- 1Department of Pathology and Laboratory Medicine, Oregon Health & Science University, Portland, OR, United States
- 2Department of Medicine, Division of Nephrology and Hypertension, Oregon Health & Science University, Portland, OR, United States
Neural epidermal growth factor-like 1 (NELL1) membranous nephropathy (MN) is notable for its segmental deposit distribution, IgG1 dominant deposits, and comparatively high rate of spontaneous remission. It has been associated with a variety of exposures and secondary conditions, specifically use of thiol-containing medications – including lipoic acid, bucillamine, and tiopronin – as well as traditional indigenous medications (TIM) particularly those with high mercury content, and non-steroid anti-inflammatory drugs (NSAIDs). Malignancies, graft vs. host disease (GVHD), infection, and autoimmune conditions have also been associated with NELL1 MN. Herein, we provide a detailed summary of the clinicopathologic features of NELL1 and associations with underlying conditions, with a focus on treatment and outcomes. Rare cases of dual NELL1 and phospholipase A2 receptor (PLA2R) positive MN are reviewed. Genome-wide association study of NELL1, role of NELL1 in other physiologic and pathologic processes, and connection between NELL1 MN and malignancy with relevance of NELL1 tumor staining are examined. Finally, relationships and potential disease mechanisms of thiol- and mercury- associated NELL1 MN are discussed.
Introduction
Membranous nephropathy (MN) is an autoimmune disease characterized by autoantibodies directed against podocyte antigens (1–3). MN is the most common cause of primary nephrotic syndrome (urinary protein loss > 3.5 grams per day with edema, hypoalbuminemia, and hyperlipidemia) in adults, with a prevalence estimated between 2-17 million cases per year (4–7). Patients with MN experience significant morbidity related to manifestations of nephrotic syndrome and chronic kidney disease. Although most patients have preserved kidney function at diagnosis, untreated disease can progress to end-stage kidney disease (ESKD) in up to a third of patients (4). The 2021 Kidney Disease Improving Global Outcomes (KDIGO) guidelines therefore advise consideration of immunosuppressive therapy in patients with moderate and high-risk disease (8).
Although falling under the same diagnostic terminology, clinical and pathologic features of “idiopathic” versus “secondary” MN have long been recognized. In 2009, Beck et al. discovered the major antigen involved in disease pathogenesis of idiopathic MN, the phospholipase A2 receptor (PLA2R) (2). Subsequently, with the use of mass spectrometry to identify proteins enriched in glomerular immune deposits, the known autoantigens in MN have rapidly expanded in the last 10 years, leading to autoantigen-based MN classification (9). The most common MN autoantigen remains PLA2R, which has been extensively phenotyped and provides a framework by which to study newly discovered antigens. Other notable MN antigens have been described and reviewed elsewhere (10), but include: thrombospondin type-1 domain containing 7A (THSD7A, associated with primary MN and malignancy) (11), neural epidermal growth factor-like 1 (NELL1, described below) (12, 13), protocadherin 7 (no definite association; subset with autoimmunity or malignancy) (14), high-temperature requirement A serine peptidase (HTRA1, no identified association) (15), Semaphorin 3B (associated with childhood MN) (16), Netrin G1 (no identified association) (17), FAT1 (associated with graft vs. host disease, GVHD) (18), contactin 1 (associated with chronic inflammatory demyelinating polyneuropathy, CIDP) (19), neuron derived neurotrophic factor (NDNF, associated with syphilis) (20), proprotein convertase subtilisin/kexin type 6 (PCSK6, associated with use of nonsteroidal anti-inflammatory drugs, NSAIDs) (21), and lupus MN associated antigens: exostosin 1/2 (22), neural cell adhesion molecule (NCAM1) (23), transforming growth factor beta receptor 3 (TGFBR3) (24) and others (25).
Given their relative rarity and more recent recognition, data for some of these antigens is based on fewer patients and is actively evolving. However, NELL1 has emerged as the second most common autoantigen in MN after PLA2R, with some distinct clinical associations and pathologic features. In this review we discuss the clinicopathologic characteristics of NELL1 MN. In addition, we summarize cases of MN expressing both PLA2R and NELL1, and we review mechanistic theories in NELL1 MN. As with other forms of MN, the factors driving development of anti-NELL1 autoantibodies are poorly understood but necessary to elucidate if we are to better treat and prognosticate patients with NELL1 MN.
Clinicopathologic studies of NELL1 MN
In 2019, two groups identified neural epidermal growth factor-like 1 (NELL1) podocyte antigen in a subset of MN with distinct histologic and immunologic features, generally segmental glomerular capillary loop subepithelial deposits with IgG1 dominant staining (12, 13). Although initially suggested to be largely a primary MN comprising 16% of PLA2R negative MN (12), secondary associations were subsequently discovered and account for the majority of cases in some studies (up 89% in our recent series) (26). The strength of these associations, disease prevalence and epidemiologic characteristics, and clinical outcomes vary by study and location, and are summarized in Table 1.
On presentation, patients with NELL1 MN have nephrotic range proteinuria (approximately 35-80%), often preserved kidney function (in 70-84%), and are usually adults with a median age in their 60’s although adolescent cases have been reported (Table 1) (12, 13, 26, 43). NELL1 MN has been reported in wide spectrum of racial and ethnic backgrounds; taken together, larger studies show a relatively similar male to female distribution although this varies significantly by study and underlying association (Table 1). Kidney biopsies (Figure 1) show the characteristic MN pattern of subepithelial immune deposits but these are usually segmental to incomplete (55-94%, rather than global as with PLA2R MN), and may be subtle by light microscopy (12, 13, 26). The immune deposits are usually IgG1 (co)dominant (86-96%), and Stage I-II by electron microscopy (82-93%); mesangial (7 - 24%) and occasional subendothelial and/or extraglomerular deposits of IgG may be present (12, 13, 26, 43). The immune deposits are positive for NELL1 by immunohistochemistry, confirming the diagnosis; corresponding anti-NELL1 antibodies have also been identified in the sera of affected patients (12, 13). Although anti-PLA2R serum testing can be used in select patients for a non-invasive diagnosis and monitoring of PLA2R MN (46, 47), anti-NELL1 serum testing is not yet widely commercially available. Notably, NELL1 MN has relatively similar pathologic features regardless of underlying etiology. However, clinical outcomes are impacted by the details of – or discovery of – the underlying exposure or condition (26), as discussed below.
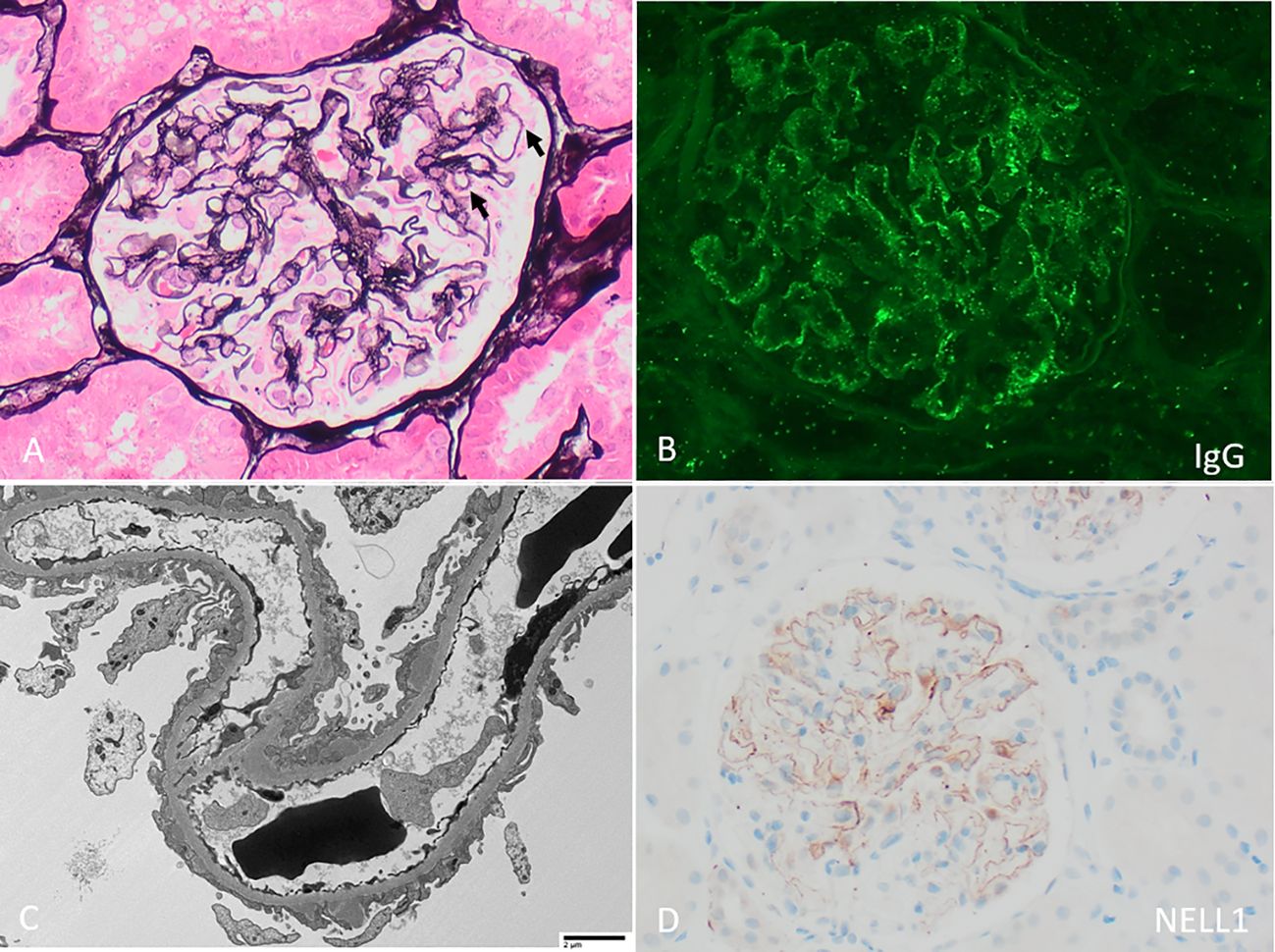
Figure 1 Neural epidermal growth factor-like 1 (NELL1) membranous nephropathy with (A) “stiff” appearing capillary loops with faint rarefactions of the glomerular basement membrane and subepithelial immune deposits (arrows, Jones stain 400x). (B) By immunofluorescence, there is segmentally distributed granular peripheral capillary wall staining for polyclonal IgG. (C) By electron microscopy, irregularly distributed subepithelial immune deposits are present, with associated podocyte foot process effacement (direct magnification 1900x). (D) By immunohistochemistry, there is corresponding incomplete capillary wall staining of immune deposits for NELL1.
NELL1 MN is associated with use of thiol-containing medications, namely lipoic acid (31, 32), bucillamine (38), and tiopronin (42), and it is plausible that NELL1 was the causative auto-antigen for many cases historically associated with thiol compounds. Traditional indigenous medications (TIM) (36) and skin products (44) are associated with NELL1 MN, and tested medications and compounds have had high mercury content with corresponding elevated blood mercury levels in affected individuals (36). The largest series of TIM-associated MN was reported from India, where NELL1 MN comprised 34% of all MN and 88% of TIM-MN (36), again suggesting that auto-reactivity to the NELL1 antigen specifically may account for most historically reported mercury-associated MN. The remission rate of NELL1 MN in patients on TIM (74% at 3.5 months) (36) or lipoic acid (88% at 11 months, without immunosuppression) (26) is quite high, and significantly higher than other NELL1 MN associations (26), likely related to relative simplicity of discontinuing the exposure (i.e. compared with treating a complex underlying condition). Thus there is a potentially large subset of patients with NELL1 MN secondary to thiol-containing medications or heavy metal exposures who have an excellent prognosis and likely do not require immunosuppression.
Another major underlying condition is malignancy, reported in in 0-33% of patients depending on study (13, 27, 36), substantially higher than that seen with PLA2R MN (~4%) (4, 13, 48). Of reported patients, 9 of 12 (75%) with oncologic remission also had remission of proteinuria (13, 42), supporting this link. Other conditions include infections – such as human immune deficiency virus (HIV) (35), hepatitis B and C (HBV, HCV) (40), tuberculosis (43). MN in the setting of hematopoietic stem cell transplant and graft-versus-host disease (GVHD) can occur with the NELL1 antigen (29) although is much more commonly associated with the protocadherin FAT1 antigen (18). Autoimmune disease, seen in 0-31% of reported patients (13, 26, 36, 37), including sarcoidosis (40, 41), rheumatoid arthritis (especially patients treated with bucillamine) (37–39) and others may also be associated with NELL1 MN. Finally, NSAIDs have also been associated with NELL1 MN (26, 34, 40) (up to 27%), though are also associated with the PCSK 6 antigen (21) and clinical correlations between NSAID cessation and remission are currently less well-documented than for thiol- or mercury- associated NELL1 MN.
Notably, series of NELL1 MN from India (36) and China (27) generally lack the associations with autoimmune disease or malignancy reported from the US, Europe, and Japan, and additional underlying genetic and/or environmental factors may contribute to disease development in different groups. Higher rates of remission tend to be seen in association with thiol or mercury exposures, and potentially with oncologic remission (13).
Dual PLA2R and NELL1 positive MN
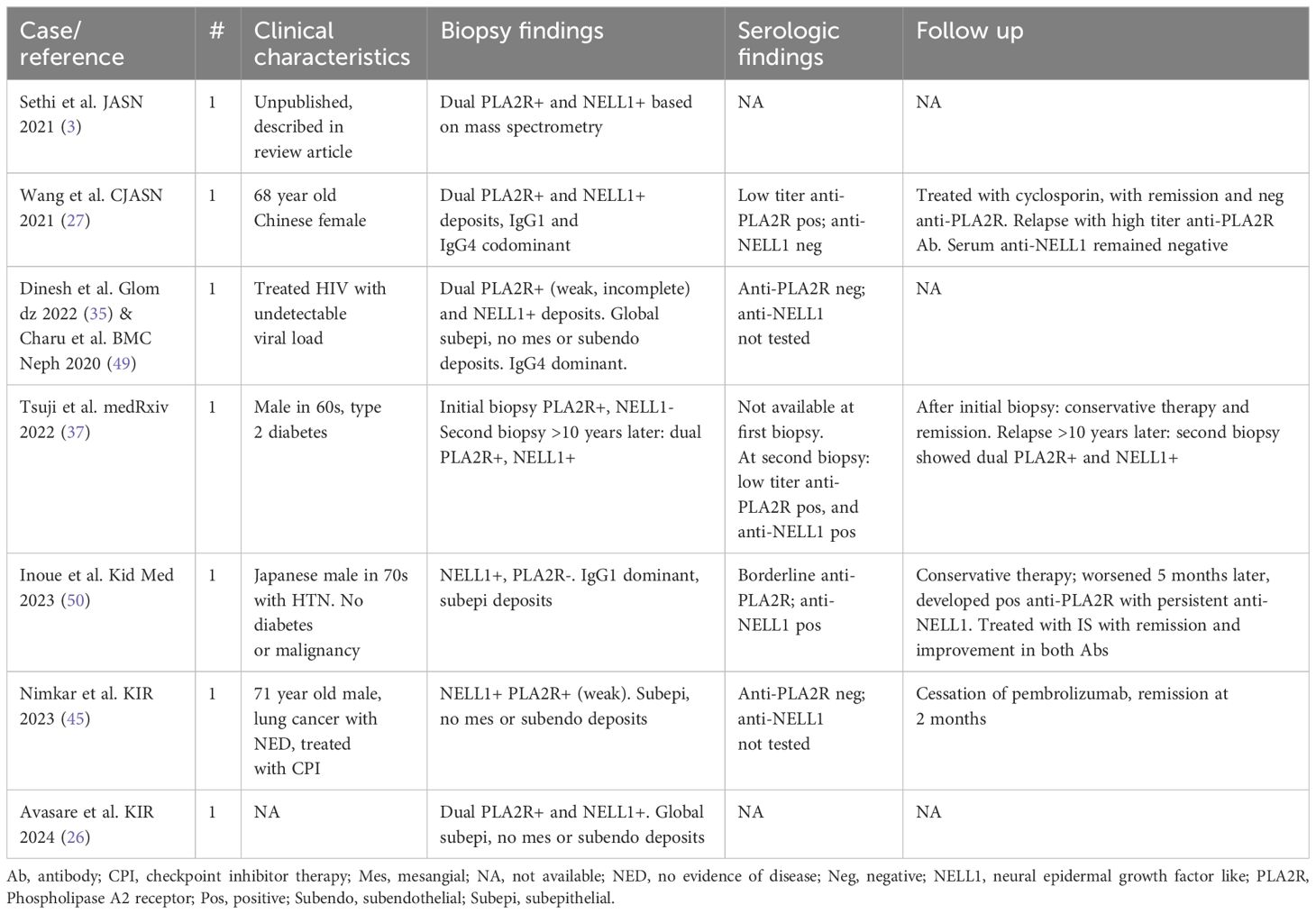
PLA2R and NELL1 are the most commonly implicated antigens in MN and have distinct clinical associations, but rare cases have been reported as dual positive (summarized in Table 2). Of seven cases, two describe patients with a low-titer (27) or borderline (50) serum anti-PLA2R antibodies at time of biopsy, one with positive tissue PLA2R (27), one with negative tissue PLA2R (50). The first went into remission but relapsed with high-titer anti-PLA2R antibodies (without anti-NELL1 antibodies) (27), and the second progressed to have a positive serum anti-PLA2R antibody (with persistent anti-NELL1 antibodies) (50). A third patient had an initial biopsy with PLA2R+ NELL1- MN (no serum available), treated conservatively, with relapse >10 years later and biopsy demonstrating dual PLA2R+ NELL1+ MN with corresponding serum antibodies to both PLA2R and NELL1 (37). In two cases, PLA2R glomerular staining is described as weak with an incomplete capillary loop distribution and was associated with a negative serum anti-PLA2R antibodies at the time of biopsy (35, 45, 49), which may represent false-positive tissue reactivity.
Taken together, limited data from these case reports suggest that MN with weak incomplete tissue PLA2R and negative serum anti-PLA2R antibodies results may benefit from additional MN antigen testing including NELL1. Patients with borderline or low-titer anti-PLA2R serum antibodies may experience a rise in anti-PLA2R titer or disease recurrence, even if the biopsy shows MN with NELL1+ and PLA2R- deposits. Finally, dual PLA2R and NELL1 tissue staining with corresponding autoantibodies may rarely exist or evolve over time. Although epitope spreading is well-described within the PLA2R molecule (51–54), mechanisms for relationships with other MN antigens are unknown.
NELL1 in physiology and disease
The NELL1 gene is located on chromosome 11 and encodes an 810 amino acid protein with a secretory signal peptide, N-terminal thrombospondin-1-like molecule (also called laminin G domain), five von Willebrand factor-like repeats with associated cysteine residues, and six epidermal growth factor (EGF)-like repeats (55–57). It is glycosylated, secreted as a 400 kDa homotrimer, and acts as cell-signaling molecule which binds to and is phosphorylated by protein kinase C-β1 (PKC- β1) (56). In human bulk tissue RNA-seq studies, NELL1 transcripts are enriched in brain, kidney, prostate, and testis (55). NELL1 also appears conserved across species, with 95% nucleotide homology between mice and humans (58–60). The function(s) of NELL1 in the kidney are not known. A single cell RNA-seq study of healthy human kidney, identified NELL1 transcripts in loop of Henle and distal tubular cells; in the glomerulus, it appears to be expressed by podocytes without significant expression by mesangial or endothelial cells (61–63) Future studies examining if and how autoimmunity and injury affect NELL1 expression within the kidney will be informative.
With regard to other human diseases, NELL1 is best studied for its role in osteoblast differentiation. Overexpression of NELL1 was identified within the prematurely fused coronal sutures in patients with non-familial, non-syndromic craniosynostosis (58, 64). Transgenic mice overexpressing the Nell1 gene recapitulate this phenotype, with overgrowth of skull bones and premature suture closure (58). In oral health, it may also play a role in angiogenesis in human dental pulp stem cells (65), contribute to bone regeneration in periodontitis (66), and promote progression in osteosarcoma (67).
Conversely, downregulation of Nell1 inhibits osteoblast differentiation (58). Mice with Nell1 point mutations and severe loss of expression have skeletal defects in cranial vault, vertebrae, and ribs (56), supporting the protein’s role in intramembranous and endochondral ossification. In these mice, loss of Nell1 function leads to reduced expression of genes encoding extracellular matrix proteins, the most severe of which include collagen 5 alpha 3 subunit (Col5a3), tenascin (Tnxb), proteoglycan 4 (Prg4), thrombospondin 3 (Thbs3) (56). Chondrocyte-specific Nell1 inactivation impedes growth and mineralization of the appendicular skeleton (68), highlighting its importance in endochondral ossification outside the skull. Notably, kidney disease is not described in mouse models of overexpression or loss of expression of Nell1, although these studies were predominantly focused on the musculoskeletal system (56, 58).
In human genetic studies, a review (60) of genome wide association studies (GWAS) highlighted NELL1 single nucleotide polymorphisms (SNPs) of genome wide significance (P < 5 x 10-8) or suggestive significance (P < 5 x 10-5) in triglyceride metabolism, autism, multiple sclerosis, inflammatory bowel disease (IBD), chronic periodontitis, non-small cell lung cancer (60), as well as in osteoporosis (68, 69). NELL1 deletion has also been described in a 3-year-old with short stature, macrocephaly, and delayed fontanelle closure (70). Overall, a specific association with kidney disease in patients with NELL1 SNPs has not been identified. However, these genetic studies corroborate the discussed roles of NELL1 in bone and dental health, and raise questions about potential genetic predispositions in the small subset of patients with NELL1 MN in the setting of multiple sclerosis (31) or IBD (13).
Mechanistic theories in NELL1 MN
The major clinical associations in NELL1 MN point to potentially differing underlying mechanisms, specifically those linked to exposure to thiol-containing medications or mercury, and malignancy. There is less data regarding potential mechanisms for NELL1 MN associated with autoimmune disease, GVHD, NSAIDs, and idiopathic NELL1 MN, and these will not be further discussed here.
The reduced form of lipoic acid (dihydrolipoic acid) contains two thiol, or sulfhydryl groups, each of which consists of a sulfur atom bonded to hydrogen and an alkyl group (R-S-H). Structurally similar thiol groups are present in D-penicillamine, captopril, bucillamine, and tiopronin (42). Of the thiol-containing medications associated with NELL1 MN, lipoic acid is potentially unique in that it is an antioxidant naturally synthesized by various plant and mammal species, including humans, which functions in the mitochondria as an enzyme cofactor (71). Lipoic acid is chiral and natural forms exist as the R enantiomer, whereas ex vivo synthesized lipoic acid supplements contain a racemic mixture of both R and S enantiomers (71–73), which have potentially differing biologic effects. Neither specific dose toxicity nor specific manufacturers have been connected to lipoic acid associated NELL1 MN (26, 31). Adverse immune events associated with thiol compounds also include pemphigus, rash, oral mucosal ulcers, and, for lipoic acid, insulin autoimmune syndrome in genetically susceptible individuals (HLA-DRB1*0406 and DRB1*0403) (42, 74–77). Mechanisms for thiol-induced MN are likely related to thiol-disulfide exchange, altering tertiary and quaternary protein structure and potentially generating neo-epitopes (42). Anti-PLA2R auto-antibodies also bind primarily to a cysteine-rich domain of PLA2R containing disulfide bones (78), and this and other auto-antibodies in MN have been identified specifically under non-reducing conditions (2, 4) (except for Semaphorin 3B), suggesting the importance of these disulfide bonds in MN autoantigen development outside the setting of thiol exposure.
Mercury-associated NELL1 MN may have a related mechanism, as mercury shows high-affinity binding to sulfhydryl groups (79). A variety of conditions, autoimmunity, and autoantibodies have been linked to mercury (80) (associations and mechanisms recently reviewed elsewhere (79)). Autoantibody development to laminins in particular is a common finding in experimental mercury exposure (80) and is particularly relevant given the role of these extracellular matrix glycoproteins in the glomerular basement membrane (81) and the presence of a laminin G domain in the NELL1 protein (57). However, laminins are a large family of proteins, and in one study of workers with chronic mercury exposure, no significant differences were found among mercury workers with anti-laminin antibodies (n=8) vs. mercury exposed without anti-laminin antibodies (n=54) vs. controls (n=60) for kidney parameters (including proteinuria, albuminuria, creatinine, etc.), nor was there a dose relationship between mercury exposure (in blood and urine) and development of anti-laminin antibodies (82). Although small and with older techniques, this study provides some evidence against a direct mechanistic relationship between anti-laminin antibodies and mercury-associated MN, and suggests that mercury-associated autoimmunity in humans is not dose-dependent and is impacted by other, modifying factors (82).
Similar to THSD7A (83, 84), the association between NELL1 MN and malignancy is made further intriguing by the presence of tumor staining in reported cases, usually of solid tumors like breast (13, 85) or prostate cancer for NELL1. However, these initial reports often lack examination of a control group (i.e., NELL1 immunostaining in tumors from patients without NELL1 MN). NELL1 immunoreactivity has been documented in wide variety of carcinomas, including but not limited to the colon, breast, prostate, lung, liver, as well as in melanoma (86). Larger studies focusing on neoplasms identified THSD7A expression in a wide variety of neoplasms (87), raising questions about how well immunohistochemically-detected tumor antigen expression will associate with development of MN in an individual patient. Future studies of NELL1 immunoreactivity in tumors may be useful in this regard. Additionally, demonstration of an association between NELL1 MN activity and/or anti-NELL1 antibodies with tumor regression would further support the association.
Discussion and future directions
In addition to defining the presenting clinical and pathological associations, outcome studies in NELL1 MN have revealed that remission may be largely influenced by underlying association, highlighting the importance of an etiology-based as well as antigen-based classification of MN. In the case of certain thiol- or mercury- containing medications, the relative ease of identifying and removing the exposure may account, at least in part, for the high remission rate. This attractive theory does not explain all cases, however, such as patients that continue or restart the same medication without persistence or recurrence of NELL1 MN. Nor does it account for the relatively low burden of detected disease despite the broad use of many of these supplements which, in the case of lipoic acid, has a generally innocuous safety profile in large studies (31, 88). It is likely that genetic factors play a role in disease development, and future GWAS studies accounting for the geographic and/or ethnic heterogeneity of NELL1 MN may further elucidate genetic susceptibility in patients with exposure-related NELL1 MN.
The driving mechanisms in NELL1 likely vary according to associated condition, and differences in remission rates may also reflect complexity or reversibility of these biologic mechanisms (i.e. in addition to simplicity of removing an exposure). Future studies correlating anti-NELL1 serum titers to underlying association, disease activity, and details of epitope antigenicity – similar to studies for PLA2R with the added focus of underlying association in some patients – may inform how these clinically-identified etiologies shape the immunologic aspects of disease.
Though the mechanistic underpinnings of NELL1 MN are not fully elucidated, observational findings on clinical associations have already had a significant impact on patient care. In our practice, those diagnosed with NELL1 MN undergo a thorough review of medication and supplement use, and malignancy screening. Because of the high remission rate reported in the literature, those with otherwise low risk disease are managed conservatively with non-immunosuppressive anti-proteinuric therapy for a period of 3-6 months. If the disease persists despite addressing possible secondary associations, then immunosuppressive strategies are discussed. We anticipate the development of serum anti-NELL1 antibodies will further guide the decision to escalate therapy, assuming anti-NELL1 antibodies have similar diagnostic and prognostic characteristics to anti-PLA2R antibodies. Furthermore, as mechanistic understanding increases, we anticipate the rise of specific therapies targeted to MN-antigen types with the overarching hope that patients may one day have more specific and less toxic therapies.
Author contributions
NA: Writing – original draft, Writing – review & editing. VK: Writing – review & editing. RA: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. (2011) 364:689–90. doi: 10.1056/NEJMc1011678
2. Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
3. Sethi S. New 'Antigens' in membranous nephropathy. J Am Soc Nephrol. (2021) 32:268–78. doi: 10.1681/ASN.2020071082
4. Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. (2021) 7:69. doi: 10.1038/s41572-021-00303-z
5. Okpechi IG, Ameh OI, Bello AK, Ronco P, Swanepoel CR, Kengne AP. Epidemiology of histologically proven glomerulonephritis in Africa: A systematic review and meta-analysis. PLoS One. (2016) 11:e0152203. doi: 10.1371/journal.pone.0152203
6. Li H, Yu X, Lan P, Wang Z, Yang S, Feng J, et al. Spectrum of biopsy-proven kidney diseases in northwest China: a review of 30 years of experiences. Int Urol Nephrol. (2022) 54:2609–16. doi: 10.1007/s11255-022-03168-7
7. Subramanian P, Kumar H, Tiwari B, Barwad A, Bagchi S, Bagga A, et al. Profile of Indian patients with membranous nephropathy. Kidney Int Rep. (2020) 5:1551–7. doi: 10.1016/j.ekir.2020.06.024
8. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. (2021) 100:753–79. doi: 10.1016/j.kint.2021.05.015
9. Sethi S, Beck LH Jr., Glassock RJ, Haas M, De Vriese AS, Caza TN, et al. Mayo clinic consensus report on membranous nephropathy: proposal for a novel classification. Mayo Clin Proc. (2023) 98(11):1671–84. doi: 10.1016/j.mayocp.2023.08.006
10. Avasare R, Andeen N, Beck L. Novel antigens and clinical updates in membranous nephropathy. Annu Rev Med. (2024) 75:219–332. doi: 10.1146/annurev-med-050522-034537
11. Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. (2014) 371:2277–87. doi: 10.1056/NEJMoa1409354
12. Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. (2020) 97:163–74. doi: 10.1016/j.kint.2019.09.014
13. Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, et al. NELL1 is a target antigen in Malignancy-associated membranous nephropathy. Kidney Int. (2021) 99:967–76. doi: 10.1016/j.kint.2020.07.039
14. Sethi S, Madden B, Debiec H, Morelle J, Charlesworth MC, Gross L, et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol. (2021) 32:1249–61. doi: 10.1681/ASN.2020081165
15. Al-Rabadi LF, Caza T, Trivin-Avillach C, Rodan AR, Andeen N, Hayashi N, et al. Serine protease HTRA1 as a novel target antigen in primary membranous nephropathy. J Am Soc Nephrol. (2021). doi: 10.1681/ASN.2020101395
16. Sethi S, Madden B, Casal Moura M, Singh RD, Nasr SH, Hou J, et al. Membranous nephropathy in syphilis is associated with neuron-derived neurotrophic factor. J Am Soc Nephrol. (2023) 34(3):374–84.
17. Reinhard L, Machalitza M, Wiech T, Groene HJ, Lasse M, Rinschen M, et al. Netrin G1 is a novel target antigen in primary membranous nephropathy. J Am Soc Nephrol. (2022) 33:1823–31. doi: 10.1681/ASN.2022050608
18. Sethi S, Madden B, Casal Moura M, Nasr SH, Klomjit N, Gross L, et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol. (2022) 33:1033–44. doi: 10.1681/ASN.2021111488
19. Le Quintrec M, Teisseyre M, Bec N, Delmont E, Szwarc I, Perrochia H, et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. (2021) 100:1240–9. doi: 10.1016/j.kint.2021.08.014
20. Sethi S MV, Fervenza F, Haas M. Neuron-Derived Neurotrophic Factor (NDNF) Is a Novel Protein Associated With Membranous Nephropathy (MN). Orlando, FL (2022).
21. Sethi S, Casal Moura M, Madden B, Debiec H, Nasr SH, Larsen CP, et al. Proprotein convertase subtilisin/kexin type 6 (PCSK6) is a likely antigenic target in membranous nephropathy and nonsteroidal anti-inflammatory drug use. Kidney Int. (2023) 104:343–52. doi: 10.1016/j.kint.2023.04.006
22. Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol. (2019) 30:1123–36. doi: 10.1681/ASN.2018080852
23. Caza TN, Hassen SI, Kuperman M, Sharma SG, Dvanajscak Z, Arthur J, et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. (2021) 100:171–81. doi: 10.1016/j.kint.2020.09.016
24. Caza TN, Hassen SI, Kenan DJ, Storey A, Arthur JM, Herzog C, et al. Transforming growth factor beta receptor 3 (TGFBR3)-associated membranous nephropathy. Kidney360. (2021) 2:1275–86. doi: 10.34067/KID.0001492021
25. Caza TN, Storey AJ, Hassen SI, Herzog C, Edmondson RD, Arthur JM, et al. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int. (2023) 103:593–606. doi: 10.1016/j.kint.2023.01.001
26. Avasare RS, Clark S, Spain RI, Wusirika R, Rope R, Gurley SB, et al. Characteristics and outcomes of NELL1 membranous nephropathy (MN) in lipoic acid users and non-users. Kidney Int Rep (accepted). (2024). doi: 10.1016/j.ekir.2024.02.1401
27. Zhu S, Liu X, Yue S, Luo B, Song Z, Xu X, et al. Segmental membranous glomerulopathy in adults. Kidney Dis (Basel). (2023) 9(6):507–16. doi: 10.1159/000533294
28. Kudose S, Santoriello D, Debiec H, Canetta PA, Bomback AS, Stokes MB, et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. (2021) 99:247–55. doi: 10.1016/j.kint.2020.06.014
29. Kudose S, Sekulic M, Mehring CJ, Santoriello D, Batal I, Stokes MB, et al. NELL1-associated membranous glomerulopathy after hematopoietic stem cell transplantation. Kidney Int Rep. (2021) 6:1992–5. doi: 10.1016/j.ekir.2021.04.033
30. Munch J, Kruger BM, Weimann A, Wiech T, Reinhard L, Hoxha E, et al. Posttransplant nephrotic syndrome resulting from NELL1-positive membranous nephropathy. Am J Transpl. (2021) 21:3175–9. doi: 10.1111/ajt.16610
31. Spain RI, Andeen NK, Gibson PC, Samuels MH, Morris CD, Solomon AJ, et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. Kidney Int. (2021) 100:1208–13. doi: 10.1016/j.kint.2021.10.010
32. Caza TN, Larsen CP. Lipoic acid in neural epidermal growth factor-like 1-associated membranous nephropathy: more than a coincidence? Kidney Int. (2022) 101:418–9. doi: 10.1016/j.kint.2021.12.001
33. Miller P, Lei L, Charu V, Higgins J, Troxell M, Kambham N. Clinicopathologic features of non-lupus membranous nephropathy in a pediatric population. Pediatr Nephrol. (2022) 37:3127–37. doi: 10.1007/s00467-022-05503-7
34. Iwakura T, Ema C, Isobe S, Fujikura T, Ohashi N, Kato A, et al. Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: a single-center retrospective study in Japan. Sci Rep. (2022) 12:2967. doi: 10.1038/s41598-022-07037-2
35. Dinesh KP, Charu V, Troxell ML, Andeen NK. NELL1-positive HIV-associated lupus-like membranous nephropathy with spontaneous remission. Glomerular Dis. (2022) 2:184–8. doi: 10.1159/000525541
36. Kurien AA, Prema Ks J, Walker PD, Caza TN. Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. (2022) 102:1424–6. doi: 10.1016/j.kint.2022.09.001
37. Tsuji T, Iwasaki S, Makita K, Furukawa S, Watanabe-Kusunoki K, Takeda-Otera S, et al. Bucillamine use for rheumatoid arthritis and type 2 diabetes mellitus are associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. medRxiv (2022). doi: 10.1101/2022.12.02.2228261.
38. Miyazaki R, Ueda H, Hayashi A, Okabe M, Katsuma A, Shimizu A, et al. Neural epidermal growth factor-like 1-positive membranous nephropathy with rheumatoid arthritis. Kidney Int Rep. (2023) 8:921–4. doi: 10.1016/j.ekir.2022.12.026
39. Takahashi-Kobayashi M, Usui J, Kawanishi K, Saito C, Ebihara I, Yamagata K. Adding cases to the study of Bucillamine-associated neural epidermal growth factor-like1-positive membranous nephropathy. Kidney Int Rep. (2023) 8:1698–9. doi: 10.1016/j.ekir.2023.05.032
41. Zubidat D, Madden B, Kudose S, Nasr SH, Nardelli L, Fervenza FC, et al. Heterogeneity of target antigens in Sarcoidosis-associated membranous nephropathy. Kidney Int Rep. (2023) 8:1213–9. doi: 10.1016/j.ekir.2023.03.019
42. Santoriello D, Ramaswamy R, Kudose S, Markowitz GS. Segmental NELL-1 membranous nephropathy complicating tiopronin therapy. Kidney Int Rep. (2023) 8:1683–6. doi: 10.1016/j.ekir.2023.05.023
43. Shuangshuang Zhu XL, Yue S, Luo B, Song Z, Xu X, Wang L, et al. Segmental membranous glomerulopathy in adults. Kidney Dis (Basel). (2023) 9(6):507–16. doi: 10.1159/000533294
44. Amar Sultan DM, Thakare S, Rojekard A, Jamale T. Mercury-associated neural epidermal growth factor-like 1 protein (NELL-1) positive membranous nephropathy after use of skin lightening creams. Clin Toxicol (Phila). (2023) 61(5):387–91. doi: 10.1080/15563650.2023.2188141
45. Nimkar A, Mehtabdin K, Wang X, Jhaveri KD. Pembrolizumab-associated nerve epidermal growth factor-like 1 membranous nephropathy. Kidney Int Rep. (2023) 8:676–9. doi: 10.1016/j.ekir.2022.12.018
46. Bobart SA, Han H, Tehranian S, De Vriese AS, Roman JCL, Sethi S, et al. Noninvasive diagnosis of PLA2R-associated membranous nephropathy: A validation study. Clin J Am Soc Nephrol. (2021) 16:1833–9. doi: 10.2215/CJN.05480421
47. Ronco P, Plaisier E, Debiec H. The role of PLA2R antibody monitoring: what we know and what we do not know. Nephrol Dial Transpl. (2023) 38:826–33. doi: 10.1093/ndt/gfab356
48. Bobart SA, Tehranian S, Sethi S, Alexander MP, Nasr SH, Moura Marta C, et al. A target antigen-based approach to the classification of membranous nephropathy. Mayo Clin Proc. (2021) 96:577–91. doi: 10.1016/j.mayocp.2020.11.028
49. Charu V, Andeen N, Walavalkar V, Lapasia J, Kim JY, Lin A, et al. Membranous nephropathy in patients with HIV: a report of 11 cases. BMC Nephrol. (2020) 21:401. doi: 10.1186/s12882-020-02042-x
50. Inoue D, Uchida T, Komatsu S, Sugisaki K, Yamada M, Ogawa H, et al. Anti-PLA(2)R antibody development during NELL1-associated membranous glomerulonephritis treatment: A case report. Kidney Med. (2023) 5:100625. doi: 10.1016/j.xkme.2023.100625
51. Seitz-Polski B, Dolla G, Payre C, Girard CA, Polidori J, Zorzi K, et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol. (2016) 27:1517–33. doi: 10.1681/ASN.2014111061
52. Beck LH Jr. The dominant humoral epitope in phospholipase A2 receptor-1: presentation matters when serving up a slice of pi. J Am Soc Nephrol. (2015) 26:237–9. doi: 10.1681/ASN.2014090877
53. Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol. (2015) 26:291–301. doi: 10.1681/ASN.2013121315
54. Fresquet M, Jowitt TA, Gummadova J, Collins R, O'Cualain R, McKenzie EA, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. (2015) 26:302–13. doi: 10.1681/ASN.2014050502
55. Medicine NLo. NELL1 neural EGFL like 1 [ Homo sapiens (human) ] . Available online at: https://www.ncbi.nlm.nih.gov/gene/4745.
56. Desai J, Shannon ME, Johnson MD, Ruff DW, Hughes LA, Kerley MK, et al. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. (2006) 15:1329–41. doi: 10.1093/hmg/ddl053
57. Bokui N, Otani T, Igarashi K, Kaku J, Oda M, Nagaoka T, et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. (2008) 582:365–71. doi: 10.1016/j.febslet.2007.12.006
58. Zhang X, Kuroda S, Carpenter D, Nishimura I, Soo C, Moats R, et al. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. (2002) 110:861–70. doi: 10.1172/JCI200215375
59. Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, et al. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. (1996) 38:273–6. doi: 10.1006/geno.1996.0628
60. Cheng X, Shi J, Jia Z, Ha P, Soo C, Ting K, et al. NELL-1 in genome-wide association studies across human diseases. Am J Pathol. (2022) 192:395–405. doi: 10.1016/j.ajpath.2021.11.006
61. Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. (2018) 23:869–81.e868. doi: 10.1016/j.stem.2018.10.010
62. Lab H. Kidney Interactive Transcriptomics (2021). Available online at: http://humphreyslab.com/SingleCell/.
63. Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun. (2021) 12:2190. doi: 10.1038/s41467-021-22368-w
64. Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, et al. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. (1999) 14:80–9. doi: 10.1359/jbmr.1999.14.1.80
65. Li M, Wang Q, Han Q, Wu J, Zhu H, Fang Y, et al. Novel molecule nell-1 promotes the angiogenic differentiation of dental pulp stem cells. Front Physiol. (2021) 12:703593. doi: 10.3389/fphys.2021.703593
66. Wang L, Li X, Song Y, Zhang L, Ye L, Zhou X, et al. NELL1 augments osteogenesis and inhibits inflammation of human periodontal ligament stem cells induced by BMP9. J Periodontol. (2022) 93:977–87. doi: 10.1002/JPER.20-0517
67. Qin Q, Gomez-Salazar M, Tower RJ, Chang L, Morris CD, McCarthy EF, et al. NELL1 regulates the matrisome to promote osteosarcoma progression. Cancer Res. (2022) 82:2734–47. doi: 10.1158/0008-5472.CAN-22-0732
68. Qi H, Kim JK, Ha P, Chen X, Chen E, Chen Y, et al. Inactivation of nell-1 in chondrocytes significantly impedes appendicular Skeletogenesis. J Bone Miner Res. (2019) 34:533–46. doi: 10.1002/jbmr.3615
69. Karasik D, Hsu YH, Zhou Y, Cupples LA, Kiel DP, Demissie S. Genome-wide pleiotropy of osteoporosis-related phenotypes: the Framingham Study. J Bone Miner Res. (2010) 25:1555–63. doi: 10.1002/jbmr.38
70. Dateki S, Watanabe S, Kinoshita F, Yoshiura KI, Moriuchi H. Identification of 11p14.1-p15.3 deletion probably associated with short stature, relative macrocephaly, and delayed closure of the fontanelles. Am J Med Genet A. (2017) 173:217–20. doi: 10.1002/ajmg.a.37978
71. Salehi B, Berkay Yilmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of alpha-lipoic acid for therapeutic purposes. Biomolecules. (2019) 9. doi: 10.3390/biom9080356
72. Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol. (2009) 54:391–8. doi: 10.1097/FJC.0b013e3181be7554
73. Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. (2011) 34:2054–60. doi: 10.2337/dc11-0503
74. Ishida Y, Ohara T, Okuno Y, Ito T, Hirota Y, Furukawa K, et al. Alpha-lipoic acid and insulin autoimmune syndrome. Diabetes Care. (2007) 30:2240–1. doi: 10.2337/dc07-0689
75. Yamada T, Imai J, Ishigaki Y, Hinokio Y, Oka Y, Katagiri H. Possible relevance of HLA-DRB1*0403 haplotype in insulin autoimmune syndrome induced by alpha-lipoic acid, used as a dietary supplement. Diabetes Care. (2007) 30:e131. doi: 10.2337/dc07-1636
76. Jaffe IA. Adverse effects profile of sulfhydryl compounds in man. Am J Med. (1986) 80:471–6. doi: 10.1016/0002-9343(86)90722-9
77. Ruocco V, De Angelis E, Lombardi ML. Drug-induced pemphigus. II. Pathomechanisms and experimental investigations. Clin Dermatol. (1993) 11:507–13. doi: 10.1016/0738-081X(93)90158-9
78. Fresquet M, Lockhart-Cairns MP, Rhoden SJ, Jowitt TA, Briggs DC, Baldock C, et al. Structure of PLA2R reveals presentation of the dominant membranous nephropathy epitope and an immunogenic patch. Proc Natl Acad Sci U S A. (2022) 119:e2202209119. doi: 10.1073/pnas.2202209119
79. Caza TN, Al-Rabadi LF. What can mercury teach us about membranous nephropathy and minimal change disease? Kidney Int Rep. (2022) 7:1157–60. doi: 10.1016/j.ekir.2022.04.078
80. Schwent M, Klein R, Templeton D. Immunological effects of mercury (IUPAC Technical Report). Pure Appl Chem. (2009) 81:153–67. doi: 10.1351/PAC-REP-08-04-02
81. Miner JH. The glomerular basement membrane. Exp Cell Res. (2012) 318:973–8. doi: 10.1016/j.yexcr.2012.02.031
82. Lauwerys R, Bernard A, Roels H, Buchet JP, Gennart JP, Mahieu P, et al. Anti-laminin antibodies in workers exposed to mercury vapour. Toxicol Lett. (1983) 17:113–6. doi: 10.1016/0378-4274(83)90045-0
83. Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al. A mechanism for cancer-associated membranous nephropathy. N Engl J Med. (2016) 374:1995–6. doi: 10.1056/NEJMc1511702
84. Zhang Z, Gong T, Rennke HG, Hayashi R. Duodenal Schwannoma as a rare association with membranous nephropathy: A case report. Am J Kidney Dis. (2019) 73:278–80. doi: 10.1053/j.ajkd.2018.09.003
85. Miyazaki R UH, Okabe M, Shimizu A, Joh K, Tsuboi N, Yokoo T. Neural epidermal growth factor-like 1 protein (NELL1)-associated membranous nephropathy with heterogeneous underlying diseases: A case report. Res Square. (2003). doi: 10.1007/s13730-023-00826-2
86. The human protein atlas: NELL1 . Available online at: https://www.proteinatlas.org/ENSG00000165973-NELL1/tissue.
87. Stahl PR, Hoxha E, Wiech T, Schroder C, Simon R, Stahl RA. THSD7A expression in human cancer. Genes Chromosomes Cancer. (2017) 56:314–27. doi: 10.1002/gcc.22440
Keywords: NELL1, membranous nephropathy, lipoic acid, NSAID, glomerular disease, drug-induced kidney disease
Citation: Andeen NK, Kung VL and Avasare RS (2024) NELL1 membranous nephropathy: clinical associations provide mechanistic clues. Front. Nephrol. 4:1323432. doi: 10.3389/fneph.2024.1323432
Received: 17 October 2023; Accepted: 04 March 2024;
Published: 26 March 2024.
Edited by:
Duvuru Geetha, Johns Hopkins Medicine, United StatesReviewed by:
David Andrew Fulcher, Australian National University, AustraliaCopyright © 2024 Andeen, Kung and Avasare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole K. Andeen, YW5kZWVuQG9oc3UuZWR1
 Nicole K. Andeen
Nicole K. Andeen Vanderlene L. Kung
Vanderlene L. Kung