
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 02 April 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00295
This article is part of the Research TopicStroke Editor's Pick 2021View all 10 articles
Researchers have sought to understand how language is processed in the brain, how brain damage affects language abilities, and what can be expected during the recovery period since the early 19th century. In this review, we first discuss mechanisms of damage and plasticity in the post-stroke brain, both in the acute and the chronic phase of recovery. We then review factors that are associated with recovery. First, we review organism intrinsic variables such as age, lesion volume and location and structural integrity that influence language recovery. Next, we review organism extrinsic factors such as treatment that influence language recovery. Here, we discuss recent advances in our understanding of language recovery and highlight recent work that emphasizes a network perspective of language recovery. Finally, we propose our interpretation of the principles of neuroplasticity, originally proposed by Kleim and Jones (1) in the context of extant literature in aphasia recovery and rehabilitation. Ultimately, we encourage researchers to propose sophisticated intervention studies that bring us closer to the goal of providing precision treatment for patients with aphasia and a better understanding of the neural mechanisms that underlie successful neuroplasticity.
Stroke affects 15 million people worldwide and approximately 800,000 people in the United States, with an estimated one third (35%) of stroke survivors left with aphasia in the chronic stage (2). Furthermore, post-stroke aphasia significantly negatively impacts quality of life (3, 4) and has a greater negative effect than other common diseases such as cancer, Alzheimer's and Parkinson's disease (5). It is therefore important that research advances our understanding of ways to alleviate the social isolation and lack of autonomy in individuals with aphasia. With advances in neuroimaging and other technologies, a large literature has emerged in the past two decades focused on the brain and language recovery, adding substantially to what we know, challenging early ideas about aphasia recovery and providing early and promising results for neuroplasticity in chronic stroke survivors. In this paper we review this work, emphasizing that the age-old nature, nurture dichotomy extends to recovery from aphasia.
Animal models of recovery from brain damage as well as early studies focused on sensory and motor learning in a variety of mammalian species have illuminated our understanding of the adaptive capacity of the brain (6, 7). Extension of this work to human brains has shown that neural networks are dynamic constructs which undergo remodeling throughout the lifespan based on experience. Further, when the brain is damaged, as in stroke-induced aphasia, experience is crucial for rewiring of neural networks (8–10).
One of the most important facts about language recovery after stroke is that it is a non-linear process, with differences in recovery processes and patterns associated with the age of the stroke. While it is well–documented that the greatest changes in the neural architecture for language occur in early stages of recovery, neuroplasticity occurs even in chronic aphasia, when neurophysiological repair processes have largely been completed (11). Three epochs of recovery have been identified including two early phases, the acute phase and the subacute, and a third chronic phase (12).
In the acute recovery period, beginning immediately after stroke lasting several hours, a series of events associated with disruption of neurophysiological and metabolic processes both proximal and distal to the infarct results in detrimental but dynamic changes in the brain. Edema may result in a shift of midline structures, affecting several bilateral cortical, and subcortical regions with associated multiple behavioral deficits. Excitotoxicity, the abnormal release of neurotransmitters such as glutamate in the synapse results in high levels of calcium ions in neurons, which results in cell death. Ultimately, since healthy neurons are deprived of input from destroyed neurons via interruptions in intra- or inter-hemispheric pathways, this cascading process results in additional cell death and transneuronal degeneration. Further, focal infarcts result in hypometabolism in intact but remote regions due to abnormal connections that affects behavior (diaschisis) (13) but there is also evidence that suggests that remote regions show increased activity as a consequence of the stroke (14). Changes in blood flow (perfusion) also result in hypoperfusion in both cerebral hemispheres and particularly in the penumbral (perilesional) region within the first 24 h following stroke onset (15).
The subacute phase begins a few days after stroke and lasts several weeks, during which the brain undergoes several changes enabling spontaneous recovery and repair. In this phase, edema is resolved and several abnormal processes return to more normal state. In addition, as the injured tissue recovers from detrimental events, several brain-repair-related events occur. Synaptogenesis results in new connections that may either involve unmasking of previously latent pathways or formation of new pathways (12, 16). Additionally, if the cell body remains functional, axons and dendrites may regenerate leading to axonal and collateral sprouting, which expand existing synaptic connections and consequent neurogenesis in cortical tissue adjacent, and remote, to the infarcted tissue (17). These processes reach peak levels during this period, resulting in a shift from initial increases in excitation of contralesional tissue due to decreases in inhibition from the lesioned hemisphere, to upregulation of functionally viable brain tissue (12). In the motor recovery literature, several studies have noted functional improvement and concomitant engagement and/or reengagement of both ipsilesional and contralateral connections during this period [see (16) for a review]. With respect to language, Saur (18) found significantly increased contralesional (right) and reduced ipsilesional (left) hemisphere fMRI activation (as compared to neurotypical adults) immediately following stroke onset, followed ~12 days after stroke by an upregulation of spared tissue in regions within left as well as right hemisphere regions homologous to left language areas. By 1-year post-stroke, peak activation decreased in the right hemisphere, shifting to greater activation in the left. The relation between these activation shifts and recovery of function, however, is unclear since the natural history of language recovery and associated neural activation has not been charted across early and chronic phases of recovery. We return to this issue below as it relates to the role of the right hemisphere in language recovery.
The third phase of recovery, and most pertinent to this paper, is the chronic phase, which may span months to years after stroke. Although physiological changes occurring during the repair phase have generally subsided as the brain reaches a stable state, mechanisms that facilitate plasticity (i.e., synaptic sprouting) remain at play and are adaptable to environmental experience. Notably, it is now known that brain changes occur throughout the life span, particularly associated with experience (8, 9, 19). For many stroke survivors with aphasia, treatment improves language abilities and associated neural processing of language several years post-stroke (20). However, not all patients show the same degree of recovery over time. This is partly because, among other reasons that influence recovery, the neural sequela of stroke persists into this phase of recovery (21) and interact with experience.
There are several factors that influence neural plasticity, including both biological and environmental and, as with any learning system, recovery from aphasia involves a synergistic relation between them (22, 23). We refer to these as organism intrinsic and organism extrinsic factors. Intrinsic factors consist of behavioral and neural variables related to the stroke itself, including lesion characteristics and patterns of impaired language, whereas extrinsic factors include environmental factors such as treatment (see Figure 1). Recognizing the importance of other intrinsic factors such as personality traits and domain-general cognitive functions (e.g., attention, memory, executive function) as well as extrinsic psychosocial variables (e.g., participation in social and work-related activities, and support systems), here we constrain our discussion to neural and treatment variables.
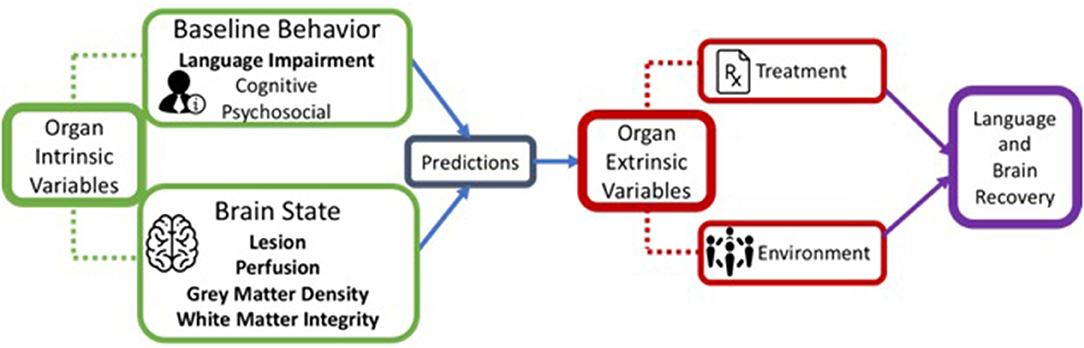
Figure 1. A schematic representation of organism intrinsic variables and organism extrinsic variables that influence language recovery.
The neural factors that impact recovery include (but are not limited to) age at the time of stroke, lesion volume and location, vascular pathophysiology, and white matter integrity.
The age of the brain at the time of stoke is well-known to impact recovery, in that younger brains are more plastic than older brains. Thus, childhood stroke-induced aphasia has better overall outcomes than adults, although learning and other cognitive skills may remain compromised (24, 25). In adults, the picture is quite different although the stroke-aphasia literature suggests a tendency for older patients to recover less well-than younger patients (26). This may, in part, be related to findings that language and other domain-general cognitive processes shift from being left lateralized to becoming more bilateral in the older brain during the aging process (27). Future research will need to examine whether older adults with aphasia show the same extent of recovery and reorganization as younger adults with aphasia and how/if brain age interacts with other organism internal predictors of recovery.
The relation between lesion characteristics and recovery of function has been addressed by several research groups. In general, large left hemisphere lesions are typically associated with poorer recovery (28, 29), whereas smaller lesions suggest better language recovery. Presumably, this is because smaller left hemisphere lesions leave a greater volume of tissue available for remapping of the language network, whereas large left hemisphere lesions limit tissue available for reorganization of function. Also, larger lesions are more likely to affect a greater number of language operations as well as domain-general systems engaged for language processing (29). However, the relation between lesion volume and recovery is not straight forward. More recent research suggests that while overall lesion volume may explain the degree of language recovery, the location of the lesion is equally if not more important in predicting recovery (30). In the motor domain, damage to specific descending tracts more accurately predict recovery than overall lesion volume (31) and lesions to the corona radiata, internal capsule, and insula impact functional outcome on the modified Rankin scale to a greater extent than lesion volume alone (32). Likewise, with respect to language recovery, Hillis found that damage to left posterior superior temporal gyrus and fibers that included superior longitudinal fasciculus and arcuate fasciculus negatively influenced the degree of recovery of naming skills (33) in acute and chronic patients with aphasia, and these findings were independent of total lesion volume. Correspondingly, sparing of tissue in left temporal parietal regions, for example, has been associated with improvements in naming resulting from treatment (34, 35).
However, other studies have found no relationships between recovery of naming skills and damage in any left hemisphere region (36). In a different analysis, Skipper-Kallal and colleagues found that total lesion volume in the left hemisphere predicted right hemisphere activation for both overt naming and covert word retrieval (37). However, no relationships were found between naming skills and damage in any given LH region when the authors controlled for total lesion volume and no relationships were observed between naming skills and right anterior activation suggesting that damage to LH IFG regions does not necessite activation in the corresponding RH regions. These results suggest that reorganization of language, while dependent on lesion size and site, may be more complex than the simple reengagement of homologous regions. Another recent study focused on recovery of sentence processing, however, found that although greater left hemisphere lesions within language-specific networks was associated with recovery and concomitant recruitment of right hemisphere neural networks, lesser damage to left domain-general network predicted recovery (38).
It should be noted that while aforementioned studies have specifically examined language processing and recovery, a number of studies have examined specific lesion locations that are associated with specific language impairments. The reader is referred to recent extensive work utilizing Voxel-based Lesion Symptom Mapping (VLSM) (39–43) and other quantitative approaches (39, 44) that provide insight into gray matter regions that are crucial for language functions by establishing the relationship between damaged/infarcted tissue and impairments in naming, semantic processing, phonological processing, spelling, and sentence processing. VLSM and other lesion-behavior approaches provide important information about which regions of the brain are critical for performance of certain language functions, but provide little insight into the brain mechanisms that may be recruited for recovery of language.
Like most other unresolved debates in aphasia recovery, the issue of the influence of lesion size and location may ultimately require large amounts of data to account for the inherent heterogeneity in stroke brains. Promising ongoing work such as the Predicting Language Outcome and Recovery After Stroke (PLORAS) database, which is a data repository of speech and language performance measures from standardized assessments and MRI scans, will provide important insights into individualized predictions of behavioral recovery based on lesion size and location information (29, 45, 46). For instance, a recent analysis of 818 stroke patients, which developed predictive models of naming scores as well-language scores, showed that disruption in connectivity between regions in the brain is associated with overall lesion location, but lesion location was sufficient to predict specific language outcomes (47). It may be that future work in this area should first focus on identifying the best methodology to delineate the singular or compounded effect of lesion location and the extent of the disruption before we can draw firm conclusions on this topic.
Until recently, the integrity of non-infarcted tissue has received little consideration with regard to remapping of language networks in chronic aphasia. Hypoperfusion (decreased blood flow) in acute stages of recovery has been well-studied, particularly within the ischemic penumbra, which is hypoperfused but still viable in the cortical tissue surrounding the irreversibly damaged ischemic core. Blood flow in this region is delayed more than 2 s beginning 3 to 6 h after stroke onset and lasting up to 72 h after stroke (48). If the penumbral region is reperfused within this time window it can be salvaged (49, 50). Hillis et al. have elegantly demonstrated that reperfusion of this at-risk tissue is possible, saving it from eventual fusion into the lesion core which increases lesion volume (51–55).
Importantly, recent research indicates that abnormal perfusion continues into the chronic phase of recovery (56). Even after ischemic penumbra resolution, perilesional hypoperfusion persists. Using arterial spin labeling to evaluate peak perfusion values in various parts of the brain, Richardson and colleagues (57) found a decrease in perfusion in the tissue surrounding the lesion in patients with chronic aphasia. Thompson and colleagues (58) replicated this finding, showing hypoperfusion within perilesional space, with the greatest hypoperfusion in tissue closest to the lesion. In addition, they found abnormal perfusion distal to the lesion in the left as well as in the right hemisphere. In another study, hypoperfused tissue negatively correlated with changes in blood oxygen level dependent (BOLD) signal in regions engaged to support treatment-induced language recovery (59). Nonetheless, the extent to which shifts in perfusion influence recovery of the neural networks for language is still under investigation.
The degree of white matter integrity also influences the extent of behavioral recovery after stroke. Notably, Catani (60) showed that most middle cerebral artery strokes impair not only gray matter, but also extensively impair subcortical white matter tracts in the infarcted hemisphere. Hence, it is not surprising that the few published studies specifically investigating the relation between white matter tract integrity and language abilities in people with stroke-induced aphasia have found that disruption of and/or impairments in [as indicated by abnormal fractional anisotropy or signal intensity in diffusion tensor imaging (DTI)] at least some white matter tracts impact language. Specifically, relationships have been found between disruption of the left hemisphere tracts and language, i.e., between the arcuate fasciculus and speech fluency (35, 61–63), the superior longitudinal fasciculus and naming (64, 65). The integrity of the left uncinate fasciculus (UF) also may play a role, although mixed findings have been reported. Whereas, one large study found a strong relationship between the UF and object naming (64), others have not found this association (61, 65, 66). Even less studied are tracts such as the extreme capsule (that includes UF and IFOF) which also appear to be related to language skills (67, 68). It follows, then, that left hemisphere white matter integrity may be a strong predictor of language recovery following stroke (69). Indeed, as noted above, Hillis et al. noted that damage to left posterior superior temporal gyrus and superior longitudinal fasciculus and arcuate fasciculus negatively influenced naming recovery (33). In another study, integrity of the left inferior longitudinal fasciculus and left inferior fronto-occipital fasciculus was related to baseline and recovered naming skills in a group of treated patients (70). Additional studies examining the relation between white matter integrity and language impairments across language domains (and tracts) will help to clarify the impact of damaged structural connections between crucial gray matter regions on language breakdown and recovery.
The integrity of white matter tracts in the contralesional (i.e., right) hemisphere is also likely to be associated with recovery. Notably, cognitively healthy people show interhemispheric differences in white matter connections between Broca's and Wernicke's areas (i.e., the long segment of the arcuate fasciculus) (71). Based on diffusion tensor MRI (DT-MRI) and tractography analyses with 40 healthy young participants (20 males), 50% of participants showed extreme leftward lateralization, whereas, and only 17.5% showed white matter tract symmetry across the two hemispheres. They further showed sex differences in the degree of lateralization, with 85% of males, but only 40% of females, showing leftward asymmetry. First, these results highlight the high degree of variability in white matter pathway structures across individuals which critically impacts our interpretation of how white matter pathways are altered after a stroke. Second, these findings suggest that the premorbid presence and volume of right hemisphere white matter tracts likely play a role in whether or not the right hemisphere is engaged to support language in post-stroke aphasia. Forkel et al. (60) showed this in a study with 16 patients with stroke-induced aphasia during the acute stage of recovery (i.e., from 2 weeks to 6 months post-stroke). Using performance on the Revised Western Aphasia Battery [WAB-R, (72)] as an index of language ability, the results showed that the volume of the right hemisphere long segment of the arcuate fasciculus predicted language severity scores at 6 months post-stroke.
A few recent studies have examined changes in white matter connectivity as a result of language recovery (66, 73). For instance, Van Hees et al. examined changes in the generalized fractional anisotropy (GFA) values for the left hemisphere arcuate fasciculus in patients who received treatment for anomia and found that prior to treatment GFA was lower for patients relative to healthy controls but after treatment, no differences were observed between patients and controls (74). Likewise, Schlaug et al. (75) found changes in the right arcuate fasciculus in six patients with chronic aphasia resulting from a course of Melodic Intonation Therapy. Finally, Wan and colleagues showed that language therapy results in changes in the FA values in the right hemisphere, specifically in white matter regions underlying frontal, posterior superior temporal and cingulate regions (76).
In summary, even in chronic stages of recovery, lesion variables continue to influence language recovery. We have identified a few that have received the most attention in the aphasia recovery literature, but point out that there is likely an interplay between these and other factors. Further, the relation between these factors and language recovery will likely differ (a) for different areas and tracts of the brain, (b) for different language functions, and (c) as a function of lesion volume, lesion and chronological age and other factors. Future research will need to address these issues as well as how/if these and other brain variables interact with one another to impact recovery.
The fact that environmental factors affect recovery of neural networks following brain damage in both animals and humans is undisputed (8, 77–81). With respect to recovery of language networks, Thompson (82) posed questions about the role of experienced-based neural plasticity in aphasia, that is treatment-induced plasticity, in a special (millennium) issue of Brain and Language focused on key research issues for the 21st century. Among the questions posed at the time were: “does treatment influence reorganization of the language network…or does language reorganize in a…biologically predisposed manner, considering site and extent of lesion and other variables?” (p. 245). Since then, a large number of people with aphasia have been entered into studies examining this question, with results indicating that treatment does influence language reorganization in the brain, although most studies are single case reports or have included few participants, and they have not always monitored the natural history of neural change in untreated patients (i.e., in aphasic controls) or considered the reliability of repeated scans, leaving the reported results unclear. Most studies in the aphasia treatment literature also have tested the effects of naming therapy, with far fewer focused on other language impairments and no studies have examined the differential effects of treatment applied to different language domains. A strong case in support of treatment-dependent neural recovery would require this. In addition, neuroimaging methods have significantly advanced in recent years, and will continue to advance, perhaps calling into question the results of early neuroimaging studies of aphasia recovery.
The second question is far more difficult to answer. Neuroimaging studies of aphasia treatment using primarily functional magnetic resonance imaging (fMRI) have shown pre- to post-treatment changes in activation in undamaged tissue within the lesioned (usually left) hemisphere, the contralesional hemisphere, or both. A review of the aphasia treatment literature between 1996 and 2016 identified a total of 41 studies, which included 628 aphasic participants across studies (83). Of those, 90 study participants showed upregulation of neural activation in the right hemisphere (46, 59, 84–88), suggesting a transfer of language function from the left to the right, 99 participants showed increased activity in the left hemisphere (89–92) and 439 patients showed bilateral recruitment of neural tissue associated with treatment-induced language improvement (59, 93–98). In addition to increases in activation, patients in several studies also have shown downregulation of neural activation associated with successful treatment outcome, possibly reflecting increased processing efficiency (99–101).
Despite increasing evidence that therapy-induced reorganization may engage bilateral neural tissue, the best candidates for supporting language recovery in the chronic phase remains an unresolved issue. Some argue that neural tissue within the left hemisphere has greater potential to support language recovery (34). Because in most humans language is primarily processed in the left, this tissue likely has a biological capacity to support recovery. There is also a strong basis for the right hemisphere to be engaged to support language recovery, given that regions within it are engaged (albeit to a lesser extent than left hemisphere) for normal language processing in healthy adults (102), particularly as adults age (27). Reports of language recovery in left-brain-damaged patients who, after a second right-brain stroke loose the language recovered (103, 104) suggest a predisposition for language in the right hemisphere. Further, as noted above, many studies of language recovery in chronic stroke show post-treatment right hemisphere activation (96, 98, 105).
A strong theory supporting left hemisphere recruitment for recovery suggests that activation of tissue in the contralesional (right) hemisphere may be maladaptive and reflect increases in inhibitory processes exerted by the right hemisphere on the left. The excitatory/inhibitory imbalance between hemispheres have been clearly shown in early phases of recovery, with greater activation in the right hemisphere, due to decreased inhibition from the damaged left hemisphere (18). Although this asymmetry resolves in later epochs of recovery and is associated with shifts from right to left hemisphere activation, Heiss and Thiel's (106) theory of interhemispheric inhibition suggests that an imbalance between the hemispheres persists into chronic states of recovery and that right hemisphere recruitment is associated with poorer, rather than improved language performance. Studies using noninvasive brain stimulation (NIBS) have shown that inhibitory stimulation (e.g., low frequency transcranial magnetic stimulation) applied to right hemisphere regions improves language ability (107, 108), suggesting that this diminishes the inhibitory effects of the right hemisphere and enhances left hemisphere recruitment for language. Notably, however, most of these studies have shown very small changes in language [see (109), for review]. In addition, several studies using NIBS applied to the left hemisphere also have found bilateral increases in neural activation associated with language improvement (96).
Regions recruited across participants, even within language domain, vary greatly and involve left perisylvian, extrasylvian and right hemisphere homologous-region activation for various language processes. Thus, the left vs. right hemisphere debate may be an oversimplification in that recruitment of neural tissue in both hemispheres may be beneficial for recovery, which likely reflects a complex and dynamic interplay between the two. As we elaborate in the next section, resolution of these issues in future research may be best accomplished by using a network approach to study the potential neural substrates for language recovery, rather than focusing on individual regions within the language network.
To underscore this point, another line of evidence has shown activation outside of the core fronto-temporal language network during language processing in patients, including in parts of the middle frontal gyrus, the precentral gyrus, and inferior parietal cortex (98, 110–113). While in many cases activation is observed when damage to the fronto-temporal language network is substantial (110) other studies have shown activation in these domain-general regions even when frontal and/or temporal regions are spared (98, 112). Work in healthy adults has shown that these domain general regions, associated with attention, working memory, cognitive control and fluid intelligence (114), are engaged for effortful language processing (115) such as understanding or producing complex syntactic structures or ambiguous words (116, 117). Importantly, activation in these regions has been associated with the hypothesis that stroke patients recruit domain-general regions due to the increased cognitive effort required for processing language after stroke (113, 118, 119). This premise is in line with one principle of neuroplasticity—that novel functions can be assumed by tissue that was previously not engaged in those functions (9, 12). What is not yet known is whether engagement of these domain general regions, in conjunction with language regions, is associated with better language recovery in patients. Future work will need to confirm or refute this hypothesis.
It is now well-documented that language processing involves a complex network of left and right hemisphere regions that are often structurally and/or functionally connected (120–123). It seems reasonable to posit that language recovery engages the same complex network of left and right hemisphere regions with specific regions in the network becoming preferentially involved during the course of recovery depending on organism intrinsic and extrinsic factors, including lesion age, size, site, and treatment success. Indeed, the notion of changes in functional networks in motor and cognitive systems, altered proximally and distally by focal lesions has received substantial validation (124–127). As noted, above, language processing in PWA is also associated with activity in domain-general regions (113, 118, 119). Within the domain of language, changes in structural and functional networks also have received recent attention, examined using different approaches, including (a) structural networks (i.e., DTI), (b) task-based fMRI, and (c) resting state fMRI. Figure 2 illustrates a schematic of reduced functional connectivity (both structural and functional) in a patient with a large left temporal lesion and aphasia, compared to healthy controls, in both the left and right hemsipheres. Per the discussion above, differences in connectivity are reflected in language-specific regions as well as domain general regions. Studies also are emerging showing treatment-induced changes in connectivity (see later).
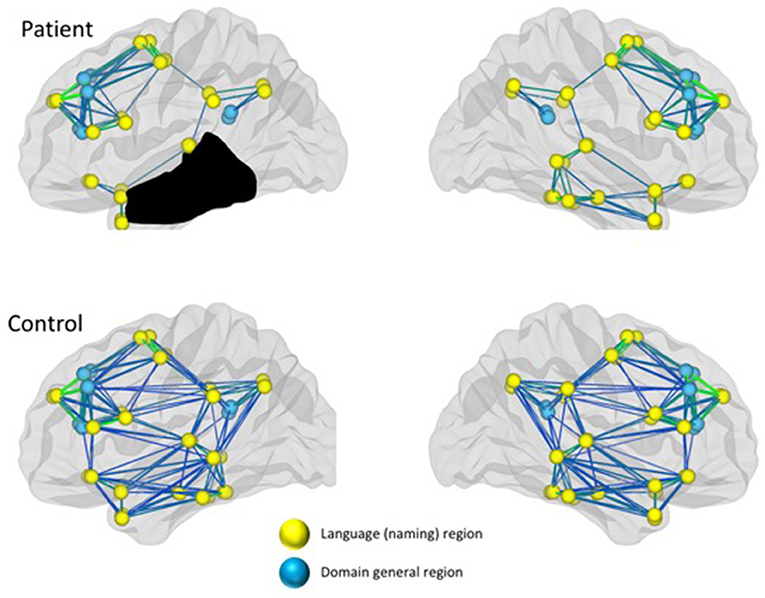
Figure 2. Reduced connectivity in both language and domain-general networks in a patient with aphasia compared to healthy controls.
Recent work by Bonilha et al. highlights the notion that disruption of structural connectivity, that is, white matter tracts, also have far-reaching impact on language and consequently language recovery (128–132). In one study, preservation of global network integrity and, in particular, connectivity of the left middle temporal lobe was associated with greater naming treatment outcomes (34). This finding implicates the integrity of the left arcuate fasciculus as important for recovery since temporal lobe structural connectivity engages this tract. In addition, it emphasizes how network-level measures of gray and white matter regions converge to inform language recovery.
Other studies have used task-based fMRI to examine functional connectivity in language networks to understand language recovery. Examining recovery of naming, one study found changes in connectivity among four left hemisphere language areas (IFG, MTG, insula, and IPL) and their right hemisphere homologs, which were strengthened for trained but not untrained items (133). Another naming recovery study found a similar pattern, with treatment-induced network changes noted in right and left hemisphere IFG and MFG (98). Additionally, across patients, the left IFG was the most modulated region in the network as a function of rehabilitation. Another study found that the neural networks for trained and untrained items that improved with treatment showed similar changes in connectivity among language nodes. Conversely, for items that did not improve with treatment (i.e., to which generalization was not observed) the network appeared similar to networks in patients who had not been trained (111). These results indicate that networks that support training-related naming improvements differ from those engaged when naming is unsuccessful.
Task-free MRI [i.e., resting-state (rsFMRI)] data also have been used to examine the effects of brain damage on language (134–137) as well as network changes associated with recovered language. While evidence is still emerging and the studies published in this domain have utilized different approaches, all indicate that rsFMRI networks are abnormal after stroke. One study showed reduced local regional connectivity in contralesional hippocampal/para hippocampal and ipsilesional parietal and occipital regions in acute post-stroke aphasic patients (134). Another study showed hypoconnectivity across a variety of networks including attention, motor and salience networks, with the greatest reduction in connectivity within the semantic network (138). A few studies also have examined changes in resting state connectivity over time. One examined four patients who suffered posterior cerebral artery strokes over three time points (i.e., in the first week, between 3 to 5 weeks, and between 5 to 7 months) and found within and across hemisphere increases in functional connectivity associated with improved language function (139). Another study examined 14 acute stroke patients 1 and 2 months after their stroke and found that lower language comprehension was associated with lower connectivity at the first time point but at the second time point, when language skills had improved, increases in connectivity were noted (140). Improved connectivity also was reported in a study with over 100 stroke patients by Siegel and colleagues. Early post-stroke most networks in the brain were disrupted, however, the degree of local and between network integration improved over time and was associated with improved behavioral recovery (141). Similarly, Van Hees et al. (142) examined changes in amplitude of low frequency fluctuations (ALFF) (a measure of spontaneous fluctuations in fMRI signal intensity) and found reduced connectivity (correlations in ALFF) between left MTG and STG that normalized after treatment and greater connectivity between left MTG and IFG after treatment. Yet another study reported a shift in resting state modularity following treatment, compared to pre-treatment, and these changes correlated with improved language production after treatment (143). While it is premature to draw strong conclusions about what studies examining resting state networks in aphasia explicate with respect to the nature of reorganization in the brain, more work on this topic will be undoubtedly important.
To summarize, a surge in research examining the neural correlates of treatment-induced recovery, identifying regions of the brain that may be recruited to support recovery of language, and detailing both structural and functional changes in brain networks underlying reorganization have informed basic notions of neural plasticity in the aphasic brain. In due course, as this line of research becomes more and more sophisticated, it will lead to a comprehensive account or model of therapy-induced reorganization in the brain and will provide the foundation for targeted therapies to capitalize on this. Until then, in addition to intervention approaches that have been well-researched, with documentation of both the behavioral and neural effects of treatment, researchers and clinicians may rely on basic principles for promoting neural plasticity derived primarily from animal studies and research in the domain of recovery from stroke-induced motor impairments, as discussed below.
As highlighted earlier in this paper, results of animal studies have served to inform what we know about experience-induced plasticity following brain damage. Based on this research, Kleim and Jones (1) summarized 10 principles shown to be important for recovery that may also enhance plasticity and language reorganization in people with aphasia. Given the large literature that has accumulated since then examining the neural mechanisms of language recovery, we condense these into six basic principles relevant to aphasia treatment (see Table 1). We also add a new principle that has emerged in the aphasia treatment literature concerning complexity in language learning and recovery. We discuss these as well as the extant data and, where appropriate, approaches to aphasia treatment that support them.
Principle 1 is based on the premises that “training that drives a specific brain function can lead to an enhancement of that function” and, conversely, that “failure to drive specific brain functions can result in functional degradation” (Kleim and Jones, p. s227). In the domain of language recovery, these principles suggest that (1) treatment focused on impaired language processes may lead to recovery of the underlying neural mechanisms associated with those processes, and (2) underuse of “specific” language systems following stroke may lead to a decrease in the ability to engage existing or new neural networks that support it. These principles argue for the use of treatment approaches that target impaired language processes.
In the domain of motor recovery, constraint induced therapy (CIT) is an approach fitting Principle 1 (144). For example, patients with unilateral upper extremity hemiparesis are provided with physical constraint of the unimpaired extremity, forcing use of the impaired limb. Some researchers have introduced a form of this for treatment for aphasia—Constraint Induced Language Therapy (CILT), which requires patients to use spoken language responses during therapy, rather than gesture, writing, or other modalities to augment communication. Although similar in principle to CIT, forcing language production per se may not drive specific brain functions, thus treatment may not affect specific language processes (e.g., phonological, lexical-semantic, syntactic networks). Indeed, the majority of studies of CILT have used general outcome measures of word-retrieval, repetition, and auditory comprehension as evidence for its effectiveness (66, 145–148), and although this evidence is strong, other approaches have been found to be equally or more effective (149). For example, a recent study examining the effects of CILT compared to other treatments showed that semantically-based treatment resulted in greater improvement (150). This finding suggests that treatment that targets a specific language domain may result in the strongest outcomes. This point is further discussed in the next section on the principle of treatment specificity.
In keeping with Principle 1, Principle 2 suggests that treatment specificity rebuilds specific language networks, pointing to the use of treatment focused on primary impairments that exploit what is known about normal language representation and processing. In the aphasia treatment literature, several treatments based on psycholinguistic and cognitive neuropsychological research and models of language have emerged in recent years, which focus on improving specific language processes. In general, studies examining the effects of these treatments show positive treatment outcomes. Further, several of these impairment-based approaches also have shown neural changes associated with improved language processing, as reviewed earlier in this paper (97, 98, 105, 111).
We mention a few of these treatments here. Semantic Feature Analysis (151, 152) exploits what is known about semantic knowledge and how this knowledge connects words with one another in the mental lexicon. Similarly, phonomotor treatment (153) targets phonological networks, controlling properties of speech sounds in trained and untrained words to promote word retrieval ability. In the domain of verbs and sentence production, Verb Network Strengthening Treatment (154), focuses on improving verb production by training the lexical selection properties of verbs. Treatment of Underlying Forms (155) also makes use of the lexical properties of verbs to improve sentence comprehension and production, using a set of metalinguistic steps focused on thematic role assignment and syntactic mapping. These treatments hold promise for rebuilding language networks in stroke-induced aphasia. Research is needed to further identify the effects of these treatments on both the neural and cognitive mechanisms associated with language processing as well as the differential effects of these and other approaches. Finally, research is needed to develop and test additional treatments that exploit normal language processes.
The salience of experience also influences neural recovery. This refers to the extent to which training invokes neural systems that promote encoding, including motivation and attention. In the animal literature, studies show that when reward is coupled, for example, with an auditory tone of a particular frequency, an increase in cortical representation of that tone, but not others, occurs, indicating that motivation enhances neural plasticity (156). Attention also modulates neural activity as demonstrated in motor learning studies with both humans and animals. For example, when attention is directed away from muscle stimulation, motor encoding fails (157). This principle has not been well-explored in the context of language rehabilitation. However, several studies using fMRI have shown increased activation in domain-general mechanisms (i.e., attention/executive function) associated with treatment-induced recovery of language in patients with aphasia (118, 119). The principle of salience implies that language reorganization in aphasia may be boosted by training that couples specific treatment with methods for enhancing motivation and attention, such as using functionally significant training stimuli and/or training in functionally relevant contexts as espoused by functional- and participant-oriented treatment approaches (158, 159).
Repetition and intensity of treatment were posed as two separate principles for promoting neuroplasticity (1). We combine these into a single principle due to the lack of a clear distinction between the two. The animal learning and sensorimotor learning literature suggest that repetition is important for facilitating neuroplasticity. Indeed, neural connections have been shown to develop through repetition, making the acquired behavior resistant to decay (160). For example, rats require repeated training trials focused on skilled-reaching behavior before measurable changes in the strength or number of synapses (161) motor map reorganization (162) are manifest. The animal learning literature also indicates the need for repeated training trials over time to facilitate maximal neural plasticity (161). Notably, principles of aphasia rehabilitation also have historically emphasized repetition. Schuell advocated “restimulation” of the language system with sufficient repetitions and providing many opportunities to respond to or produce target language behaviors both within individual treatment sessions and over time (163, 164).
The number of trials required for learning (or re-learning) and the time over which learning trials are delivered, however, is unclear. Intense training schedules have been reported to promote language recovery in aphasia, with studies indicating that higher intensity stimulation results in greater treatment gains than lower intensity stimulation (165, 166). However, the results of some other studies suggest otherwise, finding that intensive vs. less intensive treatment does not result in differential outcomes (167). Further, recent studies have shown that even though intensive treatment (i.e., massed practice) may aid with acquisition of treatment sets, maintenance of treatment effects may be boosted by non-intensive treatment, spaced over time (168, 169). Based on these studies, the benefits of intensive language treatment on facilitating optimal neuroplasticity are not yet conclusive, however, the lack of clear evidence depends partly on how intensity is defined across studies. It is unclear, for instance, whether intensity refers to the number of training hours [e.g., 2 vs. 4 h (168, 170)], the duration or spacing of training (e.g., 5 or 50 weeks of treatment with a total of 100 h) (171), or the actual number of treatment hours provided [e.g., 19 vs. 26 h (172)]. Further, studies have not explicitly addressed the content of training provided on these schedules, i.e., whether intense or spaced training schedules involve repeated training trials for improving a particular function (e.g., naming trials) or if a variety of language tasks are provided within training intervals. These differences in how intensity is quantified complicates our understanding of what ‘intensive treatment’ means and how it is related to repetition in promotion of learning and neuroplasticity. There is an emerging acknowledgment of this shortcoming (173, 174) and future studies should quantify and compare varying levels of repetition and intensity to provide more insights into this topic.
Principle 5 concerns generalization, referring to the idea that “plasticity in response to training experiences can enhance the acquisition of similar behaviors” [(126); p s227]. Interference denotes the opposite effect, which potentially restricts generalization.
Generalization to untrained behaviors is an overarching goal of aphasia treatment. Indeed, aphasia treatment studies show that generalization from trained to untrained behaviors is enhanced when there is a fundamental relation between the two, for example, when trained and untrained words share common semantic, phonological, and/or orthographic features (152, 153, 175–177) or when sentences share common grammatical processes (178). These generalization patterns suggest that the same neural circuits engaged during training of target items/structures, are engaged for related untrained items.
On the flipside of this is the principle of interference: that “plasticity in response to one experience can interfere with the acquisition of other behaviors,” indicating that environmentally- or treatment-induced recruitment of, for example, non-linguistic strategies for completion of a language task may interfere with learning and generation of optimal neural networks to support linguistic processing. This principle comes mainly from studies in the motor domain showing the maladaptive effect of learned misuse (or nonuse) and its influence on motor reorganization in the brain (144, 179, 180). An emerging area of evidence for interference in the language domain comes from treatment of bilingual aphasia. In one example, a trilingual patient showed increases in interference from the trained language into the non-trained language (L2 interference to L3, and L3 interference into L2) when L2 and L3 were trained in separate 10 week phases (181). While the overall conclusion of this study impacted the nature of cognitive control in bilingual aphasia, treatment-induced cross-language interference negatively influenced the overall outcomes in the study. Future work will provide more evidence for the presence and prevalence of treatment induced interference that impacts neural plasticity.
The construct of complexity has emerged as a general principle that is relevant to treating a range of language disorders in both children and adults (177, 178, 182–184). While challenging the longstanding clinical notion that treatment should begin with simple structures, the Complexity Account of Treatment Efficacy (CATE); (185) points to the facilitative effects of using more complex structures as a starting point for treatment. In the aphasia treatment literature, the complexity effect in language learning has been found across language domains. For example, training atypical members of semantic categories (e.g., “chicken” within the category of “birds”) improves naming of typical members (e.g., “robin”) and training abstract words (e.g., “justice”) results in generalization to concrete semantically related words (e.g., “jury”) (152, 186, 187). Similarly, in the domain of morphosyntax, training complex syntactic structures (e.g., object relative or full passive) results in improved comprehension and production of less complex, linguistically related structures (e.g., object wh-questions, truncated passives, active sentences with unaccusatives verbs) (38, 59). Notably, training simpler words within lexical categories or sentence structures does not promote generalization to more complex words or structures. From a neuroplasticity perspective, these findings suggest that the neural mechanisms engaged for complex linguistic processes are also engaged for simpler ones, if/when the complex and simple material is related. Processing both abstract and concrete words, for example, engages lexical-semantic processing networks, however, concrete, but not abstract works recruit additional sensorimotor processing nodes. Thus, training abstract words within a semantic category strengthens connections within the entire network (169). Similarly, within the domain of sentence processing, complex sentences entail processes inherent in simpler, linguistically related sentence (e.g., thematic role assignment and syntactic mapping). Hence, strengthen the neural network for processing complex sentences unlocks networks required for processing simpler ones.
Recovery from stroke-induced aphasia reflects a complex interplay between organism internal and external factors. The impact of nature, that is, the neural sequela of stroke is well-known to influence recovery—a non-linear process affected by neurophysiological changes in brain states from acute to chronic phases of stroke. The influence of nurture on neuroplasticity is less well-understood, however, the fact that the brain is sculpted in an experience-dependent manner throughout the lifespan argues strongly that the environment affects recovery of language networks. Emerging work has identified several organism internal and external factors that affect recovery, however, we emphasize that continued research is needed to completely understand the limits of brain malleability, particularly in chronic aphasia, and what variables are related to it.
On the nature side, we have highlighted research showing the influence of several lesion variables associated with stroke-induced language impairments, however, further research examining these and other variables as well as how, or if, they interact with one another to affect recovery is needed. The integrity of domain-general cognitive (e.g., attention, memory and executive function) and metacognitive impairments and associated neural networks also may be central to recovery and to date have received little attention in the aphasia recovery literature. Similarly, little work has focused in intrinsic psychological factors such as motivation and other personality traits. Research in these, and related domains, will be informative as to the role they play in neuroplasticity.
On the nurture side, several treatments for aphasia that exploit psycholinguistic and cognitive neuropsychological models of normal language representation and processing have been developed and studied. Indeed, these treatments show positive influences on both behavioral and neural processing. However, research in this arena is in its infancy. Research is needed not only to develop new treatments targeting specific language domains, but also to identify the differential effects of these treatments. In addition, research examining how, or if, the effects of these treatments may be enhanced, for example, by coupling non-invasive neural stimulation such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) with behavioral treatment, will help to inform the limits of environmental manipulations on the brain and language recovery. Finally, it is important to point out that research focused on the effects of functional social-based treatments for aphasia on neural plasticity is sorely needed. These treatments for example, may impact motivational systems, which in turn may influence language networks.
As this research unfolds, the field will move closer to developing a model of treatment-induced neural reorganization, which will provide the foundation for treatment selection and application for people with aphasia. Indeed, such a precision medicine approach is the overarching goal for clinical treatment of aphasia: to prescribe treatment and predict its outcome based on the neurocognitive deficits a patient presents, with treatment optimized for specific language deficits.
SK and CT equally contributed to the article. Both were involved in the conceptualization of the paper and in the content presented in the paper.
This work was supported by the NIH/NIDCD Clinical Research Center Grant, P50DC012283 (PI: CT; Subproject 1 PI: SK; Subproject 3 PI: CT).
SK is a scientific consultant for The Learning Corporation but there is no scientific overlap with this manuscript.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. (2008) 51:S225–39. doi: 10.1044/1092-4388(2008/018)
2. Berthier ML. Poststroke aphasia. Drugs Aging. (2005) 22:163–82. doi: 10.2165/00002512-200522020-00006
3. Koleck M, Gana K, Lucot C, Darrigrand B, Mazaux JM, Glize B. Quality of life in aphasic patients 1 year after a first stroke. Qual Life Res. (2017) 26:45–54. doi: 10.1007/s11136-016-1361-z
4. Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, et al. Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2016) 97:2188–201 e8. doi: 10.1016/j.apmr.2016.03.006
5. Lam JMC, Wodchis WP. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in ontario hospital-based long-term care residents. Med Care. (2010) 48:380–7. doi: 10.1097/MLR.0b013e3181ca2647
6. Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Ann Rev Neurosci. (1998) 21:149–86. doi: 10.1146/annurev.neuro.21.1.149
7. Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. (1993) 13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993
8. Kerr AL, Cheng SY, Jones TA. Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord. (2011) 44:538–48. doi: 10.1016/j.jcomdis.2011.04.011
9. Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. (2011) 44:521–8. doi: 10.1016/j.jcomdis.2011.04.006
10. Hartwigsen G, Saur D. Neuroimaging of stroke recovery from aphasia - Insights into plasticity of the human language network. Neuroimage. (2017). doi: 10.1016/j.neuroimage.2017.11.056
11. Teasell R, Mehta S, Pereira S, McIntyre A, Janzen S, Allen L, et al. Time to rethink long-term rehabilitation management of stroke patients. Topics Stroke Rehabili. (2012) 19:457–62. doi: 10.1310/tsr1906-457
12. Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. (2008) 63:272–87. doi: 10.1002/ana.21393
14. Mohajerani MH, Aminoltejari K, Murphy TH. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc Natl Acad Sci USA. (2011) 108:E183–91. doi: 10.1073/pnas.1101914108
15. Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. (1997) 41:581–9. doi: 10.1002/ana.410410506
16. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. (2004) 22:281–99.
17. Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. (2003) 9:64–75. doi: 10.1177/1073858402239592
18. Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. (2006) 129:1371–84. doi: 10.1093/brain/awl090
19. Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PMR. (2010) 2:S208–19. doi: 10.1016/j.pmrj.2010.10.016
20. Allen L, Mehta S, McClure JA, Teasell R. Therapeutic interventions for aphasia initiated more than six months post stroke: a review of the evidence. Topics Stroke Rehabili. (2012) 19:523–35. doi: 10.1310/tsr1906-523
21. Seghier ML, Ramsden S, Lim L, Leff AP, Price CJ. Gradual lesion expansion and brain shrinkage years after stroke. Stroke. (2014) 45:877–9. doi: 10.1161/STROKEAHA.113.003587
22. Karmiloff-smith A. Language and cognitive processes from a developmental perspective. Language Cognit Process. (1985) 1:61–85. doi: 10.1080/01690968508402071
23. Karmiloff-Smith A. Nativism versus neuroconstructivism: Rethinking the study of developmental disorders. Dev Psychol. (2009) 45:56–63. doi: 10.1037/a0014506
24. Ghotra SK, Johnson JA, Qiu W, Newton A, Rasmussen C, Yager JY. Age at stroke onset influences the clinical outcome and health-related quality of life in pediatric ischemic stroke survivors. Dev Med Child Neurol. (2015) 57:1027–34. doi: 10.1111/dmcn.12870
25. Dick AS, Raja Beharelle A, Solodkin A, Small SL. Interhemispheric functional connectivity following prenatal or perinatal brain injury predicts receptive language outcome. J Neurosci. (2013) 33:5612–25. doi: 10.1523/JNEUROSCI.2851-12.2013
26. Watila MM, Balarabe SA. Factors predicting post-stroke aphasia recovery. J Neurol Sci. (2015) 352:12–8. doi: 10.1016/j.jns.2015.03.020
27. Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. (2002) 17:85–100. doi: 10.1037/0882-7974.17.1.85
28. Plowman E, Hentz B, Ellis C, Jr. Post-stroke aphasia prognosis: a review of patient-related and stroke-related factors. J Evaluat Clin Pract. (2012) 18:689–94. doi: 10.1111/j.1365-2753.2011.01650.x
29. Hope TM, Seghier ML, Leff AP, Price CJ. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage Clin. (2013) 2:424–33. doi: 10.1016/j.nicl.2013.03.005
30. Payabvash S, Kamalian S, Fung S, Wang Y, Passanese J, Souza LC, et al. Predicting language improvement in acute stroke patients presenting with aphasia: a multivariate logistic model using location-weighted atlas-based analysis of admission CT perfusion scans. AJNR. (2010) 31:1661–8. doi: 10.3174/ajnr.A2125
31. Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. (2011) 42:421–6. doi: 10.1161/STROKEAHA.110.599340
32. Cheng B, Forkert ND, Zavaglia M, Hilgetag CC, Golsari A, Siemonsen S, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke. (2014) 45:1695–702. doi: 10.1161/STROKEAHA.114.005152
33. Hillis AE, Beh YY, Sebastian R, Breining B, Tippett DC, Wright A, et al. Predicting recovery in acute poststroke aphasia. Ann Neurol. (2018) 83:612–22. doi: 10.1002/ana.25184
34. Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair. (2016) 30:266–79. doi: 10.1177/1545968315593808
35. Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage. (2012) 60:854–63. doi: 10.1016/j.neuroimage.2011.12.057
36. Skipper-Kallal LM, Lacey EH, Xing S, Turkeltaub PE. Right hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Neural Plast. (2017) 2017:8740353. doi: 10.1155/2017/8740353
37. Skipper-Kallal LM, Lacey EH, Xing S, Turkeltaub PE. Functional activation independently contributes to naming ability and relates to lesion site in post-stroke aphasia. Human Brain Mapping. (2017) 38:2051–66. doi: 10.1002/hbm.23504
38. Barbieri E, Mack J, Chiappetta B, Europa E, Thompson CK. Recovery of offline and online sentence processing in aphasia: language and domain-general neuroplasticity. Cortex. (under revision).
39. Butler RA, Lambon Ralph MA, Woollams AM. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain. (2014) 137:3248–66. doi: 10.1093/brain/awu286
40. Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: evidence from voxel-based lesion symptom mapping. Aphasiology. (2012) 26:338–54. doi: 10.1080/02687038.2011.602391
41. Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol. (2014) 76:738–46. doi: 10.1002/ana.24266
42. Geva S, Jones PS, Crinion JT, Price CJ, Baron JC, Warburton EA. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain. (2011) 134:3071–82. doi: 10.1093/brain/awr232
43. Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun. (2015) 6:6762. doi: 10.1038/ncomms7762
44. Halai AD, Woollams AM, Lambon Ralph MA. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: Revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex. (2017) 86:275–89. doi: 10.1016/j.cortex.2016.04.016
45. Seghier ML, Patel E, Prejawa S, Ramsden S, Selmer A, Lim L, et al. The PLORAS database: a data repository for predicting language outcome and recovery after stroke. Neuroimage. (2016) 124:1208–12. doi: 10.1016/j.neuroimage.2015.03.083
46. Hope TMH, Leff AP, Prejawa S, Bruce R, Haigh Z, Lim L, et al. Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain. (2017) 140:1718–28. doi: 10.1093/brain/awx086
47. Hope TMH, Leff AP, Price CJ. Predicting language outcomes after stroke: Is structural disconnection a useful predictor? NeuroImage Clin. (2018) 19:22–9. doi: 10.1016/j.nicl.2018.03.037
48. Heiss W-D. Ischemic penumbra: evidence from functional imaging in man. J Cerebral Blood Flow Metabol. (2000) 20:1276–93. doi: 10.1097/00004647-200009000-00002
49. Baron JC. How healthy is the acutely reperfused ischemic penumbra? Cerebrovascular Dis. (2005) 20:25–31. doi: 10.1159/000089354
50. Heiss WD, Kracht LW, Thiel A, Grond M, Pawlik G. Penumbral probability thresholds of cortical flumazenil binding and blood flow predicting tissue outcome in patients with cerebral ischaemia. Brain. (2001) 124:20–9. doi: 10.1093/brain/124.1.20
51. Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Language. (2001) 79:495–510. doi: 10.1006/brln.2001.2563
52. Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, et al. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. (2001) 50:561–6. doi: 10.1002/ana.1265
53. Wityk RJ, Hillis A, Beauchamp N, Barker PB, Rigamonti D. Perfusion-weighted magnetic resonance imaging in adult moyamoya syndrome: characteristic patterns and change after surgical intervention: case report. Neurosurgery. (2002) 51:1499–505; discussion 1506. doi: 10.1097/00006123-200212000-00023
54. Reineck LA, Agarwal S, Hillis AE. Diffusion-clinical mismatch is associated with potential for early recovery of aphasia. Neurology. (2005) 64:828–33. doi: 10.1212/01.WNL.0000152983.52869.51
55. Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. (2006) 26:8069–73. doi: 10.1523/JNEUROSCI.2088-06.2006
56. Brumm KP, Perthen JE, Liu TT, Haist F, Ayalon L, Love T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. Neuroimage. (2010) 51:995–1005. doi: 10.1016/j.neuroimage.2010.03.008
57. Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, Fridriksson J. Cerebral perfusion in chronic stroke: implications for lesion-symptom mapping and functional MRI. Behav Neurol. (2011) 24:117–22. doi: 10.1155/2011/380810
58. Thompson CK, Walenski M, Chen Y, Caplan D, Kiran S, Rapp B, et al. Intrahemispheric perfusion in chronic stroke-induced aphasia. Neural Plast. (2017) 2017:2361691. doi: 10.1155/2017/2361691
59. Thompson CK, den Ouden DB, Bonakdarpour B, Garibaldi K, Parrish TB. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. (2010) 48:3211–27. doi: 10.1016/j.neuropsychologia.2010.06.036
60. Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. (2008) 44:953–61. doi: 10.1016/j.cortex.2008.04.002
61. Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke. (2011) 42:2251–6. doi: 10.1161/STROKEAHA.110.606103
62. Pani E, Zheng X, Wang J, Norton A, Schlaug G. Right hemisphere structures predict poststroke speech fluency. Neurology. (2016) 86:1574–81. doi: 10.1212/WNL.0000000000002613
63. Wang J, Marchina S, Norton AC, Wan CY, Schlaug G. Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci. (2013) 7:831. doi: 10.3389/fnhum.2013.00831
64. Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain. (2013) 136:2952–65. doi: 10.1093/brain/awt205
65. Ivanova MV, Isaev DY, Dragoy OV, Akinina YS, Petrushevskiy AG, Fedina ON, et al. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex. (2016) 85:165–81. doi: 10.1016/j.cortex.2016.04.019
66. Breier JI, Juranek J, Papanicolaou AC. Changes in maps of language function and the integrity of the arcuate fasciculus after therapy for chronic aphasia. Neurocase. (2011) 17:506–17. doi: 10.1080/13554794.2010.547505
67. Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci. (2011) 31:16949–57. doi: 10.1523/JNEUROSCI.2725-11.2011
68. Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. (2008) 105:18035–40. doi: 10.1073/pnas.0805234105
69. Hope TM, Seghier ML, Prejawa S, Leff AP, Price CJ. Distinguishing the effect of lesion load from tract disconnection in the arcuate and uncinate fasciculi. Neuroimage. (2016) 125:1169–73. doi: 10.1016/j.neuroimage.2015.09.025
70. Meier E, Johnson J, Pan Y, Kiran S. The utility of lesion classification in predicting language and treatment outcomes in chronic stroke-induced aphasia. Brain Imaging Behav. (under revision).
71. Catani MM, Allin PG, Husain M, Pugliese L, Mesulam MM, Murray RM, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. (2007) 104:17163–8. doi: 10.1073/pnas.0702116104
73. Meinzer M, Mohammadi S, Kugel H, Schiffbauer H, Floel A, Albers J, et al. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. Neuroimage. (2010) 53:283–90. doi: 10.1016/j.neuroimage.2010.06.004
74. van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair. (2014) 28:325–34. doi: 10.1177/1545968313508654
75. Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N York Acad Sci. (2009) 1169:385–94. doi: 10.1111/j.1749-6632.2009.04587.x
76. Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca's aphasia. Brain Lang. (2014) 136:1–7. doi: 10.1016/j.bandl.2014.03.011
77. Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol. (2011) 53:466–75. doi: 10.1002/dev.20557
78. Kolb B, Muhammad A, Gibb R. Searching for factors underlying cerebral plasticity in the normal and injured brain. J Commun Disord. (2011) 44:503–14. doi: 10.1016/j.jcomdis.2011.04.007
79. Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. (2007) 38:840–5. doi: 10.1161/01.STR.0000247943.12887.d2
80. Nudo RJ. Neural bases of recovery after brain injury. J Commun Disord. (2011) 44:515–20. doi: 10.1016/j.jcomdis.2011.04.004
81. Overman JJ, Carmichael ST. Plasticity in the injured brain: more than molecules matter. Neuroscientist. (2014) 20:15–28. doi: 10.1177/1073858413491146
82. Thompson CK. Neuroplasticity: evidence from aphasia. J Commun Disord. (2000) 33:357–66. doi: 10.1016/S0021-9924(00)00031-9
83. Thompson CK. The “right” hemisphere and language recovery in aphasia. Paper presented at the Clinical Aphasiology Conference. Snowbird, UT (2017).
84. Abo M, Senoo A, Watanabe S, Miyano S, Doseki K, Sasaki N, et al. Language-related brain function during word repetition in post-stroke aphasics. Neuroreport. (2004) 15:1891–4. doi: 10.1097/00001756-200408260-00011
85. Fridriksson J, Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology. (2005) 19:239–50. doi: 10.1080/02687030444000714
86. Benjamin ML, Towler S, Garcia A, Park H, Sudhyadhom A, Harnish S, et al. A behavioral manipulation engages right frontal cortex during aphasia therapy. Neurorehab Neural Rep. (2014) 28:545–53. doi: 10.1177/1545968313517754
87. Mohr B, Difrancesco S, Harrington K, Evans S, Pulvermuller F. Changes of right-hemispheric activation after constraint-induced, intensive language action therapy in chronic aphasia: fMRI evidence from auditory semantic processing1. Front Human Neurosci. (2014) 8:e00919. doi: 10.3389/fnhum.2014.00919
88. Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. (2008) 70:290–8. doi: 10.1212/01.wnl.0000287115.85956.87
89. Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci. (2010) 30:11558–64. doi: 10.1523/JNEUROSCI.2227-10.2010
90. Christie J, Ginsberg JP, Steedman J, Fridriksson J, Bonilha L, Rorden C. Global versus local processing: seeing the left side of the forest and the right side of the trees. Front Human Neurosci. (2012) 6:28. doi: 10.3389/fnhum.2012.00028
91. Rochon E, Leonard C, Burianova H, Laird L, Soros P, Graham S, et al. Neural changes after phonological treatment for anomia: An fMRI study. Brain Language. (2010) 114:164–79. doi: 10.1016/j.bandl.2010.05.005
92. Fridriksson J, Morrow-Odom L, Moser D, Fridriksson A, Baylis G. Neural recruitment associated with anomia treatment in aphasia. Neuroimage. (2006) 32:1403–12. doi: 10.1016/j.neuroimage.2006.04.194
93. Abutalebi J, Della Rosa PA, Tettamanti M, Green DW, Cappa SF. Bilingual aphasia and language control: a follow-up fMRI and intrinsic connectivity study. Brain Language. (2009) 109:141–56. doi: 10.1016/j.bandl.2009.03.003
94. Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. (2003) 15:444–61. doi: 10.1162/089892903321593153
95. Fridriksson J, Moser D, Bonilha L, Morrow-Odom KL, Shaw H, Fridriksson A, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. (2007) 45:1812–22. doi: 10.1016/j.neuropsychologia.2006.12.017
96. Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, et al. Imaging short- and long-term training success in chronic aphasia. BMC Neurosci. (2009) 10:118. doi: 10.1186/1471-2202-10-118
97. Thompson CK, Riley EA, den Ouden D-B, Meltzer-Asscher A, Lukic S. Training verb argument structure production in agrammatic aphasia: Behavioral and neural recovery patterns. Cortex. (2013) 49:2358–76. doi: 10.1016/j.cortex.2013.02.003
98. Kiran S, Meier EL, Kapse KJ, Glynn PA. Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Front Hum Neurosci. (2015) 9:316. doi: 10.3389/fnhum.2015.00316
99. Abel S, Weiller C, Huber W, Willmes K, Specht K. Therapy-induced brain reorganization patterns in aphasia. Brain. (2015) 138:1097–112. doi: 10.1093/brain/awv022
100. Leger A, Demonet JF, Ruff S, Aithamon B, Touyeras B, Puel M, et al. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. (2002) 17:174–83. doi: 10.1006/nimg.2002.1238
101. Richter MW, Miltner HR, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. (2008) 131:1391–401. doi: 10.1093/brain/awn043
102. Vigneau M, Beaucousin VP, Herve Y, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. (2006) 30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002
103. Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia. Two Case Studies Cortex. (1989) 25:555–66. doi: 10.1016/S0010-9452(89)80017-6
104. Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, et al. The right hemisphere is not unitary in its role in aphasia recovery. Cortex. (2012) 48:1179–86. doi: 10.1016/j.cortex.2011.06.010
105. van Hees S, McMahon K, Angwin A, de Zubicaray G, Copland DA. Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain Langauge. (2014) 129:47–57. doi: 10.1016/j.bandl.2013.12.004
106. Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Langauge. (2006) 98:118–23. doi: 10.1016/j.bandl.2006.02.002
107. Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Langauge. (2005) 93:95–105. doi: 10.1016/j.bandl.2004.08.004
108. Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, et al. Improved naming after TMS treatments in a chronic, global aphasia patient – case report. Neurocase. (2005) 11:182–93. doi: 10.1080/13554790590944663
109. Norise C, Hamilton RH. Non-invasive brain stimulation in the treatment of post-stroke and neurodegenerative aphasia: parallels, differences, and lessons learned. Front Human Neurosci. (2017) 10:00675. doi: 10.3389/fnhum.2016.00675
110. Turkeltaub PE, Messing S, Norise C, Hamilton RH. Are networks for residual language function and recovery consistent across aphasic patients? Neurology. (2011) 76:1726–34. doi: 10.1212/WNL.0b013e31821a44c1
111. Sandberg CW, Bohland JW, Kiran S. Changes in functional connectivity related to direct training and generalization effects of a word finding treatment in chronic aphasia. Brain Language. (2015) 150:103–16. doi: 10.1016/j.bandl.2015.09.002
112. Meier EL, Kapse KJ, Kiran S. The relationship between frontotemporal effective connectivity during picture naming, behavior, and preserved cortical tissue in chronic aphasia. Front Hum Neurosci. (2016) 10:109. doi: 10.3389/fnhum.2016.00109
113. Sims J, Kapse K, Glynn P, Sandberg C, Kiran S. The relationship between the amount of spared tissue, percent signal change and accuracy in language recovery in aphasia. Neuropsychologia. (2016) 84:113–26. doi: 10.1016/j.neuropsychologia.2015.10.019
114. Woolgar A, Parr A, Cusack R, Thompson R, Nimmo-Smith I, Torralva T, et al. Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proc Natl Acad Sci USA. (2010) 107:14899–902. doi: 10.1073/pnas.1007928107
115. Fedorenko E. The role of domain-general cognitive control in language comprehension. Front Psychol. (2014) 5:335. doi: 10.3389/fpsyg.2014.00335
116. Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. (2010) 14:172–9. doi: 10.1016/j.tics.2010.01.004
117. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. (2000) 23:475–83. doi: 10.1016/S0166-2236(00)01633-7
118. Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJ. Cognitive control and its impact on recovery from aphasic stroke. Brain. (2014) 137:242–54. doi: 10.1093/brain/awt289
119. Geranmayeh F, Brownsett SL, Wise RJ. Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain. (2014) 137:2632–48. doi: 10.1093/brain/awu163
120. Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. (2011) 5:1. doi: 10.3389/fnsys.2011.00001
121. Tomasi D, Volkow ND. Language network: segregation, laterality and connectivity. Mol Psychiatry. (2012) 17:759. doi: 10.1038/mp.2012.99
122. Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, et al. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex. (2015) 25:680–702. doi: 10.1093/cercor/bht260
123. Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. (2011) 106:1125–65. doi: 10.1152/jn.00338.2011
124. Carrera E, Tononi G. Diaschisis: past, present, future. Brain. (2014) 137:2408–22. doi: 10.1093/brain/awu101
125. Rehme AK, Grefkes C. Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol. (2013) 591:17–31. doi: 10.1113/jphysiol.2012.243469
126. Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. (2010) 67:365–75. doi: 10.1002/ana.21905
127. Westlake KP, Nagarajan SS. Functional connectivity in relation to motor performance and recovery after stroke. Front Syst Neurosci. (2011) 5:8. doi: 10.3389/fnsys.2011.00008
128. Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J Neurosci. (2016) 36:6668–79. doi: 10.1523/JNEUROSCI.4396-15.2016
129. Gleichgerrcht E, Kocher M, Nesland T, Rorden C, Fridriksson J, Bonilha L. Preservation of structural brain network hubs is associated with less severe post-stroke aphasia. Restor Neurol Neurosci. (2015) 34:19–28. doi: 10.3233/RNN-150511
130. Fridriksson J, den Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, et al. (2018). Anatomy of aphasia revisited. Brain. 141:848–62. doi: 10.1093/brain/awx363
131. Bonilha L, Gleichgerrcht E, Fridriksson J, Rorden C, Breedlove JL, Nesland T, et al. Reproducibility of the structural brain connectome derived from diffusion tensor imaging. PLoS ONE. (2015) 10:e0135247. doi: 10.1371/journal.pone.0135247
132. Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front Hum Neurosci. (2014) 8:845. doi: 10.3389/fnhum.2014.00845
133. Vitali P, Tettamanti M, Abutalebi J, Ansaldo AI, Perani D, Cappa SF, et al. Generalization of the effects of phonological training for anomia using structural equation modelling: A multiple single-case study. Neurocase. (2010) 16:93–105. doi: 10.1080/13554790903329117
134. Yang M, Li J, Yao D, Chen H. Disrupted intrinsic local synchronization in poststroke aphasia. Medicine. (2016) 95:e3101. doi: 10.1097/MD.0000000000003101
135. Zhu Y, Bai L, Liang P, Kang S, Gao H, Yang H. Disrupted brain connectivity networks in acute ischemic stroke patients. Brain Imaging Behav. (2017) 11:444–53. doi: 10.1007/s11682-016-9525-6
136. Balaev V, Petrushevsky A, Martynova O. Changes in functional connectivity of default mode network with auditory and right frontoparietal networks in poststroke aphasia. Brain Connect. (2016) 6:714–23. doi: 10.1089/brain.2016.0419
137. Dijkhuizen RM, Zaharchuk G, Otte WM. Assessment and modulation of resting-state neural networks after stroke. Curr Opin Neurol. (2014) 27:637–43. doi: 10.1097/WCO.0000000000000150
138. Sandberg CW. Hypoconnectivity of resting-state networks in persons with aphasia compared with healthy age-matched adults. Front Hum Neurosci. (2017) 11:91. doi: 10.3389/fnhum.2017.00091
139. Sebastian R, Long C, Purcell JJ, Faria AV, Lindquist M, Jarso S, et al. Imaging network level language recovery after left PCA stroke. Restor Neurol Neurosci. (2016) 34:473–89. doi: 10.3233/RNN-150621
140. Zhu D, Chang J, Freeman S, Tan Z, Xiao J, Gao Y, et al. Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Front Behav Neurosci. (2014) 8:167. doi: 10.3389/fnbeh.2014.00167
141. Siegel JS, Seitzman BA, Ramsey LE, Ortega M, Gordon EMN, Dosenbach NUF, et al. Re-emergence of modular brain networks in stroke recovery. Cortex. (2018) 101:44–59. doi: 10.1016/j.cortex.2017.12.019
142. van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Hum Brain Mapp. (2014) 35:3919–31. doi: 10.1002/hbm.22448
143. Duncan ES, Small SL. Changes in dynamic resting state network connectivity following aphasia therapy. Brain Imaging Behav. (2017) 12:1141–9. doi: 10.1007/s11682-017-9771-2
144. Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following constraint-induced movement therapy. Phys Med Rehabil Clin N Am. (2003) 14:S77–91. doi: 10.1016/S1047-9651(02)00052-9
145. Maher LM, Kendall D, Swearengin JA, Rodriguez A, Leon SA, Pingel K, et al. A pilot study of use-dependent learning in the context of constraint induced language therapy. J Int Neuropsychol Soc. (2006) 12:843–52. doi: 10.1017/S1355617706061029
146. Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. (2008) 51:1282–99. doi: 10.1044/1092-4388(2008/07-0206)
147. Johnson ML, Taub E, Harper LH, Wade JT, Bowman MH, Bishop-McKay S, et al. An enhanced protocol for constraint-induced aphasia therapy II: a case series. Am J Speech-Language Pathol Am Speech-Language-Hearing Assoc. (2014) 23:60–72. doi: 10.1044/1058-0360(2013/12-0168)
148. Meinzer M, Elbert T, Djundja D, Taub E, Rockstroh B. Extending the constraint-induced movement therapy (cimt) approach to cognitive functions: constraint-induced aphasia therapy (CIAT) of chronic aphasia. NeuroRehabilitation. (2007) 22:311–8.
149. Barthel G, Meinzer M, Djundja D, Rockstroh B. Intensive language therapy in chronic aphasia: Which aspects contribute most? Aphasiology. (2008) 22:408–21. doi: 10.1080/02687030701415880
150. Wilssens I, Vandenborre D, van Dun K, Verhoeven J, Visch-Brink E, MariÃ≪n P. Constraint-induced aphasia therapy versus intensive semantic treatment in fluent aphasia. Am J Speech-Lang Pathol. (2015) 24:281–94. doi: 10.1044/2015_AJSLP-14-0018
151. Boyle M. Semantic feature analysis treatment for aphasic word retrieval impairments: what's in a name? Top Stroke Rehabil. (2010) 17:411–22. doi: 10.1310/tsr1706-411
152. Kiran S, Thompson CK. The role of semantic complexity in treatment of naming deficits: training semantic categories in fluent aphasia by controlling exemplar typicality. J Speech Language Hear Res. (2003) 46:773–87. doi: 10.1044/1092-4388(2003/061)
153. Kendall DL, Oelke M, Brookshire CE, Nadeau SE. The influence of phonomotor treatment on word retrieval abilities in 26 individuals with chronic aphasia: an open trial. J Speech Language Hear Res. (2015) 58:798. doi: 10.1044/2015_JSLHR-L-14-0131
154. Edmonds LA, Mammino K, Ojeda J. Effect of verb network strengthening treatment (VNeST) in persons with aphasia: extension and replication of previous findings. Am J Speech-Language Pathol. (2014) 23:S312–S329. doi: 10.1044/2014_AJSLP-13-0098
155. Thompson CK, Shapiro LP. Treating agrammatic aphasia within a linguistic framework: treatment of underlying forms. Aphasiology. (2005) 19:1021–36. doi: 10.1080/02687030544000227
156. Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. (2004) 5:279–90. doi: 10.1038/nrn1366
157. Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. (2004) 92:66–72. doi: 10.1152/jn.00383.2003
158. Kagan AN, Simmons-Mackie Rowland A, Huijbregts M, Shumway E, McEwen S, et al. Counting what counts: A framework for capturing real-life outcomes of aphasia intervention. Aphasiology. (2008) 22:258–80. doi: 10.1080/02687030701282595
159. Stahl B, Mohr B, Dreyer FR, Lucchese G, Pulvermuller F. Using language for social interaction: Communication mechanisms promote recovery from chronic non-fluent aphasia. Cortex. (2016) 85:90–9. doi: 10.1016/j.cortex.2016.09.021
160. Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. (2005) 11:471–83. doi: 10.1177/1073858405278015
161. Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. (2004) 125:329–36. doi: 10.1016/j.neuroscience.2004.01.048
162. Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. (2004) 24:628–33. doi: 10.1523/JNEUROSCI.3440-03.2004
163. Schuell H. Aphasia Theory and Therapy: Selected Lectures and Papers of Hildred Schuell. Baltimore, MD: Park Press Baltimore (1974).
164. Schuell H, Carroll V, Street BS. Clinical treatment of aphasia. J Speech Hear Disord. (1955) 20:43. doi: 10.1044/jshd.2001.43
165. Godecke E, Rai T, Ciccone N, Armstrong E, Granger A, Hankey GJ. Amount of therapy matters in very early aphasia rehabilitation after stroke: a clinical prognostic model. Semin Speech Langauge. (2013) 34:129–41. doi: 10.1055/s-0033-1358369
166. Off CA, Griffin JR, Spencer KA, Rogers M. The impact of dose on naming accuracy with persons with aphasia. Aphasiology. (2016) 30:983–1011. doi: 10.1080/02687038.2015.1100705
167. Stahl B, Mohr B, Büscher V, Dreyer FR, Lucchese G, Pulvermüller F. Efficacy of intensive aphasia therapy in patients with chronic stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry. (2017) 89:586–92. doi: 10.1136/jnnp-2017-315962
168. Sage K, Snell C, Lambon Ralph MA. How intensive does anomia therapy for people with aphasia need to be? Neuropsychol Rehabili. (2011) 21:26–41. doi: 10.1080/09602011.2010.528966
169. Raymer AM, Kohen FP, Saffell D. Computerised training for impairments of word comprehension and retrieval in aphasia. Aphasiology. (2006) 20:257–68. doi: 10.1080/02687030500473312
170. Harnish SM, Morgan J, Lundine JP, Bauer A, Singletary F, Benjamin ML, et al. Dosing of a cued picture-naming treatment for anomia. Am J Speech-Language Pathol Am Speech-Language-Hear Assoc. (2014) 23:S285–S299. doi: 10.1044/2014_AJSLP-13-0081
171. Martins IP, Leal G, Fonseca I, Farrajota L, Aguiar M, Fonseca J, et al. A randomized, rater-blinded, parallel trial of intensive speech therapy in sub-acute post-stroke aphasia: the SP-I-R-IT study. Int J Lang Commun Disord. (2013) 48:421–31. doi: 10.1111/1460-6984.12018
172. Bakheit AM, Shaw S, Barrett L, Wood J, Carrington S, Griffiths S, et al. A prospective, randomized, parallel group, controlled study of the effect of intensity of speech and language therapy on early recovery from poststroke aphasia. Clin Rehabil. (2007) 21:885–94. doi: 10.1177/0269215507078486
173. Hinckley J, Carr T. Comparing the outcomes of intensive and non-intensive context-based aphasia treatment. Aphasiology. (2005) 19:965–74. doi: 10.1080/02687030544000173
174. Dignam JK, Rodriguez AD, Copland DA. Evidence for intensive aphasia therapy: consideration of theories from neuroscience and cognitive psychology. PMR. (2016) 8:254–67. doi: 10.1016/j.pmrj.2015.06.010
175. Kiran S. Typicality of inanimate category exemplars in aphasia treatment: further evidence for semantic complexity. J Speech Language Hear Res. (2008) 51:1550. doi: 10.1044/1092-4388(2008/07-0038)
176. Gray T, Kiran S. The relationship between language control and cognitive control in bilingual aphasia. Bilingual Lang Cogn. (2016) 19:433–52. doi: 10.1017/S1366728915000061
177. Riley EA, Thompson CK. Training pseudoword reading in acquired dyslexia: a phonological complexity approach. Aphasiology. (2015) 29:129–50. doi: 10.1080/02687038.2014.955389
178. Thompson CK, Shapiro LP. Complexity in treatment of syntactic deficits. Am J Speech-Language pathol Am Speech-Language-Hear Assoc. (2007) 16:30–42. doi: 10.1044/1058-0360(2007/005)
179. Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. (2006) 19:21–33. doi: 10.1097/00146965-200603000-00003
180. Takeuchi N, Izumi S. Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plast. (2012) 2012:359728. doi: 10.1155/2012/359728
181. Keane C, Kiran S. The nature of facilitation and interference in the multilingual language system: insights from treatment in a case of trilingual aphasia. Cogn Neuropsychol. (2015) 32:169–94. doi: 10.1080/02643294.2015.1061982
182. Gierut JA, Champion AH. Syllable onsets II: three-element clusters in phonological treatment. J Speech Lang Hear Res. (2001) 44:886–904. doi: 10.1044/1092-4388(2001/071)
183. Kiran S. Complexity in the treatment of naming deficits. Am J Speech-Language pathol Am Speech-Language-Hear Assoc. (2007) 16:18–29. doi: 10.1044/1058-0360(2007/004)
184. Van Horne AJO, Fey M, Curran M. Do the hard things first: a randomized controlled trial testing the effects of exemplar selection on generalization following therapy for grammatical morphology. J Speech Lang Hear Res. (2017) 60:2569–88. doi: 10.1044/2017_JSLHR-L-17-0001
185. Thompson CK, Shapiro LP, Kiran S, Sobecks J. The role of syntactic complexity in treatment of sentence deficits in agrammatic aphasia: The complexity account of treatment efficacy (CATE). J Speech Language Hear Res. (2003) 46:591–607. doi: 10.1044/1092-4388(2003/047)
186. Kiran S, Sandberg C, Abbott K. Treatment for lexical retrieval using abstract and concrete words in persons with aphasia: Effect of complexity. Aphasiology. (2009) 23:835–53. doi: 10.1080/02687030802588866
Keywords: stroke, aphasia, neuroimaging (anatomic and functional), plasticity, recovery
Citation: Kiran S and Thompson CK (2019) Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 10:295. doi: 10.3389/fneur.2019.00295
Received: 04 September 2018; Accepted: 06 March 2019;
Published: 02 April 2019.
Edited by:
Paola Marangolo, University of Naples Federico II, ItalyReviewed by:
Gesa Hartwigsen, Max Planck Institute for Human Cognitive and Brain Sciences, GermanyCopyright © 2019 Kiran and Thompson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Swathi Kiran, a2lyYW5zQGJ1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.