- 1Faculty of Natural Sciences and Mathematics, University of Maribor, Maribor, Slovenia
- 2Faculty of Medicine, University of Maribor, Maribor, Slovenia
The firing patterns of neuronal populations often exhibit emergent collective oscillations, which can display substantial regularity even though the dynamics of individual elements is very stochastic. One of the many phenomena that is often studied in this context is coherence resonance, where additional noise leads to improved regularity of spiking activity in neurons. In this work, we investigate how the coherence resonance phenomenon manifests itself in populations of excitatory and inhibitory neurons. In our simulations, we use the coupled FitzHugh-Nagumo oscillators in the excitable regime and in the presence of neuronal noise. Formally, our model is based on the concept of a two-layered network, where one layer contains inhibitory neurons, the other excitatory neurons, and the interlayer connections represent heterotypic interactions. The neuronal activity is simulated in realistic coupling schemes in which neurons within each layer are connected with undirected connections, whereas neurons of different types are connected with directed interlayer connections. In this setting, we investigate how different neurophysiological determinants affect the coherence resonance. Specifically, we focus on the proportion of inhibitory neurons, the proportion of excitatory interlayer axons, and the architecture of interlayer connections between inhibitory and excitatory neurons. Our results reveal that the regularity of simulated neural activity can be increased by a stronger damping of the excitatory layer. This can be accomplished with a higher proportion of inhibitory neurons, a higher fraction of inhibitory interlayer axons, a stronger coupling between inhibitory axons, or by a heterogeneous configuration of interlayer connections. Our approach of modeling multilayered neuronal networks in combination with stochastic dynamics offers a novel perspective on how the neural architecture can affect neural information processing and provide possible applications in designing networks of artificial neural circuits to optimize their function via noise-induced phenomena.
Introduction
Ensembles of neurons demonstrate a rich variety of coherent dynamics at the macroscopic scale which results from the input of multiple oscillatory signals and random perturbations at the microscopic scale. How neuronal populations adjust their dynamical responses to the superposition of these local noisy signals is of fundamental importance for information processing and plays a vital role in a variety of cognitive, motoric, and linguistic tasks (Riehle et al., 1997; Oram et al., 1999; Horwitz and Braun, 2004). Importantly, neuronal activity does not only depend on intrinsic neuronal properties and network architecture but is also heavily influenced by random fluctuations (Stein et al., 2005; Chialvo, 2010). Neuronal noise is a natural and unavoidable factor that originates from random opening and closing of ionic channels, stochastic nature of neuronal mechanisms, and noisy biochemical processes that underlie synaptic transmission (Faisal et al., 2008; Guo et al., 2018). While in most of the systems noise is mostly an undesirable component, it is now widely accepted that its presence is crucial to the proper functioning of neurons and can even enhance information processing capabilities and the regularity of neuronal activity (Lindner et al., 2004; McDonnell and Ward, 2011; Guo et al., 2018). This has led to a tremendous interest in investigating the sources and impact of intrinsic fluctuations in the nervous system and stems from the advances in experimental methods for identifying it as well as from a growing body of computational works demonstrating its functional consequences.
Neuronal noise has been shown to give rise to various collective dynamical behaviors, such as stochastic synchronization (Yang and Cao, 2009; Zakharova et al., 2013) or the induction of stochastic bifurcations (Hänggi and Bartussek, 1996; Gosak et al., 2008; Zakharova et al., 2010) and chimera states (Semenova et al., 2016; Majhi et al., 2019). Perhaps, the most famous examples of the so-called stochastic facilitation in neuronal systems are stochastic and coherence resonance. The former refers to the scenario where an appropriate intensity of noise evokes the best correlation between a weak periodic deterministic stimulus and the system's response (Longtin, 1993; McDonnell and Abbott, 2009; Calim et al., 2021), whereas the later encompasses a noise-induced enhancement in regularity of excited oscillations without external driving (Pikovsky and Kurths, 1997; Lee et al., 1998). Coherence is significant for communication within the brain (Deco and Kringelbach, 2016), and coherence resonance was recently suggested as a mechanism for improving neural communication (Pisarchik et al., 2019) and visual information processing (Itzcovich et al., 2017). Most importantly, this phenomenon is not only physiologically important but also theoretically appealing and challenging to understand. As a result, numerous computational models have been developed to elucidate the underlying mechanisms (Pradines et al., 1999; Ushakov et al., 2005; Beggs and Timme, 2012; Yu et al., 2017a; Guan et al., 2020; Baspinar et al., 2021). While primal investigations of coherence resonance in neuronal systems have scrutinized mainly systems with relatively small numbers of degrees of freedom, the scope has been shifting to coupled and spatially extended systems with many degrees of freedom (Lindner et al., 2004; Sagués et al., 2007; Kim et al., 2015), particularly to such complex networks describing connections between the individual units. Specifically, previous endeavors have revealed that the coherence resonance phenomenon can be modulated by the network size (Toral et al., 2003; Wang et al., 2005), as well as by the structure of the underlying network (Kwon and Moon, 2002; Gosak et al., 2010). Moreover, incorporating realistic neurophysiological features into neuronal network models, such as heterogeneity, spike-timing-dependent plasticity, and information transmission delays, was found to crucially affect the coherence resonance and can lead to very interesting dynamical behavior (Ozer and Uzuntarla, 2008; Li et al., 2009; Yu et al., 2013, 2015, 2017b; Semenova et al., 2016; Masoliver et al., 2017; Marhl and Gosak, 2019).
Neurons communicate mainly by two modalities of synaptic transmission, that is, chemical and electrical synapses (Pereda, 2014; Alcamí and Pereda, 2019), as well as by other means of communication, such as autaptic connections (Bacci and Huguenard, 2006) or presumably via magnetic fields (Guo S. et al., 2017; Ma and Tang, 2017). In a plethora of previous theoretical and experimental studies, it has been studied how the interplay between electrical and chemical coupling (Yilmaz et al., 2013; Yu et al., 2020), autapses (Wang et al., 2014; Uzun et al., 2017), and electromagnetic induction (Jia et al., 2019) affect the collective neuronal dynamics, including the coherence resonance (Balenzuela and García-Ojalvo, 2005; Yilmaz et al., 2016; Liu and Yang, 2018; Jia et al., 2021). Moreover, in recent years the research interest is shifting toward neuronal networks composed of two populations, one excitatory and the other inhibitory. In such networks neuronal communication can be of either excitatory or inhibitory nature, and they have been often used as simplified models of local networks in neocortex, hippocampus, as well as other structures (Brea et al., 2009; Isaacson and Scanziani, 2011; Hahn et al., 2019). Most importantly, several studies have shown that inhibition is not only responsible for diminishing neural activity but also leads to complex dynamical patterns that are inaccessible in systems with purely excitatory connectivity (Assisi et al., 2005; Stefanescu and Jirsa, 2008; Ledoux and Brunel, 2011; Bittner et al., 2017; Kim and Lim, 2017; Mongillo et al., 2018; Zhang and Liu, 2019; Rich et al., 2020; Xu et al., 2021). Along these lines, particular attention was given to the interplay between neuronal noise and the excitatory/inhibitory balance. Namely, the presence of inhibitory neurons generates additional nonlinear effects, which can lead to a rich variety of coherent network dynamics that emerges from noisy perturbations in a non-monotonous way (Li et al., 2009; Kawaguchi et al., 2011; Kim et al., 2015; Sancristóbal et al., 2016) and that a fine excitation-inhibition balance can improve the response of the neuronal network (Wang et al., 2012; Guo D. et al., 2017; Yu et al., 2018).
Due to the inherently compound dynamics and multiple facets of interactions that characterize neuronal assemblies, the standard network approach focusing on single networks in isolation might be insufficient to assess the underlying complex activity patterns. Recently, the multilayer network formalism has emerged as a new research direction to engage with such multidimensional systems (Boccaletti et al., 2014; Kivelä et al., 2014; Gosak et al., 2018; Battiston et al., 2020; Torres et al., 2021), including in the field of neuroscience (Bassett and Sporns, 2017; De Domenico, 2017; Maertens et al., 2021). In the context of neuronal networks, different types of interactions can be represented as a multiplex network, where the nodes in all layers are the same, whereas the connections in each layer signify different means of intercellular communication (Gosak et al., 2015; Nicosia and Latora, 2015; Nicosia et al., 2017). These concepts are gaining popularity in the field of computational neuroscience as multiplexing and different forms of interactions between layers were reported to evoke a rich variety of collective phenomena (Nicosia et al., 2013; Jalan and Singh, 2016; Majhi et al., 2017; Ge et al., 2018; Rakshit et al., 2018; Kundu et al., 2019; Parastesh et al., 2019; Sawicki et al., 2019; Bahramian et al., 2021; Kumar Verma and Ambika, 2021). As another option, the multilayer formalism can be used to characterize interactions between different neuronal subpopulations (Andreev et al., 2021) or to characterize heterologous interactions between neurons and other cell types (Virkar et al., 2016; Maertens et al., 2021). In this case, multilayered neuronal interaction schemes represent interdependent networks. Some recent computational studies have focused explicitly on the constructive role of noise in multilayered neuronal networks. It has been shown that multiplexing can give rise to coherence resonance in a two-layered network of excitable neurons (Semenova and Zakharova, 2018). Moreover, the coherence resonance and self-induced stochastic resonance in a multiplex neuronal network can be controlled by the network topology as well as by the intra- and interlayer time-delayed couplings (Yamakou and Jost, 2019; Yamakou et al., 2020). In a specific scenario where only one layer displays noise-induced spiking activity, a weak coupling between two neuronal populations in a multiplexed configuration was found to lead to coherence, anticoherence, and inverse stochastic resonances, as specified by the characteristics of interlayer links (Masoliver et al., 2021).
In this study, we make use of the multilayer network formalism to examine the coherence resonance phenomenon in populations of excitatory and inhibitory neurons. To that purpose, we represent this neuronal population as a two-layered network, where one layer contains inhibitory neurons, the other excitatory neurons, and the interlayer connections represent heterotypic interactions. This framework enables us to systematically investigate how different neurophysiological determinants influence the coherence resonance. Specifically, we investigate how the proportion of inhibitory neurons, the proportion of excitatory interlayer axons, and the architecture of interlayer connections between inhibitory and excitatory neurons affect the nature of the noise-induced dynamics.
Computational Models and Methods
We study a two-layered network of FitzHugh-Nagumo neurons in the excitable regime with noise. The equations that describe the neuronal dynamics of the ith neuron are as follows:
where Vi mimics the membrane potential and wi the recovery variable of the ith neuron, a, b, and c are constant parameters related to neuron properties, chosen to be a = 0.8, b = 0.9, and c = 4.5. The last term in Equation 1 represents noise, whereby D is noise intensity and η stands for Gaussian white noise with zero mean and unit variance.
We use a multilayer representation of the neuronal population so that one layer is populated by excitatory neurons and the other with inhibitory neurons. Formally, interactions between neurons within the same layer are portrayed by homotypic intralayer connections, whereas the coupling between different types of neurons is characterized by heterotypic interlayer connections. To model interactions between individual neurons, we introduce four different coupling strength coefficients: KEE, KEI, KIE, and KII. First and second indices refer to the type of presynaptic and postsynaptic neuron, respectively (E, excitatory and I, inhibitory), as schematically presented in Figure 1. We set the standard value of all coupling coefficients to be 0.2. We add coupling terms to Equation 1 and use different coupling coefficients depending on the type of postsynaptic neuron:
for excitatory neurons, and
for inhibitory neurons. Equation 2 remains the same for all neurons. and represent sums of all stimuli that the ith neuron receives from all coupled excitatory and inhibitory neurons, respectively, and are calculated as follows:
where N is the number of all neurons and NE the number of excitatory neurons. Mji is the element of the binary coupling matrix M, which indicates a connection between the jth and ith neuron. Indices with i ≤ NE are assigned to excitatory neurons and the rest to inhibitory neurons. The algorithm to generate the two-layered neuronal network composed of homotypic and heterotypic interneuronal connections is explained in more detail in continuation.
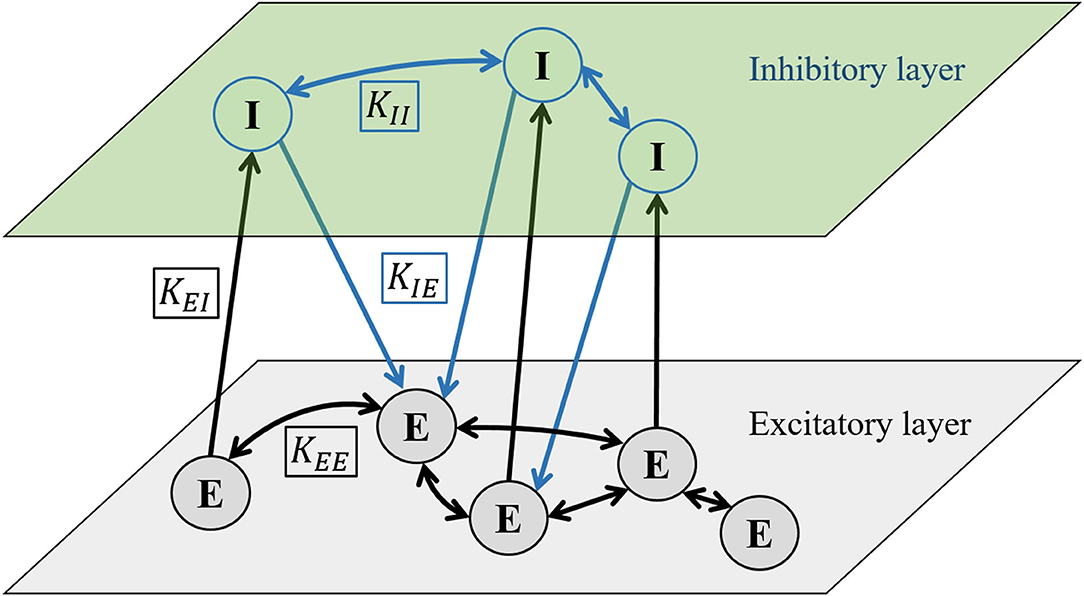
Figure 1. Schematic diagram showing the two-layered network formalism to model the interactions between excitatory (E) and inhibitory (I) neurons. The upper layer is populated by inhibitory neurons and the lower layer by excitatory neurons. Intralayer connections between excitatory neurons KEE and KII are bidirectional, whereas the interlayer connections KEI and KIE between different neuron types are directed. The directionality of interlayer connections is determined by the parameter ξ, which indicates the probability that the connection will be directed from the excitatory to the inhibitory layer (see main text for details).
Before constructing a two-layered network, we define a parameter γI, which describes the proportion of inhibitory neurons so that the number of inhibitory and excitatory neurons (NI and NE) can be expressed as
and
In our calculations, the number of neurons is set at N = 200. Both layers are populated with the proper number of neurons given a particular value of γI. We set the standard fraction of inhibitory neurons to γI = 0.2 since inhibitory neurons were found to be less common inside a typical cortex volume compared to excitatory neurons. The positions of neurons in the xy-plane are assigned randomly between 0 and 1, with the lower- and upper-layer harboring excitatory and inhibitory neurons, respectively. Connections within the same layer are modeled as a random geometric network where two neurons are connected bidirectionally if the distance between them is less than Rth. We determine the value of Rth such that in an average network with γI = 0.1 the average value of intralayer connections per neuron is k = 8.0. We find the appropriate value as Rth = 0.126. The same value is chosen for both layers so that the standard values lead to more sparsely connected neurons in the inhibitory layer, as is the case in realistic neuronal assemblies (Börgers and Kopell, 2003).
To construct interlayer connections, we made use of the vertex fitness network model (Caldarelli et al., 2002; Morita, 2006). Each neuron is assigned a fitness fi as follows:
where β defines the slope of potential fitness distribution and was set to β = 2.5. After assigning fitnesses to neurons deterministically based on their indices, the fitness values are randomly shuffled between neurons to ensure a well-mixed arrangement across both layers. Whether a connection between an ith neuron from the excitatory layer and jth neuron from the inhibitory layer exists is determined by the following condition:
where Lij is the distance between neurons in the xy-plane, Θ a connectivity threshold, and δ a network parameter that determines the nature of interlayer connections. Small values of δ lead to heterogeneous distribution of interlayer connections, while larger values prefer short-range connections, making the interlayer connectivity more homogeneous. This model represents an alternative to the preferential attachment mechanism (Barabási and Albert, 1999) as it does not rely on network growth and the links are established solely on the assigned fitness values. It should be noted that in our study no correlation between the node importance and dynamical characteristics of neurons was assumed, although this might have been a valid upgrade for heterogeneous neuronal populations. The total number of interlayer connections depends on the value of Θ. We set the average number of interlayer connections per neuron to be = 2.0 and iteratively adjust the threshold value accordingly for each generated network. All interlayer connections are directed whereby their directions are set randomly, as defined by the parameter ξ, which describes the proportion of excitatory interlayer axons or the probability that a given connection will be directed from excitatory to inhibitory layer. For each pair of excitatory and inhibitory neurons that satisfies the connectivity condition (Equation 10), we generate a random number r between 0 and 1. If r ≤ ξ, the connection is directed toward inhibitory layer representing an excitatory axon coupled to an inhibitory neuron, whereas if r > ξ the connection is directed toward the excitatory layer representing an inhibitory axon coupled to an excitatory neuron. We chose the standard fraction of excitatory interlayer axons to be ξ = 0.5 such that both directionalities are equally common. In Figure 2, we show two representative multilayer networks generated at two different values of δ, along with the corresponding interlayer node degree distributions. Evidently, for δ = 0.5, the arrangement of interlayer connections is very heterogeneous, as characterized by a scale-free-like degree distribution. In contrast, for δ = 10 the interlayer connections are established only between neurons that are adjacent in the xy-plane and the corresponding degree distribution follows a Poissonian distribution, indicating thereby a homogeneous interaction pattern between both layers.
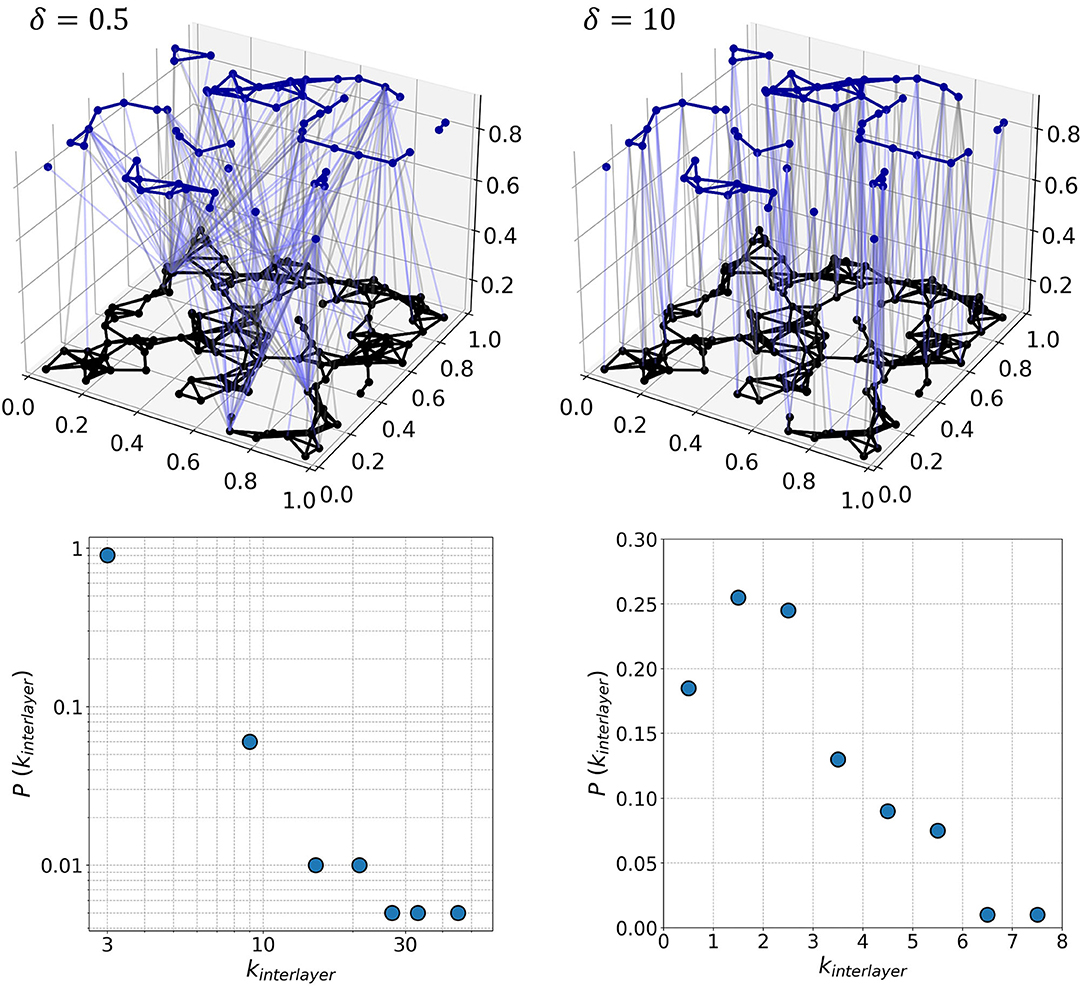
Figure 2. Two-layered network representation of neuronal communication patterns in populations with excitatory (black; lower layer) and inhibitory (upper layer, blue) neurons for two values of the interlayer connectivity parameters δ (upper row) and the corresponding degree distributions of interlayer connections (lower row). For δ = 0.5, there are many long-range connections between both layers and the architecture is very heterogeneous, as indicated by the scale-free character of the interlayer node degree distribution. In contrast, for δ = 10 the distribution of the interlayer excitatory and inhibitory axons is more homogeneous, and the corresponding degree distribution obeys a Poisson distribution. The network consists of 200 neurons, of which 30% were inhibitory (γI = 0.3). The directionality of interlayer connections was assigned at random, as specified by the parameter ξ, which was set at the standard value ξ = 0.5.
To determine the amount of coherence in the dynamics of the neuronal population, we computed the autocorrelation function for each neuron (Pikovsky and Kurths, 1997):
where τ is time lag and i deviation of membrane potential Vi from the temporal mean 〈Vi〉 at a particular time: i = Vi − 〈Vi〉. We qualify the coherence of an ith neuron with characteristic correlation time Ti, defined as (Pikovsky and Kurths, 1997),
We then calculated the average values of T for all neurons , excitatory neurons , and inhibitory neurons accordingly:
Higher values of correlation times indicate a higher degree of periodicity in the autocorrelation functions, which signifies a more coherent neuronal activity. In our simulations, the quantification of the coherence for a given set of parameters was computed on the basis of the average of , , and across 50 independent realizations in order to reduce fluctuations due to stochastic dynamics and from randomness incorporated in network constructions.
Results
First, we examine how coherence resonance manifests itself in the two-layered network composed of excitatory and inhibitory neurons. In the upper four panels of Figure 3, we show space-time plots of neuronal activity obtained at different noise intensities. It can be observed that the behavior reflects typical hallmarks of coherence resonance. Low noise intensity evokes seldom and irregular firings, high noise intensity leads to an erratic activity, whereas intermediate values of noise evoke the most coherent spatiotemporal patterns of neuronal dynamics. To quantify the observed behavior, we computed the normalized autocorrelation function (Equation 11) and the corresponding correlation times (Equation 12). The results in the lower row of Figure 3 clearly demonstrate that the stronger the periodic components of the autocorrelation function and the correlation times are the highest at intermediate noise intensities, corroborating thereby the existence of coherence resonance. In this simulation, we used the standard values of all parameters, namely, D, γI, ξ, KEE, KII, KEI, KIE, and δ. In what follows, we will investigate how variations of those parameters that refer principally to the characteristics of the multilayer neuronal network affect the noise-induced dynamics and the collective response of the system.
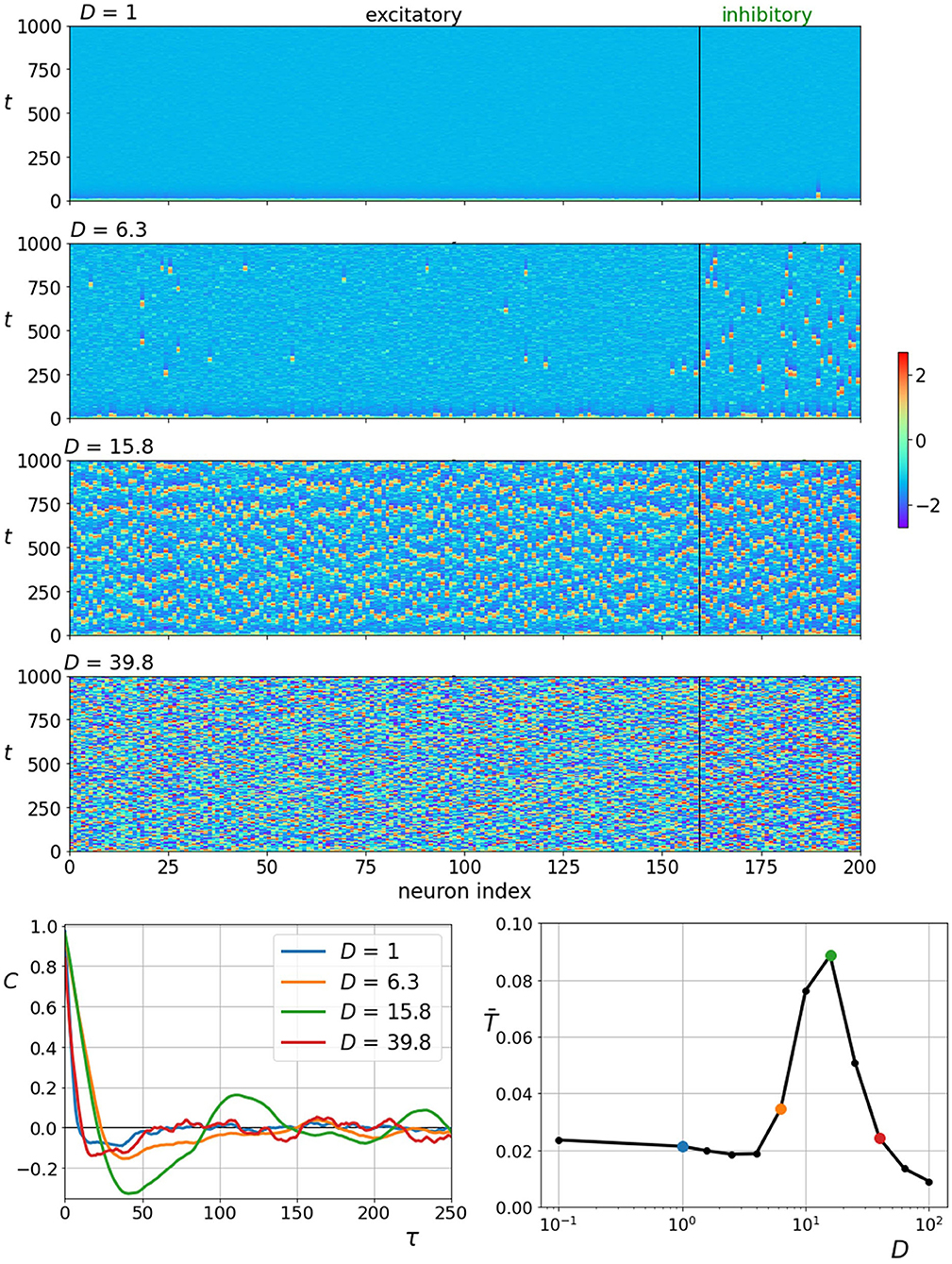
Figure 3. Raster plots of neuronal activity at different noise intensities D (upper four panels) with corresponding autocorrelation function plots of an individual neuron (bottom left panel). Average characteristic correlation time as a function of noise intensity D (bottom right panel). Colored points on the resonance curve represent specific noise intensities for which raster plots and autocorrelation plots are shown. Most coherent spiking is observed at around D = 15.
We start by analyzing how the fraction of inhibitory neurons γI influences neuronal dynamics. The upper two panels of Figure 4 show space-time plots of neuronal activity for two different fractions of γI. It is apparent that the activity of inhibitory neurons is intrinsically higher and increases further when their proportion is high (γI = 0.7). In addition, the spatiotemporal patterns appear to be more ordered when the number of inhibitory neurons is high. The lower four panels of Figure 4 show the color-coded values of average correlation times in dependence on the fraction of inhibitory neurons as well as noise intensity. It can be seen that increasing the fraction of inhibitory neurons improves the coherence in both layers. Moreover, as the proportion of inhibitory neurons is unrealistically high, the regularity of noise-induced oscillations begins to increase already at lower noise intensities. To visualize the role of the number of inhibitory neurons in further detail, we show in the lowermost panel on the right all correlation times as a function of γI near the optimal noise intensity.
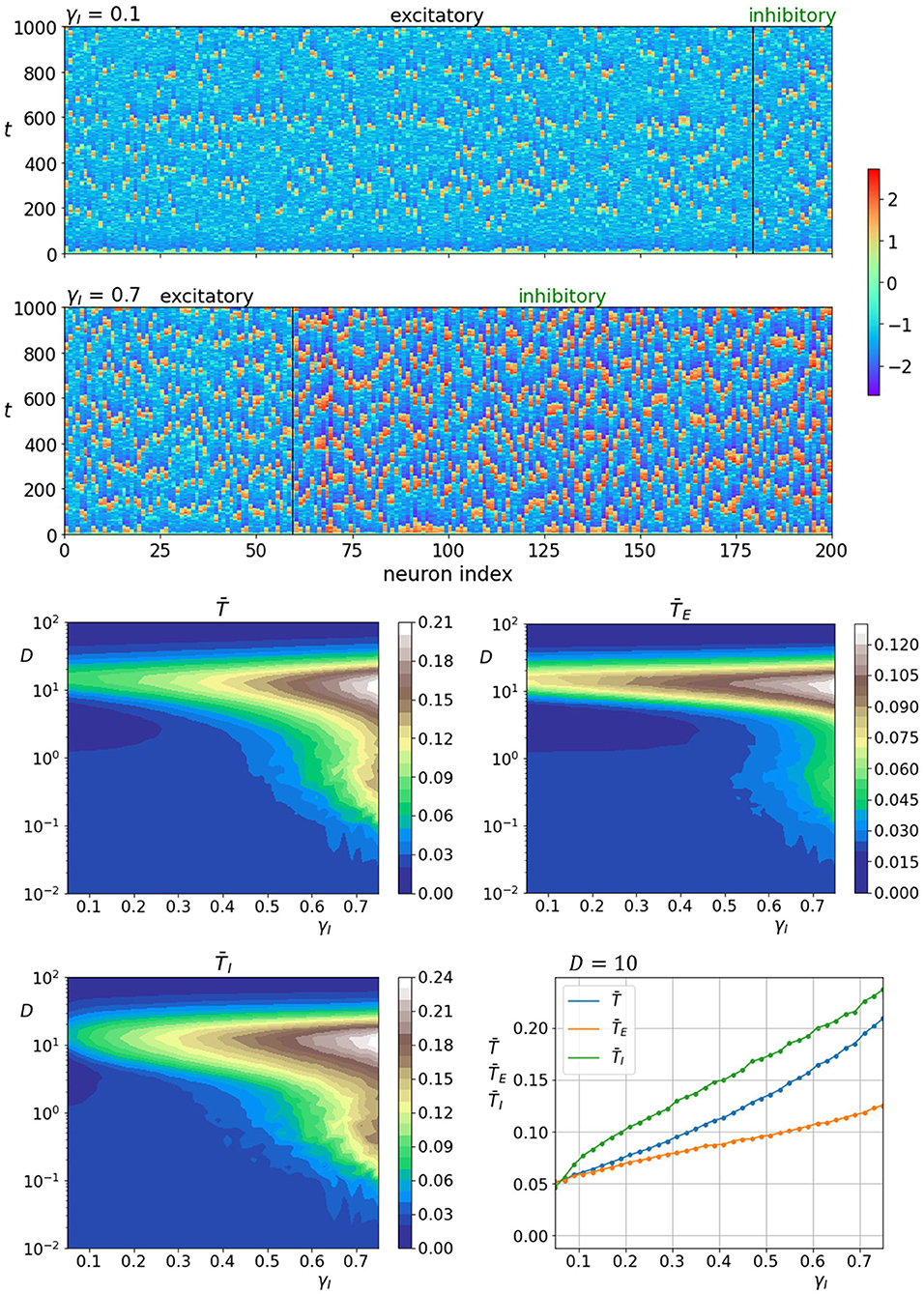
Figure 4. Raster plots of neuronal activity for two different proportions of inhibitory neurons γI (upper two panels). Quantification of the coherence resonance phenomenon in dependence on the noise intensity D and the fraction of inhibitory neurons γI (lower four panels). The contour plots show the color-coded average values of correlation times for all neurons , excitatory neurons , and inhibitory neurons . The lowermost panel on the right represents the average correlation times values near the optimal noise intensity values (D = 10).
Next, we examine how the nature of the neuronal dynamics depends on the fraction of excitatory interlayer axons ξ. The upper two panels of Figure 5 show space-time plots of neuronal activity for two different values of ξ. Lower fractions of excitatory axons seem to increase the activity in both layers. The same trend is observed for the coherence as seen on the lower four panels of Figure 5 that show average correlation times in dependence on the fraction of excitatory interlayer axons as well as noise intensity. The lowermost right panel showing average correlations time near the optimal noise intensity (D = 10) also indicates a similar increase in the coherence of noise-induced oscillation in both layers.
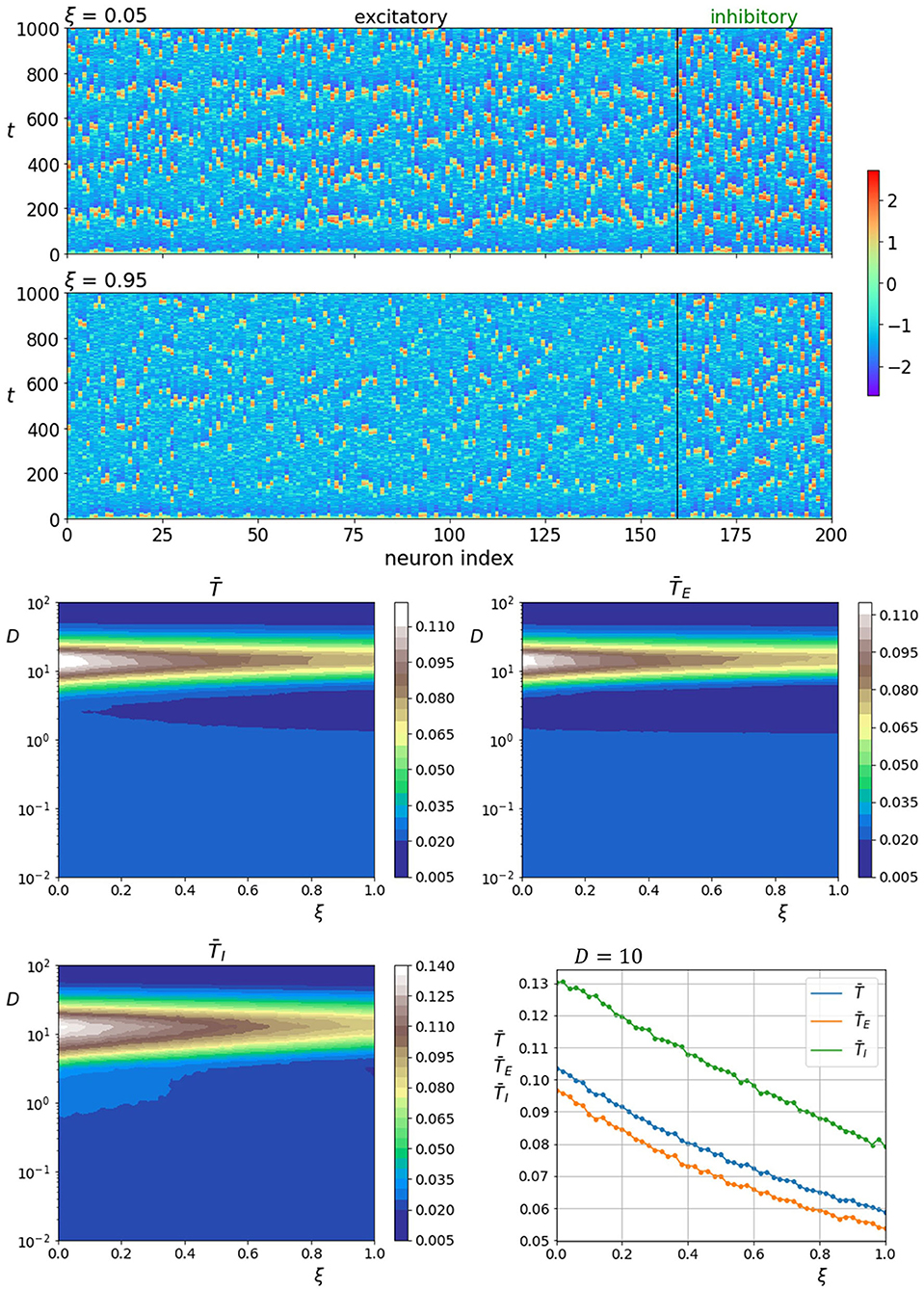
Figure 5. Raster plots of neuronal activity for two different interlayer proportions of excitatory axons ξ (upper two panels). Quantification of the coherence resonance phenomenon in dependence on the noise intensity D and the interlayer proportion of excitatory axons ξ (lower four panels). The contour plots show the color-coded average values of correlation times for all neurons , excitatory neurons , and inhibitory neurons . The lowermost panel on the right represents the average correlation times values near the optimal noise intensity values (D = 10).
We continue with the analysis of interlayer coupling strengths KEI and KIE. The upper two panels of Figure 6 show space-time plots of neuronal activity for two different coupling strengths of inhibitory interlayer axons KIE, where coupling strength of excitatory interlayer axons is held constant KEI = 0.2. The lower four panels show average correlation time values as a function of both interlayer coupling strength, whereas the lowermost right panel shows them only in dependence on inhibitory axons coupling strength KIE. We see an obvious increase in regularity when the coupling of inhibitory axons to excitatory layer gets stronger. Notably, the increase in coherence affects the excitatory layer more profoundly as the relative gain in average correlation time in the inhibitory layer is significantly lower. In contrast, enhancing the coupling strength of excitatory interlayer axons decreases the level of regularity in both layers.
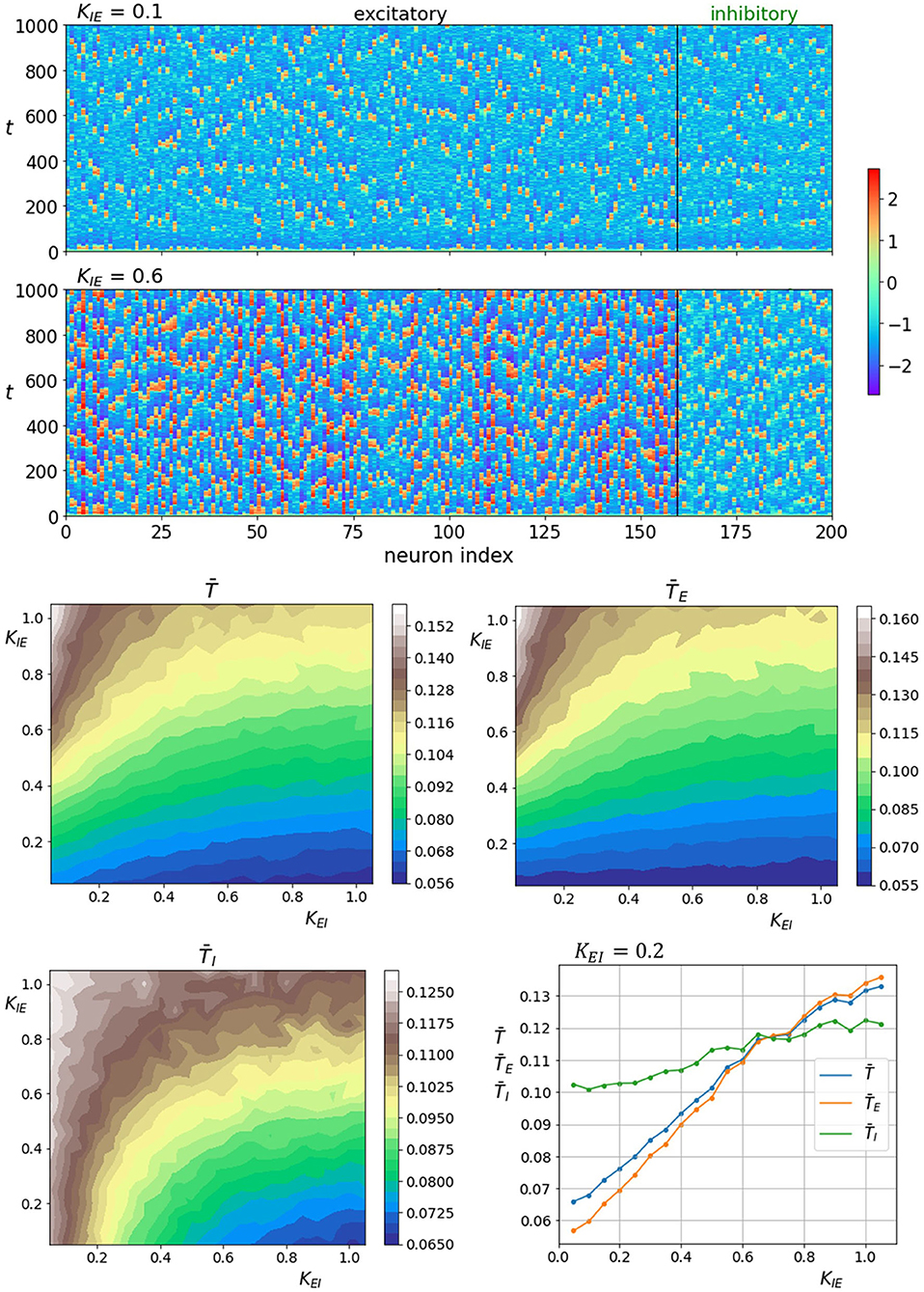
Figure 6. Raster plots of neuronal activity for two different interlayer coupling strengths KIE where KEI = 0.2 (upper two panels). Quantification of the coherence resonance phenomenon in dependence on the interlayer coupling strengths KEI and KIE (lower four panels). The contour plots show the color-coded average values of correlation times for all neurons , excitatory neurons , and inhibitory neurons . The lowermost panel on the right represents the average correlation times values in dependence on coupling strength KIE at a constant value of KEI = 0.2.
Finally, we investigate how neuronal activity is affected by the interlayer network parameter δ. The upper two panels of Figure 7 show space-time plots of neuronal activity for two different values of parameter δ. The lower four panels show average correlation times in dependence on parameter δ and noise intensity, whereas the lowermost panel on the right shows the relation between the correlation times and interlayer network parameter δ near optimal noise intensity. It can be seen that varying the parameter δ affects the noise-induced neuronal dynamics so that a higher degree of regularity is attained when the interlayer connectivity is heterogeneous (δ < 1). The effect is slightly more pronounced for the inhibitory layer. These results in combination with previous reported findings seem to point toward a general pattern where the overall coherence of neuronal dynamics can be enhanced by a stronger damping of the excitatory layer. This can be achieved by either a larger fraction of inhibitory neurons, larger fraction of inhibitory interlayer axons, a stronger coupling of inhibitory axons with excitatory layer, or a more efficient interaction patterns between both types of neurons.
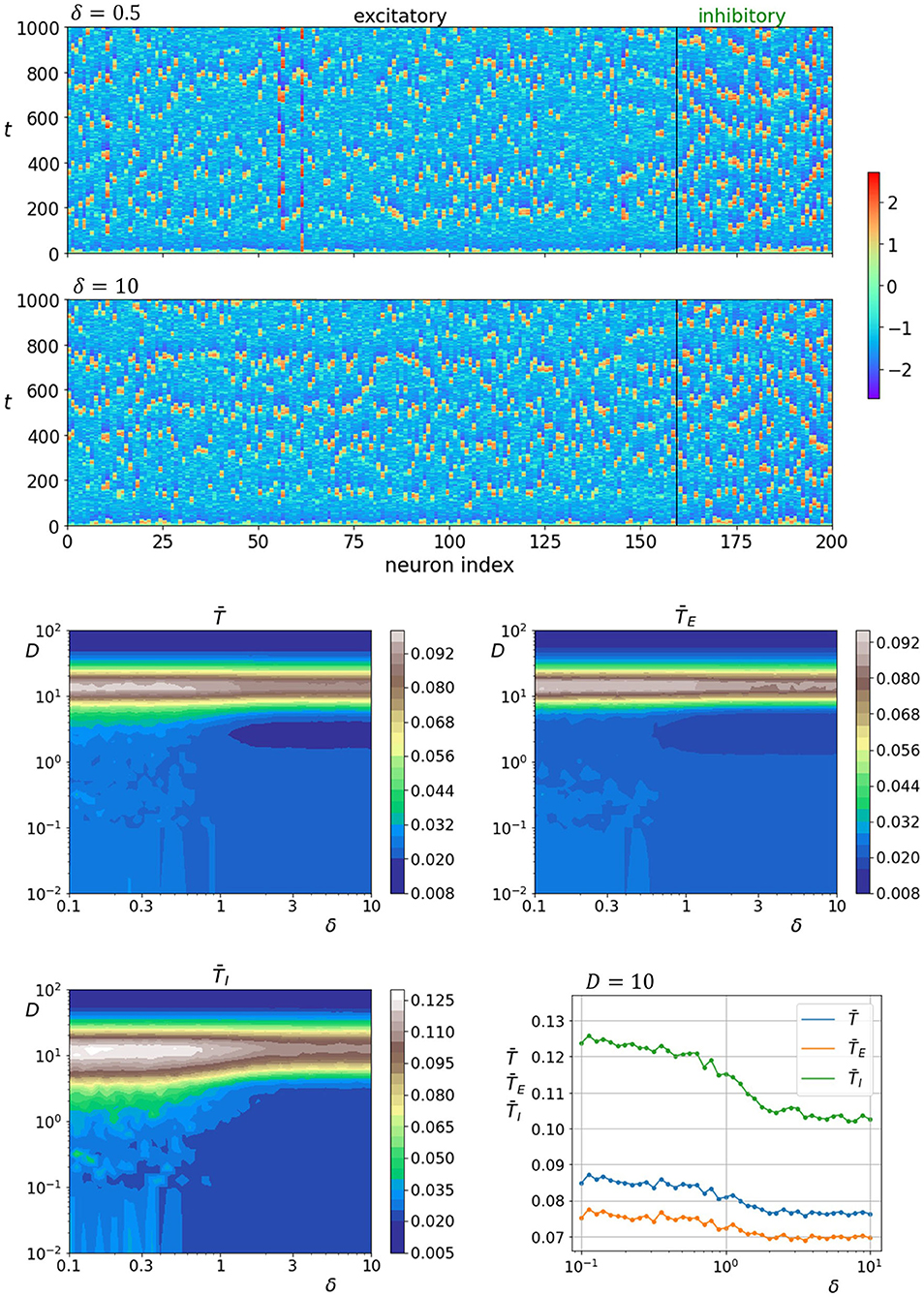
Figure 7. Raster plots of neuronal activity for two different values of parameter δ (upper two panels). Quantification of the coherence resonance phenomenon in dependence on the noise intensity D and parameter δ (lower four panels). The contour plots show the color-coded average values of correlation times for all neurons , excitatory neurons , and inhibitory neurons . The lowermost panel on the right represents the average correlation times values near the optimal noise intensity values (D = 10).
Discussion
Coherent neuronal activity is a putative mechanism whereby neuronal populations subserving specific functions communicate for the purpose of establishing dynamical patterns that accomplish perception, cognition, and action (Riehle et al., 1997; Oram et al., 1999; Horwitz and Braun, 2004). It is nowadays a well-established fact that the creation of the neuronal dynamics relies significantly on random fluctuations (Stein et al., 2005; Faisal et al., 2008; Chialvo, 2010; McDonnell and Ward, 2011; Guo et al., 2018). Previous research encompassing tissue slice preparations, whole brains, and computational models has revealed that neural synchronization can be facilitated by the addition of optimal amounts of neuronal noise, i.e., neurons can exploit noise to enhance the regularity of their pulsing dynamics. The numerous experimental evidence showing the constructive role of noise at the microscopic (Douglass et al., 1993; Gluckman et al., 1996; Gu et al., 2002; Manjarrez et al., 2002; Ward et al., 2010; Sancristóbal et al., 2016) and macroscopic (Collins et al., 1996; Simonotto et al., 1997; Russell et al., 1999; Hidaka et al., 2000; Kitajo et al., 2003; Itzcovich et al., 2017) levels of neuronal organization has evoked immense interest of computational neuroscientists to investigate the underlying mechanisms and functional implications (Lindner et al., 2004; Sagués et al., 2007; McDonnell and Abbott, 2009; Calim et al., 2021). In recent years, the models are becoming increasingly comprehensive as they incorporate several types of neuronal populations, different types of interactions, information transmission delays, plasticity in connectivity patterns, etc. Moreover, along with the developments in the field of network science, the scope is shifting to multilayer networks as this novel concept offers a more comprehensive framework to assess the complex interactions across multiple facets of neurophysiological relationships and the resulting dynamical phenomena (Boccaletti et al., 2014; Kivelä et al., 2014; Gosak et al., 2018, 2022; Battiston et al., 2020; Torres et al., 2021).
In this study, we aimed to extend the scope of coherence resonance in a multilayer network model of neuronal dynamics. We considered a mixed heterogeneous and excitable neural population composed of excitatory and inhibitory neurons. The multilayer network formalism was used to represent these subpopulations so that each neuron type occupied a separate layer and the directed interlayer connections represented excitatory or inhibitory axons. This setting enabled us to systematically investigate how different neurophysiological determinants affected the characteristics of the noise-driven neuronal dynamics. We focused specifically on the proportions of inhibitory neurons and excitatory interlayer axons as well as on the strength and structure of the interlayer connectivity. Our numerical calculations have revealed that the proposed setup represents a viable route for the realization of coherence resonance (Figure 3). The resonant behavior was found to be quite immensely affected by the number of inhibitory neurons so that a higher fraction of inhibitory units enhanced the regularity of the entire system (Figure 4). Moreover, a higher fraction of excitatory axons was found to reduce the regularity of noise-induced oscillations (Figure 5). The accuracy of neuronal excitations was also found to be affected by the interplay between the coupling strength between the excitatory in inhibitory layer, whereby the coherence resonance was more pronounced when the influence of the inhibitory layer was promoted. Interestingly, the regularity in the excitatory layers turned out to be affected more (Figure 6). Finally, we investigated how neuronal activity depends on the structure of the heterotypic interactions between the excitatory and inhibitory neurons. By varying the parameter for interlayer connectivity, the structure of connections between both layers was smoothly altered between a highly heterogeneous scale-free-like structure and a rather homogeneous organization with no substantial differences in the number of connections between individual neurons (Figure 2). It turned out that the more heterogeneous configuration evoked a higher degree of regularity in the neuronal dynamics (Figure 7). Apparently, the more heterogeneous and scale-free-like interlayer connectivity structure represents a more efficient setting for information transmission as the homogeneous interlayer connectivity pattern (Vragović et al., 2005) and as such enhances the communication between both layers, which makes the influence of the inhibitory neurons more pronounced. To sum up, our findings indicate that giving prominence to the inhibitory layer, either by increasing the number of inhibitory neurons or enhancing the coupling strength or efficiency from the inhibitory to the excitatory layer, always acts as a promoter of regular neuronal activity.
Previous computational studies have already investigated collective activity in networks of interconnected excitatory and inhibitory neurons as such networks are ubiquitous in the brain (Best et al., 2007; Sukenik et al., 2021). In the last decade, computational models and analyses have played an important role in identifying the nature of neuronal rhythmicity that orientates from the complex interplay between subpopulations of inhibitory and excitatory cells (Jadi and Sejnowski, 2014; Hahn et al., 2019; Sukenik et al., 2021). It has been shown that the presence of inhibitory neurons can facilitate rhythmic activity in neural networks (Shimokawa and Shinomoto, 2006; Cheng and Cao, 2017; Ma and Tang, 2017) and that the presence of heterogeneous inhibitory neurons can optimize their responsiveness to external stimuli (Di Volo and Destexhe, 2021). In particular, the architecture of the neuronal network was reported to play a major role in this respect (Rich et al., 2020).
Furthermore, in the context of stochastic facilitation, it has been reported that fine-tuning of inhibitory synapses can improve frequency-difference-dependent stochastic resonance in neuronal networks (Guo D. et al., 2017) and that the presence of inhibitory chemical synapses at the intralayer levels optimizes stochastic resonance in multiplex neural networks (Yamakou et al., 2020). It has also been shown that the presence of inhibitory neurons can enhance the coherence resonance behavior in neuronal networks, but the effect is rather complex and depends on the interplay between the coupling characteristics and noise (Yu et al., 2018). Along similar lines, inhibitory autapses have been recently recognized as another suppressive agent that can facilitate coherence resonance in neuronal networks (Jia et al., 2021).
Conclusion
Our results demonstrate another example of how the influence of inhibitory neurons can lead to an improved regularity of the noise-driven neuronal dynamics, which we expand to a multilayer network concept. The proposed computational model enabled us to systematically investigate the role of different neuronal network parameters and allowed us to gain a better understanding on how the architecture of interlayer connections between inhibitory and excitatory neurons affected the nature of neuronal activity patterns, even though future studies will be required to gain further insights into the underlying mechanisms. Furthermore, interesting future research directions on the topic would be to use more realistic neuronal models that could incorporate a detailed description of neuronal heterogeneity, synaptic plasticity, information transmission delays, and signaling via autaptic connections. All these features are genuine neurophysiological determinants that have been shown to affect the collective dynamics of neuronal networks. Most importantly, the proposed multilayer network representation of the neuronal subpopulations can serve as a solid ground for further upgrades in this direction.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
DR and MG conceived the research, designed the model, reviewed and edited the manuscript, and gave final approval for publication. DR performed calculations and prepared the figures. MG wrote the initial draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the financial support from the Slovenian Research Agency (grant no. P3-0396, I0-0029, J1-2457, J3-2525, N3-0133, and J3-3077).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcamí, P., and Pereda, A. E. (2019). Beyond plasticity: the dynamic impact of electrical synapses on neural circuits. Nat. Rev. Neurosci. 20, 253–271. doi: 10.1038/s41583-019-0133-5
Andreev, A. V., Maksimenko, V. A., Pisarchik, A. N., and Hramov, A. E. (2021). Synchronization of interacted spiking neuronal networks with inhibitory coupling. Chaos Solitons Fractals 146:110812. doi: 10.1016/j.chaos.2021.110812
Assisi, C. G., Jirsa, V. K., and Kelso, J. A. S. (2005). Synchrony and clustering in heterogeneous networks with global coupling and parameter dispersion. Phys. Rev. Lett. 94, 1–4. doi: 10.1103/PhysRevLett.94.018106
Bacci, A., and Huguenard, J. R. (2006). Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron 49, 119–130. doi: 10.1016/j.neuron.2005.12.014
Bahramian, A., Parastesh, F., Pham, V. T., Kapitaniak, T., Jafari, S., and Perc, M. (2021). Collective behavior in a two-layer neuronal network with time-varying chemical connections that are controlled by a Petri net. Chaos 31:45840. doi: 10.1063/5.0045840
Balenzuela, P., and García-Ojalvo, J. (2005). Role of chemical synapses in coupled neurons with noise. Phys. Rev. E Stat. Nonlinear, Soft Matter Phys. 72:021901. doi: 10.1103/PhysRevE.72.021901
Barabási, A.-L., and Albert, R. (1999). Emergence of scaling in random networks. Science 286:509, doi: 10.1126/science.286.5439.509
Baspinar, E., Schülen, L., Olmi, S., and Zakharova, A. (2021). Coherence resonance in neuronal populations: mean-field versus network model. Phys. Rev. E 103, 1–15. doi: 10.1103/PhysRevE.103.032308
Bassett, D. S., and Sporns, O. (2017). Network neuroscience. Nat. Neurosci. 20, 353–364. doi: 10.1038/nn.4502
Battiston, F., Cencetti, G., Iacopini, I., Latora, V., Lucas, M., Patania, A., et al. (2020). Networks beyond pairwise interactions: structure and dynamics. Phys. Rep. 874, 1–92. doi: 10.1016/j.physrep.2020.05.004
Beggs, J. M., and Timme, N. (2012). Being critical of criticality in the brain. Front. Physiol. 3:163. doi: 10.3389/fphys.2012.00163
Best, J., Park, C., Terman, D., and Wilson, C. (2007). Transitions between irregular and rhythmic firing patterns in excitatory-inhibitory neuronal networks. J. Comput. Neurosci. 23, 217–235. doi: 10.1007/s10827-007-0029-7
Bittner, S. R., Williamson, R. C., Snyder, A. C., Litwin-Kumar, A., Doiron, B., Chase, S. M., et al. (2017). Population activity structure of excitatory and inhibitory neurons. PLoS ONE 12, 1–27. doi: 10.1371/journal.pone.0181773
Boccaletti, S., Bianconi, G., Criado, R., del Genio, C. I., Gómez-Gardeñes, J., Romance, M., et al. (2014). The structure and dynamics of multilayer networks. Phys. Rep. 544, 1–122. doi: 10.1016/j.physrep.2014.07.001
Börgers, C., and Kopell, N. (2003). Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput. 15, 509–538. doi: 10.1162/089976603321192059
Brea, J. N., Kay, L. M., and Kopell, N. J. (2009). Biophysical model for gamma rhythms in the olfactory bulb via subthreshold oscillations. Proc. Natl. Acad. Sci. U. S. A. 106, 21954–21959. doi: 10.1073/pnas.0910964106
Caldarelli, G., Capocci, A., De Los Rios, P., and Muñoz, M. A. (2002). Scale-free networks from varying vertex intrinsic fitness. Phys. Rev. Lett. 89:258702. doi: 10.1103/PhysRevLett.89.258702
Calim, A., Palabas, T., and Uzuntarla, M. (2021). Stochastic and vibrational resonance in complex networks of neurons. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 379:236. doi: 10.1098/rsta.2020.0236
Cheng, L., and Cao, H. (2017). Synchronization dynamics of two heterogeneous chaotic rulkov neurons with electrical synapses. Int. J. Bifurc. Chaos 27:1730009. doi: 10.1142/S0218127417300099
Chialvo, D. R. (2010). Emergent complex neural dynamics. Nat. Phys. 6, 744–750. doi: 10.1038/nphys1803
Collins, J. J., Imhoff, T. T., and Grigg, P. (1996). Noise-enhanced tactile sensation. Nature 383, 770–770. doi: 10.1038/383770a0
De Domenico, M. (2017). Multilayer modeling and analysis of human brain networks. Gigascience 6, 149–152. doi: 10.1093/gigascience/gix004
Deco, G., and Kringelbach, M. L. (2016). Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci. 39, 125–135. doi: 10.1016/j.tins.2016.01.001
Di Volo, M., and Destexhe, A. (2021). Optimal responsiveness and information flow in networks of heterogeneous neurons. Sci. Rep. 11:17611. doi: 10.1038/s41598-021-96745-2
Douglass, J. K., Wilkens, L., Pantazelou, E., and Moss, F. (1993). Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365, 337–340. doi: 10.1038/365337a0
Faisal, A. A., Selen, L. P. J., and Wolpert, D. M. (2008). Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303. doi: 10.1038/nrn2258
Ge, M., Jia, Y., Kirunda, J. B., Xu, Y., Shen, J., Lu, L., et al. (2018). Propagation of firing rate by synchronization in a feed-forward multilayer Hindmarsh–Rose neural network. Neurocomputing 320, 60–68. doi: 10.1016/j.neucom.2018.09.037
Gluckman, B. J., Netoff, T. I., Neel, E. J., Ditto, W. L., Spano, M. L., and Schiff, S. J. (1996). Stochastic resonance in a neuronal network from mammalian brain. Phys. Rev. Lett. 77, 4098–4101. doi: 10.1103/PhysRevLett.77.4098
Gosak, M., Dolenšek, J., Markovič, R., Slak Rupnik, M., Marhl, M., and StoŽer, A. (2015). Multilayer network representation of membrane potential and cytosolic calcium concentration dynamics in beta cells. Chaos Solitons Fractals 80, 76–82. doi: 10.1016/j.chaos.2015.06.009
Gosak, M., Korošak, D., and Marhl, M. (2010). Optimal network configuration for maximal coherence resonance in excitable systems. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 81, 1–7. doi: 10.1103/PhysRevE.81.056104
Gosak, M., Marhl, M., and Perc, M. (2008). Chaos out of internal noise in the collective dynamics of diffusively coupled cells. Eur. Phys. J. B 62, 171–177. doi: 10.1140/epjb/e2008-00132-y
Gosak, M., Markovič, R., Dolenšek, J., Slak Rupnik, M., Marhl, M., StoŽer, A., et al. (2018). Network science of biological systems at different scales: a review. Phys. Life Rev. 24, 118–135. doi: 10.1016/j.plrev.2017.11.003
Gosak, M., Milojević, M., Duh, M., Skok, K., and Perc, M. (2022). Networks behind the morphology and structural design of living systems. Phys. Life Rev. 41, 1–21. doi: 10.1016/j.plrev.2022.03.001
Gu, H., Yang, M., Li, L., Liu, Z., and Ren, W. (2002). Experimental observation of the stochastic bursting caused by coherence resonance in a neural pacemaker. Neuroreport 13, 1657–1660. doi: 10.1097/00001756-200209160-00018
Guan, L., Gu, H., and Jia, Y. (2020). Multiple coherence resonances evoked from bursting and the underlying bifurcation mechanism. Nonlinear Dyn. 100, 3645–3666. doi: 10.1007/s11071-020-05717-0
Guo, D., Perc, M., Liu, T., and Yao, D. (2018). Functional importance of noise in neuronal information processing. EPL 124, 1–7. doi: 10.1209/0295-5075/124/50001
Guo, D., Perc, M., Zhang, Y., Xu, P., and Yao, D. (2017). Frequency-difference-dependent stochastic resonance in neural systems. Phys. Rev. E 96, 1–6. doi: 10.1103/PhysRevE.96.022415
Guo, S., Xu, Y., Wang, C., Jin, W., Hobiny, A., and Ma, J. (2017). Collective response, synapse coupling and field coupling in neuronal network. Chaos Solitons Fractals 105, 120–127. doi: 10.1016/j.chaos.2017.10.019
Hahn, G., Ponce-Alvarez, A., Deco, G., Aertsen, A., and Kumar, A. (2019). Portraits of communication in neuronal networks. Nat. Rev. Neurosci. 20, 117–127. doi: 10.1038/s41583-018-0094-0
Hänggi, P., and Bartussek, R. (1996). “Brownian rectifiers: how to convert brownian motion into directed transport,” in Nonlinear Physics of Complex Systems, eds J. Parisi, S. C. Müller, and W. Zimmermann (Berlin: Springer), 294–308. Available online at: https://link.springer.com/chapter/10.1007/BFb0105447
Hidaka, I., Nozaki, D., and Yamamoto, Y. (2000). Functional stochastic resonance in the human brain: noise induced sensitization of baroreflex system. Phys. Rev. Lett. 85, 3740–3743. doi: 10.1103/PhysRevLett.85.3740
Horwitz, B., and Braun, A. R. (2004). Brain network interactions in auditory, visual and linguistic processing. Brain Lang. 89, 377–384. doi: 10.1016/S0093-934X(03)00349-3
Isaacson, J. S., and Scanziani, M. (2011). How inhibition shapes cortical activity. Neuron 72, 231–243. doi: 10.1016/j.neuron.2011.09.027
Itzcovich, E., Riani, M., and Sannita, W. G. (2017). Stochastic resonance improves vision in the severely impaired. Sci. Rep. 7, 1–8. doi: 10.1038/s41598-017-12906-2
Jadi, M. P., and Sejnowski, T. J. (2014). Cortical oscillations arise from contextual interactions that regulate sparse coding. Proc. Natl. Acad. Sci. U. S. A. 111, 6780–6785. doi: 10.1073/pnas.1405300111
Jalan, S., and Singh, A. (2016). Cluster synchronization in multiplex networks. EPL 113:2. doi: 10.1209/0295-5075/113/30002
Jia, Y., Gu, H., Li, Y., and Ding, X. (2021). Inhibitory autapses enhance coherence resonance of a neuronal network. Commun. Nonlin. Sci. Numer. Simul. 95:105643. doi: 10.1016/j.cnsns.2020.105643
Jia, Y., Lu, B., and Gu, H. (2019). Excitatory electromagnetic induction current enhances coherence resonance of the FitzHugh–Nagumo neuron. Int. J. Mod. Phys. B 33:1950242. doi: 10.1142/S0217979219502424
Kawaguchi, M., Mino, H., and Durand, D. M. (2011). Stochastic resonance can enhance information transmission in neural networks. IEEE Trans. Biomed. Eng. 58, 1950–1958. doi: 10.1109/TBME.2011.2126571
Kim, J. H., Lee, H. J., Min, C. H., and Lee, K. J. (2015). Coherence resonance in bursting neural networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 92, 1–6. doi: 10.1103/PhysRevE.92.042701
Kim, S. Y., and Lim, W. (2017). Dynamical responses to external stimuli for both cases of excitatory and inhibitory synchronization in a complex neuronal network. Cogn. Neurodyn. 11, 395–413. doi: 10.1007/s11571-017-9441-5
Kitajo, K., Nozaki, D., Ward, L. M., and Yamamoto, Y. (2003). Behavioral stochastic resonance within the human brain. Phys. Rev. Lett. 90:218103. doi: 10.1103/PhysRevLett.90.218103
Kivelä, M., Arenas, A., Barthelemy, M., Gleeson, J. P., Moreno, Y., and Porter, M. A. (2014). Multilayer networks. J. Compl. Netw. 2, 203–271. doi: 10.1093/comnet/cnu016
Kumar Verma, U., and Ambika, G. (2021). Emergent dynamics and spatio temporal patterns on multiplex neuronal networks. Front. Comput. Neurosci. 15:109. doi: 10.3389/fncom.2021.774969
Kundu, S., Majhi, S., and Ghosh, D. (2019). Chemical synaptic multiplexing enhances rhythmicity in neuronal networks. Nonlinear Dyn. 98, 1659–1668. doi: 10.1007/s11071-019-05277-y
Kwon, O., and Moon, H. T. (2002). Coherence resonance in small-world networks of excitable cells. Phys. Lett. Sect. A Gen. At. Solid State Phys. 298, 319–324. doi: 10.1016/S0375-9601(02)00575-3
Ledoux, E., and Brunel, N. (2011). Dynamics of networks of excitatory and inhibitory neurons in response to time-dependent inputs. Front. Comput. Neurosci. 5, 1–17. doi: 10.3389/fncom.2011.00025
Lee, S. G., Neiman, A., and Kim, S. (1998). Coherence resonance in a Hodgkin-Huxley neuron. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 57, 3292–3297. doi: 10.1103/PhysRevE.57.3292
Li, X., Zhang, J., and Small, M. (2009). Self-organization of a neural network with heterogeneous neurons enhances coherence and stochastic resonance. Chaos 19:3076394. doi: 10.1063/1.3076394
Lindner, B., García-Ojalvo, J., Neiman, A., and Schimansky-Geier, L. (2004). Effects of noise in excitable systems. Phys. Rep. 392, 321–424. doi: 10.1016/j.physrep.2003.10.015
Liu, X., and Yang, X. (2018). Coherence resonance in a modified FHN neuron with autapse and phase noise. Int. J. Mod. Phys. B 32, 6–8. doi: 10.1142/S0217979218503320
Longtin, A. (1993). Stochastic resonance in neuron models. J. Stat. Phys. 70, 309–327. doi: 10.1007/BF01053970
Ma, J., and Tang, J. (2017). A review for dynamics in neuron and neuronal network. Nonlinear Dyn. 89, 1569–1578. doi: 10.1007/s11071-017-3565-3
Maertens, T., Schöll, E., Ruiz, J., and Hövel, P. (2021). Multilayer network analysis of C. elegans: looking into the locomotory circuitry. Neurocomputing 427, 238–261. doi: 10.1016/j.neucom.2020.11.015
Majhi, S., Bera, B. K., Ghosh, D., and Perc, M. (2019). Chimera states in neuronal networks: a review. Phys. Life Rev. 28, 100–121. doi: 10.1016/j.plrev.2018.09.003
Majhi, S., Perc, M., and Ghosh, D. (2017). Chimera states in a multilayer network of coupled and uncoupled neurons. Chaos 27:073109. doi: 10.1063/1.4993836
Manjarrez, E., Rojas-Piloni, J. G., Méndez, I., Martinez, L., Vélez, D., Vázquez, D., et al. (2002). Internal stochastic resonance in the coherence between spinal and cortical neuronal ensembles in the cat. Neurosci. Lett. 326, 93–96. doi: 10.1016/S0304-3940(02)00318-X
Marhl, U., and Gosak, M. (2019). Proper spatial heterogeneities expand the regime of scale-free behavior in a lattice of excitable elements. Phys. Rev. E 100:62203. doi: 10.1103/PhysRevE.100.062203
Masoliver, M., Malik, N., Schöll, E., and Zakharova, A. (2017). Coherence resonance in a network of FitzHugh-Nagumo systems: interplay of noise, time-delay, and topology. Chaos 27:101102. doi: 10.1063/1.5003237
Masoliver, M., Masoller, C., and Zakharova, A. (2021). Control of coherence resonance in multiplex neural networks. Chaos Solitons Fractals 145:110666. doi: 10.1016/j.chaos.2021.110666
McDonnell, M. D., and Abbott, D. (2009). What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 5:e1000348. doi: 10.1371/journal.pcbi.1000348
McDonnell, M. D., and Ward, L. M. (2011). The benefits of noise in neural systems: bridging theory and experiment. Nat. Rev. Neurosci. 12, 415–425. doi: 10.1038/nrn3061
Mongillo, G., Rumpel, S., and Loewenstein, Y. (2018). Inhibitory connectivity defines the realm of excitatory plasticity. Nat. Neurosci. 21, 1463–1470. doi: 10.1038/s41593-018-0226-x
Morita, S. (2006). Crossovers in scale-free networks on geographical space. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 73, 3–5. doi: 10.1103/PhysRevE.73.035104
Nicosia, V., and Latora, V. (2015). Measuring and modeling correlations in multiplex networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 92, 1–20. doi: 10.1103/PhysRevE.92.032805
Nicosia, V., Skardal, P. S., Arenas, A., and Latora, V. (2017). Collective phenomena emerging from the interactions between dynamical processes in multiplex networks. Phys. Rev. Lett. 118, 1–6. doi: 10.1103/PhysRevLett.118.138302
Nicosia, V., Valencia, M., Chavez, M., Díaz-Guilera, A., and Latora, V. (2013). Remote synchronization reveals network symmetries and functional modules. Phys. Rev. Lett. 110, 1–5. doi: 10.1103/PhysRevLett.110.174102
Oram, M. W., Wiener, M. C., Lestienne, R., and Richmond, B. J. (1999). Stochastic nature of precisely timed spike patterns in visual system neuronal responses. J. Neurophysiol. 81, 3021–3033. doi: 10.1152/jn.1999.81.6.3021
Ozer, M., and Uzuntarla, M. (2008). Effects of the network structure and coupling strength on the noise-induced response delay of a neuronal network. Phys. Lett. A 372, 4603–4609. doi: 10.1016/j.physleta.2008.05.003
Parastesh, F., Chen, C.-Y., Azarnoush, H., Jafari, S., and Hatef, B. (2019). Synchronization patterns in a blinking multilayer neuronal network. Eur. Phys. J. Spec. Top. 228, 2465–2474. doi: 10.1140/epjst/e2019-800203-3
Pereda, A. E. (2014). Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 15, 250–263. doi: 10.1038/nrn3708
Pikovsky, A. S., and Kurths, J. (1997). Coherence resonance in a noise-driven excitable system. Phys. Rev. Lett. 78, 775–778. doi: 10.1103/PhysRevLett.78.775
Pisarchik, A. N., Maksimenko, V. A., Andreev, A. V., Frolov, N. S., Makarov, V. V., Zhuravlev, M. O., et al. (2019). Coherent resonance in the distributed cortical network during sensory information processing. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-54577-1
Pradines, J. R., Osipov, G. V., and Collins, J. J. (1999). Coherence resonance in excitable and oscillatory systems: The essential role of slow and fast dynamics. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 60, 6407–6410. doi: 10.1103/PhysRevE.60.6407
Rakshit, S., Bera, B. K., and Ghosh, D. (2018). Synchronization in a temporal multiplex neuronal hypernetwork. Phys. Rev. E 98, 1–15. doi: 10.1103/PhysRevE.98.032305
Rich, S., Zochowski, M., and Booth, V. (2020). Effects of neuromodulation on excitatory–inhibitory neural network dynamics depend on network connectivity structure. J. Nonlinear Sci. 30, 2171–2194. doi: 10.1007/s00332-017-9438-6
Riehle, A., Grün, S., Diesmann, M., and Aertsen, A. (1997). Spike synchronization and rate modulation differentially involved in motor cortical function. Science 278, 1950–1953. doi: 10.1126/science.278.5345.1950
Russell, D. F., Wilkens, L. A., and Moss, F. (1999). Use of behavioural stochastic resonance by paddle fish for feeding. Nature 402, 291–294. doi: 10.1038/46279
Sagués, F., Sancho, J. M., and García-Ojalvo, J. (2007). Spatiotemporal order out of noise. Rev. Mod. Phys. 79, 829–882. doi: 10.1103/RevModPhys.79.829
Sancristóbal, B., Rebollo, B., Boada, P., Sanchez-Vives, M. V., and Garcia-Ojalvo, J. (2016). Collective stochastic coherence in recurrent neuronal networks. Nat. Phys. 12, 881–887. doi: 10.1038/nphys3739
Sawicki, J., Ghosh, S., Jalan, S., and Zakharova, A. (2019). Chimeras in multiplex networks: interplay of inter- and intra-layer delays. Front. Appl. Math. Stat. 5, 1–6. doi: 10.3389/fams.2019.00019
Semenova, N., and Zakharova, A. (2018). Weak multiplexing induces coherence resonance. Chaos 28:051104. doi: 10.1063/1.5037584
Semenova, N., Zakharova, A., Anishchenko, V., and Schöll, E. (2016). Coherence-resonance chimeras in a network of excitable elements. Phys. Rev. Lett. 117, 1–6. doi: 10.1103/PhysRevLett.117.014102
Shimokawa, T., and Shinomoto, S. (2006). Inhibitory neurons can facilitate rhythmic activity in a neural network. Phys. Rev. E 73:066221. doi: 10.1103/PhysRevE.73.066221
Simonotto, E., Riani, M., Seife, C., Roberts, M., Twitty, J., and Moss, F. (1997). Visual perception of stochastic resonance. Phys. Rev. Lett. 78, 1186–1189. doi: 10.1103/PhysRevLett.78.1186
Stefanescu, R. A., and Jirsa, V. K. (2008). A low dimensional description of globally coupled heterogeneous neural networks of excitatory and inhibitory neurons. PLoS Comput. Biol. 4:219. doi: 10.1371/journal.pcbi.1000219
Stein, R. B., Gossen, E. R., and Jones, K. E. (2005). Neuronal variability: noise or part of the signal? Nat. Rev. Neurosci. 6, 389–397. doi: 10.1038/nrn1668
Sukenik, N., Vinogradov, O., Weinreb, E., Segal, M., Levina, A., and Moses, E. (2021). Neuronal circuits overcome imbalance in excitation and inhibition by adjusting connection numbers. Proc. Natl. Acad. Sci. U. S. A. 118:e2018459118. doi: 10.1073/pnas.2018459118
Toral, R., Mirasso, C. R., and Gunton, J. D. (2003). System size coherence resonance in coupled FitzHugh-Nagumo models. Europhys. Lett. 61, 162–167. doi: 10.1209/epl/i2003-00207-5
Torres, L., Blevins, A. S., Bassett, D., and Eliassi-Rad, T. (2021). The why, how, and when of representations for complex systems. SIAM Rev. 63, 435–485. doi: 10.1137/20M1355896
Ushakov, O. V., Wünsche, H. J., Henneberger, F., Khovanov, I. A., Schimansky-Geier, L., and Zaks, M. A. (2005). Coherence resonance near a Hopf bifurcation. Phys. Rev. Lett. 95, 2–5. doi: 10.1103/PhysRevLett.95.123903
Uzun, R., Yilmaz, E., and Ozer, M. (2017). Effects of autapse and ion channel block on the collective firing activity of Newman–Watts small-world neuronal networks. Phys. A Stat. Mech. Appl. 486, 386–396. doi: 10.1016/j.physa.2017.05.049
Virkar, Y. S., Shew, W. L., Restrepo, J. G., and Ott, E. (2016). Feedback control stabilization of critical dynamics via resource transport on multilayer networks: how glia enable learning dynamics in the brain. Phys. Rev. E 94, 1–8. doi: 10.1103/PhysRevE.94.042310
Vragović, I., Louis, E., and Díaz-Guilera, A. (2005). Efficiency of informational transfer in regular and complex networks. Phys. Rev. E 71:036122. doi: 10.1103/PhysRevE.71.036122
Wang, H., Ma, J., Chen, Y., and Chen, Y. (2014). Effect of an autapse on the firing pattern transition in a bursting neuron. Commun. Nonlin. Sci. Numer. Simul. 19, 3242–3254. doi: 10.1016/j.cnsns.2014.02.018
Wang, M., Hou, Z., and Xin, H. (2005). Optimal network size for Hodgkin-Huxley neurons. Phys. Lett. Sect. A Gen. At. Solid State Phys. 334, 93–97. doi: 10.1016/j.physleta.2004.11.014
Wang, Q., Zhang, H., Perc, M., and Chen, G. (2012). Multiple firing coherence resonances in excitatory and inhibitory coupled neurons. Commun. Nonlin. Sci. Numer. Simul. 17, 3979–3988. doi: 10.1016/j.cnsns.2012.02.019
Ward, L. M., MacLean, S. E., and Kirschner, A. (2010). Stochastic resonance modulates neural synchronization within and between cortical sources. PLoS ONE 5:e14371. doi: 10.1371/journal.pone.0014371
Xu, K., Maidana, J. P., and Orio, P. (2021). Diversity of neuronal activity is provided by hybrid synapses. Nonlinear Dyn. 105, 2693–2710. doi: 10.1007/s11071-021-06704-9
Yamakou, M. E., Hjorth, P. G., and Martens, E. A. (2020). Optimal self-induced stochastic resonance in multiplex neural networks: electrical vs. chemical synapses. Front. Comput. Neurosci. 14:62. doi: 10.3389/fncom.2020.00062
Yamakou, M. E., and Jost, J. (2019). Control of coherence resonance by self-induced stochastic resonance in a multiplex neural network. Phys. Rev. E 100, 1–14. doi: 10.1103/PhysRevE.100.022313
Yang, X., and Cao, J. (2009). Stochastic synchronization of coupled neural networks with intermittent control. Phys. Lett. Sect. A Gen. At. Solid State Phys. 373, 3259–3272. doi: 10.1016/j.physleta.2009.07.013
Yilmaz, E., Ozer, M., Baysal, V., and Perc, M. (2016). Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 6, 1–14. doi: 10.1038/srep30914
Yilmaz, E., Uzuntarla, M., Ozer, M., and Perc, M. (2013). Stochastic resonance in hybrid scale-free neuronal networks. Phys. A Stat. Mech. Appl. 392, 5735–5741. doi: 10.1016/j.physa.2013.07.011
Yu, H., Galán, R. F., Wang, J., Cao, Y., and Liu, J. (2017a). Stochastic resonance, coherence resonance, and spike timing reliability of Hodgkin–Huxley neurons with ion-channel noise. Phys. A Stat. Mech. Appl. 471, 263–275. doi: 10.1016/j.physa.2016.12.039
Yu, H., Guo, X., and Wang, J. (2017b). Stochastic resonance enhancement of small-world neural networks by hybrid synapses and time delay. Commun. Nonlin. Sci. Numer. Simul. 42, 532–544. doi: 10.1016/j.cnsns.2016.06.021
Yu, H., Guo, X., Wang, J., Deng, B., and Wei, X. (2015). Spike coherence and synchronization on Newman-Watts small-world neuronal networks modulated by spike-timing-dependent plasticity. Phys. A Stat. Mech. Appl. 419, 307–317. doi: 10.1016/j.physa.2014.10.031
Yu, H., Li, K., Guo, X., Wang, J., Deng, B., and Liu, C. (2020). Firing rate oscillation and stochastic resonance in cortical networks with electrical–chemical synapses and time delay. IEEE Trans. Fuzzy Syst. 28, 5–13. doi: 10.1109/TFUZZ.2018.2889022
Yu, H., Wang, J., Du, J., Deng, B., Wei, X., and Liu, C. (2013). Effects of time delay and random rewiring on the stochastic resonance in excitable small-world neuronal networks. Phys. Rev. E 87:052917. doi: 10.1103/PhysRevE.87.052917
Yu, H., Zhang, L., Guo, X., Wang, J., Cao, Y., and Liu, J. (2018). Effect of inhibitory firing pattern on coherence resonance in random neural networks. Phys. A Stat. Mech. Appl. 490, 1201–1210. doi: 10.1016/j.physa.2017.08.040
Zakharova, A., Feoktistov, A., Vadivasova, T., and Schöll, E. (2013). Coherence resonance and stochastic synchronization in a nonlinear circuit near a subcritical Hopf bifurcation. Eur. Phys. J. Spec. Top. 222, 2481–2495. doi: 10.1140/epjst/e2013-02031-x
Zakharova, A., Vadivasova, T., Anishchenko, V., Koseska, A., and Kurths, J. (2010). Stochastic bifurcations and coherencelike resonance in a self-sustained bistable noisy oscillator. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 81, 1–6. doi: 10.1103/PhysRevE.81.011106
Keywords: neuronal dynamics, coherence resonance, excitatory neurons, inhibitory neurons, neural network, multilayer network, interlayer connectivity
Citation: Ristič D and Gosak M (2022) Interlayer Connectivity Affects the Coherence Resonance and Population Activity Patterns in Two-Layered Networks of Excitatory and Inhibitory Neurons. Front. Comput. Neurosci. 16:885720. doi: 10.3389/fncom.2022.885720
Received: 28 February 2022; Accepted: 24 March 2022;
Published: 18 April 2022.
Edited by:
Soumen Majhi, Bar-Ilan University, IsraelReviewed by:
Sajad Jafari, Amirkabir University of Technology, IranQing Yun Wang, Beihang University, China
Copyright © 2022 Ristič and Gosak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marko Gosak, bWFya28uZ29zYWtAdW0uc2k=
 David Ristič1
David Ristič1 Marko Gosak
Marko Gosak