
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 04 April 2018
Sec. Neuroepidemiology
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.00149
 Aung Zaw Zaw Phyo1
Aung Zaw Zaw Phyo1 Thibaut Demaneuf1
Thibaut Demaneuf1 Alysha M. De Livera1,2
Alysha M. De Livera1,2 George A. Jelinek1
George A. Jelinek1 Chelsea R. Brown1
Chelsea R. Brown1 Claudia H. Marck1
Claudia H. Marck1 Sandra L. Neate1
Sandra L. Neate1 Keryn L. Taylor1
Keryn L. Taylor1 Taylor Mills1
Taylor Mills1 Emily O’Kearney1
Emily O’Kearney1 Amalia Karahalios2
Amalia Karahalios2 Tracey J. Weiland1*
Tracey J. Weiland1*
Background: Multiple sclerosis (MS) is a complex, demyelinating disease of the central nervous system. Fatigue is commonly reported by people with MS (PwMS). MS-related fatigue severely affects daily activities, employment, socioeconomic status, and quality of life.
Objective: We conducted this systematic review and meta-analysis to determine whether psychological interventions are effective in managing fatigue in PwMS.
Data sources: We performed systematic searches of Medline, EMBASE, PsycINFO, and CINAHL to identify relevant articles published from database inception to April 5, 2017. Reference lists from relevant reviews were also searched.
Study selection and design: Two independent reviewers screened the papers, extracted data, and appraised the included studies. A clinical psychologist verified whether interventions were psychological approaches. A narrative synthesis was conducted for all included studies. For relevant randomized controlled trials that reported sufficient information to determine standardized mean differences (SMDs) and 95% confidence intervals (CIs), meta-analyses were conducted using a random-effects model.
Results: Of the 353 identified articles, 20 studies with 1,249 PwMS were included in this systematic review. Narrative synthesis revealed that psychological interventions reduced fatigue in PwMS. Meta-analyses revealed that cognitive behavioral therapy decreased levels of fatigue compared with non-active controls (SMD = −0.32; 95% CI: −0.63 to −0.01) and compared with active controls (relaxation or psychotherapy) (SMD = −0.71; 95% CI: −1.05 to −0.37). Meta-analyses further showed that both relaxation (SMD = −0.90; 95% CI: −1.30 to −0.51), and mindfulness interventions (SMD = −0.62; 95% CI: −1.12 to −0.12), compared with non-active control, decreased fatigue levels. The estimates of heterogeneity for the four meta-analyses varied between none and moderate.
Conclusion: This study found that the use of psychological interventions for MS-related fatigue management reduced fatigue in PwMS. While psychological interventions are generally considered first-line therapy for MS-related fatigue, further studies are needed to explore the long-term effect of this therapy.
Multiple sclerosis (MS) is a complex, demyelinating disease of the central nervous system (1, 2). Demyelinated nerve fibers can produce altered sensations and impairments in bodily functions. As a result, people with MS (PwMS) can experience a range of symptoms including numbness, double vision, cognitive difficulties, bladder problems, paralysis, blindness, and fatigue (3).
Fatigue is commonly reported by PwMS and is often one of the first symptoms of MS. Fatigue can be defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities” (4). It may affect up to 80% of PwMS (5–7) and can be severe in up to 65–70% (8, 9). The prevalence of MS-associated fatigue is greater than other MS symptoms, such as difficulty within balance, weakness, and numbness (7). While fatigue is common in a range of chronic diseases, the nature of MS-related fatigue is thought to be more profound than that experienced by either healthy people or individuals with other types of illnesses (6, 10).
Multiple sclerosis-related fatigue is described as primary or secondary fatigue. Primary fatigue results from damage to the central nervous system: neuronal dysfunction, demyelination, and inflammation. Secondary fatigue arises due to other factors such as mood disorders, lack of sleep, and medication to manage MS and related symptoms (6, 11). Since fatigue is a subjective symptom, its evaluation is difficult (12, 13). Fatigue can be measured subjectively or objectively. Subjective fatigue measurement involves the use of validated scales to investigate perceived levels of fatigue. Objective measurement of fatigue uses scales to quantify the severity and impact of fatigue on daily physical, cognitive, and psychosocial activities through various parameters (6, 14–16).
Multiple sclerosis-associated fatigue adversely affects functioning, activities of daily living, employment, socialization, and quality of life (6, 17). For PwMS, fatigue may contribute to early retirement, reduced working hours, and unemployment (17–21). The level of unemployment in PwMS has been shown to be as high as 80% (22). Consequently, fatigue and associated-unemployment has significant socioeconomic implications for PwMS and their families (6, 17, 19).
There are two forms of MS-related fatigue management: non-pharmacological approaches and pharmacological management. It has been argued that non-pharmacological interventions should be considered first-line treatment (6). These can include physical, psychological and cognitive, and mixed interventions (23, 24). Physical approaches include aerobic exercises, resistance training, electromagnetic-field therapy, and cooling therapy. Psychological approaches include cognitive behavioral therapy (CBT), relaxation therapy, psychotherapy, energy conservation education, progressive muscle relaxation, mindfulness, and educational counseling (23).
Several published articles indicate that non-pharmacological interventions are effective in improving fatigue of PwMS (23, 25–30). There are comprehensive systematic reviews (25, 31, 32) in the MS literature demonstrating the effectiveness of pharmacological interventions and physical training on fatigue. Several studies have demonstrated that psychological treatments such as CBT, mindfulness, relaxation, and educational counseling, decreased the fatigue level of PwMS (33–35). However, to date, there is only one systematic review (36) exploring effects of psychological interventions. It focused exclusively on the efficacy of CBT for MS-related fatigue and it did not provide an assessment of the effects of all psychological interventions on fatigue in PwMS. Therefore, there is a need for a broader approach to investigate the efficacy of other types of psychological interventions such as mindfulness and relaxation on MS-associated fatigue management. A broad and more comprehensive systematic review concerning psychological interventions may assist in the development of clinical and research recommendations for psychological approaches for fatigue management. Our aim of this study was to determine the efficacy of psychological interventions in improving fatigue in PwMS.
To determine the efficaciousness of psychological intervention in managing fatigue in PwMS, we defined terms of interest as follow: (a) the population of interest was PwMS who were aged 18 years or older; (b) interventions were psychological interventions; (c) comparators were non-active/active controls; (d) the outcome was fatigue; and (e) study designs included all types of studies except reviews, case reports, case series, and qualitative studies.
This systematic review and meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses statement (37). The review is registered with PROSPERO, registration number-CRD42017060497. The following electronic bibliographic databases were searched for articles published from database inception to April 5, 2017: Medline (Ovid), EMBASE, PsycINFO, and CINAHL. We consulted with a professional librarian for assistance with search strings. Search terms included those related to MS, fatigue, and psychological interventions (Table A1 in Appendix). As preliminary searches indicated few papers published in this area, we made no restrictions on language, year of publication, or publication type in our search. In addition, we also used Web of Science to search publications which cited our included studies and we undertook a hand-search of the reference lists of a relevant systematic review (36).
Articles were included if they: (a) included participants with MS who were aged 18 years or older; (b) included participants who had self-reported neurologist-diagnosed MS, or doctor-diagnosed MS, or recruitment of PwMS from MS society, clinic, and hospital; (c) assessed interventions involving psychological therapy, CBT (including self-management), stress reduction techniques, meditation, mindfulness, relaxation, guided imagery, progressive muscle relaxation, or educational counseling; (d) had a comparison group (baseline (within group) or standard-care or non-active/active-control group) or single psychological intervention group; (e) included an outcome measure for fatigue assessed using a validated tool; (f) were written in English; and (g) were full text article. In addition, we deviated from our original protocol and made a posteriori decision to include pilot studies in this review given that small studies can contribute meaningful information to meta-analyses.
We excluded papers not written in English, and studies with multi-component interventions that did not isolate the psychological therapy in design or analysis, literature reviews (including systematic reviews and meta-analyses), case reports, case series, or reported qualitative findings only. We contacted primary authors and co-authors when the methods described did not enable us to determine whether the inclusion criteria were met.
All abstracts identified through the search were independently screened for eligibility by title and abstract by two authors (Aung Zaw Zaw Phyo and Thibaut Demaneuf). All relevant full text articles were evaluated for eligibility against inclusion criteria. Two authors independently completed the eligibility assessments. This was followed by a consensus round where disagreements between the two authors (Aung Zaw Zaw Phyo and Thibaut Demaneuf) were resolved by further consensus with authors Tracey J. Weiland and Alysha M. De Livera.
The following information was extracted independently by two authors (Aung Zaw Zaw Phyo and Thibaut Demaneuf): primary author (year of publication); country where the study took place; study design; participant characteristics (i.e., age and sex of the participants); interventions assessed; scales used to assess the outcomes; findings; and study limitations. We recorded the outcome data (i.e., mean and SD) at baseline (pre-test); end of intervention; and follow-up assessments as reported in the studies.
Two authors (Aung Zaw Zaw Phyo and Thibaut Demaneuf) evaluated the quality of included studies using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies (Hamilton Tool) (38). The EPHPP evaluates six domains: (a) selection bias; (b) study design; (c) confounders; (d) blinding; (e) data collection method; and (f) withdrawals/dropouts. The EPHPP guidelines recommend that each domain is rated as strong; moderate; or weak (38). To ascertain a global rating for each study, we gave a rating of strong when there were no weak ratings across the six domains; a rating of moderate if there was one weak rating across the six domains; and a rating of weak when an article had two or more weak ratings across the six domains (38). Disagreements between reviewers were resolved through discussion and consensus with authors Tracey J. Weiland and Alysha M. De Livera. This step was not used to exclude papers from this review.
In this review, all 20 included studies were presented in a narrative synthesis. Table A2 in Appendix outlines reasons why studies were not included in this systematic review. Twelve studies (13 articles) (35, 39–50) with sufficient data were included in our meta-analyses. Table A3 in Appendix outlines reasons why the remaining eight studies (nine articles) (33, 34, 51–57) were not included in the meta-analyses.
We used post-treatment means and SDs to calculate standardized mean differences (SMDs) and 95% confidence intervals (CIs). A random-effects model was fitted using the DerSimonian and Laird estimator for the between-study variance (58). In this analysis, a negative SMD implies that fatigue is reduced in the intervention groups compared with the active/non-active control groups. According to Cohen’s definition (59), we interpreted SMDs to have a small effect if the magnitude was 0.2–0.5; moderate effect if the magnitude was 0.51–0.8, and large effect if the magnitude was >0.8. We used the I2 statistic to assess statistical heterogeneity and we interpreted these results according to the Cochrane guidelines (60) (0–40% = no heterogeneity; 30–60% = moderate heterogeneity; 50–90% = substantial heterogeneity; and 75–100% = considerable heterogeneity). Publication bias was assessed using funnel plots and Egger’s test (61).
We conducted four meta-analyses: a comparison of CBT and non-active controls; a comparison of CBT and active controls [e.g., relaxation therapy or supportive-expressive group psychotherapy (SEGP)]; a comparison of relaxation and non-active controls; and a comparison of mindfulness interventions and non-active controls. Non-active controls included waitlist, current local practice that included general advice and information provision about MS-fatigue from a variety of health professionals, standard care or treatment as usual in which the participants did not receive any psychological interventions. Data analysis was performed using Stata statistical software, version 13.0 (StataCorpLP, College Station, TX, USA).
We identified 343 articles from the database search and an additional 10 articles were identified from other sources (Figure 1). Of these, 228 remained for review after removal of duplicates. In total, 193 articles were deemed irrelevant based on the title and abstract. We assessed the full-text of the remaining 35 articles for eligibility. 22 articles met all inclusion criteria and were selected for this systematic review (Figure 1). Two published articles by Thomas et al. (47, 48) which reported the results of the same randomized controlled trial (RCT) were combined as one study. Similarly, two published articles by Jongen et al. (56, 57) on the same observational study were combined as one study. In total, there were 20 studies of which 1,249 PwMS were included. Excluded studies with the main reasons for exclusion are shown in Table A2 in Appendix. The quality of studies ranged from weak to strong; 10 studies (40–46, 49, 52, 53), 4 studies (35, 47, 48, 50, 55), and 6 studies (33, 34, 39, 51, 54, 56, 57) had a global rating of strong, moderate, and weak, respectively (Table 1).
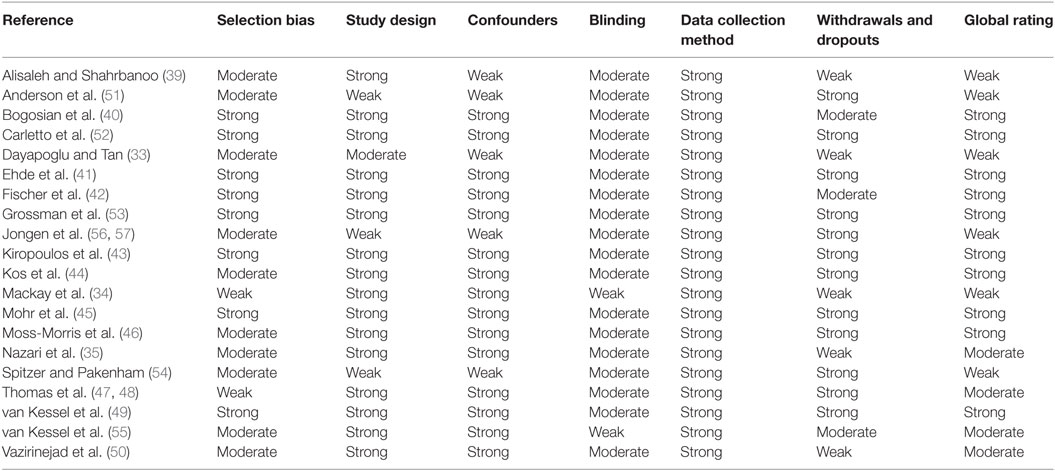
Table 1. Quality of evidence rating for included studies based on the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies.
The included studies were published between 2003 and 2017 and reported 11 RCTs (34, 35, 41–45, 47–50, 52, 53), 4 pilot RCTs (40, 43, 46, 55), 1 experimental and control groups pre-test/post-test pilot study (39), 1 observational study (56, 57), 1 single group pre-test and post-test pilot study (54), and 2 single-group pre-test and post-test studies (33, 51). The countries where the included studies took place were Turkey (33), the United States of America (41, 45), Germany (42), Switzerland (53), Belgium (44), Australia (34, 43, 54), Italy (52), Iran (35, 39, 50), United Kingdom (40, 46–48, 51), Netherlands (56, 57), and New Zealand (49, 55).
Of the 16 included intervention and control studies, 10 studies (35, 39–43, 46–48, 50, 53) compared psychological interventions against non-active controls and 6 studies (34, 44, 45, 49, 52, 55) compared psychological interventions against active controls. In four studies, two psychological interventions of interest were compared: CBT and supportive-expressive group (psychotherapy) (45); self-management occupational therapy (CBT) and relaxation therapy (44); and CBT and relaxation (49); and eye movement desensitization and reprocessing (EMDR) and relaxation therapy (52); respectively. Table 2 presents a summary of the 20 included studies, and Table 3 displays the characteristics of participants of each included study. Table 4 shows the proportion of the study sample that completed the follow-up assessment.
Alisaleh and Shahrbanoo (39) conducted a pre-test/post-test pilot study to examine the effectiveness of mindfulness-based stress reduction (MBSR) on stress and fatigue in PwMS. A total of 30 PwMS were randomly allocated into either the MBSR group or control group (no intervention—waiting list). The MBSR intervention consisted of eight sessions (2 h per session). The fatigue severity measurement was performed at baseline and at post-intervention by using the Fatigue Severity Scale (FSS). The mean (SD) of FSS for treatment group and control group after intervention were 3.52 (0.80) and 4.23 (1.01), respectively. The authors found that there were significant differences between the two groups in terms of fatigue severity after intervention [P-value < 0.001—as reported by Alisaleh and Shahrbanoo (39)] The validity of this study was weakened by the small sample size which may have limited generalizability and reliability. Furthermore, no information about the randomization process, participation rate, and blinding was provided. Hence, it is difficult to determine the quality of the study.
Anderson et al. (51) performed a single-group pre-test/post-test study to describe the development and feasibility of the help to overcome problems effectively self-management intervention for PwMS. In total, 21 PwMS received a self-management intervention which included positive psychology theory practices and CBT for 6 weeks. The severity of fatigue was measured by the FSS at baseline and after the intervention (6 weeks). The authors found a decrease in FSS mean for the intervention group [baseline: 49.4 (13.3), and post-treatment: 41.4 (14.4)]. However, the quality of these findings may have been negatively affected by some limitations such as lack of randomization, self-selected sample, and small sample size. It is possible that the self-selected sample have a natural setup to answer favorably to a positive psychological intervention for MS treatment.
Bogosian et al. (40) conducted a pilot RCT to assess the potential effectiveness of mindfulness training for progressive MS. A total of 40 PwMS were randomly assigned into two groups (mindfulness intervention n = 19 and waiting list control group n = 21). The mindfulness intervention of this study was delivered in 8 h-long sessions over an 8-week period through Skype video conferences. Fatigue was assessed using the FSS and measurements were collected at baseline, post-intervention, and 3-month follow-up. The mean (SD) FSS at three time points for the intervention and control groups were 39.91 (14.45), 43.98 (14.20), 43.87 (13.39), and 48.29 (12.24), 49.98 (10.18), and 49.08 (12.43), respectively. In this study, according to the nature of the intervention, it was not feasible to keep staff (clinical supervisors) or participants blind to treatment assignments. Hence, over-performance may have existed among participants from mindfulness intervention and a Hawthorne effect could have occurred. Moreover, the outcome measures were dependent on the participants’ self-reported data. Therefore, such weaknesses may have negatively affected the validity of the study.
Carletto et al. (52) reported a RCT to compare the efficacy of EMDR and relaxation therapy in PwMS. A total of 42 PwMS were included in this study (EMDR n = 20; and relaxation therapy n = 22). EMDR intervention was in fact the psychological intervention. Relaxation therapy consisted of a series of relaxation techniques such as diaphragmatic breathing, progressive muscle relaxation, visualization, cue-controlled relaxation, and rapid relaxation. Participants from both groups received 10 individual 60-min-long sessions for 12–15 weeks. Fatigue levels, assessed with the FSS, were measured pre-test and post-test. The mean FSS was 43.10 (15.10) at pre-test and 37.60 (19.67) at post-test for EMDR group and 43.95 (13.79) at pre-test and 39.18 (15.94) at post-test for relaxation therapy group, respectively. The authors concluded that both relaxation therapy and EMDR were significantly effective for fatigue among PwMS (P-value = 0.03). Although the considerable numbers of PwMS that were screened, the study was underpowered due to the small sample size. Therefore, this limitation may have affected the quality of this study.
Dayapoglu and Tan (33) conducted a single-group pre-test/post-test study in a neurology clinic to investigate the effect of Progressive Muscle Relaxation Technique (PMRT) on fatigue and sleep quality in PwMS. In total, 32 PwMS who met inclusion criteria were provided an intervention involving approximately 1 h of one-to-one patient education about PMRT, and listening to a compact disk on relaxation exercises. After the education, each patient was asked to perform the exercises at home once a day for 6 weeks. Data collection was conducted at two points: pre-intervention and post-intervention (6 weeks after the completion of education). The FSS was used for fatigue measurement. A statistically significant difference was found between the average FSS score pre- and post-intervention (P-value < 0.001). The validity of these results, however, may have been affected by several weaknesses including lack of randomization, participants and assessors knowing their types of intervention (no blinding), and no follow-up to ensure patients were correctly using the PMRT at home.
Ehde et al. (41) conducted a RCT to evaluate the efficacy of a telephone-delivered self-management intervention-(T-SM) for fatigue in PwMS and compared T-SM against non-active intervention (telephone-delivered parallel education—T-ED). A total of 163 patients were randomly allocated to either 8-week T-SM or T-ED. The T-SM consisted of evidence-based CBT and positive psychology strategies. Telephone outcome assessments were conducted at baseline, post-test, and at 6- and 12-month post-randomization. Fatigue was measured by using the Modified Fatigue Impact Scale (MFIS). In both groups, there were significant improvements from baseline to post-treatment in the fatigue outcome measure. (Within groups 95% CIs of T-SM and T-ED were 7.01–13.8 and 5.27–12.0, respectively.) Research assistants knowing a participant’s allocation and participants being aware of their interventions may have negatively affected the validity of the study.
Fischer et al. (42) presented a RCT to investigate the efficacy of an Internet-based CBT program, “Deprexis,” among PwMS. This study compared the “Deprexis” program against the waitlist (non-active control). In total, 90 PwMS were randomly allocated into two groups (45 participants in each group) for 9 weeks. An online questionnaire including a measure of fatigue was completed at baseline, 9 weeks after enrollment, and 6 months after the intervention. The Fatigue Scale for Motor and Cognitive Function (FSMC) was used to assess fatigue. The authors reported that the intervention decreased the FSMC-total scores from 74.18 (baseline) to 70.15 (post-treatment). The authors concluded that improvement with intervention versus control was only found in the motor fatigue subscale. Weaknesses of this study include participants not being blinded to their allocated interventions, which may have led to a Hawthorne effect. Moreover, the lack of a data monitoring committee to assess adverse events, one of the investigators knowing the treatment allocation, and small sample size posed high risks of bias and may have affected the validity of the findings.
Grossman et al. (53) conducted a RCT to examine the effects of a mindfulness-based intervention (MBI) compared with non-active control (usual care—UC) among PwMS. The MBI was a structured 8-week program of mindfulness training. A total of 150 PwMS were randomly allocated into two groups (76 patients in MBI and 74 patients in UC). All patient-reported outcome measures were administered at pre-intervention, post-intervention, and 6-month follow-up. For fatigue measurement, the MFIS was used. The direct post-intervention change and 6-month follow-up change of MBI group were 6.65 (95% CI: 4.14–9.16) and 6.58 (95% CI: 3.63–9.53), respectively. The authors concluded that these positive changes indicated that MBI was beneficial in managing MS-related fatigue and MBI compared with UC improved fatigue up to 6-month post-treatment. The significant weakness of this study was the lack of information about blinding of participants, which is important to determine the validity of the study. When the participants are aware of the alternative arm, results may be subject to contamination. Therefore, a Hawthorne effect could have occurred. Participants from the intervention arm may have over-performed, and the control arm participants may have felt increased levels of hopelessness or depression (if dissatisfied with their allocation group) resulting in greater fatigue symptom. These weaknesses may have negatively affected the validity of the study.
Jongen et al. (56, 57) conducted an observational study to examine the effect of a multidisciplinary, 3-day, social cognitive wellness program with the participation of support partners (social cognitive can do program—SCDP). SCDP was primarily a sociologically oriented approach to reduce the stressors that confine PwMS to their physical, psychological, or social roles. In total, 47 PwMS included in this program and the fatigue outcome assessments were conducted using MFIS (5-item version) at baseline, 1, 3, 6, and 12 months after the program. The authors categorized the study participants into two groups: (a) people with relapsing remitting MS and (b) people with progressive types of MS. The mean (SD) of MFIS at baseline, 1, 3, 6, and 12 months after the program were 12.72 (3.16), 11.00 (3.31), 10.94 (3.59), 11.89 (3.55), and 9.95 (3.77) for people with relapsing remitting MS, and 12.09 (4.08), 12.19 (3.53), 11.77 (3.95), 12.05 (3.50), and 11.93 (3.36) for people with progressive type of MS, respectively. The authors concluded that the intervention had no statistically significant decrease in the level of MS-related fatigue. This study was limited by its small sample size and the outcome assessment using self-report questionnaires which may have affected the quality of the study.
Kiropoulos et al. (43) reported a pilot RCT to examine the acceptability and effectiveness of an 8-week individual tailored CBT for depressive symptoms in PwMS. Thirty PwMS were randomly allocated into either CBT or the control group (treatment as usual). The 5-item MFIS was used to measure fatigue impact. The outcome assessments were measured at baseline, post-intervention, and 20-week follow-up. The mean (SD) of MFIS of both CBT and control groups were 12.13 (3.58), 8.73 (3.58), 8.06 (3.03), and 12.26 (3.84), 11.93 (4.38), 11.06 (4.74), respectively. The authors found that CBT produced a significant reduction in fatigue level among PwMS. This pilot RCT had limitations which may affected the quality of the study such as lack of blinding to treatment allocation of participants and staff; clinicians delivering the interventions also administered assessment questionnaires to participants; small sample size; and reliance on self-reported measures may affect the quality of the study.
Kos et al. (44) undertook a single-blind RCT to evaluate the effectiveness of an individual face-to-face SMOoTh intervention program versus relaxation among PwMS. Thirty-one PwMS were randomly allocated to two groups (17 patients in SMOoTh and 14 in relaxation). Both interventions consisted of three individual sessions of 60–90 min for three consecutive weeks. While SMOoTh included the component of partial-CBT, relaxation involved education on the role of stress management and practicing relaxation techniques. By using questionnaires, a researcher blinded to participants’ treatment allocation performed assessments at baseline, post-intervention, and 3-month follow-up. MFIS was used for fatigue measurement. According to the findings, the means of SMOoTh and relaxation groups were 43.5 (8.5) and 44.9 (14.3), respectively, at baseline. Both interventions decreased fatigue levels and the means (SD) were 33.9 (11.4) and 39.3 (13.1), respectively, at post-intervention. This study was weakened by the small sample size which may have limited generalizability and reliability. No information about participation rate was provided, and patients were aware of their assigned interventions. This may have increased the risk of bias affecting the validity of the results.
Mackay et al. (34) conducted a RCT in three sites in Sydney, Australia to investigate the effect of biofeedback in PwMS. It consisted of two interventions: (a) relaxation, mindfulness, social support, and education (RMSSE) intervention and (b) RMSSE plus biofeedback. Forty participants (20 per group) were randomly assigned to either the RMSSE or RMSSE plus biofeedback group for 3 weeks. Assessments were conducted at baseline, post-treatment, and 3-month follow-up. The FSS was used for fatigue measurement. The authors found that at post-treatment, the FSS mean scores for each of the groups were 3.96 (PMSSE-plus-biofeedback) and 4.38 (RMSSE). The biofeedback group revealed significant pre- to post-treatment improvement in fatigue (1.00; 95% CI 0.14–1.86; P-value = 0.02). The weaknesses of this study were small sample size, less than half of the cohort responding to the 3-month follow-up, participants in the biofeedback group knew their intervention status, less than 60% of selected individuals agreed to participate, and lack of blinding to assessors. These limitations affect the quality of the study and may have led to decreased validity of estimates.
Mohr et al. (45) performed a clinical trial to investigate the effects of treatment on fatigue in PwMS. Sixty patients with a relapsing form of MS and moderate-to-severe depression were randomly allocated to one of three validated 16-week treatments for depression: (a) individual CBT; (b) SEGP; (c) sertraline: antidepressant medication. Outcome assessments were undertaken before and after treatment. The Global Fatigue Severity subscale of Fatigue Assessment Instrument (FAI) was used for fatigue measurement. According to the findings, the mean FAI score in the CBT and SEGP groups were 58.2 (8.67) and 60.7 (8.83), respectively, at baseline. Post-intervention both groups had decreased fatigue levels and the means (SD) were 52.5 (12.52) and 61.3 (9.89), respectively. The authors found fatigue severity were significantly reduced over the course of depression treatment [P-value < 0.02 as reported by Mohr et al. (45)]. Lack of information about follow-up and the fact that the treatment assignment strategy was not purely random were the limitation of this study which may have affected the validity of this study.
Moss-Morris et al. (46) conducted a pilot RCT of an Internet-based CBT self-management for fatigue treatment in PwMS. A total of 40 PwMS were included in this study (Web-based CBT self-management MSInvigor8 n = 23 and standard care controls n = 17). The MSInvigor8 (web-based CBT) consisted of eight weekly sessions each taking between 25 and 50 min on average. Fatigue severity was measured using the original version of the Fatigue Scale and fatigue impact was assessed by the MFIS. The outcome assessments were taken at baseline and post-test (10 weeks). The authors found the web-based CBT intervention decreased MS-related fatigue and the mean (SD) fatigue score for both the web-based CBT group and control group were 21.39 (4.30), 12.39 (6.84), and 21.53 (3.62), 19.57 (5.20) respectively. For fatigue impact, the mean (SD) MFIS score of both groups were 13.17 (3.81) and 12.69 (3.89) at baseline and 9.00 (3.75) and 12.88 (3.89) at post-treatment, respectively. The authors concluded that the CBT group had significantly lower scores on both the fatigue scale and the MFIS compared with the control group. This study was limited by being a pilot/feasibility trial and recruitment occurred through the Internet which may decrease the validity of the study.
Nazari et al. (35) undertook a single-blinded randomized controlled clinical trial aiming to compare the effects of reflexology and relaxation on fatigue in women with MS. A total of 75 participants were randomly assigned to three groups: reflexology, relaxation, and control groups (25 PwMS per group). The intervention of relaxation was performed for 4 weeks (twice a week for 40 min in each session). Outcome assessments were done before, immediately after, and 2 months after intervention. The FSS was used for fatigue measurement. The author reported a significant difference was found in the fatigue mean scores in all three measurements (before, immediately after, and 2 months after intervention) of the relaxation group [P-value < 0.001 as reported by Nazari et al. (35)]. The mean fatigue severity scores immediately after intervention was significantly lower in relaxation group than the control group [P-value = 0.01 as reported by Nazari et al. (35)]. Limitations of this study were lack of blinding to participants, use of simple, non-random sampling before random assignment into three groups, and lack of information about follow-up rates. Since participants were aware of their related arm, contamination among participants may have been possible. Although participants were asked not to use the technique alone at home until the end of the study, there was no monitoring to establish whether or not this occurred. These biases may have affected the validity of the study.
Spitzer and Pakenham (54) presented a pilot study with single-group pre-test/post-test study design with the aim to evaluate a community-based mindfulness intervention. Twenty-three PwMS received a mindfulness program for 5 weeks (one 2-h session per week). The fatigue assessments were taken at pre-intervention, post-intervention, and 8-week follow-up using the MFIS. The mean (SD) of MFIS was 2.32 (0.90) at pre-intervention, 2.17 (0.73) at post-intervention, and 2.33 (0.77) at follow-up. The authors reported that the mindfulness intervention had no significant impact on fatigue. The validity of the results of the study may have been limited by the small sample size, non-random sampling, and reliance on self-report including MS course and diagnosis, and lack of information about blinding.
Thomas et al. (47, 48) carried out a multi-center RCT to assess the effectiveness of a group-based fatigue management program (FACETS) for PwMS. The 164 participants were randomly allocated into two groups (84 participants in FACETS and 80 participants in a control group—current local practice). The FACETS program is mainly based on CBT and energy effectiveness techniques. Two experienced health professionals delivered the FACETS program to groups of 6–12 participants over six weekly 90-min sessions. Fatigue severity was measured at baseline, 1 month, 4 months, and 12 months after the final FACETS session. The study reported less fatigue for those undertaking the FACETS program at the 4- and 12-month follow-up (change from baseline = −0.36; 95% CI: −0.63 to −0.08; P-value = 0.01 at 4-month follow-up; change from baseline = −0.30; 95% CI: −0.61 to 0.01; P-value = 0.06 at 12-month follow-up). Lack of information about treatment fidelity across different centers and participants knowing their interventions may have affected the validity of this study.
van Kessel et al. (49) presented a RCT to assess the efficacy of CBT for MS-related fatigue. A total of 72 PwMS were randomly allocated to receive eight weekly sessions of CBT (35 participants) or relaxation training (RT) (37 participants). Self-rated outcome measures were collected at four points (pre- and post-treatment, 3 months and 6 months after post-treatment). Fatigue was measured by using the Fatigue Scale. In this study, a normative approach was used and fatigue scale data for a matched-healthy group (72 healthy participants) were collected during the baseline assessment. According to the findings, in both groups, there were reductions in fatigue at post-treatment and follow-up. In addition, the author mentioned that the CBT group showed significantly greater reductions in fatigue level than the RT-group at the end of treatment and follow-up periods [P-value < 0.02—as reported by van Kessel et al. (49)]. At the end of treatment, the CBT group showed a significantly lower level of fatigue compared with the healthy comparison group [P-value < 0.001—as reported by van Kessel et al. (49)] and fatigue levels of the RT group were equivalent to those of the matched-healthy group. It was also difficult to determine the validity of the results of this study as the authors did not provide details regarding the blinding of assessors and participants.
van Kessel et al. (55) described a pilot RCT to compare the efficacy of a web-based CBT self-management program with and without the use of email support (therapeutic contact). A total of 39 PwMS were randomly allocated into either an Internet-based CBT with email support from a clinical psychologist (MSInvigor8-Plus) or an Internet-based CBT without email support (MSInvigor8-Only). MSInvigor8 included eight sessions of between 25 and 50 min. The outcome measures included fatigue severity (Fatigue Scale) and impact (MFIS) and were conducted at baseline and at 10 weeks. The authors found that the MSInvigor8-Plus intervention significantly reduced in fatigue severity [P-value < 0.01—as reported by van Kessel et al. (55)] and fatigue impact [P-value < 0.02—as reported by van Kessel et al. (55)] when compared with the MSInvigor8-Only group. In this study, there was no blinding for group allocation of participants and researchers. A Hawthorne effect could have existed since the participants knew their allocated interventions. Furthermore, only participants who were interested and able to use Internet-based CBT were included in this pilot RCT and only 45% of the MSInvigor8-Only group completed post-treatment follow-up. These limitations of the study may decrease the validity of the findings.
Vazirinejad et al. (50) presented a placebo-controlled clinical trial to evaluate the effectiveness of psychological training with gradual muscle relaxation technique on fatigue in PwMS. A total of 60 participants were randomly allocated to two groups: intervention and control group (no intervention) (30 PwMS per group). The intervention group received 12 sessions of psychological training with gradual muscle relaxation technique (2 sessions per week). Outcome assessments were done before, immediately after, and 3 months following intervention. The FSS was used for fatigue measurement. According to the finding, the means of intervention group were 42.83 (8.36) at baseline and 33.9 (7.07) at immediately after the intervention. The authors found a significant reduction in the FSS in the intervention group [P-value ≤ 0.001—as reported by Vazirinejad et al. (50)]. No information about randomization, blinding, and follow-up rate was provided in this study. Therefore, it is difficult to determine the validity of estimates.
There were 12 studies (35, 39–50) (n = 745) included in 4 meta-analyses. In the first meta-analysis, five studies (41–43, 46–48) (n = 429) compared CBT against non-active controls (i.e., waitlist, standard care, or current local practice). In the second meta-analysis, three studies (44, 45, 49) (n = 141) compared CBT interventions or SMOoTh (CBT) against active controls (i.e., relaxation therapy or SEGP). In the third meta-analysis, two studies (35, 50) (n = 110) compared relaxation therapy against non-active controls (routine treatment or no intervention) and in the fourth meta-analysis, two studies (39, 40) (n = 65) compared mindfulness intervention against non-active controls (i.e., waitlist group).
The CBT intervention was associated with decreased fatigue (pooled SMD: −0.32; 95% CI: −0.63 to −0.01). There was moderate heterogeneity between studies (I2 = 54.4%; P-value = 0.07) (Figure 2). Visual inspection of the funnel plot did not indicate presence of small study effects (Figure 3) and Egger’s regression asymmetry test did not suggest any small study effects (P-value = 0.09).
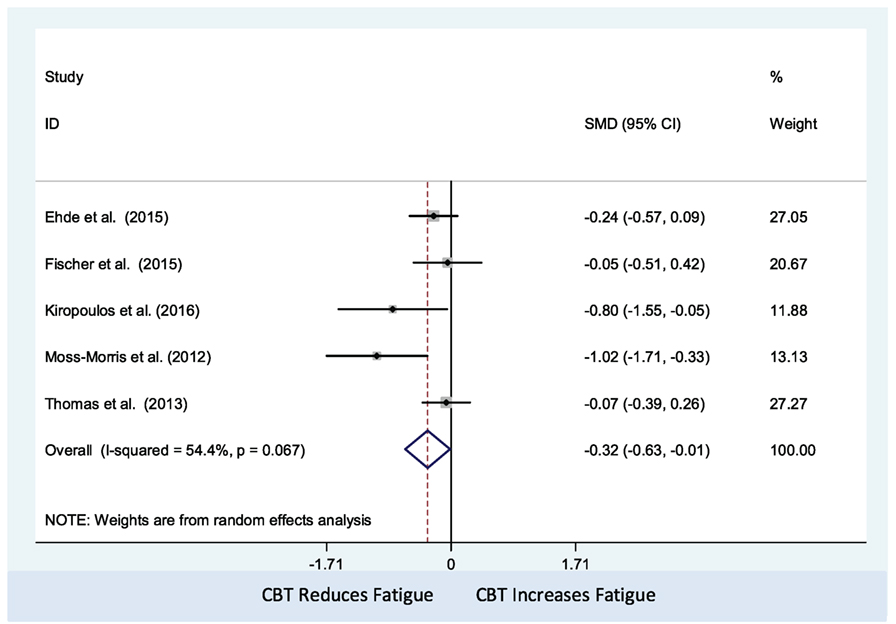
Figure 2. Comparison of cognitive behavioral therapy interventions and non-active controls on multiple sclerosis-related fatigue.
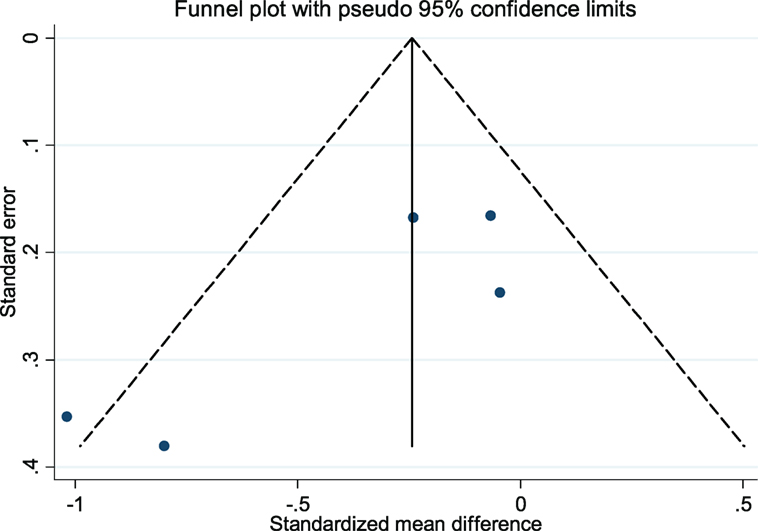
Figure 3. Funnel plot with pseudo 95% confidence limits for the meta-analysis of studies that reported a comparison of cognitive behavioral therapy interventions and non-active controls on multiple sclerosis-related fatigue. Solid vertical lines correspond to pooled standardized mean difference (SMD), dotted lines corresponds to the pseudo 95% confidence limits, and solid dots correspond to each of the SMD from the included five studies.
The CBT intervention was associated with decreased fatigue (pooled SMD = −0.71, 95% CI: −1.05 to −0.37). There was no observed statistical heterogeneity between the three studies (I2 = 0%; P-value = 0.77) (Figure 4). Only three studies were included in this meta-analysis, which precluded us from assessing the presence of small study effects using funnel plots or Egger’s test (62).
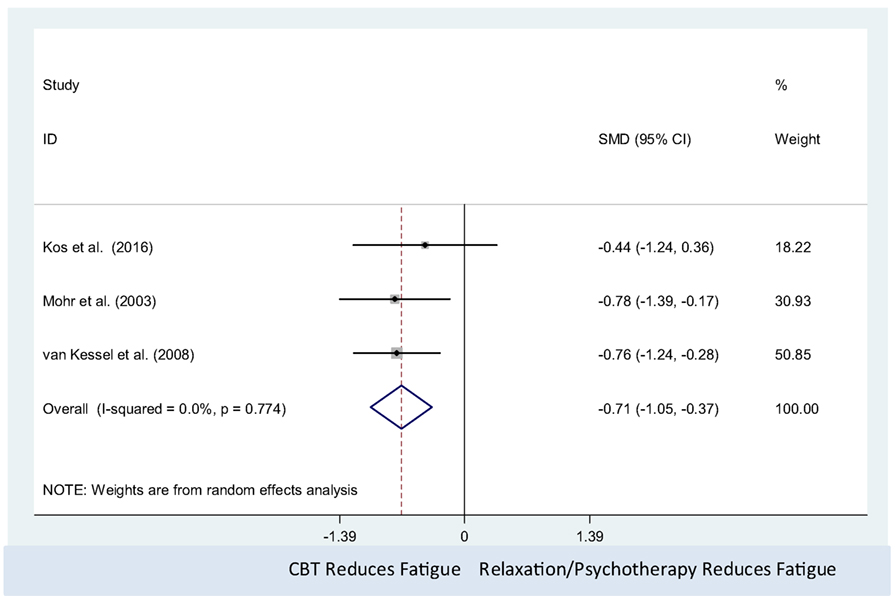
Figure 4. Comparison of cognitive behavioral therapy interventions and active controls (relaxation or psychotherapy) on multiple sclerosis-related fatigue.
The relaxation therapy was associated with decreased fatigue (pooled SMD: −0.90; 95% CI: −1.30 to −0.51). There was no observed statistical heterogeneity between studies (I2 = 0%; P-value = 0.37) (Figure 5). Only two studies were included in this meta-analysis, which precluded us from assessing the presence of small study effects using funnel plots or Egger’s test (62).
The mindfulness intervention was associated with decrease in fatigue level (pooled SMD: −0.62; 95% CI: −1.12 to −0.12). There was no observed statistical heterogeneity between studies (I2 = 0%; P-value = 0.59) (Figure 6). Only two studies were included in this meta-analysis, which precluded us from assessing the presence of small study effects using funnel plots or Egger’s test (62).
The aim of this systematic review and meta-analysis was to determine the efficacy of psychological interventions in improving fatigue in PwMS. While others have undertaken a review (36) of the effect of CBT on fatigue in PwMS, our systematic review takes a broader perspective, needed to inform clinical guidelines. This review investigated 20 studies (22 articles) (33–35, 39–57) which assessed the following psychological interventions: CBT and CBT-related psychological treatment, relaxation, mindfulness, psychological training, intensive social cognitive wellness program, and progressive muscle relaxation. We include the studies which were excluded from the meta-analysis in this review since our focus was to inform the more comprehensive view of psychological interventions in MS-related fatigue management. Results of our narrative synthesis and meta-analysis suggest that psychological interventions, particularly CBT, relaxation therapy, mindfulness, and progressive muscle relaxation, are associated with reduced fatigue in PwMS. Interestingly, our meta-analysis of three papers (44, 45, 49) indicated CBT was most effective in reducing fatigue when compared with active controls (relaxation or SEGP). It should be noted that for all studies reviewed there was no differentiation of types of fatigue, i.e., whether fatigue was considered to be primary or secondary fatigue. In addition, Asano and Finlayson (30) undertook meta-analysis of three types of fatigue management interventions for PwMS. In the study of Asano and Finlayson, they used the label “education” to describe CBT, relaxation therapy, mindfulness therapy, and energy conservative course which was not the interest of our review and grouped all educational intervention together in one meta-analysis. In this review, we included only psychological interventions and performed separate meta-analysis for different types of psychological interventions.
This review suggests that CBT is an effective psychological method of treating MS-related fatigue. Among the 20 studies included in this review, in total, 7 studies (41–43, 46–48, 51, 55) delivered CBT as a psychological intervention for fatigue treatment among PwMS. Among them, five studies (41–43, 46–48) examined the effect of CBT against non-active controls such as telephone-delivered education intervention, waitlist, current local practices, and standard care, whereas Anderson et al. (51) assessed the efficacy of CBT through the single group pre-test and post-test study design and van Kessel et al. (55) compared the effect of a web-based CBT with or without email support from clinical psychologists. In all seven studies, CBT decreased the fatigue level of PwMS. In the study of Ehde et al. (41), Kiropoulos et al. (43), Moss-Morris et al. (46), and Thomas et al. (47, 48), the authors concluded that CBT resulted in clinically significant decrease in MS-associated fatigue and was effective in managing fatigue. In the study of van Kessel et al. (55), the Internet-based CBT with an email support from a skilled clinical psychologist had significantly greater reduction in fatigue level compared with the Internet-based CBT without an email support.
The results of our meta-analysis of five studies (41–43, 46–48) indicated that CBT, compared with non-active control, had a significant effect in reducing MS-related fatigue and we found moderate heterogeneity between studies. This might be associated with the ways in which CBT was delivered, as these were slightly different across the studies. In the study of Ehde et al. (41), the intervention was a telephone-delivered self-management program consisting of CBT and positive psychology methods, whereas in the pilot RCT of Moss-Morris et al. (46), the authors used an Internet-based CBT self-management program. By contrast, Fischer et al. (42) used an online program based on principles of CBT with a focus on reducing depression, and Kiropoulous et al. (43) delivered a tailored CBT-based intervention for depressive symptoms among newly diagnosed PwMS. In the trial by Thomas et al. (47, 48), the intervention was a group-based fatigue management session in which CBT components were dominant.
The trials of Kos et al. (44), van Kessel et al. (49), and Mohr et al. (45) investigated the effect of CBT against active controls, which were relaxation therapy or psychotherapy. Three studies showed CBT compared with active controls was effective in treating fatigue. Among the three studies (44, 45, 49) which compared CBT against active controls such as relaxation therapy in fatigue treatment, van Kessel et al.’s study (49) and Mohr et al.’s study (45) demonstrated that both CBT and active controls (relaxation therapy or psychotherapy) showed clinically significant decreases in MS-associated fatigue. The meta-analysis revealed that CBT produced statistically significant decreases in fatigue level. Therefore, we can conclude that CBT is more effective for fatigue treatment compared with active controls such as relaxation therapy and SEGP, this is in line with the findings from a meta-analysis conducted in 2016 by van den Akker (36). van den Akker found that the use of CBT had a moderately positive effect on MS-related fatigue management. There are differences between the study of van den Akker et al. (36) and the present study. The review of van den Akker et al. focused exclusively on CBT and included only RCTs while our review assessed a wider range of psychological interventions; and included a single-group pre-test/post-test design study, RCTs, and randomized clinical trials.
Three studies (35, 50, 52) delivered relaxation therapy and psychological training for PwMS. Across the three studies, it was found that relaxation and psychological training was significantly effective in reducing fatigue. In Carletto et al.’s study (52), the psychological interventions (relaxation therapy and EMDR) were designed to treat post-traumatic stress disorder in PwMS, whereas Nazari et al. (35) and Vazirinejad et al. (50) delivered relaxation and psychological training with gradual muscle relaxation technique for MS-related fatigue treatment. The results of our meta-analysis of two studies (35, 50) revealed that relaxation therapy, compared with non-active control, had a significant effect in reducing MS-related fatigue.
Furthermore, in our study, a total of four studies (39, 40, 53, 54) used mindfulness interventions as psychological interventions for PwMS. In all four studies, mindfulness interventions decreased the fatigue of PwMS. However, only two studies, Alisaleh and Shahrbanoo (39) and Grossman et al. (53) showed that mindfulness interventions were significantly effective in reducing fatigue. The results of our meta-analysis of two studies (39, 40) showed that mindfulness intervention, compared with non-active control, had a significant effect in reducing fatigue among PwMS.
Moreover, this review included one study (33) which examined the effect of PMRT through a single-group pre-test/post-test design study. In that study, PMRT significantly decreased MS-related fatigue. In the trial conducted by Mackay et al. (34), RMSSE were delivered to both intervention and control groups and biofeedback was applied to the intervention arm. Both groups showed reduction in fatigue levels, but a statistically significant difference was found only in the intervention group. In addition, our systematic review included one observational study (56, 57) in which the effect of an intensive social cognitive wellness program on fatigue of PwMS was examined; however, no effect on fatigue was found.
Across the studies included in this review, the characteristics of participants were reasonably homogeneous. It is unlikely therefore that the findings observed can be attributed to differences in participant’s characteristics. The fatigue scales used by the studies reviewed, were not consistent between studies and the nature of each fatigue measurement scale were also slightly different. The FSS measures the severity of fatigue and its effect on a person’s activities and lifestyle (14). The FSS has moderate reliability (ICC = 0.751) (63), concurrent validity (Cronbach’s alpha = 0.86) (64), high internal consistency (Cronbach’s alpha 0.95) (65), and it correlates well with MFIS (r = 0.754) (63). The MFIS provides an assessment of the effects of fatigue in terms of physical, cognitive, and psychosocial functioning (15). The global fatigue severity subscale of the FAI is the tool that is used to differentiate normal fatigue from fatigue by medical disorders (66). The FSMC is a patient reported outcome measure for measuring mental and physical fatigue, and it is the scale with high internal consistency (Cronbach’s alpha > 0.91) that was tested against other fatigue scales and provide graduation of cognitive and motor fatigue (67). The fatigue scale measures the severity of physical and mental fatigue, and it has high degree of internal consistency and the validation coefficients were sensitivity 75.5 and specificity 74.5 (68). Clearly, it is likely therefore that these tools measure different aspect of fatigue and are not directly comparable. This should be considered when interpreting results of this systematic review. While this can be accounted for to some extent in meta-analysis using SMD, the use of different scales makes comparison between studies included in our narrative review difficult.
The psychological interventions used in the included studies were also quite varied. In the trials of Mohr et al. (45), Kos et al. (44), and van Kessel et al. (49), CBT and self-management therapy (CBT) were used as psychological interventions and the effects of psychological treatments in improving MS-related fatigue were compared against an active-control such as relaxation therapy and SEGP which were also the psychological interventions. Due to this design, the effect of CBT might be underestimated. In the study of Thomas et al. (47, 48), the authors used a pragmatic parallel arm multi-center RCT to investigate the effect of a group-based fatigue management intervention which was mainly based on CBT, but treatment fidelity between centers was not formally assessed. In five studies (39, 41–43, 45, 53), psychological interventions were used separately for reducing not only MS-associated fatigue but also depression or stress. Therefore, the intervention approaches of these six studies might be slightly different in comparison with other trials which were focused on fatigue treatment.
In addition, with respect to follow-up rates and attrition rates of all included studies in this review, approximately three-fourths (33, 40–44, 46–49, 51–57) of included studies reported follow-up rates in detail. Attrition across the included studies except van Kessel et al. (55) was low, with only 45% of the control group completed follow-up with unknown reasons.
This review employed a rigorous methodological strategy to search and appraise the literature. We used a broad search strategy that was developed in consultation with a librarian, and hand-searched relevant systematic review to identify relevant studies. Two reviewers were independently involved in the screening, data extraction, and appraisal of studies suitable for inclusion. When required, disagreements were resolved by further consensus with authors Tracey J. Weiland and Alysha M. De Livera. Two reviewers independently assessed the quality of included studies in accordance with the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies (Hamilton Tool) (38). We also contacted primary authors for missing information or required data for our narrative review and meta-analyses.
We limited included articles to those published in English which might have excluded relevant studies published in other languages. As well, only 12 studies (35, 39–50) were included in the meta-analyses. In addition, the follow-up lengths of the included studies were inconsistent and most of them were not longer than 12 months. Therefore, no meta-analysis was conducted to investigate the long-term effectiveness of psychological interventions. Finally, since there were fewer than 10 studies included in the meta-analysis, caution should be taken when interpreting the results from Egger’s test (62).
The finding of this review can be used to inform practice as well on clinical recommendations for psychological approaches in MS-related fatigue management. Psychological interventions such as CBT, mindfulness-based therapy, and relaxation therapy were effective in the treatment of MS-related fatigue. Most importantly, CBT was more effective in reducing fatigue levels compared with other psychological interventions such as relaxation therapy and supportive-expressive group psychotherapy. More studies are needed to investigate the efficacy of each of mindfulness, relaxation therapy, and progressive muscle relaxation for fatigue treatment; and compare the effectiveness of these kinds of psychological interventions with CBT in fatigue management. A thorough examination of the convergent validity of the fatigue scales is also warranted. Furthermore, an exploration of the long-term effect of all types of psychological interventions for fatigue management for PwMS is needed.
AZZP contributed to study design, undertook searches, review of abstracts and papers, quality appraisal, meta-analyses, and drafted the manuscript. TD contributed to review of abstracts and papers for relevance, and quality appraisal, and reviewed and approved the final manuscript. AL contributed to study design, supervised meta-analyses, and edited the final manuscript. GJ, CB, CM, SN, KT, TM, AK, and EO’K contributed to interpretation and editing of the final manuscript. EO’K provided advice on meta-analyses and edited the final manuscript. TW conceived the project and provided overall supervision for the project, and edited and approved the final manuscript.
GJ receives royalties from the book “Overcoming Multiple Sclerosis,” and has received remuneration for running overcoming MS retreats, and is Chief Editor for Frontiers in Neurology (Neuroepidemiology Section); SN and KT have received remuneration for running overcoming multiple sclerosis retreats, and are both review editors for Frontiers in Neurology; TW is an associate editor for Frontiers in Neurology (Neuroepidemiology Speciality); CM is a review editor for Frontiers in Neurology (Neuroepidemiology Speciality).
We thank all participants who provided their data for the study and the authors of the included studies in this review. The authors also thank Dr. Jim Berryman for his technical support to the review.
CBT, cognitive behavioral therapy; EPHPP, Effective Public Health Practice Project; FACETS, fatigue: applying cognitive behavioral and energy effectiveness techniques to lifestyle; FSMC, fatigue scale for motor and cognitive function; FSS, Fatigue Severity Scale; MBI, mindfulness-based intervention; MFIS, Modified Fatigue Impact Scale; PMRT, progressive muscle relaxation technique; PRISMA, preferred reporting items for systematic reviews and meta-analyses; PwMS, people with MS; RMSSE, relaxation, mindfulness, social support, and education; RT, relaxation training; SMOoTh, self-management occupational therapy; T-SM, telephone-delivered self-management intervention; T-ED, telephone-delivered parallel education; UC, usual care.
1. Gajofatto A, Calabrese M, Benedetti MD, Monaco S. Clinical, MRI, and CSF markers of disability progression in multiple sclerosis. Dis Markers (2013) 35(6):687–99. doi:10.1155/2013/484959
2. Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology (2002) 59(7):1006–10. doi:10.1212/WNL.59.7.1006
3. Pietrangelo A, Higuera V. Healthline Media. Multiple Sclerosis by the Numbers: Facts, Statistics, and You. Available from: http://www.healthline.com/health/multiple-sclerosis/facts-statistics-infographic (accessed April 17, 2017).
4. MS Council for Clinical Practice Guidelines. Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans Association (1998).
5. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka longitudinal multiple sclerosis study: methods and sample characteristics. Mult Scler (2006) 12(1):24–38. doi:10.1191/135248506ms1262oa
6. Krupp LB. Fatigue in Multiple Sclerosis: A Guide to Diagnosis and Management. New York: Demos (2004).
7. Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil (1984) 65(3):135–8.
8. Branas P, Jordan R, Fry-Smith A, Burls A, Hyde C. Treatments for fatigue in multiple sclerosis: a rapid and systematic review. Health Technol Assess (2000) 4(27):1–61.
9. Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes (2008) 6:100. doi:10.1186/1477-7525-6-100
10. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol (1988) 45(4):435–7. doi:10.1001/archneur.1988.00520280085020
11. MS Society. What Causes Fatigue? Available from: https://www.mssociety.org.uk/what-is-ms/signs-and-symptoms/fatigue/causes-of-fatigue (accessed April 19, 2017).
12. Flachenecker P, Kümpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler (2002) 8(6):523–6. doi:10.1191/1352458502ms839oa
13. Chahin S, Miller D, Sakai RE, Wilson JA, Frohman T, Markowitz C, et al. Relation of quantitative visual and neurologic outcomes to fatigue in multiple sclerosis. Mult Scler Relat Disord (2015) 4(4):304–10. doi:10.1016/j.msard.2015.05.005
14. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol (1989) 46(10):1121–3. doi:10.1001/archneur.1989.00520460115022
15. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci (1994) 21(1):9–14. doi:10.1017/S0317167100048691
16. Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother (2010) 10(9):1437–47. doi:10.1586/ern.10.99
17. Smith MM, Arnett PA. Factors related to employment status changes in individuals with multiple sclerosis. Mult Scler (2005) 11(5):602–9. doi:10.1191/1352458505ms1204oa
18. Messmer Uccelli M, Specchia C, Battaglia MA, Miller DM. Factors that influence the employment status of people with multiple sclerosis: a multi-national study. J Neurol (2009) 256(12):1989–96. doi:10.1007/s00415-009-5225-0
19. Julian LJ, Vella L, Vollmer T, Hadjimichael O, Mohr DC. Employment in multiple sclerosis. Exiting and re-entering the work force. J Neurol (2008) 255(9):1354–60. doi:10.1007/s00415-008-0910-y
20. Edgley K, Sullivan M, Dehoux E. A survey of multiple sclerosis: part 2. Determinants of employment status. Can J Rehabil (1991) 4:127–32.
21. Genevie L, Kallos JE, Struening EL. Job retention among people with multiple sclerosis. J Neurol Rehabil (1987) 1:131–5.
22. Scheinberg L, Holland N, Larocca N, Laitin P, Bennett A, Hall H. Multiple sclerosis; earning a living. N Y State J Med (1980) 80(9):1395–400.
23. Tur C. Fatigue management in multiple sclerosis. Curr Treat Options Neurol (2016) 18(6):26. doi:10.1007/s11940-016-0411-8
24. Perry M, Swain S, Kemmis-Betty S, Cooper P. Multiple sclerosis: summary of NICE guidance. BMJ (2014) 349:g5701. doi:10.1136/bmj.g5701
25. Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med (2013) 75(6):575–80. doi:10.1097/PSY.0b013e31829b4525
26. Ahmadi A, Nikbakh M, Arastoo A, Habibi A-H. The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J Hum Kinet (2010) 23(1):71. doi:10.2478/v10078-010-0009-2
27. Dettmers C, Sulzmann M, Ruchay-Plössl A, Gütler R, Vieten M. Endurance exercise improves walking distance in MS patients with fatigue. Acta Neurol Scan (2009) 120(4):251–7. doi:10.1111/j.1600-0404.2008.01152.x
28. Geddes EL, Costello E, Raivel K, Wilson R. The effects of a twelve-week home walking program on cardiovascular parameters and fatigue perception of individuals with multiple sclerosis: a pilot study. Cardiopulm Phys Ther J (2009) 20(1):5–12.
29. Svenningsson A, Falk E, Celius EG, Fuchs S, Schreiber K, Berko S, et al. Natalizumab treatment reduces fatigue in multiple sclerosis. results from the TYNERGY trial; a study in the real life setting. PLoS One (2013) 8(3):e58643. doi:10.1371/journal.pone.0058643
30. Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int (2014) 2014:798285. doi:10.1155/2014/798285
31. Kargarfard M, Etemadifar M, Baker P, Mehrabi M, Hayatbakhsh R. Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil (2012) 93:1701–8. doi:10.1016/j.apmr.2012.05.006
32. Miller P, Soundy A. The pharmacological and non-pharmacological interventions for the management of fatigue related multiple sclerosis. J Neurol Sci (2017) 381:41–54. doi:10.1016/j.jns.2017.08.012
33. Dayapoglu N, Tan M. Evaluation of the effect of progressive relaxation exercises on fatigue and sleep quality in patients with multiple sclerosis. J Altern Complement Med (2012) 18(10):983–7. doi:10.1089/acm.2011.0390
34. Mackay AM, Buckingham R, Schwartz RS, Hodgkinson S, Beran RG, Cordato DJ. The effect of biofeedback as a psychological intervention in multiple sclerosis: a randomized controlled study. Int J MS Care (2015) 17(3):101–8. doi:10.7224/1537-2073.2014-006
35. Nazari F, Shahreza MS, Shaygannejad V, Valiani M. Comparing the effects of reflexology and relaxation on fatigue in women with multiple sclerosis. Iran J Nurs Midwifery Res (2015) 20(2):200–4.
36. van den Akker LE, Beckerman H, Collette EH, Eijssen ICJM, Dekker J, de Groot V. Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Psychosom Res (2016) 90:33–42. doi:10.1016/j.jpsychores.2016.09.002
37. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med (2009) 6(7):e1000100. doi:10.1371/journal.pmed.1000100
38. National Collaborating Centre for Methods and Tools. Quality Assessment Tool for Quantitative Studies. Hamilton, ON: McMaster University (2008). Available from: http://www.nccmt.ca/resources/search/14 (accessed April 10, 2017).
39. Alisaleh E, Shahrbanoo G. Effectiveness of mindfulness-based stress reduction (MBSR) in stress and fatigue in patients with multiple sclerosis (MS). Int J Med Res Health Sci (2016) 5(7S):486–91.
40. Bogosian A, Chadwick P, Windgassen S, Norton S, McCrone P, Mosweu I, et al. Distress improves after mindfulness training for progressive MS: a pilot randomised trial. Mult Scler (2015) 21(9):1184–94. doi:10.1177/1352458515576261
41. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil (2015) 96(11):1945–58.e2. doi:10.1016/j.apmr.2015.07.015
42. Fischer A, Schroder J, Vettorazzi E, Wolf OT, Pottgen J, Lau S, et al. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. Lancet Psychiatry (2015) 2(3):217–23. doi:10.1016/S2215-0366(14)00049-2
43. Kiropoulos LA, Kilpatrick T, Holmes A, Threader J. A pilot randomized controlled trial of a tailored cognitive behavioural therapy based intervention for depressive symptoms in those newly diagnosed with multiple sclerosis. BMC Psychiatry (2016) 16(1):435. doi:10.1186/s12888-016-1152-7
44. Kos D, Duportail M, Meirte J, Meeus M, D’Hooghe MB, Nagels G, et al. The effectiveness of a self-management occupational therapy intervention on activity performance in individuals with multiple sclerosis-related fatigue: a randomized-controlled trial. Int J Rehabil Res (2016) 39(3):255–62. doi:10.1097/MRR.0000000000000178
45. Mohr DC, Hart SL, Goldberg A. Effects of treatment for depression on fatigue in multiple sclerosis. Psychosom Med (2003) 65(4):542–7. doi:10.1097/01.PSY.0000074757.11682.96
46. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther (2012) 50(6):415–21. doi:10.1016/j.brat.2012.03.001
47. Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry (2013) 84(10):1092–9. doi:10.1136/jnnp-2012-303816
48. Thomas PW, Thomas S, Kersten P, Jones R, Slingsby V, Nock A, et al. One year follow-up of a pragmatic multi-centre randomised controlled trial of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. BMC Neurol (2014) 14(1):1–11. doi:10.1186/1471-2377-14-109
49. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med (2008) 70(2):205–13. doi:10.1097/PSY.0b013e3181643065
50. Vazirinejad R, Jafarzadeh A, Yassini SM, Rahimdel A, Sayadi AR. Effectiveness of psychological training with gradual muscle relaxation technique on fatigue in multiple sclerosis patients. Acta Medica Mediterranea (2016) 32(4):987–90.
51. Anderson JK, Turner A, Clyne W. Development and feasibility of the help to overcome problems effectively (HOPE) self-management intervention for people living with multiple sclerosis. Disabil Rehabil (2017) 39(11):1114–21. doi:10.1080/09638288.2016.1181211
52. Carletto S, Borghi M, Bertino G, Oliva F, Cavallo M, Hofmann A, et al. Treating post-traumatic stress disorder in patients with multiple sclerosis: a randomized controlled trial comparing the efficacy of eye movement desensitization and reprocessing and relaxation therapy. Front Psychol (2016) 7:526. doi:10.3389/fpsyg.2016.00526
53. Grossman P, Kappos L, Gensicke H, D’Souza M, Mohr DC, Penner IK, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology (2010) 75(13):1141–9. doi:10.1212/WNL.0b013e3181f4d80d
54. Spitzer E, Pakenham KI. Evaluation of a brief community-based mindfulness intervention for people with multiple sclerosis: a pilot study. Clin Psychologist (2016). doi:10.1111/cp.12108
55. van Kessel K, Wouldes T, Moss-Morris R. A New Zealand pilot randomized controlled trial of a web-based interactive self-management programme (MSInvigor8) with and without email support for the treatment of multiple sclerosis fatigue. Clin Rehabil (2016) 30(5):454–62. doi:10.1177/0269215515584800
56. Jongen PJ, Ruimschotel R, Heerings M, Hussaarts A, Duyverman L, van der Zande A, et al. Improved self-efficacy in persons with relapsing remitting multiple sclerosis after an intensive social cognitive wellness program with participation of support partners: a 6-months observational study. Health Qual Life Outcomes (2014) 12:40. doi:10.1186/1477-7525-12-40
57. Jongen PJ, Heerings M, Ruimschotel R, Hussaarts A, Duyverman L, van der Zande A, et al. Intensive social cognitive treatment (Can Do treatment) with participation of support partners in persons with relapsing remitting multiple sclerosis: observation of improved self-efficacy, quality of life, anxiety and depression 1 year later. BMC Res Notes (2016) 9(1):375. doi:10.1186/s13104-016-2173-5
58. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7(3):177–88. doi:10.1016/0197-2456(86)90046-2
59. Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol (1962) 65(3):145–53. doi:10.1037/h0045186
60. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011). Available from: http://training.cochrane.org/handbook (Accessed: April 20, 2017).
61. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) (1997) 315(7109):629–34. doi:10.1136/bmj.315.7109.629
62. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol (2000) 53(11):1119–29. doi:10.1016/S0895-4356(00)00242-0
63. Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci (2013) 331(1–2):102–7. doi:10.1016/j.jns.2013.05.023
64. Lerdal A, Kottorp A. Psychometric properties of the Fatigue Severity Scale – Rasch analyses of individual responses in a Norwegian stroke cohort. Int J Nurs Stud (2011) 48(10):1258–65. doi:10.1016/j.ijnurstu.2011.02.019
65. Rosti-Otajärvi E, Hämäläinen P, Wiksten A, Hakkarainen T, Ruutiainen J. Validity and reliability of the Fatigue Severity Scale in Finnish multiple sclerosis patients. Brain Behav (2017) 7(7):e00743. doi:10.1002/brb3.743
66. Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res (1993) 37(7):753–62. doi:10.1016/0022-3999(93)90104-N
67. Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The fatigue scale for motor and cognitive functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler (2009) 15(12):1509–17. doi:10.1177/1352458509348519
68. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res (1993) 37(2):147–53. doi:10.1016/0022-3999(93)90081-P
69. Askey-Jones S, Silber E, Shaw P, Gray R, David AS. A nurse-led mental health service for people with multiple sclerosis. J Psychosom Res (2012) 72(6):463–5. doi:10.1016/j.jpsychores.2012.01.020
70. Bisht B, Darling WG, Grossmann RE, Shivapour ET, Lutgendorf SK, Snetselaar LG, et al. A multimodal intervention for patients with secondary progressive multiple sclerosis: feasibility and effect on fatigue. J Altern Complement Med (2014) 20(5):347–55. doi:10.1089/acm.2013.0188
71. Burschka JM, Keune PM, Oy UH, Oschmann P, Kuhn P. Mindfulness-based interventions in multiple sclerosis: beneficial effects of Tai Chi on balance, coordination, fatigue and depression. BMC Neurol (2014) 14:165. doi:10.1186/s12883-014-0165-4
72. Cosio D, Jin L, Siddique J, Mohr DC. The effect of telephone-administered cognitive-behavioral therapy on quality of life among patients with multiple sclerosis. Ann Behav Med (2011) 41(2):227–34. doi:10.1007/s12160-010-9236-y
73. Finlayson M, Preissner K, Cho C, Plow M. Randomized trial of a teleconference-delivered fatigue management program for people with multiple sclerosis. Mult Scler (2011) 17(9):1130–40. doi:10.1177/1352458511404272
74. Kinsinger SW, Lattie E, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology (2010) 24(5):573–80. doi:10.1037/a0019222
75. Knoop H, van Kessel K, Moss-Morris R. Which cognitions and behaviours mediate the positive effect of cognitive behavioural therapy on fatigue in patients with multiple sclerosis? Psychol Med (2012) 42(1):205–13. doi:10.1017/S0033291711000924
76. Kos D, Duportail M, d’Hooghe MB, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler (2007) 13(8):996–1003. doi:10.1177/1352458507078392
77. Levin AB, Hadgkiss EJ, Weiland TJ, Marck CH, van der Meer DM, Pereira NG, et al. Can meditation influence quality of life, depression, and disease outcome in multiple sclerosis? Findings from a large international web-based study. Behav Neurol (2014) 2014:916519. doi:10.1155/2014/916519
78. Mohr DC, Hart S, Vella L. Reduction in disability in a randomized controlled trial of telephone-administered cognitive-behavioral therapy. Health Psychol (2007) 26(5):554–63. doi:10.1037/0278-6133.26.5.554
79. Nejati S, Rajezi Esfahani S, Rahmani S, Afrookhteh G, Hoveida S. The effect of group mindfulness-based stress reduction and consciousness yoga program on quality of life and fatigue severity in patients with MS. J Caring Sci (2016) 5(4):325–35. doi:10.15171/jcs.2016.034
80. Tesar N, Bandion K, Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis: a randomised controlled trial. Wien Klin Wochenschr (2005) 117(21–22):747–54. doi:10.1007/s00508-005-0470-4
81. Thomas S, Thomas PW, Nock A, Slingsby V, Galvin K, Baker R, et al. Development and preliminary evaluation of a cognitive behavioural approach to fatigue management in people with multiple sclerosis. Patient Educ Couns (2010) 78(2):240–9. doi:10.1016/j.pec.2009.07.001
Keywords: fatigue, multiple sclerosis, CBT, review, meta analysis
Citation: Phyo AZZ, Demaneuf T, De Livera AM, Jelinek GA, Brown CR, Marck CH, Neate SL, Taylor KL, Mills T, O’Kearney E, Karahalios A and Weiland TJ (2018) The Efficacy of Psychological Interventions for Managing Fatigue in People With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front. Neurol. 9:149. doi: 10.3389/fneur.2018.00149
Received: 10 December 2017; Accepted: 28 February 2018;
Published: 04 April 2018
Edited by:
Bianca Weinstock-Guttman, Jacobs School of Medicine and Biomedical Sciences, United StatesReviewed by:
Jennifer Graves, University of California, San Francisco, United StatesCopyright: © 2018 Phyo, Demaneuf, De Livera, Jelinek, Brown, Marck, Neate, Taylor, Mills, O’Kearney, Karahalios and Weiland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tracey J. Weiland, dHdlaWxhbmRAdW5pbWVsYi5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.