- 1Department of Neurosurgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
- 2Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bangalore, India
- 3NIHR Global Health Research Group on Neurotrauma, University of Cambridge, Cambridge, United Kingdom
Decompressive craniectomy (DC) has become the definitive surgical procedure to manage medically intractable rise in intracranial pressure due to stroke and traumatic brain injury. With incoming evidence from recent multi-centric randomized controlled trials to support its use, we could expect a significant rise in the number of patients who undergo this procedure. Although one would argue that the procedure reduces mortality only at the expense of increasing the proportion of the severely disabled, what is not contested is that patients face the risk of a large number of complications after the operation and that can further compromise the quality of life. Decompressive craniectomy (DC), which is designed to overcome the space constraints of the Monro Kellie doctrine, perturbs the cerebral blood, and CSF flow dynamics. Resultant complications occur days to months after the surgical procedure in a time pattern that can be anticipated with advantage in managing them. New or expanding hematomas that occur within the first few days can be life-threatening and we recommend CT scans at 24 and 48 h postoperatively to detect them. Surgeons should also be mindful of the myriad manifestations of peculiar complications like the syndrome of the trephined and neurological deterioration due to paradoxical herniation which may occur many months after the decompression. A sufficiently large frontotemporoparietal craniectomy, 15 cm in diameter, increases the effectiveness of the procedure and reduces chances of external cerebral herniation. An early cranioplasty, as soon as the brain is lax, appears to be a reasonable choice to mitigate many of the late complications. Complications, their causes, consequences, and measures to manage them are described in this chapter.
Introduction
In medicine, there is increasing awareness that outcome must be evaluated in terms of quality of life and cost effectiveness, rather than merely extending the survival of a patient. Such considerations are especially important in decompressive craniectomy (DC), which is performed in certain cases of ischemic stroke, traumatic brain injury, and subarachnoid hemorrhage, to alleviate (ICP) and massive brain swelling (1–3). ICP reduction can lead to improvements in cerebrovascular compliance, cerebral oxygenation, and cerebral perfusion (4). Though many studies have shown long-term beneficial effects after DC (1, 5–7) it is still regarded as a salvage surgery. Long-term, deleterious neurocognitive, and psychosocial effects resulting in poor quality of life, and economical burden are well known (6, 8).
Anticipating a possible rise in the frequency with which decompressive craniectomies are likely to be carried out, based on the strength of recent, strong, supportive, level-one evidence in both traumatic brain injury (9) and stroke (10, 11), complication avoidance should become the new focus in surgical management and research. Currently, there is only low-quality evidence to choose the kind of interventions to avoid complications. Understanding the type and burden of the potential complications, the timeline of their appearance and the reasons why they develop will hold the key to designing good quality randomized controlled trials in the future.
After DC, cranioplasty has to be done (7) using autologous skull, or costly synthetic materials (12). Apart from its own set of complications, cranioplasty creates serious economical burden (13) in low-to-middle income countries (LMICs). They are described in detail in another chapter.
In this chapter, we classify and describe the complications of DC and suggest management techniques that can reduce the risks.
Complications
Decompressive Craniectomy
Decompressive craniectomy has many known complications. The overall complication rates range up to 53.9% (14).
Classification
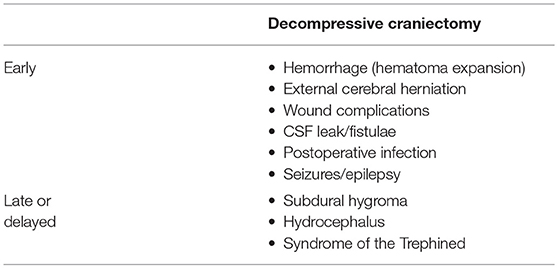
We suggest that complications be classified as those that occur in the first 4 weeks (early) and those that manifest later (late or delayed). Early complications, which occur in the first 4 weeks, are likely to happen while the patients is still at the hospital. Specific complications tend to occur during particular time periods and awareness of that information helps anticipate and treat them efficiently. Kurland et al. classified them as (i) hemorrhagic, (ii) infectious/inflammatory, and (iii) disturbances of the CSF compartment (15). They tabulated the overall average frequency of each of the complications from a total of 142 eligible reports of thousands of patients who underwent decompressive procedures. They found that one in ten patients who underwent DC develop a complication that required additional medical and/or neurosurgical intervention.
Timeline of Various Complications
Ban et al. reported, from their analysis of 89 patients, that specific complications occurred in a sequential fashion (14). Complications like cerebral contusion expansion (2.2 ± 1.2 days), newly appearing subdural or epidural hematoma contralateral to the craniectomy defect (1.5 ± 0.9 days), epilepsy (2.7 ± 1.5 days), CSF leakage through the scalp incision (7.0 ± 4.2 days), and external cerebral herniation (5.5 ± 3.3 days) occurred early. Subdural effusion (10.8 ± 5.2 days) and postoperative infection (9.8 ± 3.1 days) developed between 1 and 4 weeks postoperatively. Syndrome of the trephined and post-traumatic hydrocephalus developed after 1 month postoperatively (at 79.5 ± 23.6 and 49.2 ± 14.1 days, respectively).
Risk Factors For Developing Complications
Patient-specific risk factors for developing complications include poor neurological status and age. A low preoperative GCS (below eight) has been shown to increase the possibility of all types of complications (16). Age over 65 years is another risk factor (14). Though these risk factors are not modifiable, the surgical team should identify these risk groups to diligently look for emerging complications.
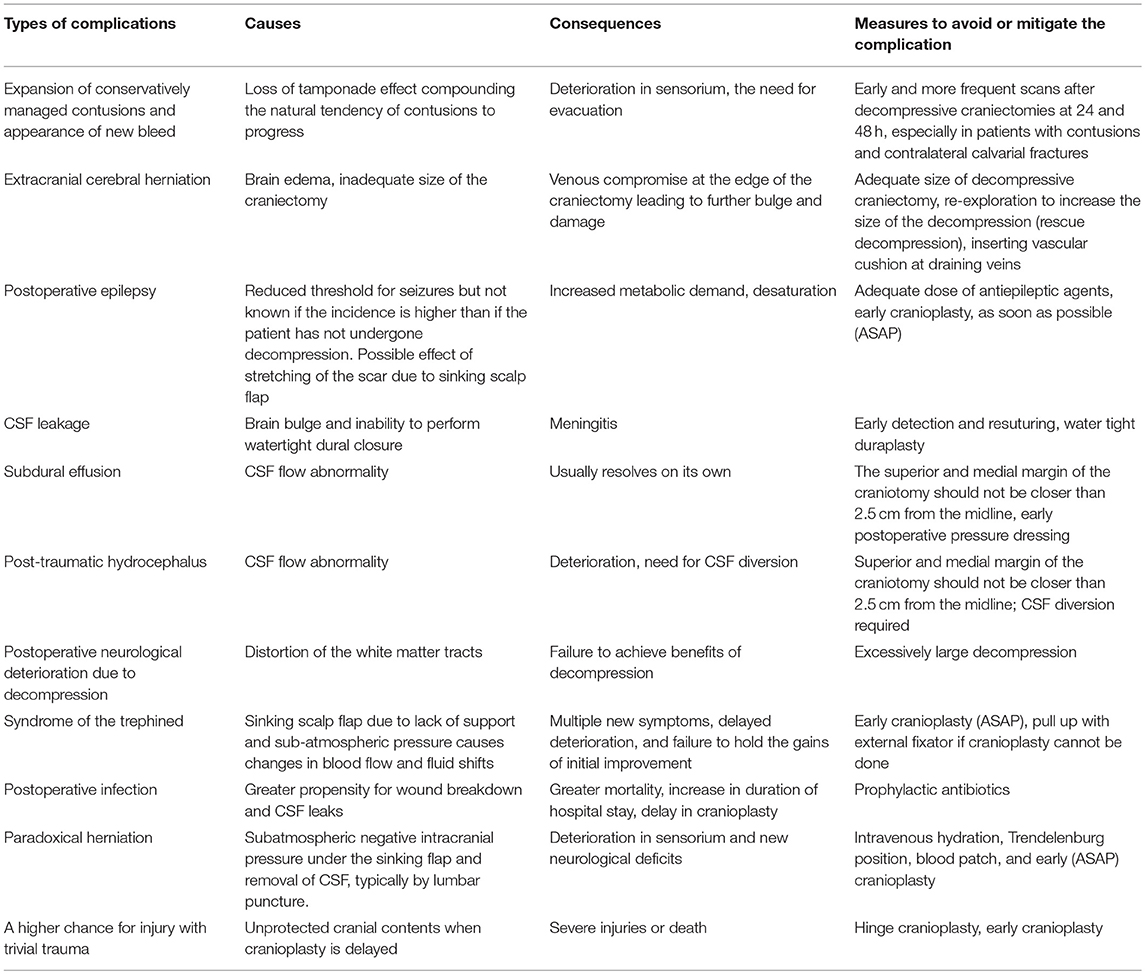
An overview of the complications is provided in Table 1, 2 summarizes probable causes, consequences, and management options.
Early complications
Hemorrhage
Expansion of conservatively managed contusions and other bleeds are major issues that occur early after the DC (Figure 1). Most expansions occur acutely after surgery and cause clinical deterioration, prolonged hospital stay, and can even prove fatal. One theory is that the hemostatic (or tamponade) effect is lost when removing the bone, and that, along with reduction in ICP facilitates the expansion mostly on the ipsilateral side. (17–19). This hypothesis is supported by the report from Flint et al. where the propensity was higher on the side of the decompression. In their series of 40 patients, new or expanded hemorrhagic contusions were observed in 23 (58%) of 40 patients and 80% of that occurred ipsilaterally (20). Other kinds of hematomas like extradural hematomas and acute subdural hematomas can either appear de novo or increase in size. Expansion or evolution of new, remotely located extradural hematomas, typically occur at a fracture site (21). Expansion of hematoma contralateral or remote from the side of the craniectomy has not been commonly reported in stroke patients.
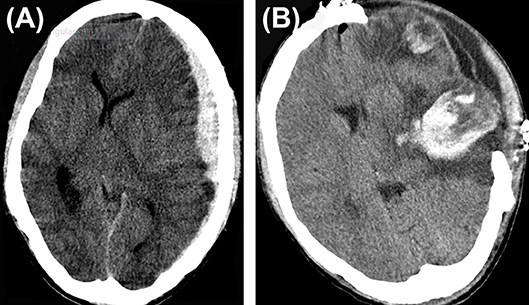
Figure 1. Hematoma expansion. (A) A case of traumatic brain injury depicting subdural hematoma (B), hematoma expansion, and subdural collection post craniectomy.
A contralateral hematoma developed an average of 2.1 days after the primary decompression surgery (16) and an ipsilateral one happened after a mean of 1.5 days (14). In the multivariate analysis of the complications in 89 consecutive patients who underwent DC, only contusion expansion led to poor outcome (14). Hemorrhagic progression of infarcts occur at a frequency of about 23.7% (123/519) of malignant stroke patients who underwent DC (15).
We suggest mandatory CT scan(s) in the first 48 h after DC to help detect this complication quickly and limit the damage.
External Cerebral Herniation
External cerebral herniation appears during the first week after surgery (Figure 2). Yang defined it as more than 1.5 cm of herniated brain tissue through the center of the craniectomy defect (16). The incidence is up to 25%. It is thought to be caused by the edema induced by cerebral re-perfusion and increased hydrostatic gradient from the capillaries, after decompression (17). Brain edema causes bulging of the brain and kinking of the draining veins at the edges of the craniectomy which in turn causes venous congestion, infarcts, further herniation, and brain parenchymal lacerations (22). Adequately large craniotomies and augmentative duraplasty avoid herniation (14). The Brain Trauma Foundation recommends that a large frontotemporoparietal DC (not less than 12 × 15 or 15 cm diameter) is needed over a small frontotemporoparietal DC for reduced mortality and improved neurologic outcomes in patients with severe TBI (23). Placing two small gelfoam pledgets on either side of drains at the craniectomy may prevent venous occlusion.
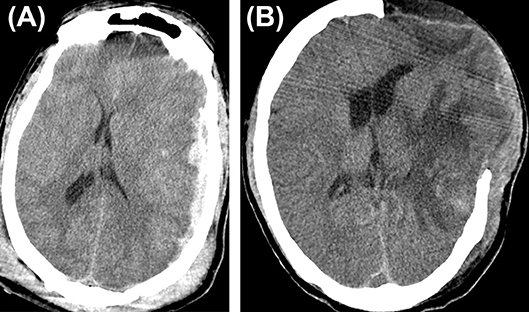
Figure 2. Cerebral herniation. (A) A case of traumatic brain injury depicting cerebral herniation (B) from the craniectomy site.
Paradoxical herniation is an unusual complication that tends to occur when there is negative, sub-atmospheric intracranial pressure under the caved-in scalp flap causing the brain to herniate when procedures like lumbar puncture CSF removal (24), ventriculoperitoneal shunt, subdural fluid drainage (25), or even making the patient assume a vertical position for postoperative mobilization is done (26). Intravenous hydration and Trendelenburg position has been used to successfully reverse the herniation.
Wound Complications
Wound complications following DC or cranioplasty after DC have been classified as dehiscence, ulceration, or necrosis (27). The large size of the scalp flap and the increased probability of injury to the superficial temporal artery during emergency surgery predispose the wound edges to ischemia at the posterior parietal and temporal areas. The pressure of the brain bulge aggravates the ischemia. The underlying, exposed, injured, or ischemic brain is especially vulnerable to infective complications once the wound breaks down.
Meticulously preserving the superficial temporal artery and limiting the posterior extent of the flap to no more than 5 cm behind the ear could reduce chance of ischemic flap breakdown. A retrospective comparison of patients operated using an n-shaped incision with those who were operated using the conventional question mark flap showed that the former technique could accomplish greater bony decompression, allows more brain protrusion and is faster to perform (28). We have noticed that making a retroauricular incision could also reduce flap necrosis.
CSF Leak/Fistulae
The overall prevalence of CSF leak/fistulae due to DC has been shown to be up to 6.3% (15). In patients undergoing DC for cerebral venous sinus thrombosis (CVST), it was seen in 2.9% (29). It appears intuitive that a meticulous augmentative duraplasty and watertight scalp closure would prevent the exodus of CSF from the wound and reduce infection risk. However, a recent randomized controlled trial where watertight duraplasty was compared with rapid-closure DC without watertight duraplasty, there was no statistically significant difference in complications like CSF leak between the two groups of 29 patients each (30). Rapid closure DC without water tight duraplasty was on an average 31 min faster and hence cheaper. Though the authors claim that both procedures are equivalent, the trial was never powered or designed to prove non-inferiority of the test procedure and hence the results should be taken with caution (31).
Postoperative Infections
Superficial wound infections including wound breakdown, necrosis, surgical site infection, sub-galeal collections, and wound breakdown occurred in about 10% of patients and incidence of deeper infections like an epidural abscess, and subdural empyema was just under 4% (15). Figure 3 shows a brain abscess which developed 2 months after DC for CVST (Figure 3C).
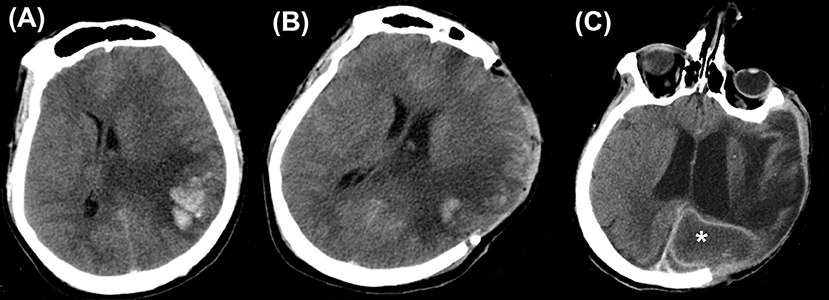
Figure 3. Infections. Computed tomography depicting (A) a case of cerebral venous sinus thrombosis. (B) Post craniectomy showed a reduction in the midline shift. (C) However, this patient developed brain abscess (asterisk) 2 months later.
The incidence of meningitis and ventriculitis is 4% probably due to the higher chances of CSF leaks. Early detection by looking for signs of meningeal irritation and guarded lumbar puncture CSF analysis is warranted.
Apart from the scalp wound complications, wound breakdown, and infection can occur when the bone flap is preserved in an abdominal pouch (Figure 4).
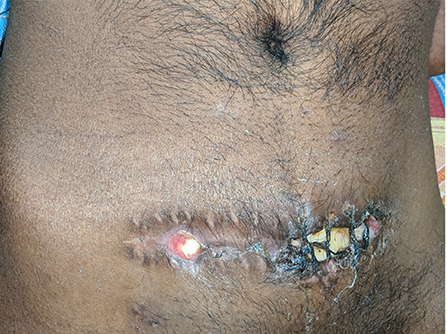
Figure 4. Abdominal wound infection. A partially exposed bone flap is seen through the gaped abdominal storage site, predisposing to infections.
Seizures/Epilepsy
Postoperative epilepsy has been documented in a varying proportion of patients who have undergone DC (14, 32–36). Suggested mechanisms include graded increases in hyperexcitability and a reduced epileptogenic threshold (14, 37). Creutzfeldt et al. retrospectively assessed 55 patients who underwent DC for malignant middle cerebral artery infarction. Of these, 49% of the patients developed seizures within the first week and 45% developed epilepsy within 1 year of surgery (32). Similarly, Santamarina et al. observed occurrence of seizures in 47.5% of all patients and in 53.7% of survivors undergoing DC for malignant MCA infarction. Logistic regression revealed that only prolonged delay from the onset of stroke to decompression (>42 h) independently predicted the development of epilepsy (34). In another study, Brondani et al. reported the prevalence of seizures in 61% (21 out of 36) of the patients with malignant MCA infarction undergoing DC. Furthermore, 59% (19 out of 34) patients developed epilepsy (33). Although a non-significant difference existed between TBI patients with or without seizures (incidence of 10.8%), the hospital stay prolonged significantly in the former group (35). Identifying the key risk factors predisposing to seizures and their effect on clinical outcomes needs more prospective studies.
In the case of TBI, Ban et al. reported that only about 3% developed seizures despite the use of anticonvulsants. Seizures disappeared in all the patients after increasing the dosage or after adding other antiepileptic drugs and that is a reasonable approach to follow in the first 2 weeks post injury (14). An early cranioplasty might serve to mitigate their occurrence, however, studies addressing this issue are currently lacking. Phenytoin and levetiracetam can be considered as antiepileptic drugs.
Late Complications
Subdural Hygroma
Subdural hygroma formation is another widely encountered complication after DC occurring in 27.4% (723/2,643) of patients with TBI and 12.5% (42/336) of patients with malignant infarction treated with DC in the total frequency calculation done by Kurland et al. (15). The putative mechanisms seems to be due to CSF flow abnormalities that develop after decompression possibly because of a disruption of the subarachnoid CSF pathways either due to trauma or surgical manipulation (38), or due to increased cerebral perfusion pressure (39). The common locations are the subdural, subgaleal, or interhemispheric areas (16, 40, 41), Though there is a speculative relationship with the development of hydrocephalus, subdural hygromas usually resolve spontaneously. But it has been shown to be associated with a worse neurological outcome (42). Effusions are thought to be reduced by a duraplasty.
Early pressure dressing applied 7–10 days after DC has been shown to reduce this complication in a small randomized controlled trial (43). A tense collection of fluid can rarely cause pressure on the brain due to a ball valve effect and has been termed external brain tamponade and such hygromas require drainage (16, 44, 45).
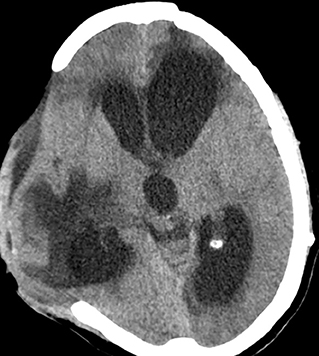
Hydrocephalus
Communicating hydrocephalus is another non-trivial complication of decompressive procedures because of the perturbation of CSF flow dynamics (Figure 5). Depending on the diagnostic criteria the incidence ranges from 0.7 to 86% (42). Bonis et al. showed by logistic regression analysis that the only factor that seemed to be associated with both subdural hygroma and hydrocephalus was if the superior margin of the craniectomy was closer than 2.5 cm to the midline (42). Development of hydrocephalus is also known to predict an unfavorable outcome (46). An early cranioplasty seems to mitigate the risk of post-traumatic hydrocephalus in a retrospective cohort study of 91,583 patients <21 years with TBI, in whom 846 developed post-traumatic hydrocephalus (47). Craniectomy without early cranioplasty was associated with markedly increased adjusted odds of post-traumatic hydrocephalus (aOR 3.67, 95% CI 2.66–5.07), an effect not seen in those undergoing cranioplasty within 30 days (aOR 1.19, 95% CI 0.75–1.89).
Syndrome of the Trephined
Syndrome of trephined has an overall prevalence of 10% (15). It was initially described by Grant and Norcross in 1939 (48). The sinking of the scalp due to lack of bony support (Figure 6) causes cerebral blood flow anomaly and dysfunction in the underlying cortex. Motor syndrome of the trephined is hypothesized to occur in patients who have had contusion induced low-density parenchymal areas. Delayed fluid shifts occur due to impaired CSF flow dynamics and this goes on to produce cerebral blood flow abnormalities and impaired motor function in a previously unaffected limb many months later (49). The syndrome can manifest in myriad ways and the most common symptoms identified in a recent systematic review were motor weakness (61.1%) followed by cognitive deficits (44.4%), language deficits (29.6%), altered level of consciousness (27.8%), headache (20.4%), psychosomatic disturbances (18.5%), seizures or electroencephalographic changes (11.1%), and cranial nerve deficits (5.6%) (50). It manifests either as new symptoms causing deterioration of the patient condition or as failure to retain the early gains. It could manifest as early as 3 days to as late as 7 years (with an average of 5 months). We must be mindful of the fact that only motor symptoms are obvious and it is quite easy to miss the diagnosis of syndrome of the trephined when non-motor symptoms like cognitive alterations occur. These symptoms, as well as, cerebral blood flow abnormalities improve dramatically after a cranioplasty. Yang has suggested it is safe to do early cranioplasty within 5–8 weeks to mitigate this risk (51) and a recent meta-analysis of observational studies involving 528 patients seems to support the possibility that neurological improvement is better in that group (52). If cranioplasty cannot be done due to a reason like infection and the patient is suffering from the effects of the sunken scalp flap, then a novel method of long standing scalp retraction using an external frame can be tried as described by Kim et al. (53).
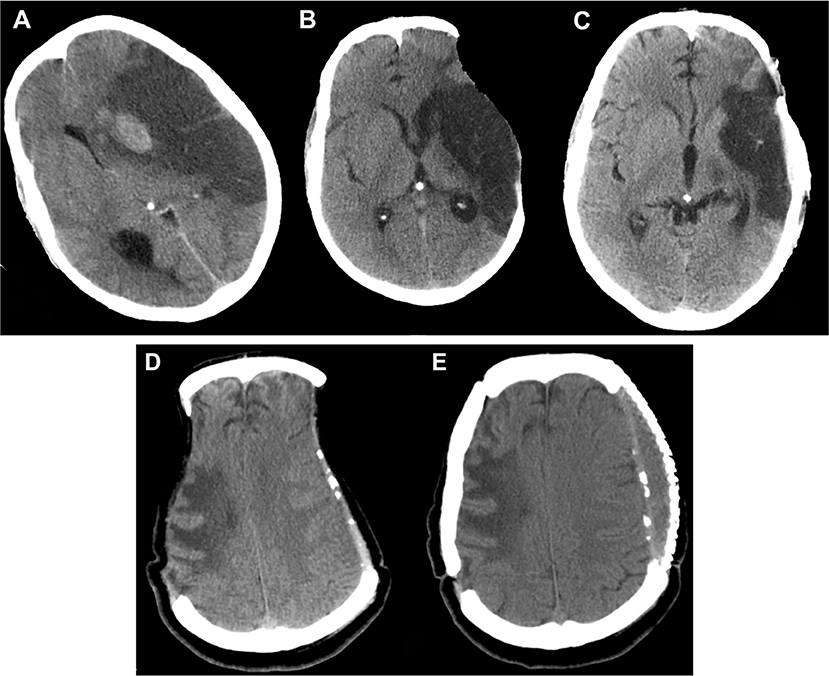
Figure 6. Sunken flap syndrome. Computed tomography depicting (A) malignant hemispheric infarction, (B) sunken flap syndrome after 6 months, which improved (C) post cranioplasty. In a different patient (D), bilateral sunken flap syndrome was observed 25 months post DC, and (E) improved after cranioplasty. DC, decompressive craniectomy.
Undue delay in cranioplasty and resorption of the bone flap after cranioplasty causes unsightly depression of the scalp. Temporal hollowing and chewing difficulty arises due to extensive dissection of the temporalis muscle to get good decompression at the temporal base. A technique of en bloc detachment and anteroinferiorly turning of the temporal muscle using a clover leaf scalp incision has been described by Missori et al., in 21 patients undergoing DC. They reported good aesthetic results and all eligible patients reported normal chewing ability (54).
Summary
Decompressive craniectomy for intractable intracranial hypertension due to stroke or traumatic brain injury is a proven treatment for reducing mortality and there is some evidence, albeit controversial (55), that it improves the fraction of good grade survivors. But the therapy is fraught with multiple, non-trivial complications that need to be anticipated and treated early (see Table 1 for an overview). Doing a sufficiently large cranioplasty to avoid cerebral herniation and having a low threshold diagnosing for progression of bleeds in the immediate postoperative period cannot be over emphasized. An early cranioplasty, preferably within 12 weeks, as soon as the brain is lax, is advisable to prevent long-term complications of DC.
Author Contributions
MG, NS prepared, edited, structured, revised, and critically reviewed the manuscript. DS, SK, and DB critically reviewed and accepted the final draft. BD edited, critically reviewed, and accepted the final draft.
Funding
BD and NS are supported by the NIHR Global Health Research Group on Neurotrauma that was commissioned by the NIHR using official development assistance (ODA) funding (Project 16/137/105). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Honeybul S, Ho KM. The current role of decompressive craniectomy in the management of neurological emergencies. Brain Inj. (2013) 27:979–91. doi: 10.3109/02699052.2013.794974
2. Honeybul S, Ho KM, Gillett GR. Reconsidering the role of decompressive craniectomy for neurological emergencies. J Crit Care (2017) 39:185–9. doi: 10.1016/j.jcrc.2017.03.006
3. Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg. (2012) 117:749–54. doi: 10.3171/2012.6.JNS111140
4. Hossain-Ibrahim MK, Wasserberg J. Decompressive craniectomy–time for a change? Br J Neurosurg. (2011) 25:538–9. doi: 10.3109/02688697.2011.584641
5. Servadei F. Clinical value of decompressive craniectomy. N Engl J Med. (2011) 364:1558–9. doi: 10.1056/NEJMe1102998
6. van Middelaar T, Nederkoorn PJ, van der Worp HB, Stam J, Richard E. Quality of life after surgical decompression for space-occupying middle cerebral artery infarction: systematic review. Int J Stroke (2015) 10:170–6. doi: 10.1111/ijs.12329
7. Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nat Rev Neurol. (2013) 9:405–15. doi: 10.1038/nrneurol.2013.106
8. McKenna A, Wilson FC, Caldwell S, Curran D, Nagaria J, Convery F. Long-term neuropsychological and psychosocial outcomes of decompressive hemicraniectomy following malignant middle cerebral artery infarctions. Disabil Rehabil. (2012) 34:1444–55. doi: 10.3109/09638288.2011.644024
9. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. (2016) 375:1119–30. doi: 10.1056/NEJMoa1605215
10. Alexander P, Heels-Ansdell D, Siemieniuk R, Bhatnagar N, Chang Y, Fei Y, et al. Hemicraniectomy versus medical treatment with large MCA infarct: a review and meta-analysis. BMJ Open (2016) 6:e014390. doi: 10.1136/bmjopen-2016-014390
11. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. (2009) 8:326–333. doi: 10.1016/S1474-4422(09)70047-X
12. Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg Focus (2014) 36:E19. doi: 10.3171/2014.2.FOCUS13561
13. Chaturvedi J, Botta R, Prabhuraj AR, Shukla D, Bhat DI, Devi BI. Complications of cranioplasty after decompressive craniectomy for traumatic brain injury. Br J Neurosurg. (2016) 30:264–8. doi: 10.3109/02688697.2015.1054356
14. Ban SP, Son Y-J, Yang H-J, Chung YS, Lee SH, Han DH. Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc. (2010) 48:244–50. doi: 10.3340/jkns.2010.48.3.244
15. Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V, et al. Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care (2015) 23:292–304. doi: 10.1007/s12028-015-0144-7
16. Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir. (2008) 150:1241–7. doi: 10.1007/s00701-008-0145-9
17. Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus (2009) 26:E7. doi: 10.3171/2009.4.FOCUS0965
18. Yadav YR, Basoor A, Jain G, Nelson A. Expanding traumatic intracerebral contusion/hematoma. Neurol India (2006) 54:377–81. doi: 10.4103/0028-3886.28109
19. Carnevale JA, Segar DJ, Powers AY, Shah M, Doberstein C, Drapcho B, et al. Blossoming contusions: identifying factors contributing to the expansion of traumatic intracerebral hemorrhage. J Neurosurg. (2018) 1–12. doi: 10.3171/2017.7.JNS17988
20. Flint AC, Manley GT, Gean AD, Hemphill JC, Rosenthal G. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma (2008) 25:503–12. doi: 10.1089/neu.2007.0442
21. Talbott JF, Gean A, Yuh EL, Stiver SI. Calvarial fracture patterns on CT imaging predict risk of a delayed epidural hematoma following decompressive craniectomy for traumatic brain injury. AJNR Am J Neuroradiol. (2014) 35:1930–5. doi: 10.3174/ajnr.A4001
22. Schirmer CM, Ackil AA, Malek AM. Decompressive craniectomy. Neurocrit Care (2008) 8:456–470. doi: 10.1007/s12028-008-9082-y
23. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury. 4th ed. Neurosurgery (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
24. Oyelese AA, Steinberg GK, Huhn SL, Wijman CAC. Paradoxical cerebral herniation secondary to lumbar puncture after decompressive craniectomy for a large space-occupying hemispheric stroke: case report. Neurosurgery (2005) 57:E594. doi: 10.1227/01.NEU.0000170437.79760.DF
25. Nasi D, Dobran M, Iacoangeli M, Di Somma L, Gladi M, Scerrati M. Paradoxical brain herniation after decompressive craniectomy provoked by drainage of subdural hygroma. World Neurosurg. (2016) 91:673.e1–4. doi: 10.1016/j.wneu.2016.04.041
26. Fields JD, Lansberg MG, Skirboll SL, Kurien PA, Wijman CAC. ”Paradoxical" transtentorial herniation due to CSF drainage in the presence of a hemicraniectomy. Neurology (2006) 67:1513–4. doi: 10.1212/01.wnl.0000242889.02957.b6
27. Di Rienzo A, Pangrazi PP, Riccio M, Colasanti R, Ghetti I, Iacoangeli M. Skin flap complications after decompressive craniectomy and cranioplasty: proposal of classification and treatment options. Surg Neurol Int. (2016) 7:S737–45. doi: 10.4103/2152-7806.193724
28. Yang HS, Hyun D, Oh CH, Shim YS, Park H, Kim E. A faster and wider skin incision technique for decompressive craniectomy: n-shaped incision for decompressive craniectomy. Korean J Neurotrauma. (2016) 12:72–6. doi: 10.13004/kjnt.2016.12.2.72
29. Rajan Vivakaran TT, Srinivas D, Kulkarni GB, Somanna S. The role of decompressive craniectomy in cerebral venous sinus thrombosis. J Neurosurg. (2012) 117:738–44. doi: 10.3171/2012.6.JNS11102
30. Vieira E, Guimarães TC, Faquini IV, Silva JL, Saboia T, Andrade RVCL, et al. Randomized controlled study comparing 2 surgical techniques for decompressive craniectomy: with watertight duraplasty and without watertight duraplasty. J Neurosurg. (2017) 1–7. doi: 10.3171/2017.4.JNS152954
31. Sasidharan GM. Letter to the Editor. A nonsignificant trial result does not mean that two procedures are equal. J Neurosurg. (2018) 1–2. doi: 10.3171/2017.11.JNS172972
32. Creutzfeldt CJ, Tirschwell DL, Kim LJ, Schubert GB, Longstreth WT, Becker KJ. Seizures after decompressive hemicraniectomy for ischaemic stroke. J Neurol Neurosurg Psychiatry (2014) 85:721–5. doi: 10.1136/jnnp-2013-305678
33. Brondani R, Garcia de Almeida A, Abrahim Cherubini P, Mandelli Mota S, de Alencastro LC, Antunes ACM, et al. High risk of seizures and epilepsy after decompressive hemicraniectomy for malignant middle cerebral artery stroke. Cerebrovasc Dis Extra (2017) 7:51–61. doi: 10.1159/000458730
34. Santamarina E, Sueiras M, Toledo M, Guzman L, Torné R, Riveiro M, et al. Epilepsy in patients with malignant middle cerebral artery infarcts and decompressive craniectomies. Epilepsy Res. (2015) 112:130–136. doi: 10.1016/j.eplepsyres.2015.02.016
35. Huang Y-H, Liao C-C, Chen W-F, Ou C-Y. Characterization of acute post-craniectomy seizures in traumatically brain-injured patients. Seizure (2015) 25:150–4. doi: 10.1016/j.seizure.2014.10.008
36. Ramakrishnan V, Dahlin R, Hariri O, Quadri SA, Farr S, Miulli D, et al. Anti-epileptic prophylaxis in traumatic brain injury: a retrospective analysis of patients undergoing craniotomy versus decompressive craniectomy. Surg Neurol Int. (2015) 6:8. doi: 10.4103/2152-7806.149613
37. Chen JWY, Ruff RL, Eavey R, Wasterlain CG. Posttraumatic epilepsy and treatment. J Rehabil Res Dev. (2009) 46:685. doi: 10.1682/JRRD.2008.09.0130
38. Aarabi B, Hesdorffer DC, Simard JM, Ahn ES, Aresco C, Eisenberg HM, et al. Comparative study of decompressive craniectomy after mass lesion evacuation in severe head injury. Neurosurgery (2009) 64:927–39. doi: 10.1227/01.NEU.0000341907.30831.D2
39. Lang JK, Ludwig HC, Mursch K, Zimmerer B, Markakis E. Elevated cerebral perfusion pressure and low colloid osmotic pressure as a risk factor for subdural space-occupying hygromas? Surg Neurol. (1999) 52:630–7. doi: 10.1016/S0090-3019(99)00144-5
40. Ki HJ, Lee H-J, Lee H-J, Yi J-S, Yang J-H, Lee I-W. The risk factors for hydrocephalus and subdural hygroma after decompressive craniectomy in head injured patients. J Korean Neurosurg Soc. (2015) 58:254–61. doi: 10.3340/jkns.2015.58.3.254
41. Kaen A, Jimenez-Roldan L, Alday R, Gomez PA, Lagares A, Alén JF, et al. Interhemispheric hygroma after decompressive craniectomy: does it predict posttraumatic hydrocephalus? J Neurosurg. (2010) 113:1287–93. doi: 10.3171/2010.4.JNS10132
42. De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M, et al. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg. (2013) 115:1308–12. doi: 10.1016/j.clineuro.2012.12.011
43. Xu G-Z, Li W, Liu K-G, Wu W, Lu W-C, Zhang J-F, et al. Early pressure dressing for the prevention of subdural effusion secondary to decompressive craniectomy in patients with severe traumatic brain injury. J Craniofac Surg. (2014) 25:1836–9. doi: 10.1097/SCS.0b013e3182a21056
44. Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. (2006) Issue 1. CD003983. doi: 10.1002/14651858.CD003983.pub2.
45. Chauhan NS, Banday IA, Morey P, Deshmukh A. External brain tamponade: a rare complication of decompressive craniectomy. Intern Emerg Med. (2017) 12:117–8. doi: 10.1007/s11739-016-1436-4
46. Honeybul S, Ho KM. Decompressive craniectomy for severe traumatic brain injury: the relationship between surgical complications and the prediction of an unfavourable outcome. Injury (2014) 45:1332–39. doi: 10.1016/j.injury.2014.03.007
47. Bonow RH, Oron AP, Hanak BW, Browd SR, Chesnut RM, Ellenbogen RG, et al. Post-Traumatic hydrocephalus in children: a retrospective study in 42 pediatric hospitals using the pediatric health information system. Neurosurgery (2018) 83:732–9. doi: 10.1093/neuros/nyx470
48. Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. (1939) 110:488–512. doi: 10.1097/00000658-193910000-00002
49. Stiver SI, Wintermark M, Manley GT. Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg. (2008) 109:245–54. doi: 10.3171/JNS/2008/109/8/0245
50. Ashayeri K, M Jackson E, Huang J, Brem H, R Gordon C. Syndrome of the trephined: a systematic review. Neurosurgery (2016) 79:525–34. doi: 10.1227/NEU.0000000000001366
51. Yang NR, Song J, Yoon K-W, Seo EK. How early can we perform cranioplasty for traumatic brain injury after decompressive craniectomy? A retrospective multicenter study. World Neurosurg. (2018) 110:e160–7. doi: 10.1016/j.wneu.2017.10.117
52. Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G, et al. Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery (2018) 82:278–88. doi: 10.1093/neuros/nyx182
53. Kim MS, Park IS. Long-standing scalp retraction technique using an external fixator for sunken skin flap syndrome. Oper Neurosurg. (2017) 13:E28–32. doi: 10.1093/ons/opx036
54. Missori P, Paolini S, Ciappetta P, Seferi A, Domenicucci M. Preservation of the temporal muscle during the frontotemporoparietal approach for decompressive craniectomy: technical note. Acta Neurochir (2013) 155:1335–9. doi: 10.1007/s00701-013-1695-z
Keywords: decompressive craniectomy, hemorrhage expansion, infections, cerebral herniation, seizures, hydrocephalus, syndrome of the trephined
Citation: Gopalakrishnan MS, Shanbhag NC, Shukla DP, Konar SK, Bhat DI and Devi BI (2018) Complications of Decompressive Craniectomy. Front. Neurol. 9:977. doi: 10.3389/fneur.2018.00977
Received: 30 July 2018; Accepted: 30 October 2018;
Published: 20 November 2018.
Edited by:
Stephen Honeybul, Sir Charles Gairdner Hospital, AustraliaReviewed by:
Corrado Iaccarino, Azienda Ospedaliero-Universitaria di Parma, ItalyRita Formisano, Fondazione Santa Lucia (IRCCS), Italy
Copyright © 2018 Gopalakrishnan, Shanbhag, Shukla, Konar, Bhat and Devi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: B. Indira Devi, YmlkZXZpZHJAZ21haWwuY29t
 M. S. Gopalakrishnan
M. S. Gopalakrishnan Nagesh C. Shanbhag
Nagesh C. Shanbhag Dhaval P. Shukla
Dhaval P. Shukla Subhas K. Konar
Subhas K. Konar Dhananjaya I. Bhat
Dhananjaya I. Bhat B. Indira Devi
B. Indira Devi