
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nephrol., 24 October 2022
Sec. Clinical Research in Nephrology
Volume 2 - 2022 | https://doi.org/10.3389/fneph.2022.1024667
This article is part of the Research TopicSocial Determinants of Kidney Health: A Global PerspectiveView all 7 articles
The social determinants of health (SDoH) are the non-medical factors that influence kidney health outcomes directly or indirectly in a substantial manner and include conditions in which people are born, grow, work, live, and age. Many such challenges in lower- and middle- income countries have an unfavourable impact on kidney health. These conditions potentially influence economic policies and systems, development agendas, social norms, social policies, and political systems. In addition, many political and legal factors also determine and modify the ultimate outcome in patients with kidney disease. Legal factors that ensure universal health care, promote gender and racial equality, prevent malpractices and regulate strict laws in the field of kidney transplantation are the paramount determinants for the provision of necessary kidney care. Converging lines of evidence have supported the impact of social variables such as socioeconomic resources, social inclusion, housing conditions, educational attainment, and financial status on kidney health, particularly affect vulnerable and disadvantaged groups and result in challenges in kidney care delivery. Furthermore, the climate is an important SDoH that plays a crucial role in the occurrence, prevalence, and progression of kidney diseases as highlighted by the presence of higher prevalence of chronic kidney disease in hot tropical countries. The rising incidence of water and vector-borne diseases causing acute kidney injury is another consequence of disruptive environmental and climate change which is detrimental to kidney health. Political risk factors such as conflict also have a devastating influence on kidney health. The relationship between SDoH and kidney health outcomes requires more clarity. Gaps in the current knowledge need to be identified to inform the development of appropriate interventions to address upstream socio-economic risk factors for kidney disease.
During the past two decades, increasing evidence has highlighted the strong relationship between social determinants of health (SDoH) and health outcomes. As per the World Health Organisation (WHO), SDoH are the “conditions in which people are born, grow, live, work, and age.” (1) Socioeconomic conditions, psychosocial factors, environmental factors, and cultural-political drivers are the main domains of SDoH. Variables such as poverty, education attainment, unemployment, housing and working conditions, racial discrimination, and cultural beliefs are the key mediators of the overall health status of any country. Social and environmental factors are highly consequential and can result in remarkable variation in health status (2). The SDoH influence implementation of many social policies associated with medical care that have the potential to alter health-related outcomes (3). Lower educational attainment can make it difficult for the patients to navigate the complex healthcare system. Utilization and access to primary health care are impeded by a lack of health insurance causing roadblocks in detecting and treating risk factors for kidney disease, such as hypertension and diabetes mellitus. SDoH also foster an environment that perpetuates kidney health inequities. Disparities related to SDoH have a key role in the progression of chronic kidney diseases (CKD), its management, and as a risk factor (4). Legal factors (the laws of the local government addressing socio-economic, gender, and ethnic inequities) and the political climate of the country (policies of the government, its interaction with various international agencies, presence of conflict, etc.) affect the social determinants of kidney health. This review aims to address the primary domains of SDoH with their implications on kidney health and provides a focus on how the enhanced understanding of the SDoH can be utilized to improve treatment plans and kidney outcomes.
Key Social determinants of health are summarized in Figure 1.
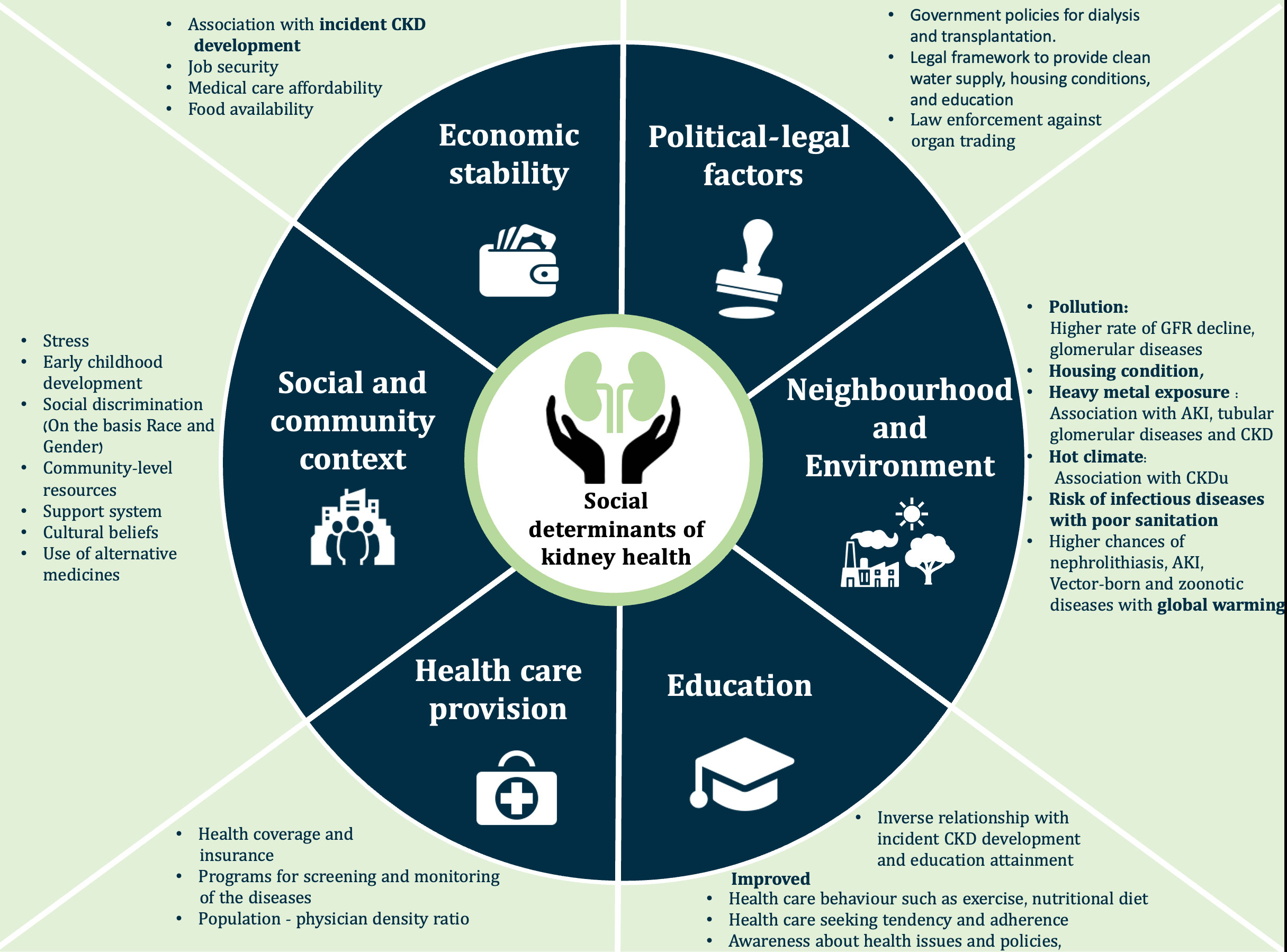
Figure 1 The social determinants of health. AKI, Acute kidney injury; CKD, Chronic kidney disease; CKDu, Chronic kidney disease of undetermined origin; GFR, Glomerular filtration rate.
Economic status is one of the most significant domains of the SDoH. Current data shows that approximately 22% of the world’s population residing in 107 developing countries is affected by multidimensional poverty (5). Around 85% of the population affected by poverty resides in sub-Saharan Africa and South Asia (5). Education, unemployment, social security, food insecurity, inability to pay for medical care, income, and living conditions are the important domains of poverty (6). There is a strong association between low economic status and prevalence and outcomes of kidney diseases (7), as poverty is the main hindrance to accessing health care. Reduced access to healthcare facilities, non-affordability, limited follow-up care, and delayed treatment have considerable ramifications for adults and children living under poor socioeconomic conditions (8, 9). Even in high-income settings such as Canada, children aged four to 11 years from lowest-income families had a 2.5 times higher risk of impaired functional health (e.g., vision, hearing, speech or mobility) as compared to highest-income families (10). Poverty is also likely contributing to the swift rise in chronic diseases such as diabetes mellitus and cardiovascular diseases being observed in Low and Low Middle-Income Countries (LMIC) and Middle-Income Countries (MIC) (11). In India, the uneven delivery of kidney healthcare, especially in children, where the rates of morbidity and mortality are dependent on the socio-economic status of the parents raises many moral and ethical issues (12). Similarly, de facto rationing of dialysis in Africa is related to poverty. The moral distress of all stakeholders - patients, families, and clinicians -resulting from the inability to access or deliver care must be addressed and acknowledged (13). It is high time for a global call to action against poverty. Legal and political frameworks must be developed to expand programs within existing social assistance systems to tackle poverty and mitigate poverty’s effect on kidney health.
Another SDoH is malnutrition. Socioeconomic status and malnutrition are intertwined. The prevalence of malnutrition is higher in low- and middle-income countries (14).
Malnutrition has both direct and indirect effects on kidney health. Maternal nutrition directly influences the kidney development of the offspring. Perturbations to maternal nutrition during pregnancy are shown to be linked with a higher likelihood of metabolic, cardiovascular, and kidney disease in the offspring (15–17). A high prevalence of protein-energy malnutrition (PEM) is seen in patients with advanced CKD and those on dialysis. PEM is also accompanied by a high inflammatory process. The “malnutrition-inflammation complex syndrome” (MICS) has a strong association with atherosclerotic cardiovascular disease. MICS is mostly attributed to factors such as reduced dietary intake, nutrient loss in dialysis, metabolic acidosis, oxidative stress and protein breakdown. Malnutrition in CKD patients is prevalent in both developed and developing countries and is a substantial cause of mortality and morbidity (18). It is, therefore, necessary to advocate for improving food security for the vulnerable and underprivileged population. In one such instance, “The Uganda Food and Nutrition Policy in Uganda” is promoting nutritional status through multi-sectoral and coordinated interventions to provide food security and better nutrition for the population (19).
Several studies have shown an association between incident CKD development and educational attainment (20). In the Indian CKD cohort, a significant percentage of patients were illiterate (26.9%). This subgroup of patients, when asked about simple aspects of kidney disease (symptoms, availability of treatment etc) had lower levels of awareness about CKD (21).
In another analysis, an allele score–based Mendelian randomization indicated that higher education attainment was associated with a decreased CKD risk (22). In the German CKD (GCKD) cohort higher all-cause mortality, and poor kidney and cardiovascular outcomes were reported in people with low educational attainment (23). The potential effect of education status on kidney disease warrants targeted public interventions by government and policymakers to mitigate the growing burden of the CKD population. The “National Education Policy” in India focused on providing universal access to school education, is certainly a step forward on this issue (24).
Homelessness and unstable housing are well-established determinants that result in adverse health consequences (25, 26). Housing insecurity is described as unaffordable housing payments and unsafe or overcrowded living conditions that impede general self-care and independence (27). Apart from the increased risk of infectious diseases in overcrowded living conditions, poor housing conditions are associated with non-communicable chronic diseases such as diabetes, and hypertension (28).
Individuals residing in LIC and LMIC undergoing rapid urbanisation have faster kidney function decline (29). In the US, housing insecurity was also linked to subsequent albuminuria at a median follow-up of 3.5 years (30). A study from San Francisco revealed that homeless adults were at 1.28 times greater risk of death or kidney failure than individuals with stable housing (31). Neglected medical care along with a stressful lifestyle results in recurrent hospitalization, increased disease progression, and higher mortality. People with unstable housing often miss their dialysis sessions (32).
The stress of patients with kidney disease is augmented by other factors related to improper housing conditions such as poor water quality, higher occurrence of infectious diseases, mental health problems, and substance abuse (26).
Addressing housing is an important SDoH and managing it with advocacy and program development is imperative. Housing and human settlements development policies in different countries have already made tremendous progress in facilitating sustainable housing development, and extending such policies to kidney patients might help to overcome unstable housing issues in such patients (33).
Gender discrimination in society drives inequalities in healthcare. Gender disparity has resulted in unequal health opportunities, differential health-seeking behaviours, vulnerabilities to diseases, and biases in medical research (34). There is an ongoing failure to maintain gender equality in the field of research. For instance, in a study from a metropolitan city in south India, of the 2158 participants in an educational and screening program for CKD amongst youth, only 32% were females (35). Gender-specific differences are observed in the aetiology, progression, and epidemiology of CKD. A higher prevalence of CKD and a slower rate of progression occurs in females as compared to males (36). However, females face more challenges like late nephrology referrals and delayed initiation of dialysis (37). And, even when initiated on dialysis women are less likely to receive optimal dialysis (38). It is not yet clear in lower-income settings, if the differences in RRT initiation between men and women are related purely to differences in the rate of CKD progression or are also impacted by nonbiological factors, such as unequal access to care or personal preference (39). What is clear, however, is that the quality of dialysis care in women, however, appears to be lower. For example, the prevalence of anaemia and malnutrition is higher in female CKD patients (40). Women also have lower odds of having arteriovenous (AV) access as compared to males for hemodialysis initiation (41). The gender-specific disparities are partly explained by the fact that women are less frequently employed, have other social responsibilities and often give low priority to their health (42). Population studies had shown that a substantial proportion (up to 27%) of women of reproductive age especially from areas like Africa may have the presence of pre-existing risk factors for renal disease such as hypertension that may be revealed during the physiological stress period of pregnancy (43, 44).
Studies have projected that elderly women opt more for conservative treatment rather than dialysis (45). Women are also disadvantaged as far as transplantation is concerned - the majority of living kidney donors are females, yet they are less likely to receive a kidney transplant (46). In various kidney transplant waiting list databases, there are fewer women than men (47). In a study from India analysing more than 5000 transplants women fare very poorly. They form the major donor pool and hardly ever receive an organ. In this study, only 32% of deceased donor kidney transplant recipients were females (48). This disparity is universal (49) but much more pronounced in African and Asian countries with socio-cultural practices, higher economic dependency and higher illiteracy rate in women being the most plausible explanations (50).
Autoimmune diseases preferentially affect women and are major causes of morbidity in females (51). Adverse effects on reproductive health and pregnancy are another pressing issue for female CKD patients. There is an increased risk of pre-eclampsia, premature delivery, and fetal complications (52).
Government policies like Janani Shishu Suraksha Karyakaram in India are a step forward in overcoming difficulties faced by pregnant women by providing completely free services to pregnant women (53). Supervised deliveries and timely management of complications in pregnant women can halt and prevent further complications like pregnancy-related acute kidney injury (AKI). Women with or at risk of CKD can be identified during pregnancy and referred for follow-up. Offspring of these higher risk pregnancies should also be identified and received life-long follow up. Mitigation of gender disparity in nephrology does not only involve enhancing education or poverty alleviation, but it needs a deeper and vigorous analysis of traditional gender roles and the place of a woman in society. An equitable deceased donor allocation system that prioritizes female recipients, especially those who are difficult to match requires discussion, but may be an example of a policy that could address current disparities.
Worldwide, discrimination because of race and ethnicity is inextricably related to adverse health outcomes. Racism confers inequalities in opportunities, income, access to health care, employment, education, health insurance, food insecurity, and community-level resources (54). For the majority of patients in the United States, kidney replacement therapy (KRT) is covered by Medicare, but this facility is not covered for undocumented immigrants (55). Accumulating evidence suggests that African Americans and Hispanics are at a 2.6 and 1.5-fold, respectively higher risk of developing kidney failure and have faster progression than white individuals (56). There is an inequitable higher prevalence of co-morbidities like diabetes and hypertension in these populations (57).
Besides genetic predisposition (58), adverse kidney outcomes in African Americans are further driven by late nephrology referrals. Most of them initiate hemodialysis with a catheter, rather than a surgically placed AV fistula (AVF). In a cohort of 396,075 patients, white patients were more likely to be initiated on hemodialysis with an AVF as compared to black patients or Hispanic patients (18.3% vs 15.5% and 14.6%, respectively; P < .001) (59) They are also less likely to receive kidney transplantation compared to Caucasians (60).
This disadvantage is not restricted to those of African origin, even indigenous ethnic minorities cannot access suitable and optimal healthcare. This is reflected in the higher incidence of kidney disease and poorer access to healthcare in indigenous populations (61). The life expectancy is lower in indigenous Australians and even now there are limitations in accessing healthcare for vulnerable groups such as the Aboriginal and Torres Strait Islander (indigenous) population (62). Subtle racial discrimination is also present in India where a complex caste substructure exists in the society. People are divided into castes which historically indicated their vocation. With the development of “superior” and inferior” vocations, Indians of the lower castes still have limited access to healthcare, especially in rural India (63). Also in India, there is a discrepancy in life expectancy at birth as there is a complex interplay between caste, religion and economic development of the region (64). Caste system is not uncommonly inextricably linked to socioeconomic status. Even the benefits of caste-based jobs, education opportunities, and health-related schemes are mostly availed by better-off people within the caste group. Despite improvements and multiple affirmative actions, the life expectancy of the lower castes is still lower. Studies are required to see whether this caste system has any impact on kidney health in Indians.
Another area where race impacts kidney care is the misclassification of the estimated glomerular filtration rate (eGFR) due to the race coefficient. The inclusion of race in the eGFR equation had indirectly deprived African Americans of timely nephrology care especially at advanced stages, compounding the disadvantage (65). Excluding race from eGFR calculations is necessary to address the impact of structural racism on patients with kidney diseases and improve clinical care and outcomes (66).
The footprints of unrestrained human activities such as deforestation, pollution, and rapid urbanization are progressively causing a rise in global temperature (67). Rising temperatures pose a huge threat to human health (68). As a consequence of climate change, the incidence of kidney disorders is also expected to rise globally. Hot weather causes an increase in core body temperatures resulting in dehydration, rhabdomyolysis, hyperosmolality, and inflammation-mediated recurrent kidney injury (69). A higher frequency of urinary tract infections and nephrolithiasis have also been reported with global warming (70). In recent years, the world is facing another pressing challenge chronic kidney diseases of unknown origin (CKDu) (71, 72). The cases of heat stress nephropathy have been reported worldwide (73–75). The renal histology usually shows tubulointerstitial nephritis (76).
Climate change and environmental disruptions have also been increasingly connected to a higher frequency of infectious diseases (77). Increased extreme weather events (floods, drought etc) are associated with increased incidences of vector-borne and zoonotic diseases (leptospirosis, malaria, dengue, hantavirus nephropathy scrub typhus, diarrheal illness etc) which consequently have a detrimental impact on kidney health (78). These infections are the most common preventable causes of AKI typically in tropical and subtropical regions (79) (Figure 2)
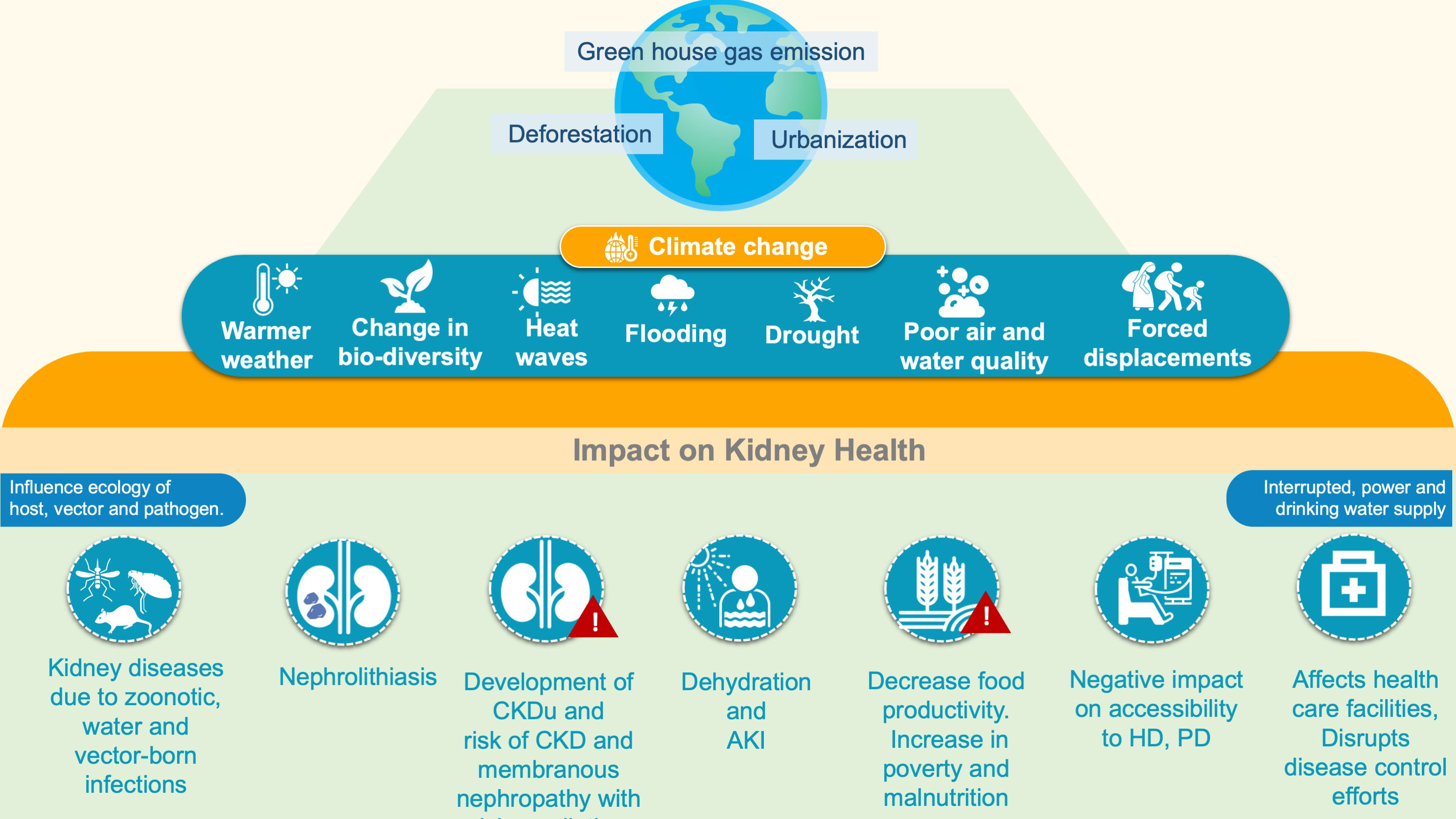
Figure 2 Climate and kidney Health. AKI, Acute kidney injury; CKD, Chronic kidney disease; CKDu, Chronic kidney disease of undetermined origin; GFR, Glomerular filtration rate; HD, Hemodialysis; PD, Peritoneal dialysis.
Epidemiological evidence suggests the role of environmental toxins in kidney diseases. Heavy metals like lead, mercury, arsenic, and cadmium are associated with CKD, AKI, tubular disorders (Fanconi syndrome), and glomerular diseases (80). Kuźma et al. demonstrated an association between eGFR decline and CKD development with medium- and short-term exposure to higher air pollution levels (81). Continuous efforts are required to prevent further environmental degradation, including improving sanitation and reducing pollution worldwide (82).
The most obvious example of politics impacting kidney health is the risk imposed by residence of patients in areas of conflict. This is becoming increasingly relevant as many parts of the world are enmeshed in civil wars, external invasions and humanitarian crises, often superimposed on pre-existing challenges in LIC and LMIC countries. Persistent long drawn conflicts not only affect the overall physical and mental health of its citizens, but also make delivery of care extremely challenging as the infrastructure becomes heavily affected. In Northwest Syria for example, as a result of the conflict, by 2020 more than half of health facilities had closed and many others were being attacked (83), while in in the Tigray region of Ethiopia, six months into the war that started in November 2020, only 27.5% of hospitals and 17.5% of health centres were still functional (84) Moreover, in that region, war and blockade have caused reduced access, funding and severe supply shortages to the only hemodialysis facility that serves a population of 9,000,000 people, condemning many patients with treatable illnesses to die (85). In addition, wars can lead to the exodus of skilled healthcare workers as they can be directly targeted by weapons (86). A notorious example is the Syrian conflict which has been estimated to have led to the exodus of more than 70% of the healthcare workforce (87). Furthermore, humanitarian crises and unstable political environments can also drive emigration as in Venezuela where 30000 physicians of which 150 are nephrologists have migrated to other countries over the past five years (88) and in Lebanon where the ongoing economic crisis coupled with the Beirut port explosion has already driven at least 1000 out of 15000 physicians out of the country (89). For those who remain, burnout can be a serious concern and constitute an additional barrier to optimal care delivery (87, 90). Moreover, the lack of adequate infrastructure and workforce can weaken the medical education system and impede both its graduate and post-graduate components, sabotaging not only the present but also the future of healthcare (87). In Iraq, a country that has long suffered of political instability, a recent cross-sectional survey identified that most medical students intended to leave the country after graduation (91). Finally, massive population displacement could also disrupt the healthcare care systems of the host countries, jeopardize the well-being of both the refugees and the local population and foster outbreaks of different types of diseases (92). A qualitative study conducted in Lebanon among nurses and nursing directors in regions in regions with a high concentration of Syrian refugees, identified as repercussions of their influx, fatigue, burnout, and depleted compassionate care at the individual level; rationing and stressed interpersonal relationships at the practice level and shortage in resources and poor performance at the healthcare system level (93). The war in Ukraine has led to the fastest number of migrating refugees since World war II (https://data.unhcr.org/en/situations/ukraine). Both their number and their rapid influx have made it challenging for neighboring countries to provide the adequate medical services not only for the migrants but for locals as well (94). Therefore, the World Health Organization (WHO) has recognized that maintainable health is largely determined by policies that guide actions beyond the health sector and has called all its members to integrate Health as part of policies directed at achieving sustainable development goals such as education, economy, transport, and housing (https://www.who.int/activities/promoting-health-in-all-policies-and-intersectoral-action-capacities).
Most of the social factors impacting kidney health are modifiable and can be improved upon. Housing, gender disparity, education, and socio-economic inequities are in a continuous flux the world over. The regional differences in the magnitude of the impact of social determinants on kidney health is not only an issue to be addressed by the healthcare officials, but is also a responsibility of the state. Nations can do a lot in improving health of its citizen by promulgating laws protecting various aspects of the “right to health”. All nations have promulgated laws ensuring “Universal Health Care” (UHC). But many lower and middle- income countries lag behind in offering basic health care to its populace. In addition, there are layers of discrimination in the basic delivery of health care. Governments also fail to provide any legal protection to the most under served population (95). To achieve UHC, there are three core legal determinants-a) health laws should address all social determinants of health, b) health and ancillary systems should be well governed, and c) public health officials should abide by the law (96). A strong legal protection to the right to health also has a major impact on kidney health as a strong legal framework is responsible for just and equitable access to treatment of kidney diseases (97). In the Indian perspective, various policies and programs are in place (some with legal protection) which have made a difference (both directly and indirectly) in promotion of kidney health (Table 1). An instance wherein a strong legal system has impacted kidney health positively in India is the rigorous implementation of the medical termination of pregnancy (MTP) act. This has led to a drastic reduction in illegal abortions which in turn has had a salutary impact on the incidence of pregnancy related acute kidney injury (105). However, as a nation, India and other LMICs have a long way to go in mitigating various social, political and legal factors which directly and/or indirectly affect kidney health (106).
Table 2 summarizes impact of each SDoH on kidney health outcomes and adaptive measures.
SDoH are the circumstances that have a large impact on an individual’s well-being and health. Variables such as socio-economic status, racial/gender discrimination, community support, and environmental conditions in conjecture with legal and political factors play a key role in influencing a person’s health. They are crucial determinants of the quality of life in CKD patients, especially in low-resource settings. SDoH has the potential to modulate the occurrence, prevalence, progression, and management of kidney disease Deliberations on the complex interplay of these factors are prudent, as addressing these affairs shall enhance the outcomes of kidney disease patients. Public health care providers should unite with the policymakers to develop health-promotion strategies and make them reach the individual level maintaining equity in health care delivery. Finally, further research to facilitate a better understanding of SDoH and their implications on kidney health is warranted to design novel interventions.
UA was responsible for the conceptualizing the manuscript and for writing up the sections of social determinants of Health (along with PM) PM wrote the social determinants of health and the final corrections were done by her. SK contributed to the political determinants of health section VL was the senior author whose ideas were incorporated in writing this manuscript. She went through the whole manuscript and suggested corrections. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization (WHO). Social determinants of health. Available at: https://www.who.int/social_determinants/en/.
2. Donkin A, Goldblatt P, Allen J, Nathanson V, Marmot M. Global action on the social determinants of health. BMJ Global Health (2018) 3:e000603. doi: 10.1136/bmjgh-2017-000603
3. Braveman P, Gottlieb L. The social determinants of health: It's time to consider the causes of the causes. Public Health Rep (2014) 129 Suppl 2:19–31. doi: 10.1177/00333549141291S206
4. Quiñones J, Hammad Z. Social determinants of health and chronic kidney disease. Cureus (2020) 12:e10266. doi: 10.7759/cureus.10266
5. The World Bank. Poverty net. Available at: https://www.worldbank.org/en/topic/poverty/overview.
6. Oshio T, Kan M. Multidimensional poverty and health: Evidence from a nationwide survey in Japan. Int J Equity Health (2014) 13:128. doi: 10.1186/s12939-014-0128-9
7. Norris KC, Beech BM. Social determinants of kidney health: Focus on poverty. Clin J Am Soc Nephrol (2021) 16:809–11. doi: 10.2215/cjn.12710820
8. Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: Neighborhood economic structure and its implications for health. Soc Sci Med (2003) 57:843–60. doi: 10.1016/s0277-9536(02)00457-4
9. McCulloch M, Luyckx VA, Cullis B, Davies SJ, Finkelstein FO, Yap HK, et al. Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol (2021) 17(1):33–45. doi: 10.1038/s41581-020-00338-7
10. Gupta RP, de Wit ML, McKeown D. The impact of poverty on the current and future health status of children. Paediatr Child Health (2007) 12(8):667–72. doi: 10.1093/pch/12.8.667
11. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world. Cardiovasc Diabetol (2018) 17:83. doi: 10.1186/s12933-018-0728-6
12. Pais P, Wightman A. Addressing the ethical challenges of providing kidney failure care for children: A global stance. Front Pediatr (2022) 10:842783. doi: 10.3389/fped.2022.842783
13. Ashuntantang G, Miljeteig I, Luyckx V. Bedside rationing and moral distress in nephrologists in sub-Saharan Africa. BMC Nephrol (2022) 23:196. doi: 10.1186/s12882-022-02827-2
14. Seferidi P, Hone T, Duran AC, Bernabe-Ortiz A, Millett C. Global inequalities in the double burden of malnutrition and associations with globalisation: a multilevel analysis of demographic and health surveys from 55 low-income and middle-income countries, 1992-2018. Lancet Glob Health (2022) 10(4):e482–90. doi: 10.1016/S2214-109X(21)00594-5
15. Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes — a global concern. Nat Rev Nephrol (2015) 11:135–49. doi: 10.1038/nrneph.2014.251
16. Young MF, Ramakrishnan U. Maternal undernutrition before and during pregnancy and offspring health and development. Ann Nutr Metab (2021) 1:1–13. doi: 10.1159/000510595
17. Gjerde A, Skrunes R, Reisaeter AV, Marti H-P, Vikse BE. Familial contributions to the association between low birthweight and risk of CKD in adult life. Kidney Int Rep (2021) 6:2151–58. doi: 10.1016/j.ekir.2021.05.032
18. Wright M, Southcott E, MacLaughlin H, Wineberg S. Clinical practice guideline on undernutrition in chronic kidney disease. BMC Nephrol (2019) 20:370. doi: 10.1186/s12882-019-1530-8
19. The Uganda food and nutrition policy. Available at: https://extranet.who.int/nutrition/gina/en/node/8236.
20. Thio CHL, Vart P, Kieneker LM, Snieder H, Ron RT, Bultman U. Educational level and risk of chronic kidney disease: Longitudinal data from the PREVEND study. Nephrol Dial Transplant (2020) 1211–18. doi: 10.1093/ndt/gfy361
21. Kumar V, Yadav AK, Sethi J, Ghosh A, Sahay M, Prasad N, et al. The Indian chronic kidney disease (ICKD) study baseline characteristics. Clin Kidney J (2022) 15:60–9. doi: 10.1093/ckj/sfab149
22. Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, et al. Causal effects of education on chronic kidney disease: A mendelian randomization study. Clin Kidney J (2020) 14:1932–8. doi: 10.1093/ckj/sfaa240
23. Winitzki D, Zacharias HU, Nadal J, Baid-Agrawal S, Schaeffner E, Schmid M, et al. Educational attainment is associated with kidney and cardiovascular outcomes in the German CKD (GCKD) cohort. Kidney Int Rep (2022) 14:1004–15. doi: 10.1016/j.ekir.2022.02.001
24. National Education Policy. Ministry of human resource development government of India (2020). Available at: https://www.education.gov.in/sites/upload_files/mhrd/files/.
25. Nyamathi AM, Salem BE. The impact of unstable housing on health. Lancet Public Health (2021) 6:e265–6. doi: 10.1016/S2468-2667(21)00035-9
26. Salhi BA, White MH, Pitts SR, Wright DW. Homelessness and emergency medicine: a review of the literature. Acad Emerg Med (2018) 25:577–93. doi: 10.1111/acem.13358
27. Rolfe S, Garnham L, Godwin J, Anderson I, Seaman P, Donaldson C. Housing as a social determinant of health and wellbeing: Developing an empirically-informed realist theoretical framework. BMC Public Health (2020) 20:1138. doi: 10.1186/s12889-020-09224-0
28. Vijayaraghavan M, Jacobs EA, Seligman H, Fernandez A. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved (2011) 22:1279–91. doi: 10.1353/hpu.2011.0131
29. Jagannathan R, Patzer RE. Urbanization and kidney function decline in low and middle-income countries. BMC Nephrol (2017) 18:276. doi: 10.1186/s12882-017-0685-4
30. Novick TK, Omenyi C, Han D, Zonderman AB, Evans MK, Crews DC. Housing insecurity and risk of adverse kidney outcomes. Kidney 360 (2020) 1:241–47. doi: 10.34067/KID.0000032019
31. Hall YN, Choi AI, Himmelfarb J, Chertow GM, Bindman AB. Homelessness and CKD: A cohort study. Clin J Am Soc Nephrol (2012) 7:1094–102. doi: 10.2215/CJN.00060112
32. Novick TK, Baweja M. American Society of nephrology healthcare justice committee advocacy and scholarship work group; American society of nephrology healthcare justice committee advocacy and scholarship work group are Housing: A critical contributor to kidney disease disparities. J Am Soc Nephrol (2022) 33(8):1471–3. doi: 10.1681/ASN.2022040424
33. Human settlements. Department of Human Settlements: Republic of South Africa Date: Last accessed on September 22, 2022. Available at https://www.gov.za/about-sa/humansettlements.
34. Alcalde-Rubio L, Hernández-Aguado I, Parker LA, Bueno-Vergara E, Chilet-Rosel E, et al. Gender disparities in clinical practice: Are there any solutions? Scoping review of interventions to overcome or reduce gender bias in clinical practice. Int J Equity Health (2020) 19:166. doi: 10.1186/s12939-020-01283-4
35. Rao PS, Wright Nunes JA, Gillespie BW, Perlman RL, Ravichandran R. Education and screening for chronic kidney disease in Indian youth: Pilot program result. Int J Nephrol Renovascular Dis (2017) 10:85–90. doi: 10.2147/IJNRD.S128417
36. Tomlinson LA, Clase CM. Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol (2019) 14:1557–9. doi: 10.2215/CJN.11030919
37. Minutolo R, Gabbai FB, Chiodini P, Provenzano M, Borrelli S, Garofalo C, et al. Collaborative study group on the conservative treatment of CKD of the Italian society of nephrology: Sex differences in the progression of CKD among older patients: A pooled analysis of 4 cohort studies. Am J Kidney Dis (2020) 75:30–8. doi: 10.1053/j.ajkd.2019.05.019
38. John R, Webb M, Young A, Stevens PE. Un referred chronic kidney disease: A longitudinal study. Am J Kidney Dis (2004) 43:825–35. doi: 10.1053/j.ajkd.2003.12.046
39. Carrero J, Hecking M, Chesnaye N, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol (2018) 14:151–64. doi: 10.1038/nrneph.2017.181
40. Shiferaw WS, Akalu TY, Aynalem YA. Risk factors for anemia in patients with chronic renal failure: A systematic review and meta-analysis. Ethiop J Health Sci (2020) 30:829–42. doi: 10.4314/ejhs.v30i5.23
41. Shah S, Leonard AC, Meganathan K, Christianson AL, Thakar CV. Gender and racial disparities in initial hemodialysis access and outcomes in incident end-stage renal disease patients. Am J Nephrol (2018) 48:4–14. doi: 10.1159/000490624
42. Crosthwaite A, Kerr PG. CKD-where have all the women gone? Kidney Int Rep (2022) 7:375–77. doi: 10.1016/j.ekir.2021.11.032
43. Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global regional and national levels: A population-based study. BMC Preg Childbirth (2021) 21:364. doi: 10.1186/s12884-021-03809-2
44. Maule SP, Ashworth DC, Blakey H, Osafo C, Moturi M, Chappell LC, et al. CKD and pregnancy outcomes in Africa: A narrative review. Kidney Int Rep (2020) 5(8):1342–9. doi: 10.1016/j.ekir.2020.05.016
45. Sparke C, Moon L, Green F, Mathew T, Cass A, Chadban S, et al. Estimating the total incidence of kidney failure in Australia including individuals who are not treated by dialysis or transplantation. Am J Kidney Dis (2013) 61:413–9. doi: 10.1053/j.ajkd.2012.10.012
46. Sheikh SS, Locke JE. Gender disparities in transplantation. Curr Opin Organ Transplant (2021) 26:513–20. doi: 10.1097/MOT.0000000000000909
47. Melk A, Babitsch B, Borchert-Mörlins B, Claas F, Dipchand AI, Eifert S, et al. Equally interchangeable? how sex and gender affect transplantation. Transplantation (2019) 103:1094–110. doi: 10.1097/TP.0000000000002655
48. Kute VB, Chauhan S, Navadiya V, Meshram HS, Patel HV, Engineer D, et al. India: Gender disparities in organ donation and transplantation. Transplantation (2022) 106:1293–7. doi: 10.1097/TP.0000000000003960
49. Vinson AJ. Gender disparities in access to kidney transplant: Inequities in the inequity. Kidney Int Rep (2022) 7(6):1145–8. doi: 10.1016/j.ekir.2022.03.034
50. Sahay M. Men are from Mars, women are from Venus: Gender disparity in transplantation. Indian J Transplant (2019) 13:237–9. doi: 10.4103/ijot.ijot_72_19
51. Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: A narrative review. Cureus (2020) 12(5):e8094. doi: 10.7759/cureus.8094
52. Dvořák J, Koucký M, Jančová E, Mysliveček M, Tesař V, Pařízek A. Chronic kidney disease and pregnancy outcomes. Sci Rep (2021) 11:21299. doi: 10.1038/s41598-021-00670-3
53. Janani shishu suraksha karyakaram. Available at: https://www.nhp.gov.in/janani-shishu-suraksha-karyakaram-jssk.
54. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev (2000) 21:75–90.
55. Kevin Tucker J. Social justice as a tool to eliminate inequities in kidney disease. Semin Nephrol (2021) 41(3):203–10. doi: 10.1016/j.semnephrol.2021.05.001
56. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal data system 2019 annual data report: Epidemiology of kidney disease in the united states. Am J Kidney Dis (2020) 75(Suppl 1):A6–7. doi: 10.1053/j.ajkd.2019.09.003
57. Centers for Disease Control and Prevention. Diabetes Report Card. 2019 CDC , Atlanta , USA: US Dept of Health and Human Services. Last accessed on September 9, 2022. Available at https://www.cdc.gov/diabetes/library/reports/reportcard.html
58. Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol (2013) 24(9):1484–91. doi: 10.1681/ASN.2013010113
59. Zarkowsky DS, Arhuidese IJ, Hicks CW, Canner JK, Qazi U, Obeid T, et al. Racial/Ethnic disparities associated with initial hemodialysis access. JAMA Surg (2015) 150(6):529–36. doi: 10.1001/jamasurg.2015.0287
60. Purnell TS. Association of race and ethnicity with live donor kidney transplantation in the united states from 1995 to 2014. JAMA (2018) 319:49–61. doi: 10.1001/jama.2017.19152
61. Chaturvedi S, Ullah S, LePage AK, Hughes JT. Rising incidence of end-stage kidney disease and poorer access to kidney transplant among Australian aboriginal and Torres strait islander children and young adults. Kidney Int Rep (2021) 6:1704–10. doi: 10.1016/j.ekir.2021.02.040
62. Ring IT, Griffiths K. Life expectancy for indigenous people is improving, but closing the gap remains unacceptably slow. Med J Aust (2022) 217(1):26–7. doi: 10.5694/mja2.51606
63. Gupta A, Sudharsanam N. Large And persistent life expectancy disparities between india’s social groups. In: Population and development review 2022. doi: 10.1111/padr.12489
64. Kumari M, Mohanty SK. Caste, religions and regional differentials in life expectancy at birth in India: Cross-sectional estimates from recent national family health survey. BMJ Open (2020) 10:e035392. doi: 10.1136/bmjopen-2019-035392
65. Tsai JW, Cerdena JP, Goedel WC, Asch WS, Grubbs V, Mendu ML. Evaluating the impact and rationale of race-specific estimations of kidney function: Estimations from US NHANES 2015-2018. EClinicalMedicine (2021) 42:101197. doi: 10.1016/j.eclinm.2021.101197
66. Hsu CY, Yang W, Parikh RV, Anderson AH, Chen TK, Cohen DL, et al. CRIC study investigators. race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med (2021) 385:1750–60. doi: 10.1056/NEJMoa2103753
67. IPCC. Managing the risks of extreme events and disasters to advance climate change adaptation. In: A special report of working groups I and II of the intergovernmental panel on climate change. Cambridge, UK, and New York, NY, USA: Cambridge University Press (2012).
68. Rocque RJ, Beaudoin C, Ndjaboue R, Cameron L, Poirier-Bergeron L, Poulin-Rheault RA, et al. Health effects of climate change: An overview of systematic reviews. BMJ Open (2021) 11:e046333. doi: 10.1136/bmjopen-2020-046333
69. Johnson RJ, Sánchez-Lozada LG, Newman LS, Lanaspa MA, Diaz HF, Lemery J, et al. Climate change and the kidney. Ann Nutr Metab (2019) 74 Suppl 3:38–44. doi: 10.1159/000500344
70. Fakheri RJ, Goldfarb DS. Ambient temperature as a contributor to kidney stone formation: Implications of global warming. Kidney Int (2011) 79(11):1178–85. doi: 10.1038/ki.2011.76
71. Borg M, Bi P, Nitschke M, Williams S, McDonald S. The impact of daily temperature on renal disease incidence: An ecological study. Environ Health (2017) 16:114. doi: 10.1186/s12940-017-0331-4
72. Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med (2019) 380:1843–52. doi: 10.1056/NEJMra1813869
73. Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin J Am Soc Nephrol (2016) 11:1472–83. doi: 10.2215/CJN.13841215
74. Rajapakse S, Shivanthan MC. Selvarajah m chronic kidney disease of unknown etiology in Sri Lanka. Int J Occup Environ Health (2016) 22:259–64. doi: 10.1080/10773525.2016.1203097
75. Abraham G, Agarwal SK, Gowrishankar S, Vijayan M. Chronic kidney disease of unknown etiology: Hotspots in India and other Asian countries. Semin Nephrol (2019) 39:272–77. doi: 10.1016/j.semnephrol.2019.02.005
76. Anupama YJ, Sankarasubbaiyan S, Taduri G. Chronic kidney of unknown etiology : Case definition for India-a perspective. Indian J Nephrol (2020) 30:236–40. doi: 10.4103/ijn.IJN_327_18
77. Polgreen PM, Polgreen EL. Infectious diseases, weather, and climate. Clin Infect Dis (2018) 66(6):815–7. doi: 10.1093/cid/cix1105
78. Borg MA, Bi P. The impact of climate change on kidney health. Nat Rev Nephrol (2021) 17:294–5. doi: 10.1038/s41581-020-00365-4
79. Bharati J, Zavaleta-Cortijo C, Bressan T, Shingada A, Obrador G, Sola L, et al. The environment and kidney health: Challenges and opportunities. Salud Publica Mex (2022) 64(Supl 1):S46–55. doi: 10.21149/12799
80. Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis (2010) 17:254–64. doi: 10.1053/j.ackd.2010.03.011
81. Kuźma Ł, Małyszko J, Bachórzewska-Gajewska H, Kralisz P, Dobrzycki S. Exposure to air pollution and renal function. Sci Rep (2021) 11:11419. doi: 10.1038/s41598-021-91000-0
82. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet (2016) 388(10053):1659–724. doi: 10.1016/S0140-6736(16)31679-8
83. Abbara A, Rayes D, Khalil M, Kewara M, Tarakji A. Humanitarian catastrophe for civilians in northwest Syria. BMJ (2020) 368:m451. doi: 10.1136/bmj.m451
84. Gesesew H, Berhane K, Siraj ES, Siraj D, Gebregziabher M, Gebre YG, et al. The impact of war on the health system of the tigray region in Ethiopia: An assessment. BMJ Glob Health (2021) 6:e007328. doi: 10.1136/bmjgh-2021-007328
85. Berhe E, Paltiel O, Teka H, Gebrearegay H, Abraha HE, Tequare MH, et al. Ethiopia’s tigray dialysis service cut due to dwindling supplies amid war. Kidney Int Rep (2022) 7:1136–7. doi: 10.1016/j.ekir.2022.02.024
86. Fouad FM, Sparrow A, Tarakji A, Alameddine M, El-Jardali F, Coutts AP, et al. Health workers and the weaponisation of health care in Syria: a preliminary inquiry for the lancet-American university of Beirut commission on Syria. Lancet (2017) 390:2516–26. doi: 10.1016/S0140-6736(17)30741-9
87. Bdaiwi Y, Rayes D, Sabouni A, Murad L, Fouad F, Zakaria W, et al. Challenges of providing healthcare worker education and training in protracted conflict: A focus on non-government-controlled areas in north west Syria. Conflict Health (2020) 14:42. doi: 10.1186/s13031-020-00287-9
88. Bellorin-Font E, Carlini RG. Kidney disease in Venezuela: the impact of lingering humanitarian crisis. Nat Rev Nephrol (2021) 17:507–8. doi: 10.1038/s41581-021-00403-9
89. Islam Z, Gangat SA, Mohanan P, Rahmat ZS, Chbib DE, Marfani WB, et al. Mental health impacts of lebanon’s economic crisis on healthcare workers amidst COVID-19. Int J Health Plann Manage (2022) 37:1160–65. doi: 10.1002/hpm.3324
90. Youssef D, Youssef J, Abou-Abbas L, Kawtharani M, Hassan H. Prevalence and correlates of burnout among physicians in a developing country facing multi-layered crises: A cross-sectional study. Sci Rep (2022) 12:12615. doi: 10.1038/s41598-022-16095-5
91. Barnett-Vanes A, Hassounah S, Shawki M, OA I, Fung C, Kedia T, et al. Impact of conflict on medical education: A cross-sectional survey of students and institutions in Iraq. BMJ Open (2016) 16:e010460. doi: 10.1136/bmjopen-2015-010460
92. Lewtak K, Kanecki K, Tyszko P, Gorynski P, Bogdan M, Nitsch-Osuch A. Ukraine War refugees -threats and new challenges for healthcare in Poland. J Hosp Infect (2022) 125:37–43. doi: 10.1016/j.jhin.2022.04.006
93. Dumit NY, Honein-AbouHaidar G. The impact of the Syrian refugee crisis on nurses and the healthcare system in Lebanon: A qualitative exploratory study. J Nurs Scholarsh (2019) 51:289–98. doi: 10.1111/jnu.12479
94. Kardas P, Babicki M, Krawczyk J, Mastalerz-Migas A. War in Ukraine and the challenges it brings to the polish healthcare system. Lancet Reg Health Eur (2022) 15:100365. doi: 10.1016/j.lanepe.2022.100365
95. Gostin LO. The legal determinants of health: How can we achieve universal health coverage and what does it mean? Int J Health Policy Manag (2021) 10:1–4. doi: 10.34172/ijhpm.2020.64
96. O’Neil Institute for National and Global Health Law. UHC legal solutions network. Available at: https://oneill.law.georgetown.edu/uhc-legal-solutions-network.
97. Uberoi D, Fosman L. What role can the right to health play in advancing equity in kidney care? Semin Nephrol (2021) 41:220–9. doi: 10.1016/j.semnephrol.2021.05.003
98. Essue BM, Jha V, John O, Knight J, Jan S. Universal health coverage and chronic kidney disease in India. Bull World Health Organ (2018) 96:442. doi: 10.2471/BLT.18.208207
99. Jha V. Setting up a national dialysis service in India: Change, choice and principles. Nephrology (Carlton) (2016) 21(11):913–5. doi: 10.1111/nep.12803
100. Medical termination of pregnancy act (2021). Available at: https://www.who.int/india/news/detail/13-04-2021-india-s-amended-law-makes-abortion-safer-and-more-accessible.
101. Bhaktwani A. The PC-PNDT act in a nutshell. Indian J Radiol Imaging (2012) 22:133–4. doi: 10.4103/0971-3026.101114
102. Pandve HT, Fernandez K, Chawla PS, Singru SA. Some initiatives for promoting environmental sanitation in India. Indian J Occup Environ Med (2011) 15:76–7. doi: 10.4103/0019-5278.90379
103. Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. National program for prevention and control of fluorosis (NPPCF) revised guidelines (2014). Available at: https://www.mohfw.nic.in.
104. Pandve HT. India’s national action plan on climate change. Indian J Occup Environ Med (2009) 13:17–9. doi: 10.4103/0019-5278.50718
105. Prakash J, Prakash S, Ganiger VC. Changing epidemiology of acute kidney injury in pregnancy: A journey of four decades from a developing country. Saudi J Kidney Dis Transpl (2019) 30:1118–30. doi: 10.2471/BLT.18.208207
Keywords: poverty, climate change, gender inequity, dialysis, kidney health
Citation: Anandh U, Meena P, Karam S and Luyckx V (2022) Social, political and legal determinants of kidney health: Perspectives from lower- and middle-income countries with a focus on India. Front. Nephrol. 2:1024667. doi: 10.3389/fneph.2022.1024667
Received: 22 August 2022; Accepted: 21 September 2022;
Published: 24 October 2022.
Edited by:
Patrick Mark, University of Glasgow, United KingdomReviewed by:
Ikechi Okpechi, University of Alberta, CanadaCopyright © 2022 Anandh, Meena, Karam and Luyckx. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Urmila Anandh, dWFuYW5kaEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.