- 1Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL, United States
- 2Sleep Disorders Center, Miller School of Medicine, Bascom Palmer Eye Institute, University of Miami, Miami, FL, United States
- 3Department of Medicine, Miller School of Medicine, University of Miami, Miami, FL, United States
- 4Department of Otolaryngology – Head and Neck Surgery, Miller School of Medicine, University of Miami, Miami, FL, United States
- 5Sleep Disorders Center, Bruce W. Carter VA Medical Center, Miami, FL, United States
Obstructive sleep apnea (OSA) is a chronic and heterogeneous disorder that leads to early mortality, stroke, and cardiovascular disease (CVD). OSA is defined by the apnea–hypopnea index, which is an index of OSA severity that combines apneas (pauses in breathing) and hypopneas (partial obstructions in breathing) associated with hypoxemia. Yet, other sleep metrics (i.e., oxygen nadir, arousal frequency), along with clinical symptoms and molecular markers could be better predictors of stroke and CVD outcomes in OSA. The recent focus on personalized medical care introduces the possibility of a unique approach to the treatment of OSA based on its phenotypes, defined by pathophysiological mechanisms and/or clinical presentation. We summarized what is known about OSA and its phenotypes, and review the literature on factors or intermediate markers that could increase stroke risk and CVD in patients with OSA. The OSA phenotypes where divided across three different domains (1) clinical symptoms (i.e., daytime sleepiness), (2) genetic/molecular markers, and (3) experimental data-driven approach (e.g., cluster analysis). Finally, we further highlight gaps in the literature framing a research agenda.
Introduction
Obstructive sleep apnea (OSA) is a chronic disorder that leads to early mortality, stroke, and cardiovascular disease (CVD) (1–5). OSA affects 34% of men and 17% of women of all ages, with an estimated cost of $149.6 billion in 2015 from untreated OSA (6–9). Obstruction of the upper airway during sleep causes hypoxemia, sleep fragmentation, and sympathetic nervous system activation (6–8). OSA may lead to stroke through its associations with potent vascular risk factors, such as hypertension, diabetes mellitus, obesity, and atrial fibrillation. OSA may also increase stroke and CVD risk through reduction in cerebral blood flow, altered cerebral autoregulation, impaired endothelial function, increased platelet activation, inflammation, and oxidative stress related to the intermittent hypoxemia–reoxygenation and arousals associated to increased sympathetic tone (10). The treatment of OSA has been considered an important intervention for reducing the morbidity and mortality associated with stroke and CVD (11). However, treatment of OSA has not consistently reduced cardiovascular risk (12, 13); results partly explained by methodological limitations, such as suboptimal adherence to positive airway pressure therapy. Importantly, some limitations may lie on the need to better identify the subset of individuals who respond more favorably to OSA therapy, with the potential of a greater reduction in adverse outcomes, for further review consider the article by Drager et al. (14). Recently, the emphasis on personalized medical care introduces the possibility of a unique approach to the treatment of OSA based on its pathophysiological mechanisms and/or clinical presentation (15, 16). Personalized medicine can lead to targeted clinical and public health strategies for reducing stroke and CVD risks associated with OSA.
Historically, OSA has been considered a uniform condition defined by an apnea-hypopnea index (AHI) ≥5 events per hour of sleep (17). The AHI combines apneas and hypopneas associated with hypoxemia. Yet, other sleep metrics (i.e., oxygen nadir, arousal frequency), along with clinical symptoms and molecular markers could be better predictors of stroke and CVD outcomes in OSA (17–19). The heterogeneity of clinical presentations leads to the evaluation of OSA phenotypes as a strategy to identify at-risk individuals for stroke and CVD (18, 19). As defined in the article by Zinchuk et al., a phenotype is “a category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life)” (20). The authors further define clinical phenotypes based on a priori categories according to the presence or absence of symptoms (e.g., daytime sleepiness). Molecular-genetic phenotypes are based on molecular features (i.e., DNA and RNA). Empirical or data-driven phenotypes are based on statistical analyses (e.g., cluster) examining a multitude of sleep symptoms, demographics, clinical, and physiological data to define distinct OSA subtypes (20).
The high prevalence of undiagnosed OSA and its resultant stroke and CVD risk provide a strong impetus to develop better risk-stratification strategies (18, 19). There is a need to further define the OSA phenotypes that may differentially affect stroke and or CVD risk. Thus, the focus of this systematic review is to summarize what is known about OSA and its phenotypes, in relation to the intermediate markers that increase stroke and CVD risk in OSA. We further highlight gaps in the literature that frame a research agenda.
Methods
We performed a systematic review by searching the PubMed and Embase databases using the PRISMA guidelines (21). The search strategy was constructed using MeSH terms. The initial search was conducted with the terms “obstructive sleep apnea” or “sleep disordered breathing” and “phenotype” or “phenotypes.” Also articles were searched with the key word “obstructive sleep apnea phenotypes.” The first author evaluated the abstract for articles that included “cardiovascular disease,” or “stroke,” or “cerebrovascular disease.” The search included articles written from January 1, 2007 until November 1, 2017.
To be included in the review, articles had to be primary research studies, published in peer-reviewed journals, written in English and included adults (≥18 years old). Articles were excluded if they were (1) animal studies; (2) a pediatric population; (3) written in languages other than English; (4) conference abstracts; (5) case reports or case series; and (6) commentary or review articles.
A total of 153 articles were retrieved and the abstract were reviewed by the lead author for inclusion and exclusion criteria. A total of 14 articles met the inclusion criteria and were independently reviewed for additional information by the authors (Alberto R. Ramos, Pedro Figueredo, and Douglas M. Wallace). The lead author conducted ancestry search of all retrieved articles’ reference lists. The authors (Alberto R. Ramos, Douglas M. Wallace) also searched the major sleep journals including Sleep Medicine Reviews, Sleep, the Journal of Clinical Sleep Medicine, Sleep Medicine, CHEST, Thorax, and Sleep and Breathing. This resulted in two additional articles. Article tracking software (COVIDENCE) was used to manage the retrieved literature.
Results
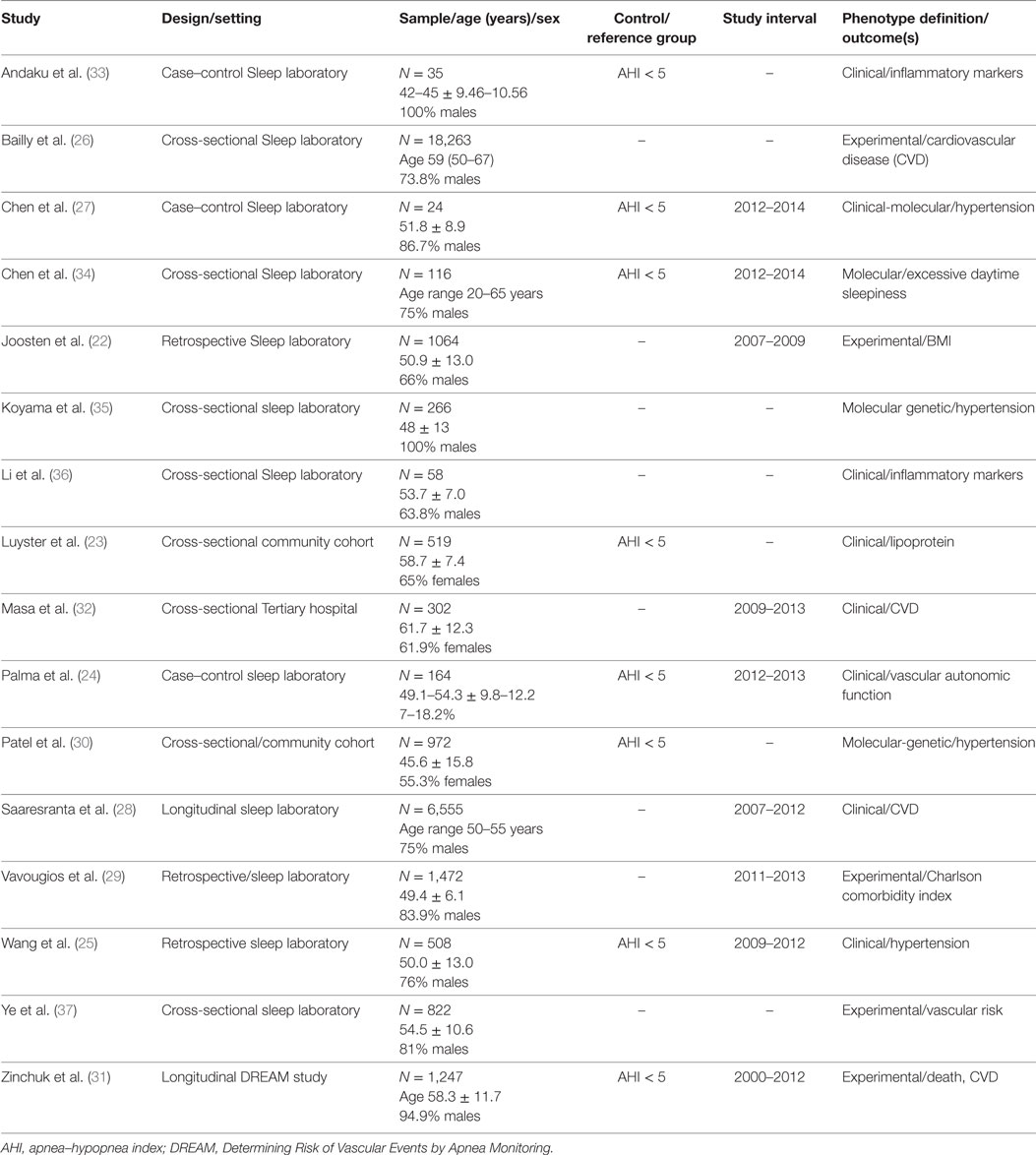
The majority of articles used clinical symptoms (n = 8) to define OSA phenotypes followed by experimental or data-driven approach (n = 5) and by molecular-genetic studies (n = 4). Most of the articles were cross-sectional analyses, case–control, or retrospective studies from sleep disorder centers (22–29). Two studies, Patel et al. (30) and Luyster et al. (23) were from community or population-based samples. The study from, Saaresranta et al. (28) and Zinchuk et al. (31) were longitudinal studies. Most studies comprised male participants, except for the two population based studies, Patel et al. (30) and Luyster et al. (23), and Masa et al. (32), which had 55 and 65, and 65% of females, respectively (Table 1).
Clinically Defined Phenotypes
The most common clinical phenotype (OSA subtype) was the patient with OSA and excessive sleepiness (EDS). In most studies, the patient with OSA and EDS had increased hypertension, inflammatory markers, and cardiovascular comorbidities (33). For example, the study from Andaku et al. (33) evaluated patients with metabolic syndrome and OSA. The authors compared patients with and without subjective daytime sleepiness. These were also compared to a control group with metabolic syndrome but without OSA and without daytime sleepiness. The authors described higher inflammatory markers (e.g., CRP) in participants with OSA and daytime sleepiness compared to the other groups adjusting for waist circumference, HOMA-IR, and triglycerides, without differences in oxidative stress markers. In a different cohort (36), 58 OSA patients (67% males), had measures of subjective sleepiness (using Epworth sleepiness scale and Stanford Sleepiness scale), and objective sleepiness with four consecutive nights of polysomnography followed by multiple sleep latency test (MSLT). In this sample, lower MSLT scores were associated with increased night time and daytime levels of interleukin-6, while subjective sleepiness was not associated with increased inflammation. In contrast, the largest cohort (n = 6,555) of OSA patients (28) divided participants into four groups based on the presence of insomnia (yes vs no) and daytime sleepiness (yes vs no). Different to other studies, daytime sleepiness was not associated to excess CVD risk.
Of interest, the study by Masa et al. (32), evaluated a 302 patients with OSA and comorbid obesity hypoventilation syndrome. The OSA phenotypes were defined by tertiles of oxygen desaturation index. Patients in the highest tertile, had increased daytime sleepiness, obesity, mostly males and more symptomatic. Paradoxically, the highest tertile had decreased cardiovascular comorbidities, such as stroke, arrhythmia, and pulmonary hypertension, among others.
Molecular-Genetic Studies
The four studies reviewed, evaluated the genetic and epigenetic markers associated with OSA clinical phenotypes and hypertension (27, 30, 34). Chen et al. (27) observed that alteration of the natriuretic peptide receptor 2 and its pathways was associated with the clinical phenotype of daytime sleepiness in OSA patients. In a different study from Chen et al. (34), decreased expression of BIRC3 gene was associated with the development of hypertension in OSA patients. In a relatively large sample (N = 972) of the Cleveland Family Study, Patel et al. (30) showed that insertion (I)/deletion (D) polymorphisms of the angiotensin converting enzyme were associated with hypertension in OSA patients with AHI > 30. Of interest, participants with a DD genotype had 37% reduction in the odds of hypertension compared to II genotype. However, Koyama et al. (35) found that among those with hypertension the II genotype was inversely associated with OSA severity [OR 0.27 (95% CI 0.09–0.80); p = 0.017] (Table 2).
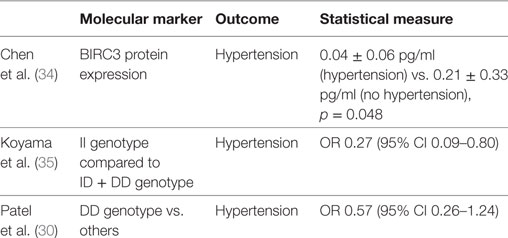
Table 2. Genetic and molecular markers associated to hypertension in patients with obstructive sleep apnea.
Experimental-Analytical
Experimental-analytical (data driven) studies suggest a heterogeneity of clinical phenotypes (22, 29, 37). The Icelandic Sleep Apnea Cohort (n = 822) of patients with moderate to severe sleep apnea (AHI ≥ 15) described three different phenotypic clusters. Cluster 1 had predominantly insomnia symptoms, cluster 2 had minimal symptoms compared to the other clusters, and cluster 3 was characterized by excessive daytime sleepiness (EDS). Interestingly, 42% of patients were in cluster 3, hence the majority of patients did not report EDS. Also patients from cluster 2 had the highest odds of hypertension (OR = 1.38 p ≤ 0.001) and CVD (OR = 1.67; p < 0.001) compared to cluster 3. These clusters were not clinically different in age, sex, BMI, AHI, or oxygen desaturations (37). A different, cluster analysis identified six phenotypic clusters associated with a number of medical and psychiatric comorbidities. The highest amount of vascular comorbidities was found in the middle-aged OSA patient with multiple sleep complaints (e.g., snoring, apnea, sleepiness) and in the obese-older patient with minimal sleep symptoms (29).
An analysis of a large sample from the French National registry of sleep apnea (N = 18, 263) included participants with moderate to severe sleep apnea (AHI ≥ 15), mean age of 59 years and predominance of males (73.8%). A hierarchical cluster analysis identified six clusters that varied by age, sleep symptoms, and medical comorbidities. Two different clusters had increased prevalence of stroke, cardiac arrhythmias, hypertension, and diabetes mellitus. Patients from cluster 3 were older patients (mean age 66) with less daytime sleepiness, while patients from cluster 6 were symptomatic middle-aged patients (mean age 60) with higher levels of sleepiness. These two clusters had the highest AHI and worse oxygen desaturations when compared to the other clusters (26). A longitudinal analysis of the 1,247 participants of the multi-center DREAM study, Determining Risk of Vascular Events by Apnea monitoring, used K-means analysis to create clusters based on polysomnography variables. The authors also used Cox proportional hazards models to evaluate the clusters associated with the composite outcome of acute coronary syndrome, transient ischemic attack, stroke, or death. The authors identified seven clusters. Most of them had increased risk of adverse cardiovascular events. However, the two clusters with the highest risk were labeled the “PLMS” cluster and the “arousal and poor sleep” cluster (31). The “PLMS” cluster had higher periodic limb movements in sleep with relatively mild AHI. This cluster had a hazard ratio (HR) of 2.36 [95% confidence interval (CI) of 1.61–3.46] of the composite outcome. The “Arousal and poor sleep” cluster was characterized by severe AHI, without hypoxic burden, but predominantly poor sleep architecture and many arousals. These cluster was associated with lower use of positive airway pressure and increase prevalence vascular risk factors. This cluster had a HR of 2.33 with 95% CI of 1.32–4.10. Of interest, sleep apnea was not associated with adverse cardiovascular events when using the AHI cutoffs of mild (<15), moderate (15 ≤ 30) and severe (≥30).
Discussion
The purpose of this review was to systematically evaluate manuscripts with concurrent definition of sleep apnea phenotypes and measures of vascular disease. As previously described, a priori symptom classification showed that the OSA-excessively sleepiness (EDS) phenotype, had increased mortality and adverse vascular outcomes. Interestingly, the OSA-EDS phenotype was associated with increased inflammatory markers, such as CRP and interleukin-6. Similarly, increased inflammation is associated with atherogenesis, hence increased inflammation could mediate the association between OSA-EDS phenotype and atherosclerotic disease. Epidemiological studies show that EDS is observed in up to 50% of patients from sleep centers; therefore, many patients do not endorse EDS. While these is not fully understood, the study from Chen et al. (27), suggest that hypomethylation of genes involved in the natriuretic peptide receptor-2 pathways could lead to EDS in OSA (27). These findings, coupled with the increased in CVD among the OSA-EDS phenotype, suggest a genetic susceptibility to the effects of hypoxia (26). Interestingly, the study from Masa et al. (32) suggests that severe hypoxemia could be protective at least in certain populations (obesity hypoventilation). A possible explanation is ischemic precondition, an adaptive mechanism where low levels of hypoxemia/ischemia leads to vascular and endothelial changes that “protects” against worse vascular events and possibly explaining the lower mortality rates of older age groups with OSA (38).
Interestingly, studies using empirically or data-driven analytical methods such as cluster analysis, observed increased cardiovascular outcomes in patients without daytime sleepiness, but rather clusters that were defined as having “poor,” “fragmented” sleep predominantly with insomnia symptoms or periodic limb movements had increased cardiovascular outcomes. Using a data-driven approach provide an avenue to further identify at at-risk individuals that otherwise may not be treated using AHI cutoffs. As noted in the study by Zinchuk et al. (31), the OSA using the AHI cuttoffs did not predict cardiovascular outcomes. This is consistent with other studies were the AHI did not correlate strongly with most relevant OSA comorbidities, such as hypertension (39). However, as noted in these studies (22, 26, 29, 37), the “classic” OSA patient (snoring, obese, daytime sleepiness) is not the most common phenotype.
In our systematic review, most studies sampled middle-aged males from sleep centers. Of importance, studies derived solely from sleep centers are of limited external validity and generalizability due to possible selection bias. Consequently, there is a need to develop tools for health-care professionals, sleep specialist, and scientist to further recognize and risk-stratify different OSA phenotypes; particularly in older-adults and females (40, 41). Importantly, population-based studies can help further evaluate age, sex and ethnic differences in OSA phenotypes; allowing clinicians and researchers to recognize participants an increased risk of stroke and CVD (40, 41), especially in populations with large burden of CVD that may benefit from inclusion in treatment studies.
Most of studies did not measured the physiologic OSA phenotypes or endotypes, as described by Eckert (42). An anatomical predisposition for upper airway collapse is necessary for OSA; however, these phenotypic classification describe non-anatomical pathways to upper airway obstruction, such a low respiratory arousal threshold, and instability of ventilator control causing an increase in “loop gain.” Of interest, a case–control study compared differences in cardiac autonomic tone in three different groups, OSA patients with hypoxia, OSA without hypoxia and controls (AHI < 5) (24). The authors observed an increased sympathetic contribution in patients with OSA and hypoxia. As noted by the authors, patients with increased loop gain can have partial airway obstruction leading to “apneas” without hypoxia. In contrast, patients with decreased loop gain coupled with factors that worsens upper airway collapse, could increase the length of apneas and arousal thresholds leading to periods of hypoxia.
Limitations
We may have not included all studies describing OSA phenotypes. We only considered studies with OSA phenotypes, which also had information on risk factors, vascular measures or comorbidities (e.g., hypertension, diabetes) known to increase the risk of stroke and CVD. In addition, articles describing OSA phenotypes, but did not label the study as such, may have not being included in this review as part of our search strategy.
Future Directions
This review provides a framework to further understand clinical, molecular and genetic information using data-driven approaches to reveal OSA subtypes. There are several important clinical phenotypes that have not been studied comprehensively. For example, the clinical presentation of OSA in pre- and post-menopausal women has been described to be significantly different than that of men (18). Similarly, race–ethnic differences may exist for OSA but have yet to be explored (43). Novel methods for diagnosis of sleep apnea (home sleep test) and use of commercially available wearables could provide further venues to define and identify patients at risk. In addition, phenotyping body position and sleep architecture needs to be incorporated into the current paradigms, as this may differently affect vascular disease and provide different intervention strategies such as positional therapy (22). There is a paucity of longitudinal studies examining whether OSA subtypes are stable over time. In addition, there are no studies determining whether OSA subtypes respond differentially to OSA treatments. Future studies should consider different OSA phenotypes with the intent to evaluate them against clinical outcomes, especially to optimize the use of clinical trials (14). Determining and evaluating the OSA phenotypes at risk for stroke or CVD can provide the structure for clinical trials in the treatment of OSA and reduction of CVD morbidity and mortality. Importantly, novel genetic and molecular markers are necessary. For example, micro-RNAs have been associated to increased atherosclerosis and CVD and recently have been shown to cause endothelial dysfunction associated to the intermittent hypoxia of patients with OSA (44). Future efforts should consider analyzing large-scale cohorts, in collaboration with geneticist and use of expertise in informatics and big data science.
Author Contributions
Drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis and interpretation of data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R21AG056952. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med (2005) 172(11):1447–51. doi:10.1164/rccm.200505-702OC
2. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med (2005) 353(19):2034–41. doi:10.1056/NEJMoa043104
4. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med (2010) 182(2):269–77. doi:10.1164/rccm.200911-1746OC
5. Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke (2012) 7(3):231–42. doi:10.1111/j.1747-4949.2011.00760.x
6. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med (2002) 165(9):1217–39. doi:10.1164/rccm.2109080
7. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc (2008) 5(2):136–43. doi:10.1513/pats.200709-155MG
8. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation (2008) 118(10):1080–111. doi:10.1161/CIRCULATIONAHA.107.189375
9. Watson NF. Health care savings: the economic value of diagnostic and therapeutic care for obstructive sleep apnea. J Clin Sleep Med (2016) 12(8):1075–7. doi:10.5664/jcsm.6034
10. Ramos AR, Seixas A, Dib SI. Obstructive sleep apnea and stroke: links to health disparities. Sleep Health (2015) 1(4):244–8. doi:10.1016/j.sleh.2015.09.005
11. Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA (2012) 307(20):2161–8. doi:10.1001/jama.2012.4366
12. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med (2016) 375(10):919–31. doi:10.1056/NEJMoa1606599
13. McEvoy RD, Neal B, Anderson CS. CPAP in obstructive sleep apnea. N Engl J Med (2016) 375(23):2302–3. doi:10.1056/NEJMc1613219
14. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, Initiative I. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation (2017) 136(19):1840–50. doi:10.1161/CIRCULATIONAHA.117.029400
15. Pack AI. Application of personalized, predictive, preventative, and participatory (P4) medicine to obstructive sleep apnea. A roadmap for improving care? Ann Am Thorac Soc (2016) 13(9):1456–67. doi:10.1513/AnnalsATS.201604-235PS
16. Lim DC, Pack AI. Obstructive sleep apnea: update and future. Annu Rev Med (2017) 68:99–112. doi:10.1146/annurev-med-042915-102623
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med (2015) 3(5):397–403. doi:10.1016/S2213-2600(15)00051-X
18. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol (2017) 69(7):841–58. doi:10.1016/j.jacc.2016.11.069
19. Redline S. Screening for obstructive sleep apnea: implications for the sleep health of the population. JAMA (2017) 317(4):368–70. doi:10.1001/jama.2016.18630
20. Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev (2016) 35:113–23. doi:10.1016/j.smrv.2016.10.002
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi:10.1016/j.jclinepi.2009.06.006
22. Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology (2012) 17(1):99–107. doi:10.1111/j.1440-1843.2011.02037.x
23. Luyster FS, Kip KE, Drumheller OJ, Rice TB, Edmundowicz D, Matthews K, et al. Sleep apnea is related to the atherogenic phenotype, lipoprotein subclass B. J Clin Sleep Med (2012) 8(2):155–61. doi:10.5664/jcsm.1768
24. Palma JA, Iriarte J, Fernandez S, Valencia M, Alegre M, Artieda J, et al. Characterizing the phenotypes of obstructive sleep apnea: clinical, sleep, and autonomic features of obstructive sleep apnea with and without hypoxia. Clin Neurophysiol (2014) 125(9):1783–91. doi:10.1016/j.clinph.2014.01.029
25. Wang Q, Zhang C, Jia P, Zhang J, Feng L, Wei S, et al. The association between the phenotype of excessive daytime sleepiness and blood pressure in patients with obstructive sleep apnea-hypopnea syndrome. Int J Med Sci (2014) 11(7):713–20. doi:10.7150/ijms.7487
26. Bailly S, Destors M, Grillet Y, Richard P, Stach B, Vivodtzev I, et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One (2016) 11(6):e0157318. doi:10.1371/journal.pone.0157318
27. Chen YC, Chen TW, Su MC, Chen CJ, Chen KD, Liou CW, et al. Whole genome DNA methylation analysis of obstructive sleep apnea: IL1R2, NPR2, AR, SP140 methylation and clinical phenotype. Sleep (2016) 39(4):743–55. doi:10.5665/sleep.5620
28. Saaresranta T, Hedner J, Bonsignore MR, Riha RL, McNicholas WT, Penzel T, et al. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS One (2016) 11(10):e0163439. doi:10.1371/journal.pone.0163439
29. Vavougios GD, George DG, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res (2016) 25(1):31–8. doi:10.1111/jsr.12344
30. Patel SR, Larkin EK, Mignot E, Lin L, Redline S. The association of angiotensin converting enzyme (ACE) polymorphisms with sleep apnea and hypertension. Sleep (2007) 30(4):531–3. doi:10.1093/sleep/30.4.531
31. Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax (2017). doi:10.1136/thoraxjnl-2017-210431
32. Masa JF, Corral L, Romero A, Caballero C, Terán-Santos J, Alonso-Álvarez ML, et al. Protective cardiovascular effect of sleep apnea severity in obesity hypoventilation syndrome. CHEST J (2016) 150(1):68–79. doi:10.1016/j.chest.2016.02.647
33. Andaku DK, D’Almeida V, Carneiro G, Hix S, Tufik S, Togeiro SM. Sleepiness, inflammation and oxidative stress markers in middle-aged males with obstructive sleep apnea without metabolic syndrome: a cross-sectional study. Respir Res (2015) 16:3. doi:10.1186/s12931-015-0166-x
34. Chen YC, Chen KD, Su MC, Chin CH, Chen CJ, Liou CW, et al. Genome-wide gene expression array identifies novel genes related to disease severity and excessive daytime sleepiness in patients with obstructive sleep apnea. PLoS One (2017) 12(5):e0176575. doi:10.1371/journal.pone.0176575
35. Koyama RG, Drager LF, Lorenzi-Filho G, Cintra FD, Pereira AC, Poyares D, et al. Reciprocal interactions of obstructive sleep apnea and hypertension associated with ACE I/D polymorphism in males. Sleep Med (2009) 10(10):1107–11. doi:10.1016/j.sleep.2008.12.012
36. Li Y, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Pejovic S, et al. Objective, but not subjective, sleepiness is associated with inflammation in sleep apnea. Sleep (2016) 40(2):zsw033. doi:10.1093/sleep/zsw033
37. Ye L, Pien GW, Ratcliffe SJ, Bjornsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J (2014) 44(6):1600–7. doi:10.1183/09031936.00032314
38. Lavie L, Lavie P. reduced cardiovascular morbidity in obesity-hypoventilation syndrome: an ischemic preconditioning protective effect? Chest (2016) 150(1):5–6. doi:10.1016/j.chest.2016.02.659
39. Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest (2016) 149(1):16–9. doi:10.1378/chest.14-2261
40. Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, Blumenthal RS, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis (2011) 219(2):963–8. doi:10.1016/j.atherosclerosis.2011.08.021
41. Shah N, Hanna DB, Teng Y, Sotres-Alvarez D, Hall M, Loredo JS, et al. Sex-specific prediction models for sleep apnea from the Hispanic community health study/study of Latinos. Chest (2016) 149(6):1409–18. doi:10.1016/j.chest.2016.01.013
42. Eckert DJ. Phenotypic approaches to obstructive sleep apnoea – new pathways for targeted therapy. Sleep Med Rev (2016). doi:10.1016/j.smrv.2016.12.003
43. Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med (2016) 18:96–102. doi:10.1016/j.sleep.2015.01.015
Keywords: obstructive sleep apnea, phenotype, stroke, cardiovascular disease, sleep disordered breathing
Citation: Ramos AR, Figueredo P, Shafazand S, Chediak AD, Abreu AR, Dib SI, Torre C and Wallace DM (2017) Obstructive Sleep Apnea Phenotypes and Markers of Vascular Disease: A Review. Front. Neurol. 8:659. doi: 10.3389/fneur.2017.00659
Received: 05 September 2017; Accepted: 22 November 2017;
Published: 05 December 2017
Edited by:
David Gozal, University of Chicago, United StatesReviewed by:
Giora Pillar, Technion – Israel Institute of Technology, IsraelThomas Penzel, Charité Universitätsmedizin Berlin, Germany
Copyright: © 2017 Ramos, Figueredo, Shafazand, Chediak, Abreu, Dib, Torre and Wallace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto R. Ramos, YXJhbW9zJiN4MDAwNDA7bWVkLm1pYW1pLmVkdQ==
 Alberto R. Ramos
Alberto R. Ramos Pedro Figueredo
Pedro Figueredo Shirin Shafazand2,3
Shirin Shafazand2,3 Alejandro D. Chediak
Alejandro D. Chediak Douglas M. Wallace
Douglas M. Wallace