- 1Division of Periodontics, Department of Diagnostic Sciences and Surgical Dentistry, School of Dental Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 2Center for Kidney Disease Innovation Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Baystate Medical Center, Springfield, MA, United States
- 4Department of Orofacial Sciences, School of Dentistry, University of California, San Francisco, San Francisco, CA, United States
1 Introduction
Chronic kidney disease (CKD) is characterized by persistent alterations in kidney structure and impaired excretory renal function and represents a public health burden affecting ~14.5% of the U.S. adult population (1). Even at early disease stages, increased urea concentration is associated with elevated serum levels of various pro-inflammatory mediators, including high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNFα) (1–4). These changes become even more pronounced as CKD progresses to end-stage renal disease, ESRD (2, 3). Given the importance of pro-inflammatory biomarkers as predictors of all-cause (5) and cardiovascular disease (CVD) (6) mortality in patients with ESRD, reductions in their levels have been proposed as critical target outcomes in this population (3, 4). Several anti-inflammatory strategies, including pharmacological (4) and non-pharmacological (mostly nutritional) (3, 4, 7, 8) interventions and the concurrent therapy of systemic comorbidities (9), have been utilized in that direction.
Hemodialysis (HD) is the primary treatment modality in ESRD. The most recent national data demonstrated that in >70% of patients with ESRD, HD was initiated with a central venous catheter (CVC) (10), which could later be replaced with an arteriovenous fistula (AVF) or arteriovenous graft (AVG) (11). Although AVF was associated with various complications, it provided the lowest rate of mortality, fatal infections, and levels of pro-inflammatory mediators compared to AVG and CVC (11–13). Even in the absence of infection, patients with CVC had significantly higher serum hs-CRP levels than those with AVF independent of sex, race, and diabetes mellitus (DM) status (12). Conversely, the CVC-to-AVF switch significantly reduced serum levels of pro-inflammatory mediators (12) and all-cause mortality rates (11). The accumulating evidence on the CVC-to-AVF switch to optimize the HD outcomes led to the development of the national ESRD Network Initiative called “Fistula First”, sometimes referred to as “Fistula First, Catheter Last” (14).
Peritoneal dialysis (PD) is another treatment modality in ESRD that offers comparable patient survival outcomes, reduced risk of septicemia, improved health-related life quality, and a more flexible lifestyle than HD. Consequently, other countries have developed the “PD first” initiative for access to care and affordability reasons, which increased the number of patients receiving PD (15). In the U.S., the 2019 Advancing American Kidney Health Executive Order contributed to the increased utilization of PD (16), and the developed North American PD Catheter Registry has offered an extensive dataset of PD outcomes (17).
Kidney transplant (KT) from living and deceased donors is another kidney replacement modality, which in the absence of contraindications offers an increased survival rate (18) and life quality (19) compared to HD. Paradoxically, oral health becomes relevant only at the pre-transplant stages, when clearance is required to proceed with the transplant process, while ignored during CKD/ESRD stages.
2 Oral cavity as an additional source of inflammation in patients with CKD/ESRD
Oral tissues are continuously exposed to ~800 bacterial species (20), and the chronic inflammatory infiltrate is present even in clinically healthy tissues (21). Therefore, it is critical to recognize that the oral cavity, an important modifiable source of inflammation in patients with ESRD, is frequently overlooked (22).
Among oral inflammatory conditions, this perspective focused on periodontitis, a polymicrobial multifactorial inflammatory disease of tooth-supporting tissues that affects 42% of the U.S. adult population (23). The Global Burden of Disease study confirmed the burden of periodontitis as the sixth most prevalent disease, with an estimated 54 billion USD/year cost of productivity loss worldwide (24–26). Once developed, it is characterized by the progressive, life-long destruction of connective tissue and alveolar bone surrounding teeth, which often leads to tooth loss (27).
3 The conceptual model of the increased prevalence of periodontitis in patients with CKD/ESRD
Using the National Health and Nutrition Examination Survey III database, we demonstrated significantly higher periodontitis prevalence in patients with CKD compared to the general population accentuated by racial disparities in CKD (35.28% (28) vs. 7.8% (29), respectively). Despite the recognition of periodontitis as a critical public health problem (30, 31), its awareness in CKD/ESRD populations remains low (32). Therefore, oral health promotion, effective treatment modalities, and disease prevention focused on reducing oral and systemic inflammation and oxidative stress should be prioritized in these populations. Our conceptual model describes the underlying mechanisms of the increased prevalence of periodontitis in patients with CKD/ESRD, assesses the contribution of periodontitis to the systemic inflammatory burden, and determines the extent of anti-inflammatory effects of standard-of-care non-surgical periodontal therapy (NSPT) in these patients (Figure 1).
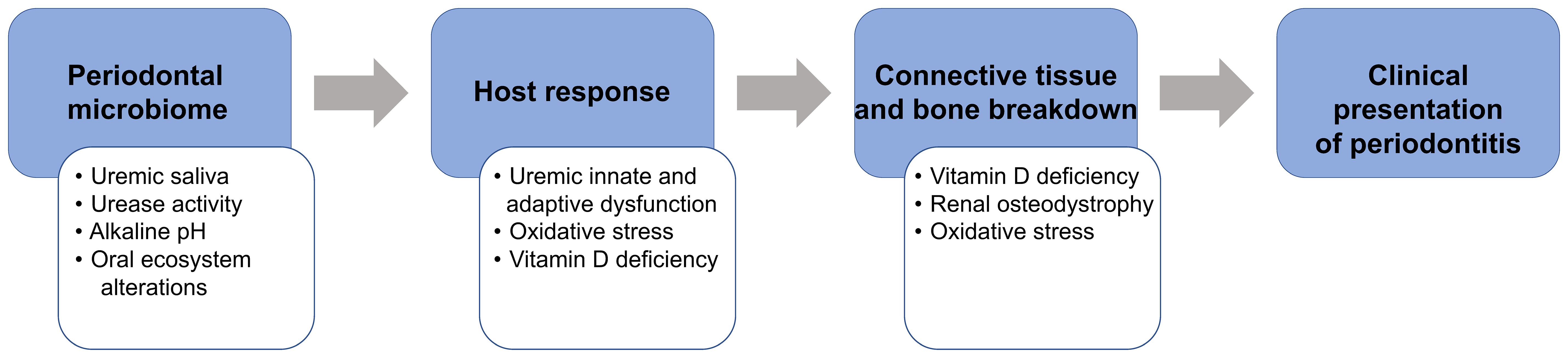
Figure 1 The proposed conceptual model that explains the modifying ESRD role (white boxes) on the pathogenesis of periodontitis (blue boxes).
3.1 Biologic plausibility for the increased prevalence of periodontitis in patients with CKD/ESRD
Periodontitis involves the interaction of periodontal pathogens in subgingival dental biofilm with the susceptible host and environmental factors, resulting in destructive changes within the connective tissue and alveolar bone. In CKD/ESRD, several mechanisms, including uremic effects on the oral environment and host immune response and behavioral factors (33, 34), can contribute to the pathogenesis of periodontitis. With the progressive loss of glomerular filtration rate (GFR), uremic solutes/toxins, normally eliminated with the urine, are retained in the body (35), contributing to anemia, immunosuppression, inflammation, infection, CVD morbidity, and mortality. Deleterious effects of increased urea toxins are mostly pronounced in ESRD (36). The proposed conceptual model, which explains the modifying role of ESRD (Figure 1, white boxes) on the pathogenesis of periodontitis (Figure 1, blue boxes), is summarized below. As urea concentration becomes pronounced (uremia), salivary urea levels dramatically increase from 7.5 to 17-26 μmol/L (37), altering the oral ecosystem and promoting the shift from microbial symbiosis to dysbiosis (38–40), similar to the uremia-related changes observed in the gut environment (41). Additional effects of the uremic milieu on innate and adaptive immunity (42) alter host response and increase infection susceptibility, as confirmed by a high prevalence of chronic infection associated with Chlamydia pneumoniae and Mycobacterium tuberculosis (43), the high relative risk for tuberculosis (44, 45), and the high prevalence of periodontitis. Also, vitamin D deficiency plays a dual role in abnormal bone breakdown/turnover (renal osteodystrophy) (46) and alteration of both innate (monocyte activation and phagocytosis) and adaptive immunity (modulation of cytokine production) (47), which could further promote periodontal tissue breakdown.
3.2 Periodontitis and the inflammatory burden in patients with CKD/ESRD
Within the last decades, the high inflammatory burden in ESRD was attributed to the “uremic puzzle.” The pieces included risk factors related to inflammation, endothelial function, vascular ossification markers, and uremic CVD markers, developing and connecting intricately (48), and contributing to CVD mortality. We now know that the complexity of the “uremic puzzle” extends past the Framingham CVD risk factors involving systemic inflammation and oxidative stress as the variables strongly associated with poor CVD outcomes in CKD (48). Overwhelming evidence indicated that the enzymatic myeloperoxidase activity linked oxidative stress and inflammation in uremia, emphasizing the importance of both events in the atherosclerotic process (49, 50). Consequently, research became focused on identifying dialysis-related factors (such as membrane bio-incompatibility, type of access, and impure dialysate) that contribute to systemic inflammation and oxidative stress at various levels (51). At the patient level, additional comorbid factors, including bacterial infections, volume overload, inadequate dialysis, and depression, were implicated in the elevation of hs-CRP and targeted in anti-inflammatory therapeutic efforts (52).
In the same context, the association between periodontitis and systemic inflammation was evidenced by higher levels of pro-inflammatory mediators in patients with periodontitis. Using a parsimonious regression model, our group previously showed that periodontitis led to the increased extent of inflammation in patients with CKD, as evidenced by an ~100% increase in the odds of elevated serum hs-CRP levels (odds ratio, OR, 2.0, 95% confidence interval, CI, 1.2, 3.6; P = 0.02) (53). Moreover, the impact of periodontitis on serum hs-CRP levels in patients with ESRD significantly correlated with the periodontitis severity (2.4, 4.2, and 4.4 mg/L in the slight, moderate, and severe periodontal tissue breakdown, respectively) (54). Recent evidence suggested that periodontitis could contribute to renal structural alterations (55), possibly via increased local and systemic oxidative stress and a compromised antioxidant capacity (56). However, precise mechanisms linking these events remain to be explored further.
As confirmed by analogy (57), bacterial infections increased levels of inflammatory mediators in patients undergoing HD, thus contributing to increased mortality (58, 59). Therefore, the CKD/ESRD - periodontitis relationship might be bi-directional in a way that CKD/ESRD potentiates the incidence of periodontitis, and periodontitis contributes to the sustained inflammation and poor outcomes in CKD/ESRD. This is indirectly confirmed by the meta-analysis of seventeen studies that showed a higher risk of developing CKD in patients with periodontitis than those without periodontitis (OR 1.60; 95% CI 1.44, 1.79; P = 0.11) (60). At the same time, patients with CKD had a similarly higher risk of developing periodontitis compared to control patients without CKD (OR 1.69; 95% CI 0.84, 3.40; P < 0.001) (60).
3.3 Oral and systemic effects of NSPT
NSPT involves the mechanical removal of dental biofilm (the microbial etiology) and calculus deposits (the main predisposing factor) from supra- and subgingival tooth surfaces (27). The treatment response is based on evaluating clinical periodontal determinants 4-6 weeks post-NSPT, and the disease progression is controlled through regular periodontal maintenance visits (typically every three months) (27). Systemic anti-inflammatory effects of NSPT first require a positive treatment response at the local/oral level with improved periodontal measures (61). In both systemically healthy and ESRD populations, NSPT improved periodontal clinical parameters and levels of systemic inflammatory markers (62).
Only short-term (<6-month follow-up) randomized controlled trials (RCTs) have examined the effects of NSPT on hs-CRP levels in patients undergoing HD and/or PD. The meta-analysis of these studies showed that NSPT significantly but moderately decreased serum hs-CRP levels (standardized mean difference, SMD, -1.53; 95% CI -2.95, -0.11; P < 0.001) (63). This reduction in hs-CRP following the NSPT appeared to be of a similar magnitude to the hs-CRP reduction observed following the CVC-to-AVF switch (OR 1.43; 95% CI 1.15, 1.68; P ≤ 0.05) (58). The levels of other pro-inflammatory mediators (IL-6 and TNFα) were not affected by NSPT (SMD, -0.23; 95% CI -0.78, 0.33; P > 0.05), even when examined at later time points (63).
It is important to highlight several key limitations in the current evidence of the matter. First, a similar decrease in hs-CRP protein concentration does not necessarily translate into clinical effects. Therefore, rigorous studies comparing the effects of NSPT and the CVC-to-AVF switch are needed. Second, as mentioned above, the systemic anti-inflammatory effect of NSPT requires a positive treatment response at the local/oral level with improved periodontal measures. Therefore, the efficacy of NSPT in trials that do not achieve clinically acceptable periodontal endpoints before evaluating systemic endpoints should be questioned. Third, as NSPT requires periodontal maintenance to minimize periodontal tissue breakdown, the episodic treatment approach with erratic maintenance visits is ineffective enough to promote optimal oral hygiene, prevent reactivation of periodontitis, and control systemic inflammation. Fourth, only short-term RCTs examined the effects of NSPT on the levels of systemic pro-inflammatory markers, which does not allow for assessing the sustainable anti-inflammatory therapeutic effects. Fifth, the discrepancies in the prevalence of systemic inflammatory conditions (such as DM) in the treatment vs. control arms and a well-documented association between periodontitis and DM make interpreting the results challenging. Finally, study design limitations include a moderate overall risk of bias (defined by low or unclear allocation concealment, blinding of participants, personnel, and outcomes) and lack of essential information about the included cohorts (such as the dialysis duration).
4 Discussion
In the present work, we proposed the conceptual model of the association between periodontitis and CKD/ESRD and provided evidence that outcomes of NSPT could be as effective in reducing inflammatory burden in patients with CKD/ESRD as the CVC-to-AVF switch. We argue that effective anti-inflammatory strategies in patients with CKD/ESRD should include multidisciplinary collaboration, oral health promotion, effective treatment strategies, and disease prevention focused on reducing oral and systemic inflammation. In this regard, outpatient dialysis centers could serve as an archetype for continuous in-center oral health maintenance care delivery. Future research is needed to examine systematic and repeated oral health delivery models in dialysis units to improve the periodontal status and modulate systemic inflammation in patients with ESRD.
Author contributions
KP: Writing – original draft, Writing – review & editing. JH: Conceptualization, Writing – review & editing, Writing – original draft. GF: Writing – review & editing. EI: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the R21 grant (DK108076) from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD, USA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Primers. (2017) 3:17088. doi: 10.1038/nrdp.2017.88
2. Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, et al. The systemic nature of CKD. Nat Rev Nephrol. (2017) 13:344–58. doi: 10.1038/nrneph.2017.52
3. Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. (2015) 39:84–92. doi: 10.1159/000368940
4. Nowak KL, Chonchol M. Does inflammation affect outcomes in dialysis patients? Semin Dial. (2018) 31:388–97. doi: 10.1111/sdi.12686
5. Sun J, Axelsson J, Machowska A, Heimburger O, Barany P, Lindholm B, et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol. (2016) 11:1163–72. doi: 10.2215/cjn.10441015
6. Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol. (2013) 26:243–53. doi: 10.5301/jn.5000169
7. Zheng HJ, Guo J, Wang Q, Wang L, Wang F, Zhang F, et al. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2021) 61:577–98. doi: 10.1080/10408398.2020.1740645
8. Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. (2018) 2018:2180373. doi: 10.1155/2018/2180373
9. Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. (2016) 129:153–162.e7. doi: 10.1016/j.amjmed.2015.08.025
10. National Institute of Diabetes and Digestive and Kidney Diseases. United States Renal Data System. 2023 Annual Data Report. Available online at: https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/4-vascular-access (Accessed April 1, 2024).
11. Arhuidese IJ, Orandi BJ, Nejim B, Malas M. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg. (2018) 68:1166–74. doi: 10.1016/j.jvs.2018.01.049
12. Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC. Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int. (2009) 76:1063–9. doi: 10.1038/ki.2009.303
13. Dukkipati R, Molnar MZ, Park J, Jing J, Kovesdy CP, Kajani R, et al. Association of vascular access type with inflammatory marker levels in maintenance hemodialysis patients. Semin Dial. (2014) 27:415–23. doi: 10.1111/sdi.12146
14. Agarwal AK, Haddad NJ, Vachharajani TJ, Asif A. Innovations in vascular access for hemodialysis. Kidney Int. (2019) 95:1053–63. doi: 10.1016/j.kint.2018.11.046
15. Li PK, Chow KM. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis. (2013) 62:993–1005. doi: 10.1053/j.ajkd.2013.03.038
16. Executive Order on Advancing American Kidney Health. Available online at: https://trumpwhitehouse.archives.gov/presidential-actions/executive-order-advancing-american-kidney-health/.
17. Oliver MJ, Perl J, McQuillan R, Blake PG, Jain AK, McCormick B, et al. Quantifying the risk of insertion-related peritoneal dialysis catheter complications following laparoscopic placement: Results from the North American PD Catheter Registry. Perit Dial Int. (2020) 40:185–92. doi: 10.1177/0896860819893813
18. Strohmaier S, Wallisch C, Kammer M, Geroldinger A, Heinze G, Oberbauer R, et al. Survival benefit of first single-organ deceased donor kidney transplantation compared with long-term dialysis across ages in transplant-eligible patients with kidney failure. JAMA Netw Open. (2022) 5:e2234971. doi: 10.1001/jamanetworkopen.2022.34971
19. Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PloS Med. (2012) 9:e1001307. doi: 10.1371/journal.pmed.1001307
20. The Expanded Human Oral Microbiome Database. Available online at: http://www.homd.org (Accessed April 1, 2024).
21. Lang NP, Bartold PM. Periodontal health. J Periodontol. (2018) 89 Suppl 1:S9–s16. doi: 10.1002/jper.16-0517
22. Grocock R, Holden B, Robertson C. The missing piece of the body? Oral health knowledge and confidence of doctors. Br Dent J. (2019) 226:427–31. doi: 10.1038/s41415-019-0077-1
23. Eke PI, Thornton-Evans G, Wei L, Borgnakke WS, Dye B, Genco RJ. Periodontitis in US adults. National health and nutrition examination survey 2009-2014. J Am Dent Assoc. (2018) 149:576–88. doi: 10.1016/j.adaj.2018.04.023
24. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. doi: 10.1177/0022034514552491
25. Listl S, Galloway J, Mossey PA, Marcenes W. Global economic impact of dental diseases. J Dent Res. (2015) 94:1355–61. doi: 10.1177/0022034515602879
26. Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. (2013) 92:592–7. doi: 10.1177/0022034513490168
27. Armitage GC, Xenoudi P. Post-treatment supportive care for the natural dentition and dental implants. Periodontol 2000. (2016) 71:164–84. doi: 10.1111/prd.12122
28. Ioannidou E, Swede H. Disparities in periodontitis prevalence among chronic kidney disease patients. J Dent Res. (2011) 90:730–4. doi: 10.1177/0022034511402209
29. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. (2015) 86:611–22. doi: 10.1902/jop.2015.140520
30. Centers for Disease Control and Prevention. National Center for Health Statistics. Healthy People 2020 (2010). Available online at: https://www.cdc.gov/nchs/healthy_people/hp2020.htm (Accessed April 1, 2024).
31. Healthy People 2030. US Department of Health and Human Services. Washington, DC: Office of Disease Prevention and Health Promotion (2030). Available at: https://health.gov/healthypeople (Accessed April 1, 2024).
32. Grubbs V, Plantinga LC, Tuot DS, Powe NR. Chronic kidney disease and use of dental services in a United States public healthcare system: a retrospective cohort study. BMC Nephrol. (2012) 13:16. doi: 10.1186/1471-2369-13-16
33. Marinoski J, Bokor-Bratic M, Mitic I, Cankovic M. Oral mucosa and salivary findings in non-diabetic patients with chronic kidney disease. Arch Oral Biol. (2019) 102:205–11. doi: 10.1016/j.archoralbio.2019.04.021
34. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. (2013) 9:255–65. doi: 10.1038/nrneph.2013.44
35. Vanholder R, Van Laecke S, Glorieux G. The middle-molecule hypothesis 30 years after: lost and rediscovered in the universe of uremic toxicity? J Nephrol. (2008) 21:146–60.
36. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. (2002) 62:1524–38. doi: 10.1046/j.1523-1755.2002.00600.x
37. Tomas I, Marinho JS, Limeres J, Santos MJ, Araujo L, Diz P. Changes in salivary composition in patients with renal failure. Arch Oral Biol. (2008) 53:528–32. doi: 10.1016/j.archoralbio.2008.01.006
38. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. (2015) 15:30–44. doi: 10.1038/nri3785
39. Araújo MV, Hong BY, Fava PL, Khan S, Burleson JA, Fares G, et al. End stage renal disease as a modifier of the periodontal microbiome. BMC Nephrol. (2015) 16:80. doi: 10.1186/s12882-015-0081-x
40. Hong BY, Furtado Araujo MV, Strausbaugh LD, Terzi E, Ioannidou E, Diaz PI. Microbiome profiles in periodontitis in relation to host and disease characteristics. PloS One. (2015) 10:e0127077. doi: 10.1371/journal.pone.0127077
41. Liu W, Huang J, Liu T, Hu Y, Shi K, Zhou Y, et al. Changes in gut microbial community upon chronic kidney disease. PloS One. (2023) 18:e0283389. doi: 10.1371/journal.pone.0283389
42. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. (2008) 3:1526–33. doi: 10.2215/cjn.00950208
43. Stenvinkel P, Heimburger O, Jogestrand T, Karnell A, Samuelsson A. Does persistent infection with Chlamydia pneumoniae increase the risk of atherosclerosis in chronic renal failure? Kidney Int. (1999) 55:2531–2. doi: 10.1046/j.1523-1755.1999.00422.x
44. Chia S, Karim M, Elwood RK, FitzGerald JM. Risk of tuberculosis in dialysis patients: a population-based study. Int J Tuberc Lung Dis. (1998) 2:989–91.
45. Simon TA, Paul S, Wartenberg D, Tokars JI. Tuberculosis in hemodialysis patients in New Jersey: a statewide study. Infect Control Hosp Epidemiol. (1999) 20:607–9. doi: 10.1086/501679
46. Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. (2006) 69:1945–53. doi: 10.1038/sj.ki.5000414
47. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). (2012) 76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x
48. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. (2008) 3:505–21. doi: 10.2215/cjn.03670807
49. Himmelfarb J. Oxidative stress in hemodialysis. Contrib Nephrol. (2008) 161:132–7. doi: 10.1159/000130658
50. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. (2019) 34:975–91. doi: 10.1007/s00467-018-4005-4
51. Stenvinkel P. Inflammation in end-stage renal disease: the hidden enemy. Nephrol (Carlton). (2006) 11:36–41. doi: 10.1111/j.1440-1797.2006.00541.x
52. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease–what have we learned in 10 years? Semin Dial. (2010) 23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x
53. Ioannidou E, Swede H, Dongari-Bagtzoglou A. Periodontitis predicts elevated C-reactive protein levels in chronic kidney disease. J Dent Res. (2011) 90:1411–5. doi: 10.1177/0022034511423394
54. Chen LP, Chiang CK, Peng YS, Hsu SP, Lin CY, Lai CF, et al. Relationship between periodontal disease and mortality in patients treated with maintenance hemodialysis. Am J Kidney Dis. (2011) 57:276–82. doi: 10.1053/j.ajkd.2010.09.016
55. Franca LFC, Vasconcelos A, da Silva FRP, Alves EHP, Carvalho JS, Lenardo DD, et al. Periodontitis changes renal structures by oxidative stress and lipid peroxidation. J Clin Periodontol. (2017) 44:568–76. doi: 10.1111/jcpe.12729
56. Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. (2017) 8:910. doi: 10.3389/fphys.2017.00910
57. Hill AB. The environment and disease: association or causation? Proc R Soc Med. (1965) 58:295–300. doi: 10.1177/003591576505800503
58. Banerjee T, Kim SJ, Astor B, Shafi T, Coresh J, Powe NR. Vascular access type, inflammatory markers, and mortality in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. (2014) 64:954–61. doi: 10.1053/j.ajkd.2014.07.010
59. Valdivielso JM, Rodriguez-Puyol D, Pascual J, Barrios C, Bermudez-Lopez M, Sanchez-Nino MD, et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. (2019) 39:1938–66. doi: 10.1161/atvbaha.119.312705
60. Kapellas K, Singh A, Bertotti M, Nascimento GG, Jamieson LM. Periodontal and chronic kidney disease association: A systematic review and meta-analysis. Nephrol (Carlton). (2019) 24:202–12. doi: 10.1111/nep.13225
61. Armitage GC. Effect of periodontal therapy on general health–is there a missing component in the design of these clinical trials? J Clin Periodontol. (2008) 35:1011–2. doi: 10.1111/j.1600-051X.2008.01327.x
62. Luthra S, Orlandi M, Hussain SB, Leira Y, Botelho J, MaChado V, et al. Treatment of periodontitis and C-reactive protein: A systematic review and meta-analysis of randomized clinical trials. J Clin Periodontol. (2023) 50:45–60. doi: 10.1111/jcpe.13709
63. Yue H, Xu X, Liu Q, Li X, Xiao Y, Hu B. Effects of non-surgical periodontal therapy on systemic inflammation and metabolic markers in patients undergoing haemodialysis and/or peritoneal dialysis: a systematic review and meta-analysis. BMC Oral Health. (2020) 20:18. doi: 10.1186/s12903-020-1004-1
Keywords: CKD/ESRD, fistula, inflammation, periodontal therapy, interdisciplinary treatment approach
Citation: Parsegian K, Himmelfarb J, Fares G and Ioannidou E (2024) Fistula first, catheter last: can the mouth be second? Front. Nephrol. 4:1385544. doi: 10.3389/fneph.2024.1385544
Received: 13 February 2024; Accepted: 15 April 2024;
Published: 23 May 2024.
Edited by:
Yuri Battaglia, University of Verona, ItalyReviewed by:
Andrzej Jaroszyński, Jan Kochanowski University, PolandCopyright © 2024 Parsegian, Himmelfarb, Fares and Ioannidou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karo Parsegian, a2Fyby5wYXJzZWdpYW5AY3VhbnNjaHV0ei5lZHU=
†ORCID: Karo Parsegian, orcid.org/0000-0002-5440-6036
Jonathan Himmelfarb, orcid.org/0000-0002-3319-1224
Effie Ioannidou, orcid.org/0000-0003-1954-5900
 Karo Parsegian
Karo Parsegian Jonathan Himmelfarb2†
Jonathan Himmelfarb2† Effie Ioannidou
Effie Ioannidou