- 1Department of Neurology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 2Institute of Brain Science, National Yang-Ming University, Taipei, Taiwan
- 3Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan
- 4Brain Research Center, National Yang-Ming University, Taipei, Taiwan
- 5Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan
- 6Faculty of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
- 7Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan
- 8Department of Radiology, National Yang-Ming University, Taipei, Taiwan
- 9Department of Radiology, Taipei Veterans General Hospital, Taipei, Taiwan
- 10Department of Radiology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 11Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan
Background: Migraine is frequently comorbid with restless legs syndrome (RLS), both displaying functional connectivity (FC) alterations in multiple brain networks, although the neurological basis of this association is unknown.
Methods: We performed resting-state functional magnetic resonance imaging and network-wise analysis of FC in migraine patients with and without RLS and healthy controls (CRL). Network-based statistics (NBS) and composite FC matrix analyses were performed to identify the patterns of FC changes. Correlation analyses were performed to identify associations between alterations in FC and clinical profiles.
Results: NBS results revealed that both migraine patients with and without RLS exhibited lower FC than CRL in the dorsal attention, salience, default mode, cingulo-opercular, visual, frontoparietal, auditory, and sensory/somatomotor networks. Further composite FC matrix analyses revealed differences in FC of the salience, default mode to subcortical and frontoparietal, auditory to salience, and memory retrieval networks between migraine patients with and without RLS. There was a trend toward a negative association between RLS severity and cross-network abnormalities in the default mode to subcortical network.
Discussion: Migraine patients with and without RLS exhibit disruptions of brain FC. Such findings suggest that these disorders are associated with differential neuropathological mechanisms and may aid in the future development of neuroimaging-driven biomarkers for these conditions.
Introduction
Migraine is a primary headache disorder affecting 10–20% of the general population, which occurs predominantly in women (female-to-male ratio, 2–3:1). It is characterized by recurrent headaches associated with moderate-to-severe pulsating pain and sensory hypersensitivity (1). Accumulating evidence suggests that restless legs syndrome (RLS) is a common comorbidity in patients with migraine (2–10). Furthermore, it has been reported that periodic limb movement disorders (including RLS) may influence the clinical presentation, severity, frequency, and treatment efficacy of migraine in children (6). RLS is a sensorimotor disorder characterized by a deeply unpleasant sensation in the legs when at rest, especially at or near bedtime, and motor restlessness that can only be relieved by voluntary movement. The prevalence of RLS in patients with migraine is higher than that in the general population (5, 10). Although some researchers have proposed that migraine and RLS share pathophysiological characteristics, such as abnormalities in dopaminergic or iron systems as well as genetic variations (2, 11), the neural mechanisms governing the comorbidity of migraine and RLS remain largely unknown.
Recent evidence suggests that disruption of coordinated activity between and within brain networks may be involved in the pathogenesis of various neuropsychological disorders (12). Resting-state functional magnetic resonance imaging (rs-fMRI) studies have suggested that the pathophysiology of migraine is associated with altered functional connectivity (FC) in several brain networks, such as the default mode network (DMN), visual, executive control, attention, and salience networks. These functional disturbances may also relate to the sensory, affective, and cognitive components of pain processing in migraine patients (13–15). In contrast, the presence of functional abnormalities in patients with RLS remains controversial. Previous studies have reported dysfunction of multiple brain networks such as the thalamic, sensory-motor, limbic/nociceptive networks, and the DMN in patients with RLS (16–19). Although previous evidence supports the existence of functional network disturbances in patients with migraine and RLS, it remains unknown whether these disturbances are associated with the comorbidity of migraine and RLS. Furthermore, most previous studies of migraine and RLS have examined connectivity with a single seed region or via pairwise coupling, which may fail to elucidate the role of such connections within the greater network. Moreover, these methods do not provide insight into how migraine with or without RLS (MIG w or w/o RLS) is associated with the restructuring of functional networks, particularly at the connectome level. In contrast, network-based statistics (NBS) provide a general framework for the characterization of specific network components, which may help identify whether and which subnetworks exhibit significant functional differences between patients with MIG w or w/o RLS (20). Such an understanding of the patterns of FC alterations between and within networks may aid in elucidating the neuropathological mechanisms underlying the comorbidity of migraine and RLS.
Therefore, to address these issues, we performed a comprehensive investigation of the whole-brain functional connectome to identify the patterns of FC changes in migraine patients with and without RLS. Using rs-fMRI, we aimed to demonstrate the association between connectivity patterns and diagnosis in such patients and to examine the following hypotheses: (1) brain FC is altered in migraine patients with or without RLS when compared to controls; (2) migraine patients with or without RLS show changes in FC patterns; and (3) changes in FC correlate with clinical variables.
Materials and Methods
Participants
The present study was approved by the Ethics Committee of Taipei Veterans General Hospital (TVGH) and Tri-Service General Hospital (TSGH) in Taiwan. All methods were carried out in accordance with the approved guidelines. All participants signed a written informed consent form after a full written and verbal explanation of the study. Patients with episodic migraine were recruited from the Headache Clinic at TVGH and TSGH. All participants met the diagnostic criteria for episodic migraine based on the third edition of the International Classification of Headache Disorders (21). None had concomitant primary or secondary headache disorders. Patients were further classified based on the presence or absence of a diagnosis of primary RLS, which was made in accordance with criteria outlined by the International Restless Legs Syndrome Study Group (IRLSSG) (22).
All patients underwent a detailed history, physical examination, laboratory testing, and electromyography to exclude secondary forms of RLS. No patients had a history of psychiatric symptoms, and none were treated with psychotropic medications, except for two patients with MIG w RLS taking a dopaminergic agent (ropinirole). Other clinical data such as sex, age, duration of RLS, duration of migraine, and sleep quality were recorded. RLS severity was assessed using the IRLSSG rating scale (23). Patients were asked to stop taking migraine-preventive medications (B-blocker or flunarizine) at least 2 days prior to imaging. All patients were symptoms free (migraine or RLS) for at least 2 days before the imaging scan. No acute migraine or RLS attacks occurred during the scanning sessions.
The healthy control (CRL) group was composed of volunteers without RLS or migraine matched for age, sex, and handedness. Control participants were recruited through community advertisements and hospital patient pools. Exclusion criteria for control participants included a family history of RLS or migraine, prior diagnosis of a primary or secondary headache disorder, and any chronic pain condition. Statistical analysis of demographic data was performed using SPSS software version 20 (SPSS, Chicago, IL, USA). Our study included 22 patients with migraine without idiopathic RLS (3 men, 19 women; mean age: 32.4 years; and age range: 20–54 years) and 22 patients with migraine with idiopathic RLS (2 men, 20 women; mean age: 33.0 years; and age range: 21–51 years). The control group was composed of 19 age- and sex-matched adults (2 men, 17 women; mean age: 33.3 years; and age range: 23–54 years) with no history of neurologic or psychiatric disease.
Data Collection
All images were collected at TVGH using a 3T MRI scanner (Discovery MR750; General Electric Healthcare, Milwaukee, WI, USA) equipped with an eight-channel phase array head coil. A whole-brain axial T1-weighted anatomical scan was acquired using a three-dimensional inversion recovery prepared fast spoiled gradient recalled sequence [repetition time (TR) = 9.2 ms, echo time (TE) = 3.7 ms, inversion time = 450 ms, flip angle = 12°, number of excitations (NEX) = 1, field-of-view (FOV) = 256 mm × 256 mm, 1 mm isotropic resolution, and 172 slices without interslice gap]. Whole-brain spontaneous activity was measured using a T2*-weighted blood oxygenation level-dependent contrast-sensitive gradient-echo echo-planar imaging sequence (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, NEX = 1, FOV = 222 mm × 222 mm, voxel size = 3.47 mm × 3.47 mm × 3.5 mm, and 43 interleaved axial slices without interslice gap). Two-hundred image volumes were acquired for each participant. During the rs-fMRI acquisition, the participants were instructed to remain relaxed and awake with their eyes closed. An experienced radiologist evaluated each brain anatomical scan for gross abnormalities.
Data Preprocessing of rs-fMRI
Data preprocessing was conducted using AFNI (Analysis of Functional Neuroimages1), FSL v.5.0.9 (Functional MRI of the Brain Software Library2), and SPM8 (Statistical Parametric Mapping software3). All functional images were first preprocessed in the original native space and then spatially normalized into the standard Montreal Neurological Institute (MNI) space for further brain functional network analysis. Prior to the preprocessing, the first 10 volumes of the acquired rs-fMRI dataset were discarded for magnetic homogenization. After removing the unwanted volumes, every individual data set underwent the following standard preprocessing: head movement correction (MCFLIRT), slice-timing correction (slicetimer), large transient and polynomial trend removal (3dDespike and 3dDetrend), non-brain tissue removal (BET), and spatial smoothing (Gaussian kernel with a 6-mm full-width at half-maximum, fslmaths). None of the participants were excluded based on the exclusion criterion of a maximum displacement larger than 2 mm and a rotation greater than 2° in any direction. To further minimize the potential contributions of non-neuronal physiological fluctuations, cardiac and respiration profiles were estimated by motion-corrected time series data using the PESTICA toolbox (24). Finally, band-pass filtering (0.01–0.08 Hz) and nuisance signal regression were combined into a single regression step (3dTproject) to remove the confounding signals from the time course of each voxel. The nuisance signals in the current study were modeled by an averaging cerebrospinal fluid time series, a local whiter matter time series (25), the Friston 24-motion-parameter model, and estimated cardiac and respiration fluctuations. The mean global signal was not included among the nuisance variables to avoid artificially induced anti-correlation patterns of brain functional networks (26). After completing the abovementioned preprocessing pipeline, the resulting rs-fMRI data sets were registered into standard MNI space using an EPI template (spm_normalise) and then resampled to a 2-mm isotropic resolution.
Motion-Related Analysis of rs-fMRI Data Set
For motion-related issues of rs-fMRI, we not only performed the subject-level motion control but also managed group-level micromovements by additionally modeling the individual mean framewise displacements (FD, calculated by fsl_motion_outliers). According to the FD exclusion criteria, subjects with the following two conditions were acceptable to be further excluded: (1) mean FD was larger than 0.25 mm, and (2) over 40% of the image volume was removed after censoring all time points with FDs > 0.25 mm (27). Combining with Friston 24-motion regressors in the subject-level nuisance regression, this two-level regression approach and conservative exclusion criteria for head motion minimized the potential influence of motion on both subject-level connectivity estimation and the group-level results interpretation.
Node and Edge Definitions in Brain Functional Networks
The analytical steps of the present study are summarized in Figure 1. To investigate FC differences among groups, we utilized a scheme that integrates fMRI meta-analysis findings using MNI coordinates to define nodes within large-scale networks (28). The edges were estimated based on the statistical dependence between nodes in residual time series data. Specifically, these nodes were subdivided into 12 large-scale functional subsystems across cortical and subcortical areas: dorsal attention, ventral attention, subcortical, salience, frontoparietal, visual, default mode, auditory, cingulo-opercular, somatomotor, memory retrieval, and cerebellar networks. (Coordinates of the 236 nodes are listed in Table S1 in Supplementary Material.) A 10-mm-diameter sphere centered at each coordinate represented each node and was used to extract the corresponding mean time series data. A 236 × 236 inter-regional FC matrix was constructed for each participant by calculating the Pearson correlation coefficient between all pairs of nodes. Finally, Fisher’s r-to-z transformation was used to improve the normality of the correlation estimates. Then, the unthresholded FC matrices (including both positive and negative z-values) were used for further network-level analysis.
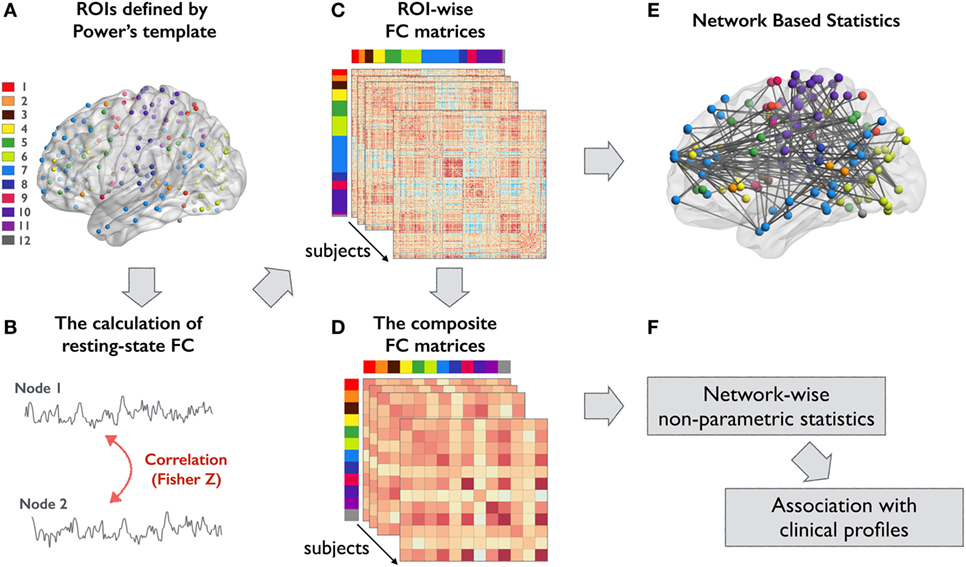
Figure 1. Overview of the analytical steps in this study. (A) ROIs defined by Power’s template were used to form ROI-wise FC matrix for all participants. Each color represents a different large-scale functional subsystem: 1, dorsal attention; 2, ventral attention; 3, subcortical; 4, salience; 5, frontoparietal task control; 6, visual; 7, default mode; 8, auditory; 9, cingulo-opercular task control; 10, sensory/somatomotor; 11, memory; and 12, cerebellar network (B) FC between an ROI pair was computed using Pearson correlation coefficient and then transformed to z-value. (C) An example of a connectivity matrix from a single subject. (D) Composite z-score approach was used to integrate the high-resolution connectivity matrices into 12 functional subsystems. (E,F) The corresponding statistical approaches for analyzing (C,D), respectively. Abbreviations: FC, functional connectivity; ROIs, regions of interest.
Analysis of Whole-Brain FC Changes Using NBS
We performed NBS with analyses of covariance to identify network-wise FC changes (20). Specifically, the primary cluster-defining threshold (t > 4, p < 5 × 10−4, one-tailed) was first applied to identify a set of suprathreshold edges, at which time all connected components and their sizes were determined. Five-thousand permutations were then used to generate the empirical null distribution for the size of the largest connected component and to estimate the significance of each identified component. Only components whose size exceeded the family-wise error (FWE)-corrected p-value of <0.05 were deemed to be statistically significant. Participant age, sex, and mean FD were regarded as nuisance variables. Since NBS results are highly dependent on the initial cluster-defining threshold, we performed an additional series of NBS analyses with different initial cluster-defining thresholds (Figure S1 in Supplementary Material). Because NBS aims to identify network-level FC changes across the whole brain, we then performed further pairwise connectivity analyses to localize the connectivity changes within or between brain functional subsystems.
Pairwise Connectivity Analysis of Brain Functional Subsystems
To localize the FC changes within and between the 12 functional subsystems, the individual 236 × 236 interregional FC matrix was integrated into a 12 × 12 FC matrix using composite z-score approach. The composite z-score was calculated by averaging the z-values of all nodes residing in the corresponding network pairs. These integrated matrices represent the intra- and inter-FC strengths of the 12 functional subsystems. A non-parametric statistical analysis with 5,000 permutations and the same statistical design was used to identify between-group differences in the aggregated FC matrices. The threshold of significance was set at a false discovery rate (FDR)-corrected p-value of <0.05. We further performed a conjunction analysis to identify the shared intra- and interconnectivity changes in functional subsystems in each patient group. This analysis was performed by searching for the union-section of the FDR-thresholded matrices from patient–control comparisons (29). Effect size was computed using Cohen’s d for each statistical comparison (30).
Correlations of Connectivity Measures with Clinical Profiles
Spearman’s rank correlation analyses with nuisance covariates (age, sex, and mean FD) were used to identify associations between the strengths of abnormal connections and clinical variables (duration of migraine, headache frequency, duration of RLS, IRLSSG severity scale, Pittsburgh Sleep Quality Index, Hospital Anxiety and Depression Scale, and Beck Depression Inventory score). Only connections identified using composite FC matrix analysis for both patient groups were included for correlation analyses.
Results
Patient’s Characteristics and rs-fMRI Motion Profiles
There were no significant differences in sex (p = 0.89) or age (p = 0.92) between patients and control participants. Demographic and clinical characteristics are summarized in Table 1. During the rs-fMRI scans, no participants exhibited maximum rotations ≥ 2.0° or displacements ≥ 2.0 mm in any direction. No significant differences in mean FD were observed between patients and controls (p = 0.88).
Group Differences in Whole-Brain FC Assessed Using NBS
Network-based statistics analyses revealed that migraine patients without RLS exhibited decreased FC relative to CRL, with eight nodes and seven edges (PFWE-corrected = 0.027), in the network composed of the dorsal attention, salience, default mode, visual, and frontoparietal networks (Figure 2A). Migraine patients with RLS exhibited decreased FC relative to CRL, with nine nodes and eight edges (PFWE-corrected = 0.023, corrected), in the network composed of the default mode, cingulo-opercular, auditory, and sensory/somatomotor networks (Figure 2B). Importantly, all connections were weaker in patients than controls. However, no differences in disrupted FC components were observed between migraine patients with and those without RLS.
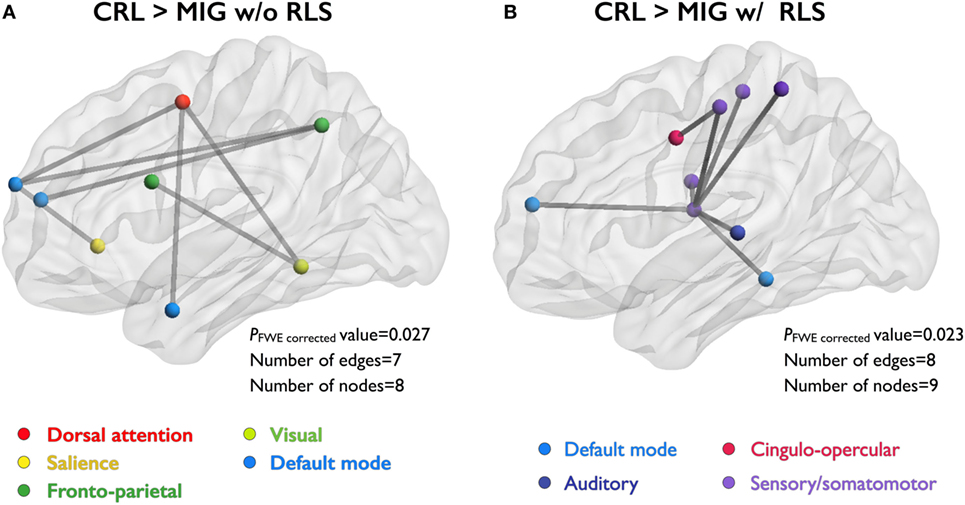
Figure 2. NBS identify the set of abnormal functional connections between study groups. Illustration of the network with reduced FC in two patient groups (compared to CRL group). Node colors correspond with the different functional subsystems. (A) Compared to CRL, MIG w/o RLS showed reduced FC in the network with eight nodes and seven edges, and (B) MIG w RLS showed reduced FC in the network with nine nodes and eight edges. Abbreviations: CRL, healthy controls; FC, functional connectivity; MIG w/o RLS, migraine without restless legs syndrome; MIG w RLS, migraine with restless legs syndrome; NBS, network-based statistics; FWE, family-wise error.
Group Differences in Network FC between Migraine Patients with and without RLS
Pairwise connectivity analyses revealed that patients with migraine, regardless of RLS diagnosis, exhibited lower FC than CRL in the following networks: salience, default mode, sensory/somatomotor, dorsal attention to salience, visual, auditory and cingulo-opercular, ventral attention to default mode, salience to visual, cingulo-opercular and cerebellar, frontoparietal to default mode, sensory/somatomotor to auditory, cingulo-opercular to cerebellar, and memory retrieval to cerebellar (PFDR-corrected < 0.05; Table 2; Figures 3A,B).
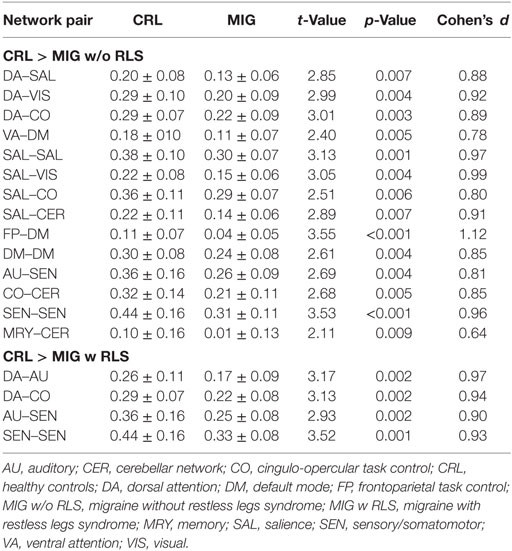
Table 2. Mean composite z scores and statistical results for network pairs between migraine patients with or without RLS and CRL.
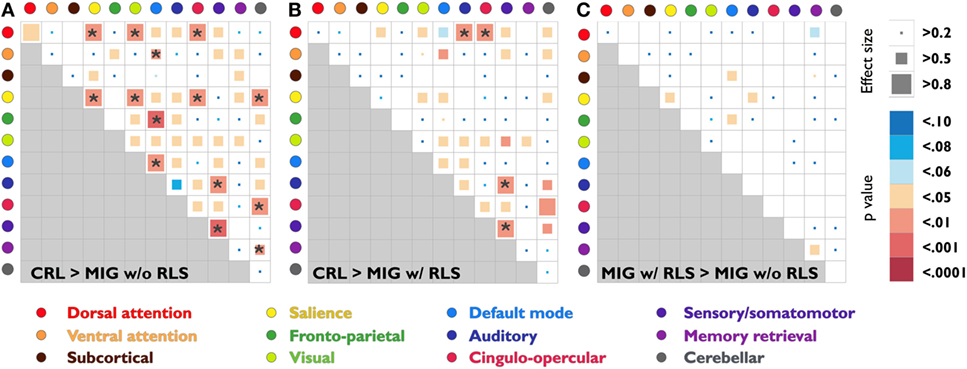
Figure 3. Statistical analysis and effect size estimation of composite z-score FC matrices among three study groups (A)–(C). Circles with different colors represent 12 functional subsystems. Inside the matrices, the squares with different colors indicate seven statistical levels of matrixwise comparisons between study groups (uncorrected p-values); the squares with different sizes indicate the corresponding effect size, which was calculated by Cohen’s d. The asterisk indicates that the p-value is less than the FDR corrected p-value 0.05. Abbreviations: CRL, healthy controls; FC, functional connectivity; FDR, false discovery rate; MIG w/o RLS, migraine without restless legs syndrome; MIG w/RLS, migraine with restless legs syndrome.
Further conjunction analyses revealed altered connectivity in the sensory/somatomotor, sensory/somatomotor to auditory, and dorsal attention to cingulo-opercular networks in migraine patients with RLS and without RLS groups. Direct exploratory group comparisons revealed changes in connectivity in the salience, default mode to subcortical and frontoparietal, auditory to salience, and memory retrieval networks between the two patient groups (Puncorrected < 0.05; Table 3; Figure 3C).
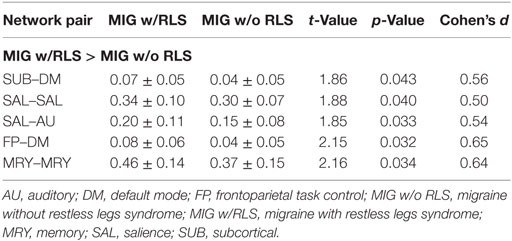
Table 3. Mean composite z-scores and statistical results for network pairs between migraine patients with and without RLS.
Associations between Clinical Variables and Network Metrics in Migraine Patients with and without RLS
There was a trend for a negative association between cross-network abnormalities in the default mode to subcortical network and RLS severity as assessed using the IRLSSG (r = −0.42, p = 0.08) in patients with MIG w RLS.
Discussion
The present study provides evidence for FC alterations in patients with MIG w and w/o RLS. Patients had FC patterns that were different from those of CRL across several networks. These included the dorsal attention, salience, default mode, cingulo-opercular, visual, frontoparietal, auditory, and sensory/somatomotor networks. We also observed similar patterns of FC changes in the sensory/somatomotor, sensory/somatomotor to auditory, and dorsal attention to cingulo-opercular networks in the two patient groups, suggesting that there may be an underlying pathological mechanism common to both groups. Comparison of the two patient groups revealed FC changes in the salience, default mode to subcortical and frontoparietal, auditory to salience, and memory retrieval networks. Finally, we observed trend-level significance for a negative association between RLS severity and cross-network abnormalities of connectivity between the DMN and subcortical network.
In our study, the intra- and interconnectivities of functional networks were significantly lower in patients than in controls, suggesting disruption of functional network organization in both patient groups. Such disruption may result in diffusively impaired functional brain connections. In migraine patients without RLS, the most affected brain functional networks were the dorsal attention, salience, default mode, visual, and frontoparietal networks. Previous studies have demonstrated that dorsal attention network alterations may manifest as memory deficits during and between attacks in patients with migraine (31). Other studies have reported alterations in prefrontal areas associated with attention and the cognitive aspects of pain processing in patients with migraine (14, 32).
Alterations in the DMN have been reported in migraine patients in a number of fMRI studies (14, 33). The DMN includes the precuneus, posterior cingulate cortex, medial prefrontal cortex, medial temporal lobe, and angular gyrus, which are connected and active when a person is at wakeful rest and not performing an attention-demanding task. Alterations in the DMN may be related to dysfunction in the integration of and attention to pain processing in migraine patients (14, 33). The salience network—a core resting-state network that includes the paralimbic–limbic regions—may be involved in the detection of external stimuli and the assignment of attentional resources. FC changes in this network have been reported in fMRI studies of migraine patients without aura (14, 34). In the present study, half of the patients with MIG w/o RLS experienced migraine auras, while previous studies have reported altered FC between the salience and visual networks in such patients (32). These findings may partially explain the observed functional alterations in the visual networks. Previous imaging studies have demonstrated decreased frontoparietal gray matter and frontoparietal network FC in migraine patients and that such changes are associated with attention-related task performance (35). There may thus be a relationship between attention and the frontoparietal network in patients with migraine. Collectively, the involvement of the aforementioned brain networks in patients with MIG w/o RLD is consistent with the reported changes in attention, cognitive function, and pain processing in patients with migraine.
We also observed that migraine patients with RLS exhibited decreased FC in the default mode, cingulo-opercular, auditory, and sensory/somatomotor networks relative to CRL. Functional changes in the DMN have been reported in previous fMRI studies, even during symptom-free periods. These changes may result in difficulties in maintaining a resting state and may involve internal stimuli that urge the patient to move his or her legs while at rest (18). Previous brain imaging studies have indicated that structural and functional changes in the sensorimotor network may be associated with sensory and motor symptoms of the legs in RLS (36, 37).
The cingulo-opercular network is composed of the anterior insula/operculum, anterior cingulate cortex, and thalamus and may be involved in the maintenance of alertness, attentional control, and sensory integration of internal and external stimuli (38). Previous PET studies have also observed altered dopaminergic and opioid activity in brain structures that serve the medial nociceptive system. These structures include the thalamus, anterior cingulate cortex, and insula, which may be involved in the affective-motivational component of pain in patients with RLS (39, 40). A previous neurophysiologic study documented changes in auditory startle responses in patients with RLS, suggesting that the disinhibition of reticulospinal pathways—which likely originate from dysfunctional rostral regions and project to the lower brainstem—is involved in the pathogenesis of RLS (41). Collectively, we observed alterations in these functional networks in patients with MIG w RLS. These changes may be associated with disruptions in attentional control and the management of sensory input information. Our results thus support the hypothesis that RLS is a disorder of somatosensory processing.
Conjunction analysis in both patient groups indicated common patterns of decreased FC relative to control participants in the sensory/somatomotor, sensory/somatomotor to auditory, and dorsal attention to cingulo-opercular networks. These observations indicate that patients with migraine exhibit decreased FC in networks associated with sensory regulation and attention. Indeed, previous studies have revealed that the sensory/somatomotor network in particular is crucial in sensory and pain processing in patients with migraine (42). We also observed that FC between the sensory/somatomotor to auditory and dorsal attention to cingulo-opercular networks was decreased relative to that of controls in both patient groups. Patients with migraine are hypersensitive to sensory stimuli, both during and between attacks (43, 44). Previous physiologic studies on interictal migraine patients indicate the presence of persistent hypersensitivity in the form of lower auditory discomfort thresholds and lower pain thresholds when compared with CRL (45, 46). Habituation deficits and exacerbated attention orientation to auditory event-related potentials have also been reported in patients with migraine, suggesting the abnormal involvement of attention-related networks (47, 48). Additional studies have revealed that auditory startle responses are altered in patients with RLS (41). Our results may partially support the shared clinical characteristic of hypersensitivity to sensory and auditory stimuli in patients with migraine and RLS (41, 46).
In the present study, we observed differences in FC between the two patient groups (migraine patients with and without RLS) with regard to the salience, default mode to subcortical and frontoparietal, salience to auditory, and memory retrieval networks. Most of the observed changes were associated with attentional modulation, sensory, anti-nociception, and limbic networks. Furthermore, it has been reported that children with both migraine and periodic limb movement disorders (including RLS) may have more severe migraine clinical presentation, severity, frequency, and other disabling aspects than children with migraine without periodic limb movement disorder (6). Therefore, these functional differences between the two patient groups may be associated with altered attentional nociceptive control of sensory inputs, which may be responsible for the clinical differences between the two groups, and the survival of undesirable sensations and abnormal sensory-motor integration in patients with RLS (14, 18). Future research is warranted to examine this speculation.
Furthermore, among the patients with RLS, the IRLSSG severity score exhibited a trend-level significance for a negative association with cross-network abnormalities between the default mode and subcortical networks. Weaker connection between these two networks may be associated with higher RLS severity. Migraine is phenotypically different from RLS, as it is characterized by a prominent worsening of symptoms during physical movement. As a result, patients with migraine avoid movement during attacks. In contrast, motor restlessness can only be relieved by voluntary movement in patients with RLS (23). Such findings indicate that RLS may be associated with cortical and subcortical functional connectome anomalies in patients with migraine. Although differences between the two groups in this study did not survive corrections for multiple comparisons, the effect sizes were medium to large, suggesting that our preliminary/exploratory findings can be used to guide future large-scale investigations.
In the present study, we identified the patterns of FC changes in patients with MIG w and w/o RLS. Our findings may aid in the development of neuroimaging-driven biomarkers in future studies. However, the present study possesses some limitations of note. First, the data in this study were obtained using a cross-sectional design. Thus, further longitudinal studies are required to determine whether the functional alterations are dynamic. Second, since most brain networks exhibiting an alteration in the present study are associated with higher cortical function, future studies should employ comprehensive cognitive testing to evaluate the relationships between these brain network alterations and cognition. Third, our sample size was not very large; however, we used a strict definition for diagnosis to ensure high quality of our investigation. Fourth, migraine prevalence peaks between the ages of 20 and 40 years, whereas RLS prevalence peaks after the age of 50 years (1, 5, 10). Furthermore, previous studies have reported that patients with RLS were older than those with comorbid migraine and RLS (49). Therefore, it is difficult to recruit a group of patients with only RLS whose mean age matches to that of migraine patients with RLS. Future studies should examine differences in FC by including an additional group of patients with RLS only. Fifth, to date, no fMRI-detectable brain activity changes have been reported due to the usage of flunarizine, as a preventive agent of migraine. However, topiramate- and propranolol-related differences in blood oxygenated level-dependent signals in fMRI have been reported in epilepsy (50) and autism (51) patients, respectively. Although the patients were asked to stop taking migraine-preventive medications (propranolol, topiramate, or flunarizine) at least 2 days prior to imaging in this study, we cannot fully exclude the potential impact of preventive medications on our findings. Finally, FC analysis does not specify the direction of information propagation in the underlying connected networks, and the contributions of excitatory vs. inhibitory neuronal coupling are unclear (52). We thus cannot determine whether these alterations are part of the pathogenesis or a consequence of the disorders. Future studies involving causality analyses are thus warranted.
In conclusion, we observed disrupted FC in patients with MIG w and w/o RLS when compared to CRL. Both groups displayed a common pattern of FC changes that predominantly affected sensorimotor and attention-related networks. The two groups exhibited FC changes most prominently in the attentional nociceptive control and sensory-related networks. Certain functional network metrics also exhibited a trend-level significance for correlation with RLS severity. Thus, our findings may aid in the development of potential connectome-based biomarkers for MIG w and w/o RLS.
Ethics Statement
The present study was approved by the Ethics Committee of TVGH and TSGH in Taiwan. All methods were carried out in accordance with the approved guidelines. All participants signed a written informed consent form after a full written and verbal explanation of the study.
Author Contributions
F-CY, K-HC, A-LH, and SJW participated in data collection, analyzed the data, and drafted the manuscript. F-CY, K-HC, A-LH, J-LF, J-FL, H-WK, C-PL, and S-JW participated in the study design, collected the data, and helped to draft the manuscript. C-PL and S-JW supervised the study, conceptualized and designed the study, and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This project was supported in part by grants from the Ministry of Science and Technology of Taiwan (MOST 106-2321-B-010-009-, MOST 106-2221-E-010-011-, MOST 104-2745-B-010-003-, MOST 104-2314-B-010-015-MY2, MOST 106-2314-B-016-007-MY2, MOST 105-2314-B-016-004-, MOST 104-2314-B-010-015-MY2, and MOST 103-2321-B-010-017-); Tri-Service General Hospital, Taiwan (TSGH-C101-159 and TSGH-C106-068); Taipei Veterans General Hospital (VGHUST105-G7-1-1, V105C-127, V105E9-001-MY2-1, and VTA105-V1-1-1); the Brain Research Center, National Yang-Ming University, Ministry of Health and Welfare, Taiwan (MOHW 104-TDU-B-211-113-003, MOHW 105-TDU-B-211-113-003, and MOHW 106-TDU-B-211-113001); and a grant from the Ministry of Education, Aim for the Top University Plan. The funders had no involvement in the study design, data collection, data analysis and interpretation, writing of the manuscript, or the decision to submit this article for publication.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fneur.2018.00025/full#supplementary-material.
Figure S1. Illustration of the network with reduced functional connectivity in two disease groups (compared to health control group) under different initial threshold settings with NBS analyses. Abbreviations: CRL, healthy controls; FWE, family-wise error; MIG w/o RLS, migraine without restless legs syndrome; MIG w RLS, migraine with restless legs syndrome; NBS, network-based statistics.
Footnotes
References
1. Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain (2010) 11:289–99. doi:10.1007/s10194-010-0217-0
2. Fuh JL, Chung MY, Yao SC, Chen PK, Liao YC, Hsu CL, et al. Susceptible genes of restless legs syndrome in migraine. Cephalalgia (2016) 36:1028–37. doi:10.1177/0333102415620907
3. Lin GY, Lin YK, Lee JT, Lee MS, Lin CC, Tsai CK, et al. Prevalence of restless legs syndrome in migraine patients with and without aura: a cross-sectional, case-controlled study. J Headache Pain (2016) 17:97. doi:10.1186/s10194-016-0691-0
4. Schürks M, Winter A, Berger K, Kurth T. Migraine and restless legs syndrome: a systematic review. Cephalalgia (2014) 34:777–94. doi:10.1177/0333102414537725
5. Winter AC, Schurks M, Berger K, Buring JE, Gaziano JM, Kurth T. Migraine and restless legs syndrome in men. Cephalalgia (2013) 33:130–5. doi:10.1177/0333102412466965
6. Esposito M, Parisi P, Miano S, Carotenuto M. Migraine and periodic limb movement disorders in sleep in children: a preliminary case–control study. J Headache Pain (2013) 14:57. doi:10.1186/1129-2377-14-57
7. Yang FC, Lin TY, Chen HJ, Lee JT, Lin CC, Huang WY, et al. Increased risk of restless legs syndrome in patients with migraine: a nationwide population-based cohort study. Medicine (Baltimore) (2016) 95:e2646. doi:10.1097/MD.0000000000002646
8. Chen PK, Fuh JL, Wang SJ. Bidirectional triggering association between migraine and restless legs syndrome: a diary study. Cephalalgia (2016) 36:431–6. doi:10.1177/0333102415596444
9. Chen PK, Fuh JL, Chen SP, Wang SJ. Association between restless legs syndrome and migraine. J Neurol Neurosurg Psychiatry (2010) 81:524–8. doi:10.1136/jnnp.2009.200030
10. Schurks M, Winter AC, Berger K, Buring JE, Kurth T. Migraine and restless legs syndrome in women. Cephalalgia (2012) 32:382–9. doi:10.1177/0333102412439355
11. Paulus W, Dowling P, Rijsman R, Stiasny-Kolster K, Trenkwalder C, deWeerd A. Pathophysiological concepts of restless legs syndrome. Mov Disord (2007) 22:1451–6. doi:10.1002/mds.21533
12. Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct (2016) 221:4203–19. doi:10.1007/s00429-015-1161-1
13. Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol (2011) 70:838–45. doi:10.1002/ana.22537
14. Tessitore A, Russo A, Giordano A, Conte F, Corbo D, Stefano M, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain (2013) 14:89. doi:10.1186/1129-2377-14-89
15. Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia (2013) 33:1264–8. doi:10.1177/0333102413490344
16. Liu C, Dai Z, Zhang R, Zhang M, Hou Y, Qi Z, et al. Mapping intrinsic functional brain changes and repetitive transcranial magnetic stimulation neuromodulation in idiopathic restless legs syndrome: a resting-state functional magnetic resonance imaging study. Sleep Med (2015) 16:785–91. doi:10.1016/j.sleep.2014.12.029
17. Gorges M, Rosskopf J, Müller HP, Lindemann K, Hornyak M, Kassubek J. Patterns of increased intrinsic functional connectivity in patients with restless legs syndrome are associated with attentional control of sensory inputs. Neurosci Lett (2016) 617:264–9. doi:10.1016/j.neulet.2016.02.043
18. Ku J, Lee YS, Chang H, Earley CJ, Allen RP, Cho YW. Default mode network disturbances in restless legs syndrome/Willis-Ekbom disease. Sleep Med (2016) 23:6–11. doi:10.1016/j.sleep.2016.05.007
19. Ku J, Cho YW, Lee YS, Moon HJ, Chang H, Earley CJ, et al. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med (2014) 15:289–94. doi:10.1016/j.sleep.2013.09.030
20. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage (2010) 53:1197–207. doi:10.1016/j.neuroimage.2010.06.041
21. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia (2013) 33:629–808. doi:10.1177/0333102413485658
22. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med (2014) 15:860–73. doi:10.1016/j.sleep.2014.03.025
23. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. Sleep Med (2003) 4:101–19. doi:10.1016/S1389-9457(03)00010-8
24. Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage (2007) 37:1286–300. doi:10.1016/j.neuroimage.2007.07.004
25. Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state fMRI, with artifact detection and removal. Neuroimage (2010) 52:571–82. doi:10.1016/j.neuroimage.2010.04.246
26. Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage (2016) 154:169–73. doi:10.1016/j.neuroimage.2016.11.052
27. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage (2014) 84:320–41. doi:10.1016/j.neuroimage.2013.08.048
28. Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron (2011) 72:665–78. doi:10.1016/j.neuron.2011.09.006
29. Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One (2012) 7:e43122. doi:10.1371/journal.pone.0043122
30. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale: L. Erlbaum Associates (1988). 567 p.
31. Gil-Gouveia R, Oliveira AG, Martins IP. The impact of cognitive symptoms on migraine attack-related disability. Cephalalgia (2016) 36:422–30. doi:10.1177/0333102415604471
32. Gao Q, Xu F, Jiang C, Chen Z, Chen H, Liao H, et al. Decreased functional connectivity density in pain-related brain regions of female migraine patients without aura. Brain Res (2016) 1632:73–81. doi:10.1016/j.brainres.2015.12.007
33. Zhang J, Su J, Wang M, Zhao Y, Yao Q, Zhang Q, et al. Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain (2016) 17:98. doi:10.1186/s10194-016-0692-z
34. Tso AR, Trujillo A, Guo CC, Goadsby PJ, Seeley WW. The anterior insula shows heightened interictal intrinsic connectivity in migraine without aura. Neurology (2015) 84:1043–50. doi:10.1212/WNL.0000000000001330
35. Mickleborough MJ, Ekstrand C, Gould L, Lorentz EJ, Ellchuk T, Babyn P, et al. Attentional network differences between migraineurs and non-migraine controls: fMRI evidence. Brain Topogr (2016) 29:419–28. doi:10.1007/s10548-015-0459-x
36. Rizzo G, Manners D, Vetrugno R, Tonon C, Malucelli E, Plazzi G, et al. Combined brain voxel-based morphometry and diffusion tensor imaging study in idiopathic restless legs syndrome patients. Eur J Neurol (2012) 19:1045–9. doi:10.1111/j.1468-1331.2011.03604.x
37. Spiegelhalder K, Feige B, Paul D, Riemann D, van Elst LT, Seifritz E, et al. Cerebral correlates of muscle tone fluctuations in restless legs syndrome: a pilot study with combined functional magnetic resonance imaging and anterior tibial muscle electromyography. Sleep Med (2008) 9:177–83. doi:10.1016/j.sleep.2007.03.021
38. Coste CP, Kleinschmidt A. Cingulo-opercular network activity maintains alertness. Neuroimage (2016) 128:264–72. doi:10.1016/j.neuroimage.2016.01.026
39. von Spiczak S, Whone AL, Hammers A, Asselin MC, Turkheimer F, Tings T, et al. The role of opioids in restless legs syndrome: an [11C]diprenorphine PET study. Brain (2005) 128:906–17. doi:10.1093/brain/awh441
40. Červenka S, Pålhagen SE, Comley RA, Panagiotidis G, Cselényi Z, Matthews JC, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain (2006) 129:2017–28. doi:10.1093/brain/awl163
41. Frauscher B, Löscher W, Högl B, Poewe W, Kofler M. Auditory startle reaction is disinhibited in idiopathic restless legs syndrome. Sleep (2007) 30:489–93. doi:10.1093/sleep/30.4.489
42. Zhao L, Liu J, Yan X, Dun W, Yang J, Huang L, et al. Abnormal brain activity changes in patients with migraine: a short-term longitudinal study. J Clin Neurol (2014) 10:229–35. doi:10.3988/jcn.2014.10.3.229
43. Wöber-Bingöl C, Wöber C, Karwautz A, Auterith A, Serim M, Zebenholzer K, et al. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia (2004) 24:12–7. doi:10.1111/j.1468-2982.2004.00621.x
44. Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology (2008) 70:1525–33. doi:10.1212/01.wnl.0000310645.31020.b1
45. Schwedt TJ, Zuniga L, Chong CD. Low heat pain thresholds in migraineurs between attacks. Cephalalgia (2015) 35:593–9. doi:10.1177/0333102414550417
46. Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML. Ictal and interictal phonophobia in migraine – a quantitative controlled study. Cephalalgia (2009) 29:1042–8. doi:10.1111/j.1468-2982.2008.01834.x
47. Demarquay G, Caclin A, Brudon F, Fischer C, Morlet D. Exacerbated attention orienting to auditory stimulation in migraine patients. Clin Neurophysiol (2011) 122:1755–63. doi:10.1016/j.clinph.2011.02.013
48. Morlet D, Demarquay G, Brudon F, Fischer C, Caclin A. Attention orienting dysfunction with preserved automatic auditory change detection in migraine. Clin Neurophysiol (2014) 125:500–11. doi:10.1016/j.clinph.2013.05.032
49. Cho SJ, Chung YK, Kim JM, Chu MK. Migraine and restless legs syndrome are associated in adults under age fifty but not in adults over fifty: a population-based study. J Headache Pain (2015) 16:554. doi:10.1186/s10194-015-0554-0
50. Tang Y, Xia W, Yu X, Zhou B, Wu X, Lui S, et al. Altered cerebral activity associated with topiramate and its withdrawal in patients with epilepsy with language impairment: an fMRI study using the verb generation task. Epilepsy Behav (2016) 59:98–104. doi:10.1016/j.yebeh.2016.03.013
51. Narayanan A, White CA, Saklayen S, Scaduto MJ, Carpenter AL, Abduljalil A, et al. Effect of propranolol on functional connectivity in autism spectrum disorder – a pilot study. Brain Imaging Behav (2010) 4:189–97. doi:10.1007/s11682-010-9098-8
Keywords: MRI, migraine, restless legs syndrome, functional connectivity, connectome
Citation: Yang F-C, Chou K-H, Hsu A-L, Fuh J-L, Lirng J-F, Kao H-W, Lin C-P and Wang S-J (2018) Altered Brain Functional Connectome in Migraine with and without Restless Legs Syndrome: A Resting-State Functional MRI Study. Front. Neurol. 9:25. doi: 10.3389/fneur.2018.00025
Received: 20 November 2017; Accepted: 12 January 2018;
Published: 30 January 2018
Edited by:
Vincenzo Guidetti, Sapienza Università di Roma, ItalyReviewed by:
Marco Carotenuto, Università degli Studi della Campania “Luigi Vanvitelli” Caserta, ItalyMarcelo M. Valença, Universidade Federal de Pernambuco, Brazil
Copyright: © 2018 Yang, Chou, Hsu, Fuh, Lirng, Kao, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Po Lin, Y3BsaW4mI3gwMDA0MDt5bS5lZHUudHc=;
Shuu-Jiun Wang, c2p3YW5nJiN4MDAwNDA7dmdodHBlLmdvdi50dw==
 Fu-Chi Yang
Fu-Chi Yang Kun-Hsien Chou
Kun-Hsien Chou Ai-Ling Hsu5
Ai-Ling Hsu5 Jong-Ling Fuh
Jong-Ling Fuh Shuu-Jiun Wang
Shuu-Jiun Wang