- 1Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
- 2IRCCS Istituto delle Scienze Neurologiche di Bologna (ISNB), Azienda Unità Sanitaria Locale (AUSL) di Bologna, Bologna, Italy
- 3Department of Psychology, University of Bologna, Bologna, Italy
Objectives: Regular physical activity is routinely recommended in children and adolescents suffering from narcolepsy type 1 (NT1), but controlled studies analyzing its influence on sleep/wake behavior, metabolic, and anthropometric profile in pediatric NT1 are lacking.
Methods: Fifty consecutive drug-naïve NT1 children and adolescents were assessed through actigraphic, clinical, and metabolic evaluations. Patients were compared with respect to their engagement in leisure-time physical activities (LTPA): patients engaged in LTPA (n = 30) and patients not engaged (No-LTPA, n = 20), respectively.
Results: LTPA patients presented lower BMI, with different BMI categories distribution and higher HDL cholesterol, when compared with No-LTPA subjects. Increased night-sleep duration, higher sleep quality, and reduction of nap frequency were documented through actigraphy in LTPA subjects. Subjective sleepiness, as measured by ESS-CHAD, was also lower in LTPA subjects while cataplexy frequency proved similar between the two groups.
Discussion: In pediatric NT1 patients, regular engagement in LTPA is associated with significant differences on sleepiness, anthropometric and metabolic profile and objectively assessed sleep/wake behavior. Engagement in LTPA is beneficial and should be strongly encouraged in pediatric NT1 patients.
Introduction
Narcolepsy type 1 (NT1) is a chronic neurological disease characterized by severe sleepiness, cataplexy and other REM sleep-like episodes intruding into wakefulness (namely hallucinations, sleep paralysis, automatic behaviors), and disrupted nocturnal sleep (1).
NT1 is caused by loss of hypothalamic hypocretinergic neurons, reflected by low (< 110 pg/mL) hypocretin-1 (or orexin A) levels in cerebrospinal fluid (CSF hctr-1) (2).
Disease onset often occurs in childhood and early adolescence and is most frequently accompanied by a rapid weight gain, resulting in overweight or obesity (3–6).
Although the mechanisms leading to weight gain in NT1 have yet to be elucidated (7), its management is of utmost importance as it represents an additional disease burden, negatively impacting on children's psychosocial functioning and increasing the cardiovascular and metabolic risk (5, 8).
The promotion of regular physical activity is among the most effective approaches to control weight in overweight children and adolescents (9). Although never investigated in narcolepsy patients, engagement in physical activity is associated with increased sleep duration and higher sleep quality in healthy subjects and in primary insomnia patients (10, 11).
In this study we analyzed the influence of regular physical activity on sleep/wake profile, sleepiness, metabolic and anthropometric features in a large cohort of drug-naïve NT1 children and adolescents.
Materials and Methods
Participants
Fifty drug-naïve NT1 patients ≤ 18 years of age (56% males, age = 12.58 ± 2.94 years, range 6-17) were included. All patients presented with excessive daytime sleepiness, video-documented cataplexy, mean sleep latency ≤ 8 min (3.65 ± 3.20 min) with ≥ 2 sleep onset REM periods (SOREMPs, mean = 4.56 ± 0.70) at multiple sleep latency test (MSLT), and low CSF hctr-1 levels (24.83 ± 31.90 pg/mL). All patients carried the HLA DQB1*06:02 allele. Data collection and database formation were approved by the local health trust's ethics committee (Comitato Etico Interaziendale Bologna-Imola, CE-BI, Prot. Num. 17009) and written informed consent was signed by parents of patients.
Sleep and Napping Behavior Assessment
Nocturnal sleep and diurnal naps were assessed through seven days of at-home actigraphic monitoring (Micro Motionlogger Watch, Ambulatory Monitoring Inc.). Devices were placed on the non-dominant wrist and initialized to collect and store motor activity in 1-min epochs.
Actigraphy was performed before diagnostic hospitalization, during the regular school-week. The description of the actigraphic variables considered for this work is reported in the Supplementary Material (12).
Participants also filled in a daily sleep diary and indicated nocturnal rest period and diurnal naps through the event-marker button.
Physical Activity and Cataplexy Assessment
Participants and their parents underwent a brief interview aimed to collect information on physical activity and cataplexy.
Regular engagement in leisure-time physical activity (LTPA), and whether patients performed their habitual physical activity schedule during the recording week, has been assessed by questions validated in other studies (namely “Do you usually undertake physical/sporting activities after school?” and “During the last seven days, how many times did you perform physical/sporting activities after school that increase your heart rate and make you get out of breath some of the time?” (13, 14). Information on type of activity, frequency (range 1–7 days), and duration were also collected. Cataplexy frequency and cataplexy occurrence, during or immediately after (within 15 min) performing physical/sporting activities, was also assessed through semi-structured clinical interview (12). Chronotype was assessed by means of the Italian version of the reduced Morningness-Eveningness questionnaire for Children and Adolescents (rMEQ-CA) (15); subjective sleepiness was assessed with the Epworth Sleepiness scale for children and adolescents (ESS-CHAD) (16).
Anthropometric and Metabolic Assessment
Height, weight, and body mass index (BMI) were measured for each participant by using a portable stadiometer (Harpenden Portable Stadiometer); BMI percentile was determined in comparison with the Italian growth percentile scales (17), with underweight defined as BMI < 5th percentile, normal weight as BMI >5th and < 85th percentile, overweight between 85th and 95th percentile, and obesity as BMI>95th (18). Blood samples were collected after fasting from midnight and analyzed for blood glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides.
Statistical Analyses
For statistical purposes, NT1 children and adolescents were classified either as “LTPAs” (n = 30) if they were engaged in any type of LTPA for at least 60 min twice a week or “No-LTPAs” (n = 20). Individual information on physical activity schedule is reported as Supplementary Material (Table S1).
Group differences in demographic, clinical, anthropometric/metabolic data and actigraphic parameters were analyzed by means of chi-squared and independent sample t-test, followed by effect size computation (Cohen's d). Analyses were conducted with IBM SPSS Statistics 19 software (SPSS, Inc. Chicago, Ill); p-value < 0.05 was considered statistically significant.
Results
Demographics, clinical, anthropometric, and metabolic data are reported in Table 1. Significant differences were observed in subjective sleepiness, with LTPAs presenting lower ESS-CHAD score than No-LTPAs, but not in objective sleep propensity or SOREMPs number at the MSLT. No differences were observed in cataplexy frequency. Significant differences were also observed in anthropometric/metabolic features, with LTPAs presenting lower BMI, a different BMI category distribution, and higher HDL cholesterol level than No-LTPAs.
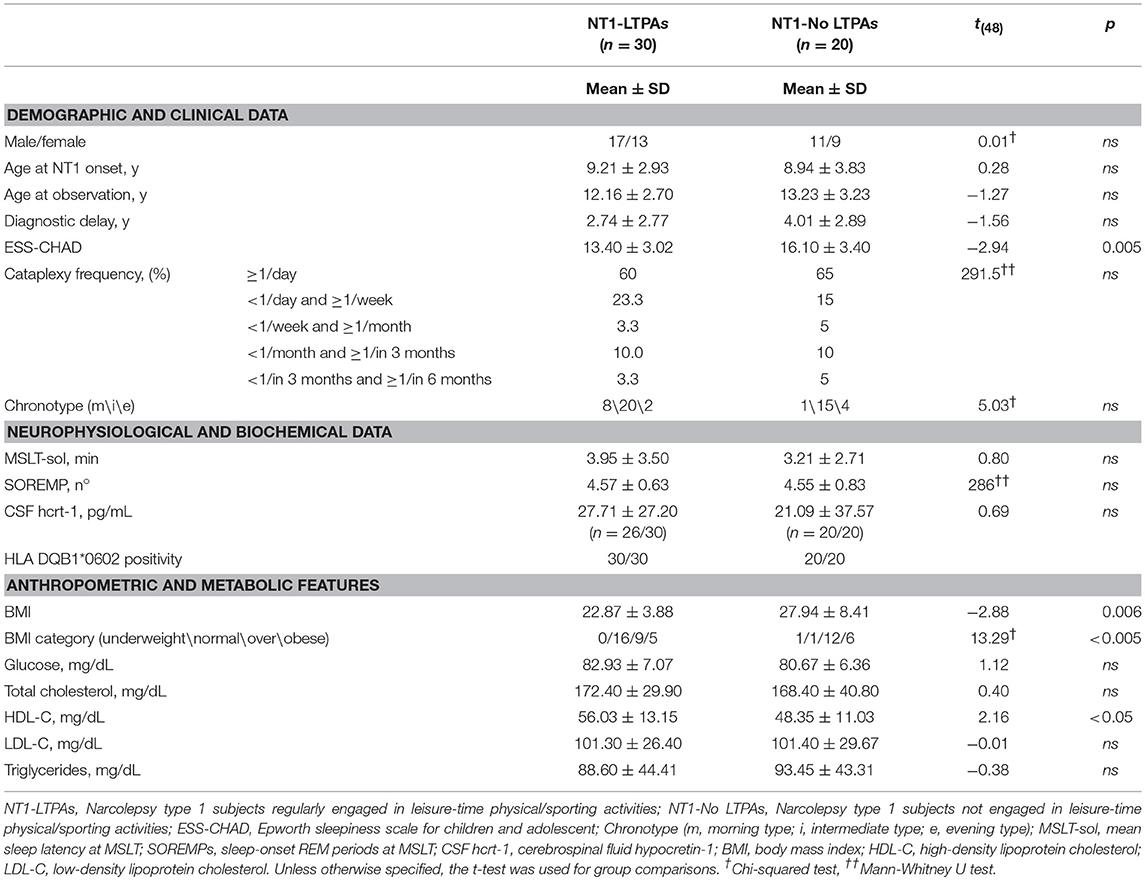
Table 1. Demographic, clinical, anthropometric and metabolic data of NT1 children and adolescents relative to their engagement in leisure-time physical/sporting activities.
Actigraphic variables are reported in Table 2. Significant differences were observed in nocturnal sleep duration and quality, with LTPAs displaying increased eTST and sleep efficiency, reduced eWASO and SMA, despite increased frequency of nocturnal awakenings when compared to No-LTPAs. Concerning the daytime period, a significant increase of DMA, reduced eDTST and naps frequency were observed in LTPAs.
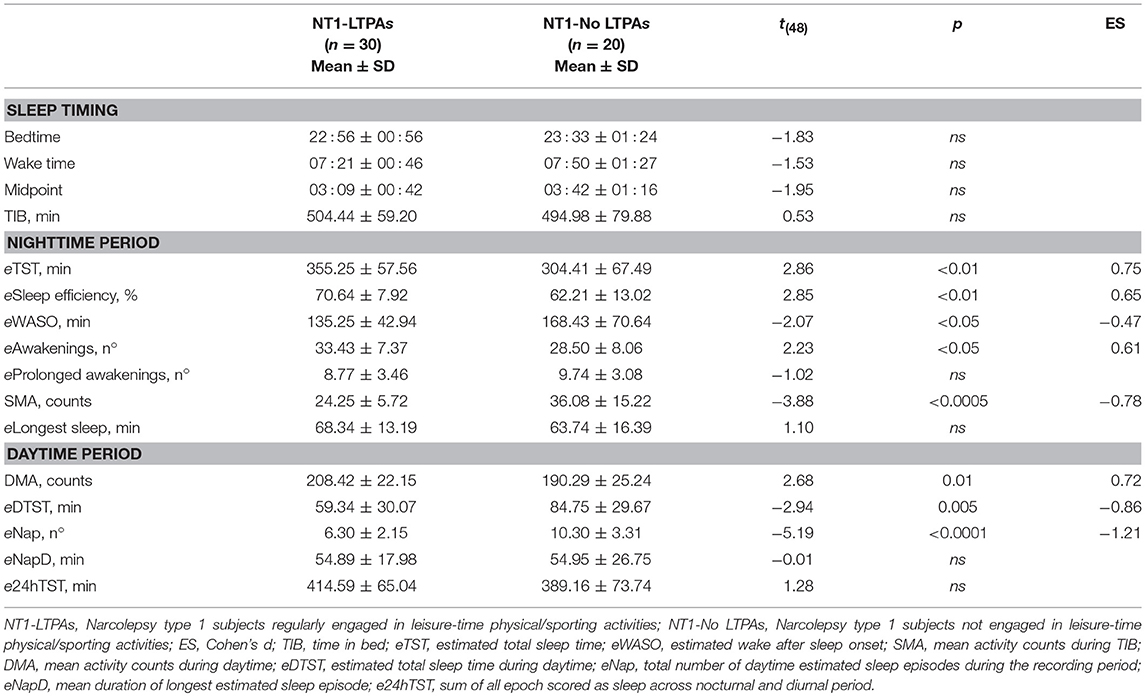
Table 2. Actigraphic nighttime and daytime parameters of NT1 children and adolescents with respect to engagement in leisure-time physical/sporting activities.
Discussion
This is the first study that investigated anthropometric/metabolic features and sleep/wake profile with respect to engagement in physical/sporting activity among pediatric NT1 patients.
Overall, our results highlight a beneficial association between regular LTPA and several disease-related aspects. Although we confirmed the over-representation of overweight and obesity among pediatric NT1 patients (42% of subjects being overweight and 22% being obese) (4, 5), our results showed that NT1 subjects regularly engaged in LTPA (60% of this cohort) presented with overall lower BMI and lower prevalence of overweight and obesity (30% overweight, 17% obese) compared to No-LTPAs (60% overweight, 30% obese).
Moreover, regular involvement in LTPA turned out to be associated with favorable differences in lipid-lipoprotein profile as LTPAs present with higher levels of HDL-C and lower levels of triglycerides when compared to No-LTPAs, although the latter difference did not reach statistical significance. Although these results are in line with previous studies on healthy children and adolescents reporting higher HDL-C and lower triglycerides levels in physically active subjects, further confirmation is required by means of interventional studies in NT1 patients (19).
Actigraphy objectively documented higher sleep quality (higher estimated sleep efficiency and lower eWASO and SMA) and increased sleep duration (higher eTST) in LTPAs compared to No-LTPAs, without significant differences in TIB and in the timing of nocturnal rest period, highlighting the positive association between regular physical activity and nocturnal sleep quality.
The abovementioned results are in line with results of previous studies in good sleepers and primary insomnia patients and, for the first time, these findings extend to patients suffering from NT1 (11, 20).
Moreover, regular engagement in LTPA was also associated with a different diurnal behavior, as LTPAs took fewer naps, spending less time asleep during daytime. Differences in subjective sleepiness levels properly confirmed the actigraphic diurnal profile, with LTPAs displaying lower ESS-CHAD score than No-LTPAs. Engagement in LTPA was not associated with differences in cataplexy frequency. However, it is of high interest to highlight that none of the children and adolescents reported to experience cataplectic attacks during or immediately after having performed physical activity. This finding could have important clinical implications, since concerns have been raised about the potential adverse effect of physical activity on cataplexy frequency (21). Indeed, in a murine model of NT1, physical exercise (wheel running) increased the total amount of wakefulness to the detriment of a negative effect on cataplexy and authors pointed out the need to optimize cataplexy control to derive benefit from physical activity (21, 22).
Our study suffers from the inherent limitations of a cross-sectional design, in particular it precludes the possibility of ruling out reverse causation (i.e., children are able to perform physical/sports activities because they are not overweight or obese) and calls for further interventional studies, in pharmacologically untreated and treated patients. Moreover, food intake was not monitored in the present study.
Nonetheless, our study showed that regular engagement in leisure-time physical/sports activity is associated with significant differences in children's anthropometric features, sleepiness and sleep/wake profile and does not seem to trigger cataplexy, thus providing a first rationale to implement regular physical activity as a complementary non-pharmacological approach to assist the management of pediatric NT1 cases.
Author Contributions
MF: conception of the study, acquisition of data, interpretation of data, statistical analyses, drafted the initial manuscript, and reviewed and revised the final version of manuscript. FP: conception of the study, interpretation of data, statistical analyses, and reviewed and revised the final version of manuscript. EA and VN: acquisition of data, interpretation of data, and reviewed and revised the final version of manuscript. PP: conception of the study, interpretation of data, and reviewed and revised the final version of manuscript. GP: conception of the study, interpretation of data, drafted the initial manuscript, and reviewed and revised the final version of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to all the children and families participating in this study, most notably the Italian Association of Narcolepsy (AIN onlus) patients. Without their contributions, this study would not have been possible. We also thank Cecilia Baroncini for editing the English text.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00707/full#supplementary-material
Abbreviations
NT1, Narcolepsy type 1; LTPA, leisure-time physical/sporting activities; CSF hctr-1, cerebrospinal fluid hctr-1; ESS-CHAD, Epworth sleepiness scale for children and adolescents; MSLT, multiple sleep latency test; SOREMPs, sleep onset REM periods; HLA, human leukocyte antigen; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TIB, time in bed; eTST, estimated total sleep time; eWASO, estimated wake after sleep onset; SMA, sleep motor activity; DMA, daytime motor activity; eDTST, daytime estimated total sleep time; e24hTST, sum of all epochs scored as sleep in both nighttime and daytime periods.
References
2. Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. (2008) 7:649–62. doi: 10.1016/S1474-4422(08)70140-6
3. Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. (2014) 15:502–7. doi: 10.1016/j.sleep.2014.01.015
4. Ponziani V, Gennari M, Pizza F, Balsamo A, Bernardi F, Plazzi G. Growing up with type 1 narcolepsy: its anthropometric and endocrine features. J Clin Sleep Med. (2016) 12:1649–57. doi: 10.5664/jcsm.6352
5. Inocente CO, Lavault S, Lecendreux M, Dauvilliers Y, Reimao R, Gustin MP, et al. Impact of obesity in children with narcolepsy. CNS Neurosci Ther. (2013) 19:521–8. doi: 10.1111/cns.12105
6. Poli F, Pizza F, Mignot E, Ferri R, Pagotto U, Taheri S, et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep (2013) 36:175–81. doi: 10.5665/sleep.2366
7. Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med. (2004) 5:147–50. doi: 10.1016/j.sleep.2003.10.006
8. Postiglione E, Antelmi E, Pizza F, Lecendreux M, Dauvilliers Y, Plazzi G. The clinical spectrum of childhood narcolepsy. Sleep Med Rev. (2018) 38:70–85. doi: 10.1016/j.smrv.2017.04.003
9. Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents pathophysiology, consequences, prevention, and treatment. Circulation (2005) 111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10
10. Chennaoui M, Arnal PJ, Sauvet F, Léger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev. (2015) 20:59–72. doi: 10.1016/j.smrv.2014.06.008
11. Passos GS, Poyares D, Santana MG, D'Aurea CV, Youngstedt SD, Tufik S, et al. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med. (2011) 12:1018–27. doi: 10.1016/j.sleep.2011.02.007
12. Filardi M, Pizza F, Antelmi E, Ferri R, Natale V, Plazzi G. In-field assessment of sodium oxybate effect in pediatric type 1 narcolepsy: an actigraphic study. Sleep (2018). doi: 10.1093/sleep/zsy050. [Epub ahead of print].
13. Ortega FB, Chillón P, Ruiz JR, Delgado M, Albers U, Alvarez-Granda JL, et al. Sleep patterns in Spanish adolescents: associations with TV watching and leisure-time physical activity. Eur J Appl Physiol. (2010) 110:563–73. doi: 10.1007/s00421-010-1536-1
14. Syväoja HJ, Kantomaa MT, Ahonen T, Hakonen H, Kankaanpää A, Tammelin TH. Physical activity, sedentary behavior, and academic performance in Finnish children. Med Sci Sports Exerc. (2013) 45:2098–104. doi: 10.1249/MSS.0b013e318296d7b8
15. Tonetti L, Adan A, Di Milia L, Randler C, Natale V. Measures of circadian preference in childhood and adolescence: a review. Eur Psychiatry. (2015) 30:576–82. doi: 10.1016/j.eurpsy.2015.01.006
16. Wang YG, Benmedjahed K, Lambert J, Evans CJ, Hwang S, Black J, et al. Assessing narcolepsy with cataplexy in children and adolescents: development of a cataplexy diary and the ESS-CHAD. Nat Sci Sleep. (2017) 9:201–11. doi: 10.2147/NSS.S140143
17. Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest. (2006) 29:581–93. doi: 10.1007/BF03344156
18. Wang Y. Cross-national comparison of childhood obesity: the epidemic and the relationship between obesity and socioeconomic status. Int J Epidemiol. (2001) 30:1129–36. doi: 10.1093/ije/30.5.1129
19. Tolfrey K, Jones AM, Campbell IG. The effect of aerobic exercise training on the lipid-lipoprotein profile of children and adolescents. Sports Med. (2000) 29:99–112. doi: 10.2165/00007256-200029020-00003
20. Kubitz KA, Landers DM, Petruzzello SJ, Han M. The effects of acute and chronic exercise on sleep. A meta-analytic review. Sports Med. (1996) 21:277–91.
21. Kotagal S. Treatment of narcolepsy and other organic hypersomnias in children. Paediatr Respir Rev. (2018) 25:19–24. doi: 10.1016/j.prrv.2017.06.012
Keywords: narcolepsy type 1, children, physical activity, actigraphy, weight control
Citation: Filardi M, Pizza F, Antelmi E, Pillastrini P, Natale V and Plazzi G (2018) Physical Activity and Sleep/Wake Behavior, Anthropometric, and Metabolic Profile in Pediatric Narcolepsy Type 1. Front. Neurol. 9:707. doi: 10.3389/fneur.2018.00707
Received: 07 June 2018; Accepted: 03 August 2018;
Published: 24 August 2018.
Edited by:
Thomas Pollmächer, Klinikum Ingolstadt, GermanyReviewed by:
Fang Han, Peking University People's Hospital, ChinaPeter Geisler, Universitätsklinikum Regensburg, Germany
Copyright © 2018 Filardi, Pizza, Antelmi, Pillastrini, Natale and Plazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Plazzi, Z2l1c2VwcGUucGxhenppQHVuaWJvLml0
 Marco Filardi
Marco Filardi Fabio Pizza
Fabio Pizza Elena Antelmi1,2
Elena Antelmi1,2 Paolo Pillastrini
Paolo Pillastrini Vincenzo Natale
Vincenzo Natale Giuseppe Plazzi
Giuseppe Plazzi