
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 23 October 2018
Sec. Neurotrauma
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.00836
 Teena Shetty1*
Teena Shetty1* Joseph T. Nguyen2
Joseph T. Nguyen2 Taylor Cogsil1
Taylor Cogsil1 Apostolos John Tsiouris3
Apostolos John Tsiouris3 Sumit N. Niogi3
Sumit N. Niogi3 Esther U. Kim1
Esther U. Kim1 Aashka Dalal4
Aashka Dalal4 Kristin Halvorsen1
Kristin Halvorsen1 Kelianne Cummings5
Kelianne Cummings5 Tianhao Zhang6
Tianhao Zhang6 Joseph C. Masdeu7
Joseph C. Masdeu7 Pratik Mukherjee8
Pratik Mukherjee8 Luca Marinelli9
Luca Marinelli9Background: Uncertainty continues to surround mild traumatic brain injury (mTBI) diagnosis, symptoms, prognosis, and outcome due in part to a lack of objective biomarkers of injury and recovery. As mTBI gains recognition as a serious public health epidemic, there is need to identify risk factors, diagnostic tools, and imaging biomarkers to help guide diagnosis and management.
Methods: One hundred and eleven patients (15–50 years old) were enrolled acutely after mTBI and followed with up to four standardized serial assessments over 3 months. Each encounter included a clinical exam, neuropsychological assessment, and magnetic resonance imaging (MRI). Chi-square and linear mixed models were used to assess changes over time and determine potential biomarkers of mTBI severity and outcome.
Results: The symptoms most frequently endorsed after mTBI were headache (91%), not feeling right (89%), fatigue (86%), and feeling slowed down (84%). Of the 104 mTBI patients with a processed MRI scan, 28 (27%) subjects had white matter changes which were deemed unrelated to age, and 26 of these findings were deemed unrelated to acute trauma. Of the neuropsychological assessments tested, 5- and 6-Digit Backward Recall, the modified Balance Error Scoring System (BESS), and Immediate 5-Word Recall significantly improved longitudinally in mTBI subjects and differentiated between mTBI subjects and controls. Female sex was found to increase symptom severity scores (SSS) at every time point. Age ≥ 25 years was correlated with increased SSS. Subjects aged ≥ 25 also did not improve longitudinally on 5-Digit Backward Recall, Immediate 5-Word Recall, or Single-Leg Stance of the BESS, whereas subjects < 25 years improved significantly. Patients who reported personal history of depression, anxiety, or other psychiatric disorder had higher SSS at each time point.
Conclusions: The results of this study show that 5- and 6-Digit Backward Recall, the modified BESS, and Immediate 5-Word Recall should be considered useful in demonstrating cognitive and vestibular improvement during the mTBI recovery process. Clinicians should take female sex, older age, and history of psychiatric disorder into account when managing mTBI patients. Further study is necessary to determine the true prevalence of white matter changes in people with mTBI.
Mild traumatic brain injury (mTBI) is defined as a traumatically induced physiological disruption of brain function (1). Although mTBI accounts for at least 75% of traumatic brain injuries and imposes an excessive societal burden (2, 3), mTBI diagnosis continues to lack objective clinical and imaging biomarkers. As of now, the best marker for severity and recovery is a subjective assessment of acute symptom burden (4). Uncertainty continues to surround mTBI diagnosis, symptoms, prognosis, and outcome for physicians and patients as reliable biomarkers remain elusive. As injury rates increase and mTBI becomes a serious public health epidemic (5), there is an increasing role for identification of potential imaging biomarkers, specific neuropsychological assessments, and validated risk factors to help guide prognoses and return to play decisions.
Given the current subjective nature of symptom burden assessment, there is a role for neuropsychological assessments in evaluating the cognitive impairment of patients after injury. The sport concussion assessment tool (SCAT) has been demonstrated as an effective tool to differentiate between mTBI subjects and controls in non-athlete populations and is widely used in mTBI studies (6–8). Tests of memory, balance, and cognition are incorporated into the SCAT (9, 10), but research has not demonstrated their effectiveness as longitudinal assessments (11). Separately, 3-word recall is commonly employed in patients with mTBI to assess memory function (12). This test is usually normal and is probably inadequate for assessing these patients.
The risk factors for mTBI severity are debated in the literature. Demographic factors commonly explored include sex, age, previous concussions, learning disability, psychiatric history, and migraine/headache history (13). Although each of these preinjury characteristics has been studied in numerous protocols, a consensus has not been reached. Further research is needed to establish the risk factors for mTBI severity so that they may be incorporated into clinical care.
Moreover, routine imaging techniques are limited in their value of serving as biomarkers of severity or prognosis in the mTBI population, and the extent of incidental magnetic resonance imaging (MRI) findings in mTBI patients also remains unclear. Conventional structural MR imaging is felt to be limited in its yield of disease severity or prognosis. Further research is necessary to investigate the anatomical characteristics of the mTBI population that present to medical attention. Better characterization of the specific abnormalities in anatomic imaging in this population is necessary.
The aim of this study was to incorporate patient history, clinical exams, imaging, and multiple neurological assessments into a prospective longitudinal study of patients presenting with an acute mild traumatic brain injury to provide guidance for hypothesis generation and future study design of mTBI research. Traditional neuropsychological assessments were developed to further attempt to detect abnormalities in patients with mTBI. Although MR imaging is not routinely performed for acute mTBI, recent advances in MRI based techniques have allowed researchers to incorporate imaging into mTBI trials. This study specifically investigated the presence of white matter hyperintensities on structural imaging. This combination of assessments and time points provided a more comprehensive and detailed assessment of symptoms and outcomes of mTBI patients than found in previous studies. This allows for identification of previously elusive potential risk factors which may influence outcome measures for mTBI populations.
This study was conducted at three sites: Hospital for Special Surgery (HSS), University of California San Francisco (UCSF), and Houston Methodist Hospital (HM). All procedures were compliant with the Health Insurance Portability and Accountability Act (HIPAA) and were approved by each institution's Institutional Review Board (IRB). All participants or guardians granted written informed consent to participate. A uniform protocol was followed at each respective site to ensure standardization of data.
Inclusion criteria were age between 15 and 50 years and a clinical diagnosis of mTBI with Glasgow Coma Scale (GCS) ≥ 13. mTBI was defined as a low-velocity injury that results in clinical symptoms but is not necessarily related to a pathological injury, in accordance with both the Zurich Guidelines and those of the American Congress of Rehabilitation Medicine (1, 14). Mechanism of injury was not an inclusion or exclusion criterion, as our population was not restricted to sport-related concussion. Patients were excluded for prior moderate or severe brain trauma, mTBI within the year, neurological or neuropsychological abnormalities, or medication that may influence assessments (detailed exclusion criteria in Appendix e-1). Patients were enrolled at either Encounter 1 (E1), within 72 h, or Encounter 2 (E2), 5–10 days post-trauma. They returned for a maximum of 4 encounters over 3 months. Encounter 3 (E3) occurred at 15–29 days and Encounter 4 (E4) at 83–97 days. All time points were chosen based on previous literature. E1 and E2 allowed us to capture the acute impairments, and E2 was used to capture the majority of individuals who are reported to recover within 10 days (15). However, studies show that comorbidities may prolong recovery for several weeks, which is captured by E3 (16). Lastly, most studies suggest complete resolution of symptoms by 3 months, which is captured by E4 (17).
At enrollment, medical and family history were collected, including psychiatric disease, learning disability, previous mTBIs not within the last year, and migraines or other headache types. Principal investigators assessed migraine by physician diagnosis. At all visits, subjects underwent a clinical examination, neurological assessments, and a multimodal MRI. MRI scans were completed within 24 h after completion of clinical neurological evaluations. After study completion, date of patient recovery was determined for subjects enrolled at HSS.
Normal control subjects were recruited from a sample similar to the patients, e.g., friends or classmates of mTBI patients, and enrolled to assess the viability of differentiating clinical neurological and MR exams against mTBI patients. Control subjects shared the same exclusion criteria as the mTBI subjects, and were matched based on age, education level, sex, and handedness. Controls completed neurological assessments and multimodal MRI at an initial and follow-up visit between 7 and 90 days, with an average of 3 weeks between the initial and follow up encounters.
All subjects were administered a Composite General Symptoms Assessment (CGSA) at each encounter. The CGSA is a comprehensive tool that combines the Sports Concussion Assessment Tool 2 (SCAT2) with other established clinical tools, such as the Balance Error Scoring System (BESS) and the concussion graded symptoms checklist (GSC) (9, 18–20). The total symptom severity score (SSS) captured ranges from 0 to 132 with higher score indicating greater symptom severity. The CGSA also includes tests of specific cognitive domains found in the SCAT: immediate memory (5-Word Recall), concentration (Digits Backward), and balance (modified BESS) (10). A full list of assessments included in the CGSA can be found in Appendix e-2.
MR imaging was performed on 3T GE Signa MR750 (GE Healthcare, Waukesha, WI) scanners with a 32-channel brain radiofrequency coil (Nova Medical, Wilmington, MA). A comprehensive imaging protocol included high-resolution volumetric structural, susceptibility and diffusion weighted imaging as well as investigational pulse sequences (described in detail in Appendix e-3). The MR data was standardized by use of a uniform protocol, scanner manufacturer and model, radiofrequency (RF) coil, and sequences as well as centralized processing.
Expert board certified neuroradiologists (AJT, PM) performed the safety reads and interpreted the structural imaging. Patients were classified with white matter abnormalities if ≥5 objective, punctate white matter foci were present. If < 5 foci were present, the white matter was classified as abnormal if lesions were >3 mm or located in atypical locations. Patients with abnormal white matter changes underwent neurological follow-up with an attending neurologist (TS) and routine clinical MRI.
In regard to age, the study population was analyzed in two groups: those aged ≥25 and those < 25 years. This dichotomization was chosen based on the age when most brain structures typically reach developmental maturity (21–23).
Statistical analysis included reporting of means and standard deviations for continuous variables and frequencies and percentages for discrete variables. Independent sample t-tests and chi-square analyses were utilized for group comparisons. Similar tests were used to compare outcomes between patients with and without white matter abnormalities. Evaluations of all outcomes were performed using generalized linear models. The modeling technique is robust enough to handle data that do and do not meet the assumption of normality. Additionally, the modeling technique also allowed for all observations from all responders at each time point to be analyzed using maximum likelihood estimations (MLE). By clustering the data into longitudinal clusters for each patient, the model controlled for the missing data at each time point. Parameter estimates were reported using maximum likelihood estimates with statistical significance at p < 0.05. Bonferroni corrections were performed for multiple comparisons.
MATLAB (Mathworks, Natick, MA) and SPSS version 23.0 (IBM Corp., Armonk, NY) were used for all analyses.
111 mTBI patients were enrolled (86 at HSS, 14 at HM, 11 at UCSF) between February 2014 and June 2015, 48 at E1 and 63 at E2. The number of observations included in encounters 1 through 4 were 48, 99, 83, and 59, respectively. Of these subjects, 48 were women (44), mean age was 23.2 ± 9.0 years, and 35 had sustained at least one previous mTBI prior to the last year. A total of 34 controls (26 HSS, 6 UCSF, 2 HM) were also enrolled. Other than number of previous mTBIs, statistical testing did not reveal any significant demographic differences between mTBI and controls (Table 1). Cause of injury for mTBI patients was as follows: 22 subjects were involved in a motor vehicle accident (20%), 73 were injured playing a sport (66%), and 18 were injured by a fall or other accident (16%). In terms of mechanism of injury, 48 subjects experienced a direct impact of their head against an object (45%), 46 had a direct impact of a blow to the head (44%), 4 had a ground level fall (4%), 1 had a fall from height <1 meter (1%), and one experienced acceleration/deceleration (non-impact) (1%).
At enrollment, the most endorsed symptoms were headache (91%), not feeling right (89%), fatigue (86%), and feeling slowed down (84%). Control patients had an average SSS of 1.68 (SD: 7.14). At enrollment, mTBI subjects scored significantly worse than controls on 5- and 6-Digit Backward Recall (p = 0.003, p = 0.006, respectively), single-leg and tandem-leg stances of the BESS (p < 0.001 for both), and Immediate 5-Word Recall (p < 0.001).
Of the 104 mTBI patients with at least one processed MRI scan, 28 subjects (27%) had white matter changes, as seen on T2 FLAIR. Of these 28 subjects, 15 (54%) had white matter changes that were deemed abnormal for age as previously described. In 26 patients (93%), these white matter findings were determined unrelated to the acute head trauma, as there were no additional supportive findings (restricted diffusion or microhemorrhages) to suggest acuity, and the findings were stable across all encounters. One patient had acute shear injury of the corpus callosum on imaging. Another patient demonstrated a focal subarachnoid hemorrhage along the right trochlear nerve with clinical findings of right trochlear nerve palsy. In the 32 controls, 9 were found to have had changes in white matter (28%), of which 3 (33%) were deemed abnormal for age. The frequency of change in white matter was not significantly different between mTBI and control populations (p = 0.840). When comparing neurological exams between patients with and without white matter abnormalities, no significant differences were found. White matter abnormalities were not associated with previous mTBIs or history of participation in contact sports. Additionally, there were no associations between personal history of migraine and white matter hyperintensities.
The average length of days since injury for each encounter was (E1) 1.98 ± 0.99, (E2) 7.82 ± 1.93, (E3) 22.11 ± 4.87, and (E4) 91.61 ± 5.53. For mTBI subjects, the average SSS for each encounter were E1 = 32.5, E2 = 32.6, E3 = 16.8, and E4 = 7.6. A significant difference is seen from E2 to E3 (p < 0.001) and E3 to E4 (p = 0.024).
Of the neurological assessments, 5-Digit Backward Recall, the modified BESS, and Immediate 5-Word Recall saw statistically significant improvement over time in the mTBI population (Figure 1). Backward Recall of 5-digits saw significant improvement over time (p = 0.009), with pairwise differences from E2 to E3 (p = 0.009) and E2 to E4 (p = 0.002) (Figure 1A). Immediate 5-Word Recall for mTBI patients saw statistically significant change across time (p = 0.007) (Figure 1B). Single-Leg, Tandem-Leg, and Double-Leg Stances as well as total score of the modified BESS saw significant changes over all 4 encounters (p < 0.05 for all) (Figure 1C). While Tandem- and Double-Leg tests improved by less than one scoring item from E1 to E4, Single-Leg Stance had the largest change from E1 to E4 (4.0 errors to 1.6 errors, p < 0.001).
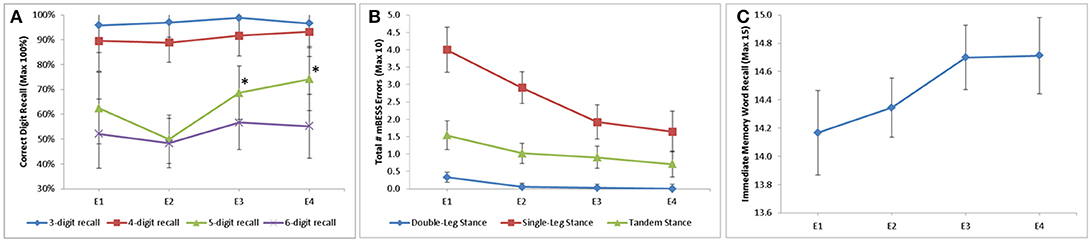
Figure 1. Longitudinal change in assessment scores stratified by (A) digit recall; (B) modified BESS; and (C) Immediate word recall. 5-Digit Backward Recall significant from E2 to E3 (50 to 69%, p = 0.009) and E2 to E4 (50 to 74%, p = 0.002). Double-Leg Stance, Single-Leg Stance, and Tandem-Leg stance of BESS all significant longitudinally (p = 0.003, p < 0.001, p = 0.028, respectively). Immediate 5 word recall statistically significant longitudinally (p = 0.007). *p ≥ 0.05.
Multiple assessments were also able to distinguish mTBI subjects from controls. 5- and 6-Digit Backward Recall were significantly different than controls at all time points (p < 0.01 for all). Single-Leg Stance differentiated between mTBI cases and controls at each time point (p < 0.001 for all encounters). Double-Leg and Tandem-Leg Stances as well as total BESS scores were also significantly different than controls across encounters, but this difference was less than one scoring item.
Backward recall of 3- and 4-digits saw no significant change over time in mTBI patients, with most correctly recalling the digits 90% of the time or better.
Multiple demographic factors were found to influence symptoms and recovery. Females reported higher symptoms scores at each time point with a significant difference at E2 (41.6 vs. 25.9, p < 0.001) (Figure 2A). Differences in clinical outcomes between sex can be found in Table 2. Patients aged ≥25 had higher symptom scores at E2 (p < 0.001), E3 (p < 0.001), and E4 (p = 0.003) (Figure 2B). Age also influenced the neuropsychological assessments. For subjects aged < 25, there was significant longitudinal improvement in 5-Digit Recall (p = 0.045), Immediate 5-Word Recall (p = 0.027), and BESS Single-Leg Stance (p = 0.023). Subjects aged ≥25 years did not significantly improve over time in any of these assessments.
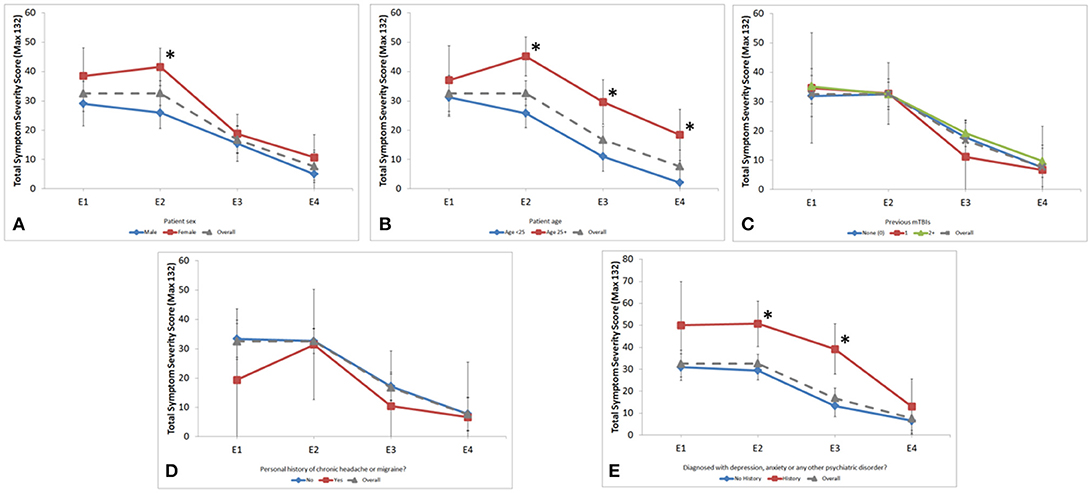
Figure 2. Longitudinal assessment of symptom severity score across encounters stratified by (A) sex; (B) age group; (C) number of previous mTBIs; (D) personal history of chronic headache or migraine and; (E) personal history of psychiatric disorder. Gender was significantly different at the E2 encounter (41.6 vs. 25.9, p < 0.001). Age was significantly different at E2 (46.0 vs. 28.0, p < 0.001), E3 (26.1 vs. 13.8, p < 0.001), and E4 (17.7 vs. 2.6, p = 0.003). Psychiatric history was significantly different at E2 (50.7 vs. 29.4, p < 0.001) and E3 (39.2 vs. 13.3, p < 0.001). *p ≥ 0.05.
Patients who reported personal history of depression, anxiety, or other psychiatric disorder had higher SSS at each time point, which was statistically significant at E2 and E3 (p = 0.003, p < 0.001 respectively; Figure 2E). This finding was not influenced by sex or age.
Number of previous mTBIs, learning disabilities, and personal history of chronic headache/migraine did not correlate with SSS or longitudinal change of SSS (Figures 2C,D).
This prospective, longitudinal study compared history, symptoms, and imaging results in a group of patients with mild TBI and non-injured controls. The acute enrollment post-trauma followed by multiple encounters over 3 months provides robust data that allows preliminary conclusions to be drawn regarding clinical symptoms, recovery, risk factors, and the usefulness of neurological assessments and MRI. To date, this is one of the first studies of this size to longitudinally assesses both clinical neurological exams and MRI data, in a design that captures more than 3 time points (24, 25). Given the young and otherwise healthy patient population, there were limited confounds in post-concussive deficits from mild cognitive impairment, other medical illness, or dementia that have historically impacted earlier mTBI studies. The demographic distribution allowed for sex- and age-stratified analyses that are not present in most other studies.
mTBI symptoms are not universal and vary tremendously between patients. However, the quantitative symptom severity score is considered a reliable approximation of the severity of a patient's injury and has previously been shown to predict prolonged recovery given that concussion currently remains a symptom-based diagnosis (26, 27). Although previous studies and meta-analyses have determined that neuropsychological deficits typically recover within 3 months, subjective report of difficulty remembering was the most frequently cited symptom at 3 months post-injury in our mTBI study cohort (17, 28). Additionally, both symptoms and neuropsychological deficits in our population were present longer than in previously cited literature (15, 16, 29). We suggest that future studies should more carefully consider longer term, subtle cognitive deficits in this population.
White matter abnormalities were seen in a larger percent of this mTBI cohort than previously documented in normal, healthy populations (27 vs. 5.3% in adults and 1.2% in adolescents) (30, 31). Direct comparison of findings however, are limited by differences in MR technologies. Due to greater signal to noise ratio, modern high-field volumetric T2 FLAIR imaging is significantly more sensitive in detecting white matter foci than reported in the historical literature (32), which may contribute to these slightly higher rates. The incidence rates of white matter abnormalities reported in the current study cannot be explained by hypertension, diabetes, demyelinating diseases as neither comorbidity was present in this study population, which was predominantly under the age of 30. Additional larger, prospective studies are needed to clarify the relationship between white matter hyperintensities and acute mTBI.
Although both 5- and 6-Digit Backward Recall differentiated between mTBI cases and controls, only 5-Digit Backward Recall was useful in evaluating mTBI patients across the first 3 months post-injury because of the noticeable longitudinal trend demonstrating improvement. mTBI patients appear to struggle with 6-Digit Backward Recall across all encounters compared to controls, suggesting that this test is helpful in diagnosing mTBI but is less helpful in tracking cognitive improvement over time. 3- and 4-digit recall may have less utility than 5- or 6-digit recall when assessing mTBI patients.
Clinicians continue to employ a simple 3-Word Recall test, dated from 1975, that does not appear to capture the memory deficit in the acute mTBI patient (12). How many words then are needed? The SCAT test has undergone multiple iterations, all of which include 5-Word Recall (10, 33). Although our research has demonstrated Immediate 5-Word Recall to be statistically significant over time, this test may not achieve the desired clinical sensitivity given the average change of one word. Clinicians may considering employing increasing numbers of words in order to better assess the memory deficit in acute mTBI.
Although all three portions of the Modified BESS assessment demonstrate significant improvement over time, the Single-Leg Stance appears to be the most sensitive in the acute window and the most advantageous position of this composite given its proportional decrease in scores across encounters.
Sex influenced symptom severity and course of recovery, with females experiencing worse symptoms at each time point, consistent with previously published literature (34–37). Studies cite that women exhibit higher rates of cognitive impairment than men after an mTBI (38). This was not captured in the assessments employed in this pilot study, suggesting a need for more sophisticated neuropsychological testing in future studies.
Age may also be a variable in mTBI severity and recovery. Data were analyzed for subjects ≥ and < 25 years, as 25 is the age when most brain structures have reached maturity (21–23). Our results show that individuals aged ≥25 endorse more symptoms after their first visit and generally take longer to recover. This is also seen in the significantly older age of the non-recovered cohort of patients. Still, the study cohort was youthful, as 80% of subjects were aged <30. These results are contrary to previous findings in the 13 to 33 age range that have shown younger age to be a possible risk factor for more severe neuropsychological deficits and longer recovery (39, 40). However, the majority of the recent mTBI literature in similar age groups focuses on sport populations. It is possible that the establishment and reinforcement of the neurocircuitry and pathways that occur between the ages of 15–25 may exert a protective effect, which remains poorly understood and needs to be addressed in future studies (41).
Studies conflict in terms of the relevance of personal psychiatric or headache history on prognosis in mTBI (13). It is interesting to note that a personal psychiatric history led to significantly worse symptoms acutely but did not independently predict recovery of patients in this study.
Recent advancements in TBI serum biomarkers have resulted in Federal Drug Administration (FDA) approval for the use of blood tests in the evaluation of mTBI (42). However, the evaluation of blood-based biomarkers was not within the scope of the current study.
Our data was meant to be hypothesis generating by reporting observations, limiting statistical power analyses. Although steps were taken to limit differentiation between institutions, there may have been slight residual instrumental variability between the enrollment sites despite standardization efforts. Our study was limited to neurocognitive assessments that are not normalized by age, although we controlled for age in our linear regression models. It is unclear if the non-significant findings of sex, age, and psychiatric history at E1 were due to timing of entry into the study at E1 or statistical power. Lastly, attrition limited longitudinal data analysis.
This study was meant to provide a priori hypotheses for future studies. The findings can help inform clinicians in their post-TBI neurologic exam and draw attention to specific risk factors for prolonged recovery. Our findings suggest that female sex is a risk factor for increased symptom burden. Similarly, age greater than 25, history of depression, anxiety, and other psychiatric disorders were associated with greater symptom severity over time. Based on our findings, we hypothesize that sex, age, and psychiatric comorbidity may influence the course of recovery from mild TBI and should be further investigated. We also hypothesize that the observed higher prevalence of white matter hyperintensities in our mTBI and control subjects is due to MR technological advancements. Further validation with larger populations of subjects is needed for all these findings. Differences in clinical neurological exams between mTBI patients and controls demonstrate valid use of our tools for the study of future mTBI neuroimaging. It is also important to note that in light of recent advancements of TBI serum biomarkers, it is critical that clinical data in tandem with both blood-based and imaging biomarkers of mTBI may lead clinicians toward a more definitive model of care.
This study was carried out in accordance with the recommendations of the Hospital for Special Surgery, Houston Methodist, and University of California San Francisco Institutional Review Boards. The protocol was approved by the Hospital for Special Surgery, Houston Methodist, and University of California San Francisco Institutional Review Boards. All subjects gave written informed consent in accordance with the Declaration of Helsinki. All minors signed an additional assent form before participating.
TS obtaining funding, study concept and design, study supervision, acquisition of data, interpretation of the data, drafting the manuscript. JN analysis and interpretation of data, drafting the manuscript. TC, AD, KH, EK, and KC interpretation of the data, drafting the manuscript. AT acquisition of data, drafting the manuscript. SN acquisition of data, drafting the manuscript for intellectual content. TZ analysis and interpretation of data, drafting of manuscript for intellectual content. JM and PM study concept and design, acquisition of data, study supervision, revising the manuscript for intellectual content. LM study concept and design, study supervision, processing of data, analysis and interpretation of data, drafting the manuscript.
TS has grants from GE and the NFL, Chembio Diagnostics, ElMindA, and Teva Pharmaceuticals. She is a member of the GE NFL Medical Advisory Board. JN has received grant support from the National Center for Advancing Translational Services (NCATS). TZ and LM are employed by General Electric. JM and PM have a grant from GE and the NFL and are members of the GE NFL Medical Advisory Board.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The funding for this study was provided by the General Electric – National Football League Head Health Initiative.
We would like to thank previous research coordinators and volunteers Dan Zuchelli, Lorena Loci, Megan Parmenter, and Zachary Gulergun, all of whom were integral in enrolling patients and documenting study visits. We would like to thank the entirety of the GE/NFL Medical Advisory Board for their continued direction as well as Dr. Victor Miranda, Ajit Shankaranarayanan, and Amy Gallenberg for their efforts. Within HSS, Tzipora Kuba's research management was necessary for the successful completion of this project. Dr. Hollis Potter and the HSS Radiology Department, specifically Joo Jung and Wendy Diesing, played an instrumental role in the guidance and execution of the imaging portion of this study. Lastly, we would like to thank the HSS Department of Neurology for providing the facility and support necessary for this investigation and specifically Virgen Gonzalez-Alakham for her efforts with enrollment. At Houston Methodist, Drs. Kenneth Podell and Howard Derman referred and studied patients, Jennifer Garrett coordinated the study, and Dr. Armen Kocharian provided MRI support. The Chao, Graham, Harrison and Natz Funds of the Houston Methodist Foundation contributed support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00836/full#supplementary-material
AUC, area under the curve; BESS, balance error scoring system; CGSA, composite general symptoms assessment; E1, encounter 1; E2, encounter 2; E3, encounter 3; E4, encounter 4; FLAIR, fluid attenuation inversion recovery; GCS, Glasgow coma scale; HIPPA, Health Insurance Portability and Accountability Act; HM, Houston Methodist; HSS, Hospital for Special Surgery; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; RF, Radiofrequency; SCAT2, sports concussion assessment tool 2nd edition; SSS, symptom severity score; TBI, traumatic brain injury; UCSF, University of California, San Francisco.
1. American Congress of Rehabilitation Medicine. Brain injury interdisciplinary special interest group, mild traumatic brain injury task force. Definition of mild traumatic brain injury. J Head Trauma Rehabil. (1993) 8:86–7.
2. Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2010).
4. Erlanger D, Kaushik T, Cantu R, Barth JT, Broshek DK, Freeman JR, et al. Symptom-based assessment of the severity of a concussion. J Neurosurg. (2003) 98:477–84. doi: 10.3171/jns.2003.98.3.0477
5. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. (2006) 21:375–8. doi: 10.1097/00001199-200609000-00001
6. Bin Zahid A, Hubbard ME, Dammavalam VM, Balser DY, Pierre G, Kim A, et al. Assessment of acute head injury in an emergency department population using sport concussion assessment tool−3rd edition. Appl Neuropsychol Adult (2016) 25, 110–9. doi: 10.1080/23279095.2016.1248765.
7. Mustafi SM, Harezlak J, Koch KM, Nencka AS, Meier T, West JD, et al. Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the NCAA-DoD CARE Consortium. J Neurotrauma (2017). doi: 10.1089/neu.2017.5158). [Epub ahead of print].
8. Silverberg ND, Luoto TM, Iverson GL. Assessment of mild traumatic brain injury with the King-Devick Test® in an emergency department sample. Brain Inj (2014) 28:1590–3. doi: 10.3109/02699052.2014.943287
9. McCrory P, Johnston K, Meeuwisse W, Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br J Sports Med. (2005) 39(Suppl. 1):i78–86. doi: 10.1136/bjsm.2005.018614
10. McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on Concussion in Sport–the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. (2009) 43:i76–90. doi: 10.4085/1062-6050-44.4.434
11. McCrea M, Iverson GL, Echemendia RJ, Makdissi M, Raftery M. Day of injury assessment of sport-related concussion. Br J Sports Med. (2013) 47:272–84. doi: 10.1136/bjsports-2013-092145
12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98.
13. Iverson GL, Gardner AJ, Ponsford JL, Sills AK, Broshek K, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. (2017) 51:941–8. doi: 10.1136/bjsports-2017-097729
14. McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorák J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. (2013) 47:250–8. doi: 10.1136/bjsports-2013-092313
15. McCrea M, Iverson GL, McAllister TW, Hammeke TA, Powell MR, Barr WB, et al. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol. (2009) 23:1368–90. doi: 10.1080/13854040903074652
16. Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics (2013) 132:8–17. doi: 10.1542/peds.2013-0432
17. Carroll L, Cassidy JD, Peloso P, Borg J, Von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. (2004) 36:84–105. doi: 10.1080/16501960410023859
18. Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the balance error scoring system. Sports Health (2011) 3:287–95. doi: 10.1177/1941738111403122
19. McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA (2003) 290:2556–63. doi: 10.1001/jama.290.19.2556
20. National Research Council. Committee on Sports-Related Concussions in Youth. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. National Academies Press (2014).
21. Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage (2008) 40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053
22. Pujol J, Vendrell P, Junqué C, Martí-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. (1993) 34:71–5.
23. Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. (1999) 2:859–61.
24. Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, Mayer AR. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. (2015) 72:530–8. doi: 10.1001/jamaneurol.2014.4778
25. Palacios EM, Yuh EL, Chang YS, Yue JK, Schnyer DM, Okonkwo DO, et al. Resting-state functional connectivity alterations associated with six-month outcomes in mild traumatic brain injury. J Neurotrauma (2017) 34:1546–57. doi: 10.1089/neu.2016.4752
26. Meehan WP, Mannix R, Monuteaux MC, Stein CJ, Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology (2014) 83:2204–10. doi: 10.1212/WNL.0000000000001073
27. Putukian M, Echemendia R, Dettwiler-Danspeckgruber A, Duliba T, Bruce J, Furtado JL, et al. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. (2015) 25:36–42. doi: 10.1097/JSM.0000000000000102
28. Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, Langhinrichsen-Rohling J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: Re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). Clin Neuropsychol. (2011) 25:608–23. doi: 10.1080/13854046.2011.565076
29. Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. (2006) 20:245–52. doi: 10.1080/02699050500487910
30. Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging (2006) 16:243–51. doi: 10.1111/j.1552-6569.2006.00047.x
31. Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Compr Psychiatry (2002) 43:361–8. doi: 10.1053/comp.2002.34636
32. Hofmann EM, Sedaghat-Kerdar M, Becker T, Warmuth-Metz M, Nadjmi M. Unspecific white matter lesions: prevalence, number and distribution in cranial MRI. Eur Radiol. (1992) 2:154–8.
33. Echemendia RJ, Meeuwisse W, McCrory P, Davis GA, Putukian M, Leddy J, et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5). Br J Sports Med. (2017) 51:848–50. doi: 10.1136/bjsports-2017-097506
34. Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg. (2009) 66:289–97. doi: 10.1097/TA.0b013e3181961da2
35. Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. (2000) 93:539–45. doi: 10.3171/jns.2000.93.4.0539
36. Kostyun RO, Hafeez I. Protracted recovery from a concussion: a focus on gender and treatment interventions in an adolescent population. Sports Health (2015) 7:52–7. doi: 10.1177/1941738114555075
37. Zuckerman SL, Apple RP, Odom MJ, Lee YM, Solomon GS, Sills AK. Effect of sex on symptoms and return to baseline in sport-related concussion. J Neurosurg Pediatr. (2014) 13:72–81. doi: 10.3171/2013.9.PEDS13257
38. Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. (2011) 30:1–17. doi: 10.1016/j.csm.2010.08.006
39. Dougan BK, Horswill MS, Geffen GM. Athletes' age, sex, and years of education moderate the acute neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. (2014) 20:64–80. doi: 10.1017/S1355617712001464
40. Pellman EJ, Lovell MR, Viano DC, Casson IR. Concussion in professional football: recovery of NFL and high school athletes assessed by computerized neuropsychological testing—part 12. Neurosurgery (2006) 58:263–74. doi: 10.1227/01.NEU.0000200272.56192.62
41. Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat (2013) 9:449–61. doi: 10.2147/NDT.S39776
Keywords: mild traumatic brain injury, age, gender, neuropsychological assessments, concussion, imaging, white matter hyperintensities, risk factors
Citation: Shetty T, Nguyen JT, Cogsil T, Tsiouris AJ, Niogi SN, Kim EU, Dalal A, Halvorsen K, Cummings K, Zhang T, Masdeu JC, Mukherjee P and Marinelli L (2018) Clinical Findings in a Multicenter MRI Study of Mild TBI. Front. Neurol. 9:836. doi: 10.3389/fneur.2018.00836
Received: 01 June 2018; Accepted: 18 September 2018;
Published: 23 October 2018.
Edited by:
Lisa Anne Brenner, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Deborah Shear, Walter Reed Army Institute of Research, United StatesCopyright © 2018 Shetty, Nguyen, Cogsil, Tsiouris, Niogi, Kim, Dalal, Halvorsen, Cummings, Zhang, Masdeu, Mukherjee and Marinelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teena Shetty, c2hldHR5dEBoc3MuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.