
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 December 2015
Sec. Neurodegeneration
Volume 6 - 2015 | https://doi.org/10.3389/fneur.2015.00263
This article is part of the Research Topic Neuropsychiatric impairment in Parkinson's Disease View all 8 articles
Extracampine hallucinations (EH), the sense of a presence or fleeting movement in the absence of an associated visual percept, have been reported in Parkinson’s disease (PD) patients but their prevalence, characteristics, and temporal relationship to visual hallucinations (VH) remain unclear. Given that, VH are predictive of cognitive impairment in PD, improved understanding of EH may have significant prognostic implications. The objective of this study is to evaluate the prevalence and characteristics of EH in a large unselected population with PD and to assess the temporal relationship between EH, VH, and memory decline. Cross-sectional data were collected from 414 PD patients using a questionnaire circulated via an online patient community. Data were obtained regarding the occurrence, timing, and characteristics of VH and EH and symptoms of PD, disease duration, disease severity, and medication history. About 50.4% of respondents reported EH and 15.5% reported VH. EH were typically experienced alongside, rather than behind, the individual (p < 0.001) without clear lateralization (p = 0.438) and were more likely to be of unfamiliar presences (p < 0.001). The occurrence of EH was associated with Hoehn and Yahr score (p = 0.002) but not disease duration (p = 0.158). EH onset was associated with VH onset (p = 0.046) and occurred after the onset of anosmia (p < 0.001), cognitive decline (p = 0.002), and sleep disturbance (p = 0.002). The reported prevalence of EH in PD patients was threefold greater than that of VH, with similar timings of onset, suggesting that EH are under-recognized and under-reported. Further work is needed to determine whether EH are predictive of cognitive decline.
Hallucinations, defined as “sensory perceptions without external stimulation of the relevant sensory organ” (1), are a common feature of Parkinson’s disease (PD) and are a poor prognostic marker associated with increased risk of nursing home placement and cognitive decline (2, 3). Various types of hallucinations have been described in PD, including formed visual hallucinations (VH), auditory hallucinations, and extracampine hallucinations (EH), the latter denoting hallucinations occurring outside the visual field (campus, from Latin, field) (4, 5).
Of these hallucinations, VH are the most commonly described with the lifetime prevalence in PD patients approaching 75% (6). The presence of VH is typically a late feature of PD (4, 7) and is predictive of cortical Lewy body pathology at post-mortem (8, 9). VH in PD are associated with the occurrence of cognitive dysfunction, daytime somnolence, and sleep disorders (4, 10, 11). Although their neural basis is not yet fully established, structural and functional neuroimaging studies have implicated a variety of brain systems, including anterior visual pathways, brainstem, and visual association regions in their pathogenesis (12). More specifically, evidence suggests a role for altered cortical visual processing in the pathogenesis of VH in PD (13).
In addition, antiparkinsonian drug therapy has been reported to precipitate psychosis (14, 15) with certain drugs, particularly dopamine agonists, being linked to VH in PD (16). However, dopaminergic drug use is not sufficient to explain the occurrence of all VH in PD (4). Furthermore, drug-induced psychosis in PD may persist even when the offending medication is withdrawn (17), and there appears to be a poor correlation between drug exposure and symptom severity (18).
In contrast to VH, EH (5) are not associated with any visual percept. Instead, EH are experienced as the sense of a presence beside or behind, or as a fleeting sense of movement alongside, the individual perceiving this phenomenon. In previous studies of hallucinoses associated with PD, EH have been classified as “minor hallucinations,” which, when combined with illusions, is associated with a prevalence ranging from 17 to 72% (4, 19–21). Several explanations exist for this wide range in reported prevalence, including heterogeneity of study populations and methodological differences in assessing the presence of these symptoms (21).
The occurrence of EH is not specific to PD; they have been reported in cerebrovascular disease, epilepsy, sleep paralysis, and traumatic brain injury (22–24). Aside from neurological disorders, EH have been described by individuals in situations associated with extreme physical hardship, such as shipwreck survivors, mountaineers, and polar explorers (25–27), perhaps most famously by Ernest Shackleton during his failed Antarctic expedition of 1914–1917.
… it seemed to me often that we were four, not three. I said nothing to my companions on the point, but afterwards Worsley said to me, ‘Boss, I had the curious feeling on the march that there was another person with us’ (25)
In the context of neurodegenerative diseases, the presence of VH is a relatively specific feature of cortical Lewy body disease (9, 28) and forms one component of the diagnostic criteria for Dementia with Lewy Bodies (DLB) and Parkinson’s Disease Dementia (8, 29). Given that, VH are predictive of future cognitive decline (30–32), it is possible that other types of hallucinosis may also have predictive value, notably those likely to be indicative of cortical involvement.
Extracampine hallucinations are by definition less overt than VH, and therefore may be both under-reported by patients and under-investigated by clinicians, who do not routinely include questions about EH in their history taking (5). Additionally, while the neural substrate of EH has yet to be clarified, their phenomenological nature would implicate higher order association cortices in EH generation beyond any reasonable doubt. As such, it is important to establish whether EH represent a cortical marker of disease in patients with PD, and thus are predictive of future cognitive and clinical decline. Such determination would have major implications for biomedical research, in terms of disease phenotyping and natural history studies, and for clinical practice, with the assessment of EH in patients with PD potentially acting as a prognostic marker alerting clinicians to impend cognitive decline. In practice, EH focused questions could be included in the routine clinical history or, in the future, could be assessed in specialist PD clinics using a validated EH questionnaire similar to those currently in use for the assessment of VH (33).
However, prior to any such studies, the first step is to obtain a better understanding of the occurrence of EH in patients with PD, in view of the limited information available at present. The primary objective of this study was therefore to determine the prevalence of EH in patients with PD. The secondary objectives are to identify the main characteristics of EH (for example, emotional content, familiarity of percept, laterality of EH) and to assess the temporal relationship between the onset of EH and other key symptoms of PD, specifically VH, cognitive impairment, and REM sleep behavior disorders. In order to obtain these data from a more representative sample of PD patients, and thus offset the potential ascertainment bias associated with sampling from a specialist PD clinics, information about EH occurrence were acquired by way of a web-based questionnaire made available to an online social networking community of patients, patientslikeme® (34).
patientslikeme® is an online patient network where members share personal health data to track their disease progression, communicate with other patients, and contribute to research studies. Members of the community are regularly invited to participate in optional questionnaire-based research studies (34). Although patients with any illness are able to sign up to the website, patientslikeme® has a large online community of individuals with PD; at the time of this study, there were 6841 such members.
All members of the patientslikeme® community who had previously self-identified as having PD were invited to participate in the study via email (Figure 1). The link provided in the email enabled potential participants to access the study information and indicate whether or not they wished to participate. Submission of the completed questionnaire was interpreted as provision of consent for study enrollment in line with ethical guidelines from The University of Sussex on the acquisition of anonymous questionnaire-based data from patient groups (35). The online survey remained open for 1 week and to verify the test–retest reliability of the EH questionnaire and, in line with routine practice in questionnaire-based surveys hosted on the patientslikeme® platform, all participants were invited by email to complete a retest phase 2 weeks later in which an identical questionnaire was administered.
Ethical approval for this study was obtained from the South East Coast – Surrey Regional Ethics Committee (reference number 12/LO/1001).
Those included in the study were adults with a self-reported diagnosis of PD. Respondents were excluded if they had self-reported Lewy body dementia, PD with associated dementia, a non-PD neurodegenerative disorder, or moderate-to-severe visual impairment (cataracts, macular degeneration, diabetic eye disease, or registered blind).
The study instrument was a novel, web-based, questionnaire, which was completed via the patientslikeme® online platform. The questions were generated by the study authors with input from patients with PD and their caregivers. Participants were informed of the nature of the study in terms of the information being requested but were blinded to the specific hypotheses being tested, in order to eliminate reporting bias. In order to increase the response rate, an email reminder was sent after the initial launch.
All data were self-reported and information was gathered regarding demographics, co-morbidities, and current and previous experiences of illusions (Q1, Table 1), VH (Q22–26), and EH (Q2–21). Two questions were used to assess whether or not participants had experienced EH (Q2 and Q6) and a participant was defined as having had an EH if they answered yes to one or both of these questions.
Participants were asked to characterize EH in terms of EH location relative to themselves (Q3), laterality (Q4), emotional content (Q28), time of day of occurrence (Q5), form (Q7), and familiarity (Q8). They were also asked about any auditory or tactile (Q9–10) components, whether their EH were stereotyped (Q11) and the effect of medications on their EH, in terms of aggravation or amelioration (Q12–14). All known trade names for PD medications were used, to help ensure correct identification of medications used by participants from different countries.
Data were also collected regarding the relationship between the onset of EH and other features of disease, such as VH, memory decline, anosmia, and sleep disturbance. Disease severity was assessed using a modified version of the Hoehn and Yahr score, already in use by patientslikeme®, in which patients identify their disease severity (Table 1).
Statistical analysis was performed using IBM SPSS Statistics Version 22.0. Descriptive statistics were used to analyze the characteristics of EH in terms of laterality (left/right), familiarity (familiar/unfamiliar), emotional content (pleasant/unpleasant/neither), associated emotional response (happy/sad/neither), and time of day (morning/afternoon/evening/night). Chi-squared testing was undertaken to determine whether there were significant differences between the expected and observed frequencies of these variables.
Respondents were divided into two groups, those with EH and those without, and the distribution of variables, such as age, gender, disease duration, and severity and presence of VH within these groups were compared using an independent t-test, Mann–Whitney U, or a chi-squared test (with or without trend) depending on whether the variable being analyzed was continuous or categorical and normally distributed. Normality of data was assessed using visual inspection of histograms, QQ plots and box plots, and Shapiro–Wilk testing.
A logistic regression analysis was undertaken to determine if a set of pre-specified variables (age, gender, disease duration, disease severity as specified by Hoehn and Yahr score, and presence of VH) were predictive of the occurrence of EH (36). These potential predictor variables were selected based on prior clinical knowledge and pre-specified hypotheses, explicitly that the prevalence of EH would not be influenced by demographic factors, such as age and sex, but would be affected by disease duration, disease severity, and VH, putative correlates of accumulating cortical Lewy Body pathology. A Box–Tidwell test was used to ensure there was linearity for continuous and transformed categorical variables. The dependent variable, the occurrence of EH, was dichotomous and assigned a value of 1 (EH occurrence) or 0 (no EH occurrence).
Data from 414 respondents were included in the study. Of the 6841 individuals with PD who were invited to participate, 569 responded indicating a response rate of 8.3%. Forty (9.7%) respondents were excluded as they had not completed the questionnaire, and 115 (20.2%) were excluded on the basis of the exclusion criteria outlined above.
The mean age of respondents was 61.9 years (standard deviation 8.2 years, range 33–88 years) and 48.2% (199/413) of the cohort were female. About 83.5% (324/388) of participants were resident in the United States or United Kingdom with others living in Europe, Canada, Australasia, South America, and the Middle East. The mean Hoehn and Yahr score was 2.11 (SD 0.99, range 1–5) with 94.9% (391/412) of participants reporting a score between 1 and 3. Mean disease duration was 7.3 years (SD 5.0 years, range 1–29 years).
About 33.9% (140/413) of respondents reported illusions and 15.5% (64/412) had experienced VH. More than half of respondents reported EH; 45.9% (190/414) reported a “feeling of movement” passing them and 24.6% (102/414) reported “feeling or imaging a presence that was not truly there.” Given that, 20.3% reported both symptoms, 50.4% (208/413) experienced either a “feeling of movement” or had “felt or imagined a presence” (Figure 2).
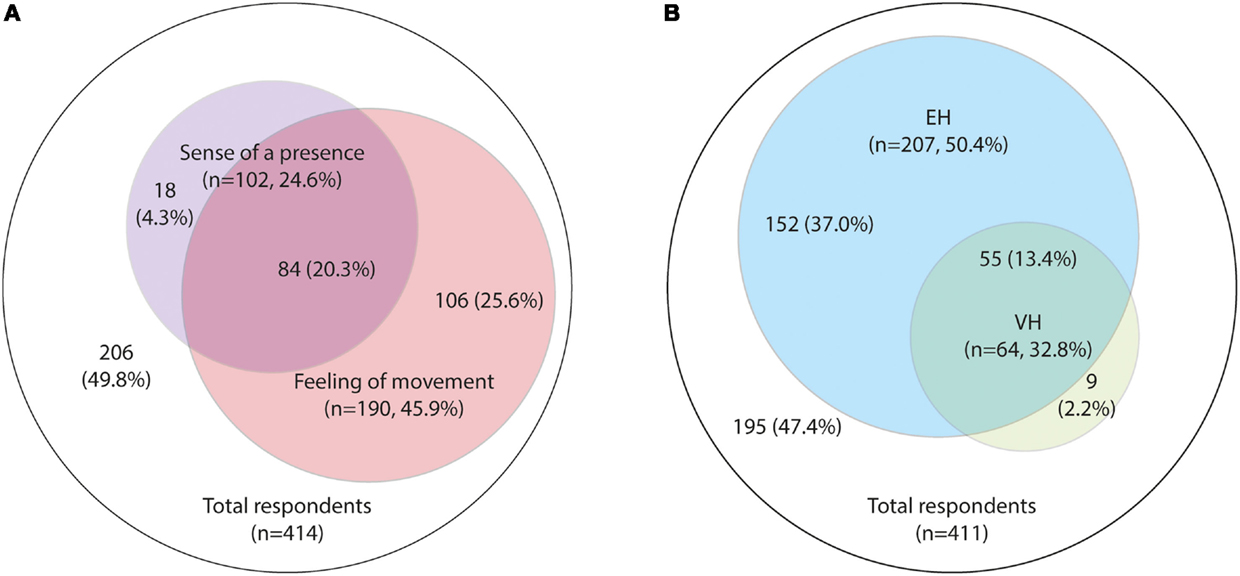
Figure 2. Prevalence of hallucinations. (A) Extracampine hallucinations (EH), “feeling or imagining a presence” versus a “feeling of movement”; (B) Prevalence of EH and visual hallucinations (VH).
Neither age nor gender was predictors for the occurrence of EH (Table 2). Mean disease duration did not differ significantly between the EH and non-EH groups (p = 0.158), but those with EH were more likely to have a higher Hoehn and Yahr Stage (p = 0.002) (Table 2).
Extracampine hallucinations were more likely to occur alongside rather than directly in front of or behind the person experiencing them (p < 0.001) (Table 3). Although EH did lateralize to the left or right in 47.0% (78/166) of cases this was a non-significant finding (p = 0.438) with a similar proportion of respondents (53.0%, 88/166) stating either that there was no left/right pattern or that they had not noticed such a pattern. Of the 47.0% (78/166) that did lateralize more were reported on the right side but this did not reach statistical significance (p = 0.070).
Extracampine hallucinations were more likely to feel “unfamiliar” than “familiar” (p = 0.001) and seemed to be emotional neutral experiences making people feel neither happy nor sad (p < 0.001), and neither pleasant nor unpleasant, whether they had reported “a feeling of movement” (p < 0.001) or the “sense of a presence” (p < 0.001) (Table 3).
In those who reported EH these were often recently experienced; 81.6% (151/185) of those who reported a “feeling of a movement passing them” and 72.3% (73/101) of those who reported a “felt or imagined presence” had experienced this phenomenon within the previous three months. EH were not associated with a particular time of day (morning/afternoon/evening/night) (p < 0.001).
Extracampine hallucinations co-existed with other PD symptoms. Of those who reported EH, 75.5% (77/102) also had anosmia, 73.3% (74/101) reported a decline in their memory or thinking, 72.5% (74/102) had restless sleep, and 77.4% (27/35) had experienced a change in their balance or walking.
The onset of anosmia, memory decline, and sleep disturbance were all more likely to precede the onset of EH in our cohort (p < 0.001, p = 0.002, and p = 0.002, respectively), but there was no clear temporal relationship between the onset of EH and the onset of motor symptoms (p = 0.221) in line with the above finding of a lack of association between EH and disease duration (Table 4).
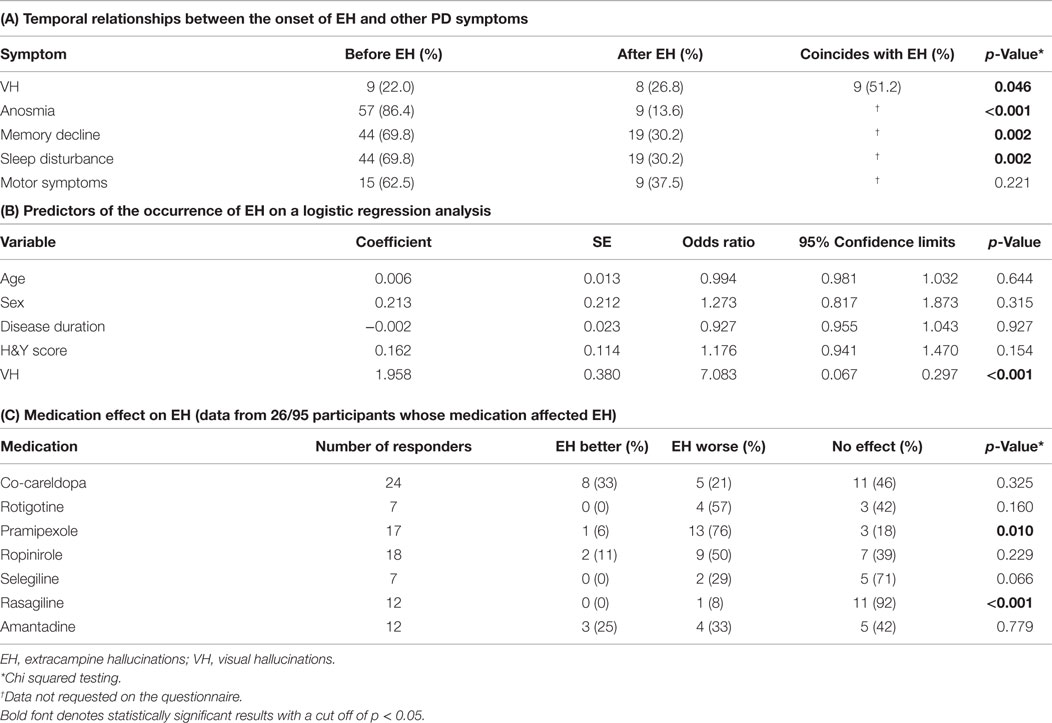
Table 4. Relationships between (A) the onset of EH and other PD symptoms, (B) EH and potential predictor variables, and (C) EH and medication.
In 52.8% (19/36) of reported cases, EH onset was described as occurring around the same time as VH, with 25% (9/36) and 22.2% (8/36), respectively, reporting EH onset before and after VH onset. This finding reached statistical significance (p = 0.046) (Table 4).
A major overlap was observed between those reporting VH and those reporting EH (Figure 2); 85.9% of those with VH also had EH. Those who reported EH were significantly more likely to report VH (26.6%, 55/207) than those who did not report EH (4.4%, 9/204) (p < 0.001) (Table 4). About 37.0% (152/411) of all respondents reported EH but no VH, and 2.2% (9/411) reported VH but no EH (Figure 2).
A binary logistic regression was performed to ascertain the effects of age, gender, disease duration, disease severity (Hoehn and Yahr score), and the presence of VH on the likelihood that the participants had experienced EH. The logistic regression model was statistically significant [χ2(5) = 44.463, p < 0.001] and explained 13.9% (Nagelkerke R2) of the variance in the presence of EH. Overall, it correctly predicted the occurrence or absence of EH in 62.4% of respondents; it correctly classified 37.9% of those experiencing EH and 87.1% of those not experiencing EH. Of these variables, only the presence of VH was found to be significantly predictive of EH occurrence (Table 4).
Participants who had experienced EH were asked if their medications made their EH better or worse. Overall medications did not appear to have an effect upon EH (p < 0.001) with 72.6% (69/95) of respondents stating they had not noticed an effect.
27.4% (26/95) reported that their medication had affected their EH and provided further data regarding individual drugs (Table 4). The following data were collected only from these 26 participants therefore the number of responses for each drug was small. For some drugs (co-benyldopa, stalevo, benzotropine, trihexyphenidyl, entacapone, rivastigmine, donepezil, and galantamine), information was obtained from fewer than five respondents, and these small sample sizes precluded meaningful interpretation; for example, only three patients in this subgroup were taking rivastigmine. Of the remaining drugs, 13/17 respondents stated that pramipexole significantly worsened EH (p = 0.010). No significant drug effect on EH was noted for other drugs (Table 4).
52.9% (301/569) of participants completed a retest phase in which they retook an identical questionnaire. Analysis of this data by patientslikeme® indicated that our questionnaire had a high test–retest reliability (Cronbachs α = 0.94).
The prevalence of EH in patients with PD, their characteristics and association with other key clinical features of PD were investigated using a web-based questionnaire circulated to the online patient community patientslikeme®. To the best of our knowledge, this is the first large cross-sectional study to focus on the occurrence of EH in patients with PD. The main finding of the study was that EH were reported by over 50% of respondents, representing a prevalence more than three times greater than that of VH (50.4 versus 15.5%). Although there is no prior study specifically exploring the prevalence of EH in PD against which these study results can be compared, this 50.4% prevalence figure falls within the range of reported prevalences of minor hallucinations in PD (4, 19–21), which includes EH.
Extracampine hallucinations were significantly more likely to be experienced as a presence alongside, rather than behind, the individual (p < 0.001), to be an unfamiliar rather than a familiar presence (p < 0.001), for the presence to be that of a person rather than an animal or inanimate object (p < 0.001), and to be emotionally neutral in content (p < 0.001). EH did not tend to be stereotypical in nature (p < 0.001), and did not tend to have an auditory or tactile component (p < 0.001). There was no significant association between occurrence of EH and time of day (p < 0.001).
With regard to other aspects of PD, there was a statistically significant (p = 0.002) association between EH occurrence and disease severity, as measured by the Hoehn and Yahr stage, but not with respondent age, gender, and disease duration of PD. The presence of VH was predictive of EH and the majority of respondents who reported EH also described VH (85.9%). In over 50% of respondents, EH and VH onset occurred around the same time.
Surprisingly, and in contrast to previous studies reporting that the onset of VH was predictive of future cognitive decline, in this study respondents stated that EH occurred after the onset of cognitive problems, as well as after anosmia and sleep disturbance. This was an unexpected finding. One possibility is that these results may be skewed by the greater difficulty in recognizing the onset of EH, given its current under-reporting and under-recognition. However, another possibility is that the Lewy body pathology may affect the cortical systems underpinning EH later than those involved in cognition. That said, this would seem a less plausible explanation when the neural substrate of EH is considered. While this is yet to be established, given that EH represent percepts that are by definition devoid of any visual form, it is probable that they are of cortical origin rather than indicative of a disorder of primary sensory pathways. This is reinforced by a recent study investigating the imaging changes associated with the “sense of a presence,” both in a small cohort of patients with neurological disorders (epilepsy, stroke but no PD patients) and healthy subjects in whom this phenomenon was induced artificially using synchronous and asynchronous stimulation (37). The brain regions most commonly associated with “the sense of a presence” were the frontoparietal cortex, insular cortex, and temporoparietal cortex. With regard to the latter, the clinicopathological observation that VH in Lewy body disease were strongly associated with a high density of Lewy bodies in the temporal lobe (9) may provide a clue as to the cortical substrate of EH. Given that, the onset of VH commonly precedes that of cognitive impairment in PD, the expectation might have been that the latter would also be preceded by the occurrence of EH. This will need to be clarified by way of future prospective natural history studies of EH in PD patients without cognitive impairment.
Insufficient data were generated to draw any firm conclusions regarding the effect of medications on EH. Although the majority of patients reported that their medications did not influence their EH, a minority did notice an effect with the only significant finding being that pramipexole worsened EH.
This study has limitations. The use of a web-based questionnaire to obtain responses from an online community of patients not diagnosed, or under the care of, the study authors means that there is no mechanism to confirm the diagnosis of PD or the Hoehn and Yahr score. Knowledge is also lacking on the demographic details of the respondent group and given the online nature of both the community and the questionnaire, it is possible that the respondents as group may not reflect the entire socio-economic breadth of the wider PD patient population. Additionally, this study is potentially vulnerable to the biases associated with all questionnaire-based research studies (selection, recall, and responder bias). With regard to disease severity of the study respondents, the mean Hoehn and Yahr score was 2.1, which may reflect the fact that patients with more severe disease could find completing online questionnaires more challenging. Finally, in order to ensure some degree of homogeneity among the test population, this study did not obtain responses from patients with PD dementia (PDD) and DLB, and no data are currently available regarding the prevalence of EH in the general population.
Future research will aim to determine the prognostic significance of EH, notably with regard to predicting later cognitive decline, as well as to establish the prevalence of EH in patients with PDD and Lewy body dementia.
The occurrence of EH in 50.4% of respondents suggests that EH is an under-reported and under-recognized symptom of PD. Given that, the occurrence of VH represents one of the core criteria for the diagnosis both of PDD and DLB, identification of EH may be of high clinical significance The likely neural substrate of this phenomenon suggests that EH may indicate cortical involvement in PD and thus be predictive of future cognitive decline.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank all the study respondents and patientslikeme® for their collaboration with this research project and in particular offer thanks to Paul Wicks, Ph.D. (Vice President of Innovation at patientslikeme®) for his assistance in setting up this collaboration, and to Magdalena Harrington, Ph.D. (Psychometrician at patientslikeme®) and Julia Braverman, Ph.D. (Research Scientist at patientslikeme®) for technical support in setting up the online questionnaire and obtaining the data. We also wish to thank Dr. Stephen Bremner, Ph.D. (Senior Lecturer in Medical Statistics at Brighton and Sussex Medical School) for his assistance with the statistical analysis.
Dr. RW is funded by the UK National Institute for Health Research (NIHR). Dr. DC is funded by the Cambridge NIHR Biomedical Research Centre and receives grant income from the UK Medical Research Council, Technology Strategy Board and the Cambridge Isaac Newton Trust. There are no other financial disclosures to make.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association (2013).
2. Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology (1993) 43:2227–9. doi: 10.1212/WNL.43.11.2227
3. Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord (2004) 19:1043–9. doi:10.1002/mds.20216
4. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain (2000) 123(Pt 4):733–45. doi:10.1093/brain/123.4.733
5. Chan D, Rossor MN. “-but who is that on the other side of you?” Extracampine hallucinations revisited. Lancet (2002) 360:2064–6. doi:10.1016/S0140-6736(02)11998-2
6. Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol (2009) 5:331–42. doi:10.1038/nrneurol.2009.62
7. Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol (2005) 4:605–10. doi:10.1016/S1474-4422(05)70146-0
8. McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology (1996) 47:1113–24. doi:10.1212/WNL.47.5.1113
9. Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain (2002) 125:391–403. doi:10.1093/brain/awf033
10. Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol (1996) 53:1265–8. doi:10.1001/archneur.1996.00550120077019
11. Gallagher DA, Parkkinen L, O’Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain (2011) 134:3299–309. doi:10.1093/brain/awr225
12. Ibarretxe-Bilbao N, Junque C, Marti MJ, Tolosa E. Cerebral basis of visual hallucinations in Parkinson’s disease: structural and functional MRI studies. J Neurol Sci (2011) 310:79–81. doi:10.1016/j.jns.2011.06.019
13. Stebbins GT, Goetz CG, Carrillo MC, Bangen KJ, Turner DA, Glover GH, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology (2004) 63:1409–16. doi:10.1212/01.WNL.0000141853.27081.BD
14. Goetz CG, Tanner CM, Klawans HL. Pharmacology of hallucinations induced by long-term drug therapy. Am J Psychiatry (1982) 139:494–7. doi:10.1176/ajp.139.4.494
15. Cummings JL. Behavioral complications of drug treatment of Parkinson’s disease. J Am Geriatr Soc (1991) 39:708–16. doi:10.1111/j.1532-5415.1991.tb03627.x
16. Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med (2000) 342:1484–91. doi:10.1056/NEJM200005183422004
17. Friedman JH. ‘Drug holidays’ in the treatment of Parkinson’s disease. A brief review. Arch Intern Med (1985) 145:913–5. doi:10.1001/archinte.145.5.913
18. Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol (1999) 56:595–601. doi:10.1001/archneur.56.5.595
19. Pacchetti C, Manni R, Zangaglia R, Mancini F, Marchioni E, Tassorelli C, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson’s disease. Mov Disord (2005) 20:1439–48. doi:10.1002/mds.20582
20. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry (2008) 79:652–5. doi:10.1136/jnnp.2007.124677
21. Fenelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci (2010) 289:12–7. doi:10.1016/j.jns.2009.08.014
22. McDonald I. Musical alexia with recovery: a personal account. Brain (2006) 129:2554–61. doi:10.1093/brain/awl235
23. Solomonova E, Nielsen T, Stenstrom P, Simard V, Frantova E, Donderi D. Sensed presence as a correlate of sleep paralysis distress, social anxiety and waking state social imagery. Conscious Cogn (2008) 17:49–63. doi:10.1016/j.concog.2007.04.007
24. Zijlmans M, van Eijsden P, Ferrier CH, Kho KH, van Rijen PC, Leijten FS. Illusory shadow person causing paradoxical gaze deviations during temporal lobe seizures. J Neurol Neurosurg Psychiatry (2009) 80:686–8. doi:10.1136/jnnp.2008.154310
27. Brugger P, Regard M, Landis T, Oelz O. Hallucinatory experiences in extreme-altitude climbers. Neuropsychiatry Neuropsychol Behav Neurol (1999) 12:67–71.
28. Bertram K, Williams DR. Visual hallucinations in the differential diagnosis of parkinsonism. J Neurol Neurosurg Psychiatry (2012) 83:448–52. doi:10.1136/jnnp-2011-300980
29. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord (2007) 22:1689–707; [quiz 1837]. doi:10.1002/mds.21507
30. Kitayama M, Wada-Isoe K, Nakaso K, Irizawa Y, Nakashima K. Clinical evaluation of Parkinson’s disease dementia: association with aging and visual hallucination. Acta Neurol Scand (2007) 116:190–5. doi:10.1111/j.1600-0404.2007.00860.x
31. Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Cognitive changes in Parkinson’s disease patients with visual hallucinations. Dement Geriatr Cogn Disord (2007) 23:281–8. doi:10.1159/000100850
32. Lee WJ, Tsai CF, Gauthier S, Wang SJ, Fuh JL. The association between cognitive impairment and neuropsychiatric symptoms in patients with Parkinson’s disease dementia. Int Psychogeriatr (2012) 24:1980–7. doi:10.1017/S1041610212001317
33. Goetz CG. Scales to evaluate psychosis in Parkinson’s disease. Parkinsonism Relat Disord (2009) 15(Suppl 3):S38–41. doi:10.1016/S1353-8020(09)70777-1
34. Smith CA, Wicks PJ. PatientsLikeMe: consumer health vocabulary as a folksonomy. AMIA Annu Symp Proc (2008) 2008:682–6.
35. Sussex. Guidelines for Completing the Online Application Form for Ethical Review. Point 14 (2012) 10 p. Available from: https://www.sussex.ac.uk/webteam/gateway/file.php?name=guidance-for-completing-online-application-form.pdf&site=377
36. Field A. Discovering Statistics Using IBM SPSS Statistics. 4th ed. Thousand Oaks, CA: SAGE (2013).
Keywords: Parkinson’s disease, hallucinations, extracampine hallucinations, cortical Lewy body disease, visual hallucinations
Citation: Wood RA, Hopkins SA, Moodley KK and Chan D (2015) Fifty Percent Prevalence of Extracampine Hallucinations in Parkinson’s Disease Patients. Front. Neurol. 6:263. doi: 10.3389/fneur.2015.00263
Received: 01 September 2015; Accepted: 03 December 2015;
Published: 21 December 2015
Edited by:
Angie A. Kehagia, King’s College London, UKReviewed by:
Greeshma Gadikota, Columbia University, USACopyright: © 2015 Wood, Hopkins, Moodley and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dennis Chan, ZGM1OThAbWVkc2NobC5jYW0uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.