- 1Division of Nephrology, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan
- 2Institute of Biomedical Sciences, Department of Medicine, MacKay Medical College, New Taipei, Taiwan
- 3Institute of Biomedical Sciences, Division of Cardiology, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan
- 5Mackay Medicine, Nursing and Management College, Taipei, Taiwan
- 6Graduate Institute of Medical Sciences and Department of Pharmacology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 7Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan
- 8Institute of Biomedical Sciences, Mackay Medical College, New Taipei, Taiwan
Background: Few studies have addressed early-stage kidney disease and preclinical cardiac structural and functional abnormalities from a large-scale Asian population. Further, the extent to which measures of myocardial function and whether these associations may vary by testing various formulas of renal insufficiency remains largely unexplored.
Objective: To explore the associations among renal function, proteinuria, and left ventricular (LV) structural and diastolic functional alterations.
Design: A cross-sectional, retrospective cohort study.
Setting: Registered data from a cardiovascular health screening program at MacKay Memorial Hospital from June 2009 to December 2012.
Participants: Asymptomatic individuals.
Measurements: Renal function was evaluated in terms of estimated glomerular filtration rate (eGFR) by both MDRD and CKD-EPI formulas and severity of proteinuria, which were further related to cardiac structure, diastolic function (including LV e’ by tissue Doppler), and circulating N-terminal pro-brain natriuretic peptide (NT-proBNP) level.
Results: Among 4942 participants (65.8% men, mean age 49.4 ± 11.2 years), the mean CKD-EPI/MDRD eGFR was 90.6 ± 15.7 and 88.5 ± 16.9 ml/min/1.73m2, respectively. Lower eGFR, estimated either by the MDRD or CKD-EPI method, and higher proteinuria were significantly associated with lower LV e’ and higher NT-proBNP (all p<0.05) even after adjusting for clinical covariates. In general, lower eGFR estimated by CKD-EPI and MDRD displayed similar impacts on worsening e’ and NT-proBNP, rather than E/e’, in multivariate models. Finally, lower LV e’ or higher composite diastolic score, rather than E/e’, demonstrated remarkable interaction with eGFR level estimated by either CKD-EPI or MDRD on circulating NT-proBNP level (p interaction <0.05).
Limitations: Proteinuria was estimated using a urine dipstick rather than more accurately by the urine protein-to-creatinine ratio. Also, pertaining drug history and clinical hard outcomes were lacking.
Conclusion: Both clinical estimate of renal insufficiency by eGFR or proteinuria, even in a relatively early clinical stage, were tightly linked to impaired cardiac diastolic relaxation and circulating NT-proBNP level. Elevation of NT-proBNP with worsening renal function may be influenced by impaired myocardial relaxation.
Introduction
Chronic kidney disease (CKD) carries an unambiguous risk for a broad spectrum of cardiovascular diseases (CVD), among which heart failure (HF) remains the most common chronic clinical manifestation in patients with CKD (1, 2). The risk of HF rises in accordance with a decline in glomerular filtration rate (GFR) and is greatest in patients with end-stage renal disease requiring dialysis (3). It has been proposed that advanced CKD is characterized by accelerated atherosclerosis (4) and large arterial remodeling, secondary to pressure or volume overload (5), and possibly indolic uremic toxins (6, 7). These factors, when taken together, may lead to unfavorable cardiac remodeling from reduced arterial compliance, increased pulse pressure, and left ventricular hypertrophy (LVH) or fibrosis closely associated with a stiffened left ventricle and impaired diastolic relaxation (2, 8). As a consequence, based on the Frank–Starling law, an acute elevation of preload can cause increased left atrial pressure and pulmonary edema despite apparently preserved ventricular systolic function (9, 10).
A number of mechanisms illustrate the bidirectional interactions between myocardial dysfunction and kidney disease (11); however, it remains unclear whether this interplay may start to take place at a relatively early, clinically asymptomatic stage. Furthermore, various estimates of GFR have been proposed (e.g., CKD Epidemiology Collaboration [CKD-EPI] (12) and four-variable Modification of Diet in Renal Disease [MDRD] (13) formulas), although their impacts on cardiac structural and functional alterations in earlier stages of renal insufficiency have not been fully explored. On the other hand, assessment of diastolic dysfunction (DD) as precursor of HF (14, 15), albeit its complexity with diversity, can be readily assessed using non-invasive echocardiography (16, 17). However, the extent to what degree these indices may be affected and whether these estimates may be equally influenced by renal insufficiency at an earlier stage remains largely unexplored in large-scale Asian population. Here, we aimed to investigate the association between renal function and echocardiographic measurement of diastolic function in asymptomatic individuals.
Methods
Data source and study population
This cross-sectional study included asymptomatic participants in an ongoing cardiovascular health screening program from June 2009 through December 2012 at a tertiary-care teaching institute in Northern Taiwan. The primary aim of this program was to examine the hypothesis that certain demographic characteristics, behavioral factors, or biochemical data are associated with subclinical cardiac dysfunction in otherwise healthy individuals. All participants underwent a thorough evaluation, including general physical examination, baseline anthropometric measurements, blood sampling, and comprehensive echocardiography on the day of appointment. As described in our previous work (18), clinical symptoms, baseline comorbidities, smoking status, and exercise habits were obtained from a detailed structured questionnaire. This study was approved by the institutional review board of MacKay Memorial Hospital (14MMHIS202), and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Baseline comorbidities collected included diabetes, hypertension, dyslipidemia, and CVD. CVD constituted a group of diseases including coronary artery disease (CAD), stroke, and peripheral arterial disease. Laboratory parameters measured included hemoglobin, fasting blood sugar, lipid profile, renal function, N-terminal pro-brain natriuretic peptide (NT-proBNP), and urinalysis. All biochemical tests were conducted using a Hitachi 7170 Automatic Analyzer (Hitachi Corp., Hitachinaka, Ibaraki, Japan), and NT-proBNP was measured using an electrochemiluminescence immunoassay “ECLIA” assay (Roche Diagnostics GmbH, D-68298, Mannheim, Germany) in a standardized central laboratory. Renal function in terms of estimated glomerular filtration rate (eGFR) was calculated using CKD-EPI and four-variable MDRD equations, and was categorized as 30 to < 60, 60 to < 90, and ≧ 90 ml/min/1.73 m2. For simplicity, eGFR is referred to as CKD-EPI eGFR if not otherwise specified. We defined proteinuria, measured with a dipstick, as negative, mild (trace to 1+), or severe (2+ to 3+). Test strips were measured using an automatic dipstick analyzer (CLINITEK Novus®, Siemens). Validation of results with quantitative urine albumin amount was good (Supplemental Figure 1). As per the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, participants were further classified based on eGFR and proteinuria categories (19). Subjects with missing data for serum creatinine or dipstick proteinuria were excluded from analysis.
Echocardiographic evaluation
Conventional echocardiography and TDI were performed on all participants, based on the American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines (20, 21) using a GE system (Vivid i, GE Vingmed Ultrasound, Norway) equipped with a 2- to 4-MHz transducer (3S-RS). LV and left atrial (LA) structural parameters measured included LV end-diastolic and end-systolic diameters, wall thickness, LA/LV volume by modified biplane Simpson’s method, and LV mass by the Devereux formula (22). Maximum LA volume (LAVmax) was measured at ventricular end-systole just before opening of the mitral valve, while minimum LA volume (LAVmin) was measured at end-diastole, just before closure of the mitral valve. LV ejection fraction (LVEF) was calculated as 100 × (maximal LV volume − minimal LV volume)/maximal LV volume. LVEF was considered abnormal if < 50%. LV mass was further indexed to body surface area (BSA) as LV mass index (LVMI), and LAV was similarly indexed to BSA. LVH was defined as an LVMI greater than 115 g/m2 in men and 95 g/m2 in women (23).
The most important modalities to evaluate diastolic function are transmitral pulsed-wave Doppler flow and tissue Doppler mitral annular velocity profile (16, 17). The former helps to assess the presence and severity of DD, which alters the relationship between peak velocity flow in early diastole (E-wave) and that in late filling (A-wave), the time taken from the maximum E to baseline (deceleration time [DT]), and the interval between closure of the aortic valve and opening of the mitral valve (isovolumetric relaxation time [IVRT]). TDI measures the velocity of mitral annular motion, characterized by peak systolic velocity (s’), early diastolic velocity (e’), and late diastolic velocity (a’) in apical four-chamber view. Average e’ was taken as the average of septal e’ and lateral e’. LV filling pressure was estimated using the E/e’ ratio (average e’). DD was defined as E/e’> 15 or average e’ <9 cm/s when E/e’ is between 8 and 15 (24). Composite diastolic score was calculated based on TDI e’ velocity, E/e’ ratio, LAV index, and pulmonary artery pressure (16). Scores ranged from 0 to 2, where 0 was normal, 2 abnormal, and 1 in-between.
All echocardiographic images were performed blind to clinical information by an experienced technician, and stored digitally and reviewed offline using proprietary software (EchoPAC version 10.8, GE Vingmed Ultrasound, Norway). The reproducibility analysis has been reported in our previous article (18). We randomly selected 50 subjects for coefficient of variation analysis of a number of measured parameters (Supplemental Table 1). For instance, the intra-class correlation coefficients for LAVmax were 92% between analyzers (interobserver) and 98.5% for the same analyzer (intraobserver).
Statistical analysis
This study analyzed the relationship between degree of renal dysfunction and cardiac deformational functional changes. The cohort was divided into eGFR and proteinuria categories. Trend tests were performed for continuous variables across categories of eGFR and proteinuria using one-way analysis of variance (ANOVA) and for categorical ones using the Cochran–Armitage test. Continuous variables are presented as mean ± standard deviation (SD); discrete variables are described as counts and percentages.
Multivariate linear regression was performed for markers of DD and renal function. Model 1 was adjusted for baseline clinical features (age and gender). Model 2 was additionally adjusted for baseline comorbidities (hypertension, diabetes, and CVD), body mass index (BMI), systolic blood pressure (SBP), current smoking, and laboratory data (fasting glucose, high-density lipoprotein [HDL], and total cholesterol). Model 3 added proteinuria to model 2. As for sensitivity tests, key echocardiographic variables (LVMI, LVEF, and stroke volume [SV]) were separately added to models 2 and 3. The final results of multivariate analyses were summarized by β-coefficient and 95% confidence intervals (CI).
Because NT-proBNP is a powerful indicator of HF (25), we also tested whether associations between renal function and diastolic parameters vary with NT-proBNP as an a priori hypothesis; therefore, possible interactions was evaluated with or without interaction terms between renal function (i.e., eGFR and proteinuria categories) and diastolic parameters (i.e., average e’, composite diastolic score, and LAV index) with NT-proBNP in factorial (two-way ANOVA in SPSS) and linear (ggplot2 package in R) designs.
All statistical analyses were carried out using Microsoft Excel 2013, IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp. Released 2013. Armonk, NY), and R (R Core Team (2022). A two-sided p-value <0.05 was considered significant.
Role of the funding source
No funding was used for this study.
Results
Baseline demographics
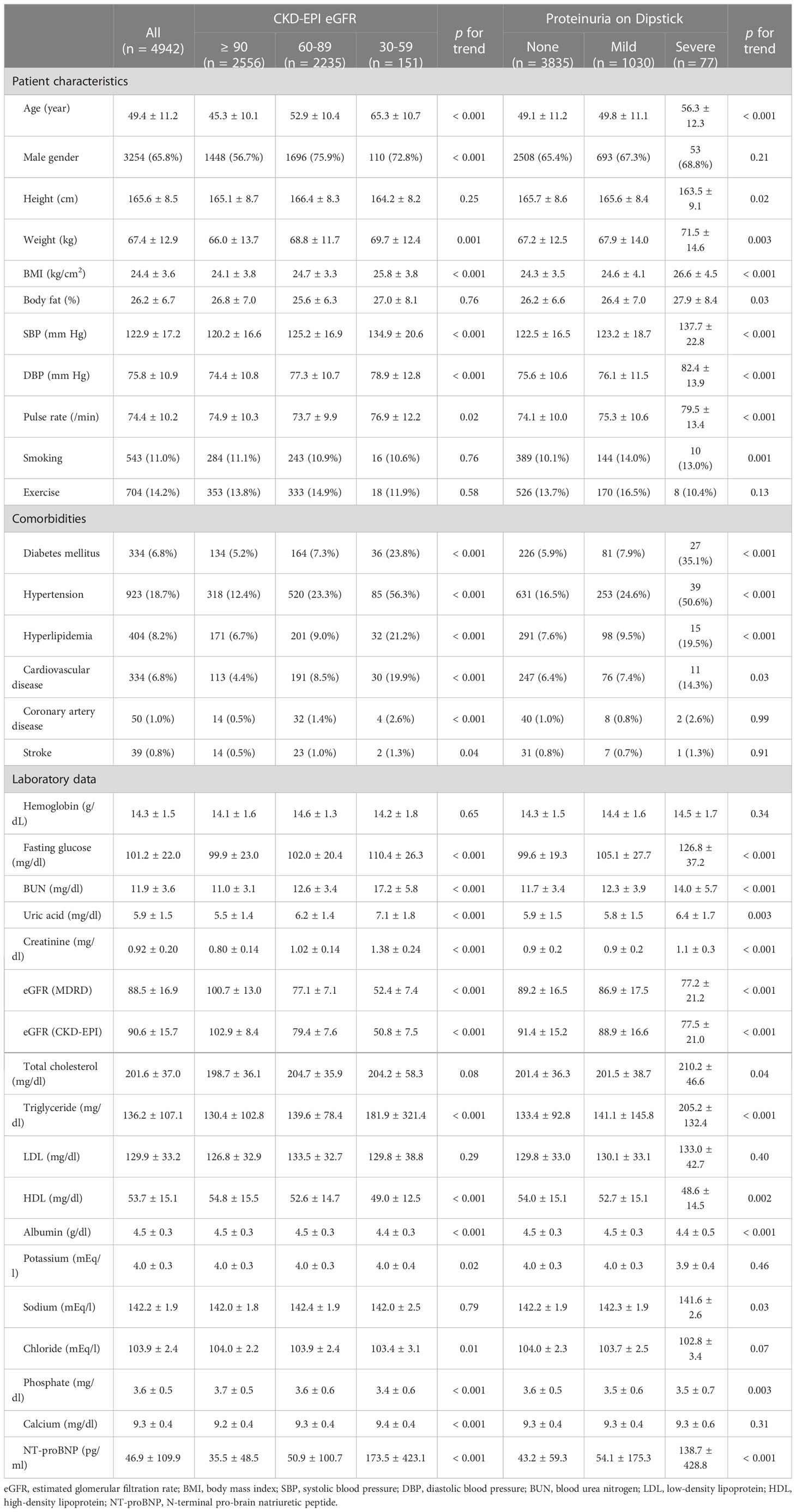
Our study included 5526 asymptomatic participants, and 584 were excluded for lack of serum creatinine or urine dipstick test (Supplemental Tables 2; 3). Among 4942 enrollees, 65.8% were men, mean age was 49.4 ± 11.2 years, and mean CKD-EPI eGFR was 90.6 ± 15.7 ml/min/1.73 m2 at enrollment (Table 1). Hypertension was the most prevalent systemic disease in this cohort, reported in 18.7% of the enrollees. All participants were categorized into three groups based on eGFR and into three groups based on proteinuria on a dipstick (Table 1). Great heterogeneity was observed between groups in terms of patient characteristics, baseline comorbidities, and laboratory data. As eGFR declined or proteinuria increased, there were trends of greater age, larger BMI, higher blood pressure, higher fasting glucose, higher uric acid, higher triglyceride, and higher NT-proBNP levels (all p for trends < 0.05).
Echocardiographic findings
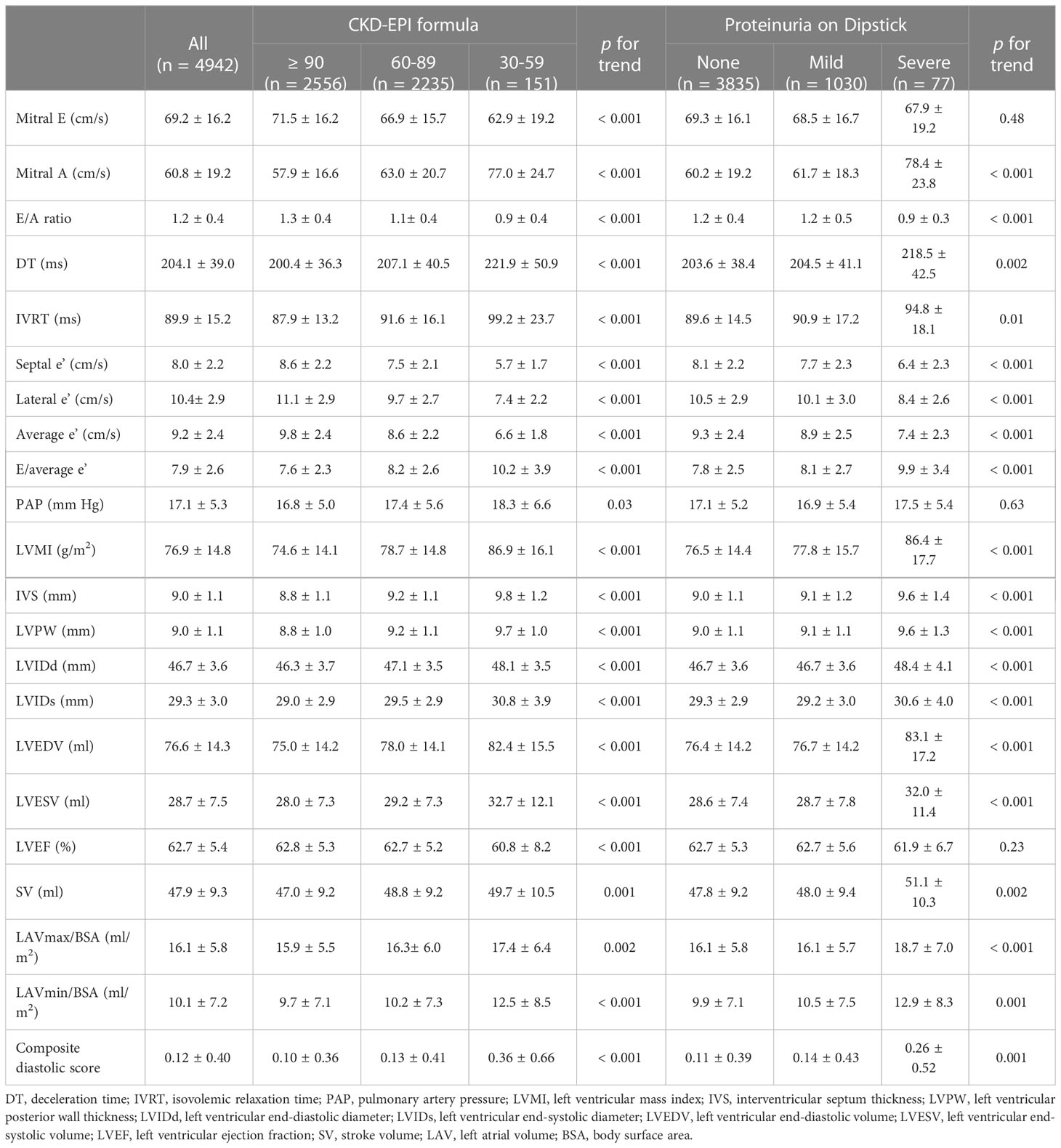
On echocardiographic assessment, the systolic function of our participants was preserved (overall LVEF was 62.7 ± 5.4%) (Table 2). Overall E/A ratio was 1.2 ± 0.4, E/average e’ 7.9 ± 2.6, septal e’ 8.0 ± 2.2 cm/s, lateral e’ 10.4 ± 2.9 cm/s, average e’ 9.2 ± 2.4 cm/s, LVMI 76.9 ± 14.8 g/m2, and NT-proBNP 46.9 ± 109.9 pg/ml. LVMI in our cohort did not meet the criteria for LVH. LV geometry differed significantly by renal function status with higher LVMI, LAV indices, and LV end-diastolic and end-systolic diameters among individuals with lower CKD-EPI eGFR (or higher proteinuria) when compared with their counterparts. In parallel with the severity of renal dysfunction, E/A ratio and e’ gradually decreased, while peak A-wave velocity, DT, IVRT, E/e’, and composite diastolic score all gradually increased (all p for trends < 0.05). Similar trends of altered cardiac structures and functions were observed across MDRD eGFR categories (Supplemental Table 4). Of note, participants in the worst categories (i.e., having eGFR between 30 and < 60 ml/min/1.73 m2 or severe dipstick proteinuria) showed the lowest TDI-determined e’ values (septal e’ < 7 cm/s, lateral e’ < 10 cm/s, and average e’ < 9 cm/s), suggestive of highly abnormal diastolic relaxation (Table 2; Supplemental Table 4) (16).
Figure 1 illustrates the levels of average e’, E/e’, LVMI, and NT-proBNP across categories of eGFR and proteinuria. We demonstrated a graded pattern of average e’, E/e’, and LVMI with the severity of renal function. In Table 3, average e’ is summarized by CKD-EPI/MDRD eGFR and proteinuria category. The levels of average e’ did not meet the risk classification for prognosis of CKD and cardiovascular mortality as per the KDIGO guidelines (19).
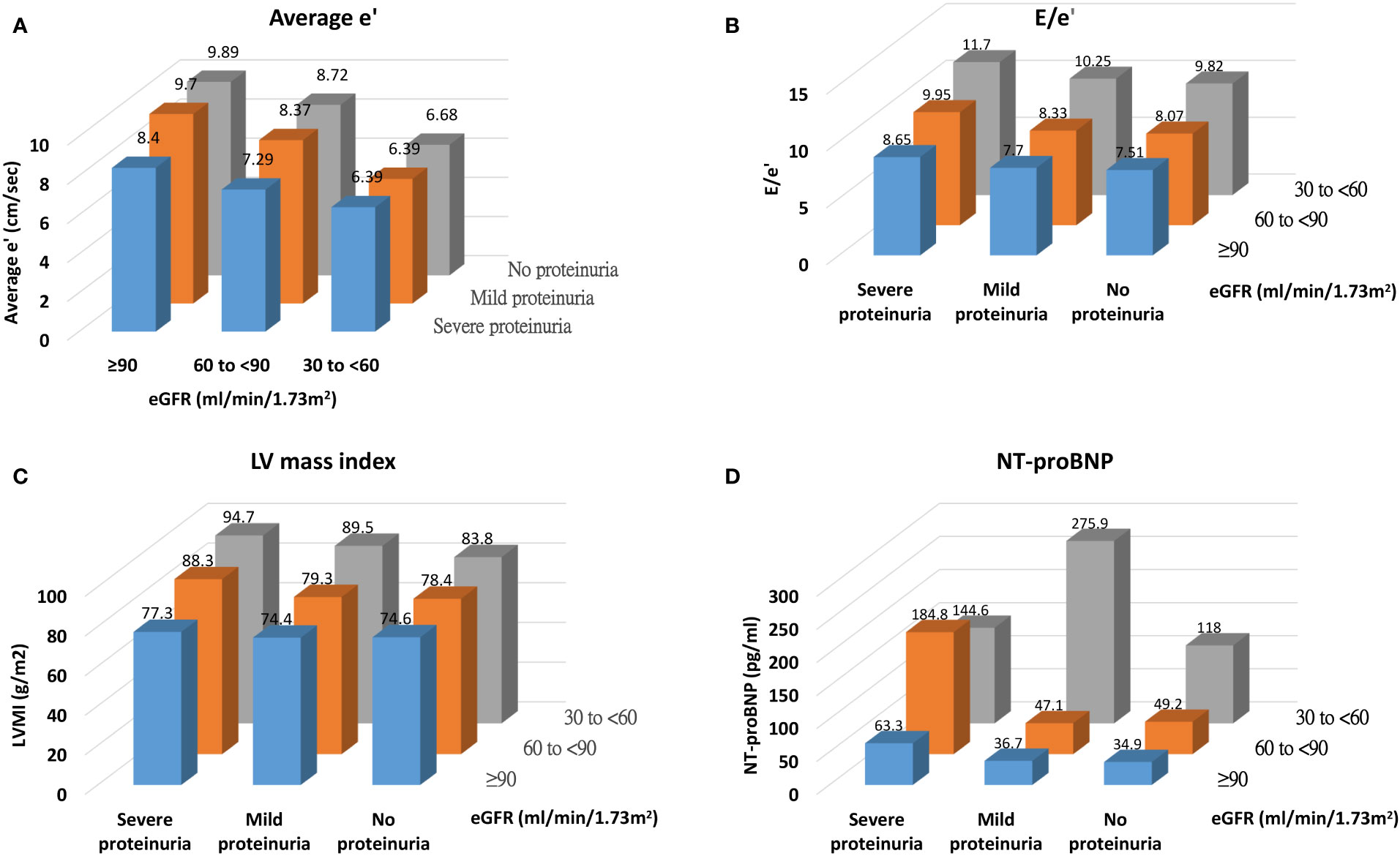
Figure 1 Distribution of cardiac structural and functional parameters [(A) average e', (B) E/e', (C) LV mass index, and (D) NT-proBNP] across categories of renal function.
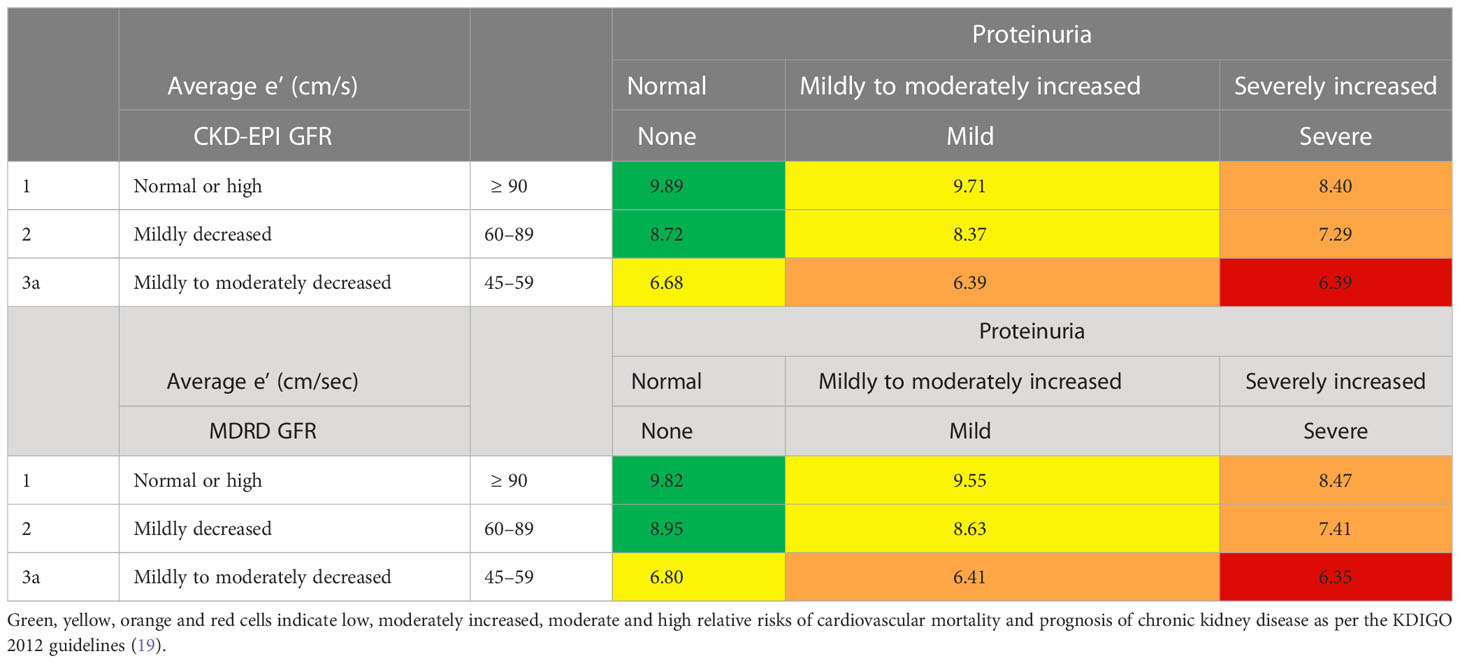
Table 3 Illustrations of average e’ of the entire cohort across graded MDRD/CKD-EPI eGFR and proteinuria categories.
Associations between cardiac diastolic function, renal insufficiency, and circulating NT-proBNP level
In several multivariate regression models adjusted for clinical risk factors, CKD-EPI eGFR was positively correlated with average e’, and negatively correlated with NT-proBNP (Table 4) and maximum LAVi (Supplemental Table 5). CKD-EPI eGFR had no significant effect on LV filling E/e’ and LVMI. The adjusted models remained significant with respect to markers of DD when CKD-EPI eGFR was replaced by MDRD eGFR (Table 4; Supplemental Table 5).
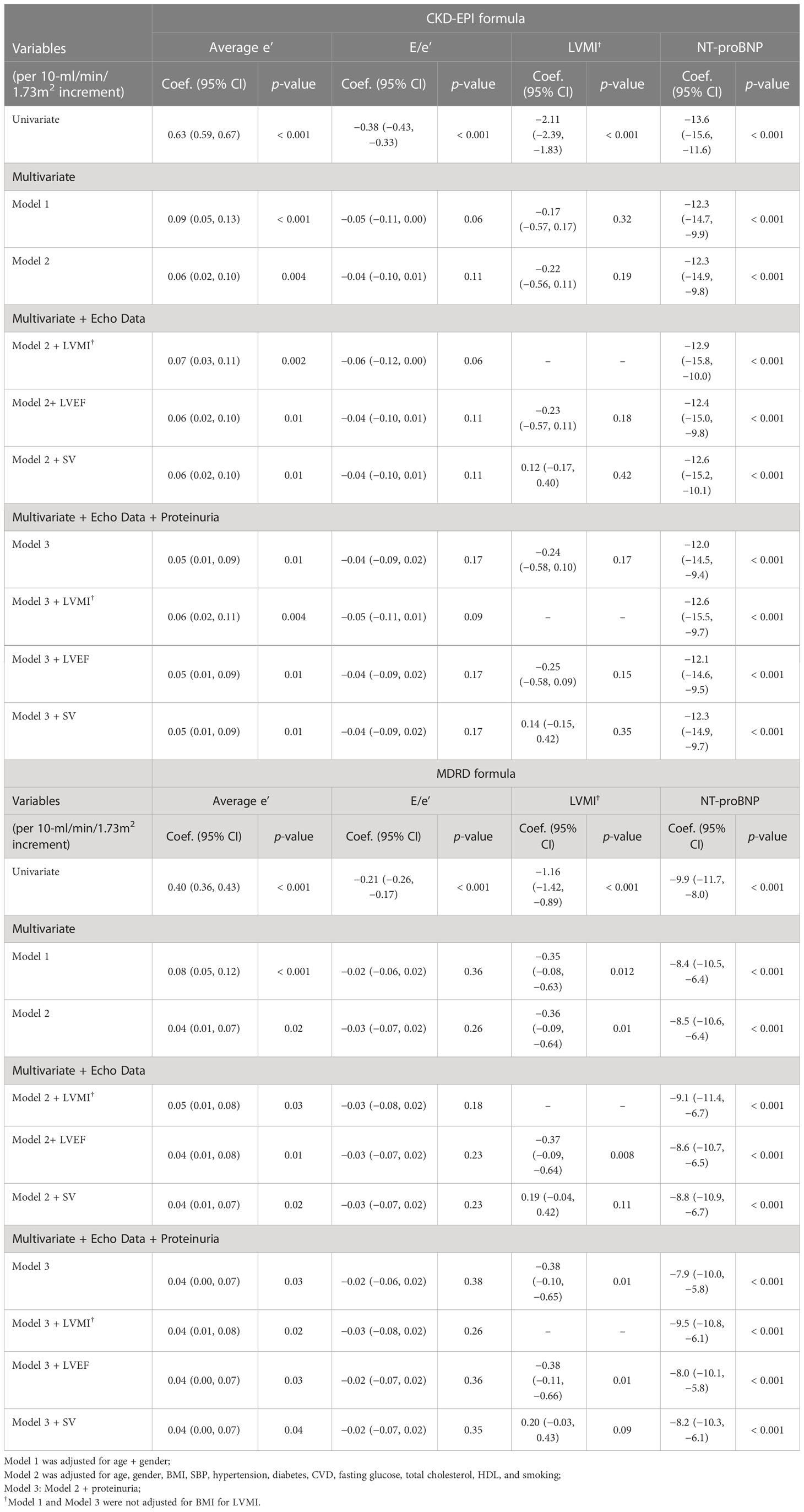
Table 4 Association of CKD-EPI and MDRD eGFR with markers of diastolic function and cardiac structure in multivariate-adjusted linear regression models.
As shown in Figure 2, a significant interaction exists between renal function and diastolic markers with reference to NT-proBNP, (interaction p < 0.05). Individuals with lower average e’ or higher composite diastolic score, rather than E/e’, present with higher NT-proBNP levels across worsening eGFR category (or having severe proteinuria) (Figure 2; Supplemental Figure 2).
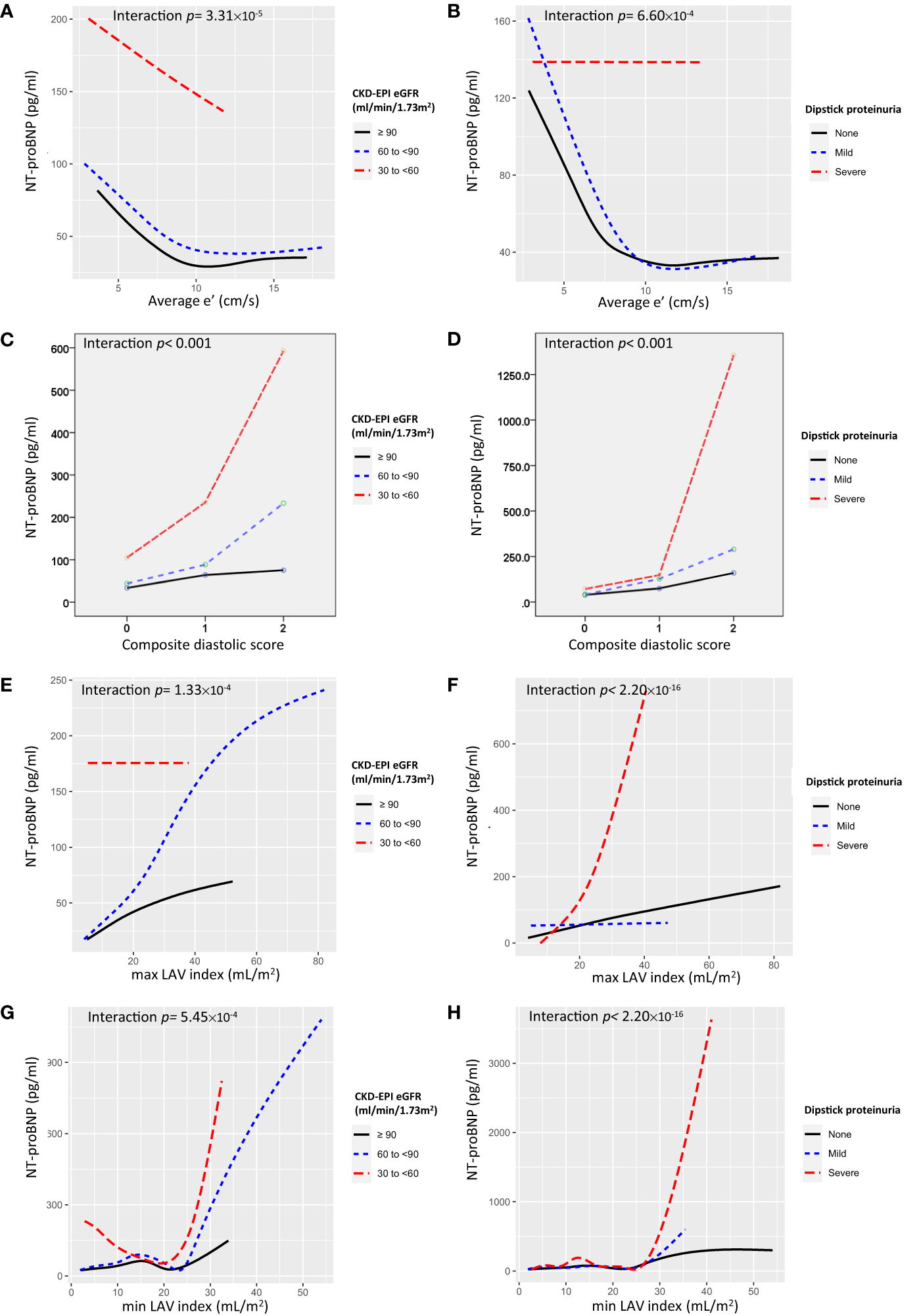
Figure 2 Interaction plots for NT-proBNP for the effects of (A) average e’ and eGFR, (B) average e’ and proteinuria, (C) composite diastolic score and eGFR, (D) composite diastolic score and proteinuria, (E) maximum LAV index and eGFR, (F) maximum LAV index and proteinuria, (G) minimum LAV index and eGFR, and (H) minimum LAV index and proteinuria.
Discussion
This observational study had a large sample size, and describes the associations between renal function and several indices of DD in a cohort without prevalent HF. The majority (97%) of our study participants had a preserved renal function (eGFR of ≥ 60 ml/min/1.73 m2). We demonstrated that in the condition of preserved LVEF, lower eGFR, estimated by either by MDRD or CKD-EPI formula, was significantly associated with lower LV e’, greater maximum LAVi, and elevated NT-proBNP, suggesting that abnormal LV structure and diastolic relaxation may be present in subjects with early stages of kidney disease and progress as renal function declines. Instead, E/e’ ratios, markers of LV filling pressures, had a lack of discriminatory power to detect subtle differences in diastolic function in subjects with mild renal impairment. Broadly, average e’ was a more sensitive alternative for the assessment of LV DD in this population.
A noteworthy strength of our study was that we analyzed LV DD with risk stratification by two-dimensional information on GFR and proteinuria. Both markers are pivotal for kidney function, and combined assessment of these two factors is better than either one solely to characterize and to prognosticate CKD progression and relevant morbidities (26). Our study provided objective evidence to demonstrate that eGFR and proteinuria both present independent and synergistic effects on LV structure and DD, even in clinically asymptomatic stages. The pathophysiological mechanism linking renal dysfunction and LV abnormalities has been extensively explored in the past decade. Aside from conventional risk factors such as older age, diabetes, hypertension, smoking, and dyslipidemia (27), some CKD-specific nonconventional factors such as albuminuria (28), LVH (29), fibroblast growth factor 23 (30), deranged mineral metabolism (31), anemia (32) and inflammation (33) may all contribute to CVD. The term cardiorenal syndrome has been increasingly used to describe that severe dysfunction of these organs often occurs in combination rather than in isolation (34). Nevertheless, CKD is a clinical continuum. Our study offered additional insight into heart–kidney interplay, which begins in the early stage of either disease when LVEF and GFR are preserved. To date, early detection of cardiorenal interaction is not easy in the clinically asymptomatic stage without the help of novel biomarkers (such as neutrophil gelatinase-associated lipocalin [NGAL], kidney injury molecule-1 [KIM-1], cystatin C, natriuretic peptides, and cardiac troponins) (35, 36).
On the other hand, NT-proBNP is of the natriuretic peptide family and has excellent in vitro stability (37) and diagnostic ability in the assessment of asymptomatic LV dysfunction in patients at risk for HF development (25). NT-proBNP levels are positively correlated with the severity of DD (25, 38); however, interpretation should always consider subjects’ age, gender (25), and renal function (39), and yet data regarding possible interactions between DD and renal function on NT-proBNP level in a large, asymptomatic Asian population remain unexplored. Using NT-proBNP as an indicator of LV DD, our interaction plots showed a marked increase in NT-proBNP in subjects in the severest categories of renal function (i.e., having eGFR between 30 and < 60 ml/min/1.73 m2 or heavy dipstick proteinuria) in comparison with those having better renal function. Moreover, an even steeper elevation in NT-proBNP was noted in subjects in the worst renal function categories with parallel lower average e’ (or in the category of composite diastolic score equal to 2 or higher LAV index), rather than E/e’, suggesting that the interaction between heart and kidneys might grow vehemently and disproportionately as either organ begins to lose some function. Of note, although prior studies have reported the utilization of CKD-EPI equation as a more applicable and useful surrogate marker than MDRD for CKD in Asians (40, 41), in our study these two equations displayed similar trends in associations with cardiac diastolic markers.
This study has several limitations. First, although proteinuria or albuminuria is more accurately assessed in terms of urinary protein-to-creatinine or albumin-to-creatinine ratio (ACR), calculated by dividing the urine protein or albumin by urine creatinine during morning urine collection, the urine dipstick test is a simple, fast, and inexpensive tool to screen and diagnose urinary tract problems, including proteinuria. Standard reagent strip dipsticks are especially sensitive to albumin, and even a dipstick test result of trace or higher identifies ACR ≥ 300 mg/g with 100% sensitivity and 83.7% specificity (42). Our study showed a graded pattern of a series of LV measurements with the severity of dipstick results, suggesting that urinalysis is a useful first step to assess proteinuria. Second, the individuals of our cohort were included in a tertiary medical center, which might introduce selection bias. Third, our cohort did not record their drug-taking history. For example, β-blockers, renin–angiotensin–aldosterone system blockers, sodium–glucose cotransporter 2 inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP1 RA) have cardioprotective and renoprotective effects, while non-steroidal anti-inflammatory drugs (NSAIDs) and contrast media may hamper renal function. However, this screening program was conducted between 2009 and 2012, when SGLT2i and GLP1 RA were unavailable. Still, certain missing drug information might elicit treatment bias. Lastly, our database did not contain clinical outcomes, and the correlations to outcomes might be more important than those to surrogate markers.
Conclusions
In conclusion, in this large cohort of participants with early CKD and without clinical HF, we found a strong association between renal function and LV structural and functional change during diastole. Average e’, instead of E/A or E/e’ ratios, was more sensitive to detect LV DD in this population. Heart–kidney crosstalk starts in the early asymptomatic stage. In this regard, renal function in terms of eGFR and dipstick proteinuria provide crude information on subjects’ LV diastolic function, and prompt interventions might be needed to hinder the devastating cardiorenal crosstalk from the perspective of preventive medicine.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the institutional review board of MacKay Memorial Hospital (14MMHIS202). The informed consent from the patients/participants was waived because of the retrospective nature of this study and the analysis used anonymous clinical data.
Author contributions
Authors’ contributions: P-CW drafted the manuscript. K-TS, J-LL and T-CH collected data. C-LH and C-JW provided the original conception and design of the study. Y-HL, C-HS and H-IY modified the statistical models critically and provided technical and statistical support during the analyses. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer Y-WW declared a past co-authorship with the authors H-IY, C-JW, and C-LH to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2023.1071900/full#supplementary-material
References
1. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. (2004) 15(5):1307–15. doi: 10.1097/01.ASN.0000123691.46138.E2
2. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. (2003) 108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80
3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med (2004) 351(13):1296–305. doi: 10.1056/NEJMoa041031
4. Drueke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. (2010) 6(12):723–35. doi: 10.1038/nrneph.2010.143
5. London GM, Marchais SJ, Guerin AP, Metivier F, Adda H. Arterial structure and function in end-stage renal disease. Nephrol Dial Transplant. (2002) 17(10):1713–24. doi: 10.1093/ndt/17.10.1713
6. Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (2014) 6(3):934–49. doi: 10.3390/toxins6030934
7. Gondouin B, Cerini C, Dou L, Sallee M, Duval-Sabatier A, Pletinck A, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int (2013) 84(4):733–44. doi: 10.1038/ki.2013.133
8. Cho GY. Diastolic dysfunction and chronic kidney disease. Korean J Intern Med (2013) 28(1):22–4. doi: 10.3904/kjim.2013.28.1.22
9. Jacob R, Dierberger B, Kissling G. Functional significance of the frank-starling mechanism under physiological and pathophysiological conditions. Eur Heart J (1992) 13 Suppl E:7–14. doi: 10.1093/eurheartj/13.suppl_E.7
10. Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the strong heart study. Am J Cardiol (2000) 86(10):1090–6. doi: 10.1016/S0002-9149(00)01165-6
11. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol (2008) 52(19):1527–39. doi: 10.1016/j.jacc.2008.07.051
12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med (2006) 145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004
14. Galderisi M. Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound. (2005) 3:9. doi: 10.1186/1476-7120-3-9
15. Lalande S, Johnson BD. Diastolic dysfunction: a link between hypertension and heart failure. Drugs Today (2008) 44(7):503–13. doi: 10.1358/dot.2008.44.7.1221662
16. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2016) 17(12):1321–60. doi: 10.1093/ehjci/jew082
17. Oh JK. Echocardiography as a noninvasive swan-ganz catheter. Circulation. (2005) 111(24):3192–4. doi: 10.1161/CIRCULATIONAHA.105.548644
18. Hung CL, Goncalves A, Lai YJ, Lai YH, Sung KT, Lo CI, et al. Light to moderate habitual alcohol consumption is associated with subclinical ventricular and left atrial mechanical dysfunction in an asymptomatic population: dose-response and propensity analysis. J Am Soc Echocardiogr (2016) 29(11):1043–51 e4. doi: 10.1016/j.echo.2016.07.014
19. Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2013) 3(1):1–150. doi: 10.1038/kisup.2012.77
20. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr (2005) 18(12):1440–63. doi: 10.1016/j.echo.2005.10.005
21. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr (2009) 22(2):107–33. doi: 10.1016/j.echo.2008.11.023
22. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol (1986) 57(6):450–8. doi: 10.1016/0002-9149(86)90771-X
23. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J Hypertens (2013) 31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc
24. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J (2012) 33(14):1787–847. doi: 10.1093/eurheartj/ehs104
25. Betti I, Castelli G, Barchielli A, Beligni C, Boscherini V, De Luca L, et al. The role of n-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. PROBE-HF study. J Card Fail (2009) 15(5):377–84. doi: 10.1016/j.cardfail.2008.12.002
26. Imai E. End-stage renal disease: GFR and albuminuria as predictors: two is better than one. Nat Rev Nephrol. (2009) 5(9):494–5. doi: 10.1038/nrneph.2009.128
27. O’Donnell CJ, Elosua R. [Cardiovascular risk factors. insights from framingham heart study]. Rev Esp Cardiol (2008) 61(3):299–310. doi: 10.1016/S1885-5857(08)60118-8
28. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. (2001) 286(4):421–6. doi: 10.1001/jama.286.4.421
29. Dubin RF, Deo R, Bansal N, Anderson AH, Yang P, Go AS, et al. Associations of conventional echocardiographic measures with incident heart failure and mortality: the chronic renal insufficiency cohort. Clin J Am Soc Nephrol. (2017) 12(1):60–8. doi: 10.2215/CJN.02700316
30. Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. (2014) 25(2):349–60. doi: 10.1681/ASN.2013050465
31. Heine GH, Nangaku M, Fliser D. Calcium and phosphate impact cardiovascular risk. Eur Heart J (2013) 34(15):1112–21. doi: 10.1093/eurheartj/ehs353
32. Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol (2002) 40(1):27–33. doi: 10.1016/S0735-1097(02)01938-1
33. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med (2004) 351(25):2599–610. doi: 10.1056/NEJMoa040967
34. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. (2010) 121(23):2592–600. doi: 10.1161/CIRCULATIONAHA.109.886473
35. Lee SR, Jeong KH. Novel biomarkers for cardio-renal syndrome. Electrolyte Blood Press (2012) 10(1):12–7. doi: 10.5049/EBP.2012.10.1.12
36. Brisco MA, Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep (2014) 11(4):485–99. doi: 10.1007/s11897-014-0226-4
37. Downie PF, Talwar S, Squire IB, Davies JE, Barnett DB, Ng LL. Assessment of the stability of n-terminal pro-brain natriuretic peptide in vitro: implications for assessment of left ventricular dysfunction. Clin Sci (1999) 97(3):255–8. doi: 10.1042/CS19990084
38. Tschope C, Kasner M, Westermann D, Gaub R, Poller WC, Schultheiss HP. The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J (2005) 26(21):2277–84. doi: 10.1093/eurheartj/ehi406
39. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis (2005) 46(4):610–20. doi: 10.1053/j.ajkd.2005.06.017
40. Jeong TD, Lee W, Chun S, Lee SK, Ryu JS, Min WK, et al. Comparison of the MDRD study and CKD-EPI equations for the estimation of the glomerular filtration rate in the Korean general population: the fifth Korea national health and nutrition examination survey (KNHANES V-1), 2010. Kidney Blood Press Res (2013) 37(4-5):443–50. doi: 10.1159/000355724
41. Jessani S, Levey AS, Bux R, Inker LA, Islam M, Chaturvedi N, et al. Estimation of GFR in south asians: a study from the general population in Pakistan. Am J Kidney Dis (2014) 63(1):49–58. doi: 10.1053/j.ajkd.2013.07.023
Keywords: chronic kidney disease, echocardiography, left ventricular diastolic dysfunction, N-terminal pro-brain natriuretic peptide, proteinuria
Citation: Wu P-C, Sung K-T, Lin J-L, Hung T-C, Lai Y-H, Su C-H, Yeh H-I, Wu C-J and Hung C-L (2023) Relation of early-stage renal insufficiency and cardiac structure and function in a large population of asymptomatic Asians: a cross-sectional cohort analysis. Front. Nephrol. 3:1071900. doi: 10.3389/fneph.2023.1071900
Received: 21 December 2022; Accepted: 13 April 2023;
Published: 12 May 2023.
Edited by:
Sara Samoni, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Nicholas Wettersten, United States Department of Veterans Affairs, United StatesYen-Wen Wu, Far Eastern Memorial Hospital, Taiwan
Copyright © 2023 Wu, Sung, Lin, Hung, Lai, Su, Yeh, Wu and Hung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Jen Wu, d2NqeWFsaUB5YWhvby5jb20udHc=; Chung-Lieh Hung, am90YXJvMzc5MUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Pei-Chen Wu
Pei-Chen Wu Kuo-Tzu Sung2,3
Kuo-Tzu Sung2,3 Cheng-Huang Su
Cheng-Huang Su Hung-I. Yeh
Hung-I. Yeh Chung-Lieh Hung
Chung-Lieh Hung