- Department of Nephrology, Loma Linda University, Loma Linda, CA, United States
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide. Recently, there have been multiple advances in the understanding of IgAN pathophysiology and therapeutic options. Despite the advent of new treatment options, individual risk stratification of the disease course and choosing the best treatment strategy for the patient remains challenging. A multitude of clinical trials is ongoing, opening multiple opportunities for enrollment. In this brief review we discuss the current approach to the management of IgAN and highlight the ongoing clinical trials.
Introduction
IgA nephropathy (IgAN) was first described in 1968 by Berger and Hinglais (1). It is the most common primary glomerular disease (2) and a leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide (3–5). While being the most prevalent glomerulopathy, it is marked by remarkable geographic and ethnic heterogeneity, with incidence varying from 39–45 cases per million population (p.m.p.) per year in Japan to 10–21 cases p.m.p. per year in the USA, and with 9.9 cases p.m.p. per year in the UK (6). It accounts for approximately 40% of all native biopsies in Japan, 25% in Europe, 12% in the USA, and less than 5% in Central Africa (3). This might be attributed to obscure genetic and environmental factors, along with intensive urine screening and a low threshold for renal biopsies in Asian countries (4).
IgAN treatment has evolved at a rapid rate in the last decade. A number of recently completed and ongoing clinical trials are leading to a shift in the treatment paradigm. We aim to review the traditional treatment strategies, Kidney Disease: Improving Global Outcomes (KDIGO) 2021 guidelines, novel therapies, and ongoing clinical trials in this ever-changing field.
Current treatment strategies
Supportive care
As per the KDIGO 2021 guidelines, supportive care (SC) for IgAN involves rigorous blood pressure control, optimal renin–angiotensin–aldosterone system inhibition (RAAS-i), assessing and addressing cardiovascular risk, and lifestyle changes, including dietary sodium restriction, smoking cessation, weight control, and exercise, as appropriate (5). Other than dietary sodium restriction, no other specific dietary intervention has been shown to affect outcomes in IgAN.
Hypertension and proteinuria are independent risk factors for disease progression in IgAN. In a prospective study of 332 patients with IgAN, the cumulative incidence of dialysis or death was much higher in patients with hypertension at disease diagnosis (defined in the study as > 140/90 mmHg) than in those without hypertension (15% vs. 3% and 41% vs. 6% at 10 and 20 years, respectively) (7). In another study involving 542 patients with IgAN, a higher mean arterial pressure was associated with a higher risk of progressive kidney disease. This effect was observed at all levels of proteinuria (8). There are no specific blood pressure targets to improve progression; however, KDIGO recommends a blood pressure target of < 120/80 mmHg for all glomerular diseases.
In a study involving 1,155 patients, there was a statistically significant improvement in 10-year kidney survival in patients with proteinuria < 1 gm/day compared with patients with proteinuria >1 gm/day, with 10-year dialysis-free survival of 94% (95% CI 90% to 98%) and 20-year dialysis-free survival of 89% (95% CI 82% to 96%). Patients with proteinuria > 1.0 gm/day were associated with a 9.4-fold risk compared with patients with proteinuria < 1.0 gm/day (p < 0.001) and a 46.5-fold risk compared with patients with proteinuria < 0.5 gm/day (p < 0.001). Moreover, patients who achieved proteinuria < 0.5 gm/day benefit much more than those with proteinuria between 0.5 and 1.0 gm/day [hazard ratio (HR) 13.1, p < 0.001] (9). Similar results were seen in an individual participant-level meta-analysis of data for 830 patients from 11 randomized controlled trials (RCTs), which showed that a reduction in proteinuria (independent of the presence or absence of hypertension) was associated with a lower risk for doubling of serum creatinine level, ESRD, or death in patients with IgAN (10).
Retrospective data from large registries have revealed that patients with IgAN treated with an angiotensin-converting enzyme inhibitor (ACEi) to control blood pressure have a lower rate of annual loss of kidney function than similar patients not treated with an ACEi or an angiotensin II receptor blocker (ARB) (10). An RCT of 109 Asian patients with IgAN showed greater proteinuria reduction and slowing of the rate of kidney deterioration with valsartan compared with placebo (11). An RCT of 44 patients with IgAN comparing enalapril with other antihypertensives (nifedipine, amlodipine, atenolol, diuretics, and doxazosin) demonstrated better kidney survival and a reduction in proteinuria (12). However, there is a lack of studies evaluating the efficacy of renin-angiotensin-aldosterone system (RAAS) blockade in IgAN with moderately increased albuminuria (30–300 mg/d) and normal blood pressure.
Therefore, KDIGO guidelines recommend that all patients with proteinuria > 0.5 gm/day, irrespective of whether or not they have hypertension, be treated with either an ACEi or an ARB as a category 1B recommendation (1: we recommend, B: moderate evidence meaning the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different). Maximally tolerated doses of RAAS inhibitors should be used. No benefit of dual blockade has been demonstrated (13). The treatment goal is proteinuria reduction to < 1 gm/day.
Sodium-glucose cotransporter-2 inhibitors
Sodium–glucose cotransporter-2 inhibitors (SGLT2 inhibitors) have shown promising results in patients with CKD, including the DIAMOND, DAPA-CKD, and EMPA-KIDNEY trials (14). A prespecified analysis of the DAPA-CKD trial in 270 patients with IgAN (with 254 biopsy-confirmed cases) showed that adding SGLT2 inhibitors to angiotensin-converting enzyme (ACE)/ARB therapy substantially reduced the risk of CKD progression. The primary outcome [composite of sustained decline in estimated glomerular filtration rate (eGFR) of > 50%, end-stage kidney disease, or death from a kidney disease-related or cardiovascular cause) occurred in 4% of participants on dapagliflozin and 15% of participants on placebo (HR 0.29; 95% CI 0.12 to 0.73). Mean rates of eGFR decline with dapagliflozin and placebo were –3.5 and –4.7 mL/min/1.73 m2/year, respectively. Dapagliflozin reduced the urinary albumin-to-creatinine ratio by 26% relative to placebo. Adverse events leading to study drug discontinuation were similar with dapagliflozin and placebo (15). The EMPA-KIDNEY trial had 1,669 patients with glomerular disease, and thus a stratified analysis based on the etiology of glomerular disease will be highly anticipated.
KDIGO 2021 guidelines do not recommend SGLT2 inhibitors because the guidelines predate the above trials. With the above results, SGLT2 inhibitors are likely to become the standard of care in IgAN worldwide; however, with the caveat that the trials (previous and ongoing) do not universally include combination of RAAS-i and sodium-glucose cotransporter-2 (SGLT2) inhibition.
Sparsentan
Sparsentan is an oral dual endothelin and angiotensin II receptor antagonist that was recently approved by the Food and Drug Administration (FDA) for high-risk IgAN patients. The Phase III PROTECT trial compared sparsentan 400 mg total with irbesartan 300 mg total in 404 patients with proteinuria ≥ 1 gm/day after a 3-month run-in period (16). The urine protein-to-creatinine ratio (UPCR) decreased by 49.8% (mean percentage decrease from baseline) in the sparsentan group compared with 15.1% in the irbesartan group at 36 weeks (between-group relative reduction of 41%, p < 0·0001). Adverse events were similar in both groups, with no cases of hepatotoxicity, heart failure, or edema-related discontinuations. Liver aminotransferases and total bilirubin should be checked prior to initiation of treatment and alanine transaminase and aspartate transaminase should be monitored monthly for 12 months followed by every 3 months during treatment. Treatment should be held if aminotransferases increase to three times the upper limit of normal. Since animal data have shown major birth defects associated with sparsentan use in pregnancy, pregnancy testing is required before, during, and after treatment, and women of child-bearing age must use effective contraception prior to initiation of treatment, during treatment, and for 1 month after treatment.
Hydroxychloroquine
Hydroxychloroquine is recommended only for Chinese patients who remain at high risk of disease progression despite optimized SC. An RCT in China showed a 48% reduction in proteinuria, compared with a 10% reduction with a placebo, after 6 months of use in patients with 0.75–3.5 gm/day proteinuria, despite optimized RAAS-inhibition (17).
Immunosuppression
Glucocorticoids
Patients with persistent proteinuria > 1 gm/day despite optimized SC (defined as treatment with a maximal tolerated or allowed daily dose of RAAS blockade and good blood pressure control) for a minimum of 3–6 months are at high risk of progressive CKD and need to be evaluated for further treatment options. The MEST-C Oxford classification (18) and International IgA Nephropathy Prediction Tool (IIgAN-PT) quantify the risk of progression to ESRD; however, there is no uniform threshold above which a patient is considered a candidate for immunosuppressive therapy.
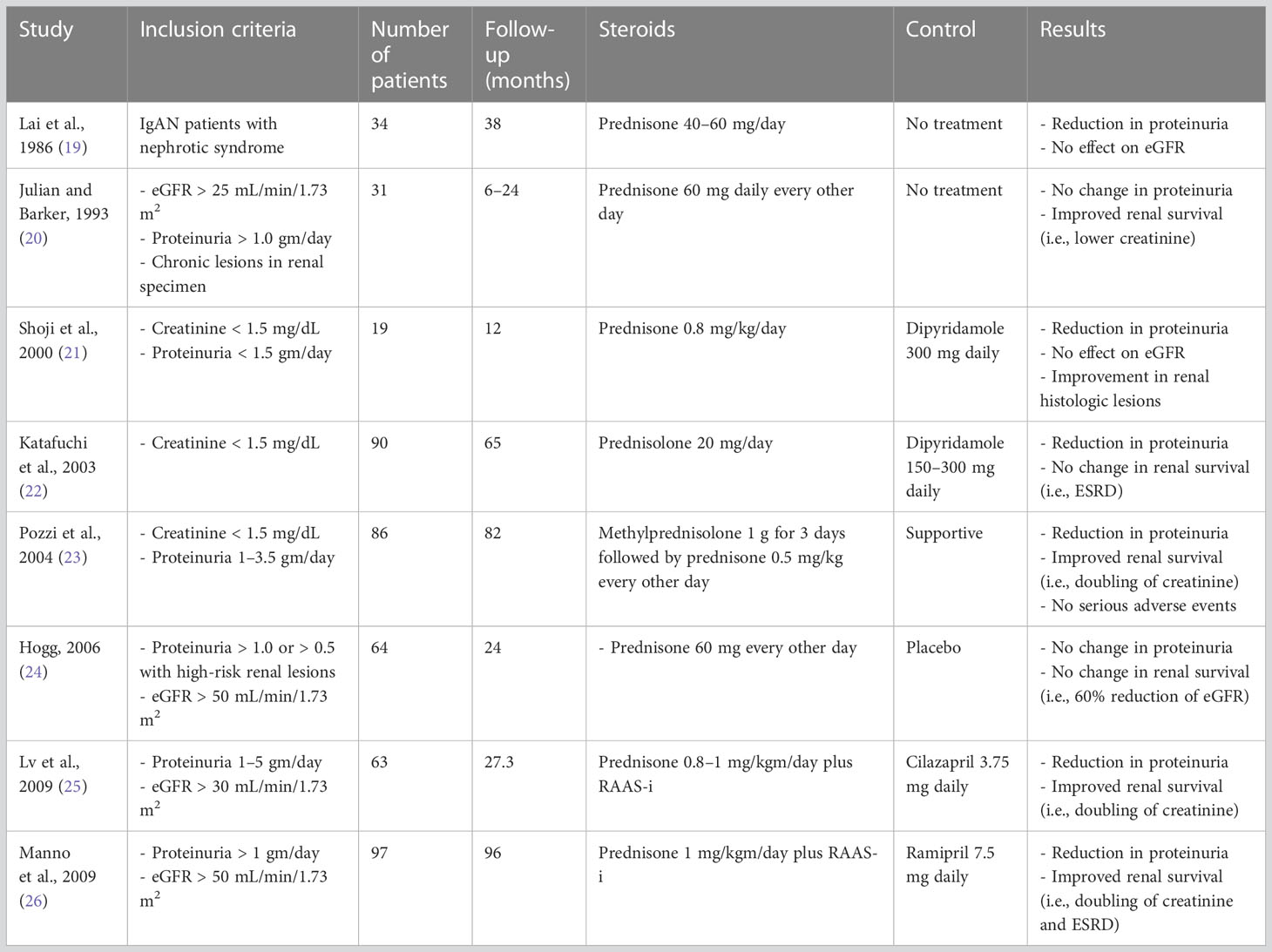
Multiple RCTs have evaluated the effect of glucocorticoids in IgAN (Table 1) with varying results. During the earlier studies, RAAS blockade was not the standard of care, and therefore some of the trial results cannot be extrapolated to the current IgAN population where RAAS-i is universal.
The two latest RCTs evaluating glucocorticoids in IgAN are the STOP-IgA and TESTING trials. The STOP-IgA trial had a 6-month run-in period followed by intravenous methylprednisolone 1 gm/day for 3 days at the start of months 1, 3, and 5, and oral prednisolone 0.5 mg/kg/48 h on the other days in patients with eGFR > 60 mL/min/1.73 m2; and used oral prednisone 40 mgm/day (tapered to 10 mgm/day over the first 3 months, 10 mgm/day during months 4–6, and 7.5 mgm/day during months 7–36), along with cyclophosphamide 1.5 mg/kgm/day for 3 months, followed by azathioprine 1.5 mg/kgm/day during months 4–36, in patients with eGFR 30–59 mL/min/1.73 m2. After a median follow-up of 7.4 years, steroids did not alter long-term outcomes; 45% of the steroid group reached the end point of 40% eGFR loss, ESRD, or death compared with 50% of the immunosuppression group (HR 1.2, 95% CI 0.75 to 1.92). ESRD developed in 23.6% of patients in the supportive group and 25.9% of patients in the immunosuppression group (27).
In the STOP-IgA trial, mean eGFR was 59 mL/min/1.73 m2and mean proteinuria was 1.7 gm/day at the start of the trial (2.2 gm/day at the initiation of run-in phase). The mean annual eGFR loss was 2.5 mL/min/1.73 m2/year compared with 5–6 mL/min/1.73 m2/year in previous RCTs, which could be attributed to intensive SC for 6 months prior to randomization and during the trial. The slow decline in eGFR also indicates that STOP-IgA patients were at low risk of progressive kidney disease. A retrospective analysis of MEST-C histologic grading in 47% of patients (histologic grading was available for 70/149 patients only) revealed that both the primary end point and ESRD were more likely in patients with M1, E1, S1, and T1/2 than in patients with zero scores in the respective MEST-C categories. However, none of the MEST-C categories was significantly associated with annual eGFR loss. According to the authors, due to statistical and analytical limitations, the study was unable to define/identify subgroups based on kidney biopsy histology grading, baseline eGFR, or proteinuria level, which might have benefited from immunosuppression (28).
The original TESTING trial compared placebo with oral methylprednisolone (0.6–0.8 mg/kgm/day for 2 months, followed by a taper of 8 mg/month for 6–8 months). The study had to be halted due to serious adverse events, including two treatment-related deaths; however, it did show clinical benefit, leading to TESTING 2.0. TESTING 2.0 used a lower dose of glucocorticoids (oral methylprednisolone 0.4 mg/kg daily) along with Pneumocystis jirovecii prophylaxis. The mean baseline eGFR was 61.5 mL/min/1.73m2 and mean proteinuria was 2.4 gm/day. Median time since kidney biopsy was 5 months (range 4–27 months). The primary composite outcome (i.e., 40% eGFR reduction, kidney failure, or death due to kidney disease) occurred less frequently in the methylprednisolone group than in the placebo group [74 (28.8%) vs. 106 (43.1%), respectively, HR 0.53 (95% CI 0.39 to 0.72), p < 0.001] over a mean follow-up of 4.2 years. A beneficial effect was observed in both the high- and reduced-dose groups when analyzed separately. Serious adverse events (hospitalization due to serious infection, gastrointestinal bleeding, clinically evident fractures, osteonecrosis, and new-onset diabetes mellitus) were significantly higher in the methylprednisolone group (37 vs. 8 total events, 10.9% vs. 2.8% subjects), including four fatalities (three in the high-dose group, one in the reduced-dose group). Among serious adverse events, 30 occurred in the high-dose group, seven in the reduced-dose group, and eight in the placebo group. Twelve patients in the high-dose group had severe infections requiring hospitalization compared with five patients in the reduced-dose group and three patients in the placebo group. All four cases of Pneumocystis pneumonia occurred in the high-dose steroid group. The reduced-dose group had no gastrointestinal bleeding, fractures, or osteonecrosis reported (29, 30).
The higher proteinuria in TESTING participants compared with STOP-IgA participants (2.4 vs. 1.7 gm/day), higher annual eGFR loss (4.97 vs. 2.68 mL/min/1.73 m2/year), and higher MEST-C scores (M1 lesions: 59.8% vs. 25.7%, E1 lesions: 25.2% vs. 17.1%) indicate a greater benefit of steroids in patients who are at high risk of disease progression.
It is important to note that in most clinical trials there is an interval time period between retrieving the kidney biopsy and clinical trial enrollment. Two specific elements related to this approach need to be considered. First, only a diagnosis of biopsy-proven IgAN was needed, without consideration of the amount of activity versus chronicity in the biopsy specimen. Second, the longer the time interval between biopsy and treatment initiation, the higher the likelihood of progression to chronic disease, which may not be as responsive to immunosuppressive therapy. Also, most of the trials require a RAAS-i/run-in phase of at least 3 months (consistent with KDIGO guidelines) prior to randomization, allowing time for possible progression to chronic histologic disease (e.g., in STOP-IgA, mean time from biopsy to trial enrollment was 5 months along with a 6-month run-in period). Allowing a longer interval period (between biopsy and treatment initiation) and mixing acute and chronic pathologic changes may bias trial results to the null, even if potentially beneficial for patients with recently diagnosed highly active IgAN.
There are a few ongoing trials that aim to minimize the interval between biopsy and treatment initiation along with stratifying patients into groups according to renal histology findings (active vs. chronic lesions). For example, the TIGER trial (NCT03188887), for which the inclusion criteria included renal biopsy within 45 days of inclusion visit, compared steroids plus RAAS-i or SGLT2 inhibitors with RAAS-i or SGLT2 inhibitors along with repeat biopsy at 12 and 24 months to compare the evolution of histologic lesions. The CLIgAN trial (NCT04662723) is composed of two RCTs; the first RCT will compare RAAS-i plus steroids with RAAS-i only in patients with active renal lesions and the second RCT will compare RAAS-i plus SGLT2 inhibitors with RAAS-i alone in patients with chronic renal lesions. Another trial is investigating the relationship between MEST-C classification and clinical remission rates after treatment (NCT05528991). The results of these trials will be highly anticipated and will help the physician in choosing the optimal candidate for steroid therapy.
The KDIGO 2021 guidelines state that patients with persistent proteinuria > 1gm/day despite optimized SC for 3–6 months should be offered clinical trial enrollment as the first step and a 6-month course of glucocorticoid therapy as the next step; however, with the caveat that the clinical benefit of glucocorticoids in IgAN is not established, and glucocorticoid therapy should be given with extreme caution or avoided entirely in patients with an eGFR of < 30 mL/min/1.73 m2, diabetes, obesity (BMI > 30 kg/m2), active peptic ulcer disease, latent infections (e.g., viral hepatitis, TB), secondary disease (e.g., cirrhosis), severe osteoporosis, and/or uncontrolled psychiatric illness (5). The KDIGO guidelines emphasize discussing the risk of steroid-related adverse effects with patients (serious infections leading to hospitalization, gastrointestinal bleeding, new-onset impaired glucose tolerance/diabetes, weight gain, osteonecrosis, and fractures), particularly those with an eGFR of < 50 mL/min/1.73 m2 (category 2B recommendation: 2: We suggest (rather than recommend), B: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.), and encourage “incorporating prophylaxis against Pneumocystis pneumonia along with gastroprotection and bone protection when using glucocorticoids (prednisone equivalent of 0.5 mg/kg/day)”. It also states that “there is no data to support efficacy or reduced toxicity of alternate-day glucocorticoid regimens, or dose-reduced protocols”. It is highly imperative to note that the 2021 KDIGO guidelines were published before TESTING 2.0 was completed and thus do not take the reduced-dose protocol results into consideration. Therefore, low-dose steroids can be considered as one of the options prior to clinical trial enrollment. Future trials evaluating the efficacy and safety of combination of steroids and SGLT2 inhibitors would be of value, since SGLT2 inhibitors are becoming the standard of care and pose an increased risk of ketoacidosis and genitourinary infections.
Mycophenolate mofetil
The use of mycophenolate mofetil (MMF) is currently recommended only in Chinese patients as a steroid-sparing agent, as per KDIGO guidelines. A randomized clinical trial conducted in China showed non-inferiority of MMF plus low-dose steroids compared with standard-dose steroids, along with significantly fewer side effects in the combination group (31). A Chinese trial comparing MMF with steroids showed greater reduction in proteinuria in the MMF group (32). However, two other RCTs conducted in Belgium and the USA showed no beneficial effects (33, 34). A longer trial in Hong Kong (40 Chinese patients, 6 years’ follow-up), which compared MMF for 6 months with SC, demonstrated a slower annual decline in eGFR, 1.12 mL/min/1.73 m2/year in the MMF group compared with 3.81 mL/min/1.73 m2/year in the SC group (p = 0.012) (35). A reduction in proteinuria in the MMF group was also observed, but only during the first 24 months.
The MAIN trial compared MMF (1.5 gm/day for the first 12 months followed by a 0.75–1.0 g maintenance dose) with SC (maximally tolerated losartan and low salt diet) in 170 Chinese adults with proteinuria > 0.75 gm/day and a eGFR between 30 and 60 mL/min/1.73 m2 after a 3-month run-in phase (36). Primary outcomes were a composite of doubling of creatinine, ESRD, death due to kidney or cardiovascular cause, and progression of CKD. The primary composite outcome was observed in six (7.1%) patients in the MMF group and 18 (21.2%) patients in the SC group [p = 0.008, HR 0.23 (95% CI 0.09 to 0.63)]. Progression of CKD occurred in seven (8.2%) patients in the MMF group and 23 (27.1%) patients in the SC group [p = 0.001, HR 0.23 (95% CI 0.10 to 0.57)]. The MMF group also had a higher reduction in proteinuria than the SC group (57.1% vs. 28.2%, p < 0.001). During the post-trial follow-up, mean annual eGFR loss was 7.1 mL/min/1.73 m2 in the SC group and 6.1 mL/min/1.73 m2 in patients who discontinued MMF after the trial compared with 4.1 mL/min/1.73 m2 in patients who continued MMF during the post-trial period. Serious adverse events occurred more frequently in the MMF group; however, they were not statistically different (p = 0.37). Infections, especially pneumonia, and elevated liver enzymes were more common in the MMF group, but also not statistically significant (p = 0.37 and p = 0.21, respectively). Gastrointestinal side effects were the only adverse effects that were statistically different between the two groups (p = 0.001).
Both the Hong Kong trial and MAIN trial showed that discontinuation of MMF compared with continuing maintenance MMF resulted in accelerated eGFR decline, underscoring the importance of continuation of therapy. The Hong Kong trial did not include patients with advanced glomerulosclerosis, tubular atrophy, or interstitial fibrosis; however, in the MAIN trial, 83.5% of patients had MEST-C score of S1 and 54.1% had a score of T2, showing benefit in patients with advanced histologic disease as well. Using low-dose MMF (1.5 gm/day for the first 12 months followed by a 0.75–1.0 g maintenance dose vs. 2 gm/day or 3 gm/day) resulted in lower infectious complications (16.5% vs. 54% and 79%, respectively) (37, 38). The role of MMF as an immunosuppressant in patients with contraindications to steroids or as a low-dose maintenance therapy needs to be explored further in non-Chinese patients.
Targeted-release budesonide
Targeted-release formulation of budesonide (TRF-budesonide) is an oral formulation of budesonide that is designed to deploy and release at the terminal ileum, thus delivering the medication locally to Peyer’s patches (gut-associated lymphoid tissue), which is a primary site for IgA production. The local delivery system minimizes systemic exposure with a potential for reduced systemic side effects of steroids (39). The FDA approved the oral, delayed-release, targeted formulation of budesonide designed for IgAN in December 2021 because of its efficacy in reducing proteinuria (long-term effects on eGFR were still to be determined since Nefigard Part B was ongoing at that time).
The Phase II Nefigan trial evaluated budesonide 8 mg or 16 mg compared with placebo for 9 months. Use of budesonide for 9 months was associated with a 24.4% decrease from baseline in mean UPCR (change in UPCR vs. placebo 0.74; 95% CI 0.59 to 0.94; p = 0.0066). At 9 months, mean UPCR had decreased by 27% in patients who received 16 mgm/day (0.71; 95% CI 0.53 to 0.94; p = 0.0092) and 21.5% in patients who received 8 mgm/day (0.76; 95% CI 0.58 to 1.01; p = 0.0290), whereas patients who received placebo had an increase in mean UPCR of 2.7%. Incidence of adverse events was similar in all groups (88% in the TRF-budesonide 16 mgm/day group, 94% in the TRF-budesonide 8 mg/day, and 84% in placebo group), with nasopharyngitis being the most common adverse event (40). The promising results led to the Phase III Nefigard trial.
Similar to its predecessor, Part A of the Nefigard trial showed that patients receiving optimized RAAS-i along with TRF-budesonide (Nefecon 16 mg) for 9 months had a 48% reduction in UPCR compared with placebo (p < 0.0001) at 12 months. After 9 months of treatment, eGFR in the Nefecon group decreased from baseline by 0.17 mL/min/1.73 m2 compared with 4.04 mL/min/1.73 m2 in the placebo group. The difference was maintained at 12 months with a 3.37 mL/min/1.73 m2 per year (p = 0.01) improvement in eGFR (41). Unlike the reduction in proteinuria, the greatest benefit in eGFR was seen in the subgroup with baseline UPCR ≥ 1.5 g/g, and there was no benefit in patients with baseline UPCR < 1.5 g/g.
Results of Part B of the Nefigard trial were recently published with findings consistent with Part A. The primary end point, eGFR over 2 years, was on average 5.05 mL/min/1.73 m2 higher with Nefecon than with placebo (p < 0.0001). Mean change in eGFR over the 2-year period was -2.47 mL/min/1.73 m2 for Nefecon 16 mg compared with -7.52 mL/min/1.73 m2 for placebo (percentage change of –11% in Nefecon group vs. –21.5% in placebo group). The percentage change in UPCR at 24 months was –30.7% in the Nefecon group compared with –1.0% in the placebo group. Treatment benefit on eGFR was observed across baseline UPCR subgroups. The drug was well tolerated, with the most common adverse events being peripheral edema, hypertension, muscle spasms, and acne. Less than 10% of patients discontinued the drug due to adverse events. The study concluded that a prolonged positive effect on proteinuria and eGFR was observed, even after 15 months of drug discontinuation, indicating a disease-modifying capability.
Other immunosuppressants
As per KDIGO guidelines, there is no documented evidence of efficacy for azathioprine, cyclophosphamide (unless rapidly progressive IgAN), calcineurin inhibitors, or rituximab (5). In the Stop-IgA trial, the arm with an eGFR of 30–59 mL/min/1.73 m2 did receive oral cyclophosphamide along with oral steroids for 3 months, but, as mentioned above, it did not have any effect on long-term outcomes (27).
Fish oil
There is conflicting evidence about the use of fish oil in IgAN. As per KDIGO guidelines, patients who wish to take fish oil should be advised of the dose and formulation used in the published clinical trials that reported efficacy. A multi-center randomized clinical trial comparing fish oil (12 g daily) with a similar dose of olive oil in patients with IgAN who had persistent proteinuria showed that, after a mean follow-up of 6.4 years, 17 patients in the fish oil group and 29 in the placebo group reached the primary end point (50% reduction in serum creatinine; p = 0.002), and eight patients in the fish oil group and 19 in the placebo group developed ESRD (p = 0.009) (42, 43). It concluded that early and prolonged treatment with fish oil slows disease progression for high-risk patients with IgAN. Another RCT tested a 6-month course of polyunsaturated fatty acids (PUFA) (3 gm/day) in a group of 30 patients with biopsy-proven IgAN and proteinuria already treated with RAAS-i compared with continuing standard therapy. At the end of the 6-month trial, the reduction in proteinuria was 72.9% in the PUFA group and 11.3% in the RAAS group (p < 0.001). A reduction of ≥ 50% of baseline proteinuria was achieved in 80.0% of PUFA patients and in 20.0% of RAAS-i patients (p = 0.002). Low- and high-dose omega-3 fatty acids (eicosapentaenoic acid (EPA) 1.88 g and docosahexaenoic acid (DHA) 1.47 g vs. EPA 3.76 g and DHA 2.94 g) have been shown to be similar in slowing the rate of renal function loss (44). Doses should be 3.3 gm/day or more of prescription-strength omega-3 fatty acids, and not over-the-counter supplements.
Tonsillectomy
As per KDIGO guidelines, tonsillectomy should not be performed as a treatment for IgAN in Caucasian patients because there is paucity of data in this population. The data that are available do not support the efficacy of tonsillectomy in Caucasian and Chinese patients. However, in Japan, it is routinely performed because multiple studies have reported improved kidney survival and partial or complete remission of hematuria and proteinuria following tonsillectomy alone or with pulsed glucocorticoids (45–47).
IgA nephropathy and pregnancy planning
All women of childbearing potential should be offered preconception counseling when appropriate. Preconception counseling should include a discussion on cessation of RAAS inhibitors and blood pressure control with alternative antihypertensives preconception. In those women at high risk of progressive CKD despite maximal supportive care, a trial of immunosuppression to reduce proteinuria prior to conception may be preferable to emergent initiation of immunosuppression during pregnancy.
Novel treatments and ongoing trials
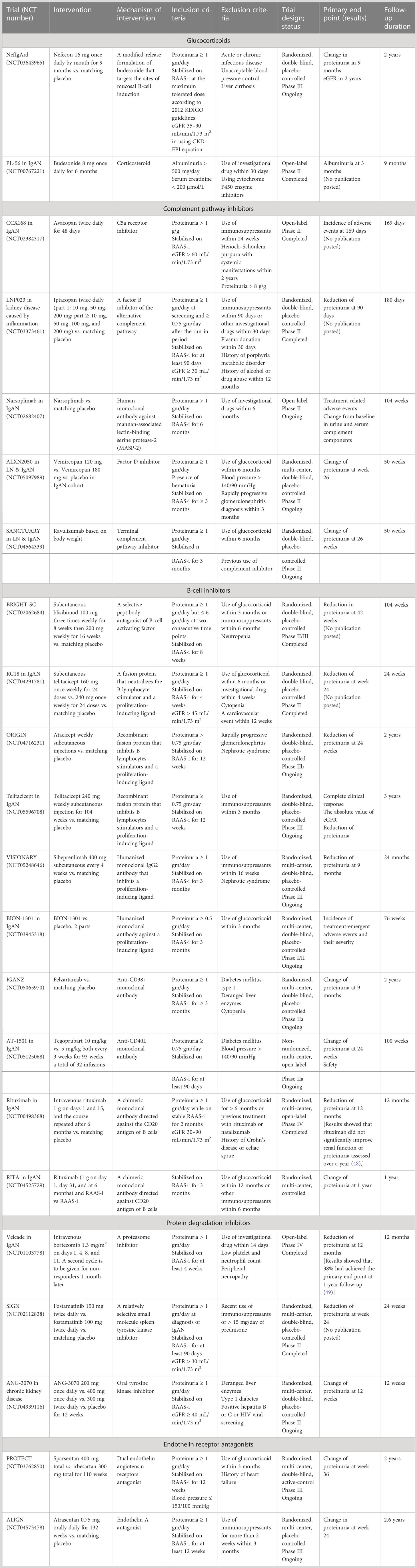
There are several novel treatments that have been discovered and are currently being studied for IgAN. A search for ongoing clinical trials in IgAN was conducted via ClinicalTrials.gov until March 2023 and has been summarized in Table 2.
Discussion
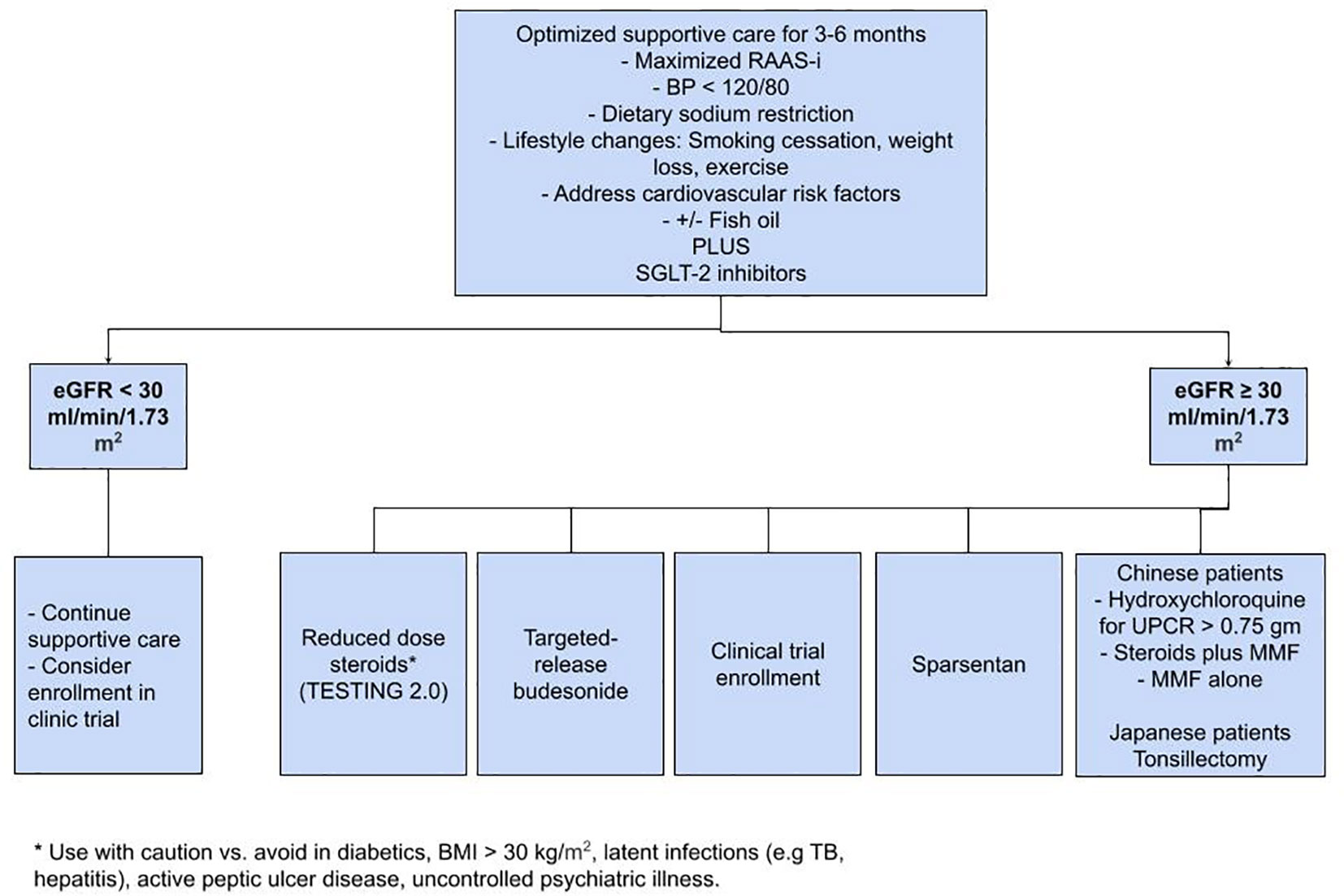
IgAN continues to be the most prevalent glomerular disease worldwide. As a first step, SC, including RAAS-i and SGLT2 inhibitors, plays a paramount role in slowing the progression of the disease. For patients at high risk of progression, low-dose steroids, targeted budesonide, and sparsentan appear promising; however, it is extremely important to discuss appropriate clinical trials and possible enrollment with the patient as well (Figure 1). The results of the trials mentioned in Table 2 could significantly alter the future of IgAN’s treatment paradigm. Commencing early supportive care, evaluating the need for immunosuppression, and timely referral to clinical trials has the potential to change the course of disease in patients with IgAN.
Author contributions
SMN wrote the review article. FA created the clinical trials table. SN wrote the abstract. SN, RM, ZZ, and AA reviewed the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE, Angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FDA, Food and Drug Administration; HR, hazard ratio; KDIGO, Kidney Disease: Improving Global Outcomes; IgAN, IgA nephropathy; MMF, mycophenolate mofetil; RAAS, renin–angiotensin–aldosterone system; RAAS-i, renin–angiotensin–aldosterone system inhibition; RCT, randomized controlled trial; SC, supportive care; SGLT2, sodium–glucose cotransporter-2; SGLT2 inhibitors, sodium–glucose cotransporter-2 inhibitor; TRF-budesonide, targeted-release formulation of budesonide; UPCR, urine protein-to-creatinine ratio.
References
1. Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) (1968) 74(9):694–5.
2. McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant (2011) 26:414–30. doi: 10.1093/ndt/gfq665
3. Woo KT, Chan CM, Chin YM, Choong HL, Tan HK, Foo M, et al. Global evolutionary trend of the prevalence of primary glomerulonephritis over the past three decades. Nephron Clin Pract (2010) 116:c337–46. doi: 10.1159/000319594
4. McQuarrie EP, Mackinnon B, Young B, Yeoman L, Stewart G, Fleming S, et al. Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant (2009) 24:1524–8. doi: 10.1093/ndt/gfn677
5. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int (2021) 100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021
6. Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol (2018) 38(5):435–42. doi: 10.1016/j.semnephrol.2018.05.013
7. Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol (2011) 22:752. doi: 10.1681/ASN.2010040355
8. Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol (2007) 18:3177. doi: 10.1681/ASN.2007050526
9. Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant (2012) 27:1479–85. doi: 10.1093/ndt/gfr527
10. Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, et al. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual patient meta-analysis. Am J Kidney Dis (2016) 68:392–401. doi: 10.1053/j.ajkd.2016.02.042
11. Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, et al. Hong Kong Study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis (2006) 47:751–60. doi: 10.1053/j.ajkd.2006.01.017
12. Praga M, Gutierrez E, Gonzalez E, Morales E, Hernández E. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol (2003) 14:1578–83. doi: 10.1097/01.ASN.0000068460.37369.DC
13. Lennartz DP, Seikrit C, Wied S, Fitzner C, Eitner F, Hilgers RD, et al. Single versus dual blockade of the renin-angiotensin system in patients with IgA nephropathy. J Nephrol (2020) 33:1231–9. doi: 10.1007/s40620-020-00836-8
14. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. For the DAPA-CKD trial committees and investigators. dapagliflozin in patients with chronic kidney disease. N Engl J Med (2020) 383:1436–46. doi: 10.1056/NEJMoa2024816
15. Wheeler DC, Toto RD. DAPA-CKD trial committees and investigators. a pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int (2021) 100(1):215–24. doi: 10.1016/j.kint.2021.03.033
16. Heerspink HJL, Radhakrishnan J, Perkovic V, PROTECT Investigators. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomized, double-blind, active-controlled clinical trial. Lancet (2023) 401(10388):1584–94. doi: 10.1016/S0140-6736(23)00569-X
17. Liu LJ, Yang YZ, Shi SF, Bao YF, Yang C, Zhu SN, et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis (2019) 74:15–22. doi: 10.1053/j.ajkd.2019.01.026
18. Markowitz G. Glomerular disease: updated Oxford classification of IgA nephropathy: a new MEST-c score. Nat Rev Nephrol (2017) 13(7):385–6. doi: 10.1038/nrneph.2017.67
19. Lai KN, Lai FM, Chan KW. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long-term controlled trial. Clin Nephrol (1986) 26(4):174–80.
20. Julian BA, Barker C. Alternate-day prednisone therapy in IgA nephropathy. preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol (1993) 104:198–206. doi: 10.1159/000422413
21. Shoji T, Nakanishi I, Tsubakihara Y. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis (2000) 35:194–201. doi: 10.1016/S0272-6386(00)70326-X
22. Katafuchi R, Ikeda K, Fujimi S. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis (2003) 41:972–83. doi: 10.1016/S0272-6386(03)00194-X
23. Pozzi C, Andrulli S, Locatelli F. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol (2004) 15:157–63. doi: 10.1097/01.ASN.0000103869.08096.4F
24. Hogg RJ, Lee J, Holub BJ, Southwest Pediatric Nephrology Study Group. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the southwest pediatric nephrology study group. Clin J Am Soc Nephrol (2006) 1:467–74. doi: 10.2215/CJN.01020905
25. Lv J, Zhang H, Wang H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis (2009) 53:26–32. doi: 10.1053/j.ajkd.2008.07.029
26. Manno C, Torres DD, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant (2009) 24:3694–701. doi: 10.1093/ndt/gfp356
27. Rauen T, Eitner F, Floege J, STOP-IgAN Investigators. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med (2015) 373(23):2225–36. doi: 10.1056/NEJMoa1415463
28. Rauen T, Wied S, Fitzner C, Eitner F, Sommerer C, Zeier M, et al. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int (2020) 98:1044–52. doi: 10.1016/j.kint.2020.04.046
29. Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA (2017) 318:432–42. doi: 10.1001/jama.2017.9362
30. Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA (2022) 327(19):1888–98. doi: 10.1001/jama.2022.5368
31. Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D, et al. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis (2017) 69:788–95. doi: 10.1053/j.ajkd.2016.11.027
32. Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control trial of mycophenolate mofetil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi (2002) 82(12):796–801.
33. Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int (2004) 65(5):1842–9. doi: 10.1111/j.1523-1755.2004.00588.x
34. Frisch G, Lin J, Appel G, Markowitz G, D'Agati V, Radhakrishnan J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dialysis Transplant (2005) 20(10):2139–45. doi: 10.1093/ndt/gfh974
35. Tang SC, Tang AW, Lai KN, Leung JC, Ho YW, Lai KN. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int (2010) 77(6):543–9. doi: 10.1038/ki.2009.499
36. Hou FF, Xie D, Wang J, Xu X, Yang X, Ai J, et al. Effectiveness of mycophenolate mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Network Open (2023) 6(2):e2254054. doi: 10.1001/jamanetworkopen.2022.54054
37. Dooley EMA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med (2011) 365(20):1886–95. doi: 10.1056/NEJMoa1014460
38. Werth VP, Joly P, Mimouni D, Maverakis E, Caux F, Lehane P, et al. Rituximab versus mycophenolate mofetil in patients with pemphigus vulgaris. N Engl J Med (2021) 384(24):2295–305. doi: 10.1056/NEJMoa2028564
39. Liao J, Zhou Y, Zhao S, Huang K, Chen P, Wu Y, et al. Current knowledge of targeted-release budesonide in immunoglobulin a nephropathy: a comprehensive review. Front Immunol (2023) 13:926517. doi: 10.3389/fimmu.2022.926517
40. Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomized, placebo-controlled phase 2b trial. Lancet (2017) 389:2117–27. doi: 10.1016/S0140-6736(17)30550-0
41. Barratt J, Lafayette R, Rovin B, Stone A, Cattran D, Floege J, et al. Results from part a of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin a nephropathy. Kidney Int (2023) 103(2):391–402. doi: 10.1016/j.kint.2022.09.017
42. Donadio JV Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo nephrology collaborative group. N Engl J Med (1994) 331:1194. doi: 10.1056/NEJM199411033311804
43. Donadio JV Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo nephrology collaborative group. J Am Soc Nephrol (1999) 10:1772. doi: 10.1681/ASN.V1081772
44. Donadio JV Jr, Larson TS, Grande JP. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol (2001) 12:791. doi: 10.1681/ASN.V124791
45. Hotta O, Taguma Y, Kurosawa K, Sudo K, Suzuki K, Horigome I. Early intensive therapy for clinical remission of active IgA nephropathy: a three-year follow-up study. Nihon Jinzo Gakkai Shi (1993) 35(8):967–73.
46. Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin a nephropathy. Nephrol Dial Transplant (2014) 29:1546–53. doi: 10.1093/ndt/gfu020
47. Kawasaki Y, Takano K, Suyama K, Isome M, Suzuki H, Sakuma H, et al. Efficacy of tonsillectomy pulse therapy versus multiple-drug therapy for IgA nephropathy. Pediatr Nephrol (2006) 21:1701–6. doi: 10.1007/s00467-006-0272-6
48. Lafayette RA, Canetta PA, Fervenza FC, Appel GB, Novak J, Nath KA, et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol (2017) 28(4):1306–3. doi: 10.1681/ASN.2016060640
Keywords: IgA nephropathy, proteinuria, SGLT2 inhibitors, glucocorticoids, budesonide, sparsentan
Citation: Noor SM, Abuazzam F, Mathew R, Zhang Z, Abdipour A and Norouzi S (2023) IgA nephropathy: a review of existing and emerging therapies. Front. Nephrol. 3:1175088. doi: 10.3389/fneph.2023.1175088
Received: 27 February 2023; Accepted: 03 May 2023;
Published: 23 May 2023.
Edited by:
Savino Sciascia, University of Turin, ItalyReviewed by:
Darren Lee, Monash University, AustraliaFrancesco Paolo Schena, University of Bari Aldo Moro, Italy
Copyright © 2023 Noor, Abuazzam, Mathew, Zhang, Abdipour and Norouzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sahibzadi Mahrukh Noor, snoor@llu.edu; Sayna Norouzi, snorouzi@llu.edu
 Sahibzadi Mahrukh Noor
Sahibzadi Mahrukh Noor