- 1Faculty of Psychology and of Engineering, Uninettuno University, Rome, Italy
- 2Laboratory of Electrophysiology for Translational neuroScience LET’S, Institute of Cognitive Sciences and Technologies ISTC, Consiglio Nazionale delle Ricerche CNR, Roma, Italy
- 3Department of Psychology, University of Milano-Bicocca, Milan, Italy
- 4IRCCS Fondazione Don Carlo Gnocchi, Milan, Italy
- 5IRCCS Centro Neurolesi “Bonino Pulejo”, Messina, Italy
- 6Engineer Freelance, Rome, Italy
- 7Department of Human Neurosciences, Sapienza University of Rome, Rome, Italy
This perspective article addresses the critical and up-to-date problem of task-specific musician’s dystonia (MD) from both theoretical and practical perspectives. Theoretically, MD is explored as a result of impaired sensorimotor interplay across different brain circuits, supported by the most frequently cited scientific evidence—each referenced dozens of times in Scopus. Practically, MD is a significant issue as it occurs over 60 times more frequently in musicians compared to other professions, underscoring the influence of individual training as well as environmental, social, and emotional factors. To address these challenges, we propose a novel application of the FeeSyCy principle (feedback-synchrony-plasticity), which emphasizes the pivotal role of feedback in guiding inter-neuronal synchronization and plasticity—the foundation of learning and memory. This model integrates with established literature to form a comprehensive framework for understanding MD as an impaired FeeSyCy-mediated relationship between the individual and their environment, ultimately leading to trauma. The proposed approach provides significant advantages by enabling the development of innovative therapeutic and preventive strategies. Specifically, it lays the groundwork for multimodal psycho-physical therapies aimed at restoring balance in the neural circuits affected by MD. These strategies include personalized psychotherapy combined with physical rehabilitation to address both the psychological and physiological dimensions of MD. This integration offers a practical and value-added solution to this pressing problem, with potential for broad applicability across similar conditions.
1 Introduction
Task-specific dystonia is a movement disorder characterized by a usually painless loss of dexterity specific to a particular motor skill (Sadnicka et al., 2018). In musicians, the disorder emerges as loss of finger motor coordination or embouchure exclusively when playing the musical instrument (Candia et al., 2003), giving rise to hand or embouchure musician’s dystonia (MD; Stahl and Frucht, 2017). MD of the hand affects musicians who play strings and plucked strings such as violinists, cellists, guitarists, pianists and more rarely wind and brass players. MD of the embouchure affects brass and woodwind players, involving the perioral, lingual, and facial musculature (Frucht et al., 2001).
The causes of MD are still not yet completely clarified, but it is believed to arise from a combination of genetic predisposition and environmental and psychic factors, affecting the brain’s motor control system. As for the genetic hypothesis, it is partly supported by evidence that up to 25% of patients with MD have another affected family member with dystonia. Furthermore, a recent genome analysis found an association with the arylsulfatase G gene (ARSG) in both musician’s hand dystonia and writer’s cramp, but a specific causal mutation within this gene has not yet been identified (Stahl and Frucht, 2017; Nibbeling et al., 2015). Possible predisposing risk factors for MD include a positive family history of dystonia, a history of musculoskeletal injury, nerve entrapment or overuse syndrome, and obsessive personality traits (Rozanski et al., 2015).
MD is the most common form of focal task-specific dystonia, with a prevalence of 1:100 compared to 1:6,600 for idiopathic dystonia (Rozanski et al., 2015). The prevalence of MD varies depending on the instrument played, with musicians playing piano, violin, guitar, and brass instruments being about 85% (Aránguiz et al., 2015), and embouchure MD accounting for 13%–14% of MD (Butler and Rosenkranz, 2006; Jabusch and Altenmüller, 2006), and cervical dystonia involving 1%–2% of musicians with MD.
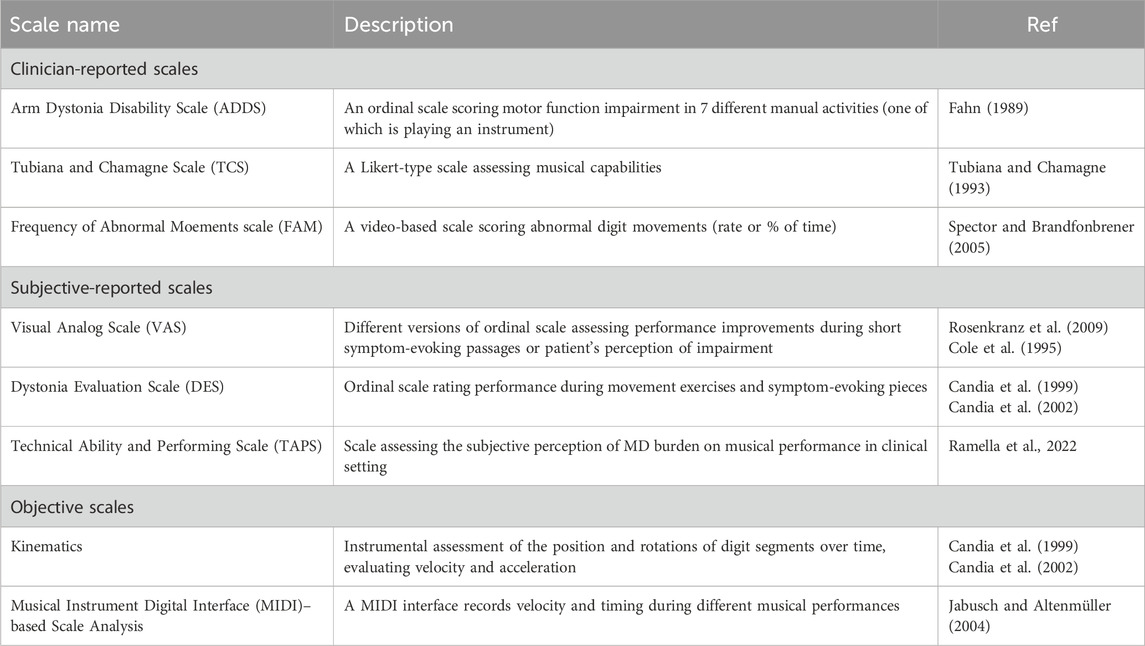
Advancements require sharing standardized measures among experts in the field to evaluate the severity of MD symptoms, for diagnosis and assessing the success of treatments. As key reference we ground on the work of Peterson and collegues (Peterson et al., 2013), which developed a wide overview of the rating scales used in MD. The vast majority of studies use subjective-reported or clinician-reported scales, while objective scales started to be introduced (see Table 1). To date, the Arm Dystonia Disability Scale (ADDS) and Tubiana and Chamagne Scale (TCS) appear to be the most used tools for evaluating dystonia severity, with the TCS being the most specific for MD (Catellani et al., 2024).
The present Perspective integrates the most robust evidence on MD pathophysiology and interventions (Section 2) with our model of neuronal network governing principles (Section 3), highlighting its alteration in MD (Section 4). This foundation supports the development of novel therapeutic strategies, which are detailed in Section 5.
2 Current treatments
Among approaches tested over the years to address MD, the most common is botulinum neurotoxin (BoNT) injections (Cohen et al., 1989; Jankovic and Schwartz, 1990; Kuru et al., 2010; Gupta and Pandey, 2021). This commonly used treatment is effective for about 12–16 weeks with the effect dose-dependent. Cautiousness is indicated, as BoNT is operator dependent; it depends on guides (electromyography/ultrasound), doses, and expertise. It is not possible to consider BoNT as a recommended treatment, but some good results are published in finger flexors dystonia (Gupta and Pandey, 2021). However, in the case of embouchure dystonia, most authors report a worsening of musical performance with the use of botulinum toxin (Hallett, 2018; Weise et al., 2019; Lee et al., 2021). Furthermore, a recent investigation (Ioannou et al., 2023) revealed that, compared to the control group, the thickness and force strength of injected flexor digitorum superficialis (FDS) and profundus (FDP) muscles were decreased by about 10%–12% with respect to the non-injected body side, with the extent of weakness and atrophy significantly predicted by the total amount of BoNT injected during the entire treatment period and the time since the last injection not predicting the amount of strength and muscle mass recovery after cessation of treatment.
Non-pharmacological approaches were developed mainly involving various neuromuscular programs (Jabusch and Altenmüller, 2004; Enke and Poskey, 2018). While recent reviews observe that most studies were applied on a very small sample of musicians with MD, with follow-up assessment absent or very short, and the evaluations often based on subjective nominal or ordinal scale, employing study designs without any type of randomization to placebo or alternative treatments or blindness, we will report here only clear indications originating in focal task specific hand dystonia and implemented in musicians.
“Sensory tricks,” also called alleviating exercises, are known to provide temporary relief of dystonic symptoms (Kaji et al., 1995; Loyola et al., 2013; Dagostino et al., 2019), usually involving the alteration of tactile or proprioceptive feedback of the dystonic districts. In examples, musicians often experience a marked improvement in fine-motor control when they play with a latex glove or when holding an object, such as chewing gum, between their fingers, thus modifying the somatosensory input information (Zeuner et al., 2005). Usually, the effects are very rapid in changing the motor pattern, but they last for a limited time. Sensory motor retuning (SMR) treatment has been introduced in a seminal study in 2002 (Candia et al., 2002) introducing the immobilization with splints of one or more fingers other than the task-specific dystonic finger. The dystonic finger/s performed repetitive exercises in coordination with the non-splinted ones for 1–2 h per day for eight consecutive days under therapist supervision. The subjects were then asked to continue the practice for 1 h per day for 1 year. The splint maintains the fingers of the person with MD in their characteristic rest position on the instrument, simulating the positions experienced during normal performance. In this way, the dystonic finger can participate in the alternating movements of the individual fingers with all the possible permutations of the other fingers of the dystonic hand. In seminal studies, the effects of the treatments were assessed by dystonic finger dexterity quantified by movement speed and accuracy: for example, pre- and post-treatment segments of the movement slopes of a patient’s right D3 (RD3) and left D3 (LD3) were assessed while performing a trill-like task at a selected fast and free speed. Before treatment, the recorded movements of the dystonic finger (RD3) were irregular and uncontrolled compared to the movements of the homologous LD3, which served as control finger. These differences are no longer present after treatment.
Sakai (2006) developed a motor control retraining technique in MD pianists, named “slow-down exercise” (SDE). During the exercise program, patients undergo basic movement training at decreased speed, ensuring that the dystonic patterns do not occur at this reduced speed. The pianists would increase metronome speed every 2 weeks as long as they could maintain a normal movement pattern. Berque and colleagues (Berque et al., 2010) developed a standardized protocol combining SMR plus SDE intervention over a 12-month period.
3 Goal-dependent feedback-synchrony-plasticity principle that governs the interaction of the person with the environment (FeeSyCy)
To overcome the limitations of current therapeutic strategies, a deeper understanding of the mechanisms underlying MD is essential. Our approach begins with a model that helps us explore the relationship between individuals and the world they inhabit, integrating both electrophysiological processes and emotional and social mechanisms in a cohesive framework.
Our description of the triadic principle of feedback, synchrony, and plasticity (FeeSyCy, Tecchio et al., 2020) (Figure 1) incorporates the dynamic nature of neuronal networks, which are driven by goal-dependent feedback loops. These loops give rise to the adaptive interaction between the body and brain with the environment, allowing goals to be identified and pursued through multiple interconnected processes within the individual.
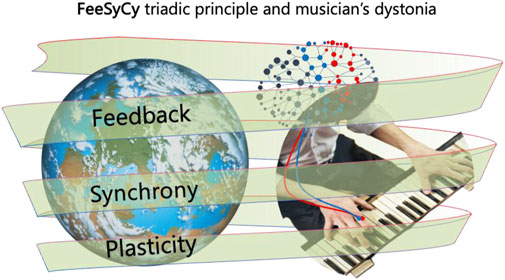
Figure 1. Neuronal network mediated alterations in musician’s dystonia. An outline of the FeeSyCy triadic principle—feedback, synchrony, plasticity—that governs the body-brain interaction with the environment and is involved in the altered sensorimotor integration seen in the musician’s dystonia.
The whole brain can be seen as an ensemble of neurons coordinating the body-brain network’s interaction with the environment. In this context, output (motor actions) depends on sensory input (via somatic, proprioceptive, visual, and auditory receptors): critically the physiology stands on input that, in turn, depends on the output (Tecchio et al., 2020; Persichilli et al., 2022). This reciprocal relationship is fundamental to the feedback loop that underlies adaptive behavior (Rossi et al., 1998). When motor actions are performed, sensory feedback from the environment is shaped by the brain in accordance with the desired goal, continually refining and adjusting the system to reduce the difference between top-down expectations and bottom-up sensory inputs (Fink et al., 2014).
Within this feedback loop, the brain’s neurons organize themselves through locally specific dynamic synchronizations among nodes of functional networks (Tecchio et al., 2008a). These synchronizations occur at the subsystem level, typically accompanied by desynchronizations with wider cortical regions, such as those giving rise to the reduction of occipital alpha power when opening the eyes. The modulation of synchrony leads to either the continued execution of planned actions or corrective adaptations, depending on the feedback received from the environment.
Central to this process is the Hebbian learning rule, which governs how neurons modify their output in response to synchronized inputs. When two input signals arrive simultaneously at a neuron, its likelihood of firing increases, thus facilitating message transmission within dynamic networks along the whole lifespan (Kerkman et al., 2022; Madadi Asl et al., 2022; Hill and Den Hartigh, 2023; Hristovski et al., 2023). This mechanism underlies key learning processes such as trial-and-error and imitation, enabling neurons to adapt their output based on the distance between the expected outcome and the current state. If this distance is small, behavioral adaptations occur within the existing network framework (working adaptation). Significant structural changes arise if the distance is large, reflecting new skill acquisition and plastic adaptation for learning. We use the term network plasticity, defined as a change of network output in response to the same input, referring to the brain’s capacity to adapt structurally and functionally, which is an ability manifested at multiple levels, including synaptic plasticity (through potentiation and depression phenomena) and neuronal structural modification, including pruning when the brain gets rid of neurons and connections that it no longer requires, and branching when new synaptic connections are made. These changes are further supported by modifications in myelin sheaths, which adjust the timing of information transmission with high precision. This multi-layered plasticity to ensure system adaptations critically requires not only active behavior but also sleep, when synaptic strengths are renormalized, maintaining high the potential for new acquisitions in the neural network (Papadakis et al., 2022; Rizzo et al., 2023).
In sum, the FeeSyCy triadic principle operates through a feedback loop in which sensory input and motor output continuously influence each other, transduced by neuronal synchronizations that drive plastic changes. These processes work together to support goal-directed behavior, via learning for the ongoing adaptation of the body-brain system to the environment. The FeeSyCy principle subtends physiological action in the environment, at multiple levels, from simply motoric, to social, emotional, and affective.
4 MD mechanisms
4.1 Neurophysiological counterpart
Impairment in sensorimotor integration, crucial in the development of task-specific dystonia, is evidenced by findings from animal studies, behavioral research, and clinical reports utilizing neurophysiological and neuroimaging techniques in humans (Quartarone et al., 2006; Breakefield et al., 2008; Lin and Hallett, 2009). A seminal animal study (Byl et al., 1997) demonstrated that repetitive finger compression led to degradation of hand representation and impaired motor control, while a less repetitive strategy preserved sensory integrity and motor function. From that moment, dystonia was constantly associated with the repetitiveness of motor patterns influencing somatosensory representation and motor execution. Following the SMR model by Candia and colleagues (Candia et al., 2002), treatment effects were studied on somatosensory hand representation. Pre-treatment, finger relations differed between affected and unaffected hands, but post-treatment, contralateral finger representations resembled those of the less affected side and were ordered more according to homuncular principles (Candia et al., 2003). These physiological changes correlate with behavioral outcomes (Candia et al., 2003). Per the FeeSyCy principle, we expect synchronization alterations in MD. Investigations employing transcranial magnetic stimulation (TMS) protocols revealed the absence of local inhibitory mechanisms during movement initiation and maintenance phases in individuals with task-specific hand dystonia, leading to disrupted cortical inhibition-excitation dynamics (Desrochers et al., 2019). This disturbance affects cortical surround inhibition critical for skill acquisition and relies on inter-hemispheric balances (Pagliara et al., 2023; Bertoli et al., 2023). Notably, patients with task-specific hand dystonia exhibit disrupted cortical surround inhibition (Hallett, 2011) even during motor preparation (Beck et al., 2008). Altered excitation and inhibition balances in local motor cortex circuits, reflecting disrupted basal ganglia input, were evidenced by reduced excitability of cortical inhibitory circuits in patients with task-specific dystonia (Ridding et al., 1995). While no differences were observed in spectral properties between patients and controls in primary motor or somatosensory hand representations, these areas demonstrated reduced functional coupling during movement, along with excessive synchronization in patients compared to controls in the ongoing gamma band around 40 Hz (Tecchio et al., 2008b; Melgari et al., 2013). Concerning plasticity (Madadi Asl et al., 2022; McGowan et al., 2014), utilizing established non-invasive models of plasticity in humans (Stefan et al., 2000), it has been observed that the motor system exhibits abnormal increases in cortico-spinal excitability and attenuated intracortical inhibitory reinforcement following central associative and peripheral stimulation (Quartarone et al., 2003). Moreover, evidence confirms alterations in long-term potentiation and depression in MD (Quartarone et al., 2006).
While we emphasized the relevance of MD in the present context, the FeeSyCy principle is broadly applicable. Its explanatory potential extends across all task-specific dystonias, which, unlike other movement disorders, are primarily characterized by dysfunctions rooted in altered sensorimotor integration rather than by degenerative processes or structural lesions affecting motor pathways.
4.2 Psycho-social counterpart
While the neurophysiological profile described above is typical of task-specific dystonia of the hand, in musicians, the occupational dimension decisively modifies the impact of the symptomatology (Rosenkranz et al., 2005) consistent with epidemiological data that indicate MD as the most incident task-specific dystonia (Ioannou et al., 2016). It is immediate to consider that for a musician whose center of professional life is making music, the body is even more immediately at the center of the relationship with the rest of the world. For musicians, health tends to acquire two key dimensions in a dynamic balance, one that concerns the parts of the body directly involved in playing the instrument (especially the hand), and another that focuses on maintaining the whole person’s health. These two dimensions must be integrated in daily attention and care, deepening the awareness that learning through experience, aimed at mutual help and communication, is the key to protecting and recovering the whole body’s wellbeing (Schoeb and Zosso, 2012).
In the context of the increasingly clear nature of the human being determined within the experience of lifelong learning or traumas, and their deep impact on personal identity, (Grifoni et al., 2023), where one of the three main dimensions that index the development of a population is the length of the course of study, the learning approaches for musicians become critical in the training years and all along life. In fact, musicians typically start their training at a young age, with the need to develop movements automated through extensive repetitions, gradually increasing complexity, with the need to balance stereotyped production and emotional participation to the story they like to tell. Given the psycho-emotional pressure intrinsic with the public engagement of a musician’s activity, affecting the respiratory, circulatory, sensorimotor, and cognitive systems (Ioannou et al., 2016), the organization of individual and orchestral training is a critical element determining future evolution managing challenges in adapting to the demands of their profession. Indeed, trauma can have far-reaching consequences, extending beyond psychological distress to impact sensorimotor functionality in musicians. These repercussions are evident in the disruption of motor coordination, manifested as hesitations, involuntary movements, or complete motor blocks, which impede the expression of artistic intentions.
Furthermore, trauma-induced negations can exert influence on cognitive processes, including memory, attention, and decision-making, exacerbating the challenges faced by musicians during performance. Such cognitive impairments may lead to errors or inconsistencies in musical execution, undermining the quality of their performances. In light of these challenges, addressing trauma and its associated negations is paramount for preserving both physical and mental wellbeing in musicians. By acknowledging and confronting these negations, musicians can embark on a psychotherapeutic path towards reclaiming agency over their bodies and artistic expression. This process fosters resilience and facilitates a more comprehensive approach to musical performance and self-care for MD.
5 Discussion
We have reported on the most stable achievements on Musician’s Dystonia (MD). Modern rehabilitation approaches aim to restore a physiological position that supports musicians’ natural gestures, primarily through botulinum neurotoxin injections, sensory-motor retuning, and its variants. These strategies emphasize body awareness and the adjustment of positions for relaxation within a dynamic training program. The consolidated neurophysiological knowledge on the alteration of sensorimotor integration in MD, which coherently with the FeeSyCy feedback-synchrony-plasticity functioning principle that governs neuronal networks, is accompanied by impairments of intra-cortical synchronizations and plasticity, has recently been complemented by the growing awareness of the impact on symptoms of socio-emotional-cognitive experience.
In response, new therapeutic strategies are being developed to alleviate symptoms that profoundly affect musicians’ quality of life and often lead to career disruption. These strategies adopt a multidisciplinary approach involving neurology (Pérez and Preciado, 2019), physiotherapy, occupational therapy, and psychology (O’Neill, 2012; Zhang et al., 2020), integrated into personalized precision treatments. Within this integrated framework, the psychological protocol Eye Movement Desensitization and Reprocessing (EMDR) emerges as a comprehensive therapeutic approach to address the emotional trauma underlying MD. Notably, an enhanced method, referred to as EMDR+ (Grifoni et al., 2024), is proposed employing bi-modal visual (Stamatis et al., 2023) and auditory intervention of bilateral alternate stimulation, along with the inclusion of music in trauma-evocative and rewarding phases. EMDR + offers a unique potential for enriching therapeutic outcomes for musicians experiencing performance anxiety (O’Connor et al., 2021).
We believe that the proposed approach holds significant relevance to the field of Network Physiology, which investigates how physiological systems and subsystems interact, synchronize their functions, and integrate into networks to generate diverse physiological states and conditions in both health and disease. The FeeSyCy principle, emerging as a pivotal element in network functioning across multiple scales—ranging from small neuronal groups to the entire brain-body system and even society as a network of individuals—highlights the transformative potential of understanding systems as networks. This perspective opens the door to profoundly novel and effective interventions for symptom relief, such as in MD, and underscores the importance of incorporating this knowledge into teaching and training programs across various disciplines to prevent dysfunction and promote resilience.
Author contributions
JG: Conceptualization, Writing–original draft, Writing–review and editing. VC: Writing–review and editing. AC: Writing–review and editing. RC: Writing–review and editing. MR: Writing–review and editing. AQ: Writing–review and editing. TL: Writing–review and editing. KA: Writing–review and editing. LP: Writing–review and editing. FP: Writing–review and editing. FT: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Current Research Funds, Ministry of Health, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aránguiz, R., Chana-Cuevas, P., Alburquerque, D., and Curinao, X. (2015). Focal dystonia in musicians: phenomenology and musical triggering factors. Med. Probl. Perform. Art. 30, 270–275. doi:10.1016/j.nrl.2013.12.024
Beck, S., Richardson, S. P., Shamim, E. A., Dang, N., Schubert, M., and Hallett, M. (2008). Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J. Neurosci. 28 (41), 10363–10369. doi:10.1523/JNEUROSCI.3564-08.2008
Berque, P., Gray, H., Harkness, C., and McFadyen, A. (2010). A combination of constraint-induced therapy and motor control retraining in the treatment of focal hand dystonia in musicians. Med. Probl. Perform. Art. 25, 149–161. doi:10.21091/mppa.2010.4032
Bertoli, M., Tataranni, A., Porziani, S., Pasqualetti, P., Gianni, E., Grifoni, J., et al. (2023). Effects on corticospinal tract homology of Faremus personalized neuromodulation relieving fatigue in multiple sclerosis: a proof-of-concept study. Brain 13 (4), 574. doi:10.3390/brainsci13040574
Breakefield, X. O., Blood, A. J., Li, Y., Hallett, M., Hanson, P. I., and Standaert, D. G. (2008). The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 9, 222–234. doi:10.1038/nrn2337
Butler, K., and Rosenkranz, K. (2006). Focal hand dystonia affecting musicians. Part II: an overview of current rehabilitative treatment techniques. Br. J. Hand Ther. 11, 79–87. doi:10.1177/175899830601100302
Byl, N. N., Merzenich, M. M., Cheung, S., Bedenbaugh, P., Nagarajan, S. S., and Jenkins, W. M. (1997). A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys. Ther. 77, 269–284. doi:10.1093/ptj/77.3.269
Candia, V., Elbert, T., Altenmüller, E., Rau, H., Schäfer, T., and Taub, E. (1999). Constraint-induced movement therapy for focal hand dystonia in musicians. Lancet 353, 42. doi:10.1016/S0140-6736(05)74865-0
Candia, V., Schäfer, T., Taub, E., Rau, H., Altenmuüller, E., Rockstroh, B., et al. (2002). Sensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch. Phys. Med. Rehabil. 83, 1342–1348. doi:10.1053/APMR.2002.35094
Candia, V., Wienbruch, C., Elbert, T., Rockstroh, B., and Ray, W. (2003). Effective behavioral treatment of focal hand dystonia in musicians alters somatosensory cortical organization. Proc. Natl. Acad. Sci. U. S. A. 100, 7942–7946. doi:10.1073/PNAS.1231193100
Catellani, I., Arcuri, P., Vita, F., Platano, D., Boccolari, P., Lanfranchi, E., et al. (2024). An overview of rehabilitation approaches for focal hand dystonia in musicians: a scoping review. Clin. Rehabil. 38, 589–599. doi:10.1177/02692155231225705
Cohen, L. G., Hallett, M., Geller, B. D., and Hochberg, F. (1989). Treatment of focal dystonias of the hand with botulinum toxin injections. J. Neurol. Neurosurg. Psychiatry 52, 355–363. doi:10.1136/JNNP.52.3.355
Cole, R., Hallett, M., and Cohen, L. G. (1995). Double-blind trial of botulinum toxin for treatment of focal hand dystonia. Mov. Disord. 10, 466–471. doi:10.1002/mds.870100411
Dagostino, S., Ercoli, T., Gigante, A. F., Pellicciari, R., Fadda, L., and Defazio, G. (2019). Sensory trick in upper limb dystonia. Park. Relat. Disord. 63, 221–223. doi:10.1016/J.PARKRELDIS.2019.01.006
Desrochers, P., Brunfeldt, A., Sidiropoulos, C., and Kagerer, F. (2019). Sensorimotor control in dystonia. Brain Sci. 9, 79. doi:10.3390/brainsci9040079
Enke, A. M., and Poskey, G. A. (2018). Neuromuscular Re-education programs for musicians with focal hand dystonia: a systematic review. Med. Probl. Perform. Art. 33, 137–145. doi:10.21091/MPPA.2018.2014
Fahn, S. (1989). “Assessment of the primary dystonias,” in Quantification of neurologic deficit. Editor T. L. Munsat (Oxford: Butterworths), 241–270.
Fink, A. J. P., Croce, K. R., Huang, Z. J., Abbott, L. F., Jessell, T. M., and Azim, E. (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509, 43–48. doi:10.1038/nature13276
Frucht, S. J., Fahn, S., Greene, P. E., O’Brien, C., Gelb, M., Truong, D. D., et al. (2001). The natural history of embouchure dystonia. Mov. Disord. 16, 899–906. doi:10.1002/MDS.1167
Grifoni, J., Crispiatico, V., Castagna, A., Quartarone, A., Converti, R. M., Ramella, M., et al. (2024). Musician's dystonia: an opinion on novel treatment strategies. Front. Neurosci. 18, 1393767. doi:10.3389/fnins.2024.1393767
Grifoni, J., Pagani, M., Persichilli, G., Bertoli, M., Bevacqua, M. G., L’Abbate, T., et al. (2023). Auditory personalization of EMDR treatment to relieve trauma effects: a feasibility study [EMDR+] personalization of EMDR treatment to relieve trauma effects: a feasibility study [EMDR+]. Brain Sci. 13, 1050. doi:10.3390/BRAINSCI13071050
Gupta, N., and Pandey, S. (2021). Treatment of focal hand dystonia: current status. Neurol. Sci. 42, 3561–3584. doi:10.1007/S10072-021-05432-7
Hallett, M. (2011). Neurophysiology of dystonia: the role of inhibition. Neurobiol. Dis. 42, 177–184. doi:10.1016/J.NBD.2010.08.025
Hallett, M. (2018). Mechanism of action of botulinum neurotoxin: unexpected consequences. Toxicon 147, 73–76. doi:10.1016/J.TOXICON.2017.08.011
Hill, Y., and Den Hartigh, R. J. R. (2023). Resilience in sports through the lens of dynamic network structures. Front. Netw. Physiol. 3, 1190355. doi:10.3389/fnetp.2023.1190355
Hristovski, R., Balagué, N., and Stevanovski, M. (2023). Long-term exercise adaptation. Physical aging phenomena in biological networks. Front. Netw. Physiol. 3, 1243736. doi:10.3389/fnetp.2023.1243736
Ioannou, C. I., Furuya, S., and Altenmüller, E. (2016). The impact of stress on motor performance in skilled musicians suffering from focal dystonia: physiological and psychological characteristics. Neuropsychologia 85, 226–236. doi:10.1016/J.NEUROPSYCHOLOGIA.2016.03.029
Ioannou, C. I., Hodde-Chriske, F. L., and Altenmüller, E. (2023). Long-term muscular atrophy and weakness following cessation of botulinum toxin type A injections in the flexor digitorum muscle of musicians with focal hand dystonia. Toxins 15 (4), 296. doi:10.3390/TOXINS15040296
Jabusch, H. C., and Altenmüller, E. (2004). Three-dimensional movement analysis as a promising tool for treatment evaluation of musicians’ dystonia. Adv. Neurol. 94, 239–245.
Jabusch, H. C., and Altenmüller, E. (2006). Epidemiology, phenomenology, and therapy of musician’s cramp. Music, Mot. Control Brain, 265–282. doi:10.1093/ACPROF:OSO/9780199298723.003.0017
Jankovic, J., and Schwartz, K. (1990). Botulinum toxin injections for cervical dystonia. Neurology 40 (2), 277–280. doi:10.1212/wnl.40.2.277
Kaji, R., Rothwell, J. C., Katayama, M., Ikeda, T., Kubori, T., Kohara, N., et al. (1995). Tonic vibration reflex and muscle afferent block in writer's cramp. Ann. Neurol. 38 (2), 155–162. doi:10.1002/ana.410380206
Kerkman, J. N., Zandvoort, C. S., Daffertshofer, A., and Dominici, N. (2022). Body weight control is a key element of motor control for toddlers' walking. Front. Netw. Physiol. 2, 844607. doi:10.3389/fnetp.2022.844607
Kuru, M., Hirayama, K., and Tanaka, T. (2010). Efficacy of treatment of focal dystonia in musicians with botulinum toxin: a review. J. Hand Ther. 23, 268–277. doi:10.1016/J.JHT.2010.08.007
Lee, A., Al-Sarea, J., and Altenmüller, E. (2021). Nonlinear changes in botulinum toxin treatment of task-specific dystonia during long-term treatment. Toxins 13 (6), 371doi. doi:10.3390/toxins13060371
Lin, P. T., and Hallett, M. (2009). The pathophysiology of focal hand dystonia. J. Hand Ther. 22, 109–113. doi:10.1016/j.jht.2008.10.008
Loyola, D. P., Camargos, S., Maia, D., and Cardoso, F. (2013). Sensory tricks in focal dystonia and hemifacial spasm. Eur. J. Neurol. 20 (4), 704–707. doi:10.1111/ene.12054
Madadi Asl, M., Vahabie, A. H., Valizadeh, A., and Tass, P. A. (2022). Spike-timing-dependent plasticity mediated by dopamine and its role in Parkinson's disease pathophysiology. Front. Netw. Physiol. 2, 817524. doi:10.3389/fnetp.2022.817524
McGowan, J., Haskins, B., and Dittman, R. (2014). The role of music in enhancing brain plasticity. Ann. N. Y. Acad. Sci. 1337, 30–36. doi:10.1111/nyas.12400
Melgari, J. M., Zappasodi, F., Porcaro, C., Tomasevic, L., Cassetta, E., Rossini, P. M., et al. (2013). Movement-induced uncoupling of primary sensory and motor areas in focal task-specific hand dystonia. Neuroscience 250, 434–445. doi:10.1016/j.neuroscience.2013.07.027
Nibbeling, E., Schaake, S., Tijssen, M. A., Weissbach, A., Groen, J. L., Altenmüller, E., et al. (2015). Accumulation of rare variants in the arylsulfatase G (ARSG) gene in task-specific dystonia. J. Neurol. 262, 1340–1343. doi:10.1007/s00415-015-7718-3
O’Connor, E. E., Bowers, M., and Carson, D. (2021). Music performance anxiety: mechanisms and management. J. Hand Ther. 11, 87–94. doi:10.1177/1758998319834524
O’Neill, S. (2012). The role of the therapeutic relationship in music therapy. Music Ther. Perspect. 30, 1–7. doi:10.1093/mtp/30.1.1
Pagliara, M. R., Cecconi, F., Pasqualetti, P., Bertoli, M., Armonaite, K., Gianni, E., et al. (2023). On the homology of the dominant and non-dominant corticospinal tracts: a novel neurophysiological assessment. Brain Sci. 13 (2), 278. doi:10.3390/brainsci13020278
Papadakis, Z., Garcia-Retortillo, S., and Koutakis, P. (2022). Effects of acute partial sleep deprivation and high-intensity interval exercise on postprandial network interactions. Front. Netw. Physiol. 2, 869787. doi:10.3389/fnetp.2022.869787
Pérez, M. C., and Preciado, J. M. (2019). Clinical update: the role of cortical mechanisms in the treatment of dystonia in musicians. Front. Neurol. 10, 1–7. doi:10.3389/FNEUR.2019.00754
Persichilli, G., Grifoni, J., Pagani, M., Bertoli, M., Gianni, E., L’Abbate, T., et al. (2022). Sensorimotor interaction against trauma. Front. Neurosci. 16, 913410. doi:10.3389/FNINS.2022.913410
Peterson, D. A., Berque, P., Jabusch, H. C., Altenmüller, E., and Frucht, S. J. (2013). Rating scales for musician's dystonia: the state of the art. J. Neurol. 81 (6), 589–598. doi:10.1212/WNL.0b013e31829e6f72
Quartarone, A., Bagnato, S., Rizzo, V., Siebner, H. R., Dattola, V., Scalfari, A., et al. (2003). Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain 126 (Pt 12), 2586–2596. doi:10.1093/brain/awg273
Quartarone, A., Siebner, H. R., and Rothwell, J. C. (2006). Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 29 (4), 192–199. doi:10.1016/j.tins.2006.02.007
Ramella, M., Converti, R. M., Giacobbi, G., Castagna, A., Saibene, E., Borgnis, F., et al. (2022). The Technical Ability and Performing Scale (TAPS): a newly developed patient-reported functional rating scale for Musician's focal dystonia. Park. Relat. Disord. 99, 79–83. doi:10.1016/j.parkreldis.2022.05.015
Ridding, M. C., Sheean, G., Rothwell, J. C., Inzelberg, R., and Kujirai, T. (1995). Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J. Neurol. Neurosurg. Psychiatry 59 (5), 493–498. doi:10.1136/jnnp.59.5.493
Rizzo, R., Wang, J. W. J. L., DePold Hohler, A., Holsapple, J. W., Vaou, O. E., and Ivanov, P. C. (2023). Dynamic networks of cortico-muscular interactions in sleep and neurodegenerative disorders. Front. Netw. Physiol. 3, 1168677. doi:10.3389/fnetp.2023.1168677
Rosenkranz, K., Butler, K., Williamon, A., and Rothwell, J. C. (2009). Regaining motor control in musician’s dystonia by restoring sensorimotor organization. J. Neurosci. 29, 14627–14636. doi:10.1523/JNEUROSCI.2094-09.2009
Rosenkranz, K., Williamon, A., Butler, K., Cordivari, C., Lees, A. J., and Rothwell, J. C. (2005). Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain 128, 918–931. doi:10.1093/BRAIN/AWH402
Rossi, S., Pasqualetti, P., Tecchio, F., Sabato, A., and Rossini, P. M. (1998). Modulation of corticospinal output to human hand muscles following deprivation of sensory feedback. Neuroimage 8, 163–175. doi:10.1006/nimg.1998.0352
Rozanski, V. E., Rehfuess, E., Bötzel, K., and Nowak, D. (2015). Task-specific dystonia in professional musicians. A systematic review of the importance of intensive playing as a risk factor. Dtsch. Arztebl Int. 21 (112), 871–877. doi:10.3238/arztebl.2015.0871
Sadnicka, A., Kornysheva, K., Rothwell, J. C., and Edwards, M. J. (2018). A unifying motor control framework for task-specific dystonia. Nat. Rev. Neurol. 14 (2), 116–124. doi:10.1038/nrneurol.2017.146
Sakai, N. (2006). Slow-down exercise for the treatment of focal hand dystonia in pianists. Med. Probl. Perform. Art. 21 (1), 25–28. doi:10.21091/mppa.2006.1005
Schoeb, V., and Zosso, A. (2012). “You cannot perform music without taking care of your body”: a qualitative study on musicians’ representation of body and health. Med. Probl. Perform. Art. 27, 129–136. doi:10.21091/mppa.2012.3024
Spector, J. T., and Brandfonbrener, A. G. (2005). A new method for quantification of musician’s dystonia: the frequency of abnormal movements scale. Med. Probl. Perform. Art. 20, 157–162. doi:10.21091/mppa.2005.4031
Stahl, C. M., and Frucht, S. J. (2017). Focal task specific dystonia: a review and update. J. Neurol. 264 (7), 1536–1541. doi:10.1007/s00415-016-8373-z
Stamatis, A., Garcia-Retortillo, S., Morgan, G. B., and Sanchez-Moreno, A. (2023). Case report: cortico-ocular interaction networks in NBA2K. Front. Netw. Physiol. 3, 1151832. doi:10.3389/fnetp.2023.1151832
Stefan, K., Kunesch, E., Cohen, L. G., Benecke, R., and Classen, J. (2000). Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123 (3), 572–584. doi:10.1093/brain/123.3.572
Tecchio, F., Bertoli, M., Gianni, E., L’Abbate, T., Paulon, L., and Zappasodi, F. (2020). To Be is to become. Fractal neurodynamics of the body-brain control system. Front. Physiol. 11, 609768. doi:10.3389/fphys.2020.609768
Tecchio, F., Melgari, J. M., Zappasodi, F., Porcaro, C., Milazzo, D., Cassetta, E., et al. (2008b). Sensorimotor integration in focal task-specific hand dystonia: a magnetoencephalographic assessment. Neuroscience 154 (2), 563–571. doi:10.1016/j.neuroscience.2008.03.045
Tecchio, F., Zappasodi, F., Porcaro, C., Barbati, G., Assenza, G., Salustri, C., et al. (2008a). High-gamma band activity of primary hand cortical areas: a sensorimotor feedback efficiency index. Neuroimage 40, 256–264. doi:10.1016/j.neuroimage.2007.11.038
Tubiana, R., and Chamagne, P. (1993). Occupational Arm ailments in musicians. Bull. Acad. Natl. Med. 177, 203–212.
Weise, D., Weise, C. M., and Naumann, M. (2019). Central effects of botulinum neurotoxin—evidence from human studies. Toxins 11 (1), 21. doi:10.3390/toxins11010021
Zeuner, K. E., Shill, H. A., Sohn, Y. H., Molloy, F. M., Thornton, B. C., Dambrosia, J. M., et al. (2005). Motor training as treatment in focal hand dystonia. Mov. Disord. 20 (3), 335–341. doi:10.1002/mds.20314
Keywords: task-specific focal dystonia, sensory-motor integration, psychic trauma, multi-sensory multimodal rehabilitation, feedback synchrony plasticity: the FeeSyCy principle governing networks, network physiology
Citation: Grifoni J, Crispiatico V, Castagna A, Converti RM, Ramella M, Quartarone A, L’Abbate T, Armonaite K, Paulon L, Panuccio F and Tecchio F (2025) Musician’s dystonia: a perspective on the strongest evidence towards new prevention and mitigation treatments. Front. Netw. Physiol. 4:1508592. doi: 10.3389/fnetp.2024.1508592
Received: 09 October 2024; Accepted: 19 December 2024;
Published: 22 January 2025.
Edited by:
Klaus Lehnertz, University of Bonn, GermanyReviewed by:
Ali Foroutannia, University of Canberra, AustraliaCopyright © 2025 Grifoni, Crispiatico, Castagna, Converti, Ramella, Quartarone, L’Abbate, Armonaite, Paulon, Panuccio and Tecchio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franca Tecchio, ZnJhbmNhLnRlY2NoaW9AY25yLml0
 Joy Grifoni
Joy Grifoni Valeria Crispiatico
Valeria Crispiatico Anna Castagna
Anna Castagna Rosa Maria Converti4
Rosa Maria Converti4 Marina Ramella
Marina Ramella Teresa L’Abbate
Teresa L’Abbate Karolina Armonaite
Karolina Armonaite Luca Paulon
Luca Paulon Francescaroberta Panuccio
Francescaroberta Panuccio Franca Tecchio
Franca Tecchio