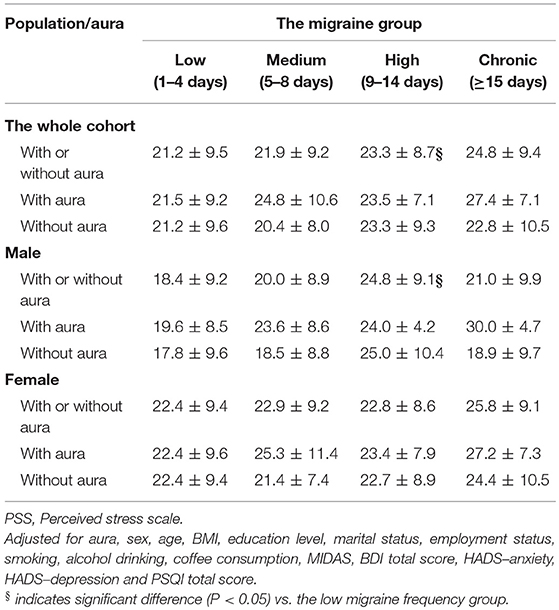
Table 3 shows the pairwise comparisons of PSS total scores among the four migraine frequency groups with adjustment for all variables listed in Table 1. Perceived stress was significantly higher in high than in low frequency migraineurs (P = 0.035, Figure 1A). After stratifying this analysis by sex, this phenomenon was observed only in male (P = 0.04) and not in female (Figure 1B) subjects. In addition, the presence or absence of aura did not modify the association between migraine frequency and perceived stress.
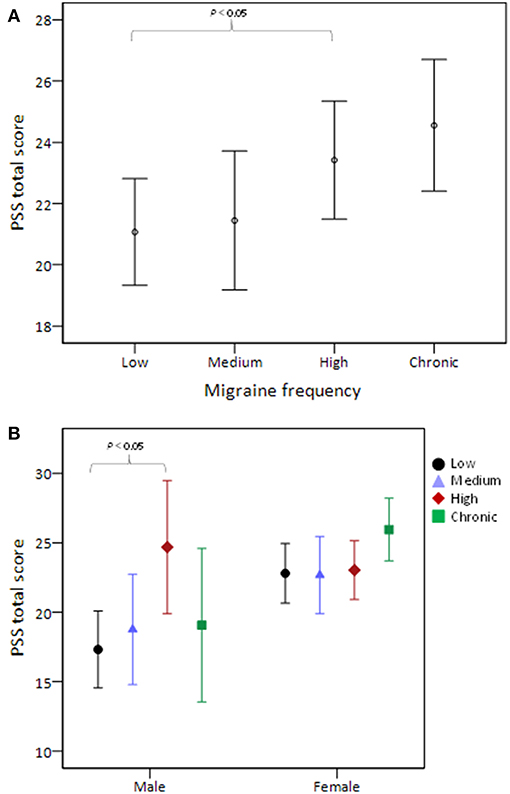
Figure 1. PSS total score stratified by migraine frequency (A) and the comparison was further stratified by sex (B). The middle horizontal line represents the mean and the error bars represent 95% confidence interval around the means. PSS, Perceived Stress Scale.
Factors Associated With PSS Total Score
Table 4 lists the results of the multivariable linear regression analysis investigating factors associated with PSS total score. Multivariable analysis identified the following variables as independent factors: high (versus low) migraine frequency, younger age, higher perceived depression levels and higher perceived anxiety levels.
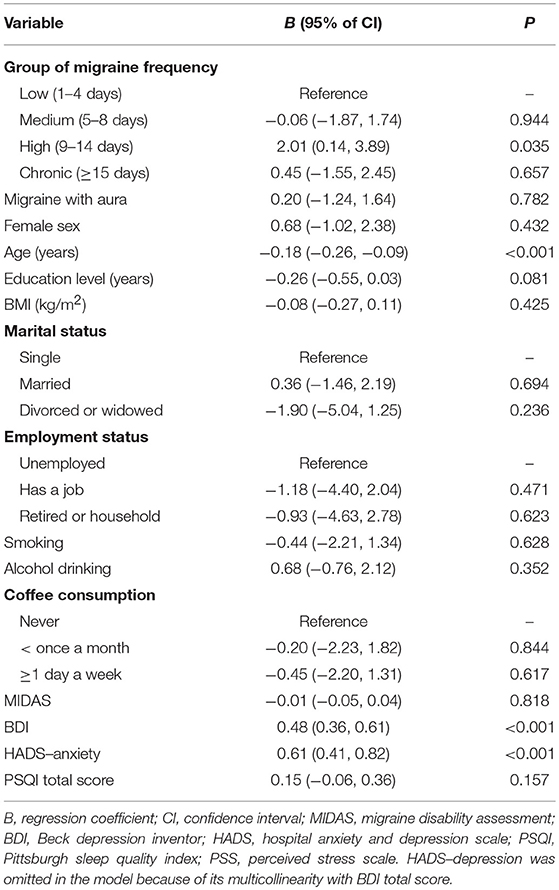
Table 4. Multivariable linear regression analysis of factors associated with PSS scores in the patients with migraine.
Discussion
In our study, after adjusting for aura, sex, age, BMI, education level, marital status, employment status, smoking, alcohol intake, coffee consumption, MIDAS, BDI total score, HADS–anxiety, HADS–depression, and PSQI total score, the perceived stress level was significantly higher in the high than in the low migraine frequency group. We also found a relationship between perceived stress and migraine frequency in the male but not in the female group. Furthermore, migraines with or without auras did not modify the association between migraine frequency and perceived stress. It is noteworthy that there were no significant differences in total PSS score between the high frequency and chronic migraine groups.
Earlier studies have reported that migraine can be triggered by stress (9, 28, 29). A higher level of perceived stress was found previously in migraineurs compared to non-migraineurs (12, 30). Hence, we initially expected to find a trend between migraine frequency and perceived stress levels. Surprisingly, our study revealed that the perceived stress level was significantly higher in the high frequency migraineurs than in the chronic migraine group. It is known that chronic migraineurs can have more mood events, sleep problems and a poorer quality of life than patients with migraine episodes or healthy patients (31, 32). In a prospective cohort study, stress was comorbid with migraine and major depression (33). Various psychosomatic and psychiatric disorders influenced perceived stress in migraineurs (34). The influence of stress in chronic migraineurs partially explained why we identified higher perceived stress levels in those who experienced migraine at a higher frequency, but this was not statistically significant in the chronic migraine group when we adjusted for all potential co-variables, including psychiatric comorbidities.
On the other hand, adaptive and maladaptive changes in the stress response also have been suggested as a mechanism underlying chronic migraine (35, 36). The hippocampus has a role in stress adaptation, and structural and functional changes in the hippocampus have been related to stressful events (36). In addition, migraine has been suggested to alter the hippocampus volume (37). A smaller hippocampal volume may result in persistent pain states (38). In a cross-sectional study of episodic migraine, a significantly larger bilateral hippocampal volume was found in the low frequency group than in the high frequency and healthy control groups. This suggests an initial adaptive plasticity related to migraine that becomes dysfunctional with increased frequency (39). The results between our high and low frequency migraine groups are consistent with this suggestion. However, another study demonstrated different results, indicating no differences in hippocampal volume between groups with a headache frequency of 1–2 or 8–14 days/month, and the volumes peaked at 5–7 days/month (40). Furthermore, our results opposed those of Moon et al., who reported higher PSS scores in chronic migraineurs (19). This discrepancy implies that the role of adaptation to persistent headache and stress with regard to different migraine frequencies remain controversial. Persistent pain may produce stress-like changes in hippocampal neurogenesis (41). We suggest that chronic migraineurs may adapt to the experience of migraine and may also be affected by other psychiatric comorbidities. These may be the reasons we did not observe a significantly higher perceived stress level in this group in our study. Thus, the ability to adapt to persistent headache may influence perceived stress in migraineurs. This may explain why we did not find a clear association between perceived stress and migraine frequency in this or prior studies.
While high frequency migraineurs had a significantly higher level of perceived stress in our study, we investigated whether sex differences existed. The influence of sex on stress and migraine is worthy of discussion. The biological response to stress is associated with activity of the hypothalamic-pituitary-adrenal (HPA) axis, which is regulated by the release of hypothalamic corticotropin releasing factor (CRF) (42). Dysregulation of CRF, which is regarded as a stress neuropeptide, is thought to contribute to the pathophysiology of stress-related disorders (43). Several prior studies have demonstrated sex differences in the response to stress (44–47). Increased acute HPA and autonomic responses have been found in men compared to women (34). Furthermore, women have been previously reported to have higher stress sensitivity and a higher risk for depression and anxiety disorders compared to men (48, 49). Table 3 shows that women had generally higher PSS scores in each migraine frequency group compared to men except for the high frequency group. As psychiatric comorbidities may influence the perceived stress level, our results implied that women had a poor response to stress and more psychosomatic and psychiatric problems, including depression and anxiety disorders, which may have contributed to the insignificant association between migraine and perceived stress.
Several strengths to our study included a large number of subjects, controlled study design, demographically similar groups, sex-differentiated subgroups, use of validated questionnaires, consideration of sleep quality, and anxiety/depression disorders, analysis of migraine subgroups (with or without auras) and robust statistical analysis. Nevertheless, there several limitations also are worth mentioning. First, we used a cross-sectional design, which limited our ability to confirm causal inferences. Future studies with the longitudinal design are necessary to explore the possible causal relationship. Second, our study population comprised patients from a single tertiary hospital, limiting the broad generalizability of our findings. In the future studies, we would involve multiple centers to enroll more patients, and a population-based study may also be warranted. Third, perceived stress was evaluated with a self-rated PSS rather than with an objective assessment. Furthermore, we used the version of the PSS with 14 items, which is one of the most frequently used tools that has great validity (50). However, several recent studies have discussed the use of modified versions of the PSS and demonstrated higher consistency and reliability in a 10-item version (51–53). Among the 14 items used in our study, scores on items 1 and 12 were not consistently greater as the migraine frequency increased. In future studies, we will evaluate different tools for greater reliability and consistency. Lastly, most high frequency and chronic migraineurs had been treated with migraine-preventing agents, such as calcium channel blockers, β-blockers, antiepileptic drugs, or even antidepressants, all of which may have effects on psychiatric comorbidities. For the benefit of the participants, we permitted use of the preventive interventions to improve their quality of life. In future studies, we will take preventive interventions into consideration and adjust for these as confounding variables.
Conclusions
In conclusion, our findings revealed that the perceived stress level was significantly higher in patients with high than in those with low migraine frequency. This also was found in men, but it was not significant in those with chronic migraine or in women. Adaptation to migraine and psychiatric comorbidities may contribute to the association between migraine frequency and perceived stress level.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
Y-CA and F-CY participated in data collection, analyzed the data, and drafted the manuscript. C-SL, J-TL, M-SL, S-JC, C-LT, G-YL, and Y-KL participated in the study design, collected the data and helped to draft the manuscript. F-CY supervised the study, conceptualized, and designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported in part by grants from the Ministry of Science and Technology of Taiwan (MOST 105-2314-B-016-004-, MOST 106-2314-B-016-007-MY2) and Tri-Service General Hospital, Taiwan (TSGH-C106-068, TSGH-C107-072, TSGH-C108-100).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all members in the lab at TSGH for interesting discussions on the manuscript.
Abbreviations
PSS, Perceived Stress Scale; BDI, Beck depression inventor; HADS, hospital anxiety and depression scale; RLS, restless legs syndrome; PSQI, Pittsburgh sleep quality index; MIDAS, Migraine Disability Assessment questionnaire; HPA, hypothalamic-pituitary-adrenal; CRF, corticotropin releasing factor.
References
1. Houle TT, Turner DP, Golding AN, Porter JAH, Martin VT, Penzien DB, et al. Forecasting individual headache attacks using perceived stress: development of a multivariable prediction model for persons with episodic migraine. Headache. (2017) 57:1041–50. doi: 10.1111/head.13137
2. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. (2007) 68:343–9. doi: 10.1212/01.wnl.0000252808.97649.21
3. Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Sem Neurol. (2010) 30:107–19. doi: 10.1055/s-0030-1249220
5. Stovner Lj, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. (2007) 27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x
6. Faedda N, Natalucci G, Calderoni D, Cerutti R, Verdecchia P, Guidetti V. Metacognition and headache: which is the role in childhood and adolescence? Front Neurol. (2017) 8:650. doi: 10.3389/fneur.2017.00650
7. Lipton RB, Buse DC, Hall CB, Tennen H, Defreitas TA, Borkowski TM, et al. Reduction in perceived stress as a migraine trigger: testing the “let-down headache” hypothesis. Neurology. (2014) 82:1395–401. doi: 10.1212/WNL.0000000000000332
8. Chu HT, Liang CS, Lee JT, Yeh TC, Lee MS, Sung YF, et al. Associations between depression/anxiety and headache frequency in migraineurs: a cross-sectional study. Headache. (2018) 58:407–15. doi: 10.1111/head.13215
9. Pellegrino ABW, Davis-Martin RE, Houle TT, Turner DP, Smitherman TA. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. (2018) 38:1188–98. doi: 10.1177/0333102417727535
10. Puca F, Genco S, Prudenzano MP, Savarese M, Bussone G, D'Amico D, et al. Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. The Italian Collaborative Group for the Study of psychopathological factors in primary headaches. Cephalalgia. (1999) 19:159–64.
11. Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol. (2010) 1:16. doi: 10.3389/fneur.2010.00016
12. Wacogne C, Lacoste JP, Guillibert E, Hugues FC, Le Jeunne C. Stress, anxiety, depression, and migraine. Cephalalgia. (2003) 23:451–5. doi: 10.1046/j.1468-2982.2003.00550.x
13. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav. (1983) 24:385–96. doi: 10.2307/2136404
14. Prior A, Vestergaard M, Larsen KK, Fenger-Grøn M. Association between perceived stress, multimorbidity and primary care health services: a Danish population-based cohort study. BMJ Open. (2018) 8:e018323. doi: 10.1136/bmjopen-2017-018323
15. Wiegner L, Hange D, Björkelund C, Ahlborg G Jr. Prevalence of perceived stress and associations to symptoms of exhaustion, depression and anxiety in a working age population seeking primary care–an observational study. BMC Family Practice. (2015) 16:38. doi: 10.1186/s12875-015-0252-7
16. Racic M, Todorovic R, Ivkovic N, Masic S, Joksimovic B, Kulic M. Self- perceived stress in relation to anxiety, depression and health-related quality of life among health professions students: a cross-sectional study from bosnia and herzegovina. Slovenian J Public Health. (2017) 56:251–9. doi: 10.1515/sjph-2017-0034
17. Guidetti V, Faedda N, Siniatchkin M. Migraine in childhood: biobehavioural or psychosomatic disorder? J Headache Pain. (2016) 17:82. doi: 10.1186/s10194-016-0675-0
18. Holm JE, Lokken C, Myers TC. Migraine and stress: a daily examination of temporal relationships in women migraineurs. Headache. (1997) 37:553–8. doi: 10.1046/j.1526-4610.1997.3709553.x
19. Moon HJ, Seo JG, Park SP. Perceived stress in patients with migraine: a case-control study. J Headache Pain. (2017) 18:73. doi: 10.1186/s10194-017-0780-8
20. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. 3rd ed. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
21. Olesen J. ICHD-3 beta is published. Use it immediately. Cephalalgia. (2013) 33:627–8. doi: 10.1177/0333102413487610
22. Lin YK, Lin GY, Lee JT, Lee MS, Tsai CK, Hsu YW, et al. Associations between sleep quality and migraine frequency: a cross-sectional case-control study. Medicine. (2016) 95:e3554. doi: 10.1097/MD.0000000000003554
23. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
25. Atkinson MJ, Allen RP, DuChane J, Murray C, Kushida C, Roth T, et al. Validation of the Restless Legs Syndrome Quality of Life Instrument (RLS-QLI): findings of a consortium of national experts and the RLS Foundation. Qual Life Res. (2004) 13:679–93. doi: 10.1023/B:QURE.0000021322.22011.d0
26. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
27. Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. (2001) 41:854–61. doi: 10.1046/j.1526-4610.2001.041009854.x
28. Sarchielli P. Trigger factors of migraine and tension-type headache. J Headache Pain. (2006) 7:172–3. doi: 10.1007/s10194-006-0304-4
29. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. (2007) 27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x
30. Eskin M, Akyol A, Çelik EY, Gültekin BK. Social problem-solving, perceived stress, depression and life-satisfaction in patients suffering from tension type and migraine headaches. Scand J Psychol. (2013) 54:337–43. doi: 10.1111/sjop.12056
31. Kim SY, Park SP. The role of headache chronicity among predictors contributing to quality of life in patients with migraine: a hospital-based study. J Headache Pain. (2014) 15:68. doi: 10.1186/1129-2377-15-68
32. Lucchesi C, Baldacci F, Cafalli M, Dini E, Giampietri L, Siciliano G, et al. Fatigue, sleep-wake pattern, depressive and anxiety symptoms and body-mass index: analysis in a sample of episodic and chronic migraine patients. Neurol Sci. (2016) 37:987–9. doi: 10.1007/s10072-016-2505-1
33. Swanson SA, Zeng Y, Weeks M, Colman I. The contribution of stress to the comorbidity of migraine and major depression: results from a prospective cohort study. BMJ Open. (2013) 3:e002057. doi: 10.1136/bmjopen-2012-002057
34. Verma R, Balhara YP, Gupta CS. Gender differences in stress response: role of developmental and biological determinants. Ind Psychiatry J. (2011) 20:4–10. doi: 10.4103/0972-6748.98407
35. Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. (2012) 73:219–34. doi: 10.1016/j.neuron.2012.01.001
36. Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. (2012) 52:102–6. doi: 10.1111/j.1526-4610.2012.02241.x
37. Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cerebral Cortex. (2011) 21:435–48. doi: 10.1093/cercor/bhq109
38. Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. (2013) 136:815–27. doi: 10.1093/brain/aws371
39. Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. (2013) 218:903–12. doi: 10.1007/s00429-012-0437-y
40. Liu HY, Chou KH, Lee PL, Fuh JL, Niddam DM, Lai KL, et al. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia. (2017) 37:1329–36. doi: 10.1177/0333102416678624
41. Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. (2006) 7:544–55. doi: 10.1016/j.jpain.2006.01.458
42. Holsboer F. Corticotropin-releasing hormone modulators and depression. Curr Opin Invest Drugs. (2003) 4:46–50.
43. Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Hormones Behav. (2005) 48:1–10. doi: 10.1016/j.yhbeh.2005.01.009
44. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. (1999) 160:1–12. doi: 10.1677/joe.0.1600001
45. Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. (2012) 32:709–23. doi: 10.1007/s10571-012-9824-4
46. Bangasser DA, Wiersielis KR. Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones (Athens). (2018) 17:5–13. doi: 10.1007/s42000-018-0002-z
47. Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. (2006) 31:642–52. doi: 10.1016/j.psyneuen.2006.02.003
48. Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. (2014) 35:320–30. doi: 10.1016/j.yfrne.2014.05.004
49. Ezzati A, Jiang J, Katz MJ, Sliwinski MJ, Zimmerman ME, Lipton RB. Validation of the Perceived Stress Scale in a community sample of older adults. Int J Geriatr Psychiatry. (2014) 29:645–52. doi: 10.1002/gps.4049
50. Lee S, Crockett MS. Effect of assertiveness training on levels of stress and assertiveness experienced by nurses in Taiwan, Republic of China. Issues Mental Health Nursing. (1994) 15:419–32. doi: 10.3109/01612849409006918
51. Leung DY, Lam TH, Chan SS. Three versions of perceived stress scale: validation in a sample of Chinese cardiac patients who smoke. BMC Public Health. (2010) 10:513. doi: 10.1186/1471-2458-10-513
52. Lee EH, Chung BY, Suh CH, Jung JY. Korean versions of the Perceived Stress Scale (PSS-14, 10 and 4): psychometric evaluation in patients with chronic disease. Scand J Caring Sci. (2015) 29:183–92. doi: 10.1111/scs.12131
Keywords: perceived stress, headache, migraine, frequency, adaptation, sex difference
Citation: An Y-C, Liang C-S, Lee J-T, Lee M-S, Chen S-J, Tsai C-L, Lin G-Y, Lin Y-K and Yang F-C (2019) Effect of Sex and Adaptation on Migraine Frequency and Perceived Stress: A Cross-Sectional Case-Control Study. Front. Neurol. 10:598. doi: 10.3389/fneur.2019.00598
Received: 18 March 2019; Accepted: 21 May 2019;
Published: 05 June 2019.
Edited by:
Uwe Reuter, Charité Medical University of Berlin, GermanyReviewed by:
Marco Carotenuto, Università degli Studi della Campania Luigi Vanvitelli Caserta, ItalyAli Sazci, Kocaeli University, Turkey
Copyright © 2019 An, Liang, Lee, Lee, Chen, Tsai, Lin, Lin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu-Chi Yang, ZnVqaS15YW5nJiN4MDAwNDA7eWFob28uY29tLnR3
 Yu-Chin An
Yu-Chin An Chih-Sung Liang
Chih-Sung Liang Jiunn-Tay Lee
Jiunn-Tay Lee Meei-Shyuan Lee4
Meei-Shyuan Lee4 Sy-Jou Chen
Sy-Jou Chen Fu-Chi Yang
Fu-Chi Yang