
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 14 March 2017
Sec. Stroke
Volume 8 - 2017 | https://doi.org/10.3389/fneur.2017.00098
This article is part of the Research Topic Stroke in Elderly: Current Status and Future Directions View all 11 articles
Background: Although it is generally thought that patients with distal middle cerebral artery (M2) occlusion have a favorable outcome, it has previously been demonstrated that a substantial minority will have a poor outcome by 90 days. We sought to determine whether assessing the Alberta Stroke Program Early CT Score (ASPECTS) infarct location allows for identifying patients at risk for a poor 90-day outcome.
Methods: We retrospectively analyzed patients with isolated acute M2 occlusion admitted to a single academic center between January 2010 and August 2012. Infarct regions were defined according to ASPECTS system on the initial head computed tomography. Discriminant function analysis was used to define specific ASPECTS regions that are predictive of the 90-day functional outcome as defined as a modified Rankin Scale score of 3–6. In addition, logistic regression was used to model the relationship between each individual ASPECT region with poor outcome; for evaluation and comparison, odds ratios, c-statistics, and Akaike information criterion values were estimated for each region.
Results: Ninety patients with isolated M2 were included in the final analysis. ASPECTS score ≤6 predicted poor outcome in this cohort (sensitivity = 0.591, specificity = 0.838, p < 0.001). Using multiple approaches, we found that infarction in ASPECTS regions M3 and M6 were strongly associated with poor functional status by 90 days.
Conclusion: Infarction in ASPECTS regions M3 and M6 are key predictors of functional outcome following isolated distal M2 occlusion. These findings will be helpful in stratifying outcomes if validated in future studies.
The site of arterial occlusion represents one of the most important factors determining outcome after anterior circulation ischemic stroke (1, 2). However, relatively little is known regarding outcome predicting variables in patients with distal middle cerebral artery (MCA) occlusion.
The Alberta Stroke Program Early CT Score (ASPECTS) was introduced to provide a structured infarct size and location analysis to aid clinical decision making (3). Its availability on initial evaluation of non-contrast head computed tomography (CT) makes it a relevant neuroimaging marker that does not require complex image post-processing. Its utility for acute treatment decision making in acute stroke patients with MCA occlusion has previously been documented (4). Specifically, stroke patients with a high pre-treatment ASPECTS are more likely to have a favorable outcome. However, because patients with distal MCA occlusion (M2) tend to have a high ASPECTS related to the sparing of subcortical tissues (5–7), a more granular understanding of the association of the ASPECTS with outcome in these patients is needed.
A better understanding of this issue is highlighted by the transformative results from the recent positive endovascular stroke trials (2, 4, 8–10). However, less than 3% of enrolled patients had an isolated M2 occlusion and the majority of patients experience a favorable outcome irrespective of endovascular therapy (4, 7, 11). Hence, identifying imaging parameters that predict poor outcome in this population may aid in the proper patient selection for therapy.
Therefore, we sought to determine whether information regarding infarct location encoded in the ASPECTS allows for defining patients at high risk for a poor functional outcome after isolated M2 occlusion in addition to the total ASPECTS score. We hypothesized that infarction in distinct ASPECTS location will be associated with a poor outcome.
We performed a retrospective analysis of consecutive acute ischemic stroke patients admitted to a single academic center from January 2010 to August 2012. Only patients with isolated M2 occlusion on admission CT-angiography (CTA) were included. This study was approved by the Institutional Review Board of the University of Massachusetts Medical School. Because this was a retrospective analysis the consent requirement was waived. Included patients have been described as part of a prior study (12).
All patients had head CT performed on presentation to the emergency room. Patient demographics, comorbidities, pre-admission medications, laboratory data, treatment modality [conservative management versus acute intervention (intravenous thrombolysis and endovascular recanalization)], and stroke etiology (according to the Trial of Org 10172 in Acute Stroke Treatment classification) (13) after completion of diagnostic evaluation, were collected on all patients. Admission National Institutes of Health Stroke Scale and modified Rankin Scale (mRS) scores were assessed at the time of presentation and at 90 days by a stroke-trained physician or study nurse certified in the mRS (12). Good outcome at 90 days was defined as mRS ≤2.
All CT sequences were obtained on a 64-row detector Philips scanner. NCCT was performed in a non-helical mode at 120 KvP and 200 mA with data reconstruction at 5 mm axial slices. CTA was performed using 64 mm × 0.625 mm detector configuration with a pitch of 0.673, from the arch of aorta to the vertex using 120 KvP, 300 mA, and 0.5-s rotation time. Patients received 60–80 mL of Isovue 370 (Bracco Diagnostics, Princeton, NJ, USA) in the antecubital vein at a rate of 4 mL/s through a power injector followed by 40 mL saline. Three-dimensional orthogonal maximum intensity projection images were created in three planes.
Image review and analysis has been described in detail earlier (12). Briefly, CT and CTA were reviewed independently by study physicians masked to clinical data, follow-up scans, patient variables, and outcomes. The M2 segment was defined as the segment extending from the bifurcation/trifurcation of the MCA to the top of the Sylvian fissure to further division (12). For the purpose of this study, we performed an infarct location analysis on the initial head CT as defined by ASPECTS system (3). These areas included the insula (I), caudate nucleus (C), lentiform nucleus (L), internal capsule (IC), superior parietal lobe (M6), precentral and superior frontal lobe (M5), anterior superior frontal lobe (M4), inferior parietal and posterior temporal lobe (M3), temporal lobe (M2), and anterior inferior frontal lobe (M1) (14). Scoring was conducted by an experienced reader (Muhib Khan) trained in ASPECTS (http://www.aspectsinstroke.com).
All analyses were conducted using SAS Software 9.4 (SAS Inc., Cary, NC, USA). Poor outcome (mRS 3–6) was modeled with ASPECTS score using multivariable logistic regression, both as an ordinal measure and dichotomized >6 versus ≤6. Discriminant function analysis (DFA) was used to derive a single statistical function from all ASPECTS regions that distinguishes between a poor and favorable outcome; ASPECT regions were then evaluated by how well each region correlated with the derived discriminant function distinguishing between a poor and favorable outcome. DFA was used as it is more robust than multivariable logistic regression for small sample sizes. DFA was produced using the CANDISC procedure. In order to confirm and quantify the relationship between each region and outcome, logistic regression was used to model the relationship between each individual ASPECT region with poor outcome using the LOGISTIC procedure; odds ratios (OR) (effect size), c-statistics [area under the receiver operating characteristics curve (AUC)], and Akaike information criterion (AIC) (AIC-relative model fit) values were, therefore, estimated for each region. In addition, sensitivity, specificity, as well as positive and negative predictive values and likelihood ratios were determined for each ASPECTS region predicting poor outcome using generalized estimating equations (GEE), which allowed multiple observations (regions) to be nested within patients, using the GLIMMIX procedure. Group differences examined in Table 1 were compared using the χ2-square test or Wilcoxon test. A two sided p < 0.05 (when applicable) was considered significant and all estimates were calculated for 95% confidence interval (95% CI).
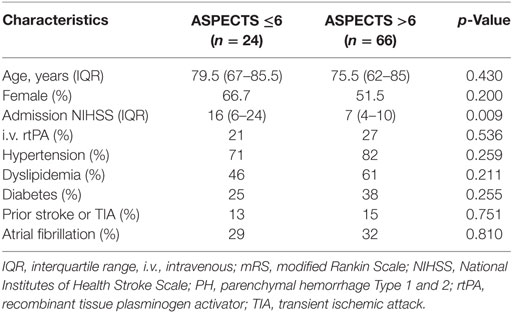
Table 1. Baseline characteristics (unadjusted) of the patient sample as stratified by Alberta Stroke Program Early CT Score (ASPECTS).
Overall, 90 patients with isolated M2 occlusions were included for analysis. Among these, 66 (73%) patients had an ASPECTS of >6 and the majority of patients (69%) had a good 90-day outcome. Baseline characteristics of included patients as stratified by ASPECTS ≤6 versus >6 (15) are summarized in Table 1.
The mean ASPECTS score was 7.1, 95% CI 6.8–7.4. An ASPECTS score ≤6 was associated with poor outcome (sensitivity = 0.591, specificity = 0.838, p < 0.001). Logistic regression analysis indicated that for every unit decrease in ASPECTS score, the odds of poor outcome increased more than twofold (OR: 2.32, 95% CI 1.50–3.59, AUC = 0.799, p < 0.001). We then sought to determine whether distinct infarct regions encoded by the ASPECTS allow for defining patients at risk for a poor functional outcome. Because more than one ASPECTS region was frequently infarcted in a given patient, DFA was used to model the relationship of region on poor outcome. Table 2 summarizes each region’s relationship with poor outcome derived from the DFA whereby larger loading and coefficient values indicate a stronger relationship with the statistically derived discriminant function that distinguished between and poor and favorable outcome. In particular, infarction in M3 had the strongest relationship with the discriminant function relative to all other regions, followed by M6. The relationship with each region, alone, was then quantified using logistic regression, which indicated that infarction in M3 and M6 had statistically significant and relatively large OR (right M3 = 115, left M3 = 14.9, right M6 = 25.7, left M6 = 10.4), indicating a relationship with poor outcome. Moreover, infarction in M3 and M6 had the largest AUCs indicating superior discrimination between good and poor outcomes, relative to all other areas. M3 and M6 also had the smallest AIC values, indicating that models of poor outcome using M3 and M6 were superior to models using other ASPECTS encoded regions, i.e., the combined analysis with logistic regression and DFA indicates that infarction in cortical M3 and adjacent M6 ASPECTS regions on either side are the strongest predictors of a poor outcome after M2 occlusion. Infarction in the left M5, right M2, and right M5 should also be considered as indicators of poor outcome as they were statistically significant or approached significance relative to the remaining regions.
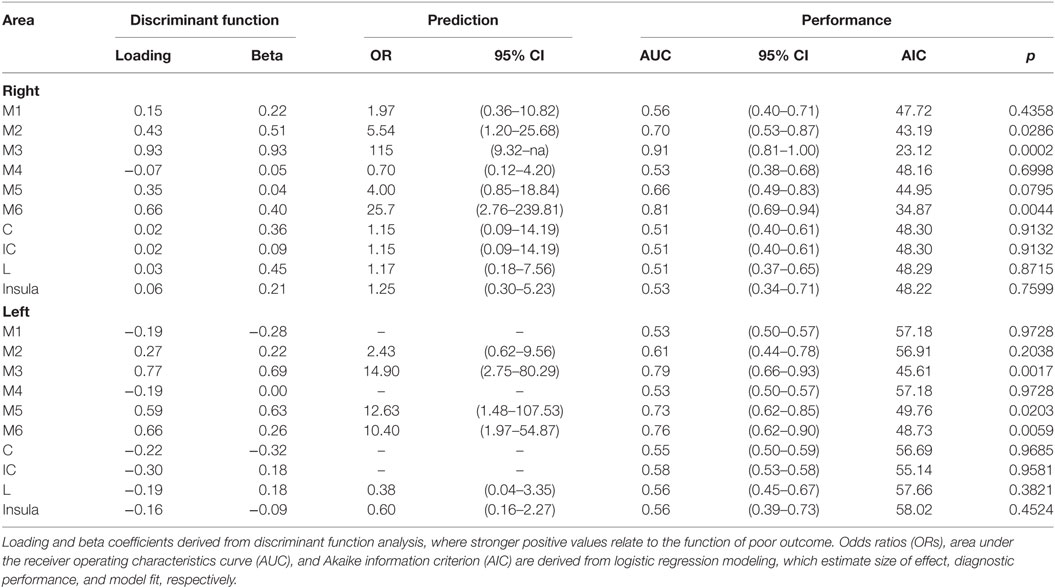
Table 2. Discriminate function analysis, prediction, and performance of individual Alberta Stroke Program Early CT Score region infarction indicative of a poor outcome.
Last, each region was evaluated for diagnostic performance of poor outcome. Using GEE, we found that M3, M5, and M6 for both the right and the left had a high sensitivity and specificity (p < 0.01) for predicting a poor outcome (Table 3). Specifically, infarction in right M3 region was highly sensitive (0.91) and specific (0.92) of poor outcome.
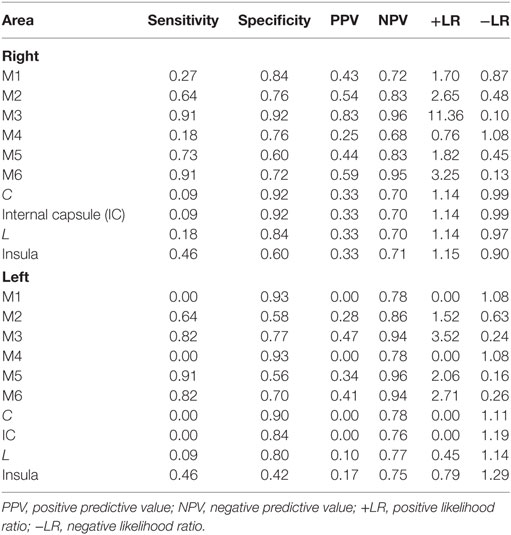
Table 3. Sensitivity, specificity, positive and negative predictive values, and likelihood ratios of individual Alberta Stroke Program Early CT Score region infarction for poor outcome (mRS >2).
The most important findings of our study are that infarction in the M3 and M6 ASPECTS region predict a poor outcome after isolated M2 occlusion. We and others have previously shown that a substantial minority of patients with M2 occlusion have a poor outcome by 3 months (7, 11, 12, 16). Our results show that the anatomical and geometric information of the ASPECTS allows for more specific identification of patients at high risk for poor outcome after isolated M2 occlusion.
The ASPECTS template for anatomical lesion mapping within the MCA territory has the advantage that it does not require elaborate volumetric image analysis and allows for identifying patients likely to have a poor outcome in the emergency setting (4, 17, 18). The dichotomized ASPECTS score has been used to predict outcome in earlier studies, which found an ASPECTS greater than 6 to 7 to be predictive of good outcome (3, 4, 17, 19). Recent multicenter cohort of M2 occlusion patients undergoing thrombectomy also showed that ASPECTS ≥6 predicts a good outcome (7). Consistent with these prior results, we found that ASPECTS ≥6 predicted a good outcome.
Using DFA, we found that in our sample, infarction in M3 and M6 locations were most predictive of a poor outcome. This is not too surprising because even relatively small infarcts in the M3 region (which includes the language centers located within the inferior parietal and posterior temporal lobes) and M6 region (which includes the primary motor cortex) may cause significant disability (20, 21) related to ensuing motor deficits and aphasia (22, 23). Of note, though infarction of subcortical structures such as lentiform nucleus and IC have been identified as predictors of outcome (20, 24, 25), we did not find such an association in our study. However, this discrepancy may be explained by the fact that our study was underpowered to detect such an association due to low number of patients with a subcortical infarction. It is possible that subcortical infarction occurred mostly in patients who originally had an occlusion of the M1-segment of the MCA [which gives rise to the lenticulostriate arteries to supply the striatocapsular region (26, 27)] and then subsequent clot migration to the M2 segment [which predominantly supplies the cerebral cortex and adjacent white matter (26–28)] by the time of CTA.
We did not find any effect on outcome based on hemispheric laterality of stroke. Earlier studies have shown an impact of hemispheric laterality on functional outcomes after ischemic stroke (29–32). We feel that smaller infarct volume and neglect might have played a role in minimizing the effect of lateralization. Further study is required to determine the effects of lesion laterality and the impact on stroke outcome. Endovascular therapy in these patients has recently been shown to improve outcomes (7). Therefore, further research is needed using an external stroke cohort evaluating our findings to improve patient selection for this particular group. We envision the development of an imaging index that incorporates assessments of ischemic core volumes and location in conjunction with markers of collateral and perfusion status. Such an index will allow for accurate and rater unbiased selection of patients for emergent recanalization therapies for M2 occlusion.
The strengths of our study relate to the analysis of a well-defined patient population based on advanced intracranial vascular imaging. We utilized multiple statistical methods in a stepwise fashion to identify the ASPECTS regions, which predict outcomes in this population. The collection of a large number of clinically relevant variables contributed to improved data quality while limiting the potential for misclassification. Limitations of our study relate to its retrospective design and relatively small sample size. However, to account for the low sample size, we used DFA instead of logistic regression alone. Williams and Titus found that, as a general rule, sample size should be about 3xP variables [in our case p = 10, for each group (30)] (33). In addition to mitigate the risk of DFA-related model overfitting, we also provided the results of simple logistic regression as well as repeated measures logistic regression with modeling of the different ASPECTS regions within each patient. Importantly, our models were stable as indicated by the consistent results across model solutions (sensitivity, specificity, loadings, odds ratio, area under the receiver operator characteristics curve, AIC, and p-Values). Further, diagnostic and therapeutic modalities were used at the treating stroke physician’s discretion, which may have introduced bias. Moreover, non-contrast CT head was used to determine ASPECTS, which might be less sensitive than newer imaging modalities. Our findings require confirmation in further, prospective studies and should only be considered hypothesis generating.
In conclusion, this study shows that infarct topography as defined by ASPECTS allows for prediction of functional outcome after M2 occlusion. These findings may be helpful for stratifying outcomes if validated in other studies.
MK: study concept and design, data acquisition, statistical analysis, interpretation of data, and drafting the article. GB: statistical analysis, interpretation of data, and drafting the article. RG: data acquisition, interpretation of data, and critical revision of the manuscript for important intellectual content. BS: interpretation of data and critical revision of the manuscript for important intellectual content. NH: data acquisition, interpretation of data, drafting the article, and critical revision of the manuscript for important intellectual content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
NH is supported by grant K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. All other authors have nothing to disclose.
1. Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke (2007) 38:948–54. doi: 10.1161/01.STR.0000257304.21967.ba
2. Broderick JP, Tomsick TA, Palesch YY. Endovascular treatment for acute ischemic stroke. N Engl J Med (2013) 368:2430–35. doi:10.1056/NEJMc1304759
3. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet (2000) 355:1670–4. doi:10.1016/S0140-6736(00)02237-6
4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med (2015) 372:1019–30. doi:10.1056/NEJMoa1414905
5. Gupta D, Sharma A, Uchino K, Alexandrov AV, Khan K, Shuaib A, et al. Accuracy of National Institutes of Health Stroke Scale score in predicting the site of arterial occlusion in acute stroke: a Transcranial Doppler Study. J Stroke Cerebrovasc Dis (2016) 25:2109–15. doi:10.1016/j.jstrokecerebrovasdis.2016.06.004
6. Hill MD, Demchuk AM, Goyal M, Jovin TG, Foster LD, Tomsick TA, et al. Alberta Stroke Program early computed tomography score to select patients for endovascular treatment: Interventional Management of Stroke (IMS)-III Trial. Stroke (2014) 45:444–9. doi:10.1161/STROKEAHA.113.003580
7. Sarraj A, Sangha N, Hussain MS, Wisco D, Vora N, Elijovich L, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol (2016) 73(11):1291–6. doi:10.1001/jamaneurol.2016.2773
8. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med (2015) 372:1009–18. doi:10.1056/NEJMoa1414792
9. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med (2015) 372:2285–95. doi:10.1056/NEJMoa1415061
10. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med (2015) 372:2296–306. doi:10.1056/NEJMoa1503780
11. Rahme R, Yeatts SD, Abruzzo TA, Jimenez L, Fan L, Tomsick TA, et al. Early reperfusion and clinical outcomes in patients with M2 occlusion: pooled analysis of the PROACT II, IMS, and IMS II studies. J Neurosurg (2014) 121:1354–8. doi:10.3171/2014.7.JNS131430
12. Khan M, Goddeau RP Jr, Zhang J, Moonis M, Henninger N. Predictors of outcome following stroke due to isolated M2 occlusions. Cerebrovasc Dis Extra (2014) 4:52–60. doi:10.1159/000360075
13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke (1993) 24:35–41. doi:10.1161/01.STR.24.1.35
14. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. The prognosis for aphasia in stroke. J Stroke Cerebrovasc Dis (2012) 21:350–7. doi:10.1016/j.jstrokecerebrovasdis.2010.09.009
15. Puetz V, Dzialowski I, Hill MD, Demchuk AM. The Alberta Stroke Program Early CT Score in clinical practice: what have we learned? Int J Stroke (2009) 4:354–64. doi:10.1111/j.1747-4949.2009.00337.x
16. Rahme R, Abruzzo TA, Martin RH, Tomsick TA, Ringer AJ, Furlan AJ, et al. Is intra-arterial thrombolysis beneficial for M2 occlusions? Subgroup analysis of the PROACT-II trial. Stroke (2013) 44:240–2. doi:10.1161/STROKEAHA.112.671495
17. Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR, et al. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke (2005) 36:2110–5. doi:10.1161/01.STR.0000181116.15426.58
18. Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke (2009) 40:2743–8. doi:10.1161/STROKEAHA.109.550111
19. Demchuk AM, Goyal M, Menon BK, Eesa M, Ryckborst KJ, Kamal N, et al. Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke (2015) 10:429–38. doi:10.1111/ijs.12424
20. Phan TG, Chen J, Donnan G, Srikanth V, Wood A, Reutens DC. Development of a new tool to correlate stroke outcome with infarct topography: a proof-of-concept study. Neuroimage (2010) 49:127–33. doi:10.1016/j.neuroimage.2009.07.067
21. Menezes NM, Ay H, Wang Zhu M, Lopez CJ, Singhal AB, Karonen JO, et al. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke (2007) 38:194–7. doi:10.1161/01.STR.0000251792.76080.45
22. Meijer R, Ihnenfeldt DS, de Groot IJ, van Limbeek J, Vermeulen M, de Haan RJ. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clin Rehabil (2003) 17:119–29. doi:10.1191/0269215503cr585oa
23. Haselbach D, Renggli A, Carda S, Croquelois A. Determinants of neurological functional recovery potential after stroke in young adults. Cerebrovasc Dis Extra (2014) 4:77–83. doi:10.1159/000360218
24. Phan TG, Demchuk A, Srikanth V, Silver B, Patel SC, Barber PA, et al. Proof of concept study: relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebrovasc Dis (2013) 35:560–5. doi:10.1159/000351147
25. Weiller C, Willmes K, Reiche W, Thron A, Isensee C, Buell U, et al. The case of aphasia or neglect after striatocapsular infarction. Brain (1993) 116(Pt 6):1509–25. doi:10.1093/brain/116.6.1509
26. Marinkovic S, Gibo H, Milisavljevic M. The surgical anatomy of the relationships between the perforating and the leptomeningeal arteries. Neurosurgery (1996) 39:72–83. doi:10.1097/00006123-199607000-00016
27. Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg (1981) 54:151–69. doi:10.3171/jns.1981.54.2.0151
28. Rangaraju S, Owada K, Noorian AR, Nogueira RG, Nahab F, Glenn BA, et al. Comparison of final infarct volumes in patients who received endovascular therapy or intravenous thrombolysis for acute intracranial large-vessel occlusions. JAMA Neurol (2013) 70:831–6. doi:10.1001/jamaneurol.2013.413
29. Di Legge S, Saposnik G, Nilanont Y, Hachinski V. Neglecting the difference: does right or left matter in stroke outcome after thrombolysis? Stroke (2006) 37:2066–9. doi:10.1161/01.STR.0000229899.66019.62
30. Yoo AJ, Romero J, Hakimelahi R, Nogueira RG, Rabinov JD, Pryor JC, et al. Predictors of functional outcome vary by the hemisphere of involvement in major ischemic stroke treated with intra-arterial therapy: a retrospective cohort study. BMC Neurol (2010) 10:25. doi:10.1186/1471-2377-10-25
31. Laufer Y, Sivan D, Schwarzmann R, Sprecher E. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabil Neural Repair (2003) 17:207–13. doi:10.1177/0888439003259169
32. Helenius J, Goddeau RP Jr, Moonis M, Henninger N. Impact of leukoaraiosis burden on hemispheric lateralization of the National Institutes of Health Stroke Scale deficit in acute ischemic stroke. Stroke (2016) 47:24–30. doi:10.1161/STROKEAHA.115.011771
Keywords: M2 occlusions, outcome, Alberta Stroke Program Early CT Score, stroke, thrombolysis
Citation: Khan M, Baird GL, Goddeau RP Jr., Silver B and Henninger N (2017) Alberta Stroke Program Early CT Score Infarct Location Predicts Outcome Following M2 Occlusion. Front. Neurol. 8:98. doi: 10.3389/fneur.2017.00098
Received: 01 November 2016; Accepted: 28 February 2017;
Published: 14 March 2017
Edited by:
Jean-Claude Baron, Université Paris Descartes, FranceReviewed by:
Daniel Strbian, Helsinki University Hospital, FinlandCopyright: © 2017 Khan, Baird, Goddeau, Silver and Henninger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhib Khan, bXVoaWIua2hhbkBzcGVjdHJ1bWhlYWx0aC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.