- 1Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 2Research & Development, James J. Peters Veterans Affairs Medical Center, Bronx, NY, USA
- 3Rehabilitation Medicine Service, James J. Peters Veterans Affairs Medical Center, Bronx, NY, USA
- 4Department of Pathology, Uniformed Service University of Health Science, Bethesda, MD, USA
- 5Fishberg Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 6Department of Radiology, Icahn School of Medicine at Mount Sinai, Translational and Molecular Imaging Institute, New York, NY, USA
- 7Department of Rehabilitation Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 8Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 9Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 10Neurology Service, James J. Peters Veterans Affairs Medical Center, Bronx, NY, USA
- 11Mental Illness Research, Education, and Clinical Center (MIRECC VISN 2 South), James J. Peters Veterans Affairs Medical Center, Bronx, NY, USA
The long-term effects of blast exposure are a major health concern for combat veterans returning from the recent conflicts in Iraq and Afghanistan. We used an optimized diffusion tensor imaging tractography algorithm to assess white matter (WM) fractional anisotropy (FA) in blast-exposed Iraq and Afghanistan veterans (n = 40) scanned on average 3.7 years after deployment/trauma exposure. Veterans diagnosed with a blast-related mild traumatic brain injury (mTBI) were compared to combat veterans with blast exposure but no TBI diagnosis. Blast exposure was associated with decreased FA in several WM tracts. However, total blast exposure did not correlate well with neuropsychological testing performance and there were no differences in FA based on mTBI diagnosis. Yet, veterans with mTBI performed worse on every neurocognitive test administered. Multiple linear regression across all blast-exposed veterans using a six-factor prediction model indicated that the amount of blast exposure accounted for 11–15% of the variability in composite FA scores such that as blast exposure increased, FA decreased. Education accounted for 10% of the variability in composite FA scores and 25–32% of FA variability in the right cingulum, such that as level of education increased, FA increased. Total blast exposure, age, and education were significant predictors of FA in the left cingulum. We did not find any effect of post-traumatic stress disorder on cognition or composite FA. In summary, our findings suggest that greater total blast exposure is a contributing factor to poor WM integrity. While FA was not associated with neurocognitive performance, we hypothesize that FA changes in the cingulum in veterans with multiple combat exposures and no head trauma prior to deployment may represent a marker of vulnerability for future deficits. Future work needs to examine this longitudinally.
Introduction
The long-term effects of blast exposure have become a growing health concern within the military due to the large number of Iraq and Afghanistan combat veterans who have experienced single or multiple blasts from improvised explosive devices, rocket propelled grenades, and mortar rounds. It is estimated that approximately 10–20% of veterans returning from these conflicts sustained blast-related mild traumatic brain injury (mTBI) in theater (1–4). The true prevalence may be even higher, given that many blast-related injuries went unrecognized both during and after deployment, particularly in the early years of these conflicts (5). Screening and diagnosing of mTBI are complicated by the nature of the condition as initial assessment heavily relies on self-report measures (2, 6, 7), and the overlap of TBI symptoms with those of post-traumatic stress disorder (PTSD), which is present in at least 30% of returning service members who have suffered a mTBI (1, 2, 4).
Blast Exposure and Cognitive Functioning
The chronic effects of blast exposure on cognitive functioning are an area of growing research interest. One study found that Marines returning from combat with self-reported concussion and symptoms consistent with mTBI showed decline of cognitive performance on a computerized cognitive battery at a 3-month follow-up; however, these deficits recovered by month 6 (8). By contrast, another group reported that veterans with blast-related mTBI and combat controls with no history of blast exposure showed no performance differences on cognitive measures including the Controlled Oral Word Association Test, Trail Making Test, Color–Word Interference Test, and Verbal Selective Reminding Test (9). The interpretation of such discrepancies is further complicated by the use of different methodologies to assess cognition (i.e., raw scores from paper and pencil tests vs. reaction time on computerized tests). A review paper by Karr et al. (10) points to inconsistencies in the conceptualization of cognitive constructs used to describe post-mTBI impairment. Moreover, the authors stipulate that the magnitude of mTBI-related effects within each cognitive domain remains unclear as the respective effect sizes for “cognitive deficits” appear especially heterogeneous across meta-analyses (i.e., d = −0.11 to 0.72) and that these higher order functions appear most susceptible to multiple mTBI [d = 0.24 (11)]. Recent reports also suggest that mTBI patients may, in fact, experience subtle cognitive deficits that reflect diminished initial acquisition of novel information (12, 13). Specifically, mTBI patients demonstrated statistically significant deficits in performance on the first trial of the California Verbal Learning Task, whereas there were no impairments on total learning or memory composite variables (12). The most relevant hypothesis is that mTBI patients may demonstrate cognitive deficits that are not often detected in standard neuropsychological assessments. In short, the nature and extent of cognitive impairment following blast exposure are varied in the literature, symptomatic complaints are common, and they do not seem to associate exclusively with any single profile.
Diffusion Imaging and Blast mTBI
More recently, neuroimaging techniques have been utilized to help elucidate the pathology of mTBI. While there have been several neuroimaging studies devoted to civilian TBI (14–17), the extent to which blast injuries are a distinct neurobiological entity remains unknown but has become the focus of much recent work [reviewed in Ref. (18)]. Diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI) method, assesses the diffusion of water molecules in order to map microscopic details about the integrity of white matter (WM) fiber structure. It is based on the principle that water moves most easily along axonal bundles because there is the smallest number of obstacles to prevent movement in this direction. Diffusion measurements along different axes are fitted to a 3D ellipsoid (tensor) at each voxel. The most common metric in DTI studies is fractional anisotropy (FA), which reflects the magnitude and directionality of water diffusion. More specifically, FA represents the fraction of the tensor that can be assigned to diffusion that is constrained along one axis only, referred to as anisotropic diffusion. FA values are scaled from 0 (isotropic) to 1 (anisotropic). FA and other DTI metrics are thought to reflect the microstructural properties of WM tracts, including abnormal axonal diameter, coherence of the fiber tracts, fiber density, and myelination, although the microstructural correlates are not entirely clear (19–23). Because DTI is considered to be sensitive to subtle forms of damage and to diffuse axonal injury, it holds promise as a sensitive tool for identifying subtle pathology in blast-related mTBI, and several studies have utilized DTI to examine blast injury across a range of TBI severity at various time intervals post-exposure (24–34). These studies have generally found decreased FA in blast-related mTBI, results that have been interpreted as suggesting that diffuse axonal injury is a major component of the structural injury associated with blast. Nevertheless, the relationship between the total amount of blast exposure, such as the number of blasts, and the development of clinical symptoms of mTBI remains unclear. More specifically, (a) there are no studies that have specifically looked at the relationship between total number of blasts, especially blasts not linked to any physical or psychological sequela; and (b) there is little consistent evidence linking blast exposure with persistent changes in brain morphology and physiology.
The current study used an optimized DTI tractography algorithm to measure FA and to characterize the effects of blast exposure on WM integrity in blast-exposed Iraq and Afghanistan veterans, who had no formally diagnosed history of head trauma prior to deployment. Loss of consciousness (LOC) and co-occurring PTSD were investigated as indicators of blast severity, and veterans who suffered a blast-related mTBI were compared to a group of combat veterans who had a history of blast exposure but did not meet criteria for a TBI diagnosis. Finally, we examined the relationship between total amount of blast exposure, WM integrity, and cognitive performance, hypothesizing that veterans exposed to more blasts would have lower FA. We also predicted that lower FA, indicative of compromised WM integrity, would be associated with poorer performance on neuropsychological tests across cognitive domains.
Methods
Participants
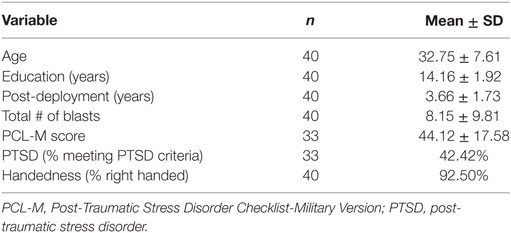
Forty Iraq and/or Afghanistan male veterans were recruited through the James J. Peters Veterans Affairs Medical Center (JJPVAMC, Bronx, NY, USA) Polytrauma/TBI Clinic through referrals from study physicians (Jessie Simantov and Gregory A. Elder), neuropsychologists (Effie M. Mitsis and Charlene Bang), as well as through flyers and advertisements posted throughout the medical center. Participants had an average age of 32.8 ± 7.6 years (range: 22–50 years) and 14.2 ± 1.9 years of education (Table 1). Participants were enrolled 3.7 ± 1.7 years post-deployment (range: 0.2–7.0 years). All participants had a history of primary blast exposure, with 24 veterans experiencing blasts severe enough to meet criteria for mTBI (Tables 2 and 3). The number of blast events experienced by study participants varied widely (range: 1–45, mean/SD 8.2 ± 9.8). Participants who met criteria for blast-related mTBI experienced an average of 4.8 ± 5.0 blast episodes during the course of their deployment, while veterans with no TBI diagnosis experienced more blasts despite not meeting threshold for a TBI diagnosis (mean/SD 13.1 ± 13.0; t = 2.85, df = 38, p < 0.01). None of the veterans studied had a formally diagnosed history of head injury prior to military service.
The PTSD Checklist-Military Version (PCL-M) was administered to evaluate PTSD symptomatology. We determined the presence of PTSD using a cutoff score of 44, in accordance with the guidelines published by the National Center for PTSD.1 Fourteen of 33 veterans (42%) who completed the PCL-M met criteria for PTSD (note: 7 participants did not complete the measure). PCL-M scores were significantly higher in veterans with blast-related mTBI (mean/SD 50.9 ± 16.7) than those without (mean/SD 36.9 ± 16.0; t = −2.45, df = 31, p = 0.02).
Exclusion criteria included any significant medical illness, neurological disease or psychiatric disorder (other than depression and/or PTSD), moderate-to-severe TBI, systemic cancer, history of psychoactive substance use/abuse and current alcohol or other drug dependence within the past year, use of psychoactive drugs, any significant lifetime history of pre-combat-related TBI with LOC or reported history of concussion requiring hospitalization, education level <10 years, or the presence of any MRI-incompatible prostheses or ferromagnetic metal. All participants had a negative urine toxicology screening for drugs of abuse on the day of their MRI scan. All study procedures were approved by the JJPVAMC and Icahn School of Medicine at Mount Sinai Institutional Review Boards. Participants provided written informed consent and were paid for their time and travel.
Clinical Assessments
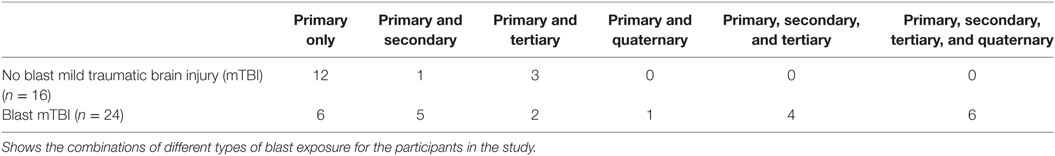
Details of blast injury were determined by self-report and review of all available clinical histories from the Veteran’s Affairs (VA) medical records of each participant (Tables 2 and 3). Primary blast injury refers to the direct effect of blast overpressure on tissue. Secondary injury results from objects propelled by the blast. Tertiary injuries are a feature of high-energy explosions and occur as a result of an individual being propelled through the air and striking other objects. Quaternary blast injuries encompass all other injuries caused by explosions, such as burns, crush injuries, and toxic inhalations. Assessments for the type of blast injury followed the accepted standards (35–37); all participants had a negative structural MRI scan indicating no brain injuries secondary to impact from foreign objects.
Prior to entry in the study, all veterans were evaluated by study physicians as part of their standard TBI screening and medical evaluation and underwent neuropsychological evaluation. A final diagnosis of blast-related mTBI was based upon Department of Defense/VA criteria (38) and determined by consensus during weekly meetings of the clinical and TBI/Polytrauma team. Study personnel obtained additional detailed history of blast exposure through interview with each veteran. The Defense and Veterans Brain Injury Center screening tool was administered to obtain information regarding any transient loss of awareness or LOC (≤30 min), post-traumatic amnesia (<24 h), as well as information on post-concussive symptoms of dizziness, confusion, headache, balance and memory problems, tinnitus, irritability, and sleep problems.
Neurocognitive Assessments; Domain and Composite Scores
Participants completed a battery of neuropsychological tests assessing cognitive domains with a particular emphasis on memory, executive function, attention, and language. These included the Wechsler Adult Intelligence Scale-Fourth Edition (39), which assesses participants’ overall intelligence; Wide Range Achievement Test 4 (40), which measures an individual’s ability to read words, comprehend sentences, spell, and compute solutions to math problems; Controlled Oral Word Association Test-FAS [COWA-FAS (41)], which is a verbal fluency test measuring spontaneous production of words belonging to the same category or beginning with some designated letter; California Verbal Learning Test (CVLT)-Second Edition (42, 43), which measures episodic verbal learning and memory and can detect sensitivity to a range of clinical conditions; and the Brief Visuospatial Memory Test-Revised (44), which measures figural learning and retention for examination of non-verbal memory (Table 4). The instruments were administered and scored by trained research assistants, and the final scoring was confirmed by a neuropsychologist. Raw scores for each test were standardized based on age- and education-adjusted normative data. Tests were grouped into three domains: attention/executive function, language/education, and memory. Domain scores were calculated by averaging the normalized test score within each cognitive domain. An overall composite score was calculated by averaging the three domain scores. This approach to calculating domain and composite scores has been used by our group and others (45, 46) in studies evaluating cognitive functioning in mildly-impaired cohorts.
Magnetic Resonance Imaging
Magnetic resonance imaging scans were conducted in the Department of Radiology at the Mount Sinai Hospital, the academic-affiliate hospital of the JJPVAMC. All MRI scans were visually inspected by a neuroradiologist at Mount Sinai blind to diagnostic group. No visible lesions were detected for any veteran, and all research MRI reports were read as normal.
All participants underwent MRI on a Siemens Allegra 3 T head-dedicated scanner for acquisition of axial structural and diffusion tensor images. A pulsed-gradient spin-echo sequence with echo planar imaging pulse sequence acquisition was used. A b-factor of 1,250 s/mm2 was chosen based on tests performed to find the optimal balance for signal-to-noise ratio and diffusion weighting. Twelve gradient directions with b = 1,250 s/mm2 were used [repetition time (TR) = 4,100 ms, echo time (TE) = 80 ms, field of view (FOV) = 21 cm, matrix 128 × 128, 28 slices, thickness = 3 mm, skip = 1 mm]. High-resolution 3D MP-RAGE images were also obtained (TR = 2,500 ms, TE = 4.4 ms, FOV = 21 cm, matrix size = 256 × 256, 208 slices with thickness = 0.82 mm). Quality assurance was performed immediately after each scan.
DTI Processing and Fiber Tracking
FSL2 was used for eddy current correction, brain extraction, and the computation of FA and mean diffusivity (MD) maps. DTI tractography processing was performed using in-house software, developed in MATLAB v2013 (The MathWorks Inc., Natick, MA, USA). A multiple region brute-force fiber tracking method was used for quantification of white mater characteristics as previously described (47). Briefly, fibers were traced using a streamline tractography algorithm from every voxel throughout the entire volume that exceeded a minimum FA (48). Tracking was terminated when FA fell below 0.1 or when the algorithm encountered a sharp angle change in the principal diffusion direction between sequential voxels (45°). Each tract was then indexed such that a queried voxel returns all streamlines that pass through it. All tracts that passed through a target voxel are then associated with that voxel. This method is more robust than streamline tractography alone, in which a single fiber emerges from a voxel.
Image Analysis
To minimize variance due to inter-subject differences in tractography seeds, all anatomical criteria were defined on the study-specific template (i.e., mean FA image of all participants in the current study) that was created with the tract-based spatial statistics (TBSS) package of FSL [as in our prior work; see Ref. (49)]. Established methods to create anatomical seed criteria (50) were used to guide diffusion tractography quantification of the following tracts: right and left cingulum bundle, inferior longitudinal fasciculus, superior longitudinal fasciculus, internal capsule, inferior fronto-occipital fasciculus, uncinate, the frontal projection of the corpus callosum (the forceps minor), and the posterior projection of the corpus callosum (the forceps major) (Figure 1). Tract integrity was quantified using a normalized line integral of FA and MD for every tract per participant (49). These results were then exported for further statistical analysis.
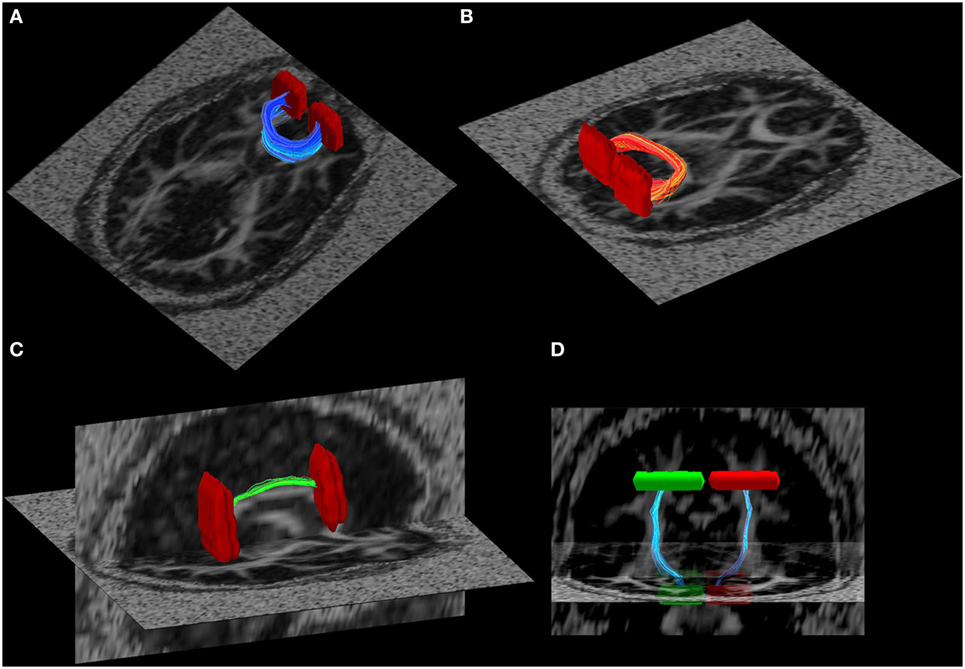
Figure 1. Illustration of some of the white matter tracts used in the tract-based fractional anisotropy quantification. (A) Forceps major, (B) forceps minor, (C) R-cingulum, and (D) cortical spinal tract. Seed regions of interest are illustrated in red and green.
In addition, we performed region-of-interest-based FA analysis. Co-registered FA images in Montreal Neurological Institute (MNI) brain space were computed using the TBSS workflow. The Johns Hopkins University ICBM-DTI-81 WM labels atlas was used to locate anatomical regions in MNI152 space. Mean FA was calculated for each atlas-defined region on the each participant’s co-registered MNI FA image computed from TBSS. Composite FA scores were also calculated for each participant (see Statistical Analysis).
Statistical Analysis
As indicated above, the main objective of this study was to examine the relationship between total amount of blast exposure, WM integrity, and cognitive performance. In order to test our hypotheses that veterans exposed to more blasts would have lower FA and that lower FA would be associated with poorer neurocognitive performance, we employed multiple linear regression models. The variables included in our models were selected based on the consensus that they were clinically important factors that influence WM integrity.
A plot of the residuals vs. predicted values was used to check the assumptions of linearity and homoscedasticity. The normality assumption was evaluated based on the residuals using a QQ plot by comparing the residuals to “ideal” normal observations. A histogram of the Cook’s distance (which is a measure of the influence of each observation on the regression coefficients) was generated to identify outliers that may or may not be influential data points. Multicollinearity was assessed by examining tolerance and variance inflation factors of each variable in the regression models.
Due to the lack of consistent reports indicating relations between altered FA in specific brain regions and specific cognitive deficits, we adopted a two-set analytical approach: (1) to examine the correlations of composite FA across all the key tracts and (2) to examine the FA values of the left and right cingulum bundle-specific cognitive domains. Therefore, we developed two sets of linear regressions. In the first set, separate models were developed with a composite value of FA as the dependent variable. This composite FA value was calculated by averaging the FA values from each participant’s WM tracts (middle cerebellar peduncle, pontine cross tract, genu of corpus callosum, body of corpus callosum, splenium, fornix, corticospinal tract, medial lemniscus, inferior cerebellar peduncle, superior cerebellar peduncle, anterior limb of the internal capsule, posterior limb of internal capsule, retrolenticular internal capsule, anterior corona radiata, superior corona radiata, posterior corona radiata, posterior thalamic radiation, sagittal stratum, external capsule, cingulum bundle, fornix striata terminalis, superior longitudinal fasciculus, superior frontal occipital fasciculus, uncinate fasciculus, and tapetum). PTSD symptom severity (PCL-M score), total blast exposure, LOC, and the four cognitive scores (memory domain score, language/education domain score, attention/executive function domain score, and overall composite score) were the primary independent variables in this set, adjusting for age and education. In the second set, separate models were estimated with the FA values of the left and right cingulum bundle as dependent variables. PTSD symptom severity (PCL-M score), total blast exposure, LOC, and the four cognitive scores (included singly for each WM tract) were the primary independent variables while adjusting for age and education. This analysis was replicated in several other WM tracts implicated in studies of blast injury utilizing diffusion imaging techniques.
A final set of regression models examined neurocognition. Separate models were developed with the domain and composite cognitive scores as dependent variables. PTSD symptom severity (PCL-M score), total blast exposure, LOC, and the composite value of FA were the primary independent variables while adjusting for age and education.
In all models, linear and quadratic terms were explored for the explanatory variables. Semi-partial R2 estimates were calculated for each of the explanatory variables in each model, and an overall R2 was calculated for each model. All models were estimated using SPSS statistical software.
Pearson product-moment correlations were used to investigate the association between total blast exposure and cognitive functioning across the entire blast-exposed population. Two-tailed, unpaired t-tests examined differences in neuropsychological testing performance, PCL-M scores, and blast exposure between blast-exposed veterans who did and did not meet criteria for mTBI. An ANCOVA (Shapiro–Wilks; covariates were age and education) was used to examine between-group differences in FA: Group (no mTBI vs. mTBI) × WM tract (middle cerebellar peduncle, pontine cross tract, genu of corpus callosum, body of corpus callosum, splenium, fornix, corticospinal tract, medial lemniscus, inferior cerebellar peduncle, superior cerebellar peduncle, anterior limb of the internal capsule, posterior limb of internal capsule, retrolenticular internal capsule, anterior corona radiata, superior corona radiata, posterior corona radiata, posterior thalamic radiation, sagittal stratum, external capsule, cingulate, gyrus, fornix striata terminalis, superior longitudinal fasciculus, superior frontal occipital fasciculus, uncinate fasciculus, and tapetum).
Results
Neurocognitive Performance
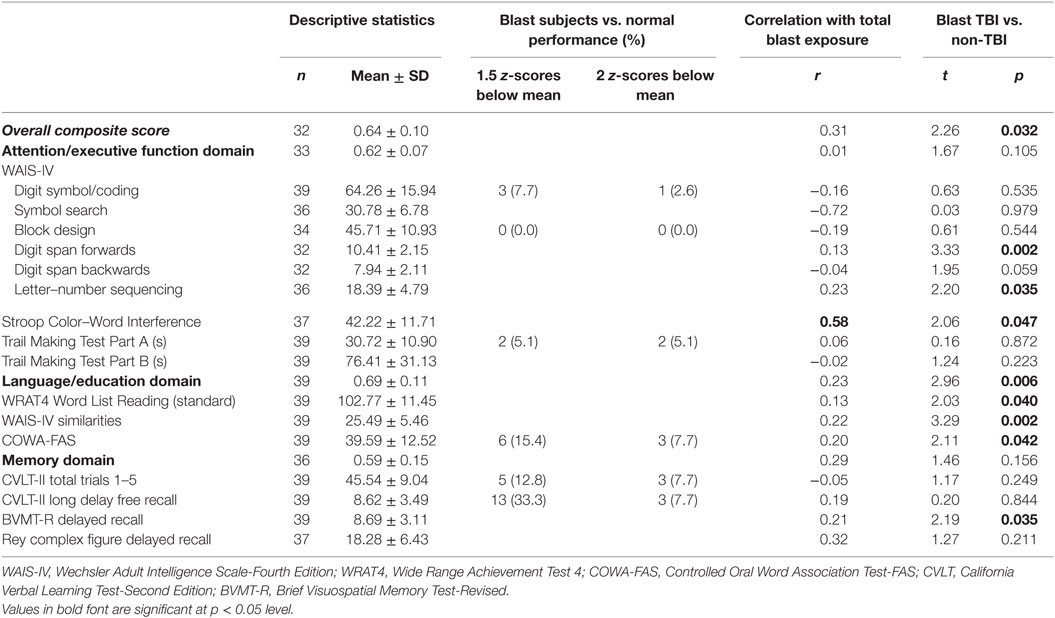
We first conducted a normative assessment comparing participant scores on certain individual neuropsychological tests to normalized datasets. The tests chosen for this analysis have well-established norms and are highly indicative of the domains outlined in Table 4. The frequency counts (i.e., number of veterans who performed below 1.5 SD below the mean and below 2 SD below the mean for these tests) are listed in the third column of Table 4. On tests of attention and executive function, participants performed close to the mean for their age. For example, not one participant (in either group) performed 1.5 SDs below the mean on block design. On language tests (COWA-FAS), a greater number of participants performed poorly. However, the worst performance was on memory tasks: one-third of participants performed 1.5 SDs or more below the mean for their age on the CVLT.
Next, we examined associations between total blast exposure and cognitive performance. Total blast exposure did not correlate well with performance on neuropsychological tests. Only the Stroop Task was significant: greater number of blasts was associated with poorer performance (all r values are reported in Table 4). Finally, on an exploratory basis, we examined whether there were differences between blast-exposed veterans who met criteria for blast-related TBI with those who did not meet criteria. Veterans with mTBI performed worse than the non-TBI veterans on every test administered. Table 4 reports t and p values.
WM Integrity
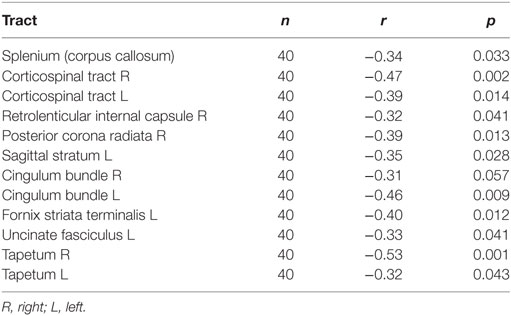
To examine the relationship between WM integrity and blast injury, we correlated the total number of blast exposures with DTI FA values across all participants. Increased blast exposure was associated with decreased FA in several WM tracts, including the corpus callosum, the corticospinal tract, the internal capsule, and the cingulum (Figure 2; Table 5). When cingulum was added as a covariate, the relationship between blasts and overall FA remained significant for the right (SE 0.0003; p = 0.04), but not for the left, cingulum (SE 0.0003; p = 0.32). This is in line with other reports suggesting that cingulum is a vulnerable area, which is often affected in blast injury (26, 51, 52) and may account for a significant portion of the findings. However, the overall FA measures survived significance for the right cingulum suggesting that the relationship between number of blasts and abnormalities in brain microstructure extends beyond that particular brain region. Additionally, the ANCOVA analyses indicated that there were no between-group differences in FA based on mTBI diagnosis (i.e., main effect for mTBI controlling for age and education was p > 0.74).
Figure 2. Example of a series of color orientation fractional anisotropy maps from one study participant where the primary colors represent principal directions of the various white-matter tracks (red: left–right, green: anterior–posterior, and blue: superior–inferior).
Predicting FA Values
Multiple linear regression analysis was employed to develop models predicting WM integrity. A first set of models predicted composite FA (i.e., averaged FA across 48 WM tracts) from PTSD symptom severity, total blast exposure, LOC, the four cognitive scores (memory domain score, language/education domain score, attention/executive function domain score, and overall composite score), age, and education. A model was developed including each cognitive score singly, resulting in four separate models of FA composite. Our results show that blast exposure predicts composite FA in the model including the attention/executive function domain score, such that as blast exposure increases, FA decreases (Table 6). Specifically, blast exposure explains 15% of the variability in composite FA scores when including the attention/executive function domain score. The six-predictor model was statistically significant, F(6,25) = 3.42, p = 0.01, and accounted for 45% of the variance in composite FA (R2 = 0.45, adjusted R2 = 0.32).
In the model of composite FA including the language domain scores, both total blast exposure and education had significant (p < 0.05) partial effects (Table 6). Total blast exposure explained 11% of the variability in this model, predicting composite FA such that as blast exposure increases, FA decreases (Figure 3). Education explained 10% of the variability in this model, predicting FA composite such that as the level of education increases, FA increases. The six-predictor model was statistically significant F(6,25) = 3.74, p < 0.01, and accounted for 47% of the variance in composite FA (R2 = 0.47, adjusted R2 = 0.35).
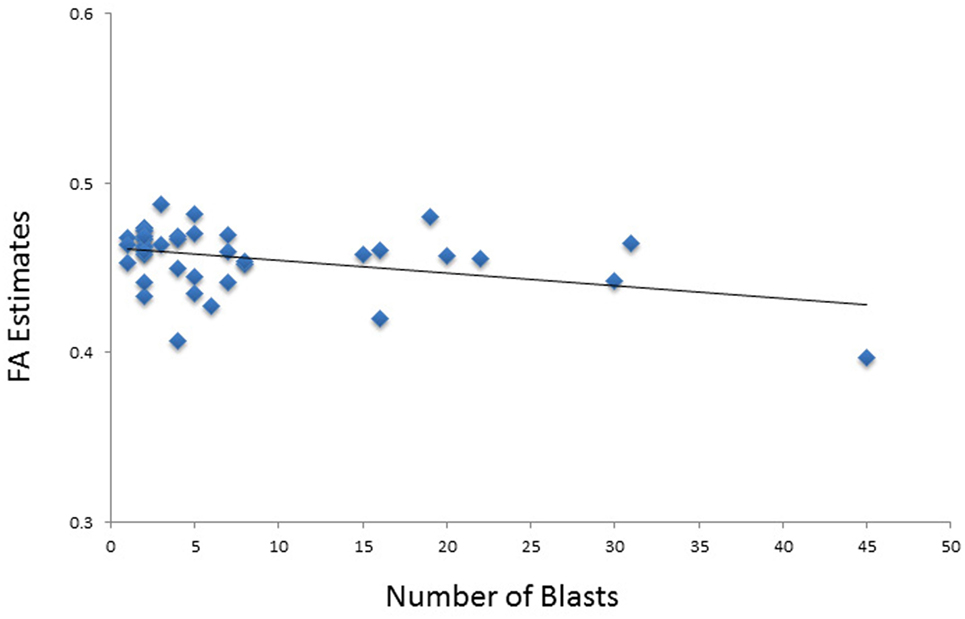
Figure 3. Scatterplot shows the relationship between total number of blasts and fractional anisotropy (FA) values.
We also examined FA individually in the right and left cingulum bundle. The independent variables included in these models were PTSD symptom severity, total blast exposure, LOC, the four cognitive scores, age, and education. Blast exposure and education had significant (p < 0.05) partial effects in two of the four models examining FA in the right cingulum bundle (Table 7). Our results indicate that education predicts FA in the right cingulum bundle such that as the level of education increases, FA increases. Education accounts for the largest share of the variability in FA in the right cingulum (25% of the model with neurocognitive composite scores and 32% with memory scores). Total blast exposure also significantly predicts FA in the right cingulum bundle, such that as blast exposure increases, FA decreases. The total number of blasts accounts for 18% of the variability of the model with the neurocognitive composite score and 19% of the variability of the model with the memory domain score.
Total blast exposure, age, and education were significant predictors of FA in the left cingulum bundle (Table 8). Blast exposure predicts FA in the left cingulum such that as the number of blasts increases, FA decreases. Specifically, blast exposure accounts for between 12 and 18% of variability in FA in the left cingulum. Age also inversely predicts FA in the left cingulum bundle, accounting for approximately 11% of variability in FA. Education accounted for the largest share of variability in FA in the left cingulum (23–27%) and predicts FA such that as the level of education increases, FA increases. When other individual WM tracts (listed in Table 5) were examined, nothing was found to be significant.
Predicting Cognitive Functioning
Multiple linear regression analysis was employed to develop models predicting cognitive functioning. Each of the four cognitive scores was examined as a dependent variable. PTSD symptom severity, total blast exposure, LOC, and FA were the primary independent variables while adjusting for age and education. We found no significant partial effects of these primary independent variables in multiple regression models predicting neurocognition.
Discussion
We examined the relationship between blast exposure and WM integrity using DTI and neurocognitive assessments in a cohort of Iraq and Afghanistan combat veterans who had multiple exposures during deployment but no formally diagnosed history of head trauma before military service. Two sets of findings emerged: first, reduced FA was associated with greater blast exposure, but not with the clinical diagnosis of mTBI. Second, while the blast-exposed veterans scored below the expected performance for their age on tasks of memory and language, participants with a clinical diagnosis of mTBI were more impaired than their blast-exposed non-TBI counterparts on these tasks, as well as on tasks of attention and executive functioning.
WM Integrity
Consistent with the hypotheses, the results suggest that WM integrity is compromised in veterans with blast exposure in relation to the dose of exposure, which corroborates the findings of a recent study by Trotter et al. (53). However, our findings are the first to show this dose–response relationship regardless of whether exposures were severe enough to result in LOC and/or clinical mTBI diagnosis. When examining FA in individual WM tracts, we found that greater blast exposure significantly predicted lower FA in the bilateral cingulum. The cingulum bundle is a complex structure that has been implicated in memory formation and executive function (54) as it contains prominent medial and dorsal prefrontal connections to the medial temporal and parietal lobes (55). It is uniquely vulnerable to cerebral trauma given its shape and location within the skull (56), and lasting changes to WM in this region following moderate–severe civilian mTBI have been reported (26, 57).
A study by Wu et al. (58) examined 12 civilian mTBI participants and 10 matched non-TBI controls. They reported that decreased FA in the bilateral cingulum bundle was associated with lower performance on an episodic verbal learning and memory task in the mTBI group, while no association was observed in controls (58). We did not find significant correlations between neuropsychological performance and FA, and cognitive domain scores were not significant predictors of WM changes either in models examining composite or individual WM tract FA. This suggests that the biological mechanisms that underlie cognitive deficits in the acute stages of mTBI (58) may differ from those still present years after deployment (our sample averaged 3.7 ± 1.9 years post-deployment). In clinical terms, this suggests that there might be a natural trajectory for the traumatized brain to recover and compensate for the effects of acute blast-related trauma as suggested by others (30). One can further hypothesize that early initiation of rehabilitation cognitive programs is warranted in veterans with history of blast exposures even if they may not meet criteria for TBI.
Blast exposure is thought to inflict injury through pressure waves transmitted in the air. Damage to the nervous system is thought to occur through biophysical mechanisms related to the shock wave’s impact on brain tissue (59–61). Blast injuries severe enough to cause moderate-to-severe TBI are without doubt a mix of injury mechanisms common to both blast and non-blast forms of injury. What is not well understood is the degree to which primary blast waves, particularly those of lesser intensity, may injure the brain directly, although studies in experimental animal models suggest that even relatively low-level blast exposure can cause direct injury (18, 62–64). This last notion potentially explains our finding that greater number of blast exposures is associated with compromised WM integrity or decreased FA, even in the absence of the clinical symptoms of mTBI. Nonetheless, FA changes in the cingulum in individuals with multiple blast exposures may represent a marker of vulnerability for future cognitive deficits.
WM Integrity and Education
Differences in WM integrity between individuals with varying levels of education may have influenced and offset the FA changes related to blast exposure. Education significantly predicted FA in both the left and the right cingulum, in the opposite direction as blast exposure. Education is thought to play a role in developing the WM microstructure and several groups have linked higher levels of education to higher FA (65, 66). Noble et al. (66) found that higher education is an age-independent predictor of WM integrity in late adolescence, and that greater educational attainment was associated with increased FA in the superior longitudinal fasciculus and in the cingulum bundle. Together, these observations suggest that higher levels of education before exposure to blast(s) could serve as a resilience factor that may help to mitigate the negative effects of blast injury on cognition. Moreover, a veteran’s education level may serve as an additional guide in developing rehabilitation programs that optimize clinical outcomes.
mTBI and PTSD
Post-traumatic stress disorder in veterans has been shown to negatively affect cognition (67, 68) and the contribution of individual disorders (e.g., TBI vs. PTSD) to cognitive deficits in veterans exposed to blast and other injuries remains a subject of debate. Our findings indicate that veterans who met criteria for mTBI indeed performed worse on a battery of neurocognitive tests in comparison to those who did not meet criteria for the clinical diagnosis of mTBI; also, we did not find a significant effect of PTSD on neuropsychological testing performance. As this study lacked a PTSD-only control group, this finding should be interpreted with caution. In clinical terms, if assumed that mTBI may account for most of the cognitive deficits in cases of comorbid mTBI and PTSD, then it is expected that improvements in cognition should be associated with TBI-focused treatments. However, as all diagnosed conditions deserve the appropriate treatment to be delivered simultaneously, the initiation of cognitive rehabilitation regardless of diagnoses seems prudent when cognitive difficulties are suspected or identified.
WM Integrity and Cognitive Functioning
Prior studies linking mTBI to decreased FA have suggested, by extension, that a loss of WM integrity could underlie cognitive deficits (18, 34, 53, 69–72). However, our hypothesis that lower FA would be associated with poorer performance on neuropsychological tests across cognitive domains was not supported. WM abnormalities did not explain the neurocognitive deficits. It is interesting in this context to note that only a minority of participants without an mTBI diagnosis (4 of the 16 veterans) had secondary or tertiary injuries, suggesting that most of these participants experienced only the effects of primary blast. By contrast, most veterans with a mTBI diagnosis experienced secondary or tertiary injuries suggesting a more mixed blast/non-blast mechanism of injury. Thus, while primary blast may alter WM integrity, the production of significant cognitive defects may require a combination of blast with non-blast secondary and tertiary injuries or a higher intensity blast exposure. This suggests considerable heterogeneity in relation to the causes for mTBI, and it is also possible that the cognitive deficits observed in veterans with mTBI were subserved by mechanisms other than changes in WM integrity. What is still unknown is the extent to which the cumulative effects of multiple blasts on WM integrity may confer vulnerability for neurological or psychological conditions and lead to the early development of neurodegenerative pathology. Further work is needed in order to elucidate the mechanism by which blast exposure affects brain structure in order to be able to develop potential therapeutics.
Study Limitations and Future Research
As is typical of neuroimaging research, there are limitations to the present study. The imaging protocol was conducted in accordance with the well-accepted and verified image acquisition techniques available at the time of the study. We acknowledge that there have been more recent improvements in the methodology of DTI image acquisition that offer certain advantages over the protocol used in this study (e.g., no gap in image acquisition). It should be emphasized, however, that all measures were taken to assure the highest possible quality of the neuroimaging data at the time of the study. Some investigators have suggested that improvements in data quality and implementation of more sophisticated tractography methods are unlikely to lead to increasingly accurate maps of human anatomical connections (73). Additionally, it is possible that the use of more specialized neuropsychological tests [or subsections of such tests, see Ref. (12, 13)] may have identified and isolated more discrete cognitive deficits. Such detailed assessments may have provided measures of cognitive impairment that might have been more associated with the biological measures of WM integrity. On the other hand, such assessments would have also increased the burden to participants. The total number of participants was limited to 40 making additional work important for replication of the study’s findings, especially with the inclusion of an additional control group with no blast exposure. Finally, the inclusion of a PTSD-only group would have helped to delineate WM profiles uniquely linked to PTSD; however, this was not a primary aim of the study. Future research is needed to address these issues.
Conclusion
This report documents new evidence that a greater total number of blast exposures is associated with lower WM integrity even in the absence of clinical TBI. Further, as the etiology of blast-related TBI is rather diverse, specific FA changes in the cingulum in veterans with multiple blast exposures is hypothesized to be a marker of vulnerability for future cognitive deficits.
Author Contributions
EMM was the PI of a VA Merit Grant that supported this research. CF drafted some sections of the paper and assisted with the data collection. II and DD wrote sections of the paper. EW and CT assisted in image processing and the statistical analysis of the neuroimaging data. JS and CB helped recruit and assess the study participants. EM consulted on the statistical analysis. GE worked on the paper and consulted on the research study. MS consulted on all aspects of the neuropsychology data. EH was a co-investigator on the VA Merit Grant, worked on the statistical analysis, data interpretation, and the paper.
Disclaimer
The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences, the Department of Defense; or, the United States Army, Navy, or Air Force (DLD).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was funded by Veteran’s Affairs (VA) Merit Awards (I01CX000190 to EMM and I01CX000261 to EH).
Abbreviations
COWA-FAS, Controlled Oral Word Association Test-FAS; CVLT, California Verbal Learning Test; DoD, Department of Defense; DTI, diffusion tensor imaging; DVBIC, Defense and Veterans Brain Injury Center; FA, fractional anisotropy; FOV, field of view; IED, improvised explosive device; ISMMS, Icahn School of Medicine at Mount Sinai; JJPVAMC, James J. Peters Veterans Affairs Medical Center; MD, mean diffusivity; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; PCL-M, PTSD Checklist-Military Version; PTSD, post-traumatic stress disorder; TBI, traumatic brain injury; TBSS, tract-based spatial statistics; TE, echo time; TR, repetition time; VA, Veteran’s Affairs; WM, white matter.
Footnotes
References
1. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med (2008) 358(5):453–63. doi:10.1056/NEJMoa072972
2. Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. Psychiatr Clin North Am (2010) 33(4):757–81. doi:10.1016/j.psc.2010.08.001
3. Tanielian T, Jaycox LH. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand Corporation (2008).
4. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol (2008) 167(12):1446–52. doi:10.1093/aje/kwn068
5. Chase RP, Nevin RL. Population estimates of undocumented incident traumatic brain injuries among combat-deployed US military personnel. J Head Trauma Rehabil (2015) 30(1):E57–64. doi:10.1097/HTR.0000000000000061
6. Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil (2009) 90(7):1084–93. doi:10.1016/j.apmr.2009.01.023
7. Lange RT, Brickell TA, Ivins B, Vanderploeg RD, French LM. Variable, not always persistent, postconcussion symptoms after mild TBI in U.S. military service members: a five-year cross-sectional outcome study. J Neurotrauma (2013) 30(11):958–69. doi:10.1089/neu.2012.2743
8. Haran FJ, Alphonso AL, Creason A, Campbell JS, Johnson D, Young E, et al. Analysis of post-deployment cognitive performance and symptom recovery in U.S. Marines. PLoS One (2013) 8(11):e79595. doi:10.1371/journal.pone.0079595
9. Troyanskaya M, Pastorek NJ, Scheibel RS, Petersen NJ, McCulloch K, Wilde EA, et al. Combat exposure, PTSD symptoms, and cognition following blast-related traumatic brain injury in OEF/OIF/OND service members and veterans. Mil Med (2015) 180(3):285–9. doi:10.7205/MILMED-D-14-00256
10. Karr JE, Areshenkoff CN, Garcia-Barrera MA. The neuropsychological outcomes of concussion: a systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology (2014) 28(3):321–36. doi:10.1037/neu0000037
11. Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc (2010) 16(2):262–7. doi:10.1017/S1355617709991287
12. Geary EK, Kraus MF, Pliskin NH, Little DM. Verbal learning differences in chronic mild traumatic brain injury. J Int Neuropsychol Soc (2010) 16(3):506–16. doi:10.1017/S135561771000010X
13. Geary EK, Kraus MF, Rubin LH, Pliskin NH, Little DM. Verbal learning strategy following mild traumatic brain injury. J Int Neuropsychol Soc (2011) 17(4):709–19. doi:10.1017/S1355617711000646
14. Rutgers DR, Fillard P, Paradot G, Tadie M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol (2008) 29(9):1730–5. doi:10.3174/ajnr.A1213
15. Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain (2008) 131(Pt 2):559–72. doi:10.1093/brain/awm294
16. Bigler ED. Mild traumatic brain injury: the elusive timing of “recovery”. Neurosci Lett (2012) 509(1):1–4. doi:10.1016/j.neulet.2011.12.009
17. Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci (2013) 7:395. doi:10.3389/fnhum.2013.00395
18. Elder GA, Stone JR, Ahlers ST. Effects of low-level blast exposure on the nervous system: is there really a controversy? Front Neurol (2014) 5:269. doi:10.3389/fneur.2014.00269
19. Inglese M, Bomsztyk E, Gonen O, Mannon LJ, Grossman RI, Rusinek H. Dilated perivascular spaces: hallmarks of mild traumatic brain injury. AJNR Am J Neuroradiol (2005) 26(4):719–24.
20. Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma (2007) 24(9):1447–59. doi:10.1089/neu.2007.0241
21. Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology (2008) 70(12):948–55. doi:10.1212/01.wnl.0000305961.68029.54
22. Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol (2010) 31(2):340–6. doi:10.3174/ajnr.A1806
23. Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol (2013) 34(11):2064–74. doi:10.3174/ajnr.A3395
24. Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, et al. Case report of a soldier with primary blast brain injury. Neuroimage (2009) 47(Suppl 2):T152–3. doi:10.1016/j.neuroimage.2009.01.060
25. Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma (2010) 27(4):683–94. doi:10.1089/neu.2009.1073
26. Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med (2011) 364(22):2091–100. doi:10.1056/NEJMoa1008069
27. Davenport ND, Lim KO, Armstrong MT, Sponheim SR. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage (2012) 59(3):2017–24. doi:10.1016/j.neuroimage.2011.10.050
28. Hayes JP, Morey RA, Tupler LA. A case of frontal neuropsychological and neuroimaging signs following multiple primary-blast exposure. Neurocase (2012) 18(3):258–69. doi:10.1080/13554794.2011.588181
29. Matthews SC, Spadoni AD, Lohr JB, Strigo IA, Simmons AN. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in Operations Enduring and Iraqi Freedom. Psychiatry Res (2012) 204(2–3):149–54. doi:10.1016/j.pscychresns.2012.04.018
30. Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry (2012) 169(12):1284–91. doi:10.1176/appi.ajp.2012.12050600
31. Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehabil (2013) 28(1):1–12. doi:10.1097/HTR.0b013e318256d3d3
32. Mac Donald C, Johnson A, Cooper D, Malone T, Sorrell J, Shimony J, et al. Cerebellar white matter abnormalities following primary blast injury in US military personnel. PLoS One (2013) 8(2):e55823. doi:10.1371/journal.pone.0055823
33. Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma (2014) 31(5):425–36. doi:10.1089/neu.2013.2952
34. Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, et al. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil (2015) 30(1):E15–25. doi:10.1097/HTR.0000000000000030
35. Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, Cantrill SV. Blast injuries. Lancet (2009) 374(9687):405–15. doi:10.1016/S0140-6736(09)60257-9
36. DePalma RG. Chap. 2: Combat TBI: history, epidemiology, and injury modes. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press (2015).
37. Elder GA, Gama Sosa MA, De Gasperi R, Stone JR, Dickstein DL, Haghighi F, et al. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front Neurol (2015) 6:48. doi:10.3389/fneur.2015.00048
38. Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev (2009) 46(6):C1–68. doi:10.1682/JRRD.2008.03.0038
39. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. Sydney, Australia: Pearson Clinical and Talent Assessment (2008).
40. Wilkinson GS, Robertson GJ. Wide Range Achievement Test-4 (WRAT-4). Lutz, FL: Psychological Assessment Resources Inc. (2006).
41. Golden CJ, Espe-Pfeifer P, Wachsler-Felder J. Neuropsychological Interpretation of Objective Psychological Tests. New York, NY: Springer Science & Business Media (2000).
42. Elwood RW. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol Rev (1995) 5(3):173–201. doi:10.1007/BF02214761
43. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition. San Antonio, TX: The Psychological Corporation (2000).
44. Benedict RH. Brief Visuospatial Memory Test-Revised. Odessa, FL: Psychological Assessment Resources, Inc. (1997).
45. Nandipati S, Luo X, Schimming C, Grossman HT, Sano M. Cognition in non-demented diabetic older adults. Curr Aging Sci (2012) 5(2):131–5. doi:10.2174/1874609811205020131
46. Post JB, Morin KG, Sano M, Jegede AB, Langhoff E, Spungen AM. Increased presence of cognitive impairment in hemodialysis patients in the absence of neurological events. Am J Nephrol (2012) 35(2):120–6. doi:10.1159/000334871
47. Huang H, Zhang J, van Zijl PC, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med (2004) 52(3):559–65. doi:10.1002/mrm.20147
48. Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol (1999) 82(1):290–300.
49. Carpenter DM, Tang CY, Friedman JI, Hof PR, Stewart DG, Buchsbaum MS, et al. Temporal characteristics of tract-specific anisotropy abnormalities in schizophrenia. Neuroreport (2008) 19(14):1369–72. doi:10.1097/WNR.0b013e32830abc35
50. Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage (2007) 36(3):630–44. doi:10.1016/j.neuroimage.2007.02.049
51. Yeh PH, Wang B, Oakes TR, French LM, Pan H, Graner J, et al. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp (2014) 35(6):2652–73. doi:10.1002/hbm.22358
52. Yeh PH, Guan Koay C, Wang B, Morissette J, Sham E, Senseney J, et al. Compromised neurocircuitry in chronic blast-related mild traumatic brain injury. Hum Brain Mapp (2017) 38(1):352–69. doi:10.1002/hbm.23365
53. Trotter BB, Robinson ME, Milberg WP, McGlinchey RE, Salat DH. Military blast exposure, ageing and white matter integrity. Brain (2015) 138(Pt 8):2278–92. doi:10.1093/brain/awv139
54. Lin YC, Shih YC, Tseng WY, Chu YH, Wu MT, Chen TF, et al. Cingulum correlates of cognitive functions in patients with mild cognitive impairment and early Alzheimer’s disease: a diffusion spectrum imaging study. Brain Topogr (2014) 27(3):393–402. doi:10.1007/s10548-013-0346-2
55. Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci (2009) 29(4):1175–90. doi:10.1523/JNEUROSCI.3328-08.2009
56. Bigler ED. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology (2007) 21(5):515–31. doi:10.1037/0894-4105.21.5.515
57. Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage (2008) 42(2):503–14. doi:10.1016/j.neuroimage.2008.04.254
58. Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma (2010) 27(2):303–7. doi:10.1089/neu.2009.1110
59. Kucherov Y, Hubler G, DePalma R. Blast induced mild traumatic brain injury/concussion: a physical analysis. J Appl Phys (2012) 112:104701–6. doi:10.1063/1.4765727
60. Gupta RK, Przekwas A. Mathematical models of blast-induced TBI: current status, challenges, and prospects. Front Neurol (2013) 4:59. doi:10.3389/fneur.2013.00059
61. Nakagawa A, Manley GT, Gean AD, Ohtani K, Armonda R, Tsukamoto A, et al. Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research. J Neurotrauma (2011) 28(6):1101–19. doi:10.1089/neu.2010.1442
62. Sosa MA, De Gasperi R, Paulino AJ, Pricop PE, Shaughness MC, Maudlin-Jeronimo E, et al. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun (2013) 1:51. doi:10.1186/2051-5960-1-51
63. Gama Sosa MA, De Gasperi R, Janssen PL, Yuk FJ, Anazodo PC, Pricop PE, et al. Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol Commun (2014) 2:67. doi:10.1186/2051-5960-2-67
64. Kamnaksh A, Budde MD, Kovesdi E, Long JB, Frank JA, Agoston DV. Diffusion tensor imaging reveals acute subcortical changes after mild blast-induced traumatic brain injury. Sci Rep (2014) 4:4809. doi:10.1038/srep04809
65. Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci U S A (2005) 102(41):14931–6. doi:10.1073/pnas.0506897102
66. Noble KG, Korgaonkar MS, Grieve SM, Brickman AM. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci (2013) 16(5):653–64. doi:10.1111/desc.12077
67. Uddo M, Vasterling JJ, Brailey K, Sutker PB. Memory and attention in combat-related post-traumatic stress disorder (PTSD). J Psychopathol Behav Assess (1993) 15(1):43–52. doi:10.1007/BF00964322
68. Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry (1995) 152(1):137–9. doi:10.1176/ajp.152.1.137
69. Adam O, Mac Donald CL, Rivet D, Ritter J, May T, Barefield M, et al. Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology (2015) 85(3):219–27. doi:10.1212/WNL.0000000000001758
70. Davenport ND, Lim KO, Sponheim SR. White matter abnormalities associated with military PTSD in the context of blast TBI. Hum Brain Mapp (2015) 36(3):1053–64. doi:10.1002/hbm.22685
71. Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. Neuroimage Clin (2015) 8:148–56. doi:10.1016/j.nicl.2015.04.001
72. Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum Brain Mapp (2016) 37(1):220–9. doi:10.1002/hbm.23022
Keywords: adult brain injury, diffusion tensor imaging, magnetic resonance imaging, cognitive function, military injury
Citation: Ivanov I, Fernandez C, Mitsis EM, Dickstein DL, Wong E, Tang CY, Simantov J, Bang C, Moshier E, Sano M, Elder GA and Hazlett EA (2017) Blast Exposure, White Matter Integrity, and Cognitive Function in Iraq and Afghanistan Combat Veterans. Front. Neurol. 8:127. doi: 10.3389/fneur.2017.00127
Received: 21 October 2016; Accepted: 17 March 2017;
Published: 21 April 2017
Edited by:
Kenneth Curley, Iatrikos Research and Development Solutions LLC, USAReviewed by:
Karin A. Rafaels, Army Research Laboratory, USAJoseph Bleiberg, Walter Reed National Military Medical Center, USA
Gerard Riedy, National Intrepid Center of Excellence (NICoE), USA
Copyright: © 2017 Ivanov, Fernandez, Mitsis, Dickstein, Wong, Tang, Simantov, Bang, Moshier, Sano, Elder and Hazlett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin A. Hazlett, ZXJpbi5oYXpsZXR0QG1zc20uZWR1
 Iliyan Ivanov
Iliyan Ivanov Corey Fernandez
Corey Fernandez Effie M. Mitsis1,3
Effie M. Mitsis1,3 Dara L. Dickstein
Dara L. Dickstein Charlene Bang
Charlene Bang Erin A. Hazlett
Erin A. Hazlett