
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 August 2014
Sec. Neurotrauma
Volume 5 - 2014 | https://doi.org/10.3389/fneur.2014.00161
This article is part of the Research Topic Understanding the mechanism of traumatic brain injury-induced energy metabolism View all 11 articles
Inconsistent gender differences in the outcome of TBI have been reported. The mechanism is unknown. In a recent male animal study, repeated stress followed by TBI had synergistic effects on brain gene expression and caused greater behavioral deficits. Because females are more likely to develop anxiety after stress and because anxiety is mediated by cannabinoid receptors (CBRs) (CB1 and CB2), there is a need to compare CB1 and CB2 expression in stressed males and females. CB1 and CB2 mRNA expression was determined in the amygdala, hippocampus, prefrontal cortex (PFC), and hypothalamus of adolescent male and female rats after 3 days of repeated tail-shock stress using qPCR. PFC CB1 and CB2 protein levels were determined using Western blot techniques. Both gender and stress had significant effects on brain CB1 mRNA expression levels. Overall, females showed significantly higher CB1 and CB2 mRNA levels in all brain regions than males (p < 0.01). Repeated stress reduced CB1 mRNA levels in the amygdala, hippocampus, and PFC (p < 0.01, each). A gender × stress interaction was found in CB1 mRNA level in the hippocampus (p < 0.05), hypothalamus (p < 0.01), and PFC (p < 0.01). Within-sex one-way ANOVA analysis showed decreased CB1 mRNA in the hippocampus, hypothalamus, and PFC of stressed females (p < 0.01, each) but increased CB1 mRNA levels in the hypothalamus of stressed males (p < 01). There was a gender and stress interaction in prefrontal CB1 receptor protein levels (p < 0.05), which were decreased in stressed females only (p < 0.05). Prefrontal CB2 protein levels were decreased in both male and female animals after repeated stress (p < 0.05, each). High basal levels of CBR expression in young naïve females could protect against TBI damage whereas stress-induced CBR deficits could predict a poor outcome of TBI in repeatedly stressed females. Further animal studies could help evaluate this possibility.
There is a growing body of literature supporting a gender effect on the acute response and long-term outcomes of TBI, yet the findings are inconsistent (1–5). Several studies suggest that gender differences in TBI outcome may be age-dependent. In a recent retrospective mortality study, involving 10,135 prepubescent (0–12 years), and 10,145 pubescent (12–18 years) hospitalized patients who sustained isolated moderate-to-severe TBI (defined as a head Abbreviated Injury Scale (AIS) score of 3 or greater). Ley et al. (6) found a significantly reduced mortality rate in prepubescent patients than in pubescent patients (5.2 vs. 8.6%, p < 0.0001). Additionally, females in the pubescent but not in the prepubescent age group showed a significantly greater decrease in mortality than males. Groswasser et al. (7) also reported a significantly better predicted-outcome for young females than for males under the age of 18 with comparable levels of TBI severity. Barr (8) reported that high school girls with TBI outperform boys of the same age on selected measures of processing speed and executive functions. Similar gender specific findings have been reported by others (9–12). However, other studies demonstrated that older women took significantly longer time than men to recover from TBI, after controlling for age, injury severity, mechanism of injury, and comorbidities (13–15). The mechanism for the inconsistent gender effect across different age groups is unknown.
Both genetic and epigenetic/environmental factors could be involved (16–19). Early stress exposure has been recognized as an important mechanism for neuropsychiatric disorders (20–22). Stress and stress-related anxiety could also influence TBI outcome as people who exhibited high levels of acute stress symptoms and anxiety had poor TBI outcome (23). A significant portion of the US military personnel returning from Iraq and Afghanistan battlefields have experienced persistent somatic pain, as well as comorbidity of mild traumatic brain injury (mTBI) and post-traumatic stress disorder (PTSD) (24–30). In a logistic regressions study of 2,348 veterans of Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) (51% female), Iverson et al. (31) reported significant associations between probable TBI, symptomatic anxiety, and symptomatic physical health in both genders. Additionally, TBI is more strongly associated with all health symptoms for females and symptomatic anxiety and physical health for male veterans without probable PTSD (31). To examine the potential influence of repeated stress on the outcome of TBI, we recently reported that repeated stress followed by TBI had synergistic effects on the expression of brain mitochondrial electron transport chain complex subunits, and caused more severe behavioral deficits in male animals (females were not examined in that study) (32).
It is not clear if stress could have an equal influence on the outcome of TBI in males and females, although a greater impact of stress on the psychological outcome of females is well known. Our recent animal model studies of PTSD have shown that brain cannabinoid receptors (CBRs) are more rapidly depleted in the cerebella and brain stems of stressed female adolescent rats than in males (32, 33). Other studies suggest that endocannabinoids (eCBs) and CBR activity are involved in the functional recovery of animal experiencing repeated stress and TBI (32–37).
From the results of these studies and other evidence, we hypothesized that CBR-mediated activity may be a critical mechanism linking PTSD and TBI and is responsible for gender difference in PTSD and TBI. We are intent on investigating neuroprotective factors in male and female rats to evaluate how this may relate to recovery following TBI. While actual TBI procedure was not part of the current study design, the findings may translate to issues regarding TBI and co-occurring stress as evidenced in diagnoses such as PTSD.
Anandamide and 2-arachidonoylglycerol (2-AG) are the main components of brain eCBs. eCBs are synthesized upon demand through enzymatic cleavage of membrane lipid precursors and immediately released into the synaptic space. Anandamide has a higher affinity for the CB1 than for CB2 receptors (38), which are highly expressed in the hippocampus, striatum, cerebellum, and cortex (39). 2-AG has a low affinity for CB1 but is more abundant than anandamide (>200-fold) in the brain.
Endocannabinoids are synthesized upon demand through enzymatic cleavage of membrane lipid precursors and are immediately released into the synaptic space. They induce complex neuroprotective, anxiolytic, and modulator effects on brain structure and function via the activation of CBRs (mainly CB1 and CB2). Anandamide and 2-arachidonoylglycerol (2-AG) are the main brain eCBs and can alleviate blood–brain barrier dysfunction, brain edema, lesion volume, neuronal death, and improve behavioral performance in rodent models of TBI through multiple mechanisms (40–45). Anandamide has a higher affinity for CB1 receptors than for CB2 receptors (38), which are highly expressed in the hippocampus, striatum, cerebellum, and cortex (39). 2-AG, on the other hand, has a low affinity for CB1 but is more abundant than anandamide (by >200-fold) in the brain. The neuroprotective effects of eCBs in TBI could also be mediated by CB1 receptor activation, which can inhibit anxiety, stress response, and the retention of aversive memories (46). Animals lacking CB1 receptors show hypersensitivity to stressful stimuli, increased anxiety-like behaviors, and higher mortality (reduced lifespan) (47–50). CB2 receptors are primarily expressed in peripheral immune cells; however, recent studies show that they are also expressed in microglia, dendritic cells, brain endothelial cells, and the subgroups of neurons in several brain regions (51–55).
Evidence supporting a role of eCBs in TBI-induced injury and/or neuroprotection includes the significantly elevated levels of 2-AG following TBI (41). When administered to mice with TBI, 2-AG decreased brain edema, inflammation and infarct volume, and improved clinical recovery (42–44). 2-AG also suppressed inflammation, tumor necrosis factor-a (TNF-a), and reactive oxygen species (ROS) in LPS-stimulated macrophages and LPS-stimulated mice (56).
In this study, we examined CB1 and CB2 receptor expression after repeated tail-shock stress in the amygdala, hypothalamus, hippocampus, and prefrontal cortex (PFC) of adolescent male and female rats to determine how the base-line CBR can be affected by chronic stress. These brain regions play key roles in stress response and emotional memory. Adolescent animals were studied because they are more sensitive to stress than adult, a trait that could have a significant influence on disease development in adulthood (57–60). Furthermore, a gender difference in TBI outcome has been shown for pubescent animals, but not for prepubescent ones (6).
Male and female Sprague–Dawley rats (n = 16, each) (Taconic Farms, Germantown, NY, USA) weighing 120–150 g (5–6 weeks old) were used in this study. Animals of the same sex were housed two per cage and raised at room temperature (22 ± 2°C) on a 12 h light–dark schedule (lights on 1800 h). Animals had ad libitum access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Uniformed Services University of the Health Sciences, and were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Animals were left undisturbed for 7-day after arrival. The stress procedure consisted of a 2-h per day session of immobilization and tail-shocks over three consecutive days as reported previously (61). In brief, half of the animals (eight per sex group) were restrained in individual Plexiglas tube and given 40 electric shocks (2 mA, 3 s duration) at varying intervals (140–180 s). The control animals were handled daily for the same time period but were not subjected to the immobilization and tail-shock stress procedures. All animals were returned to their home cages immediately after exposure to the stress or control conditions.
Following the last stress session on day 3, both the control animals and the stressed animals were decapitated after light anesthesia with halothane. The brains were rapidly removed. A Vibratome (Technical Products International, St. Louis, MO, USA) was used to cut 1.6 mm-thick transverse slices containing the whole amygdala region (Bregma −3.60 to −2.00 mm) from tissue blocks. The basolateral complex, composed mainly of the lateral and basolateral nuclei, was dissected from this slice laterally, as outlined, by the white matter tract of the external capsule (corpus callosum) and medially by the white matter tract of the longitudinal association bundle. This transverse slice (Bregma −3.60 to −2.00 mm) also contained the hippocampal dentate gyrus and CA1–CA3 regions as well as part of the hypothalamus. The PFC was similarly dissected. All tissue samples were immediately stored in pre-cooled isopentane (−40°C).
Dissected brain tissue samples were homogenized and total RNA was extracted using an RNeasy kit (Qiagen, Germany) according to the manufacturer’s protocol. One microgram of total RNA was reverse transcribed into first-strand cDNA using the RETROscript reverse transcriptase kit (Ambion, TX, USA) according to the manufacturer’s recommendations.
Fifty nanograms of the reverse transcribed RNA from the RT-reaction was used as the template for quantitative real-time PCR reaction with a final PCR reaction volume of 25 μl and a final concentration of the 5′ and 3′ PCR primers at 100 nM each. CB1 (TTTCCCACTCATTGACGAGAC, GTGAGCCTTCCAGAGAATGT) and CB2 (AAAGCACACCAACATGTAGCC, GGAACCAGCATATGAGCAGAA) qPCR primers were designed using Primer3 software (MIT, MA, USA) with the size of amplified cDNA ranging between 90 and 150 bp (34). Quantification of CB1 and CB2 mRNA expression was performed (in triplicate) using a two-step PCR reaction procedure on an iQ5 Real-Time PCR System (BioRad, CA, USA) using the SYBR Green SuperMix (BioRad, CA, USA). After initial denaturation at 95°C for 3 min, 40 cycles of primer annealing and elongation were conducted at 60°C for 45 s, followed by denaturation at 95°C for 10 s. Fluorescent emission data were captured, and mRNA levels were quantified using the threshold cycle value (Ct).
Fold change in mRNA expression was calculated using the following equation: Fold = 2(Ct control − Ct stress). To compensate for potential variations in input RNA amounts and the efficiency of reverse transcription, data for CB1 and CB2 mRNA of each sample were additionally normalized by reference to the data obtained from house keeping genes β-actin (GenBank accession no. X62085) determined from the same sample. The fold change in the compensated mRNA expression data was calculated using the equation: fold change = 2−ΔΔCt, where ΔCt = target gene Ct − housekeeping gene (β-actin) Ct, and ΔΔCt is ΔCt control − ΔCt stress (or fold change) = 2(ΔCt control − ΔCt stress).
Prefrontal cortex tissues from the stressed and control animals were homogenized and sonicated for 40 s in the T-Per tissue lysis buffer for western blot analysis (Pierce, IL, USA). Amygdala, hypothalamus, and hippocampus tissue proteins were not examined due to the limited amount of these tissues that were dissected. Protein concentrations were determined using a Bradford assay (BioRad, CA, USA). Aliquots of 20 μg proteins were separated by electrophoresis on NuPage gels (10%) and transferred to a polyvinylidene difluoride membrane before being incubated with the primary antibodies of CB1, phosphorylated-CB1, glycosylated-CB1, and CB2, diluted at 1:500 each (Santa Cruz Biotechnologies, CA, USA). The membranes were rinsed in a 0.01 M Tris-buffered saline solution (pH 7.4) containing 0.1% Triton X-100 for 30 min, blocked in 5% non-fat dry milk for 30 min and incubated overnight at 4°C with the primary antibody in a Tris-buffered saline solution containing 3% non-fat dry milk. Membranes were washed three-times with the Tris-buffered saline solution and incubated overnight at 4°C with a horseradish peroxidase-conjugated secondary antibody in the Tris-buffered saline solution containing 3% non-fat dry milk. Immunoreactive bands were visualized using horseradish peroxidase-conjugated anti-rabbit antibodies in a 1:3000 ratio, and ECL Western blotting detection reagents (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). The western blots were captured with a digital camera and the intensities quantified with NIH Image 1.62.
Data regarding the effects of gender and stress on CB1 and CB2 receptors for individual brain regions were analyzed using two-way ANOVA analyses. Because of the significant gender and stress interactions found in brain CB1 receptor expression, within-sex one-way ANOVA analyses were also conducted. A p-value of <0.05 was considered statistically significant.
Two-way ANOVA analyses revealed significant gender and stress effects on CB1 mRNA levels in the amygdala, hippocampus, and the PFC (p < 0.01, each). Overall, female animals exhibited higher basal levels of CB1 mRNA expression in the amygdala, hippocampus, and the PFC than male animals (p < 0.01, each) (Figure 1; Table 1). Stressed animals exhibited reduced CB1 mRNA levels in the amygdala, hippocampus, and the PFC when compared to those brain regions of the control animals (p < 0.01, each) (Figure 1). However, in the hypothalamus there was no significant difference between the CB1 mRNA levels in the stress and control groups (p > 0.05). A significant interaction between gender and stress on CB1 mRNA levels was found in the hippocampus (p < 0.05), hypothalamus (p < 0.01), and PFC (p < 0.01). Within-sex one-way ANOVA revealed decreased CB1 mRNA levels in the hippocampus, hypothalamus, and PFC of female animals (p < 0.01, each) but increased CB1 mRNA level in the hypothalamus of male animals after the stress (p < 0.05) (Figures 1A–D).
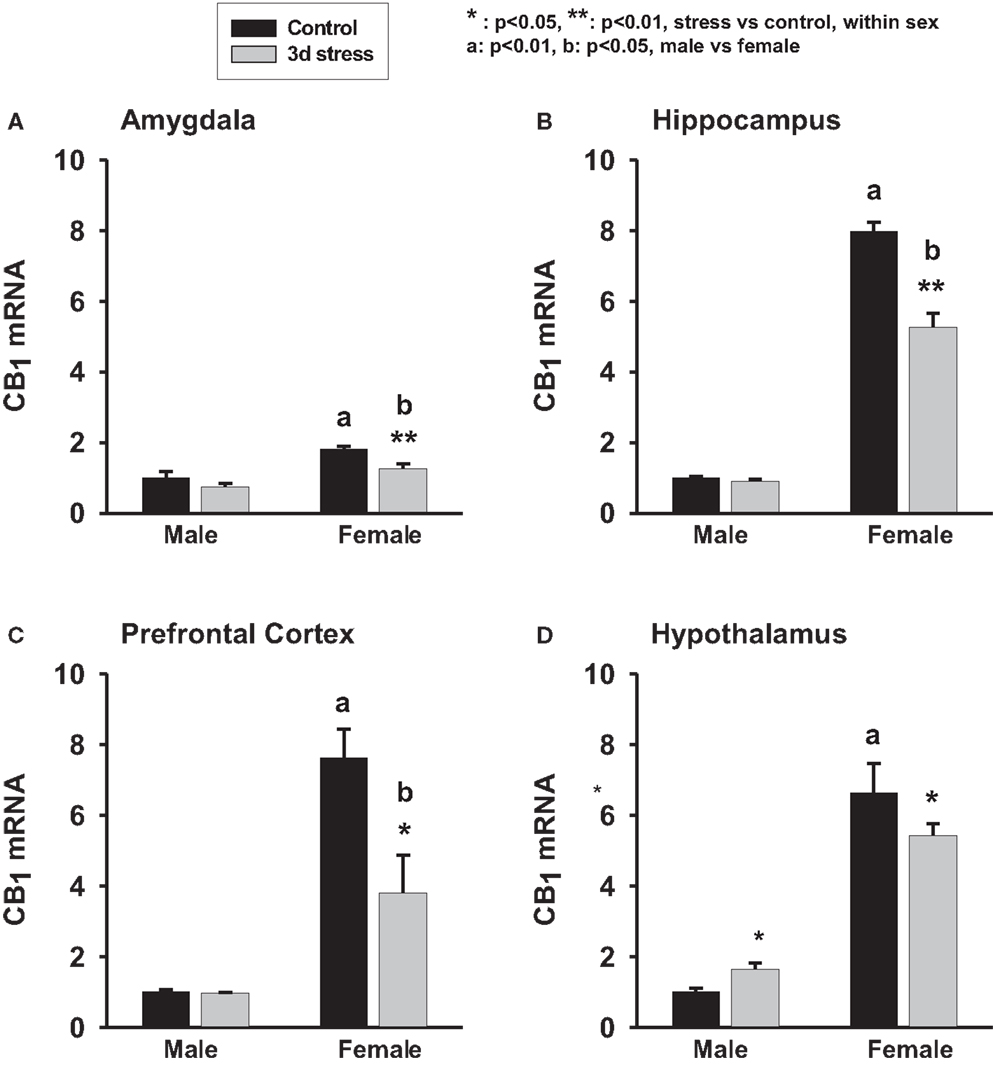
Figure 1. Two-way ANOVA show significant effects of gender and stress. Female adolescent rats show a greater baseline of CB1 mRNA expression in the amygdala, hippocampus, hypothalamus and prefrontal cortex than the males (p < 0.01, each). Three days repeated tail-shock stress significantly down-regulated CB1 mRNA levels in rat amygdala (A), hippocampus (B), prefrontal cortex (C) and hypothalamus (D), especially in the female rats. Black column: control; gray column, stressed group, a, p < 0.01, male vs. female; b, p < 0.01: control group vs. stress group; *p < 0.05; **p < 0.01, control vs. stress within sex comparison.
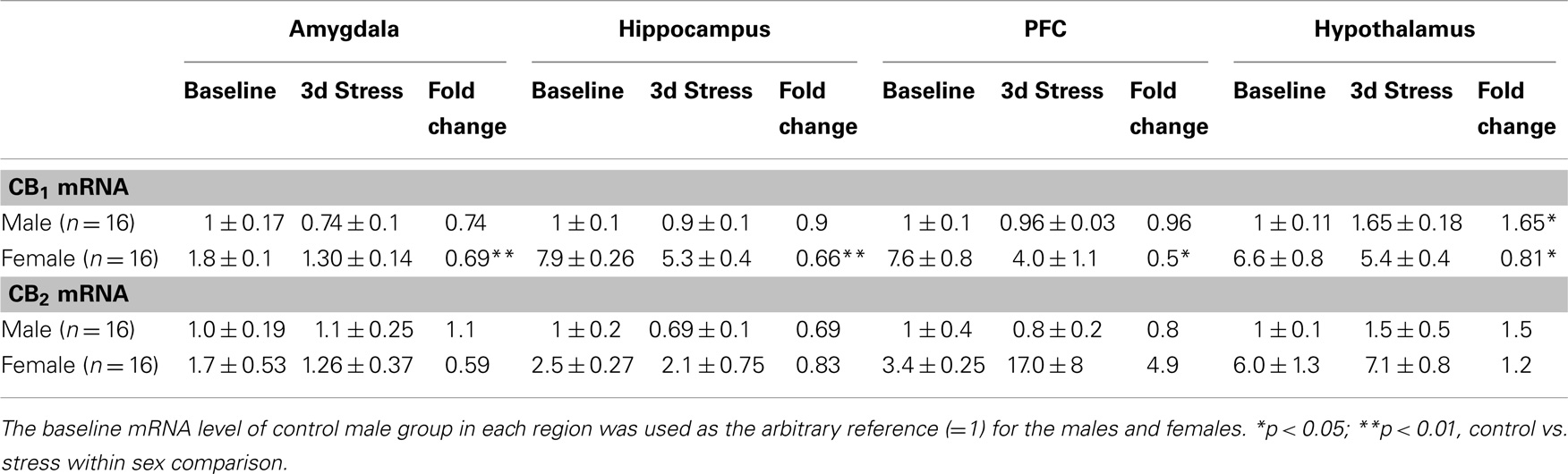
Table 1. Relative fold change (mean ± SD) in CB1 and CB2 mRNA expression levels in the amygdala, hippocampus, prefrontal cortex (PFC) and hypothalamus of male and female adolescent rats after 3 days repeated (2 h/day) tail-shock stress.
Base-line CB2 mRNA levels were significantly higher in the hippocampus, hypothalamus, and PFC of female animals than in male animals (p < 0.01, each) (Figures 2A–D). CB2 mRNA levels, however, remained unchanged following stress.
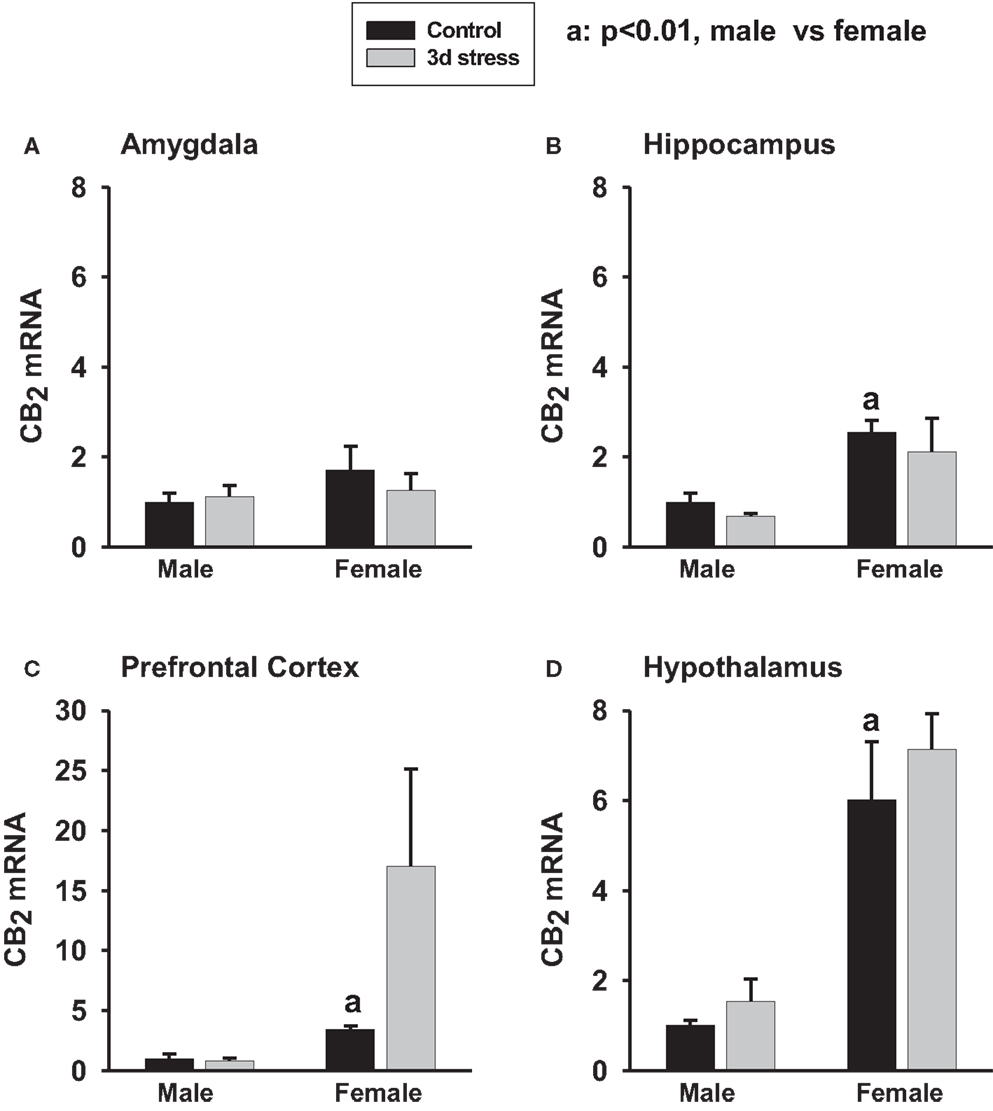
Figure 2. Two-way ANOVA show that female rats exhibited greater CB2 mRNA expression in the amygdala (A) (p < 0.1), hippocampus (B) (p < 0.01), prefrontal cortex (C) (p < 0.01) and hypothalamus (D) (p < 0.01) than male rats. Within-sex one-way ANOVA show that CB2 mRNA levels were significantly increased in the prefrontal cortex of female rats after the stress exposure (p < 0.05). Black column: control; gray column, stressed group, a, p < 0.01, male control vs. female control; *p < 0.05; **p < 0.01, control vs. stress within sex comparison.
Two-way ANOVA analyses showed no significant gender or stress effects on total CB1 proteins, phosphorylated (p-CB1), or glycosylated-CB1 (g-CB1) proteins in the PFC (Figure 3; Table 2). There were, however, significant gender-by-stress interactions in total proteins and glycosylated-CB1 proteins (p < 0.05, each). Within-sex one-way ANOVA analyses showed significantly decreased total CB1 protein levels (p < 0.05) and glycosylated-CB1 protein levels (p < 0.05) in the PFC of stressed female rats but not in stressed males.
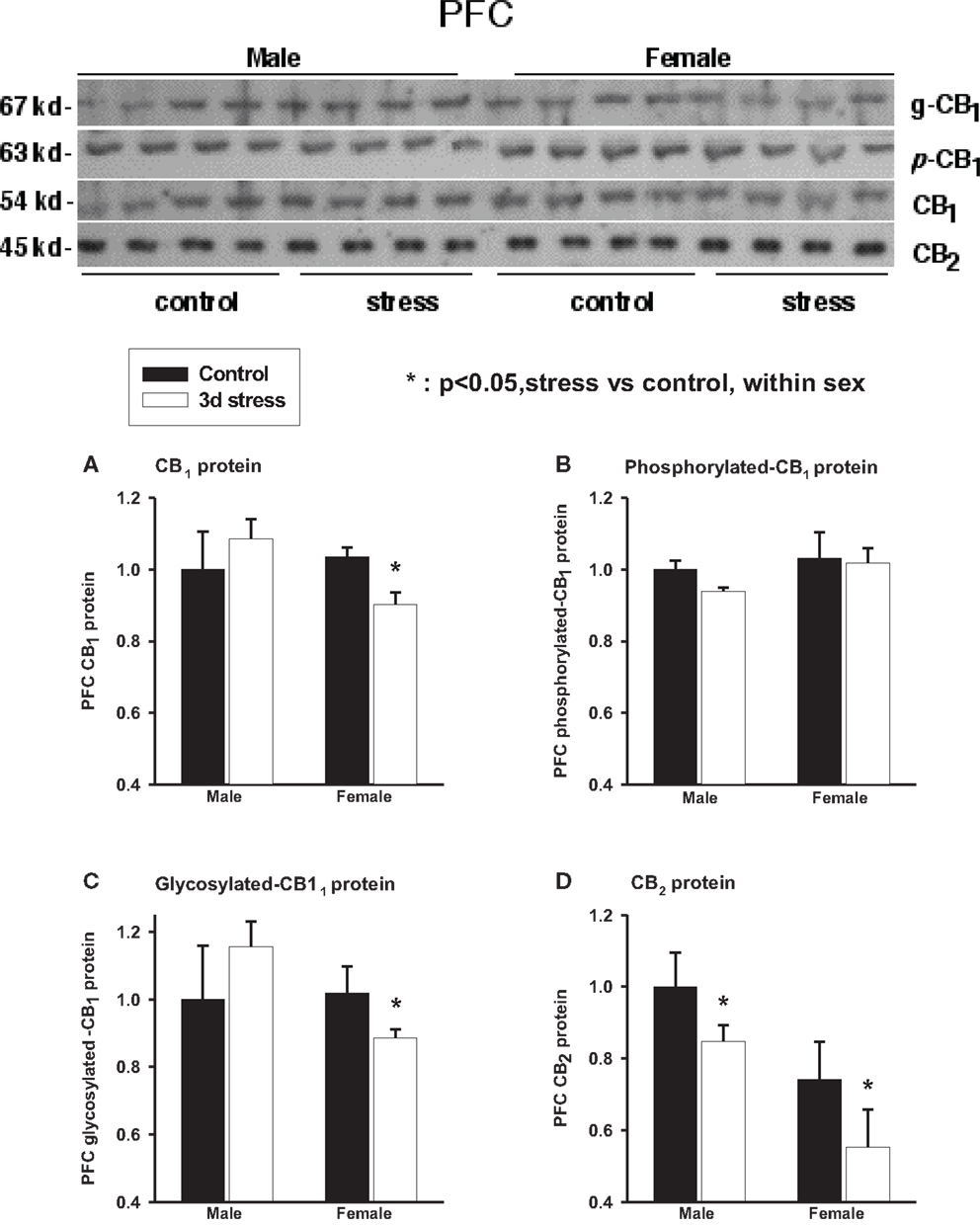
Figure 3. Upper panel: representative western blots of total CB1 and CB2 receptor proteins; glycosylated (g-CB1) and phosphorylated (p-CB1) CB1 proteins in rat prefrontal cortex tissue homogenates. Lower panel: two-way ANOVA of the western blot showing a trend level of stress × sex interaction in total CB1 proteins (A); phosphorylated CB1 proteins (p-CB1) (B) and; glycosylated-CB1 proteins (C). A within-sex one-way ANOVA showed significantly reduced total CB1 proteins and glycosylated-CB1 proteins in female prefrontal cortex (mean ± SD); CB2 protein levels were significantly reduced in the prefrontal cortex of both female and male rats after repeated stress (D). The mean value of the male control group was used as the arbitrary reference = 1, *p < 0.05, control vs. stress within sex comparison, black column, control group; blank column, stressed group.
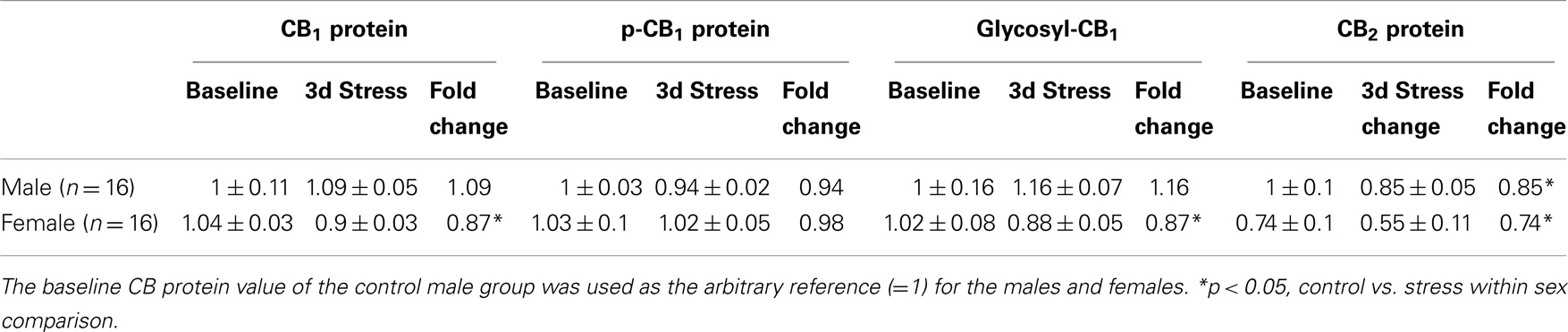
Table 2. Relative fold change (mean ± SD) in CB1 and CB2 protein expression levels in the prefrontal cortex of male adolescent rats after 3 days repeated inescapable repeated tail-shock stress and female.
Two-way ANOVA analyses showed that prefrontal CB2 protein levels were greater in males than in females (p < 0.01) and were significantly suppressed in both sexes after repeated stress (p < 0.05). There was no significant gender-by-stress interaction in prefrontal CB2 protein levels.
A recent retrospective mortality study of TBI, involving more than 20,200 prepubescent and pubescent patients with moderate-to-severe TBI, showed that mortality rates were significantly lower in the prepubescent patients than in pubescent patients (p < 0.0001) (6). Within the pubescent group, it was further found that females had a significantly lower mortality rate than males. The mechanism underlying the age-dependent gender differences in TBI outcome is unknown. Besides a potential role of female sex hormones that may have protected the pubescent females, our studies as well as others suggest that brain CBR-mediated activity could play a critical role in the age-related gender difference in TBI outcome through at least two mechanisms: anxiolytic activity and neuroprotection.
In our study, female adolescent animals showed higher base-line CB1 and CB2 mRNA expression levels in the amygdala, hippocampus, hypothalamus, and PFC than the male adolescents (Figures 1 and 2; Table 1). That difference, however, disappeared rapidly after the repeated stress induced a larger reduction in CB1 mRNA levels in the female brain. Furthermore, although repeated stress down-regulated CB1 mRNA expression in the hypothalamus of female rats, caused the up-regulation of CB1 mRNA expression in the hypothalamus of male rats. This divergent result is consistent with the selective inhibition of hypothalamic neuronal activity by CB1 agonists in female but not in male guinea pigs (62), and the observation of a greater elevation of corticosterone in females than in males after stress (63). A reduction in CB1 protein and glycosylated-CB1 protein levels was also found in the PFC of the stressed female rats whereas a trend of increased CB1 protein was found in the male animals.
The higher base-line CB1 mRNA expression in the adolescent female rat brain when compared to their male counterparts is consistent with the reports of greater CB1 mRNA expression in the white blood cells of female humans (64, 65), higher eCBs content in the brains of female rats (66) and increased CB1 mRNA expression in the cerebella and brainstems of female rats (34). Because, CB1 activity is neuroprotective and a lack of CB1 activity in CB1 knockout animals is linked with increased mortality (67), our findings support the notion that greater base-line CB1 expression in female adolescent brains may underlie the reduced mortality in pubescent compared to the females with moderate-to-severe TBI when compared with adolescent males of the same TBI severity (6). While it is not yet known why such female-specific neuroprotection is present only in the pubescent but not in the pre- or post-pubescent populations, recent studies suggest that chronic stress when combined with high levels of stress hormone production but lower levels of female sex hormones production may deplete brain CBRs more rapidly, which could result in a large eCB/CBR deficit in the affected females.
To support this, Reich et al. (68) reported a lower level of CB1 expression in the hippocampus of socially isolated adult female rats than in their male counterparts. It should be noted that Reich et al.’s study differs from this study in many aspects including: (1) differences in stress paradigms (i.e., 3 days of repeated intense stress in our study vs. 3 weeks of chronic mild heterotypic stressors); (2) controls (naïve normal controls in this study vs. socially isolated controls); (3) feeding regimes (ad libitum feeding in this study vs. a frequent 14 h food/water deprivation); (4) housing environments (same sex pair-housing in this study vs. trio-housing with frequent wet cage rotation); (5) study times (acute phase of stress in this study vs. 3 weeks after chronic stress), and (6) hormone statuses (adolescent in our study vs. adult).
Increased crowding of unisex housing has been found to be stressful for female rats but anxiolytic for males and the opposite is true under isolated rearing (69). The unisex pair-housing in our study may be more stressful for the females than for the males and thus potentiating a greater loss of base-line CB1 receptors in the females after repeated stress. In contrast, chronic mild heterotypic stressors were more stressful for male rats but anxiolytic for females reared in isolation (69).
Gender-related differences in fasting-induced lipid catabolism also exist. It has been reported that females mobilize more fat reserve and thus catabolize more lipophilic eCBs than males during short-term fasting (70, 71). Animals in this study were fed ad libitum without fasting whereas the stressed animals in the Reich’s study experienced multiple episodes of food and water deprivation (>6 times in 14 h) that may have potentiated greater eCB release and CB1 receptor depletion in the stressed adult female brain of that study.
In this study, repeated stress caused a divergent pattern of prefrontal CB1 receptor expression between the males and females. Adolescent female rats displayed a significant reduction in prefrontal CB1 receptor expression. However, prefrontal CB1 receptor expression followed a positive trend in adolescent male rats, which became significant seven afterwards (33), reinforcing the findings of the divergent CB1 gene expression patterns after stress. This increased CB11 expression in male PFC is consistent with the increased mitochondrial electron transport chain complex subunit expression in the PFC of stressed male animals (32). Because of the known anxiolytic and analgesic effects of eCBs and CB11 activation, the more-rapid loss of CB1 in stressed adolescent female brains is consistent with the clinical observations of a greater prevalence and higher severity of anxiety symptoms such as increased sensitivity to fear signals, emotional disturbance, and pain in females after chronic stress exposure (72–76).
Ley et al. (6) showed that human prepubescent, regardless of sex, are better protected against TBI-caused mortality than human pubescent (and possibly the adults as well). Although the mechanism is unknown, developmental studies have shown that the level of CB11 expression in the human PFC is highest after birth but declines rapidly during the postnatal and prepubescent periods and with age (77). Thus, potentially high levels of CB11 expression and activity during the prepubescent periods of development may have provided equally strong neuroprotection against TBI-induced brain damage and mortality in both naive prepubescent males and females (6). While developmentally regulated decline in brain CB11 expression and CB11-associated neuroprotection may be partially compensated by increased sex hormone in naïve pubescent and young adult females, this compensation may be adversely affected in stressed females.
The mechanisms for the poor reported long-term poor outcome of TBI in the older female population could be more complex (78). Again, deficient brain CB1 activity, due to chronic stress, elevated stress hormone levels, and reduced sex hormone levels could all play a role in the female brain, leaving it more vulnerable to TBI damage.
It is possible that the neuroprotective effects of sex steroids in TBI (79) may act partially by upregulating brain eCB activity and CB receptor expression (77). Sex hormones during the estrous cycle have been linked with brain CB1 receptor density, which is reduced in the limbic forebrain and hypothalamus after ovariectomy and castration but can be restored after estradiol, progesterone, and testosterone administration in intact and ovariectomized/castrated rats (80–84). High levels of stress-induced corticosteroid secretion and base-line corticosteroids as well as slow clearance of corticosteroids could lead to reduced CB1 receptor levels in stressed females (85, 86). Chronic exposure to high level of corticosterone, CB1 agonists, and cannabinoids have been reported to downregulate CB1 receptor density, CB1 receptor binding, and CB1 mRNA expression in various brain regions of male and female animals (87–91).
It is noted that although a reduction in prefrontal CB1 and CB2 mRNA expression was not immediately observed in the male animals after 3 days of repeated stress, the expression was significantly decreased in the stressed male animals 7 days following the stress (33), suggesting a delayed pattern of CBR reduction in male adolescents after repeated stress when compared to the females. Other studies showed that 10 days of mild chronic stress (30 min restraint stress per day) upregulated CB1 binding in the PFC of adolescent and adult male rats that was resolved after 40 days recovery period. Furthermore, adolescents exposed to stress were found to have a sustained downregulation of prefrontocortical CB1 receptors in adulthood (92).
CB1 receptor activation in the forebrain and amygdala is anxiolytic (46, 93, 94). The loss of CB1-mediated anxiolytic and neuroprotective activity in these brain regions of both female and males could predict enhanced amygdala-mediated fear memory, especially in the females due to a greater propensity for CB1 reduction. Indeed, loss or inhibition of CB1 receptors in the amygdala, hippocampus, and PFC have been associated with the impaired ability to extinguish fear memories (50, 95, 96). PFC is known to exert a powerful inhibitory effect on amygdala activity and on fear extinction (97, 98) and it has been observed that the amygdala and hippocampus interact to mediate emotional memories (99).
Stress-induced reduction of brain CB1 and CB2 protein expression may also contribute to a more vulnerable brain structure and function (100–111) through multiple mechanisms in response to TBI, including increased microglia activation, inflammation and apoptosis, impaired blood–brain barrier integrity, compromised neuroprotection, and neuroregeneration in response to TBI (42, 112, 113). Activation of CB1 and CB2 receptors may minimize brain damage and promote tissue repair after TBI through the attenuation of injury-stimulated inducible nitric oxide (iNOS) and ROS in microglia (114), promoting neural progenitor (NP) proliferation, and neurosphere generation (115–118). These actions are abrogated when there is a deficit of brain CB1 receptors (119–121). Altered expression levels of brain and peripheral CB1 and CB2 receptors could also underlie changes in energy metabolism and body weight loss, both of which are common phenomena resulting from TBI, due to their direct influence on feeding, glucose uptake, fatty acid synthesis and triglyceride accumulation, energy expenditure, and metabolic homeostasis (122–127).
It is tempting to speculate that brain CBR deficit, associated with stress, age, gender, and anxiety/agitation, could play a central role in the individual variations in the outcome of TBI (128). While TBI is not part of the current study, the findings of stress-induced CBR deficits may translate to issues regarding TBI and co-occurring stress as evidenced in diagnoses of comorbidity of PTSD and mTBI in military personnel returned from Iraq and Afghanistan war zone that could advance our understanding of the neuroprotection of the consequences of TBI. Strategies to reduce gender and stress-related brain CBR deficit and agents to restore CBR activity could become potentially effective therapies for TBI. Indeed, treatment with synthetic 2-AG resulted in attenuated edema formation, infarct volume, and blood–brain barrier permeability in a mouse model of TBI, an effect dose-dependently attenuated by a CB1 antagonist (41). And partial inhibition of 2-AG degradation, improved motor coordination, and working memory performance in mice model of TBI (37). Selective and highly potent cannabinoid CB1 and CB2 receptor agonist showed a pronounced neuroprotective effect in a rat TBI model (129). A potent and CB1 and CB2 receptor agonist, when applied before, during, and after transient occlusion of the middle cerebral artery, significantly and dose-dependently reduced cortical lesion sizes and motor deficits (130).
In summary, we found a higher basal value of CBRs in the forebrain of adolescent female animals, which was significantly reduced after repeated stress. Because of the known anxiolytic and neuroprotective effect of eCB and CBR activities, this high base-line CBR may provide a neuroprotective mechanism for the improved outcome of prepubescent and pubescent females with TBI. The stress-induced reduction of CBR may underlie the poor long-term outcome of older female TBI patients who may also be experiencing postmenopause-related reductions in reproductive hormones. As brain eCBs and CBR activity is implicated in age and gender-dependent difference in the outcome of TBI and PTSD, further studies in this direction are required.
The Guest Associate Editor Yumin Zhang declares that, despite being affiliated to the same institution as authors Guoqiang Xing, Janis Carlton, Xiaolong Jiang, Jillian Wen, Min Jia and He Li, the review process was handled objectively and no conflict of interest exists. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported, in part, by Lotus Biotech.com and the Center for the Study of Traumatic Stress, Uniformed Services University of the Health Sciences. Daniel Xing proofread this manuscript.
1. Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J Trauma (2009) 67:950–3. doi: 10.1097/TA.0b013e3181ba3354
2. Aaro Jonsson C, Catroppa C, Godfrey C, Smedler AC, Anderson V. Individual profiles of predictors and their relations to 10 years outcome after childhood traumatic brain injury. Brain Inj (2013) 27:831–8. doi:10.3109/02699052.2013.775493
3. Anderson V, Catroppa C, Godfrey C, Rosenfeld JV. Intellectual ability 10 years after traumatic brain injury in infancy and childhood: what predicts outcome? J Neurotrauma (2012) 29:143–53. doi:10.1089/neu.2011.2012
4. McIntyre A, Mehta S, Aubut J, Dijkers M, Teasell RW. Mortality among older adults after a traumatic brain injury: a meta-analysis. Brain Inj (2013) 27:31–40. doi:10.3109/02699052.2012.700086
5. King N. A systematic review of age and gender factors in prolonged post concussion symptoms after mild head injury. NeuroRehabilitation (2014) 34(4):741–8. doi:10.3233/NRE-141072.
6. Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, et al. Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg (2013) 75:682–6. doi:10.1097/TA.0b013e31829d024f
7. Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj (1998) 12:805–8. doi:10.1080/026990598122197
8. Barr WB. Neuropsychological testing of high school athletes. Preliminary norms and test-retest indices. Arch Clin Neuropsychol (2003) 18:91–101. doi:10.1093/arclin/18.1.91
9. Xiong Y, Mahmood A, Lu D, Qu C, Goussev A, Schallert T, et al. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res (2007) 1185:301–12. doi:10.1016/j.brainres.2007.09.052
10. Ratcliff JJ, Greenspan AI, Goldstein FC, Stringer AY, Bushnik T, Hammond FM, et al. Gender and traumatic brain injury: do the sexes fare differently? Brain Inj (2007) 21:1023–30. doi:10.1080/02699050701633072
11. Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, et al. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav Brain Res (2007) 181:200–9. doi:10.1016/j.bbr.2007.04.006
12. Niemeier JP, Marwitz JH, Lesher K, Walker WC, Bushnik T. Gender differences in executive functions following traumatic brain injury. Neuropsychol Rehabil (2007) 17:293–313. doi:10.1080/09602010600814729
13. Brown SB, Colantonio A, Kim H. Gender differences in discharge destination among older adults following traumatic brain injury. Health Care Women Int (2012) 33:896–904. doi:10.1080/07399332.2012.673654
14. Renner C, Hummelsheim H, Kopczak A, Steube D, Schneider HJ, Schneider M, et al. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj (2012) 26:1360–71. doi:10.3109/02699052.2012.667592
15. Schmidt AT, Hanten GR, Li X, Vasquez AC, Wilde EA, Chapman SB, et al. Decision making after pediatric traumatic brain injury: trajectory of recovery and relationship to age and gender. Int J Dev Neurosci (2012) 30:225–30. doi:10.1016/j.ijdevneu.2011.11.003
16. Conley YP, Okonkwo DO, Deslouches S, Alexander S, Puccio AM, Beers SR, et al. Mitochondrial polymorphisms impact outcomes after severe traumatic brain injury. J Neurotrauma (2014) 31:34–41. doi:10.1089/neu.2013.2855
17. Bulstrode H, Nicoll JA, Hudson G, Chinnery PF, Di Pietro V, Belli A. Mitochondrial DNA and traumatic brain injury. Ann Neurol (2014) 75(2):186–95. doi:10.1002/ana.24116
18. Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, et al. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology (2013) 27:438–51. doi:10.1037/a0032837
19. Ponsford J. Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation (2013) 32(4):803–15. doi:10.3233/NRE-130904
20. Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci (2014) 8:19. doi:10.3389/fnins.2014.00019
21. Karsten CA, Baram TZ. How does a neuron “know” to modulate its epigenetic machinery in response to early-life environment/experience? Front Psychiatry (2013) 4:89. doi:10.3389/fpsyt.2013.00089
22. Gudsnuk KM, Champagne FA. Epigenetic effects of early developmental experiences. Clin Perinatol (2011) 38:703–17. doi:10.1016/j.clp.2011.08.005
23. Broomhall LG, Clark CR, McFarlane AC, O’Donnell M, Bryant R, Creamer M, et al. Early stage assessment and course of acute stress disorder after mild traumatic brain injury. J Nerv Ment Dis (2009) 197:178–81. doi:10.1097/NMD.0b013e318199fe7f
24. Higgins DM, Kerns RD, Brandt CA, Haskell SG, Bathulapalli H, Gilliam W, et al. Persistent pain and comorbidity among operation enduring freedom/operation Iraqi freedom/operation new dawn veterans. Pain Med (2014) 15(5):782–90. doi:10.1111/pme.12388
25. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol (2008) 167:1446–52. doi:10.1093/aje/kwn068
26. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. US Army Med Dep J (2008):7–17; republished from New Engl J Med (2004) 351:13–22. doi:10.1056/NEJMoa040603
27. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med (2008) 358:453–63. doi:10.1056/NEJMoa072972
28. Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress (2010) 23:17–24. doi:10.1002/jts.20483
29. Carlson KF, Barnes JE, Hagel EM, Taylor BC, Cifu DX, Sayer NA. Sensitivity and specificity of traumatic brain injury diagnosis codes in United States Department of Veterans Affairs administrative data. Brain Inj (2013) 27:640–50. doi:10.3109/02699052.2013.771795
30. Brenner LA, Vanderploeg RD, Terrio H. Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehabil Psychol (2009) 54:239–46. doi:10.1037/a0016908
31. Iverson KM, Pogoda TK, Gradus JL, Street AE. Deployment-related traumatic brain injury among operation enduring freedom/operation Iraqi freedom veterans: associations with mental and physical health by gender. J Womens Health (Larchmt) (2013) 22:267–75. doi:10.1089/jwh.2012.3755
32. Xing G, Barry ES, Benford B, Grunberg NE, Li H, Watson WD, et al. Impact of repeated stress on traumatic brain injury-induced mitochondrial electron transport chain expression and behavioral responses in rats. Front Neurol (2013) 4:196. doi:10.3389/fneur.2013.00196
33. Xing G, Carlton J, Jiang X, Jia M, Sharma P, Li H. Delayed effects of repeated inescapable severe stress on brain cannabinoid receptor expression and acoustic startle response in adolescent male rats: relevance to the development of posttraumatic stress disorder and stress-related brain atrophy. In: Forman E, Fuller J, editors. PTSD: The New Research. New York, NY: Nova (2013). p. 83–108.
34. Xing G, Carlton J, Zhang L, Jiang X, Fullerton C, Li H, et al. Cannabinoid receptor expression and phosphorylation are differentially regulated between male and female cerebellum and brain stem after repeated stress: implication for PTSD and drug abuse. Neurosci Lett (2011) 502:5–9. doi:10.1016/j.neulet.2011.05.013
35. Martinez-Vargas M, Morales-Gomez J, Gonzalez-Rivera R, Hernandez-Enriquez C, Perez-Arredondo A, Estrada-Rojo F, et al. Does the neuroprotective role of anandamide display diurnal variations? Int J Mol Sci (2013) 14:23341–55. doi:10.3390/ijms141223341
36. Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res (2012) 90:2293–305. doi:10.1002/jnr.23114
37. Tchantchou F, Zhang Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma (2013) 30:565–79. doi:10.1089/neu.2012.2647
38. Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol (1995) 48:443–50.
39. Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol (2000) 422:159–71. doi:10.1002/(SICI)1096-9861(20000626)422:2<159::AID-CNE1>3.0.CO;2-1
40. van derStelt M, Veldhuis WB, van Haaften GW, Fezza F, Bisogno T, Bar PR, et al. Exogenous anandamide protects rat brain against acute neuronal injury in vivo. J Neurosci (2001) 21:8765–71.
41. Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature (2001) 413:527–31. doi:10.1038/35097089
42. Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab (2005) 25:477–84. doi:10.1038/sj.jcbfm.9600047
43. Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis (2006) 22:257–64. doi:10.1016/j.nbd.2005.11.004
44. Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol (2011) 163:1402–10. doi:10.1111/j.1476-5381.2011.01343.x
45. Cohen-Yeshurun A, Willner D, Trembovler V, Alexandrovich A, Mechoulam R, Shohami E, et al. N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J Cereb Blood Flow Metab (2013) 33:1242–50. doi:10.1038/jcbfm.2013.75
46. Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology (2005) 30:516–24. doi:10.1038/sj.npp.1300655
47. Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci (2002) 16:1395–8. doi:10.1046/j.1460-9568.2002.02192.x
48. Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) (2002) 159:379–87. doi:10.1007/s00213-001-0946-5
49. Uriguen L, Perez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology (2004) 46:966–73. doi:10.1016/j.neuropharm.2004.01.003
50. Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature (2002) 418:530–4. doi:10.1038/nature00839
51. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol (2008) 13:147–59. doi:10.1111/j.1369-1600.2008.00108.x
52. Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem (2005) 95:437–45. doi:10.1111/j.1471-4159.2005.03380.x
53. Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res (2004) 132:87–92. doi:10.1016/j.molbrainres.2004.08.025
54. Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science (2005) 310:329–32. doi:10.1126/science.1115740
55. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol (2005) 168:299–325. doi:10.1007/3-540-26573-2_10
56. Gallily R, Breuer A, Mechoulam R. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice. Eur J Pharmacol (2000) 406:R5–7. doi:10.1016/S0014-2999(00)00653-1
57. Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol (2010) 31:232–40. doi:10.1016/j.yfrne.2010.02.004
58. Widom CS, Czaja SJ, Dutton MA. Childhood victimization and lifetime revictimization. Child Abuse Negl (2008) 32:785–96. doi:10.1016/j.chiabu.2007.12.006
59. Weber K, Rockstroh B, Borgelt J, Awiszus B, Popov T, Hoffmann K, et al. Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry (2008) 8:63. doi:10.1186/1471-244X-8-63
60. Wainwright NW, Surtees PG. Childhood adversity, gender and depression over the life-course. J Affect Disord (2002) 72:33–44. doi:10.1016/S0165-0327(01)00420-7
61. Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology (2009) 34:410–23. doi:10.1038/npp.2008.71
62. Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol (2005) 94:2983–6. doi:10.1152/jn.01187.2004
63. Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci (2006) 26:6643–50. doi:10.1523/JNEUROSCI.5126-05.2006
64. Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol (2002) 127:169–76. doi:10.1016/S0165-5728(02)00113-3
65. Onaivi ES, Chaudhuri G, Abaci AS, Parker M, Manier DH, Martin PR, et al. Expression of cannabinoid receptors and their gene transcripts in human blood cells. Prog Neuropsychopharmacol Biol Psychiatry (1999) 23:1063–77. doi:10.1016/S0278-5846(99)00052-4
66. Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol (2006) 291:R349–58. doi:10.1152/ajpregu.00933.2005
67. Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A (1999) 96:5780–5. doi:10.1073/pnas.96.10.5780
68. Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res (2009) 203:264–9. doi:10.1016/j.bbr.2009.05.013
69. Westenbroek C, Snijders TA, den Boer JA, Gerrits M, Fokkema DS, Ter Horst GJ. Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Horm Behav (2005) 47:620–8. doi:10.1016/j.yhbeh.2005.01.004
70. Hill JO, Talano CM, Nickel M, DiGirolamo M. Energy utilization in food-restricted female rats. J Nutr (1986) 116:2000–12.
71. Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab (2001) 281:E1333–9.
72. Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A (2002) 99:10789–94. doi:10.1073/pnas.162356599
73. Marumo K, Takizawa R, Kawakubo Y, Onitsuka T, Kasai K. Gender difference in right lateral prefrontal hemodynamic response while viewing fearful faces: a multi-channel near-infrared spectroscopy study. Neurosci Res (2009) 63:89–94. doi:10.1016/j.neures.2008.10.012
74. Pratchett LC, Pelcovitz MR, Yehuda R. Trauma and violence: are women the weaker sex? Psychiatr Clin North Am (2010) 33:465–74. doi:10.1016/j.psc.2010.01.010
75. Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un)predictable painful electrocutaneous stimulation: evidence for mediation by fear of pain. Pain (2012) 153(5):1030–41. doi:10.1016/j.pain.2012.02.005
76. Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia (2012) 50(7):1578–93. doi:10.1016/j.neuropsychologia.2012.03.011
77. Choi K, Le T, McGuire J, Xing G, Zhang L, Li H, et al. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res (2012) 46:882–9. doi:10.1016/j.jpsychires.2012.03.021
78. Ng I, Lee KK, Lim JH, Wong HB, Yan XY. Investigating gender differences in outcome following severe traumatic brain injury in a predominantly Asian population. Br J Neurosurg (2006) 20:73–8. doi:10.1080/02688690600682259
79. Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience (2011) 191:101–6. doi:10.1016/j.neuroscience.2011.04.013
80. Busch L, Sterin-Borda L, Borda E. Effects of castration on cannabinoid cb receptor expression and on the biological actions of cannabinoid in the parotid gland. Clin Exp Pharmacol Physiol (2006) 33:258–63. doi:10.1111/j.1440-1681.2006.04355.x
81. Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci (2005) 77:2471–8. doi:10.1016/j.lfs.2005.04.019
82. Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol (2010) 160:544–8. doi:10.1111/j.1476-5381.2010.00776.x
83. González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun (2000) 270:260–6. doi:10.1006/bbrc.2000.2406
84. Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci (1994) 54:159–70. doi:10.1016/0024-3205(94)00585-0
85. Butkevich I, Mikhailenko V, Semionov P, Bagaeva T, Otellin V, Aloisi AM. Effects of maternal corticosterone and stress on behavioral and hormonal indices of formalin pain in male and female offspring of different ages. Horm Behav (2009) 55:149–57. doi:10.1016/j.yhbeh.2008.09.008
86. Caudell KA, Gallucci BB. Neuroendocrine and immunological responses of women to stress. West J Nurs Res (1995) 17:672–92. doi:10.1177/019394599501700607
87. Fan F, Tao Q, Abood M, Martin BR. Cannabinoid receptor down-regulation without alteration of the inhibitory effect of CP 55,940 on adenylyl cyclase in the cerebellum of CP 55,940-tolerant mice. Brain Res (1996) 706:13–20. doi:10.1016/0006-8993(95)01113-7
88. Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, et al. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus (2008) 18:221–6. doi:10.1002/hipo.20386
89. Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. Eur J Pharmacol (2008) 583:322–39. doi:10.1016/j.ejphar.2007.12.033
90. Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res (1993) 616:293–302. doi:10.1016/0006-8993(93)90220-H
91. Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res Mol Brain Res (1997) 46:100–8.
92. Lee TT, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB(1) receptor binding in male rats. Neuroscience (2013) 249:106–14. doi:10.1016/j.neuroscience.2012.11.017
93. Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther (2006) 318:304–11. doi:10.1124/jpet.106.101287
94. Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem (2008) 90:290–3. doi:10.1016/j.nlm.2008.04.003
95. Roche M, O’Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci (2007) 26:2643–53. doi:10.1111/j.1460-9568.2007.05861.x
96. De Oliveira Alvares L, Genro BP, Diehl F, Quillfeldt JA. Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem (2008) 90:1–9. doi:10.1016/j.nlm.2008.01.009
97. Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev (2008) 32:581–97. doi:10.1016/j.neubiorev.2007.10.003
98. Vlachos I, Herry C, Luthi A, Aertsen A, Kumar A. Context-dependent encoding of fear and extinction memories in a large-scale network model of the basal amygdala. PLoS Comput Biol (2011) 7:e1001104. doi:10.1371/journal.pcbi.1001104
99. Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem (2007) 14:318–24. doi:10.1101/lm.477007
100. Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry (2002) 52:119–25. doi:10.1016/S0006-3223(02)01359-8
101. De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry (2006) 60:697–703. doi:10.1016/j.biopsych.2006.04.035
102. Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport (2009) 20:1402–6. doi:10.1097/WNR.0b013e3283300fbc
103. Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res (2011) 192:84–90. doi:10.1016/j.pscychresns.2010.09.001
104. Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, et al. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp (2012) 34(11):2986–99. doi:10.1002/hbm.22117
105. Liu Y, Li YJ, Luo EP, Lu HB, Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One (2012) 7:e39025. doi:10.1371/journal.pone.0039025
106. Cardenas VA, Samuelson K, Lenoci M, Studholme C, Neylan TC, Marmar CR, et al. Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res (2011) 193:93–100. doi:10.1016/j.pscychresns.2011.01.013
107. Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry (2010) 71:1636–44. doi:10.4088/JCP.08m04754blu
108. Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34:1181–8. doi:10.1016/j.pnpbp.2010.06.016
109. Hedges DW, Woon FL. Premorbid brain volume estimates and reduced total brain volume in adults exposed to trauma with or without posttraumatic stress disorder: a meta-analysis. Cogn Behav Neurol (2010) 23:124–9. doi:10.1097/WNN.0b013e3181e1cbe1
110. Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry (2009) 66:1373–82. doi:10.1001/archgenpsychiatry.2009.160
111. Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res (2009) 174:210–6. doi:10.1016/j.pscychresns.2009.06.001
112. Marchalant Y, Brothers HM, Norman GJ, Karelina K, DeVries AC, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol Dis (2009) 34:300–7. doi:10.1016/j.nbd.2009.01.014
113. Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid Delta9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol (2010) 160:677–87. doi:10.1111/j.1476-5381.2010.00756.x
114. Ribeiro R, Wen J, Li S, Zhang Y. Involvement of ERK1/2, cPLA2 and NF-kappaB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat (2013) 10(0–101):1–14. doi:10.1016/j.prostaglandins.2012.11.003
115. McLaughlin CR, Abood ME. Developmental expression of cannabinoid receptor mRNA. Brain Res Dev Brain Res (1993) 76:75–8.
116. Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J (2005) 19:1704–6. doi:10.1096/fj.05-3995fje
117. Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci (2006) 26:1551–61. doi:10.1523/JNEUROSCI.3101-05.2006
118. Palazuelos J, Ortega Z, Diaz-Alonso J, Guzman M, Galve-Roperh I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J Biol Chem (2012) 287:1198–209. doi:10.1074/jbc.M111.291294
119. Czéh B, Müller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology (2007) 32:1490–503. doi:10.1038/sj.npp.1301275
120. Tishkina AO, Levshina IP, Lazareva NA, Passikova NV, Stepanichev MY, Ajrapetyanz MG, et al. Chronic stress induces nonapoptotic neuronal death in the rat hippocampus. Dokl Biol Sci (2009) 428:403–6. doi:10.1134/S0012496609050032
121. Yun J, Koike H, Ibi D, Toth E, Mizoguchi H, Nitta A, et al. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: possible involvement of a brain-specific transcription factor Npas4. J Neurochem (2010) 114:1840–51. doi:10.1111/j.1471-4159.2010.06893.x
122. Richard D, Guesdon B, Timofeeva E. The brain endocannabinoid system in the regulation of energy balance. Best Pract Res Clin Endocrinol Metab (2009) 23:17–32. doi:10.1016/j.beem.2008.10.007
123. Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT, et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia (2011) 54:3121–31. doi:10.1007/s00125-011-2302-6
124. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab (2013) 17:475–90. doi:10.1016/j.cmet.2013.03.001
125. Vettor R, Pagano C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab (2009) 23:51–63. doi:10.1016/j.beem.2008.10.002
126. Silvestri C, Ligresti A, Di Marzo V. Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord (2011) 12:153–62. doi:10.1007/s11154-011-9167-3
127. Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol (2008) 153:1272–80. doi:10.1038/sj.bjp.0707682
128. Jonsson CA, Catroppa C, Godfrey C, Smedler AC, Anderson V. Cognitive recovery and development after traumatic brain injury in childhood: a person-oriented, longitudinal study. J Neurotrauma (2013) 30:76–83. doi:10.1089/neu.2012.2592
129. Mauler F, Horváth E, De Vry J, Jäger R, Schwarz T, Sandmann S, et al. BAY 38-7271: a novel highly selective and highly potent cannabinoid receptor agonist for the treatment of traumatic brain injury. CNS Drug Rev (2003) 9:343–58. doi:10.1111/j.1527-3458.2003.tb00259.x
Keywords: stress, anxiety, brain cannabinoid receptors, sex dimorphism, TBI outcome
Citation: Xing G, Carlton J, Jiang X, Wen J, Jia M and Li H (2014) Differential expression of brain cannabinoid receptors between repeatedly stressed males and females may play a role in age and gender-related difference in traumatic brain injury: implications from animal studies. Front. Neurol. 5:161. doi: 10.3389/fneur.2014.00161
Received: 15 March 2014; Accepted: 12 August 2014;
Published online: 28 August 2014.
Edited by:
Yumin Zhang, Uniformed Services University of the Health Sciences, USAReviewed by:
Gina Signoracci, Eastern Colorado Health Care System, USACopyright: © 2014 Xing, Carlton, Jiang, Wen, Jia and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqiang Xing, Johns Hopkins University-MCC, 9601 Medical Center Drive, Suite 227, Rockville, MD 20850, USA e-mail:Z3hpbmc5OUB5YWhvby5jb20=;
He Li, Department of Psychiatry, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814-4799, USA e-mail:aGUubGlAdXN1aHMuZWR1
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.