- 1Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, United States
- 2Geriatric Research, Education and Clinical Center (GRECC), VA Puget Sound Health Care System, U.S. Department of Veterans Affairs, Seattle, WA, United States
- 3VISN 20 Northwest Network Mental Illness Research, Education, and Clinical Center (MIRECC), VA Puget Sound Health Care System, U.S. Department of Veterans Affairs, Seattle, WA, United States
The most frequent injury sustained by US service members deployed to Iraq or Afghanistan is mild traumatic brain injuries (mTBI), or concussion, by far most often caused by blast waves from improvised explosive devices or other explosive ordnance. TBI from all causes gives rise to chronic neuroendocrine disorders with an estimated prevalence of 25–50%. The current study expands upon our earlier finding that chronic pituitary gland dysfunction occurs with a similarly high frequency after blast-related concussions. We measured circulating hormone levels and accessed demographic and testing data from two groups of male veterans with hazardous duty experience in Iraq or Afghanistan. Veterans in the mTBI group had experienced one or more blast-related concussion. Members of the deployment control (DC) group encountered similar deployment conditions but had no history of blast-related mTBI. 12 of 39 (31%) of the mTBI participants and 3 of 20 (15%) veterans in the DC group screened positive for one or more neuroendocrine disorders. Positive screens for growth hormone deficiency occurred most often. Analysis of responses on self-report questionnaires revealed main effects of both mTBI and hypopituitarism on postconcussive and posttraumatic stress disorder (PTSD) symptoms. Symptoms associated with pituitary dysfunction overlap considerably with those of PTSD. They include cognitive deficiencies, mood and anxiety disorders, sleep problems, diminished quality of life, deleterious changes in metabolism and body composition, and increased cardiovascular mortality. When such symptoms are due to hypopituitarism, they may be alleviated by hormone replacement. These findings suggest consideration of routine post-deployment neuroendocrine screening of service members and veterans who have experienced blast-related mTBI and are reporting postconcussive symptoms.
Introduction
“Concussions,” a term often used synonymously with “mild traumatic brain injuries” (mTBI), accounted for 2.8 million emergency room visits or hospitalizations in the US in 2013 (1). The number of reported concussions has increased especially rapidly in high school, college, and professional athletes and deployed service members. Approximately 75% of diagnosed TBIs are mTBIs (2). In response, the focus of research and media attention on the prevalence, natural history, treatment, and prevention of mTBI in athletes has increased dramatically during the past decade. However, mTBI in military personnel and veterans has received less public notice, even though mTBI—most often caused by blasts from improvised explosive devices—is the most frequent injury sustained by US troops deployed to Iraq and Afghanistan (3–5). mTBIs constituted 82.4% of approximately 290,000 military TBIs diagnosed from 2000 to 2013. In both civilian and military populations, many mTBIs are unreported or undiagnosed, and the true incidence has been estimated to be two to five times higher than current reports (6–9).
The American Congress of Rehabilitation Medicine has defined mTBI as manifested by at least one of the following: 1. a period of loss of consciousness (LOC) of approximately 30 min or less; 2. loss of memory not greater than 24 h for events immediately before or after the incident; 3. any alteration in mental state at the time of the incident (e.g., feeling dazed, disoriented, or confused); or 4. focal neurological deficit(s) that may or may not be transient (10, 11).
A frequent consequence of TBI is chronic hypopituitarism, defined as a deficiency in one or more pituitary hormone axes. Since 2000, more than 40 research papers have characterized chronic neuroendocrine deficiencies resulting from TBI, more than half of which reported a prevalence of 25–50% after TBIs from all causes. Although review articles often assert an association of the prevalence of hypopituitarism with the severity of the injury, multiple studies that included a range of TBIs from mild to severe have found no relationship between pituitary dysfunction and severity of TBI (12–21). Repetitive mild TBIs have been shown to result in a high prevalence of hypopituitarism in several studies (22–27).
We previously published preliminary data supporting the high prevalence of chronic hypopituitarism in US military veterans deployed to Iraq or Afghanistan who sustained one or more blast-related concussions compared to similarly deployed veterans without blast exposure (27). We have now extended our preliminary findings to a larger sample and have also examined the effects of chronic hypopituitarism on measures of mood, sleep quality, symptoms of posttraumatic stress disorder (PTSD), and cognitive functioning.
Materials and Methods
Participants and Sample Acquisition
The Congressionally Directed Medical Research Programs Concept Award that funded this study prohibited direct sampling of biological fluids from human participants and access to identifiable private information. Therefore, all plasma and serum samples, demographic, and blast exposure data used in the study were obtained from an established biorepository entitled “Alzheimer’s Disease Research Center Participant Registry and Sample Repository.” The VA Puget Sound Health Care System Institutional Review Board and the U.S. Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office approved the subject protocol with a waiver of informed consent. All participants whose samples were utilized had provided written informed consent to have their samples and data used in future research of this type. No direct intervention with any participant was allowed. These conditions precluded the use of provocative testing as a part of the screening procedure.
Plasma and serum specimens and demographic and testing data were obtained from the repository for 59 male veterans with documented hazardous duty experience in Iraq and/or Afghanistan with the US Armed Forces. Thirty-nine of these individuals (the mTBI group) had sustained at least one explosive blast-induced concussion. The remaining 20 veterans (deployment control or DC group) were exposed to similar deployment conditions but had not experienced a blast-related mTBI.
Blood samples from 95 healthy male community volunteers with no evidence or history of cognitive or functional decline and no history of military service or TBI were also retrieved from the repository. These samples were used only for the establishment of normative hormone concentration ranges using our assay methods.
Exclusion Criteria and Screening
Exclusion criteria for all participants included a history of TBI with LOC greater than 30 min; penetrating head wound; seizure disorder; insulin-dependent diabetes; current or past diagnosis of schizophrenia, other psychotic disorders, bipolar disorder, or dementia with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (28); or a DSM-IV diagnosis of alcohol or other substance abuse or dependence within the previous 3 months.
Screening for study eligibility included physical and neurological examinations. The Structured Clinical Interview for DSM-IV (SCID-IV) was used to screen for exclusionary mood, psychotic, anxiety, and substance abuse disorders (29).
Demographics and Military History
Demographic, military deployment history, and blast exposure information were collected from all participants at screening. Data collected included age, education (in years), race, body mass index (BMI), number of deployments to Iraq or Afghanistan, total deployment time (in months), number of blast exposures during deployment, and time since last blast exposure.
Blast exposure and mTBI histories were obtained from participants in a semi-structured clinical interview by two expert clinicians. Specific inquiries were made about total number of blast-related mTBIs in Iraq or Afghanistan and lifetime history of non-blast head injuries accompanied by acute symptoms of mTBI. Evaluations based on these interviews determined the assignment of the participants to mTBI or DC groups.
The Wechsler Test of Adult Reading (WTAR) and the Combat Experiences Questionnaire (CEQ) were also administered. The WTAR is a measure of premorbid intellectual functioning that is thought to be resilient to mTBI. The test provides estimates of both verbal IQ and the overall level of general cognitive and intellectual functioning (Full Scale IQ) (30, 31). The CEQ is an 18-item true/false questionnaire excerpted from Hoge et al. (32) addressing the frequency and severity of experiences that participants may have been exposed to during deployment (e.g., shooting or directing fire at the enemy; being attacked, or ambushed).
Hormone Measurement
Blood samples were collected between 9:00 a.m. and 10:00 a.m. from supine participants 30 or more min after the insertion of an intravenous catheter in an antecubital vein. Samples for determination of hormones in plasma were collected in chilled tubes containing ethylenediaminetetraacetic acid, placed on ice, and centrifuged at 4°C prior to removal of the plasma fraction. Blood samples for serum hormone quantification were collected in serum-separator tubes, allowed to clot at room temperature for 10 min, and centrifuged at 4°C to isolate serum. Serum and plasma samples were aliquoted and stored at −70°C. Eleven pituitary or target-organ hormones were measured in these samples. The type, source, and performance characteristics of the assay kits used for the measurements are shown in Table 1. Adrenocorticotropin (ACTH), cortisol, thyroid-stimulating hormone (TSH), oxytocin, and vasopressin concentrations were determined in plasma; free thyroxine, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, total testosterone, and insulin-like growth factor-I (IGF-I) were measured in serum. Measurements of plasma and urine osmolality, plasma and urine Na+, plasma K+, urine specific gravity (USG), blood urea nitrogen, creatinine, and glucose were used in determining functional vasopressin insufficiency.
Criteria for Identifying Pituitary Hormone Deficiencies
Concentration percentiles based on the lognormal distribution of each hormone in blood samples from 95 community control participants were calculated. Dysfunction in each of five anterior pituitary axes and two posterior pituitary axes were defined by specific percentile levels based on the consensus of criteria used in multiple published studies of pituitary dysfunction after TBI from all causes. Due to concerns about the reliability and sensitivity of the vasopressin assay, measures of urine and plasma osmolality (Posm) and electrolytes were the primary determinants of diabetes insipidus (DI) and syndrome of inappropriate antidiuretic hormone secretion. Measurement of serum IGF-I concentration was used for growth hormone deficiency (GHD) screening. Since IGF-I concentrations decline significantly with increasing age in adults, the lognormal distribution of IGF-I levels in the reference group was adjusted for age, and the IGF-I concentration of each of the TBI and DC subjects was plotted in relation to age to provide appropriate comparisons. The criteria for deficient (or excessive) hormone levels in each axis are shown in Table 2.
PTSD Evaluation
The Clinician-Administered PTSD Scale for DSM-IV (CAPS) (33) is a structured interview designed to make a categorical diagnosis of PTSD and to assess the frequency and intensity of 17 PTSD symptoms to yield continuous severity scores for each symptom and the disorder as a whole. Administration of the CAPS requires identification of an index traumatic event to serve as the basis for symptom inquiry. In the absence of an appropriate traumatic event the symptom severity questionnaire is not administered.
Symptom Self-Report Questionnaires
The PTSD Checklist-Military Version (PCL-M), a self-report inventory of the 17 DSM-IV symptoms that define PTSD, was used to assess individual PTSD symptoms (34). The Neurobehavioral Symptom Inventory (NSI) is a 22-item questionnaire designed to assess the presence and severity of common cognitive, emotional, sensory, and somatic postconcussive symptoms (35). The Patient Health Questionnaire-9 (PHQ-9) is the 9-item depression module of the Patient Health Questionnaire that corresponds with DSM-IV criteria for depression (36). The Pittsburgh Sleep Quality Index (PSQI) is a self-report questionnaire assessing sleep quality and disturbances over a 1-month time interval (37). The Alcohol Use Disorders Identification Test-Consumption enquires about frequency and quantity of typical alcohol consumption and the frequency of episodes of heavy drinking (38).
Cognitive Measures
Trail Making Test
The Trail Making Test (TMT) consists of two parts. Trails A is a measure of processing speed (visual search and motor speed skills). Trails B has an added set-shifting component and is widely regarded as a measure of executive functioning (39). The score on each part represents the amount of time required to complete the task. Normative scores, corrected for age, education, race, and gender, are reported as T-scores.
Ruff 2&7 Selective Attention Test
The Ruff 2&7 Test is a measure of visual sustained and selective attention. Participants identify and mark target digits that are intermixed with other number distractors or capital letter distractors. This measure yields age and education corrected Total Speed (total target numbers crossed out) and Total Accuracy T scores. This measure has been shown to be sensitive to the effects of mTBI (40).
Test of Memory Malingering (TOMM)
The TOMM was used to evaluate performance validity on the neuropsychological measures in this study (41). This measure involves two learning/recognition trials and an optional retention trial following a delay. Established cutoff scores were used to determine performance validity; if participants scored below these cutoffs, their neuropsychological test scores were not included in the analyses.
Statistical Analysis
Analysis of the difference between the proportion of participants with hypopituitarism in the DC and mTBI groups was performed with a modified “N-1” chi-squared test for 2 × 2 contingency tables (42, 43). Analysis of the difference in the number of individual hormonal abnormalities between the two groups was performed with the unequal variance t-test for independent samples (44). Analysis of the relationships of mTBI and hypopituitarism with demographic, military history, symptom self-report, and cognitive testing data were performed with two-way analysis of variance (ANOVA). One-way ANOVAs were used to analyze the differences in the scores among the four participant subgroups, DC-N, DC-HP, mTBI-N, and mTBI-HP, on each of the individual items of the NSI.
Results
Identification of Abnormal Plasma/Serum Hormone Concentrations
12 of the 39 mTBI participants (31%) and 3 of the 20 DC participants (15%) screened positive for one or more hormonal disorders. Of the 15 participants with abnormal hormone levels, 8, including 2 of the DC participants, had markedly low basal serum IGF-I levels consistent with GHD. The diagonal line in Figure 1 represents the age-adjusted 10th percentile of the IGF-I concentration of the community control reference sample [equivalent to an SD score (SDS) of −1.4] used as the cutoff level for identifying probable GHD (Table 2). Basal IGF-I concentrations of four of the eight (mTBI-E, mTBI-A, mTBI-B, and DC-A) participants who screened positive fell below an SDS of −2.0 relative to the age-adjusted mean.
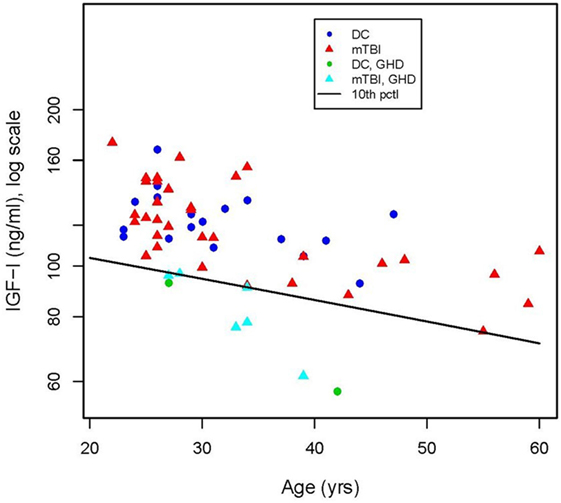
Figure 1. Concentration of insulin-like growth factor (IGF-I) in serum of deployment control (DC) (circles) and mild traumatic brain injuries (mTBI) (triangles) participants as a function of age. The criterion for a positive screen for growth hormone is an IGF-I level below the age-adjusted 10th percentile of IGF-I concentration (diagonal line) in the community control reference group. Serum IGF-I values of six of the mTBI group ( ) and two of the DC group (
) and two of the DC group ( ) fell below the cutoff line.
) fell below the cutoff line.
Hypogonadism was identified in two participants in the mTBI group. Two mTBI participants screened positive for hypothyroidism and one participant in each group screened positive for hyperprolactinemia. No participants screened positive for secondary adrenal insufficiency (sAI) (Table 3).
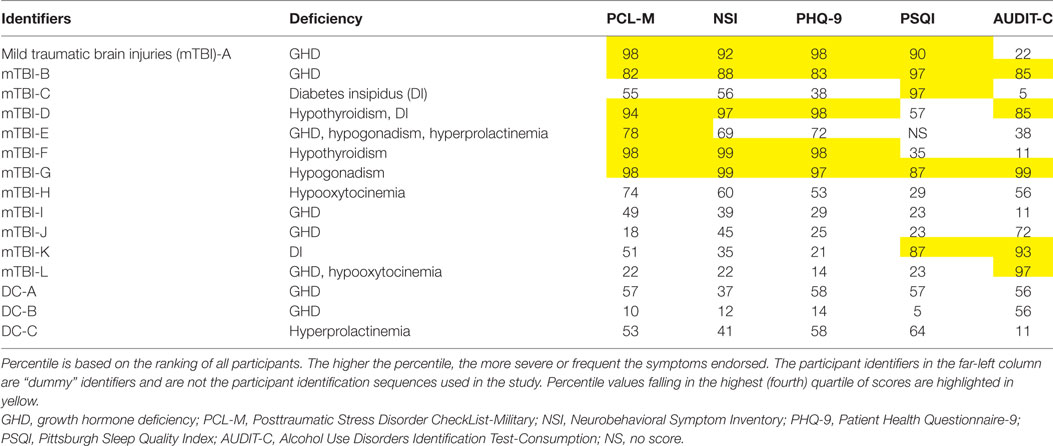
Table 3. Hormonal disorders and self-report questionnaire percentiles for individual participants who screened positive for hypopituitarism.
Five mTBI group individuals screened positive for posterior pituitary hormone deficiencies. Three were identified with DI based on multiple measures (including plasma and urine Na+, plasma and urine osmolality, and USG), and two screened positive for hypooxytocinemia. One individual, mTBI-E, screened positive for GHD, hypogonadism, and hyperprolactinemia. mTBI-D screened positive for hypothyroidism and DI, and TBI-L had hormone concentrations indicative of both GHD and hypooxytocinemia.
The proportion of individuals in each participant group who screened positive for one or more indices of hypopituitarism did not differ significantly (p = 0.094) when compared using the modified “N-1” chi-squared test for 2 × 2 contingency tables (42, 43).
When the differences between the two groups in the number of individual deficiencies were compared, a significant group difference was observed. The 39 members of the mTBI group screened positive for a total of 16 individual pituitary disorders (mean number of deficits per person = 0.413, SD = 0.715). Three of 20 (15%) DC participants showed evidence of a single pituitary impairment (mean = 0.15, SD = 0.366). There was a significant difference in total number of deficiencies between the groups by t-test of independent samples with unequal variances (t = 1.85, df = 56.99, p = 0.035) (44).
Relationships of mTBI and Hypopituitarism to Demographics and Military History
After hormonal screening, the mTBI and DC groups were divided into subgroups based on the presence or absence of hypopituitarism. The resulting four groups are: deployment controls with (DC-N) and without (DC-HP) normal pituitary function, and mTBI group members with (mTBI-N) and without (mTBI-HP) normal pituitary function. Differences in demographics, deployment history, and blast exposure of the subgroups were analyzed with two-way ANOVA (Table 4). At the time of study enrollment, the four groups of veteran participants did not differ significantly in age, education, BMI, number of deployments, total months deployed, or time since last the blast-related concussion. There also were no significant differences among subgroup scores on the WTAR. There were, however, significant group differences in combat experiences endorsed on the CEQ. Both mTBI (F = 90.9, df = 1, p < 0.0001) and hypopituitarism (F = 4.21, df = 1, p < 0.05) were significantly associated with increased numbers of combat experiences.
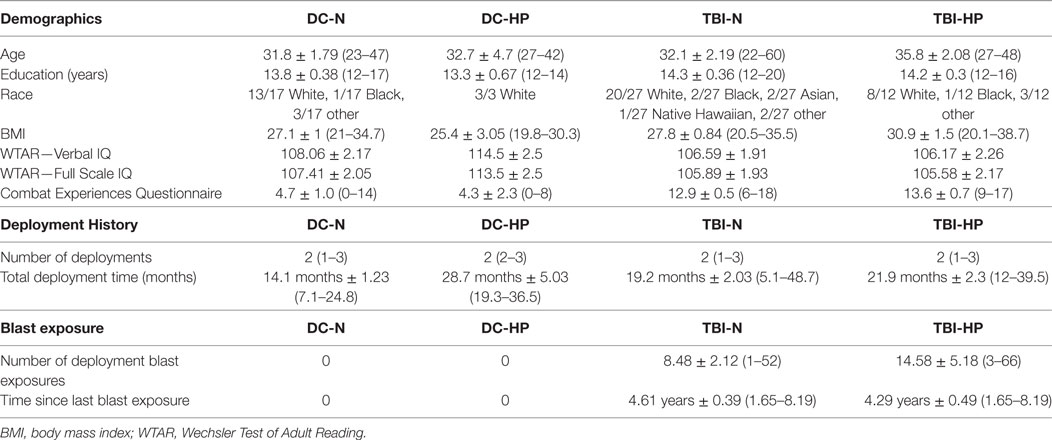
Table 4. Subgroup means ± SEM and ranges ( ) for demographic characteristics, deployment history, and blast exposure of study participants.
PTSD Diagnosis
Two of 17 participants (11.8%) in the DC-N group, 1 of 3 (33.3%) in the DC-HP group, 13 of 26 (50.0%, one unscored) in the mTBI-N group, and nine of 12 (75.0%) in the mTBI-HP group were clinically diagnosed with PTSD with the CAPS. There was no significant difference on the total CAPS score between the mTBI-N and mTBI-HP groups (Table 5) (33). The scores of the two members of the DC-N group and one of the DC-HP group who were diagnosed with PTSD were not entered in the table because the Ns were too small to include in the ANOVA.
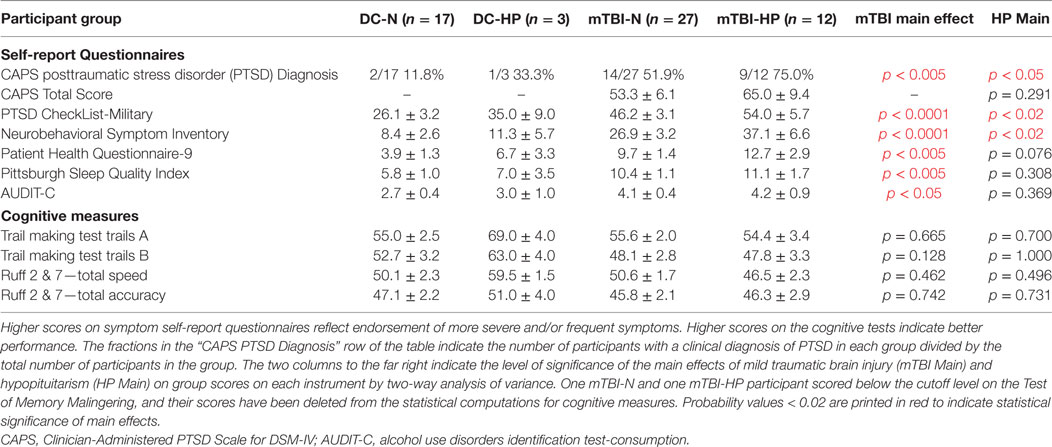
Table 5. Means and SEMs of participant group scores on symptom self-report questionnaires and cognitive instruments.
There were significant main effects of mTBI on each of five self-report questionnaires by two-way ANOVA (Table 5) and significant main effects of hypopituitarism on two of the questionnaires: those measuring PTSD symptoms and postconcussive symptoms. The mean score of the mTBI-HP group was numerically highest of the four groups on the total score of each of the self-report questionnaires.
Scores and percentile rankings on each of the self-report questionnaires were examined for individuals who screened positive for hypopituitarism to assess whether specific neuroendocrine disorders might be uniquely associated with extreme scores on a particular questionnaire (Table 3). Due to the small number of individuals in each category, it is not possible to come to any definitive conclusions. However, some interesting patterns appeared when percentiles were examined. For example, each of the two veterans who screened positive for hypothyroidism scored in the fourth quartile of the self-report questionnaires assessing PTSD symptoms, depression symptoms, postconcussive symptoms, and current alcohol use symptoms. One of the two participants reported sleep inefficiency in the fourth quartile. Of a total of eight participants in the mTBI-HP and DC-HP groups that screened positive for GHD, only two in the mTBI group, mTBI-A and mTBI-B, had scores in the fourth quartile on more than one symptom self-report questionnaire. The IGF-I serum concentrations of both fell more than 2 SDS below the age-adjusted mean. No members of the DC-HP group scored in the fourth quartile of any self-report questionnaires.
Significant group effects were found on 15 of 22 of the individual items on the NSI by one-way ANOVA. The mean score of the mTBI-HP group was the highest on all 22 of the items, as illustrated in Figure 2. The highest scores of the mTBI-HP group on the individual components of the NSI were on the “forgetfulness,” “difficulty sleeping,” and “irritability” items.
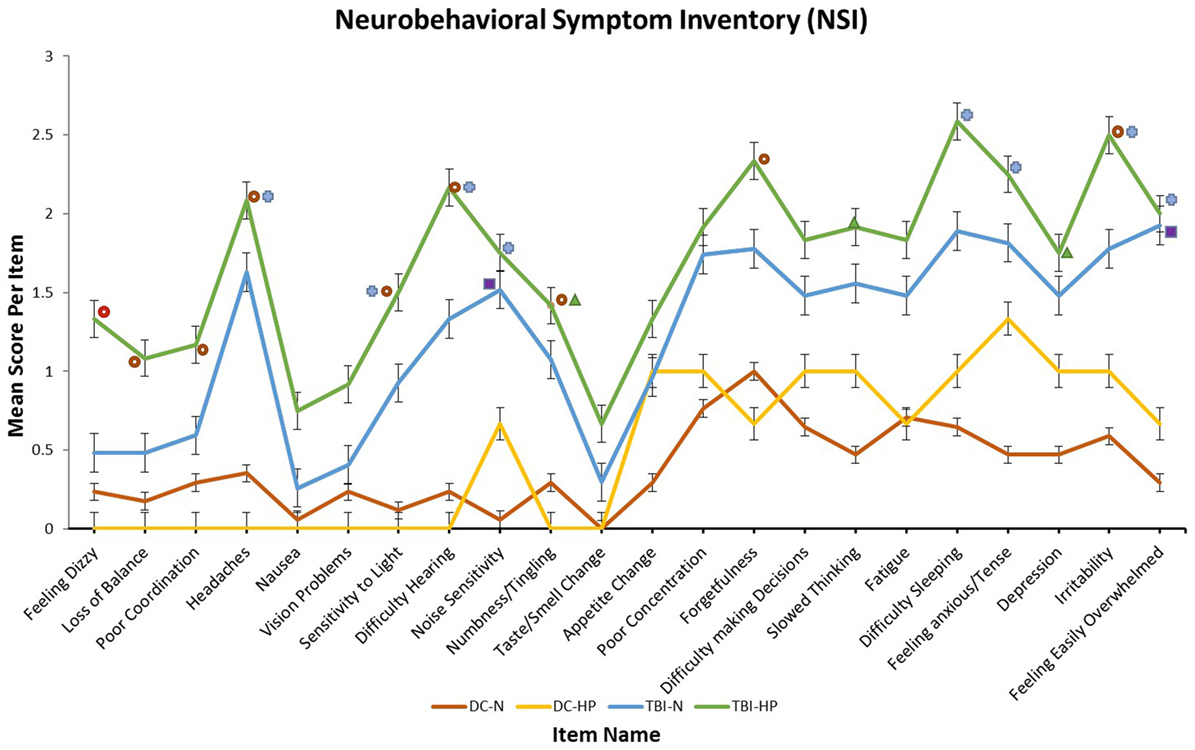
Figure 2. Mean scores of each participant group on each item of the neurobehavioral symptom inventory. T-bars indicate SEMs. The higher the score, the greater the frequency or severity of the symptom endorsed. All symbols indicate a significant main effect of participant group on that self-report item by one-way analysis of variance (ANOVA). Significant group effects were found by one-way ANOVA on 15 of 22 items. Each symbol represents a specific result of post hoc Tukey HSD tests.  No significant paired comparisons by Tukey HSD.
No significant paired comparisons by Tukey HSD.  Significant difference between mTBI-HP and DC-N by Tukey HSD.
Significant difference between mTBI-HP and DC-N by Tukey HSD.  Significant difference between mTBI-HP and DC-HP by Tukey HSD.
Significant difference between mTBI-HP and DC-HP by Tukey HSD.  Significant difference between mTBI-N and DC-HP by Tukey HSD.
Significant difference between mTBI-N and DC-HP by Tukey HSD.
Cognitive Measures
No significant participant group differences were found by two-way ANOVA of the total scores of any of the cognitive measures used in the study. Neither mTBI nor hypopituitarism had a measurable influence on the limited number of cognitive domains explored with these instruments, i.e., processing speed (TMT: trails A–T score), mental flexibility (TMT: trails B–T score), or selective attention (Ruff 2&7: total speed T score, total accuracy T score) (Table 5). All participants but two scored above the published cutoff for performance validity on the TOMM, suggesting that their performance on neuropsychological measures was an accurate reflection of their current cognitive abilities. One mTBI-N and one mTBI-HP participant scored below the cutoff level, and their scores have been deleted from the statistics for cognitive measures shown in Table 5.
Discussion
Our findings support the hypothesis that the prevalence of chronic posttraumatic hypopituitarism (PTHP) consequent to blast-related mTBI in US Armed Forces veterans is in accord with that reported in studies of pituitary dysfunction in the general population after TBI from all causes. Twelve of 39 (31%) deployed veterans who sustained blast-related concussions and three veterans in the DC group screened positive for hypopituitarism. A wide range of values (from 5 to 90%) for the prevalence of PTHP in civilians has been reported in 40-plus studies since 2000 with most finding a range of 25–50% (27, 45). The large variance is due to differences in the populations sampled, injury severity, time since injury, hormone measurement methods, and screening and clinical diagnostic criteria (46, 47). We employed clearly defined screening criteria calculated from the distribution of basal morning concentrations of each hormone in a reference population.
The hormone concentration measurements obtained in this study are valuable as screening assessments but cannot be viewed as diagnostic in the absence of provocative testing and/or clinical evaluation. They can, however, serve to direct the focus of clinical appraisal by identifying individuals most likely to suffer from clinically significant deficits. Hypogonadism, hypothyroidism, and lactotroph dysfunction can be provisionally identified by measuring basal hormone levels. However, central DI resulting from vasopressin deficiency depends upon determination of USG, electrolyte concentrations, urine and Posm, and performance of a water deprivation test for adequate screening. Although measurement of basal IGF-I, ACTH, and cortisol levels are helpful screening tools, diagnosis of GHD and sAI require provocative testing for accurate diagnosis. Since this study was restricted to the use of banked samples, provocative testing was not possible.
The finding that three of the participants in the DC group screened positive for an endocrine disorder—two for GHD and one for hyperprolactinemia—was not unexpected. First, there is a possibility that these individuals had sustained an unrecognized mTBI in their past. The DC participants whose blood samples and data were drawn from the repository for the study had undergone thorough screening and detailed interviews about their TBI history at the time of sample collection and denied having experienced a concussion. However, given the relative lack of knowledge about concussions and the unconcern about “getting your bell rung” prevalent 20 or more years ago, when these veterans were growing up, the existence of prior mTBIs from impact cannot be ruled out absolutely in either group. However, the study’s focus was on blast-related mTBI, the mechanisms of which differ substantially from those of impact mTBI (48, 49), and the screening process eliminated the possibility of prior blast-related concussions in the DC group.
Second, as stated above, hormonal screening does not provide conclusive evidence of clinically significant deficiencies in the absence of provocative testing and/or evaluation of potential symptoms. Also, the use of specific concentration cutoff criteria to identify deficiencies does not take into account relevant variables such as time of day, age, gender, body weight, or fasting state. Participant DC-C screened positive for hyperprolactinemia by exceeding our normal prolactin concentration range by only a fraction of a percentile, clearly an insufficient basis for a clinical diagnosis of hyperprolactinemia.
As is characteristic of previous studies of PTHP, we found that positive screens for GHD occurred more frequently than those for any other pituitary hormone deficiency. Single daytime measurements of serum GH are not valid indicators of somatotroph function or daily GH release. Approximately 75% of GH secretion occurs during nighttime sleep, and concentrations are markedly low during the day, with short, irregularly spaced pulses of secretion occurring at long intervals (50). However, GH-stimulated hepatic production of IGF-I can be a valuable predictor of GHD. Significantly low age-adjusted levels of IGF-I are notably indicative of GHD, but the presence of normal or elevated IGF-I levels does not preclude the diagnosis (51–56).
The diagnostic accuracy of measuring IGF-I concentrations as an alternative to provocative testing to recognize GHD has been compared with receiver operating characteristic (ROC) analysis. Corneli et al. reported that the best pair of highest sensitivity (96.6%) and specificity (74.6%) for identifying GHD was obtained with an IGF-I cutoff of −1.3 SDS (57), whereas Maghnie et al. found the best combination of sensitivity (77%) and specificity (100%) with a cutoff of −1.7 SDS (58). In the current study, an IGF-I concentration cutoff of −1.4 SDS relative to the age-adjusted means of the reference sample was used to determine a positive screen for GHD. The high specificity of IGF-I measurements reduces the likelihood of false positives, but the low sensitivity of the measurements suggests that some participants with GHD may not have been identified.
Insufficient GH secretion in adults has been shown to have negative effects in several cognitive domains and to be associated with numerous behavioral symptoms (17, 59, 60). These include reduced physical mobility, fatigue, sleep difficulties, depression, social isolation, low sexual drive, lowered metabolic rate, and reduced aerobic capacity (61, 62). Poor quality of life is also strongly associated with adult GHD, especially in terms of vitality and energy (63–66). Adult GHD results in reduced lean body mass, lipidemia, and increased adiposity. Even partial GHD is associated with adverse lipid profiles and early atherosclerosis in adult patients (67).
The mTBI group included two veteran participants who were considered hypogonadal based on our criteria: a total testosterone concentration less than the fifth percentile of the reference sample together with an LH or FSH level below the 10th percentile reference level. Hypogonadism has deleterious effects beyond those on fertility, psychosexual function, and general well-being. Male testosterone deficits are associated with muscle weakness, reduced lean body mass, decreased energy and motivation, impaired exercise tolerance, and premature mortality secondary to cardiovascular disease (68, 69).
One mTBI participant, mTBI-E, exhibited a highly elevated concentration of serum prolactin, 2.5 times higher than the next highest concentration measured in either group. Hyperprolactinemia is causally associated with hypogonadism through attenuation of LH and FSH secretion and desensitization of gonadal LH and FSH receptors. Reduction of FSH levels by excessive prolactin secretion also gives rise to hypoactive sexual desire and erectile dysfunction. The symptoms of hyperprolactinemia include gynecomastia and erectile dysfunction in men, irregular menses in women, and decreased libido, infertility, galactorrhea, and osteoporosis in both sexes (70, 71).
Prolactin is the only anterior pituitary hormone for which secretion is under primarily inhibitory control. Dopamine tonically suppresses prolactin secretion, and diminishment of this inhibition results in high levels of circulating prolactin. Antipsychotic medications that act as antagonists at dopamine D2 receptors may induce hyperprolactinemia (70). Participant mTBI-E had been taking quetiapine, a so-called dopamine-sparing antipsychotic (72). Though less likely to elevate prolactin levels than other antipsychotics (e.g., haloperidol and risperidone) hyperprolactinemia prevalence rates of up to 29% have been reported in association with its use (73, 74).
Participant mTBI-E had also been taking prazosin, an α-1 adrenoceptor antagonist. Norepinephrine, as well as dopamine, has been shown to inhibit prolactin secretion in sheep via pituitary α-1 receptors both in vivo and in vitro (75). Use of quetiapine and/or prazosin may have been responsible for the elevation of prolactin levels in this individual. Participant DC-C, who was found to have a dopamine level fractionally above the 95th percentile of the reference group, reported taking only natural supplements.
Two veterans in the mTBI group screened positive for central hypothyroidism, and no members of either group were found with sAI. TSH and ACTH deficiencies have been less frequently reported in previous studies of pituitary dysfunction after TBI than those of gonadotropins or GH (66, 76, 77). This outcome may be due in part to the anatomical location of thyrotrophs and corticotrophs in the pituitary’s protected median wedge. Vascular input to this region is carried by both long hypophyseal portal vessels and by the inferior hypophyseal artery via the short hypophyseal portal vessels. In contrast, GH-secreting somatotrophs are more susceptible to damage because they are situated in the gland’s exposed lateral wings and are almost exclusively dependent on the long hypophyseal portal vessels for their blood supply. Gonadotrophs are distributed throughout the anterior pituitary (78).
A large majority of studies of chronic PTHP have focused solely on anterior pituitary hormonal disorders. Of those studies that have included investigations of the deleterious effects of TBI on posterior pituitary function, most have reported the existence of disorders related to that lobe as well, the most common being DI (15, 22, 66, 77, 79–81).
In the current study, in addition to the 12 participants from both groups who screened positive for anomalous anterior pituitary hormone levels, five veterans with mTBI (including one growth hormone deficient and one hypothyroid individual) screened positive for posterior pituitary hormone deficiencies. Repeated assays for vasopressin gave inconsistent results and the data were deemed to be unreliable. A positive screen for DI was defined by a combination of USG less than or equal to 1.005, urine osmolality less than 200 mOsm/kg, and normal Posm.
Oxytocin levels below the fifth percentile threshold of the reference group were found in plasma of mTBI-H and mTBI-L. Apart from its important role in parturition and lactation, there is no strong evidence for the clinical significance of oxytocin deficiency. However, many animal and human studies have provided evidence for the positive association of oxytocin levels with behavior and attitudes related to social interactions such as maternal and romantic bonding, attenuation of stress responses, and mediation of social support (82, 83). Deficiencies in oxytocin appear to be associated with psychiatric conditions involving deficits in social behavior including autism spectrum disorders and schizophrenia (84–86). Although findings in the literature lack consistency, recent research on both central and peripheral actions of oxytocin have identified potential relationships with mood and anxiety (87), learning and memory (88), improvement of wound healing (89), modulation of inflammatory responses (90, 91), neuroprotection (89, 91, 92), regulation of food intake and body weight (93), and reduction of pain sensitivity (94, 95).
There was no significant difference between the numbers of blast exposures sustained by the two mTBI subgroups during Iraq/Afghanistan deployment (Table 4). Two-way ANOVA revealed significant main effects of both mTBI and hypopituitarism on the scores of the four groups on self-report questionnaires measuring PTSD (PCL-M), and postconcussive symptoms (NSI). This finding suggests an independent effect of hypopituitarism on behavioral measures.
In another study currently in progress, the blast-related concussion histories of the two mTBI groups are similar to one another, with the mTBI-N group tending to be higher than the mTBI-HP group in number of deployment mTBIs (96). Nonetheless, the TBI-HP group endorses significantly greater symptom frequency and severity than the TBI-N group on the PCL-M, NSI, PHQ-9, and the PSQI described above, as well as the Quality of Life Assessment of GHD in Adults (QoL-AGHDA) (97), and the Fatigue Severity Scale (96, 98). These preliminary findings support the hypothesis that mTBI-induced hypopituitarism has an additive effect in increasing neurobehavioral symptomatology beyond that of mTBI alone. There is an expanding literature about the wide-ranging and severe consequences of repetitive concussions (22–26), but there is thus far no evidence from our studies that the number of concussions is related to the prevalence of hypopituitarism.
There have been very few published studies of PTHP after blast-related concussion to date (21, 27, 99, 100) other than our preliminary report. Baxter and colleagues found that six of 19 (32.0%) Afghanistan-deployed male United Kingdom soldiers with moderate or severe blast-related TBI had anterior pituitary dysfunction, compared to one of 39 (2.6%) age- and gender-matched civilian control subjects with moderate-to-severe non-blast TBI (99). In another study, 5 of 20 veterans with mTBI sustained during combat (85% from explosive blast) had sub-threshold GH responses to glucagon administration (100). A study using an integrated structural magnetic resonance imaging protocol examined 834 military service members with TBI. The participants were diagnosed with primarily chronic (mean, 1,381, median, 888, days after injury), blast-related (84%), mild (92%) TBI. The results showed that 29.0% of military TBI participants had pituitary abnormalities compared with only 2.4% in a group of 42 control participants. The rates included all pituitary abnormalities noted in structural images, both before and after administration of contrast agent (21).
There is significant overlap in the symptomology of persistent postconcussive (PPCS) and PTHP, and similarity of both to PTSD. This congruity of symptoms increases the difficulty of deciphering the etiology, progression, and identifiable differences among the conditions that are essential for successful treatment, recovery, and rehabilitation (90). Lack of recognition of pituitary dysfunction may be conducive to diminished quality of life, fatigue, cognitive deficits, sleep difficulties, sexual dysfunction, psychiatric and behavioral disorders such as anxiety, irritability, depression, and social isolation, as well as deleterious modifications in body composition and metabolism leading to increased cardiovascular mortality. Unlike PTSD and PPCS, PTHP is often readily treatable once identified, and its symptoms may often be reversed or attenuated with appropriate hormone replacement therapy.
Routine endocrine evaluation of hypopituitarism after brain injury has been advocated by several investigators studying PTHP (25, 53, 76, 77, 80, 101–108). A recent review of the literature stated that because “many of the symptoms of hypopituitarism are similar to those of TBI, it is important to make clinicians caring for combat veterans aware of its occurrence … All patients who had a TBI of any severity, should undergo baseline hormonal evaluation” (109). The results of this study further support the need for endocrine evaluation in military personnel and veterans with a history of blast-related mTBI who are currently reporting postconcussive symptoms.
Ethics Statement
The Congressionally Directed Medical Research Programs Concept Award that funded this study prohibited direct sampling of biological fluids from human participants and access to identifiable private information. Therefore, all plasma and serum samples, demographic, and blast exposure data used in the study were obtained from an established biorepository entitled “Alzheimer’s Disease Research Center Participant Registry and Sample Repository.” The VA Puget Sound Health Care System Institutional Review Board and the US Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office approved the subject protocol with a waiver of informed consent. All participants whose samples were utilized had consented to have their samples and data used in future research of this type. No direct intervention with any participant was allowed. These conditions precluded the use of provocative testing as a part of the screening procedure.
Author Contributions
CW, EP, and KP contributed to the conception and design of the study. AU wrote the first draft of the manuscript. CW and JS wrote the remaining drafts and final manuscript. JS and AU compiled and organized the data. JS performed statistical analyses and literature searches and prepared the tables and figures. EC and CS performed the hormone assays and compiled the hormone data. All authors contributed to manuscript revision and read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thank you to Jane B. Shofer for statistical consultation and advice, Carol Xiang for database management, Madeleine Werhane for data entry and compilation, and Hollie Holmes for administrative support.
Funding
United States Department of Defense Congressionally Directed Medical Research Programs Concept Award Number W81XWH-11-1-0278 and United States Department of Veterans Affairs (USDVA) Rehabilitation Research and Development Service Merit Award #5 I01 RX 000509 to CW, USDVA Clinical Science Research and Development Service Career Development Award #IK2CX000516 to KP, USDVA Rehabilitation Research and Development Service Merit Award #B77421 to EP, the Geriatric Research, Education and Clinical Center, and the Research and Development Service of the VA Puget Sound Health Care System; the VA Northwest Network Mental Illness Research, Education and Clinical Center; and the University of Washington Alzheimer’s Disease Research Center NIA AG05136.
Abbreviations
ACTH, adrenocorticotropin; AUDIT-C, alcohol use disorders identification test-consumption; ACRM, American Congress of Rehabilitation Medicine; ANOVA, analysis of variance; BUN, blood urea nitrogen; BMI, body mass index; CAPS, clinician-administered PTSD scale for DSM-IV; CEQ, combat experiences questionnaire; DC, deployment control; DI, diabetes insipidus; DSM-IV, diagnostic and statistical manual of mental disorders, fourth edition; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; EDTA, ethylenediaminetetraacetic acid; FSH, follicle-stimulating hormone; GHD, growth hormone deficiency; IRMA, immunoradiometric assay; IGF-I, insulin-like growth factor-I; LOC, loss of consciousness; LH, luteinizing hormone; mTBI, mild traumatic brain injuries; NSI, neurobehavioral symptom inventory; PHQ-9, patient health questionnaire-9; PCTL, percentile; PPCS, persistent postconcussive; PSQI, Pittsburgh Sleep Quality Index; Posm, plasma osmolality; PTHP, posttraumatic hypopituitarism; PTSD, posttraumatic stress disorder; PCL-M, PTSD checklist-military version; ROC, receiver operating characteristic; RIA, radioimmunoassay; sAI, secondary adrenal insufficiency; SDS, SD score; SIADH, syndrome of inappropriate antidiuretic hormone secretion; TSH, thyroid-stimulating hormone; TMT, trail making test; Uosm, urine osmolality; USG, urine specific gravity; WTAR, Wechsler test of adult reading.
References
1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths – United States, 2007 and 2013. MMWR Surveill Summ (2017) 66:1–18. doi:10.15585/mmwr.ss6609a1
2. Voss JD, Connolly J, Schwab KA, Scher AI. Update on the epidemiology of concussion/mild traumatic brain injury. Curr Pain Headache Rep (2015) 19(7):32. doi:10.1007/s11916-015-0506-z
3. Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, MacGregor AJ, et al. Injuries from combat explosions in Iraq: injury type, location, and severity. Injury (2012) 43(10):1678–82. doi:10.1016/j.injury.2012.05.027
4. Hoencamp R, Vermetten E, Tan ECTH, Putter H, Leenen LPH, Hamming JF. Systematic review of the prevalence and characteristics of battle casualties from NATO coalition forces in Iraq and Afghanistan. Injury (2014) 45(7):1028–34. doi:10.1016/j.injury.2014.02.012
5. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil (2006) 21(5):398–402. doi:10.1097/00001199-200609000-00004
6. Powell JM, Ferraro JV, Dikmen SS, Temkin NR, Bell KR. Accuracy of mild traumatic brain injury diagnosis. Arch Phys Med Rehabil (2008) 89(8):1550–5. doi:10.1016/j.apmr.2007.12.035
7. Llewellyn T, Burdette GT, Joyner AB, Buckley TA. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin J Sport Med (2014) 24(1):76–9. doi:10.1097/01.jsm.0000432853.77520.3d
8. Meehan WP III, Mannix RC, O’Brien MJ, Collins MW. The prevalence of undiagnosed concussions in athletes. Clin J Sport Med (2013) 23(5):339–42. doi:10.1097/JSM.0b013e318291d3b3
9. Chase RP, Nevin RL. Population estimates of undocumented incident traumatic brain injuries among combat-deployed US military personnel. J Head Trauma Rehabil (2015) 30(1):E57–64. doi:10.1097/htr.0000000000000061
10. Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil (1993) 8(3):86–7. doi:10.1097/00001199-199309000-00010
11. Menon DK, Schwab K, Wright DW, Maas AI. Int interagency initiative, common position statement: definition of traumatic brain injury. Arch Phys Med Rehabil (2010) 91(11):1637–40. doi:10.1016/j.apmr.2010.05.017
12. Krewer C, Schneider M, Schneider HJ, Kreitschmann-Andermahr I, Buchfelder M, Faust M, et al. Neuroendocrine disturbances one to five or more years after traumatic brain injury and aneurysmal subarachnoid hemorrhage: data from the German database on hypopituitarism. J Neurotrauma (2016) 33(16):1544–53. doi:10.1089/neu.2015.4109
13. Rosario ER, Aqeel R, Brown MA, Sanchez G, Moore C, Patterson D. Hypothalamic-pituitary dysfunction following traumatic brain injury affects functional improvement during acute inpatient rehabilitation. J Head Trauma Rehabil (2013) 28(5):390–6. doi:10.1097/HTR.0b013e318250eac6
14. Kozlowski AJ, Pretz CR, Dams-O’Connor K, Kreider S, Whiteneck G. An introduction to applying individual growth curve models to evaluate change in rehabilitation: a national institute on disability and rehabilitation research traumatic brain injury model systems report. Arch Phys Med Rehabil (2013) 94(3):589–96. doi:10.1016/j.apmr.2012.08.199
15. Aimaretti G, Ambrosio MR, Di Somma C, Gasperi M, Cannavo S, Scaroni C, et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab (2005) 90(11):6085–92. doi:10.1210/jc.2005-0504
16. Schneider HJ, Schneider M, Saller B, Petersenn S, Uhr M, Husemann B, et al. Prevalence of anterior pituitary insufficiency 3 and 12 months after traumatic brain injury. Eur J Endocrinol (2006) 154(2):259–65. doi:10.1530/eje.1.02071
17. Popovic V, Pekic S, Pavlovic D, Maric N, Jasovic-Gasic M, Djurovic B, et al. Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. J Endocrinol Invest (2004) 27(11):1048–54. doi:10.1007/BF03345308
18. Agha A, Rogers B, Sherlock M, O’Kelly P, Tormey W, Phillips J, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab (2004) 89(10):4929–36. doi:10.1210/jc.2004-0511
19. Aimaretti G, Ambrosio MR, Di Somma C, Fusco A, Cannavo S, Gasperi M, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) (2004) 61(3):320–6. doi:10.1111/j.1365-2265.2004.02094.x
20. Silva PPB, Bhatnagar S, Herman SD, Zafonte R, Klibanski A, Miller KK, et al. Predictors of hypopituitarism in patients with traumatic brain injury. J Neurotrauma (2015) 32(22):1789–95. doi:10.1089/neu.2015.3998
21. Riedy G, Senseney JS, Liu W, Ollinger J, Sham E, Krapiva P, et al. Findings from structural MR imaging in military traumatic brain injury. Radiology (2016) 279(1):207–15. doi:10.1148/radiol.2015150438
22. Kelly DF, Chaloner C, Evans D, Mathews A, Cohan P, Wang C, et al. Prevalence of pituitary hormone dysfunction, metabolic syndrome, and impaired quality of life in retired professional football players: a prospective study. J Neurotrauma (2014) 31(13):1161–71. doi:10.1089/neu.2013.3212
23. Orrison WW, Hanson EH, Alamo T, Watson D, Sharma M, Perkins TG, et al. Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma (2009) 26(5):689–701. doi:10.1089/neu.2008.0636
24. Tanriverdi F, Unluhizarci K, Coksevim B, Selcuklu A, Casanueva FF, Kelestimur F. Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin Endocrinol (Oxf) (2007) 66(3):360–6. doi:10.1111/j.1365-2265.2006.02737.x
25. Tanriverdi F, Ulutabanca H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. Three years prospective investigation of anterior pituitary function after traumatic brain injury: a pilot study. Clin Endocrinol (Oxf) (2008) 68(4):573–9. doi:10.1111/j.1365-2265.2007.03070.x
26. Kelestimur F, Tanriverdi F, Atmaca H, Unluhizarci K, Selcuklu A, Casanueva FF. Boxing as a sport activity associated with isolated GH deficiency. J Endocrinol Invest (2004) 27(11):Rc28–32. doi:10.1007/bf03345299
27. Wilkinson CW, Pagulayan KF, Petrie EC, Mayer CL, Colasurdo EA, Shofer JB, et al. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front Neurol (2012) 3:11. doi:10.3389/fneur.2012.00011
28. American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press Inc (1994). p. 828–9.
29. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV (SCID). New York: Biometrics Research, New York State Psychiatric Institute (2002). p. 1–4.
30. Steward KA, Novack TA, Kennedy R, Crowe M, Marson DC, Triebel KL. The Wechsler test of adult reading as a measure of premorbid intelligence following traumatic brain injury. Arch Clin Neuropsychol (2017) 32(1):98–103. doi:10.1093/arclin/acw081
31. Venegas J, Clark E. Wechsler test of adult reading. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York (2011). p. 2693–4.
32. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med (2004) 351(1):13–22. doi:10.1056/NEJMoa040603
33. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician administered PTSD scale. J Trauma Stress (1995) 8(1):75–90. doi:10.1007/bf02105408
34. Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther (2001) 39(8):977–86. doi:10.1016/s0005-7967(00)00084-x
35. Cicerone KD, Kalmar K. Persistent postconcussion syndrome – the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil (1995) 10(3):1–17. doi:10.1097/00001199-199506000-00002
36. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 – validity of a brief depression severity measure. J Gen Intern Med (2001) 16(9):606–13. doi:10.1046/j.1525-1497.2001.016009606.x
37. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index – a new instrument for psychiatric practice and research. Psychiatry Res (1989) 28(2):193–213. doi:10.1016/0165-1781(89)90047-4
38. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C) – an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med (1998) 158(16):1789–95. doi:10.1001/archinte.158.16.1789
39. Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc (2006) 1(5):2277–81. doi:10.1038/nprot.2006.390
40. Cicerone KD, Azulay J. Diagnostic utility of attention measures in postconcussion syndrome. Clin Neuropsychol (2002) 16(3):280–9. doi:10.1076/clin.16.3.280.13849
42. Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med (2007) 26(19):3661–75. doi:10.1002/sim.2832
43. Richardson JT. The analysis of 2 × 1 and 2 × 2 contingency tables: an historical review. Stat Methods Med Res (1994) 3(2):107–33. doi:10.1177/096228029400300202
44. Ruxton GD. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behav Ecol (2006) 17(4):688–90. doi:10.1093/beheco/ark016
45. Tanriverdi F, Kelestimur F. Pituitary dysfunction following traumatic brain injury: clinical perspectives. Neuropsychiatr Dis Treat (2015) 11:1835–43. doi:10.2147/ndt.s65814
46. Kokshoorn NE, Wassenaar MJ, Biermasz NR, Roelfsema F, Smit JW, Romijn JA, et al. Hypopituitarism following traumatic brain injury: prevalence is affected by the use of different dynamic tests and different normal values. Eur J Endocrinol (2010) 162(1):11–8. doi:10.1530/eje-09-0601
47. Klose M, Stochholm K, Janukonyte J, Christensen LL, Cohen AS, Wagner A, et al. Patient reported outcome in posttraumatic pituitary deficiency: results from the Danish National Study on posttraumatic hypopituitarism. Eur J Endocrinol (2015) 172(6):753–62. doi:10.1530/eje-14-1069
48. Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front Neurol (2010) 1:151. doi:10.3389/fneur.2010.00151
49. Young L, Rule GT, Bocchieri RT, Walilko TJ, Burns JM, Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front Neurol (2015) 6:89. doi:10.3389/fneur.2015.00089
50. Van Cauter E, Kerkhofs M, Caufriez A, Van Onderbergen A, Thorner MO, Copinschi G. A quantitative estimation of growth hormone secretion in normal man: reproducibility and relation to sleep and time of day. J Clin Endocrinol Metab (1992) 74(6):1441–50. doi:10.1210/jcem.74.6.1592892
51. Juul A, Kastrup KW, Pedersen SA, Skakkebaek NE. Growth hormone (GH) provocative retesting of 108 young adults with childhood-onset GH deficiency and the diagnostic value of insulin-like growth factor I (IGF-I) and IGF-binding protein-3. J Clin Endocrinol Metab (1997) 82(4):1195–201. doi:10.1210/jcem.82.4.3892
52. Hadjadj S, Faure-Gerard C, Ragot S, Millet C, Duengler F, Torremocha F, et al. Diagnostic strategy for growth hormone deficiency: relevance of IGF-1 determination as a screening test. Ann Endocrinol (Paris) (2007) 68(6):449–55. doi:10.1016/j.ando.2007.08.004
53. Ho KK2007 GH Deficiency Consensus Workshop Participants. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol (2007) 157(6):695–700. doi:10.1530/eje-07-0631
54. Prodam F, Pagano L, Corneli G, Golisano G, Belcastro S, Busti A, et al. Update on epidemiology, etiology, and diagnosis of adult growth hormone deficiency. J Endocrinol Invest (2008) 31(9 Suppl):6–11.
55. Tanriverdi F, Agha A, Aimaretti G, Casanueva FF, Kelestimur F, Klose M, et al. Manifesto for the current understanding and management of traumatic brain injury-induced hypopituitarism. J Endocrinol Invest (2011) 34(7):541–3. doi:10.3275/7805
56. Zgaljardic DJ, Guttikonda S, Grady JJ, Gilkison CR, Mossberg KA, High WM Jr, et al. Serum IGF-1 concentrations in a sample of patients with traumatic brain injury as a diagnostic marker of growth hormone secretory response to glucagon stimulation testing. Clin Endocrinol (Oxf) (2011) 74(3):365–9. doi:10.1111/j.1365-2265.2010.03935.x
57. Corneli G, Di Somma C, Prodam F, Bellone J, Bellone S, Gasco V, et al. Cut-off limits of the GH response to GHRH plus arginine test and IGF-I levels for the diagnosis of GH deficiency in late adolescents and young adults. Eur J Endocrinol (2007) 157(6):701–8. doi:10.1530/eje-07-0384
58. Maghnie M, Aimaretti G, Bellone S, Bona G, Bellone J, Baldelli R, et al. Diagnosis of GH deficiency in the transition period: accuracy of insulin tolerance test and insulin-like growth factor-I measurement. Eur J Endocrinol (2005) 152(4):589–96. doi:10.1530/eje.1.01873
59. Falleti MG, Maruff P, Burman P, Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology (2006) 31(6):681–91. doi:10.1016/j.psyneuen.2006.01.005
60. Pavlovic D, Pekic S, Stojanovic M, Zivkovic V, Djurovic B, Jovanovic V, et al. Chronic cognitive sequelae after traumatic brain injury are not related to growth hormone deficiency in adults. Eur J Neurol (2010) 17(5):696–702. doi:10.1111/j.1468-1331.2009.02910.x
61. Mossberg KA, Masel BE, Gilkison CR, Urban RJ. Aerobic capacity and growth hormone deficiency after traumatic brain injury. J Clin Endocrinol Metab (2008) 93(7):2581–7. doi:10.1210/jc.2008-0368
62. Rosén T, Wirén L, Wilhelmsen L, Wiklund I, Bengtsson B-Å. Decreased psychological well-being in adult patients with growth hormone deficiency. Clin Endocrinol (Oxf) (1994) 40(1):111–6. doi:10.1111/j.1365-2265.1994.tb02452.x
63. Kelly DF, McArthur DL, Levin H, Swimmer S, Dusick JR, Cohan P, et al. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J Neurotrauma (2006) 23(6):928–42. doi:10.1089/neu.2006.23.928
64. Bushnik T, Englander J, Katznelson L. Fatigue after TBI: association with neuroendocrine abnormalities. Brain Inj (2007) 21(6):559–66. doi:10.1080/02699050701426915
65. Svensson J, Finer N, Bouloux P, Bevan J, Jonsson B, Mattsson AF, et al. Growth hormone (GH) replacement therapy in GH deficient adults: predictors of one-year metabolic and clinical response. Growth Horm IGF Res (2007) 17(1):67–76. doi:10.1016/j.ghir.2006.11.002
66. Bavisetty S, Bavisetty S, McArthur DL, Dusick JR, Wang C, Cohan P, et al. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery (2008) 62(5):1080–93; discussion 93–4. doi:10.1227/01.neu.0000325870.60129.6a
67. Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf) (2008) 69(3):347–58. doi:10.1111/j.1365-2265.2008.03292.x
68. Agha A, Thompson CJ. High risk of hypogonadism after traumatic brain injury: clinical implications. Pituitary (2005) 8(3):245–9. doi:10.1007/s11102-005-3463-4
69. Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, et al. Association between premature mortality and hypopituitarism. Lancet (2001) 357(9254):425–31. doi:10.1016/S0140-6736(00)04006-X
70. Ajmal A, Joffe H, Nachtigall LB. Psychotropic-induced hyperprolactinemia: a clinical review induced hyperprolactinemia: a clinical review. Psychosomatics (2014) 55(1):29–36. doi:10.1016/j.psym.2013.08.008
71. Capozzi A, Scambia G, Pontecorvi A, Lello S. Hyperprolactinemia: pathophysiology and therapeutic. Gynecol Endocrinol (2015) 31(7):506–10. doi:10.3109/09513590.2015.1017810
72. Carboni L, Negri M, Michielin F, Bertani S, Fratte SD, Oliosi B, et al. Slow dissociation of partial agonists from the D(2) receptor is linked to reduced prolactin release. Int J Neuropsychopharmacol (2012) 15(5):645–56. doi:10.1017/s1461145711000824
73. Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, et al. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry (2004) 65(11):1491–8. doi:10.4088/JCP.v65n1108
74. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs (2014) 28(5):421–53. doi:10.1007/s40263-014-0157-3
75. Lincoln G, Clarke I. Noradrenaline and dopamine regulation of prolactin secretion in sheep: role in prolactin homeostasis but not photoperiodism. J Neuroendocrinol (2002) 14(1):36–44. doi:10.1046/j.0007-1331.2001.00734.x
76. Blair JC. Prevalence, natural history and consequences of posttraumatic hypopituitarism: a case for endocrine surveillance. Br J Neurosurg (2010) 24(1):10–7. doi:10.3109/02688690903536637
77. Krahulik D, Zapletalova J, Frysak Z, Vaverka M. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J Neurosurg (2010) 113(3):581–4. doi:10.3171/2009.10.jns09930
78. Ben-Shlomo A, Melmed S. Hypothalamic regulation of anterior pituitary function. 3rd ed. In: Melmed S, editor. The Pituitary. London, England: Elsevier/Academic Press (2011). p. 21–47.
79. Klose M, Juul A, Struck J, Morgenthaler NG, Kosteljanetz M, Feldt-Rasmussen U. Acute and long-term pituitary insufficiency in traumatic brain injury: a prospective single-centre study. Clin Endocrinol (Oxf) (2007) 67(4):598–606. doi:10.1111/j.1365-2265.2007.02931.x
80. Leal-Cerro A, Flores JM, Rincon M, Murillo F, Pujol M, Garcia-Pesquera F, et al. Prevalence of hypopituitarism and growth hormone deficiency in adults long-term after severe traumatic brain injury. Clin Endocrinol (Oxf) (2005) 62(5):525–32. doi:10.1111/j.1365-2265.2005.02250.x
81. Kelly DF, Gonzalo IT, Cohan P, Berman N, Swerdloff R, Wang C. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a preliminary report. J Neurosurg (2000) 93(5):743–52. doi:10.3171/jns.2000.93.5.0743
82. Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol (2009) 30(4):548–57. doi:10.1016/j.yfrne.2009.05.005
83. Campbell A. Oxytocin and human social behavior. Pers Soc Psychol Rev (2010) 14(3):281–95. doi:10.1177/1088868310363594
84. Vanya M, Szucs S, Vetro A, Bartfai G. The potential role of oxytocin and perinatal factors in the pathogenesis of autism spectrum disorders-review of the literature. Psychiatry Res (2017) 247:288–90. doi:10.1016/j.psychres.2016.12.007
85. Simplicio MD, Harmer CJ. Oxytocin and emotion processing. J Psychopharmacol (2016) 30(11):1156–9. doi:10.1177/0269881116641872
86. Romano A, Tempesta B, Micioni Di Bonaventura MV, Gaetani S. From autism to eating disorders and more: the role of oxytocin in neuropsychiatric disorders. Front Neurosci (2016) 9:497. doi:10.3389/fnins.2015.00497
87. Naja WJ, Aoun MP. Oxytocin and anxiety disorders: translational and therapeutic aspects. Curr Psychiatry Rep (2017) 19(10):67. doi:10.1007/s11920-017-0819-1
88. Brambilla M, Manenti R, de Girolamo G, Adenzato M, Bocchio-Chiavetto L, Cotelli M. Effects of intranasal oxytocin on long-term memory in healthy humans: a systematic review. Drug Dev Res (2016) 77(8):479–88. doi:10.1002/ddr.21343
89. Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound heating. Psychoneuroendocrinology (2004) 29(8):1004–11. doi:10.1016/j.psyneuen.2003.10.003
90. Szeto A, Sun-Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab (2017) 312(3):E183–9. doi:10.1152/ajpendo.00346.2016
91. Lagraoui M, Sukumar G, Latoche JR, Maynard SK, Dalgard CL, Schaefer BC. Salsalate treatment fallowing traumatic brain injury reduces inflammation and promotes a neuroprotective and neurogenic transcriptional response with concomitant functional recovery. Brain Behav Immun (2017) 61:96–109. doi:10.1016/j.bbi.2016.12.005
92. Ceanga M, Spataru A, Zagrean A-M. Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neurosci Lett (2010) 477(1):15–8. doi:10.1016/j.neulet.2010.04.024
93. Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol Behav (2015) 152:438–49. doi:10.1016/j.physbeh.2015.05.023
94. Pfeifer AC, Ditzen B, Neubauer E, Schiltenwolf M. Effect of oxytocin on human pain perception. Schmerz (2016) 30(5):457–69. doi:10.1007/s00482-016-0164-z
95. Xin Q, Bai B, Liu W. The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochem Int (2017) 103:57–64. doi:10.1016/j.neuint.2016.12.021
96. Schultz JS, Burges DE, Colasurdo EA, Shofer JB, Wilkinson CW. Hypopituitarism caused by blast-related mTBI may result in PTSD-like symptoms. Abstracts of the Society for Behavioral Neuroendocrinology 21st Annual Meeting. Long Beach, CA (2017). 67 p.
97. McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, et al. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res (1999) 8(4):373–83. doi:10.1023/a:1008987922774
98. Krupp LB, Larocca NG, Muirnash J, Steinberg AD. The Fatigue Severity Scale – application to patients with multiple sclerosis and systemic lupus-erythematosus. Arch Neurol (1989) 46(10):1121–3. doi:10.1001/archneur.1989.00520460115022
99. Baxter D, Sharp DJ, Feeney C, Papadopoulou D, Ham TE, Jilka S, et al. Pituitary dysfunction after blast traumatic brain injury: the UK BIOSAP study. Ann Neurol (2013) 74(4):527–36. doi:10.1002/ana.23958
100. Ioachimescu AG, Hampstead BM, Moore A, Burgess E, Phillips LS. Growth hormone deficiency after mild combat-related traumatic brain injury. Pituitary (2015) 18(4):535–41. doi:10.1007/s11102-014-0606-5
101. Masel BE. Traumatic brain injury induced hypopituitarism: the need and hope of rehabilitation. Pituitary (2005) 8(3–4):263–6. doi:10.1007/s11102-006-6052-2
102. Schneider M, Schneider HJ, Stalla GK. Anterior pituitary hormone abnormalities following traumatic brain injury. J Neurotrauma (2005) 22(9):937–46. doi:10.1089/neu.2005.22.937
103. Urban RJ, Harris P, Masel B. Anterior hypopituitarism following traumatic brain injury. Brain Inj (2005) 19(5):349–58. doi:10.1080/02699050400004807
104. Powner DJ, Boccalandro C, Alp MS, Vollmer DG. Endocrine failure after traumatic brain injury in adults. Neurocrit Care (2006) 5(1):61–70. doi:10.1385/NCC:5:1:61
105. Behan LA, Agha A. Endocrine consequences of adult traumatic brain injury. Horm Res (2007) 68(Suppl 5):18–21. doi:10.1159/000110466
106. Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry (2008) 79(7):753–9. doi:10.1136/jnnp.2007.132837
107. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary (2010) 13(2):146–53. doi:10.1007/s11102-009-0215-x
108. Park KD, Kim DY, Lee JK, Nam HS, Park YG. Anterior pituitary dysfunction in moderate-to-severe chronic traumatic brain injury patients and the influence on functional outcome. Brain Inj (2010) 24(11):1330–5. doi:10.3109/02699052.2010.506863
Keywords: traumatic brain injury, blast, concussion, pituitary, military, posttraumatic stress disorder, growth hormone deficiency, veterans
Citation: Undurti A, Colasurdo EA, Sikkema CL, Schultz JS, Peskind ER, Pagulayan KF and Wilkinson CW (2018) Chronic Hypopituitarism Associated with Increased Postconcussive Symptoms Is Prevalent after Blast-Induced Mild Traumatic Brain Injury. Front. Neurol. 9:72. doi: 10.3389/fneur.2018.00072
Received: 30 November 2017; Accepted: 31 January 2018;
Published: 19 February 2018
Edited by:
Marco Sarà, San Raffaele Cassino, ItalyReviewed by:
Roberto Piperno, Physical Medicine and Rehabilitation, ItalyAkinobu Nakamura, Hokkaido University, Japan
Copyright: © 2018 Undurti, Colasurdo, Sikkema, Schultz, Peskind, Pagulayan and Wilkinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles W. Wilkinson, d2lsa2luc29AdXcuZWR1, Y2hhcmxlcy53aWxraW5zb25AdmEuZ292
†Present address: Arundhati Undurti, The Vancouver Clinic, Vancouver, WA, United States
 Arundhati Undurti1†
Arundhati Undurti1† Jaclyn S. Schultz
Jaclyn S. Schultz Kathleen F. Pagulayan
Kathleen F. Pagulayan Charles W. Wilkinson
Charles W. Wilkinson