- 1Nephrology Division, Kidney Transplant Program David Geffen School of Medicine at University of California at Los Angeles (UCLA), Los Angeles, CA, United States
- 2Division of Nephrology and Hypertension Olive-View University of California at Los Angeles (UCLA) Medical Center, Sylmar, CA, United States
- 3David Geffen School of Medicine at University of California at Los Angeles (UCLA), Los Angeles, CA, United States
Sodium-glucose cotransporter 2 inhibitor (SGLT2i), a glucosuric agent initially approved for use as an antidiabetic agent, was unexpectedly found to confer cardio-and reno-protective effects in individuals with or without type 2 diabetes mellitus. Despite mounting evidence suggesting that SGLT2i provides cardio- and reno-protective benefits in both diabetic and non-diabetic and in chronic kidney disease (CKD) patients in the general population, reservations for its use in the transplant setting persist due to concerns for increased risk of genital mycotic and urinary tract infections. A comprehensive review of the literature on the efficacy and safety of SGLT2i use in diabetic kidney transplant recipients is herein presented followed by authors’ opinion on its optimal use in this patient population.
Introduction
The sodium-glucose cotransporter 2 inhibitor antidiabetic drug class improves glucose control in diabetic patients by inducing glucosuria via inhibition of glucose reabsorption at proximal convoluted tubules. In patients without hyperglycemia, the glomerular filtered load of glucose is relatively low, thereby limiting the extent of glucosuria and inherently minimizes hypoglycemic risk (1). The cardiorenal protective effects of SGLT2i in subjects with or without type 2 diabetes mellitus have been demonstrated in several randomized controlled trials and systematic review and meta-analysis (2–6). The EMPEROR-Reduced randomized placebo-controlled trial designed to study the effect of empagliflozin on cardiovascular and kidney outcomes across the spectrum of kidney function demonstrated a significant reduction in cardiovascular death, heart failure hospitalization, and total heart failure hospitalization among empagliflozin-treated patients compared with their placebo-treated counterparts at a median follow-up of 16 months. A reduction in composite kidney outcome (defined as sustained profound decline in estimated glomerular filtration rate [eGFR], chronic dialysis, or transplant) was also observed among patients randomized to receive empagliflozin irrespective of baseline renal function (Hazard ratio [HR] for patients with vs. without chronic kidney disease: 0.53 vs. 0.46, respectively, p=0.78) (7). Whether the cardiorenal benefits of SGLT2i seen in the general population can be extrapolated to the transplant population remains to be elucidated. The following section provides a summary of clinical studies evaluating the efficacy and safety of SGLTi use in kidney transplant recipients with pre-existing diabetes or post-transplantation diabetes mellitus (PTDM).
Methods
A literature search using PubMed Advanced Search Builder was conducted on October 1, 2022 using medical subject headings (MeSH) ([renal OR kidney] (1,246,987 articles) AND [transplant] (968,905) AND [sodium glucose transport protein 2, SGLT2, sodium glucose cotransporter 2, SGLT2, canaglifloxin, empagliflozin, ertugliflozin, OR dapagliflozin] (11,359)) yielded 223 articles. Full-text articles selected for inclusion in our review included randomized controlled trials, retrospective studies, cohort studies, and prospective and retrospective case series. A total of 10 studies evaluating the efficacy and safety of SGLT2i in diabetic kidney transplant recipients with pre-existing type 2 diabetes or PTDM were identified. Of the 10 studies, seven were included in a systematic review and meta-analysis (7/7 studies were graded as good quality using the quality assessment tool for case series studies proposed by the National Heart, Lung and Blood Institute). One additional study included in the systematic review and meta-analysis was in abstract format and was graded as fair quality.
Studies evaluating the efficacy and safety of SGLT2i use in diabetic kidney transplant recipients
At the time of this writing, there has been only one prospective, randomized placebo-controlled trial evaluating the efficacy and safety of SGLT2i in kidney transplant recipients with post-transplantation diabetes mellitus (PTDM). Study inclusion criteria included more than one year after transplant, stable renal function (eGFR > 30 mL/min/1.73m2), and stable immunosuppressive therapy. Forty-four eligible patients with PTDM were randomized to receive either empagliflozin (n=22) or placebo (n=22) for 24 weeks. A statistically significant reduction in A1C and body weight was observed among empagliflozin-treated patients compared with their placebo-treated counterparts (-0.2% vs. 0.1%, p=0.025 and -2.5 kg vs. +1.0 kg, respectively, p=0.014). There were no significant differences in adverse events, immunosuppressive drug levels, or eGFR between the two treatment groups (8). The incidence of urinary tract infections (UTIs) was comparable between the two treatment arms. One patient in the empagliflozin group had genital mycotic infection. While randomized controlled trial provides the highest quality of evidence, the small sample size and short duration of follow-up limit the ability to evaluate the outcome of eGFR change.
Systematic review and meta-analysis of eight studies involving 132 diabetic kidney transplant recipients demonstrated that SGLT2i use resulted in a significantly lower A1C (-0.56%, p=0.007) and body weight (-2.16 kg, p < 0.001) at end of study compared with baseline levels (9–16). No significant changes in eGFR, serum creatinine, urine protein creatinine ratio, or blood pressure were observed. In total, fourteen of 132 study patients had urinary tract infections and one of 72 participants from 5 studies had genital mycosis. Other reported adverse events included one case of acute kidney injury (AKI), one case of small ulcer in the lower extremity, and one case of cellulitis. There were no reported cases of euglycemic ketoacidosis or acute rejection during the follow-up period. Of eight studies, one was randomized controlled trial, hence only patients in the treatment arm were included in the meta-analysis (8). Seven were categorized as case series. Baseline eGFR among all study patients was 64.5 ± 19.9 ml/min/1.72 m2. Mean time from transplantation varied from 3 to 20 years. Follow-up duration ranged from 6 to 24 months. Based on the study results, it was concluded that SGLT2i are effective in lowering A1C, reducing body weight, and preserving kidney function in diabetic kidney transplant recipients without serious adverse events including euglycemic ketoacidosis and acute rejection (16).
In one single-center retrospective study designed to evaluate the metabolic, electrolyte and safety outcomes of early SGLT2i utilization in diabetic kidney transplant recipients, a significant reduction in body weight (-2.95 kg, p < 0.0001) and improvement in magnesium concentration (0.13, p=0.0004) were observed at 6-month follow-up. Overall insulin requirements decreased by -3.7 units (SD 22.8, p=0.17). There was no statistically significant change in eGFR at three and six months after SGLT2i initiation (change in eGFR of -1 mL/min/1.73m2, p=0.8831 and 1 mL/min/1.73m2, p=0.1478, respectively). Of the 50 patients enrolled in the study, half were started on therapy within the first year after transplant with a median time to drug initiation of 319 days. The incidence of UTIs was not significantly different from that reported historically in this high-risk patient population (14% vs. ~ 20%, respectively). There was one case of genital yeast infection. No cases of diabetic ketoacidosis (DKA), amputations, or AKI were seen. Study inclusion criteria included absence of AKI within 30 days prior to the initiation of SGLT2i, freedom from any UTIs 6 months prior to SGLT2i initiation, and eGFR ≥ 30 mL/min/1.73m2 at the time of SGLT2i initiation (17).
A retrospective multicenter study aimed to assess the safety, tolerability, and effectiveness of SGLT2 in kidney transplant recipients with pre-existing type 2 DM or PTDM demonstrated that adding SGLT2i to a regimen containing three antihyperglycemic agents resulted in a modest reduction in A1C at 3- and 12- month follow-up (0.6% [p=0.013] and 0.4% [p=0.016], respectively). Non-statistically significant weight loss occurred in 15 patients with a recorded weight at both baseline and 3 months post SGLT2i initiation (weight loss of 1.6 kg, p=0.11). Median time from kidney transplant to SGLT2i initiation was 28 months (range 16-60 months). Of 39 patients studied, 27 remained on therapy for at least 1 year. In patients with follow-up data, no statistically significant changes in kidney function (n=35) or tacrolimus exposure was observed (n=24). Ten patients (25%) experienced an adverse event while on SGLT2i therapy, with UTI being the most common (n=6, three required hospitalization). Five of six patients with UTI had a history of UTI prior to SGLT2 initiation. Four continued therapy without UTI recurrence. Urosepsis and pyelonephritis leading to SGLT2i discontinuation occurred in patients with prior history of recurrent UTIs. One patient developed diabetic ketoacidosis (due to uncontrolled diabetes) and concurrent UTI. Two patients developed complications from diabetic foot ulcers, both of whom had known peripheral vascular disease and prior history of diabetic foot ulcer. Two patients developed AKI more than 90 days after SGLT2i initiation determined by independent investigators to be unrelated to SGLT2i therapy. No episode of Fournier gangrene, genital mycotic infection, or bone fractures were identified during the study period (18).
A retrospective multicenter cohort study conducted to evaluate the efficacy and safety of SGLT2i in kidney transplant recipients with either pre-existing or post-transplantation diabetes demonstrated that SGLT2i use confers a renoprotective effect. Of the 2083 diabetics enrolled in the study, 226 were prescribed SGLT2i for more than 90 days and were included in the study. Baseline eGFR were similar between SGLT2i users and non-users (69.8 ± 17.8 vs. 68.9 ± 20.4 mL/min/1.73m2, respectively, p=0.459). During a mean follow-up of 62.9 ± 42.2 months, a significantly lower risk of the primary composite outcome of all-cause mortality, death-censored graft failure, and serum creatinine doubling were observed among SGLT2i users compared with non-users in multivariate and propensity score-matched models (adjusted HR 0.43; p=0.006, and adjusted HR 0.45; p=0.013, respectively). All three individual components of the outcome were significantly lower in SGLT2i users compared with non-users. A transient dip of more than 10% decline in eGFR occurred in 15.6% of SGLT2i-treated group during the first month but gradually recovered thereafter. Risk factors for eGFR dip were shorter time from transplantation to SGLT2i usage and higher mean tacrolimus trough levels. Post hoc analysis showed no difference in eGFR between the dipper and non-dipper groups at all time points. A significant decrease in urine protein-to-creatinine ratio was observed in both dipper (p=0.003) and non-dipper groups (p < 0.001). The incidence of any bacterial or fungal urinary tract infections were similar between SGLT2i users and non-users and no case of euglycemic ketoacidosis was identified. The study findings suggest that SGLT2i confers a renoprotective effect in diabetic kidney transplant recipients and its use is safe in such population (19).
However, the study is not without potential confounding factors. Notably, patients who were prescribed SGLT2i for less than 90 days were excluded from the study analysis (n=29), raising concerns that SGLT2i adverse events or deterioration in allograft function may have led to the discontinuation of therapy in these patients (20). Nonetheless, data from clinical trials and propensity-matched analyses of data from clinical practice unequivocally demonstrated that SGLT2i are “kidney safe” and do not predispose to acute kidney injury (AKI) (21). In contrast, its use reduces AKI risk and protects against diabetic kidney disease progression in the non-transplant population (6, 21).
Angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) use was more common in SGLT2i users vs. non-users (48.7% vs. 33.8%, respectively, p < 0.001). Nonetheless, although the cardiorenal protective effects of ACEI and ARB have been well-described in the general population, their favorable impact on patient and graft survival in the transplant setting has not been consistently demonstrated (22).
Studies evaluating the efficacy and safety of SGLT2i use in diabetic kidney transplant recipients are summarized in Table 1.
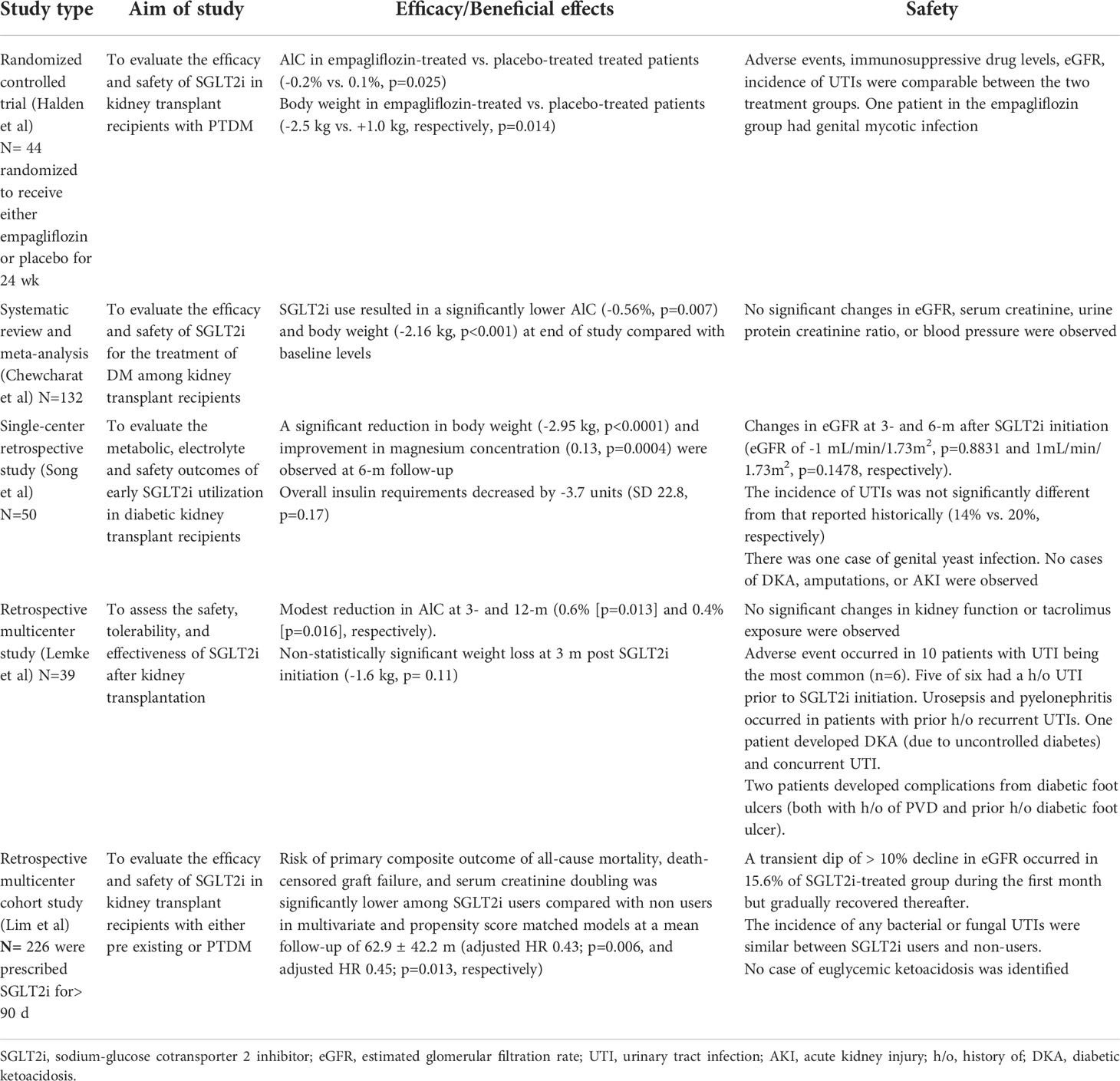
Table 1 Studies evaluating the efficacy and safety of SGLT2i use in diabetic kidney transplant recipients.
Discussion
SGLT2i use in diabetic kidney transplant recipients results in modest decrease in A1c without increased risk for genitourinary infectious complications or euglycemic DKA. However, it should also be noted that patients with history of recurrent UTIs, urosepsis or genital mycotic infections were generally excluded from these studies. Furthermore, in most studies, patients selected for SGLT2i therapy were more than 1-year post-transplantation because of concerns for fluctuating kidney allograft function and increased infection risk in the early post-transplantation period. Nonetheless, the potential beneficial effects of SGLT2i including weight loss, renoprotection, metabolic and electrolyte benefit, and lack of significant drug-drug interaction with calcineurin inhibitor render SGLT2i an attractive treatment option for kidney transplant recipients. Of interest, an experimental animal model of tacrolimus-induced diabetes demonstrated that empagliflozin improves hyperglycemia and suppressed the tacrolimus-induced twofold increase in the expression of SGLT2 (23). Furthermore, empagliflozin was found to have a direct protective effect on tacrolimus-induced renal injury. The study findings suggest that SGLT2i is suitable for use in kidney transplant recipients with tacrolimus-induced PTDM.
Similar to the general population, the use of SGLT2i in the transplant setting can cause an acute transient dip in eGFR, thought to be due to SGLT2i induced afferent arteriolar vasoconstriction. It is suggested that the natriuretic effect of SGLT2i leads to increased tubuloglomerular feedback and afferent arteriolar vasoconstriction even in denervated kidney allograft. Whether the resultant reduction in intraglomerular hypertension and hyperfiltration play a role in reducing proteinuria and preserving eGFR in diabetic kidney transplant recipients warrants further investigation.
To date, there has been no case of Fournier gangrene associated with SGLT2i use in diabetic kidney transplant recipients. One case of new diabetic foot ulcer/osteomyelitis occurring years after starting empagliflozin leading to distal limb amputation has been reported. Notably, the patient had a history of peripheral vascular disease and prior history diabetic foot ulcers (18). It has been hypothesized that the diuretic effect of SGLT2i could result in hypovolemia and hypoperfusion of distal lower-extremities, potentially leading to limb ischemia and necrosis and ultimately limb amputation (24). Clinicians should remain vigilant to such complications particularly in high risk diabetics with pre-existing foot ulcers or peripheral arterial disease.
In the authors’ opinion, SGLT2i use in diabetic kidney transplant recipients appears safe and effective in selected candidates. Based on currently available literature, optimal use of SGLT2i in kidney transplant recipients with pre-existing diabetes or PTDM is suggested in Table 2 (12, 19, 25–29). The routine recommendation for SGLT2i use in diabetic and non-diabetic kidney transplant recipients for their potential cardio- and reno-protective effects awaits further studies. Large randomized controlled trials with long-term follow-up are needed.
Author contributions
P-PT and P-PC contributed equally to searching the literature, writing the manuscript, and designing the tables. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation (2016) 134:752–72. doi: 10.1161/CIRCULATIONAHA.116.021887
2. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet (2019) 393(10166):31–9. doi: 10.1016/S0140-6736(18)32590-X
3. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-Art review. J Am Coll Cardiol (2020) 75(4):422–34. doi: 10.1016/j.jacc.2019.11.031
4. Anker SD, Butker J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
5. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: Findings from the CANVAS program. Am J Kidney Dis (2021) 77(1):23–34.e1. doi: 10.1053/j.ajkd.2020.06.018
6. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol (2019) 7(11):845–54. doi: 10.1016/S2213-8587(19)30256-6
7. Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: Insights from EMPEROR-reduced. Circulation (2021) 143(4):310–21. doi: 10.1161/CIRCULATIONAHA.120.051685
8. Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Jan Brox J, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care (2019) 42(6):1067–74. doi: 10.2337/dc19-0093
9. Rajasekeran H, Kim SJ, Cardella CJ, Schiff J, Cattral M, Cherney DZI, et al. Use of canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: A case series. Diabetes Care (2017) 40(7):e75–6. doi: 10.2337/dc17-0237
10. Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, et al. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant (2019) 19(3):907–19. doi: 10.1111/ajt.15223
11. Shah M, Virani Z, Rajput P, Shah B. Efficacy and safety of canagliflozin in kidney transplant patients. Indian J Nephrol (2019) 29(4):278–81. doi: 10.4103/ijn.IJN_2_18
12. Attallah N, Yassine L. Use of empagliflozin in recipients of kidney transplant: A report of 8 cases. Transplant Proc (2019) 51(10):3275–80. doi: 10.1016/j.transproceed.2019.05.023
13. Mahling M, Schork A, Nadalin S, Fritsche A, Heyne N, Guthoff M. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res (2019) 44(5):984–92. doi: 10.1159/000501854
14. Kong J, Joon J, Chul Y, Eun W, Hyuk K, Hyun SS, et al. Sodium/glucose cotransporter 2 inhibitor for the treatment of diabetes in kidney transplant patients. Nephrol Dial Transplant (2019) 34:SP770.
15. Alkindi f, Al-Omary HL, Hussain Q, Hakim MA, Chaaban A, Boobes Y. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. Transplant Proc (2020) 52(1):175–8. doi: 10.1016/j.transproceed.2019.11.007
16. Chewcharat A, Prasitlumkum N, Thongprayoon C, Bathini T, Medaura J, Vallabhajosuyula S, et al. Efficacy and safety of SGLT-2 inhibitors for treatment of diabetes mellitus among kidney transplant patients: A systematic review and meta-analysis. Med Sci (Basel) (2020) 8(4):47. doi: 10.3390/medsci8040047
17. Song CC, Brown A, Winstead R, Yakubu I, Demehin M, Kumar D, et al. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diabetes Metab (2020) 4(2):e00185. doi: 10.1002/edm2.185
18. Lemke A, Brokmeier HM, Leung SB, Mara KC, Mour GK, Wadei HM, et al. Sodium-glucose cotransporter 2 inhibitors for treatment of diabetes mellitus after kidney transplantation. Clin Transplant (2022) 36(8):e14718. doi: 10.1111/ctr.14718
19. Lim JH, Kwon S, Jeon Y, Kim YH, Kwon H, Kim YS, et al. The efficacy and safety of SGLT2 inhibitor in diabetic kidney transplant recipients. Transplantation (2022) 106(9):e404–12. doi: 10.1097/TP.0000000000004228
20. Francis RS. SGLT2i after kidney transplantation: Ready for prime time? Transplantation (2022) 106(9):1732–3. doi: 10.1097/TP.0000000000004227
21. Sridhar VS, Tuttle KR, Cherney DZI. We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am J Kidney Dis (2020) 76(4):454–6. doi: 10.1053/j.ajkd.2020.05.014
22. Hiremath S, Fergusson DA, Fergusson N, Bennett A, Knoll GA. Renin-angiotensin system blockade and long-term clinical outcomes in kidney transplant recipients: A meta-analysis of randomized controlled trials. Am J Kidney Dis (2017) 69(1):78–86. doi: 10.1053/j.ajkd.2016.08.018.
23. Jin J, Jin L, Luo K, Lim SW, Chung BH, Yang CW. Effect of empagliflozin on tacrolimus-induced pancreas islet dysfunction and renal injury. Am J Transplant (2017) 17(10):2601–16. doi: 10.1111/ajt.14316
24. Potier L, Mohammedi K, Velho G, Roussel R. SGLT2 inhibitors and lower limb complications: The diuretic-induced hypovolemia hypothesis. Cardiovasc Diabetol (2021) 20:107. doi: 10.1186/s12933-021-01301-x
25. Mahmoud TS, Yagan J, Hasan A, Gheith O, Mostafa M, Rida S, et al. Outcomes of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in diabetic kidney transplant recipients. Am J Transplant (2022) 22(supl 3). Available at: https://atcmeetingabstracts.com/abstract/outcomes-of-sodium-glucose-cotransporter-2-inhibitors-and-glucagon-like-peptide-1-receptor-agonists-in-diabetic-kidney-transplant-recipients/
26. Song C, Brown A, Winstead R, Sterling S, Yakubu I, Christensen J, et al. Intermediate term outcomes of SGLT2 inhibitors amongst diabetic kidney transplant recipients. Am J Transplant (2022) 22(suppl 3). Available at: https://atcmeetingabstracts.com/abstract/intermediate-term-outcomes-of-sglt2-inhibitors-amongst-diabetic-kidney-transplant-recipients/
27. Yagan J, Mahmoud TS, Hasan A, Gheith O, El-Serwy n, Rida S, et al. Sodium-glucose Co-transporter 2 inhibitors; short-term outcome in diabetic kidney transplant recipients. Am J Transplant (2022) 22(suppl 3). Available at: https://atcmeetingabstracts.com/abstract/outcomes-of-sodium-glucose-cotransporter-2-inhibitors-and-glucagon-like-peptide-1-receptor-agonists-in-diabetic-kidney-transplant-recipients
28. Marathias KP, Lambadiari VA, Markakis KP, Vlahakos VD, Bacharaki D, Raptis AE, et al. Competing effects of renin angiotensin system blockade and sodium-glucose cotransporter-2 inhibitors on erythropoietin secretion in diabetes. Am J Nephrol (2020) 51(5):349–56. doi: 10.1159/000507272
29. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia (2019) 62(7):1154–66. doi: 10.1007/s00125-019-4859-4
Keywords: sodium-glucose cotransporter 2 inhibitor, SGLT2 inhibitor, post-transplantation diabetes mellitus, PTDM, kidney transplantation, diabetic kidney disease, diabetic kidney transplant recipients
Citation: Pham P-TT and Pham P-CT (2022) Optimal use of SGLT2 inhibitors in diabetic kidney transplant recipients. Front. Nephrol. 2:1014241. doi: 10.3389/fneph.2022.1014241
Received: 08 August 2022; Accepted: 17 October 2022;
Published: 10 November 2022.
Edited by:
Masoud Sadeghi, Kermanshah University of Medical Sciences, IranReviewed by:
Ayman Al Jurdi, Massachusetts General Hospital, Harvard Medical School, United StatesChi Yuen Simon Cheung, Queen Elizabeth Hospital (QEH), Hong Kong SAR, China
Copyright © 2022 Pham and Pham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phuong-Thu T. Pham, UFBoYW1AbWVkbmV0LnVjbGEuZWR1
 Phuong-Thu T. Pham
Phuong-Thu T. Pham Phuong-Chi T. Pham2,3
Phuong-Chi T. Pham2,3