- 1Department of Neurology, Epilepsy Center, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Epilepsy Center, Cleveland Clinic, Cleveland, OH, United States
- 3Imaging Institute, Cleveland Clinic, Cleveland, OH, United States
- 4Department of Neurosurgery, Cleveland Clinic, Cleveland, OH, United States
- 5Department of Anatomic Pathology, Cleveland Clinic, Cleveland, OH, United States
Background and Purpose: Surgical management of patients with cingulate epilepsy (CE) is highly challenging, especially when the MRI is non-lesional. We aimed to use a voxel-based MRI post-processing technique, implemented in a morphometric analysis program (MAP), to facilitate detection of subtle epileptogenic lesions in CE, thereby improving surgical evaluation of patients with CE with non-lesional MRI by visual inspection.
Methods: Included in this retrospective study were 9 patients with CE (6 with negative 3T MRI and 3 with subtly lesional 3T MRI) who underwent surgery and became seizure-free or had marked seizure improvement with at least 1-year follow-up. MRI post-processing was applied to pre-surgical T1-weighted volumetric sequence using MAP. The MAP finding was then coregistered and compared with other non-invasive imaging tests (FDG-PET, SPECT and MEG), intracranial EEG ictal onset, surgery location and histopathology.
Results: Single MAP+ abnormalities were found in 6 patients, including 3 patients with negative MRI, and 3 patients with subtly lesional MRI. Out of these 6 MAP+ patients, 4 patients became seizure-free after complete resection of the MAP+ abnormalities; 2 patients didn't become seizure-free following laser ablation that only partially overlapped with the MAP+ abnormalities. All MAP+ foci were concordant with intracranial EEG ictal onset (when performed). The localization value of FDG-PET, SPECT and MEG was limited in this cohort. FCD was identified in all patients' surgical pathology except for two cases of laser ablation with no tissue available.
Conclusion: MAP provided helpful information for identifying subtle epileptogenic abnormalities in patients with non-lesional cingulate epilepsy. MRI postprocessing should be considered to add to the presurgical evaluation test battery of non-lesional cingulate epilepsy.
Introduction
Surgical management of patients with cingulate epilepsy (CE) is highly challenging, especially in the setting of negative MRI. Due to its mesial and deep location from the cerebral surface as well as the absence of unique ictal manifestations, scalp video-electroencephalography (EEG) may be misleading or non-localizable (1–4). The fast propagation of seizure activities originating from cingulate cortex (CC) within the limbic network (5), complicated functional connectivity between homotopic cingulate and sensorimotor cortex (3, 6), and diffuse bilaterally secondary synchrony of epileptiform discharges from cingulate lesions (2, 7) all contribute to the difficulty in localizing CE.
A confirmed MRI lesion can contribute directly to the identification of the epileptogenic zone (EZ) (8). When patients have no apparent lesions on the MRI, presurgical evaluation and surgical management can be particularly difficult, as seizure origin could be strongly influenced by the availability of collective expertise and experience in semiology, neurophysiological exploration, and functional imaging interpretation (4, 8). Previous studies with voxel-based MRI post-processing using a morphometric analysis program (MAP) (9) combined with visual MRI analysis indicated high sensitivity in the identification of subtle epileptic lesions (10–14); MAP+ findings was reported to provide valuable targets for invasive evaluation and resection (15). However, there was no study on the post-processing neuroimaging characteristics of CE with a normal pre-surgical MRI.
In the current study, we aimed to investigate the usefulness of voxel-based MRI post-processing to detect subtle abnormalities in CE with a negative pre-surgical MRI. In relation to the MAP findings, we examined the non-invasive electro-clinical characteristics and functional imaging findings in these patients. When possible, concordance with intracranial EEG finding was investigated.
Materials and Methods
Patients
This retrospective study was approved by the institutional review board ethical guidelines of two hospitals: Cleveland Clinic Foundation (CCF) and the Second Affiliated Hospital of Zhejiang University (SAHZU). We reviewed a consecutive series of patients who had surgery at CCF from January 2008 to December 2016 and SAHZU from January 2013 to April 2017. The inclusion criteria were as follows: (1) intracranial-EEG (ICEEG) confirmed focal cingulate ictal onset during recorded habitual seizures, or resection of the cingulate cortex with/without adjacent cortex rendered the patient seizure-free or having marked seizure improvement with 1-year follow-up; (2) preoperative MRI and postoperative MRI/CT data were available; (3) preoperative MRI was considered as negative or suspicious of a subtle lesion during the multidisciplinary patient management conferences (PMC). Patients were excluded if they (1) had poor MRI quality; (2) had a definite lesion in the cingulate cortex on MRI; and (3) seizures recurred without a marked improvement after surgery. The vertical anterior/posterior commissure lines (VAV/VPC) were used as a landmark to divide the cingulate cortex into three parts: the anterior cingulate, located rostral to the VAC; the middle cingulate, located between VAC and VPC; and the posterior cingulate, located caudal to the VPC line (2).
Presurgical Evaluation
The surgical strategy was discussed based on pre-surgical evaluation including history, semiology, video scalp-EEG, MRI, FDG-PET, subtraction ictal SPECT co-registered with MRI (SISCOM), Magnetoencephalography (MEG) and ICEEG. Semiology based on history and video-EEG was evaluated with classifications developed by Lüders et al. (16). Results of pre-surgical evaluation tests were obtained from chart reviews of the patients' clinical files.
Data Acquisition and Analyses
MRI post-processing was based with MAP07 within MATLAB 2015a (MathWorks, Natick, Massachusetts) and analyzed on a voxel basis (9) with comparison to a normal database consisting of 90 normal controls (17). Patients from CCF were scanned by 3.0-T MRI scanners (Trio or Skyra, SIEMENS, Erlangen, Germany) with T1-weighted Magnetization Prepared Rapid Acquisition with Gradient Echo images; patients from SAHZU were scanned with a 3.0-T MRI scanner (MR750, GE Healthcare) with a 3-dimensional (3D) T1-weighted Spoiled Gradient Recalled Echo sequence. Detailed parameters can be found elsewhere (18). The final outputs of MAP consisted of three feature maps, the junction, extension, and thickness maps. The junction map is sensitive to blurring of the gray-white matter junction; the extension map is sensitive to abnormal gyration and extension of gray matter into white matter; the thickness map is sensitive to abnormal cortical thickness (9). A blinded reviewer (Shan Wang) used a z-score threshold of 3 to identify candidate MAP+ regions in the junction file and then examined the suspect on extension (Z>6) and thickness (Z>4) files. The abnormality was reaffirmed by a neuroradiologist (SEJ), checking pre-operative MRI including T1-weighted, T2-weighted and FLAIR sequences to confirm MAP+ positive regions. In all MAP+ patients, we used SPM12 to co-register preoperative T1-weighted images, MAP files and postoperative MRI images in order to confirm whether the location of the MAP+ regions was included in the resection.
Pathology and Outcome
Surgical pathology, when available, was re-reviewed by dedicated neuropathologists from each hospital. The diagnosis and classification of FCD were performed according to the ILAE guidelines (19). Postoperative seizure outcomes were determined according to Engel's Classification (8). Engel Class 1 (seizure-free) and 2 (>90% reduction) were regarded as marked improvement of seizure frequency (2, 8).
Results
Patient Population
Out of the 1,518 patients with a localized resection from the CCF surgical database, 21 patients had resection of the cingulate cortex; 17 of the 21 patients had strictly non-lesional MRI or had subtle cingulate abnormalities. Ten patients were further excluded because the invasive EEG onset was not merely limited to the cingulate region, or the resection included but extended beyond the cingulate cortex, or there was no marked seizure improvement after surgery. Out of the 240 patients with a localized resection from SAHZU, 7 patients had resection of the cingulate cortex; 3 patients had strictly non-lesional MRI. One patient was further excluded because the invasive EEG onset was not merely limited to the cingulate cortex. Therefore, a total of 9 patients were identified from the two Epilepsy Centers (7 from CCF), including 5 from anterior CE, 3 from middle CE and 1 from posterior CE. Six were females; the median age at surgery was 22 (range, 14.5–38) years; the median epilepsy duration was 60 (range, 13–173) months. Six patients with negative MRI underwent ICEEG monitoring, which confirmed cingulate focal ictal onset during their habitual seizures. Subtle CC abnormalities in three patients were identified during re-review at PMC and no ICEEG was recommended per PMC consensus for these 3 patients. Detailed clinical information, results of pre-surgical evaluation, pathology, and postsurgical seizure outcomes were summarized in Table 1.
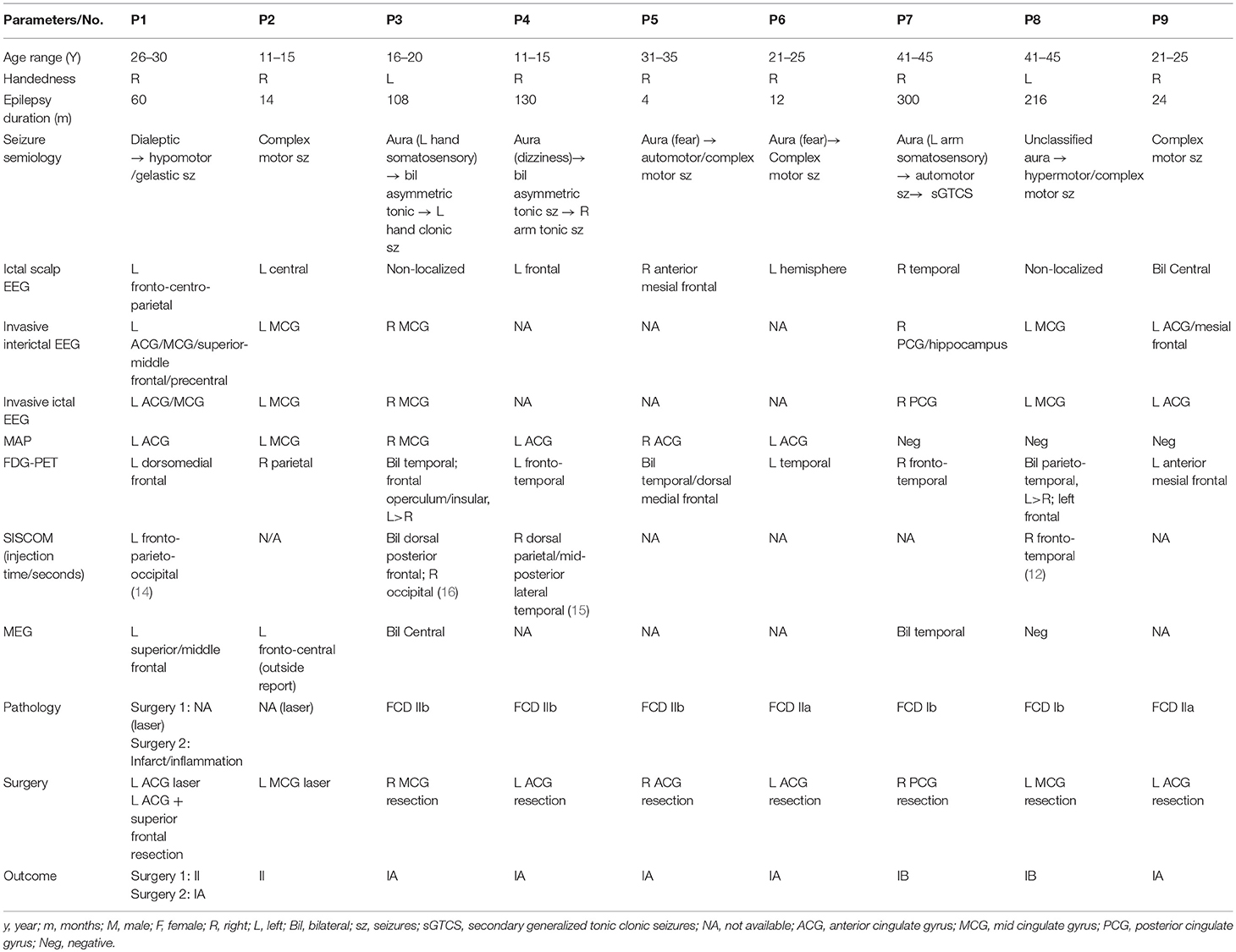
Table 1. Summary of clinical profile, presurgical evaluation data, surgery, histopathology, and postoperative seizure outcome for the 9 included patients.
Non-invasive Pre-surgical Evaluation
On scalp EEG, ictal onset lateralized to the ipsilateral hemisphere (fronto-centro-parietal = 1, central = 1, frontal = 2, temporal = 1, hemisphere = 1) in 6 of the 9 patients. FDG-PET was performed in all 9 patients; in only 2 patients, hypometabolism overlapped with (and also extended beyond) the CC (P1 and P9). Ictal SPECT was successfully obtained in 4 of the 9 patients (injection time: 12–16 s); the hyperperfusion areas contained the CC only in one patient (P1). MEG was performed in 5 of the 9 patients; positive findings were found in 4 patients, and only 2 of the 4 patients had MEG findings overlapping with the CC (P1 and P2, both loose clusters).
MAP Findings
The MAP findings are illustrated for all 9 patients in Figure 1. Single MAP+ abnormalities were found in 6 patients (P1-P6), including 3 of the 6 patients with negative MRI, and 3 patients with subtly lesional MRI. In P1-P3 who had negative MRI, MAP gray-white junction file pinpointed a subtle abnormality in the anterior or middle CC, which was found in retrospect to represent subtle blurring of gray-white matter junction in the original T1/FLAIR images, concordant with ICEEG (Figure 1). P4-P6 with subtly lesional MRI were all found to have abnormalities on MAP in the anterior CC; they did not have ICEEG as the subtle findings were identified during re-review at PMC. P7-P9 had negative MAP while their ICEEG showed focal ictal onset in the cingulate cortex. MAP extension or thickness files did not have additional yield; only in P4, a supra-threshold abnormality was seen on the extension file accompanying the junction file.
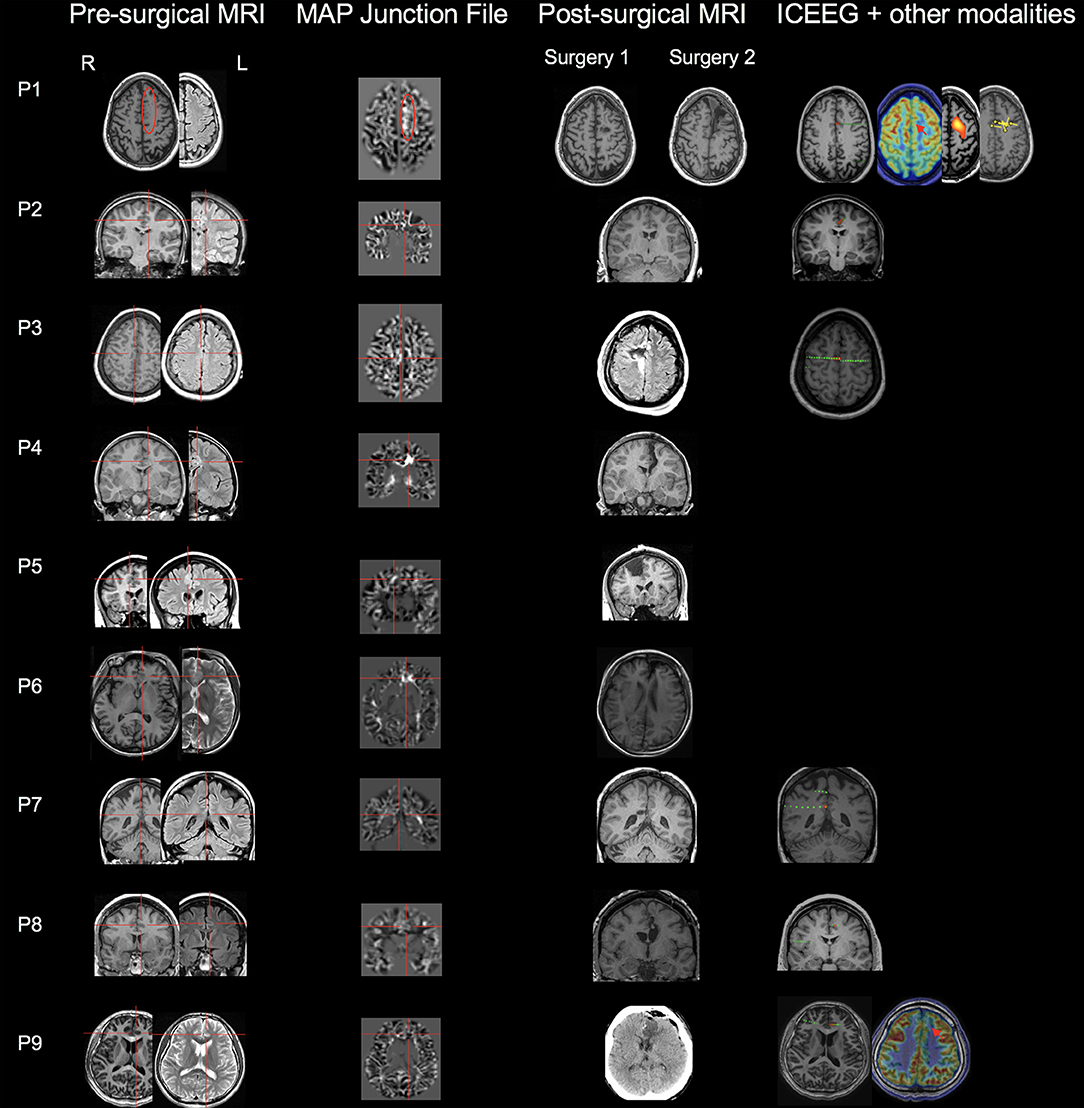
Figure 1. MAP findings illustrated for all 9 patients included in this study. Single MAP+ abnormalities were found in 6 patients (P1-P6), including P1-P3 who had negative 3T MRI by visual analyses, and P4-P6 who had subtly lesional 3T MRI by visual analyses. P7-P9 had negative MAP. First column: pre-surgical T1-weighted/FLAIR images; second column: co-registered MAP junction files; third column: post-surgical MRI indicating resection of the cingulate cortex. The red circle or cross hair shows the location of subtle abnormalities identified by MAP (in P7-P9, MAP was negative so the crosshair was set to the location of the ICEEG ictal onset). Fourth column shows ICEEG ictal onset and FDG-PET/SISCOM/MEG findings (if concordant). Red electrode contacts indicate ictal onset locations that are concordant with MAP+ findings. In P1, two surgeries were performed, 25 months apart. P1 had seizure recurrence at 15 months following laser ablation that partially overlapped with the MAP+ abnormality, and became seizure-free for 1 year after the second resection to clean up the resection margin, which included the entire MAP+ region.
Outcome, Surgery, and Pathology
Out of the 6 MAP+ patients, 4 patients (P3-P6) had the resection completely overlapping with the MAP+ region and became seizure-free; two patients (P1 and P2) didn't become seizure-free: P1 experienced seizure recurrence at 15 months following laser ablation that partially overlapped with the MAP+ abnormality, and became seizure-free for 1 year after the second resection to clean up the resection margin, which included the entire MAP+ region; in P2, who had marked improvement in seizure frequency and intensity (Class II), post-operative MRI indicated incomplete removal of the area corresponding to ICEEG and MAP. The 3 MAP-negative patients did become seizure-free (one Class Ia, two Class Ib) following resective surgery guided by ICEEG. Surgical pathology revealed FCD in 7 patients, including FCD type Ib (n = 2), type IIa (n = 2), and type IIb (n = 3). No specimen was sent to pathology examination in the two patients who had laser ablation.
Discussion
Non-lesional cingulate epilepsy is a rare form of epilepsy (2). Our current study presents the largest series of patients with surgically confirmed non-lesional cingulate epilepsy, with utility of MRI postprocessing to help identify subtle structural abnormalities in this challenging cohort. We showed that voxel-based MRI postprocessing identified subtle epileptic abnormalities in the majority of patients, while the localization value of scalp EEG, PET, ictal SPECT, and MEG was relatively limited. This finding emphasizes the practical value of adding MRI post-processing into the presurgical evaluation workflow of MRI-negative cingulate epilepsy.
Surgical management of patients with CE is challenging, as CE exhibits significant heterogeneity in its manifestations due to different seizure propagation patterns (3). Animal and human studies have demonstrated that the anterior CC is bi-directionally connected to the prefrontal and premotor areas, and the posterior CC bi-directionally connected to the mesial temporal regions (1, 3, 20, 21). Moreover, epileptic discharges from the CC often present secondary bilateral synchronous epileptiform discharges, which increases the difficulty to precisely localize (22). Not surprisingly, scalp EEG was less helpful to localize EZ located in the CC because of its low spatial resolution and inability to detect deep focus (3). Complex epileptic networks and fast propagation of discharges from the CC could account for the relatively low yields of PET and SISCOM as reported in previous studies (1, 2, 12, 23). Wong et al. (24). demonstrated that rapid spread of epileptic activities could result in widespread hypometabolism, sometimes remote to the EZ. Diffuse regions of hyperperfusion might reflect the epileptic network which includes the epileptic focus as well as the propagation pathways away from the onset, further complicating the task of localization (25). Although MEG has theoretical advantages including high spatial and temporal resolution in identifying epileptic activities from deep structures compared to scalp EEG (26), its localization seemed to be limited for CC as shown in our study, perhaps due to the CC producing radially oriented sources difficult to be detected by MEG source localization.
In 50% (3 of 6) of the patients with CE and negative MRI in our series, abnormalities were identified using MAP; in all 3 patients with CE and subtly lesional MRI, abnormalities were identified using MAP; the overall detection rate was analogous to published series whose detection rate ranged between 43 and 50% in MRI-negative epilepsies (12, 13). FCD is the most common identifiable pathology among MRI-negative epilepsies, frequently presenting blurring of the gray-white matter junction (11). Therefore, it is expected that junction map was the most helpful feature map in the current study and previous studies (11–13). The majority (4 of 5) patients with FCD type II were successfully detected by MAP in our study, while neither case with FCD type I (P7-P8) was MAP+. Therefore, the type of the underlying pathology likely contributes to the negative MAP results. It's a considerable challenge to identify and demarcate FCD type I by current MRI techniques even in patients with confirmed histopathology (27), as FCD type I is typically not as well-characterized on the MRI with less prominent features. Our previous study looked at a group of 150 MRI-negative epilepsies which mostly consisted of FCD type I; not all patients with positive pathology of FCD type I were MAP+; additionally, 5 patients with FCD type I had seizure recurrence even though resection fully overlapped with their MAP+ regions, which suggests insufficient delineation of the full extent of the FCD type I using the current technique (13). Another point worth noting is that the T1-based MAP processing, as utilized in this study, would not be able to capture subtle FCDs with a strong T2 change but no T1 change. This could be another factor contributing to negative MAP results. In the face of a completely non-lesional MRI (visual-negative and MAP-negative), ICEEG is often mandatory to explore the epileptogenic zone.
Being seizure-free is the gold standard to identify epileptogenic characteristics of MAP+ changes (11, 12). In the current study, MAP+ findings were included in the surgical resection in 4 patients with seizure freedom, suggesting that these findings were true positive findings. The two patients who didn't become seizure-free both had laser ablation which partially overlapped with their MAP+ abnormalities; the less optimal seizure outcomes might be due to the incomplete removal of the epileptic structural abnormality. The type of surgery could also be contributive; although minimally invasive, laser ablation was reported to be less effective than conventional resective surgery in a prior study on 19 pediatric patients (28).
Limitations
Patients studied here were a highly selected cohort and could not represent all patients with cingulate epilepsy. Using a combined dataset from two epilepsy centers, there might have been differences in the interpretation of presurgical evaluation tests and surgical decision. These limitations should be considered when interpreting results from our study.
Conclusion
Surgical management of patients with cingulate epilepsy is highly challenging, particularly when the MRI is negative. The localizing yield of non-invasive tests such as scalp EEG, PET, ictal SPECT and MEG in non-lesional cingulate epilepsy is relatively limited and ICEEG is often mandatory. MRI postprocessing could be incorporated into routine surgical evaluation to enhance detection of subtle epileptogenic abnormalities in this particularly challenging population.
Ethics Statement
This study was carried out in accordance with the recommendations of the institutional review board ethical guidelines of two hospitals (Cleveland Clinic Foundation and the Second Affiliated Hospital of Zhejiang University) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review board ethics committee.
Author Contributions
ShaW contributed to the conception, design the study, analysis of the data, interpretation of the results, and drafting the manuscript. BJ revising the manuscript. TA analysis of the data. MK analysis of the data. SJ revising the manuscript. BK revising the manuscript. JG-M interpretation of the results. RP analysis the data. IN and AA interpretation of the results. ShuW, MD, and ZIW interpretation of the results, drafting the manuscript, and final approval of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81671283, 81671282).
Abbreviations
CE, cingulate epilepsy; CC, cingulate cortex; MAP, morphometric analysis program; FCD, focal cortical dysplasia.
References
1. Alkawadri R, Gonzalez-Martinez J, Gaspard N, Alexopoulos AV. Propagation of seizures in a case of lesional mid-cingulate gyrus epilepsy studied by stereo-EEG. Epileptic Disord (2016) 18:418–25. doi: 10.1684/epd.2016.0874
2. Alkawadri R, So NK, Van Ness PC, Alexopoulos AV. Cingulate epilepsy: report of 3 electroclinical subtypes with surgical outcomes. JAMA Neurol. (2013) 70:995–1002. doi: 10.1001/jamaneurol.2013.2940
3. Enatsu R, Bulacio J, Nair DR, Bingaman W, Najm I, Gonzalez-Martinez J. Posterior cingulate epilepsy: clinical and neurophysiological analysis. J Neurol Neurosurg Psychiatry (2014) 85:44–50. doi: 10.1136/jnnp-2013-305604
4. Lacuey N, Davila JC, Zonjy B, Amina S, Couce M, Turnbull J, et al. Lesion-negative anterior cingulate epilepsy. Epileptic Disord. (2015) 17:134–42. doi: 10.1684/epd.2015.0749
5. Von Lehe M, Wagner J, Wellmer J, Clusmann H, Kral T. Epilepsy surgery of the cingulate gyrus and the frontomesial cortex. Neurosurgery (2012) 70:900–10; discussion 910. doi: 10.1227/NEU.0b013e318237aaa3
6. Arienzo D, Babiloni C, Ferretti A, Caulo M, Gratta C Del, Tartaro A, et al. Somatotopy of anterior cingulate cortex ( ACC ) and supplementary motor area ( SMA ) for electric stimulation of the median and tibial nerves : An fMRI study. Neuroimage (2006) 33:700–5. doi: 10.1016/j.neuroimage.2006.06.030
7. Ralston BL. Cingulate epilepsy and secondary bilateral synchrony. Electroencephalogr Clin Neurophysiol. (1961) 13:591–8. doi: 10.1016/0013-4694(61)90173-0
8. Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia (2008) 49:758–764. doi: 10.1111/j.1528-1167.2007.01428.x
9. Huppertz H-JJ, Grimm C, Fauser S, Kassubek J, Mader I, Hochmuth A, et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. (2005) 67:35–50. doi: 10.1016/j.eplepsyres.2005.07.009
10. Wong-Kisiel LC, Tovar Quiroga DF, Kenney-Jung DL, Witte RJ, Santana-Almansa A, Worrell GA, et al. Morphometric analysis on T1-weighted MRI complements visual MRI review in focal cortical dysplasia. Epilepsy Res. (2018) 140:184–91. doi: 10.1016/J.EPLEPSYRES.2018.01.018
11. Wang ZI, Ristic AJ, Wong CH, Jones SE, Najm IM, Schneider F, et al. Neuroimaging characteristics of MRI-negative orbitofrontal epilepsy with focus on voxel-based morphometric MRI postprocessing. Epilepsia (2013) 54:2195–2203. doi: 10.1111/epi.12390
12. Wang ZI, Alexopoulos AV, Jones SE, Najm IM, Ristic A, Wong C, et al. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann Neurol. (2014) 75:759–70. doi: 10.1002/ana.24169
13. Wang ZI, Jones SE, Jaisani Z, Najm IM, Prayson RA, Burgess RC, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. (2015) 77:1060–75. doi: 10.1002/ana.24407
14. Wagner J, Weber B, Urbach H, Elger CE, Rgen Huppertz H-J, Huppertz H-J. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain (2011) 134:2844–54. doi: 10.1093/brain/awr204
15. Wellmer J, Parpaley Y, Von Lehe M, Huppertz H-JJ. Integrating magnetic resonance imaging postprocessing results into neuronavigation for electrode implantation and resection of subtle focal cortical dysplasia in previously cryptogenic epilepsy. Neurosurgery (2010) 66:187–94; discussion 194–5. doi: 10.1227/01.NEU.0000359329.92781.B7
16. Lüders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, et al. Semiological seizure classification. Epilepsia (1998) 39:1006–13. doi: 10.1111/j.1528-1157.1998.tb01452.x
17. Huppertz HJ, Wellmer J, Staack AM, Altenmüller DM, Urbach H, Kröll J, et al. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia (2008) 49:772–85. doi: 10.1111/j.1528-1167.2007.01436.x
18. Jin B, Krishnan B, Adler S, Wagstyl K, Hu W, Jones S, et al. Automated detection of focal cortical dysplasia type II with surface-based magnetic resonance imaging postprocessing and machine learning. Epilepsia (2018)982–92. doi: 10.1111/epi.14064
19. Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia (2011) 52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x
20. Kubota Y, Enatsu R, Gonzalez-Martinez J, Bulacio J, Mosher J, Burgess RC, et al. In vivo human hippocampal cingulate connectivity: a corticocortical evoked potentials (CCEPs) study. Clin Neurophysiol. (2013) 124:1547–56. doi: 10.1016/j.clinph.2013.01.024
21. Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. (1987) 262:271–89.doi: 10.1002/cne.902620208
22. Iwasaki M, Nakasato N, Kakisaka Y, Kanno A, Uematsu M, Haginoya K, et al. Clinical Neurophysiology Lateralization of interictal spikes after corpus callosotomy. Clin Neurophysiol. (2011) 122:2121–7. doi: 10.1016/j.clinph.2011.04.013
23. Morales-Chacon LM, Alfredo Sanchez Catasus C, Minou Baez Martin M, Rodriguez Rojas R, Lorigados Pedre L, Estupinan Diaz B. Multimodal imaging in nonlesional medically intractable focal epilepsy. Front Biosci. (2015) 7:42–57. doi: 10.2741/E716
24. Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia (2012) 53:1333–40. doi: 10.1111/j.1528-1167.2012.03547.x
25. Von Oertzen TJ. PET and ictal SPECT can be helpful for localizing epileptic foci. Curr Opin Neurol. (2018) 31:184–91. doi: 10.1097/WCO.0000000000000527
26. Knowlton RC, Laxer KD, Aminoff MJ, Roberts TP, Wong ST, Rowley HA. Magnetoencephalography in partial epilepsy: clinical yield and localization accuracy. Ann Neurol (1997) 42:622–31. doi: 10.1002/ana.410420413
27. Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. (2008) 63:758–69. doi: 10.1002/ana.21398
Keywords: epilepsy, surgery, cingulate, MRI post-processing, non-lesional, focal cortical dysplasia
Citation: Wang S, Jin B, Aung T, Katagiri M, Jones SE, Krishnan B, Gonzalez-Martinez JA, Prayson RA, Najm IM, Alexopoulos AV, Wang S, Ding M and Wang ZI (2018) Application of MRI Post-processing in Presurgical Evaluation of Non-lesional Cingulate Epilepsy. Front. Neurol. 9:1013. doi: 10.3389/fneur.2018.01013
Received: 31 August 2018; Accepted: 09 November 2018;
Published: 27 November 2018.
Edited by:
Fernando Cendes, Universidade Estadual de Campinas, BrazilReviewed by:
Marino M. Bianchin, Universidade Federal do Rio Grande do Sul (UFRGS), BrazilXiaorong Liu, Guangzhou Medical University, China
Copyright © 2018 Wang, Jin, Aung, Katagiri, Jones, Krishnan, Gonzalez-Martinez, Prayson, Najm, Alexopoulos, Wang, Ding and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Irene Wang, d2FuZ2kyQGNjZi5vcmc=
 Shan Wang
Shan Wang Bo Jin1
Bo Jin1 Stephen E. Jones
Stephen E. Jones Jorge A. Gonzalez-Martinez
Jorge A. Gonzalez-Martinez Imad M. Najm
Imad M. Najm Meiping Ding
Meiping Ding Irene Wang
Irene Wang