- 1Department of Neurology, Loyola University Medical Center, Maywood, IL, USA
- 2Department of Pharmacy, Loyola University Medical Center, Maywood, IL, USA
Cardiogenic cerebral embolism represents 20% of all acute ischemic strokes (AISs) with one-third of these being caused by left ventricular thrombus (LVT). LVT is not a contraindication for treatment with intravenous recombinant tissue plasminogen activator (IV rtPA) for AIS. However, the subsequent treatment of a potentially unstable LVT is contraindicated for 24 h following the use of IV rtPA according to current guidelines. We present a 66-year-old man with AIS treated with IV rtPA. Echocardiogram shortly after treatment demonstrated both a large apical and septal thrombus in the left ventricle and at 12 h post IV rtPA infusion, therapeutic anticoagulation with heparin was started without complication. In practice, the action of IV rtPA outlasts its apparent half-life because of thrombin-binding and the prolonged effects and longer half-life of its product, plasmin; however, the pharmacokinetics do not warrant prolonged avoidance of therapeutic anticoagulation when clinically indicated. Our case demonstrates that anticoagulation for potentially unstable LVT can be safely initiated at 12 h following IV rtPA treatment for AIS.
Introduction
A 66-year-old man presented to our hospital with acute left sided weakness, facial droop, slurred speech, and complete left hemianopsia (NIHSS 6) within 90 min of symptom onset. His medical history was remarkable for ischemic stroke a year prior with no residual deficit and myocardial infarction (MI) 15 years prior complicated by ventricular fibrillation requiring temporary balloon pump. He had been discharged earlier that day from another hospital where evaluation for shortness of breath revealed a left ventricular thrombus (LVT). He was to initiate warfarin that evening, which he had not yet commenced. The patient had no contraindications to intravenous recombinant tissue plasminogen activator (IV rtPA) and was treated within 60 min of presentation.
Magnetic resonance imaging (MRI) demonstrated acute infarcts in both cerebral hemispheres (Figure 1) and no evidence of hemorrhage. An apical LVT (2.4 cm × 1.4 cm) and a second thrombus adjacent to the interventricular septum (1.6 cm × 2.2 cm) were confirmed on transthoracic echocardiogram (TTE) (Figure 2) within 12 h of presentation and 10 h of completion of IV rtPA infusion. Ejection fraction was 25%; regional variation of wall motion and akinesis of the left ventricle (LV) were also noted.
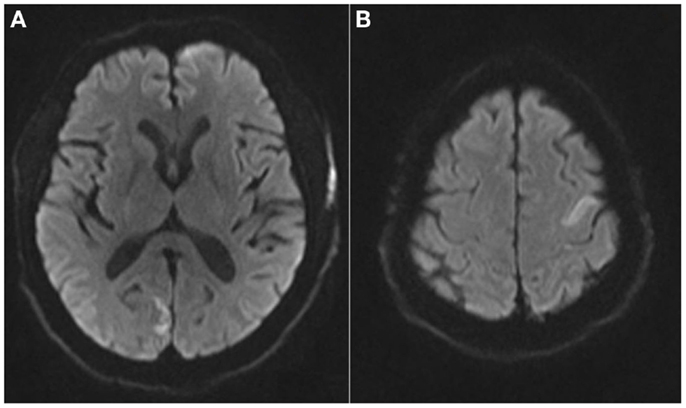
Figure 1. Magnetic resonance imaging brain axial diffusion weighted images demonstrating areas of restricted diffusion in the R occipital lobe (A) and left superior frontal lobe (B).
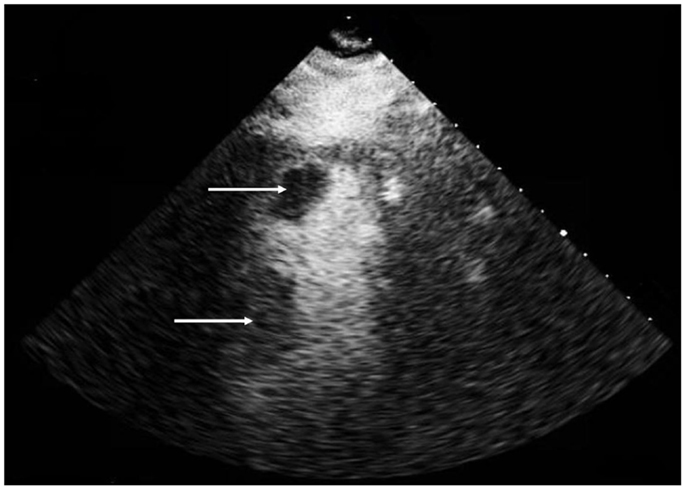
Figure 2. Transthoracic echocardiogram, apical 2 chamber view with contrast demonstrating a left ventricular apical thrombus (2.4 cm × 1.4 cm) (top arrow) and a second thrombus (1.6 cm × 2.2 cm) (bottom arrow) adherent to the interventricular septum.
This presented a therapeutic conundrum whereby the presence of a potentially high recurrent cardioembolic source required anticoagulation but was contraindicated due to recent administration of IV rtPA. The patient’s activated partial thromboplastin time (aPTT), prothrombin time (PT), and international normalized ratio (INR) were verified to be within the normal range. The pharmacokinetics of IV rtPA and its byproducts were reviewed and the decision to start therapeutic anticoagulation was made. Non-bolus IV heparin infusion was initiated 12 h after IV rtPA infusion. The patient’s neurological deficit improved and he was bridged to warfarin and discharged home with a favorable outcome (NIHSS 1, modified Rankin Scale 1).
Discussion
LVT and Cardiogenic Embolism
Cardiogenic embolism causes 20% of all ischemic strokes. While non-valvular atrial fibrillation (AF) is responsible for approximately 50% of these cases, left ventricular mural thrombus represents almost one third of cases (1). The majority of LVT have been associated with acute myocardial infarction (AMI), and also occur in patients with chronic ventricular dysfunction from coronary artery disease, hypertension, or dilated cardiomyopathy (1). Myocardial injury can lead to severe regional wall motion abnormality and hypokinesis, akinesis, dyskinesis, or aneurysmal dilatation of the LV. Patients with large anterior MI have a high risk of developing LVT and subsequent cardiogenic thromboembolism (2). The incidence of embolic complications is highest in the first 1–3 months post-MI, with an absolute risk of 9% in patients with acute MI complicated by LVT (3). Beyond the acute phase, patients with persistent myocardial dysfunction have a 5-year stroke risk of 8% (4, 5). TTE is 23% sensitive and 96% specific for LVT detection (6), improving to 92% sensitivity when a high prevalence of LVT was anticipated (7) and remains the preferred method for detection. If image quality is poor or LVT risk is high with negative or equivocal echocardiography, contrast enhanced cardiac MRI (CMR) should be considered. It has a sensitivity of 88% and specificity of 99% (6).
The 2013 American College of Cardiology Foundation/American Heart Association Guideline for the Management of ST-elevation MI recommend vitamin K antagonist therapy for 3 months (2, 8) and perhaps indefinitely (9) with a goal INR of 2.0–3.0 if the risk of thromboembolism exceeds the bleeding risk when LVT is demonstrated. This recommendation was based on observational studies reporting better outcomes and fewer cerebral emboli in patients treated with heparin and then transitioned to warfarin. No guideline for prolonged duration of anticoagulation exists and the decision is guided by follow-up echocardiography and demonstration of resolution or persistence of the clot and embolic risk as determined by the clinician.
Cardiomyopathy leading to impaired LV systolic function and reduced stroke volume creates relative stasis and risk for LVT development (5). Etiology of cardiomyopathy can be ischemia or infarction, or non-ischemic causes such as genetic or acquired structural or metabolic defects. Decreased ejection fraction and older age are both independent risk factors for stroke after MI (4). The warfarin versus aspirin in reduced cardiac ejection fraction (WARCEF) trial showed fewer ischemic strokes in follow-up for patients in sinus rhythm and ejection fraction <35% randomized to warfarin compared to aspirin 325 mg daily; however, the primary end point of ischemic stroke, intracerebral hemorrhage, or death was similar between groups (10). If LV dysfunction due to extensive regional wall motion abnormalities is demonstrated, prophylactic treatment with warfarin may be considered in patient with previous stroke or transient ischemic attack (TIA) (1). Similarly, in patients with LVT in the setting of congestive heart failure due to dilated cardiomyopathy, anticoagulation might be reasonable particularly if the embolic potential is high and the bleeding risk is low. This is supported by indirect data from observational studies in patients with LVT after MI (11).
No single clinical feature predicts embolic risk. Thrombi that demonstrate mobility, protrusion, and central echolucency are higher risk (12). A theoretical risk of embolization from destabilization of a LVT after thrombolysis exists. However, only case reports exist of post thrombolysis infarction in the contralateral hemisphere (13). The largest case series of five patients did not demonstrate subsequent re-embolization after IV thrombolysis (14). The presence of LVT is not listed as a contraindication to IV thrombolysis for acute ischemic stroke (AIS) according to current guidelines (15).
IV rtPA Kinetics
Alteplase was FDA-approved for use in acute stroke in 1996 and is also indicated for use in pulmonary embolism (PE) and AMI (16, 17). It exerts its thrombolytic effects by converting plasminogen to plasmin on clot-bound fibrin, in turn degrading fibrin clot (18). This results in a 16–36% decrease in circulating fibrin and 16–62% decrease in fibrinogen (19). Alteplase has limited activity in the absence of fibrin (20).
The elimination of alteplase is biphasic, reflecting a two-compartment circulatory model (21–23). In healthy patients, there is an alpha-phase with rapid decrease in plasma concentration due to tissue distribution. The alpha-phase half-life (t1/2) ranges from 3.3 to 6 min. The beta-phase is a slower decrease in plasma concentration due to metabolism and excretion with a t1/2 of 26–40 min (24, 25). In patients with AMI, the alpha-phase t1/2 of alteplase is <5 min and the beta-phase t1/2 is 16–88 min (23, 26, 27). The majority of alteplase pharmacokinetic studies have been conducted in AMI patients, who may have different elimination characteristics compared to patients with AIS (18). The mean age in many AMI studies is <60 years, and >45% of patients who receive alteplase for AIS are >70 years (18, 28). Alteplase metabolism may be slower in the elderly, contributing to increased hemorrhage risk (18, 29). Additionally, alteplase doses used in AMI studies differ from the 0.9 mg/kg dose for AIS.
After alteplase administration, fibrinogen concentrations return to 81% of pre-infusion values within 24 h (19, 23, 30). Plasminogen and alpha-2 antiplasmin demonstrate similar concentration trends (70 and 35% of pre-infusion concentrations at 2 h post-infusion, 83 and 88% at 24 h, respectively) (23). Fibrin degradation products are found for upwards of 7 h in patients treated with alteplase (31). Therefore, safe timing of antithrombotic initiation after alteplase treatment is uncertain. A study of 60 AIS patients who received IV rtPA followed by 2850 units nadroparin twice daily either immediately after rtPA or 24 h later found similar symptomatic intracranial hemorrhage rates at 36 h between the two groups (early group 8.6 and 4% later group OR 1.8, 95% CI 0.5–3.2) (32). Sixteen (45.7%) early treatment and nine (36%) standard treatment patients achieved a modified Rankin Scale score of 0 or 1 at 3 months (OR 1.2, 95% CI 0.5–3.2) (32). Patients with PE are generally treated with thrombolysis followed by immediate anticoagulation with heparin, and in a trial comparing this regimen to heparin alone, only one intracranial hemorrhage was observed, in a patient who had sustained head trauma (33). Larger trials investigating early versus delayed anticoagulation for AIS patients who have received IV rtPA are needed.
Conclusion
Left ventricular thrombus can complicate both AMI and CHF with dilated cardiomyopathy. These patients often have overlapping risk factors for stroke and additional factors must be considered in the management of AIS in this population. Additionally, with increasing efficiency and expedited care at advanced stroke centers, ventricular thrombi may be increasingly discovered very early in the patient’s post stroke care. LVT is not a contraindication for treatment of AIS with IV rtPA. However, subsequent use of therapeutic anticoagulation following thrombolysis within 24 h is contraindicated. This case demonstrates that early anticoagulation, 12 h post-thrombolytic infusion, can be safely administered. Further evaluation of the safety of early anticoagulation for LVT with high recurrent embolic potential following IV rtPA for AIS is needed.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2011) 42(1):227–76. doi: 10.1161/STR.0b013e3181f7d043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, et al. CHEST Supplement. Chest (2012) 141(2):e637S–e668S. doi:10.1378/chest.11-2306
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol (1993) 22(4):1004–9. doi:10.1016/0735-1097(93)90409-T
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Loh E, Sutton MSJ, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med (1997) 336(4):251–7. doi:10.1056/NEJM199701233360403
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation (2006) 113(10):e409–49. doi:10.1161/01.STR.0000199147.30016.74
6. Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J (2006) 152(1):75–84. doi:10.1016/j.ahj.2005.08.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Visser C, Kan G, David G, Lie K, Durrer D. Two dimensional echocardiography in the diagnosis of left ventricular thrombus. A prospective study of 67 patients with anatomic validation. Chest (1983) 83(2):228–32. doi:10.1378/chest.83.2.228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. de Lemos JA, Ettinger SM. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol (2013) 61(4):e78–140. doi:10.1016/j.jacc.2012.11.019
9. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol (2004) 44(3):671–719. doi:10.1016/j.jacc.2004.07.002
10. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med (2012) 366(20):1859–69. doi:10.1056/NEJMoa1202299
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Pulerwitz T, Rabbani LE, Pinney SP. A rationale for the use of anticoagulation in heart failure management. J Thromb Thrombolysis (2004) 17(2):87–93. doi:10.1023/B:THRO.0000037663.81512.71
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Billingsley I, Leong-Poi H. Left ventricular thrombus: diagnosis, prevention management. Cardiol Rounds (2005) 10(7):1–6.
13. Garg A, Yaduvanshi A, Mohindra KD. Cardioembolic stroke on unaffected side during thrombolysis for acute ischemic stroke. Neurol India (2010) 58(1):112–4. doi:10.4103/0028-3886.60419
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Derex L, Nighoghossian N, Perinetti M, Honnorat J, Trouillas P. Thrombolytic therapy in acute ischemic stroke patients with cardiac thrombus. Neurology (2001) 57(11):2122–5. doi:10.1212/WNL.57.11.2122
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2013) 44(3):870–947. doi:10.1161/STR.0b013e318284056a
16. Semba CP, Deitcher SR, Li X, Resnansky L, Tu T, McCluskey ER. Treatment of occluded central venous catheters with alteplase: results in 1,064 patients. J Vasc Interv Radiol (2002) 13(12):1199–205. doi:10.1016/S1051-0443(07)61965-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the US food and drug administration (FDA). Ann Neurol (2009) 66(1):6–10. doi:10.1002/ana.21750
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Acheampong P, Ford GA. Pharmacokinetics of alteplase in the treatment of ischaemic stroke. Expert Opin Drug Metab Toxicol (2011) 8(2):271–81. doi:10.1517/17425255.2012.652615
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Mueller HS, Rao AK, Forman SA. Thrombolysis in myocardial infarction (TIMI): comparative studies of coronary reperfusion and systemic fibrinogenolysis with two forms of recombinant tissue-type plasminogen activator. J Am Coll Cardiol (1987) 10(3):479–90. doi:10.1016/S0735-1097(87)80188-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Larrue V. Intravenous alteplase for acute ischaemic stroke. Expert Opin Pharmacother (2005) 6(15):2699–709. doi:10.1517/14656566.6.15.2699
21. Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation (1997) 96(3):761–8. doi:10.1161/01.CIR.96.3.761
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Krause J. Catabolism of tissue-type plasminogen activator (t-PA), its variants, mutants and hybrids. Fibrinolysis (1988) 2(3):133–42. doi:10.1016/0268-9499(88)90026-4
23. Tanswell P, Tebbe U, Neuhaus K, Gläsle-Schwarz L, Wojcik J, Seifried E. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol (1992) 19(5):1071–5. doi:10.1016/0735-1097(92)90297-Z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Nilsson T, Wallén P, Mellbring G. In vivo metabolism of human tissue-type plasminogen activator. Scand J Haematol (1984) 33(1):49–53. doi:10.1111/j.1600-0609.1984.tb02209.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Tanswell P, Seifried E, Su PC, Feuerer W, Rijken DC. Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects. Clin Pharmacol Ther (1989) 46(2):155–62. doi:10.1038/clpt.1989.120
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Seifried E, Tanswell P, Ellbruck D, Haerer W, Schmidt A. Pharmacokinetics and haemostatic status during consecutive infusions of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction. Thromb Haemost (1989) 61(3):497–501.
27. Garabedian HD, Gold HK, Leinbach RC, Johns JA, Yasuda T, Kanke M, et al. Comparative properties of two clinical preparations of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. J Am Coll Cardiol (1987) 9(3):599–607. doi:10.1016/S0735-1097(87)80054-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Ford GA, Ahmed N, Azevedo E, Grond M, Larrue V, Lindsberg PJ, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke (2010) 41(11):2568–74. doi:10.1161/STROKEAHA.110.581884
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Stangl MDK, Laule MDM, Tenckhoff MDB, Stangl V, Gliech MDV, Dübel MDP, et al. Fibrinogen breakdown, long-lasting systemic fibrinolysis, and procoagulant activation during alteplase double-bolus regimen in acute myocardial infarction. Am J Cardiol (1998) 81(7):841–7. doi:10.1016/S0002-9149(98)00018-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Verstraete M, Bounameaux H, de Cock F, Van de Werf F, Collen D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans. J Pharmacol Exp Ther (1985) 235(2):506–12.
31. Eisenberg PR, Sherman LA, Tiefenbrunn AJ, Ludbrook PA, Sobel BE, Jaffe AS. Sustained fibrinolysis after administration of t-PA despite its short half-life in the circulation. Thromb Haemost (1987) 57(1):35–40.
32. Mikulík R, Dufek M, Goldemund D, Reif M. A pilot study on systemic thrombolysis followed by low molecular weight heparin in ischemic stroke. Eur J Neurol (2006) 13(10):1106–11. doi:10.1111/j.1468-1331.2006.01458.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Goldhaber S, Come P, Lee R, Braunwald E, Parker J, Haire W, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet (1993) 341(8844):507–11. doi:10.1016/0140-6736(93)90274-K
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: acute ischemic stroke, cardioembolic, thrombolysis, left ventricular thrombus, anticoagulation, rtPA, alteplase, pharmacokinetics
Citation: Gill R, Donahey E and Ruland S (2015) Early administration of therapeutic anticoagulation following intravenous thrombolysis for acute cardiogenic embolic stroke caused by left ventricular thrombus: case report and topic review. Front. Neurol. 6:9. doi: 10.3389/fneur.2015.00009
Received: 14 November 2014; Paper pending published: 10 December 2014;
Accepted: 12 January 2015; Published online: 02 February 2015.
Edited by:
Rajeev Kumar Garg, Rush University Medical Center, USAReviewed by:
Shawna Cutting, Rush University Medical Center, USASarah Y. Song, Rush University Medical Center, USA
Copyright: © 2015 Gill, Donahey and Ruland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth Donahey, Department of Pharmacy, Loyola University Medical Center, 2160 South First Avenue, Maywood, IL 60153, USA e-mail:ZWRvbmFoZXlAbHVtYy5lZHU=
 Rick Gill
Rick Gill Elisabeth Donahey
Elisabeth Donahey Sean Ruland
Sean Ruland