- 1Population Health Research Institute, McMaster University, Hamilton, ON, Canada
- 2Department of Neurology, School of Medicine, The 2nd Affiliated Hospital of Zhejiang University, Hangzhou, China
- 3UCL Institute of Neurology, London, UK
- 4Hemorrhagic Stroke Research Program, Department of Neurology, Massachusetts General Hospital Stroke Research Center, Harvard Medical School, Boston, MA, USA
Intravenous thrombolytic therapy in acute ischemic stroke patients is complicated by intracerebral hemorrhage (ICH) at a site remote from the infarcted area in roughly 2–3% of cases (1, 2). Historically, the etiology underlying these was proposed to be hemorrhagic infarction at a distant unrecognized silent focus of ischemia from multiple emboli. However, the use of diffusion-weighted imaging has demonstrated clear examples of remote intracerebral hemorrhage (rICH) occurring at sites devoid of ischemia, signifying alternate contributory mechanisms (3). Cerebral microbleeds (CMBs) are markers of bleeding-prone microangiopathies – most commonly hypertensive arteriopathy and cerebral amyloid angiopathy (CAA) (4) – that are visualized on T2*-weighted magnetic resonance imaging (MRI). Pathological studies have demonstrated intact erythrocytes underlying 13% of CMBs (5) implying that a subset of these lesions reflect acute or subacute areas of microhemorrhage. Fittingly, radiographic studies have demonstrated development of new CMBs in 5–13% of acute ischemic stroke patients within the first week after symptom onset (6–8). It is hence biologically plausible that thrombolysis-induced expansion of rapidly appearing CMBs might be the cause underlying a proportion of rICH in acute ischemic stroke patients. In this Opinion piece, we explored this hypothesis by pooling available evidence from relevant MRI patient cohorts with acute ischemic stroke.
Two recent studies from east-Asian centers including a total of 345 patients have assessed the risk of rICH in patients who develop new post-stroke CMBs. Both studies used exclusively intravenous thrombolysis with rtPA: the dose used was 0.6 mg/kg in one study (7) and 0.9 mg/kg in the other (8). Overall, 129 (39%) of the patients had CMBs on pre-treatment baseline MRI and 17 (5%) developed new CMBs at 24 h post-thrombolysis. Post-thrombolysis rICH occurred in 2% (n = 7) of the entire population. In fixed effects pooled meta-analysis of the data, patients who developed new CMBs had a significantly increased risk of rICH than patients without new CMBs (odds ratio (OR) 16.15, 95% CI 3.72–70.18, p < 0.0001; Figure 1). The results were consistent in sensitivity analysis using a random effects model.
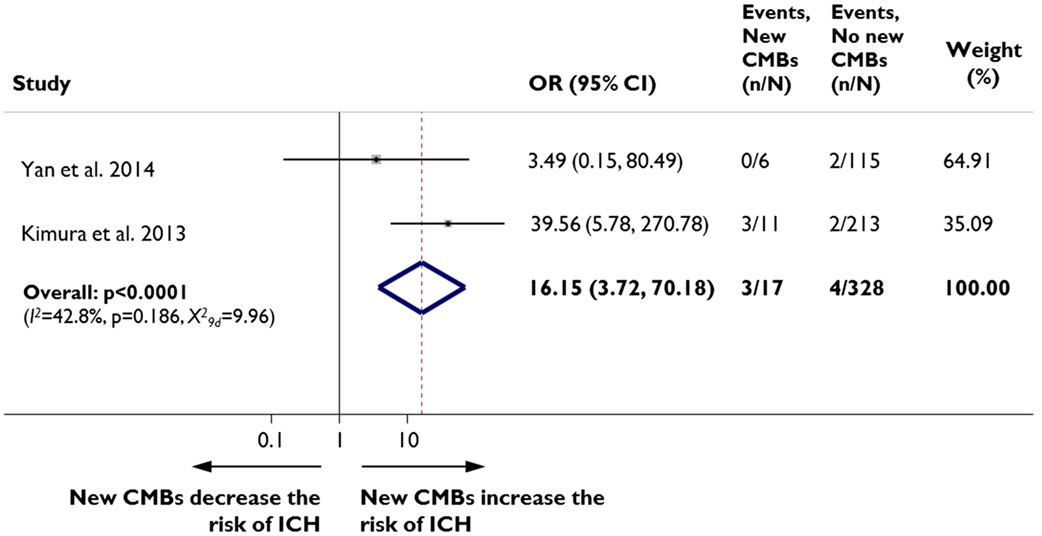
Figure 1. New cerebral microbleeds and remote (i.e., extra-ischemic) intracerebral hemorrhage. Meta-analysis of the association between symptomatic remote intracerebral hemorrhage (ICH) risk in patients with acute ischemic stroke treated with intravenous thrombolysis, in relation to the presence of new cerebral microbleeds (CMBs) on MRI. Moderate heterogeneity was detected across the different studies pooled in the meta-analysis. Two authors search PubMed and extracted relevant data for the analysis. We quantified the strength of the association between new CMBs and ICH using odds ratios (OR) and their corresponding 95% CIs, with the inverse variance method for weighting. We assessed statistical heterogeneity using I-squared statistics and also visually through inspection of the forest plot. We repeated all analyses using random effects models. Meta-analyses were performed using Stata 11.2 (StataCorp LP, Texas).
These findings, although preliminary, suggest that thrombolysis-induced expansion of new CMBs might account for a proportion of rICH in acute ischemic stroke patients. Post-thrombolysis rICH has been previously documented to occur at a site of CMB (9) and meta-analyses have suggested elevated risk of any post-thrombolysis symptomatic intracerebral hemorrhage in patients with CMBs (10, 11). However, whether these lesions – detected on baseline pre-thrombolysis MRI – were acute CMBs or simply a chronic marker of underlying hemorrhage-prone microangiopathies in the brain is uncertain, as thus far only chronic-subacute CMBs have been proposed to possibly have a distinctive MRI signature (12).
Two cohorts published in the last year have attempted to characterize clinical predictors of rICH (1, 2). In the Safe Implementation of Treatments in Stroke-International Stroke Register (SITS-ISTR) prior stroke and older age were independently associated with rICH. However, the lack of robust associations with traditional ischemic risk factors led the authors to postulate whether another undetected mechanism, including CAA (13), was at play (2). Conversely, prior transient ischemic attack (TIA) was the only clinical predictor of rICH in an Australian cohort (1). Although, this observation could support the notion that rICH occurs from hemorrhagic transformation of unrecognized acute or subacute ischemic infarcts, patients with CAA often experience transient focal neurological episodes that can mimic TIA, and are highly predictive of future lobar ICH (14).
Together, these observations raise the possibility that multiple etiologies (both primary hemorrhagic and primary ischemic) likely contribute to the pathogenesis of post-thrombolysis rICH. They also demonstrate the rapidly evolving nature of microbleeds in the acute phase of ischemic stroke, suggesting a potential role of an active small vessel microangiopathic process (15). As our pooled analysis is unadjusted, it remains to be determined whether the association between new CMBs and rICH is indeed an independent one or rather simply an indirect association due to common underlying pathophysiology, such as small vessel disease, stroke-induced acute hypertensive response, or neurovascular unit dysfunction from up regulation of inflammatory cascades. Future larger studies that incorporate a comprehensive assessment of both clinical and MRI predictors of rICH, as well as circulating markers of inflammation, would further elucidate this hypothesis.
Author Contributions
Study Concept: AS and AC. Acquisition of data: AS, SY, and AC. Statistical Analysis: AC. Drafting of the manuscript: AS; Revising the manuscript for content: AC and SY.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gao Y, Churilov L, Teo S, Yan B. Remote intracerebral haemorrhage post intravenous thrombolysis: experience from an Australian stroke centre. J Clin Neurosci (2014) 22(2):352–6. doi: 10.1016/j.jocn.2014.07.009
2. Mazya MV, Ahmed N, Ford GA, Hobohm C, Mikulik R, Nunes AP, et al. Remote or extraischemic intracerebral hemorrhage – an uncommon complication of stroke thrombolysis: results from the safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke (2014) 45:1657–63. doi:10.1161/STROKEAHA.114.004923
3. Hill MD, Barber PA, Demchuk AM, Sevick RJ, Frayne R, Buchan AM. Symptomatic hemorrhage after alteplase therapy not due to silent ischemia. BMC Neurol (2001) 1:1. doi:10.1186/1471-2377-1-1
4. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol (2009) 8:165–74. doi:10.1016/S1474-4422(09)70013-4
5. Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis (2011) 32:528–34. doi:10.1159/000331466
6. Jeon SB, Kwon SU, Cho AH, Yun SC, Kim JS, Kang DW. Rapid appearance of new cerebral microbleeds after acute ischemic stroke. Neurology (2009) 73:1638–44. doi:10.1212/WNL.0b013e3181bd110f
7. Kimura K, Aoki J, Shibazaki K, Saji N, Uemura J, Sakamoto Y. New appearance of extraischemic microbleeds on T2*-weighted magnetic resonance imaging 24 hours after tissue-type plasminogen activator administration. Stroke (2013) 44:2776–81. doi:10.1161/STROKEAHA.113.001778
8. Yan S, Chen Y, Zhang X, Liebeskind DS, Lou M. New microbleeds after thrombolysis: contiguous thin-slice 3T MRI. Medicine (Baltimore) (2014) 93:e99. doi:10.1097/MD.0000000000000099
9. Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke (2002) 33:95–8. doi:10.1161/hs0102.101792
10. Charidimou A, Fox Z, Werring DJ. Do cerebral microbleeds increase the risk of intracerebral hemorrhage after thrombolysis for acute ischemic stroke? Int J Stroke (2013) 8:E1–2. doi:10.1111/ijs.12048
11. Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke (2013) 8:348–56. doi:10.1111/j.1747-4949.2012.00869.x
12. Shoamanesh A, Catanese L, Sakai O, Pikula A, Kase CS. Diffusion-weighted imaging hyperintensities in intracerebral hemorrhage: microinfarcts or microbleeds? Ann Neurol (2013) 73(6):795–6. doi:10.1002/ana.23853
13. Charidimou A, Nicoll JA, Mccarron MO. Thrombolysis-related intracerebral hemorrhage and cerebral amyloid angiopathy: accumulating evidence. Front Neurol (2015) 6:99. doi:10.3389/fneur.2015.00099
14. Charidimou A, Peeters A, Fox Z, Gregoire SM, Vandermeeren Y, Laloux P, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke (2012) 43:2324–30. doi:10.1161/STROKEAHA.112.657759
Keywords: acute stroke, cerebral microbleeds, cerebral small vessel disease, cerebral amyloid angiopathy, intracerebral hemorrhage, thrombolysis
Citation: Shoamanesh A, Yan S and Charidimou A (2015) New cerebral microbleeds and mechanism of post-thrombolysis remote intracerebral hemorrhage: “red meets white” revisited. Front. Neurol. 6:203. doi:10.3389/fneur.2015.00203
Received: 21 July 2015; Accepted: 01 September 2015;
Published: 15 September 2015
Edited by:
Anders Fogh Christensen, Bispebjerg University Hospital of Copenhagen, DenmarkReviewed by:
Craig S. Anderson, University of Sydney, AustraliaShyam Prabhakaran, Northwestern University, USA
Ronil Vikesh Chandra, Monash Health, Australia
Copyright: © 2015 Shoamanesh, Yan and Charidimou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Charidimou,YW5kcmVhcy5jaGFyaWRpbW91LjA5QHVjbC5hYy51aw==;
Ashkan Shoamanesh,QXNoa2FuLlNob2FtYW5lc2hAcGhyaS5jYQ==
†The title is inspired by an Editorial, published after the first study revealing the rapidly evolving nature of cerebral microbleeds in the acute phase of ischemic stroke was reported. See Kidwell and Greenberg (2009), Ref. (15).
 Ashkan Shoamanesh
Ashkan Shoamanesh Shenqiang Yan
Shenqiang Yan Andreas Charidimou
Andreas Charidimou