- 1 Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA
- 2 Department of Neurology, Duke University Medical Center, Durham, NC, USA
- 3 Department of Neurology, Medical College of Wisconsin, Milwaukee, WI, USA
- 4 Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
- 5 Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
- 6 Department of Neurology, Vanderbilt University Medical Center, Memphis, TN, USA
- 7 Department of Neurology, University of Louisville Medical Center, Louisville, KY, USA
Background and Purpose: Intra-arterial reperfusion therapies are expanding frontiers in acute ischemic stroke (AIS) management but there is considerable variability in clinical practice. The use of general anesthesia (GA) is one example. We aimed to better understand sedation practices in AIS. Methods: An online survey was distributed to the 68 active members of the Society of Vascular and Interventional Neurology (SVIN). Survey development was based on discussions at the SVIN Endovascular Stroke Round Table Meeting (Chicago, IL, 2008). The final survey contained 12 questions. Questions were developed as single and multiple-item responses; with an option for a free-text response. Results: There was a 72% survey response rate (N = 49/68). Respondents were interventional neurologists in practice 1–5 years (71.4%, N = 35). The mean (±SD) AIS interventions performed per year at the respondents’ institutions was 42.5 ± 25, median 35.0 (IQR 20, 60). The most frequent anesthesia type used was GA (anesthesia team), then conscious sedation (nurse administered), monitored anesthesia care (anesthesia team), and finally local analgesia alone. There was a preference for GA because of eliminating movement (65.3% of respondents; N = 32/49), perceived procedural safety (59.2%, N = 29/49), and improved procedural efficacy (42.9%, N = 21/49). However, cited limitations to GA included risk of time delay (69.4%, N = 34), of propagating cerebral ischemia due to hypoperfusion or other complications (28.6%, N = 14), and lack of adequate anesthesia workforce (20.4%, N = 7). Conclusions: The most frequent type of anesthesia used by Neurointerventionalists for AIS interventions is GA. Prior to making GA standard of care during AIS intervention, more data are needed about effects on clinical outcomes.
Introduction
Endovascular/intra-arterial reperfusion therapies are pushing the frontiers of AIS management. These modalities hold promise for treating thousands of AIS patients who otherwise would gain limited benefit from intravenous thrombolytics (Mazighi et al., 2009). At present, the field of endovascular acute stroke therapy is faced with a limited number of evidence-based practices and significant variability in the application of various therapeutic modalities. The use of general anesthesia (GA) is one example of this variability. There is debate within the field whether or not GA should be used to facilitate delivery of neuroendovascular therapies, including AIS therapies (Qureshi et al., 2001; Abou-Chebl et al., 2006). GA is variably employed by different practitioners at different medical centers around the world. Neurointerventionalists (NI) lack the clinical evidence to support or refute the efficacy of GA in changing clinical outcome.
We designed and distributed an electronic survey to members of the Society of Vascular and Interventional Neurology (SVIN; www.svin.org). The purpose of this study was to develop the foundation for understanding the current sedation practices by NI. The SVIN members represent interventional neurologists from around the world, but predominately the United States. Here we will discuss NI’s current sedation preferences, the perceived limitations of each, and future directions in the field.
Materials and Methods
After receiving a waiver of written informed consent from the Duke University School of Medicine Institutional Review Board, an online survey was distributed to the 68 active members of the SVIN. The survey was developed as a collaborative effort of the members of the investigational team based on input received by the first and senior authors (DM and OZ) following a presentation at the 2008 SVIN Endovascular Stroke Roundtable Meeting (Chicago, IL, USA 2008).
The final survey was a set of 12 questions (Appendix 1) agreed upon by consensus from the investigational team. Questions were developed as single-item and multiple-item responses. Additionally, the option for a free-text response was included where applicable. Survey Monkey™ was used to distribute the survey and collect responses. Respondents were asked to rate some items on an ordinal scale of: never, least frequent, frequent, most frequent. In summarizing this data in Figure 1, the ordinal data was converted to a 1 through 4 numeric scale. The “average rating” in Figure 1 refers to the average score on this numeric scale. Statistical analysis was performed using SAS v9.1 for Windows software (Cary, NC, USA). Missing values were treated as null, imputation was not used.
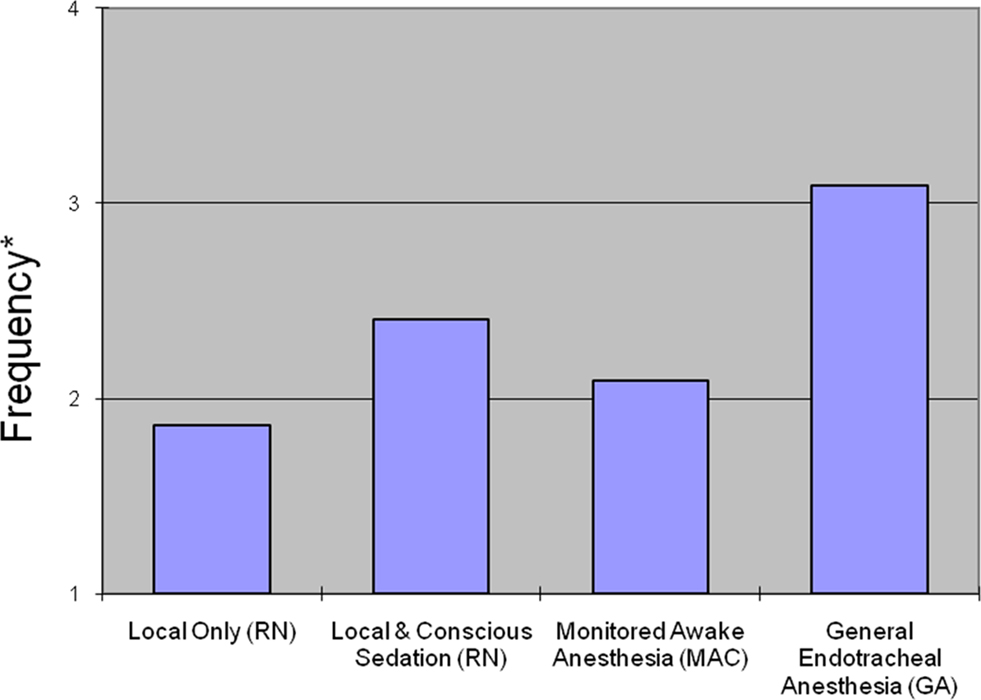
Figure 1. Averaged ratings of physician’s frequency of use for four types of anesthesia. *Treated as ordinal where 1 = Never, 2 = Least frequent, 3 = Frequent, 4 = Most frequent.
Results
Respondents’ Demographics (Survey Questions 1, 2, 3, 4)
Response rate was high at 72% (N = 49/68). Most respondents (98.0%, N = 48/49) completed their basic residency training in neurology; one respondent was a radiologist. Respondents were mentored by physicians trained in interventional neurology (30.6%, N = 15/49), endovascular neurosurgery (22.4%, N = 11/49), interventional neuroradiology (44.9%, N = 22/49), and interventional cardiology (2.0%, N = 1/49). A typical respondent had been in practice as a NI for 1–5 years (71.4%, N = 35/49), while 22.4% (N = 11/49) had been in practice for 6–10 years, and 6.1% (N = 3/49) had been practicing for more than 10 years. The number of endovascular AIS interventions performed at each institution varied tremendously [42.5 ± 25 (Mean ± SD)] procedures per year, median 35.0 (IQR 20, 60). The range varied from a minimum of 8 to a maximum of 100 procedures performed each year.
Analgesic/Anesthetic Use During Endovascular AIS Interventions? (Survey Question 5)
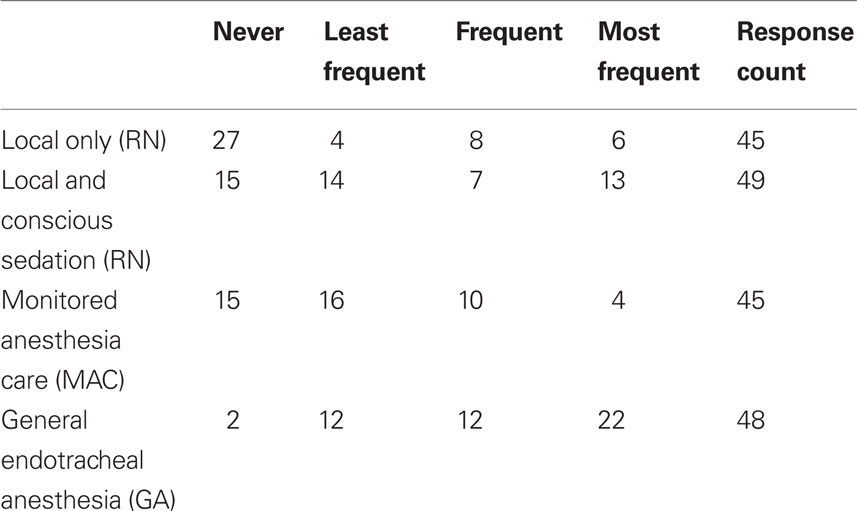
When asked to rate their preference for anesthesia type, respondents most frequently rated GA as their preferred method of anesthesia during AIS interventions (Figure 1). Local with conscious sedation (nurse administered) was the second-most preferred type of anesthesia while monitored anesthesia care (MAC; conscious sedation by an anesthesia team) was third. Local analgesia alone was least likely to be selected as the preferred type of anesthesia. 55.1% (N = 27/49) of respondents indicated that they never use local-only anesthesia in AIS interventions, and 4.1% (N = 2/49) of respondents indicated that they never use GA. Three respondents (6.1%) commented that they use GA in all AIS intervention cases. Responses are shown in Table 1. Spearman’s rank correlation coefficient was used to explore the relationship between preferences (based on frequency of use) for local versus GA. There was an inverse relationship between anesthesia type and frequency (local and GA, versus never to most frequent) in all intervention cases (r = −0.535) which remained statistically significant even after controlling for the number of years in practice (r = −0.525; p = 0.0003), demonstrating the dichotomy in sedation preferences amongst practitioners in this field.
What Type of Endovascular AIS Cases Mandate GA? (Survey Question 6)
Preference for GA was clearly associated with case type. Mechanical thrombectomy was most often considered a mandate for the use of GA (55% of respondents, N = 27/49). Patients with a National Institutes of Health Stroke Scale (NIHSS) score >15 and those with brainstem stroke elicited a GA preference for 53% (N = 26/49) and 51% (N = 25/49) of respondents respectively. Slightly more than half (50.3% overall) of the respondents were convinced that any mechanical manipulation including angioplasty and/or stenting required GA. Left hemispheric intervention was preferred to be performed under GA more often (30.6%) than were right hemispheric interventions (20.4%; p < 0.001). While only 29% (N = 14/49) thought intra-arterial chemical thrombolysis warranted GA use, 10 respondents (20%) commented that an uncooperative patient due to any reason, including respiratory distress, should be given GA.
Have You had Complications from Local Anesthesia? (Survey Question 7) or Sedation? (Survey Question 8)
Respondents attributed the following complications to local analgesia without sedation: agitation requiring acute conversion to GA (38.8%; N = 19/49), patient movement resulting in injury (16.3%; N = 8/49), aspiration (12.2%; N = 6/49), and airway loss (12.2%; N = 6/49). 43% (N = 21/49) reported no complications with local anesthesia alone.
The highest perceived complication of both conscious sedation and MAC was agitation requiring acute conversion to GA for 46.9% (N = 23/49) of respondents. Other perceived complications included aspiration (24.5%, N = 12/49), airway loss (18.4%, N = 9/49) and patient movement resulting in injury (14.3%, N = 7/49).
Have You had Complications from GA? (Survey Question 9)
In considering GA, respondents perceived that the most common complication in their practice was time delay (71.4%, N = 35/49). This was offered to respondents as a possible anesthetic complication with the idea that the time delay presumably would not have occurred had the NI not requested the involvement of the anesthesia team. Other identified complications included hypotension (44.9%, N = 22/49), tough emergence/extended recovery time (18.4%, N = 9/49), and other GA related complications (12.2%, N = 6/49).
Why Wouldn’t You Prefer GA in all of Your Cases? (Survey Question 10)
When asked to hypothetically consider GA for all cases, respondents again cited the risk of time delay (69.4%, N = 34/49), of propagating cerebral ischemia due to hypoperfusion or risk of other complications (28.6%, N = 14/49), and lack of adequate anesthesia workforce (20.4%, N = 10/49) at their institution.
Why Would You Like to Use GA in all of Your Case? (Survey Question 11)
If GA was to be considered in all cases of AIS intervention, the characteristics that made GA more appealing were eliminating movement and saving intra-operative (actual procedural) time (65.3% of respondents, N = 32/49), increased procedural safety (59.2%, N = 29/49), and increased procedural efficacy (42.9%, N = 21/49).
After GA, Would You Prefer to Keep the Patient Intubated? (Survey Question 12)
Twenty-four respondents (49%) stated a preference for extubation and liberation from mechanical ventilation immediately after an AIS procedure, and thus have the patient recover immediately. Seventeen respondents (34.7%) preferred to decide on extubation after obtaining a head CT scan, and eight respondents (16.3%) preferred to keep the patient intubated for 24 h following GA for any AIS interventional procedure.
Discussion
This is the first survey of its kind in the field of interventional endovascular AIS therapy. NI’s treating AIS with endovascular therapy were queried about their preferences for and perceptions of various sedation practices during the interventional procedure. The results show that the preferred method of sedating AIS patients is GA (more than half of the respondents). Even if we combine conscious sedation and MAC (anesthesia team) use it still does not surpass the preference for GA as the most frequent approach to sedation (Table 1).
This preference was particularly clear when the need for mechanical instrumentation was expected. The reason behind this may be related to the use of large intra-arterial catheters and devices to deliver intra-arterial AIS therapy which pose challenges in providing adequate local analgesia. Local anesthesia alone was never chosen for AIS intervention by more than half of the respondents in our survey (N = 27/49). AIS patients, some with pre-existing cognitive decline or dementia, may not be able to cooperate with the care team. Stroke patients with neglect may not realize that they have a neurologic deficit and those with global aphasia would not be able to follow commands. These factors can lead to an inability to tolerate the interventional procedure.
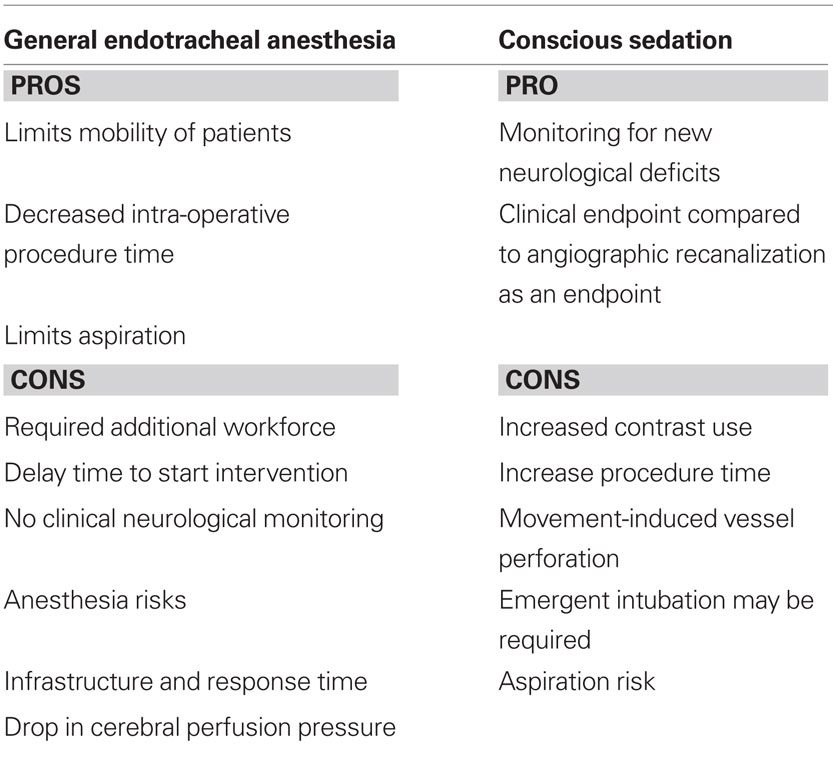
One solution for the clinical challenges related to the use of local analgesia alone is the use of conscious sedation (nurse administered) for AIS intervention. However, various concerns arise with the use of conscious sedation. Patients have not been fasted as they would for an elective cerebral endovascular procedure. This raises the concern for pulmonary aspiration of gastric contents in the setting of sedation and supine positioning (American Society of Anesthesiologist Task Force on Preoperative Fasting, 1999). Additionally, most patients with large cerebral vessel occlusion are expected to have bulbar dysfunction with dysphagia and may be unable to handle secretions or adequately protect their airways (Martino et al., 2005). Emergent conversion to GA during the endovascular procedure could result in patient injury from endovascular devices, hypoxia, or aspiration and necessitates the presence of a practitioner skilled in endotracheal intubation. The need for emergent endotracheal intubation can result from any combination of oversedation, neurologic decline, airway compromise, or patient agitation/cooperation issues. In our survey, airway loss, aspiration, and agitation requiring sedation were considered the most common complications from conscious sedation.
Finally, the management of sedation by the physician performing the endovascular therapy in cases of conscious sedation is a distraction and adds stress to an already demanding situation. The use of MAC provided by an anesthesia team is a potential solution but anesthesia providers are reluctant to provide conscious sedation to non-fasted patients. In this patient population there is often a narrow therapeutic window with potential for agitation on one end and apnea on the other. Nonetheless, conscious sedation is successfully employed in many cases. In support of conscious sedation, only 14.3% of respondents (N = 7/49) felt that the risk of injury due to patient movement was a source of complications, and 28.6% (N = 14/49) felt that they did not have complications from sedation in AIS intervention.
General anesthesia is a logical and seemingly attractive solution for the AIS patient population. However, there are limitations that can be significant. The neurologic examination is masked with sedation and the procedure must proceed to a radiographic endpoint (i.e., some degree of recanalization) rather than a clinical endpoint. Similarly, the use of GA must not delay the delivery of reperfusion therapy. The infrastructure must be in place at each medical center to allow the immediate deployment of an anesthesia team to provide care for these patients as a “level one” emergency. This is not trivial as the endovascular suite is usually apart from the main operating suites. This stretches the capability of the anesthesia team to respond, particularly at times when staffing levels are limited. Finally, the use of GA incurs some risk to the patient. While elevated intracranial pressure is rarely an issue in the first hours after AIS, there is a risk to the AIS patient under GA from any drop in cerebral perfusion pressure which could compromise collateral flow (penumbral perfusion) and extend the infarct. The anesthesiologist needs to be aware of these issues.
The use of GA offers a variety of potential therapeutic options for the patient and NI (Table 2). Detailed anesthetic management is beyond the scope of this discussion and has been recently reviewed by others (Lee et al., 2004; Young, 2007). Airway protection by endotracheal intubation prevents, or at least limits, aspiration and ensures oxygenation and even hyperoxia if desired. Sedation and neuromuscular blockade prevent movement during critical parts of the procedure. This can facilitate road mapping, clot extraction, angioplasty, and stenting. Temporary apnea is easily accomplished and hemodynamic parameters can be titrated as needed. One example would be the use of induced hypotension or even adenosine cardiac arrest to limit hemorrhage in the setting of vessel rupture (Kahn et al., 2000). Glycemic control can similarly be handled by the anesthesia team. Various cerebral protectant therapies also become available, such as propofol or barbiturate burst suppression and therapeutic hypothermia (Hemmen and Lyden, 2007). Adjunctive therapies to the endovascular stroke intervention, such as transcranial neurosonography, may also be desirable in certain settings such as monitoring reperfusion or enhancing thrombolysis (Alexandrov et al., 2004). These adjuncts may require that the patient not move and thus mandate GA.
While a small literature exists regarding standard sedation practices for elective interventional radiology procedures of various types (McDermott et al., 1993; Mueller et al., 1997), emergent acute stroke therapy is distinct. Single center case series of non-emergent procedures showed no apparent increase in complication rates in neurointerventional procedures performed in awake patients (Qureshi et al., 2001; Abou-Chebl et al., 2006). Out of 150 aneurysm coiling procedures performed under conscious sedation, only 3 (2%) required conversion to GA (Qureshi et al., 2001).
In general, the various endovascular acute stroke trials have not reported the type of sedation or anesthesia used, or complications from the use of a given anesthetic modality (Furlan et al., 1999; Smith et al., 2005, 2008). However, Nichols et al. (2010) recently reported sedation practices in the IMS (Interventional Management of Stroke) II Trial. They identified 75 patients with sedation data out of 81 total. These patients were classified on a sedation scale where 1 = no sedation, 2 = mild sedation (patient could still be examined clinically), 3 = heavy sedation (clinical exam obscured by sedation), and 4 = pharmacological paralysis (i.e., intubated and/or paralyzed). Fifty three percent (N = 40) were given no sedation (grade 1) and 23% (N = 17) were intubated/paralyzed (grade 4). Patients in the higher sedation categories had more severe strokes as evidenced by their higher baseline NIHSS’s. Patients in lower sedation categories had better outcomes, more frequent reperfusion rates, and lower mortality. The authors were unable to determine any cause and effect relationship between sedation and outcome, although sedation scale was a predictor of poor outcome on multivariate analysis controlling for baseline NIHSS. Abou-Chebl et al. (2010) recently reported the results of a retrospective review of 980 endovascular acute stroke cases performed at multiple centers. Similar to the results of Nichols et al. (2010), patients who received GA had worse neurologic outcomes [OR 2.33 (95%CI 1.63–3.44), p < 0.0001] and higher mortality [OR 1.68(95%CI 1.23–2.30), p < 0.0001] compared to those given conscious sedation. Again, cause and effect could not be determined but the results are striking and highlight the need for more research into this area. Jumaa et al. (2010), retrospectively reviewed 126 patients who had received endovascular therapy for acute stroke due to middle cerebral artery occlusion. “Non-intubated state” was associated with lower infarct volume (OR 0.25, p = 0.004), in-hospital mortality (OR 0.32, p = 0.011), and better neurologic outcome (OR 3.06, p = 0.042). All of these reports are recent and post dated our practice survey.
Our survey is based on the self-reported perceptions of a group of physicians and is subject to the limitations of such data in regards to accuracy and, in this case, recall bias. Nonetheless, it is the first attempt in the field of interventional AIS therapy to identify sedation preferences and practice. The survey was limited to 49 NI’s from the SVIN. This enhances the internal validity of the sample, but may not be representative of all NI’s who treat AIS with endovascular therapy. Further studies should incorporate a more diverse sample to enhance the external validity of the findings. Finally, our survey did not explore factors such as the NI’s involvement in determining anesthesia type, specific criteria for requesting GA, or the ventilator/critical care management by the NI during the case. All of these factors might impact outcome.
Conclusion
Neurointerventionalists perceived the choice of anesthesia to be an important factor in overall clinical outcome. Practitioners favored the use of GA during AIS intervention especially when mechanical manipulation was expected. Our survey suggests that the preference for GA is based on the assumption that limiting movement makes the interventional procedure safer and more efficacious. Although GA and immobility allow the NI to achieve greater image quality and decrease procedural time, the greatest perceived limitation to GA is time delay in starting the interventional procedure. Three reports of retrospective data in the current literature suggest that type of sedation, in particular GA, may negatively impact outcome in AIS interventions (Abou-Chebl et al., 2010; Jumaa et al., 2010; Nichols et al., 2010). There is no high level evidence to suggest cause and effect, only association. This finding, coupled with the heterogeneity in clinical practice and overall preference for GA identified in our study, indicate that the need for further research is paramount. The creation of a prospective multi-center registry for AIS patients is needed to define clinical outcomes based on anesthetic practice as well as surgical and patient factors. Future trials should consider type of anesthesia as a factor in overall therapeutic efficacy.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Funding: No external funding sources.
References
Abou-Chebl, A., Krieger, D. W., Bajzer, C. T., and Yadav, J. S. (2006). Intracranial angioplasty and stenting in the awake patient. J. Neuroimaging 16, 216–223.
Abou-Chebl, A., Lin, R., Hussain, M. S., Jovin, T. G., Levy, E. I., Liebeskind, D. S., Yoo, A. J., Hsu, D. P., Rymer, M. M., Tayal, A. H., Zaidat, O. O., Natarajan, S. K., Nogueira, R. G., Nanda, A., Tian, M., Hao, Q., Kalia, J. S., Nguyen, T. N., Chen, M., and Gupta, R. (2010). Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: Preliminary results from a retrospective multi-center study. Stroke 41, 1175–1179.
Alexandrov, A. V., Molina, C. A., Grotta, J. C., Garami, Z., Ford, S. R., Alvarez-Sabin, J., Montaner, J., Saqqur, M., Demchuk, A. M., Moyé, L. A., Hill, M. D., Wojner, A. W.; for the CLOTBUST Investigators (2004). Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N. Engl. J. Med. 351, 2170–2178.
American Society of Anesthesiologist Task Force on Preoperative Fasting. (1999). Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 90, 896–905.
Furlan, A., Higashida, R., Wechsler, L., Gent, M., Rowley, H., Kase, C., Pessin, M., Ahuja, A., Callahan, F., Clark, W. M., Silver, F., and Rivera, F. (1999). Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 282, 2003–2011.
Jumaa, M. A., Zhang, F., Ruiz-Ares, G., Gelzinis, T., Malik, A. M., Aleu, A., Oakley, J. I., Jankowitz, B., Lin, R., Reddy, V., Zaidi, S. F., Hammer, M. D., Wechsler, L. R., Horowitz, M., and Jovin, T. G. (2010). Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke 41, 1180–1184.
Kahn, R. A., Moskowitz, D. M., Marin, M. L., Hollier, L. H., Parsons, R., Teodorescu, V., and McLaughlin, M. (2000). Safety and efficacy of high-dose adenosine-induced asystole during endovascular AAA repair. J. Endovasc. Ther. 7, 292–296.
Lee, C. Z., Litt, L., Hashimoto, T., and Young, W. L. (2004). Physiologic monitoring and anesthesia considerations in acute ischemic stroke. J. Vasc. Interv. Radiol. 15, S13–S19.
Martino, R., Foley, N., Bhogal, S., Diamant, N., Speechley, M., and Teasell, R. (2005). Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36, 2756–2763.
Mazighi, M., Serfaty, J. M., Labreuche, J., Laissy, J. P., Meseguer, E., Lavallée, P. C., Cabrejo, L., Slaoui, T., Guidoux, C., Lapergue, B., Klein, I. F., Olivot, J. M., Raphaeli, G., Gohin, C., Claeys, E. S., and Amarenco P; RECANALISE investigators (2009). Comparison of intravenous alteplase with a combined intravenous–endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 8, 802–809.
McDermott, V. G., Chapman, M. E., and Gillespie, I. (1993). Sedation and patient monitoring in vascular and interventional radiology. Br. J. Radiol. 66, 667–671.
Mueller, P. R., Wittenberg, K. H., Kaufman, J. A., and Lee, M. J. (1997). Patterns of anesthesia and nursing care for interventional radiology procedures: a national survey of physician practices and preferences. Radiology 202, 339–343.
Nichols, C., Carrozzella, J., Yeatts, S., Tomsick, T., Broderick, J., and Khatri, P. (2010). Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J. Neurointerv. Surg. 2, 67–70.
Qureshi, A. I., Suri, M. F., Khan, J., Kim, S. H., Fessler, R. D., Ringer, A. J., Guterman, L. R., and Hopkins, L. N. (2001). Endovascular treatment of intracranial aneurysms by using Guglielmi detachable coils in awake patients: safety and feasibility. J. Neurosurg. 94, 880–885.
Smith, W. S., Sung, G., Saver, J., Budzik, R., Duckwiler, G., Liebeskind, D. S., Lutsep, H. L., Rymer, M. M., Higashida, R. T., Starkman, S., Gobin, Y. P.; for the Multi MERCI Investigators (2008). Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke 38, 1205–1212.
Smith, W. S., Sung, G., Starkman, S., Saver, J. L., Kidwell, C. S., Gobin, Y. P., Lutsep, H. L., Nesbit, G. M., Grobelny, T., Rymer, M. M., Silverman, I. E., Higashida, R. T., Budzik, R. F., Marks, M. P.; for the MERCI Trial Investigators (2005). Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36, 1432–1438.
Keywords: anesthesia, acute ischemic stroke, endovascular, intra-arterial, neurointerventional
Citation: Mcdonagh DL, Olson DM, Kalia JS, Gupta R, Abou-Chebl A and Zaidat OO (2010) Anesthesia and sedation practices among neurointerventionalists during acute ischemic stroke endovascular therapy. Front. Neur. 1:118. doi:10.3389/fneur.2010.00118
Received: 14 May 2010;
Paper pending published: 14 June 2010;
Accepted: 28 July 2010;
Published online: 11 November 2010.
Edited by:
Randall Edgell, St. Louis University, USAReviewed by:
Eliahu Feen, St. Louis University, USA;Sushant Kale, St. Louis University, USA;
Vora Nirav, Saint Louis University School of Medicine, USA;
Amer Alshekhlee, Case Western Reserve University, USA
Copyright: © 2010 Mcdonagh, Olson, Kalia, Gupta, Abou-Chebl and Zaidat. This is an open-access publication subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Osama O. Zaidat, Neurointerventional Program, Departments of Neurology, Radiology and Neurosurgery, Medical College of Wisconsin and Froedtert Hospital West 9200 W. Wisconsin Ave; Milwaukee, WI 53226, USA. e-mail: szaidat@mcw.edu