- 1Department of Neurological Surgery, Northwestern University, Chicago, IL, United States
- 2Department of Neurology, Northwestern University, Chicago, IL, United States
Everyday human behavior relies upon extraordinary feats of coordination within the brain. In this perspective paper, we argue that the rich temporal structure of music provides an informative context in which to investigate how the brain coordinates its complex activities in time, and how that coordination can be disrupted. We bring insights from the neuroscience of musical rhythm to considerations of timing deficits in Attention Deficit/Hyperactivity Disorder (ADHD), highlighting the significant overlap between neural systems involved in processing musical rhythm and those implicated in ADHD. We suggest that timing deficits warrant closer investigation since they could lead to the identification of potentially informative phenotypes, tied to neurobiological and genetic factors. Our novel interdisciplinary approach builds upon recent trends in both fields of research: in the neuroscience of rhythm, an increasingly nuanced understanding of the specific contributions of neural systems to rhythm processing, and in ADHD, an increasing focus on differentiating phenotypes and identifying distinct etiological pathways associated with the disorder. Finally, we consider the impact of musical experience on rhythm processing and the potential value of musical rhythm in therapeutic interventions.
Introduction
Music is pervasive across cultures and plays an important role in human interaction, development and social bonding (Cross, 2001). The temporal structure of music is integral to its functions, and the experience of music relies upon a precisely-timed orchestration of activity across the brain's sensory, cognitive, motor, and reward systems. Musical rhythms inspire us to move (Keller and Rieger, 2009; Dalla Bella et al., 2013), and movement can, in turn, shape our perception of rhythmic patterns (Phillips-Silver and Trainor, 2005, 2007). Music also facilitates interpersonal synchrony, increasing pro-social behavior (Cirelli et al., 2012, 2014) and breaking down perceived barriers between self and other by coordinating shared emotional experiences (Tarr et al., 2014). Several studies suggest that interaction with music promotes synchronous neural activity not only across brain regions, but between the brains of individuals, for example during music listening (Abrams et al., 2013) and improvisation (Müller et al., 2013).
The rewarding qualities of music are also intrinsically linked to its temporal structure, through the creation and manipulation of expectations over time (Cooper and Meyer, 1960; Huron, 2006). Within this temporal framework, the fulfillment and violation of expectations provides a rich palette of emotional expression, mediated by the reward transmitter dopamine (Schultz, 1998; Salimpoor and Zatorre, 2013). The rhythmic patterns found across a range of musical styles have been shown to exhibit an optimal balance of predictability and surprise, even in their written form (Levitin et al., 2012), and the subtle timing variations found in live musical performance further contribute to the emotional expression perceived by a listener (Repp, 1995; Palmer, 1997; Ashley, 2002; Bhatara et al., 2011). As these examples highlight, the influence of music on human experience is closely tied to its temporal structure and the coordinated neural activity it induces, both within and between individuals.
The dynamic interplay between predictive (top-down) and reactive (bottom-up) processing, exemplified in how the brain responds to musical rhythm, is also a necessary foundation for cognitive functions, such as attention (Engel et al., 2001; Raichle, 2010). For example, the ability to anticipate what is likely to happen next and streamline the allocation of neural resources accordingly must be balanced with the ability to respond to unexpected salient events in the environment. In disorders such as ADHD, this balance is disrupted, resulting in impaired attentional control and difficulties inhibiting irrelevant inputs. We have chosen to consider ADHD in particular because in addition to the core symptoms of inattention and/or hyperactivity/impulsivity, ADHD is also characterized by deficits in motor and perceptual timing (Smith et al., 2002; Fair et al., 2012; Zelaznik et al., 2012; Demers et al., 2013; Noreika et al., 2013). Recent studies have revealed rhythm-related deficits in ADHD (Hove et al., 2017; Puyjarinet et al., 2017), and much of the same neural infrastructure that supports the processing of musical rhythm is implicated in ADHD, from brain circuitry (Silk et al., 2009; Silberstein et al., 2016; Mueller et al., 2017) and neural dynamics (Başar and Güntekin, 2008; Mazaheri et al., 2014; Loo et al., 2017) to dopamine signaling, with leading genetic risk factors for ADHD including dopamine gene variants (Swanson et al., 2000; DiMaio et al., 2003). Here, we propose that insights from research on musical rhythm could offer a more nuanced understanding of timing deficits in ADHD, and potentially lead to the identification of informative phenotypes, linked to neurobiological and genetic factors.
The Neural Infrastructure of Musical Rhythm
In this section we highlight key components of the neural infrastructure involved in processing musical rhythm. Although this is by no means an exhaustive review, some basic definitions of terms may prove useful. We will use the term “rhythm” to refer to temporal patterns formed from sequences of durations or onsets, whereas “beat” refers to a periodic pulse. In a piece of music, the beat typically defines the basic unit of timing, and “meter” refers to the grouping of beats into a recurring pattern of stresses or accents, such as would differentiate the feel of a waltz vs. a march.
Sensory-Motor Integration
Studies with non-human primates and even zebrafish have shown that neural ensembles can entrain to a rhythmic stimulus (Quintana and Fuster, 1999; Sumbre et al., 2008), and it is likely that human interaction with musical rhythm is founded upon these basic entrainment mechanisms. However, it is notable that the natural human tendency to move to music, for example by tapping a foot to the beat, has proven surprisingly elusive in the animal kingdom (Patel et al., 2009).
Imaging studies have revealed that in humans, rhythm perception is associated with activation not only in auditory cortices but in frontal, parietal and motor regions, including the supplementary motor area (SMA), basal ganglia and cerebellum (Grahn and Brett, 2007; Grahn, 2012; Large et al., 2015; Merchant et al., 2015). It has been suggested that the close sensory-motor coupling necessary for synchronization of movement to music may be unique to vocal learning species (including parrots and songbirds, as well as humans), in which it is a necessary basis for learning and producing complex communication signals (Patel and Iversen, 2014). Recent evidence of successful entrainment to the musical beat in non-vocal-learning species, for example a California sea lion (Cook et al., 2013), have cast doubt on this theory. Nonetheless, it is well established that close interaction between sensory and motor systems provides a sophisticated mechanism of temporal prediction and feedback (Schroeder et al., 2010), and that this plays an important role in how humans process musical rhythm.
The extensive activation of motor areas during rhythm perception, even in the absence of overt movement (Zatorre et al., 2007; Chen et al., 2008; Grahn and Rowe, 2009), is consistent with accumulating evidence that these systems serve a broader role in temporal processing and cognition. For example, fronto-striatal and fronto-cerebellar pathways are increasingly viewed as contributing to more general pattern-detection, predictive and cognitive functions (Akshoomoff and Courchesne, 1992; Graybiel, 1997; Schubotz, 2007). It has been proposed that striatal pathways are particularly involved in generating internal representations of beat and metrical structure (Grahn and Brett, 2007; Schwartze and Kotz, 2013). On the other hand, cerebellar circuits are more involved in the precise encoding of complex sequences, fast timing features and durations (Grube et al., 2010; Schwartze and Kotz, 2013). Together, these pathways create a system that can generate complex temporal predictions while also adapting to incoming information.
Models of Rhythm Perception
In constructing computational models of rhythm perception, a major challenge is to capture not only the individual components of temporal processing that are involved, but how those mechanisms interact in real time to maintain the ongoing balance between predictive (top-down) and reactive (bottom-up) processing, discussed above (see McAuley, 2010; Grahn, 2012, for review). For example, several rule-based models have been proposed in which the regular beat and metrical structure inferred by a rhythmic pattern are maintained by an internal clock (Longuet-Higgins and Lee, 1982; Povel and Essens, 1985; Desain and Honing, 1999). However, these models do not generally account for adaptive, online predictions and instead determine a “best fit” pattern of regular intervals based on the rhythm sequence as a whole (summarized in Grahn, 2012).
Models based on the entrainment of multiple oscillators have had greater success in accounting for online prediction that is tolerant to more complex rhythmic structure while remaining sensitive to natural variations in performance (Large and Kolen, 1994; Large and Palmer, 2002; Angelis et al., 2013). Indeed, there is evidence to suggest that natural, non-random patterns of timing variability (i.e., those exhibiting fractal scaling and long-range correlations) may actually improve the accuracy of listeners' temporal predictions (Rankin et al., 2009, 2014), and this was also demonstrated by the model (Large and Palmer, 2002).
In their theory of neural resonance, Large and Snyder extend these computational models to propose that entrainment is performed in the brain by neural oscillators (Large and Snyder, 2009), and this theory is supported by evidence from imaging and EEG studies (Large and Snyder, 2009; Nozaradan et al., 2012; Tierney and Kraus, 2015). Interestingly, individual variation in the temporal characteristics of neural activity (including long-range correlations) has been shown to predict variability in motor timing behavior (Linkenkaer-Hansen et al., 2001; Smit et al., 2013). A recent paper also linked these temporal characteristics of neural activity to fluctuations in attention, and it was proposed that the typical increase in long-range correlations over the course of development may be delayed or disrupted in ADHD (Smit and Anokhin, 2017). This represents a fascinating area for future study, and a further potential link between ADHD and the temporal dynamics of brain and behavior.
Within entrainment models, different frequencies of neural oscillations serve distinct functions. For example, Large and Snyder suggest that bursts of high frequency oscillatory activity facilitate coordination across motor and sensory systems. Peaks in beta (13–30 Hz) and gamma (30–100 Hz) power were observed as an anticipatory response to rhythmic patterns (Snyder and Large, 2005; Fujioka et al., 2009), and persisted even when the sound stimulus stopped, supporting their role as self-sustaining timekeepers. Further, temporal modulations in beta activity were altered by the specific metrical structure imposed by the listener onto an ambiguous rhythm pattern, suggesting top-down modulation of oscillatory dynamics (Iversen et al., 2009). Given the association between beta oscillations and motor coordination, the modulation of beta power may provide another indication of the influence of motor systems on rhythm processing (Large et al., 2015).
Neural responses to musical rhythm may also take the form of entrainment to specific frequencies actually present in the stimulus, for example the frequency of the musical beat. Neural entrainment to the beat has been observed in a number of EEG studies in the form of increased spectral power at the frequency corresponding to the tempo of the musical beat, typically within the delta range (1–4 Hz), and even to harmonics and subharmonics of that frequency (Nozaradan et al., 2012; Tierney and Kraus, 2013, 2015; Nozaradan, 2014). The influence of motor systems on this form of neural beat entrainment was investigated in a recent lesion study (Nozaradan et al., 2017). Both cerebellar and basal ganglia patients showed reduced neural activity aligned with the beat compared with controls, with cerebellar patients showing reductions specifically with faster tempo rhythms, and basal ganglia patients showing a greater deficit with complex rhythm patterns, which the authors interpreted as relying more heavily on the internal generation of a beat. These findings suggest that variation in cerebellar and striatal function (such as observed in ADHD) may be associated with distinct rhythm processing deficits. This study therefore provides compelling evidence for distinct specializations of these two motor areas in the coordination of neural entrainment to musical rhythm, linked with dissociable deficits.
Parsing Heterogeneity in ADHD: The Search for Phenotypes
ADHD is a highly prevalent and heterogenous disorder. Despite significant research efforts, characterization of the neurobiological basis of ADHD has proven elusive: diagnosis still relies heavily on self-report questionnaires, and treatment typically takes the form of a trial-and-error pharmacological approach. It has been difficult to identify biomarkers of the disorder because there has been no clear mapping between neural measures and clinical subtypes (i.e., predominantly inattentive, predominantly hyperactive/impulsive and combined type).
Although ADHD is associated with structural and functional abnormalities, including within frontal, striatal and cerebellar pathways, these findings have generally been small, and have not always been replicated (see Rubia, 2016, for review). Similarly, profiles of oscillatory dynamics have not been consistent enough to provide a clear neural “signature” of ADHD. EEG studies reveal abnormal patterns of oscillatory activity (Başar and Güntekin, 2008; Mazaheri et al., 2014; Loo et al., 2017), including reduced power in the beta frequency range. Indeed, a clinical diagnostic device assessing the ratio between theta and beta activity was developed and approved by the FDA (USDHHS, 2013). However, a subsequent meta-analysis suggested the theta-beta ratio is only elevated within a subgroup of individuals with ADHD, and is therefore not a reliable basis for diagnosis (Arns et al., 2013). A more nuanced understanding of distinct phenotypes of ADHD could help to increase diagnostic accuracy, and improve the development of clinical tools to aid in the evaluation and monitoring of treatment.
Research in the field is shifting toward the identification of distinct phenotypes and multiple etiologies (Castellanos and Tannock, 2002; Nigg et al., 2005; Durston et al., 2011). There is evidence from neuropsychological (Rommelse et al., 2008; Fair et al., 2012; Nikolas and Nigg, 2015), electrophysiological (Başar and Güntekin, 2008; Mazaheri et al., 2014; Loo et al., 2017) and genetic studies (Shaw et al., 2007; Giedd et al., 2008; Kebir and Joober, 2011) to suggest the presence of distinct subgroups within ADHD, beyond the clinical subtypes. However, these subgroups have yet to be reconciled across methodologies to provide full characterization of etiological pathways.
Although motor and timing deficits are not included within the diagnostic criteria for ADHD, they are increasingly recognized as common symptoms (Toplak et al., 2006; Demers et al., 2013; Kaiser et al., 2015; Dahan et al., 2016), and have been identified as a promising area for future study (Rubia, 2016). Consistent with the presence of multiple phenotypes, a recent study identifying rhythm deficits in children and adults with ADHD noted significant variation in performance within the ADHD group (Puyjarinet et al., 2017). Based on neuropsychological studies, it has been suggested that deficits in temporal information processing (e.g., duration discrimination) and increased response variability may represent distinct phenotypes, linked to dysfunction in cerebellar and basal ganglia pathways, respectively (Durston et al., 2011; Fair et al., 2012). Given the distinct roles of fronto-cerebellar and fronto-striatal pathways in rhythm processing (Grahn and Brett, 2009; Grahn, 2012; Merchant et al., 2015; Nozaradan et al., 2017), including their separate influence on neural entrainment discussed in the previous section, we argue that further examination of rhythm-related deficits in ADHD could help to characterize phenotypes of ADHD, and to shed light on the different ways in which the dynamics within associated neural systems may be disrupted.
Further, genetic risk factors for ADHD include genes affecting dopaminergic transmission, which may influence timing behavior (Valera et al., 2010). This is supported by pharmacological studies in which timing deficits in ADHD are reduced by methylphenidate (which increases levels of dopamine) (Noreika et al., 2013) as well as a study in which dopamine manipulation in healthy controls was associated with impaired timing skills (Coull et al., 2012). As mentioned in the introduction, dopamine indexes temporal expectation within the context of musical rhythm. More broadly, dopamine supports neural communication within reward, motor and cognitive pathways and is involved in a wide range of functions including reward-based learning, motor coordination and cognitive control. It has been proposed that a common theme across its various functions is that dopamine coordinates neural systems to optimize responsiveness at different timescales, matching the timescales of activity in the environment (Schultz, 2007). In other words, dopamine helps to keep the brain “in sync” with the world around it. This is accomplished via multiple dopamine release mechanisms with distinct kinetic properties (Schultz, 2007). Therefore, we speculate that genetic variation in specific components of the dopaminergic system could lead to distinct deficits in neural and behavioral timing. This is consistent with evidence from animal studies, in which different genetic modifications affecting dopamine transmission in mice were associated with distinct behavioral timing deficits (Cevik, 2003; Drew et al., 2007; Balci et al., 2009, 2010), as well as evidence of dissociable timing deficits in humans linked to dopamine gene variants (Wiener et al., 2011).
Dopamine also helps to mediate the balance between inhibitory and excitatory neural activity that sustains neural oscillations, therefore genetic variations in dopaminergic signaling at different timescales may also influence temporal characteristics of oscillatory dynamics, such as the long-range correlations discussed above. Disrupted neural dynamics may in turn influence the development of cortical networks (Uhlhaas et al., 2010). Indeed, longitudinal studies have demonstrated distinct trajectories of structural brain development associated with different dopamine gene polymorphisms in ADHD (Shaw et al., 2007; Giedd et al., 2008), however the potential role of neural dynamics in mediating these developmental differences remains to be explored. Recent research indicates that ADHD, neural dynamics and timing-related behaviors are all heritable (Tye et al., 2011; Agostino and Cheng, 2016), suggesting that a “genes to behavior” approach may prove fruitful.
Effects of Expertise
Several aspects of rhythm processing that are implicated in ADHD are also strengthened in expert musicians (summarized in Table 1), suggesting the potential for these systems to be shaped by experience. Behaviorally, musicians are better than controls at rhythm perception and temporal discrimination tasks (Rammsayer and Altenmüller, 2006; Wallentin et al., 2010) and have more consistent sensorimotor timing (Repp and Su, 2013). They also demonstrate enhanced cognitive function, including attention, inhibitory control and working memory (see Benz et al., 2015, for recent review), with enhanced inhibitory control linked to more consistent sensorimotor timing (Slater et al., 2017, 2018). Researchers found that musicians had larger volumes in motor areas including the cerebellum and basal ganglia, as well as frontal and parietal regions associated with cognitive control (see Schlaug, 2015, for review), and music training has been associated with functional changes to oscillatory dynamics (Bhattacharya and Petsche, 2005; Trainor et al., 2009).
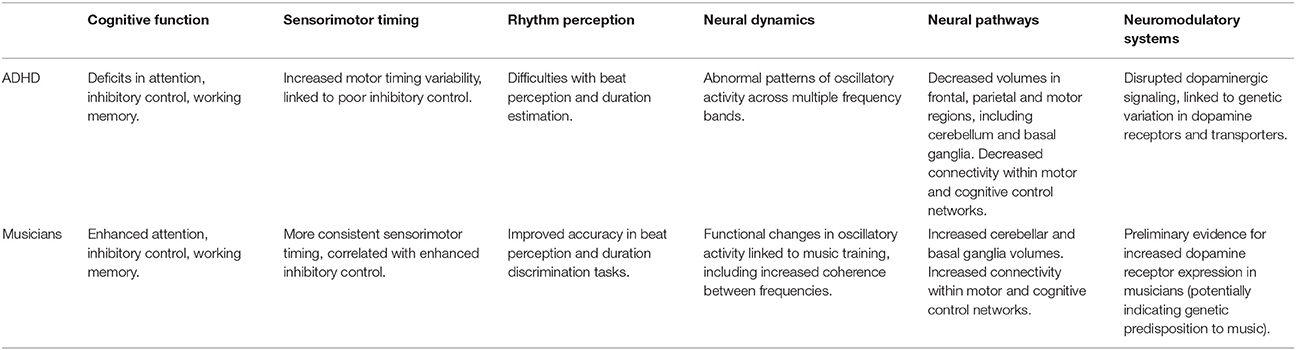
Table 1. How the infrastructure of musical rhythm processing is influenced by ADHD and musical expertise.
It is possible that group comparisons reflect innate differences in those drawn to pursue music rather than causal effects of training, in fact there is some preliminary evidence showing increased expression of dopamine receptors in musicians compared with controls, suggesting a potential genetic tendency toward musicianship (Emanuele et al., 2010). However, evidence from longitudinal studies (Moreno et al., 2011; Roden et al., 2014) as well as links between behavioral enhancements, extent of expertise (Slater et al., 2018) and specific instrument played (Krause et al., 2010) suggest that experience plays at least some role in observed differences. Further, therapies focusing on motor timing or rhythm have shown some success in ameliorating the broader symptoms of ADHD (Shaffer et al., 2001; Leisman and Melillo, 2010; Dahan et al., 2016), although more intervention studies are needed. Taken together, these findings suggest that common underlying mechanisms involved in both cognitive and motor control could potentially be strengthened by music-based interventions, building on the established use of music-based therapies in the treatment of a variety of other disorders. With a clearer understanding of distinct phenotypes, the efficacy of such interventions for ADHD could be greatly improved.
Conclusions
By considering how the brain processes musical rhythm, we force ourselves to take an integrated approach to how the brain coordinates its activities in time. Here, we argue that it is exactly this kind of integrated approach that is needed to advance understanding of a complex, heterogeneous disorder such as ADHD.
Whereas a great deal of neuroscientific research has focused on the spatial dimension—within perception itself, as well as in the localization of functions to particular brain regions—the inherently temporal nature of musical sound helps to bring mechanisms of neural coordination to the forefront. In this review, we have explored common neural infrastructure that is involved in processing musical rhythm, and implicated in ADHD. We have discussed how the heterogeneity of ADHD has hampered progress toward the identification of biomarkers and objective diagnostic tools. We suggest that further investigation of the basis of rhythm and timing deficits could ultimately help to form a more integrated view of the etiologies of ADHD, bridging the gap between genetic factors (e.g., variation in dopaminergic signaling), neural dynamics and the development of cortical networks, and the behavioral control of cognition and movement. We have also highlighted that the same neural systems are strengthened in expert musicians, suggesting the potential for neuroplasticity to have remediating effects. This novel, interdisciplinary approach could inform therapeutic strategies, harnessing the rewarding properties of music to strengthen coordination within the brain.
Author Contributions
JS conceived and wrote the article, MT contributed to the writing of the article.
Funding
Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number KL2TR001424. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrams, D. A., Ryali, S., Chen, T., Chordia, P., Khouzam, A., Levitin, D. J., et al. (2013). Inter-subject synchronization of brain responses during natural music listening. Eur. J. Neurosci. 37, 1458–1469. doi: 10.1111/ejn.12173
Agostino, P. V., and Cheng, R.-K. (2016). Contributions of dopaminergic signaling to timing accuracy and precision. Curr. Opin. Behav. Sci. 8, 153–160. doi: 10.1016/j.cobeha.2016.02.013
Akshoomoff, N. A., and Courchesne, E. (1992). A new role for the cerebellum in cognitive operations. Behav. Neurosci. 106, 731. doi: 10.1037/0735-7044.106.5.731
Angelis, V., Holland, S., Upton, P. J., and Clayton, M. (2013). Testing a computational model of rhythm perception using polyrhythmic stimuli. J. New Music Res. 42, 47–60. doi: 10.1080/09298215.2012.718791
Arns, M., Conners, C. K., and Kraemer, H. C. (2013). A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J. Atten. Disord. 17, 374–383. doi: 10.1177/1087054712460087
Ashley, R. (2002). Do [n't] change a hair for me: the art of jazz rubato. Music Percept. 19, 311–332. doi: 10.1525/mp.2002.19.3.311
Balci, F., Day, M., Rooney, A., and Brunner, D. (2009). Disrupted temporal control in the R6/2 mouse model of Huntington's disease. Behav. Neurosci. 123, 1353. doi: 10.1037/a0017650
Balci, F., Ludvig, E. A., Abner, R., Zhuang, X., Poon, P., and Brunner, D. (2010). Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain Res. 1325, 89–99. doi: 10.1016/j.brainres.2010.02.034
Başar, E., and Güntekin, B. (2008). A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 1235, 172–193. doi: 10.1016/j.brainres.2008.06.103
Benz, S., Sellaro, R., Hommel, B., and Colzato, L. S. (2015). Music makes the world go round: the impact of musical training on non-musical cognitive functions—a review. Front. Psychol. 6:2023. doi: 10.3389/fpsyg.2015.02023
Bhatara, A., Tirovolas, A. K., Duan, L. M., Levy, B., and Levitin, D. J. (2011). Perception of emotional expression in musical performance. J. Exp. Psychol. Hum. Percept. Perform. 37, 921. doi: 10.1037/a0021922
Bhattacharya, J., and Petsche, H. (2005). Phase synchrony analysis of EEG during music perception reveals changes in functional connectivity due to musical expertise. Signal Process. 85, 2161–2177. doi: 10.1016/j.sigpro.2005.07.007
Castellanos, F. X., and Tannock, R. (2002). Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Rev. Neurosci. 3, 617–628. doi: 10.1038/nrn896
Cevik, M. (2003). “Neurogenetics of interval timing,” in Functional and Neural Mechanisms of Interval Timing, ed W. H. Meck (Boca Raton, FL: CRC Press), 297–316.
Chen, J. L., Penhune, V. B., and Zatorre, R. J. (2008). Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 18, 2844–2854. doi: 10.1093/cercor/bhn042
Cirelli, L. K., Einarson, K., and Trainor, L. J. (2012). “Bouncing babies to the beat: Music and helping behaviour in infancy,” in 12th International Conference on Music Perception and Cognition (Thessaloniki).
Cirelli, L. K., Wan, S. J., and Trainor, L. J. (2014). Fourteen-month-old infants use interpersonal synchrony as a cue to direct helpfulness. Philos. Trans. R. Soc. B Biol. Sci. 369:20130400. doi: 10.1098/rstb.2013.0400
Cook, P., Rouse, A., Wilson, M., and Reichmuth, C. (2013). A California sea lion (Zalophus californianus) can keep the beat: motor entrainment to rhythmic auditory stimuli in a non vocal mimic. J. Comp. Psychol. 127, 412–27. doi: 10.1037/a0032345
Cooper, G., and Meyer, L. B. (1960). The Rhythmic Structure of Music. Chicago, IL: University of Chicago Press.
Coull, J. T., Hwang, H. J., Leyton, M., and Dagher, A. (2012). Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J. Neurosci. 32, 16704–16715. doi: 10.1523/JNEUROSCI.1258-12.2012
Cross, I. (2001). Music, cognition, culture, and evolution. Ann. N. Y. Acad. Sci. 930, 28–42. doi: 10.1111/j.1749-6632.2001.tb05723.x
Dahan, A., Ryder, C. H., and Reiner, M. (2016). Components of motor deficiencies in ADHD and possible interventions. Neuroscience 378, 34–53. doi: 10.1016/j.neuroscience.2016.05.040
Dalla Bella, S., Białunska, A., and Sowinski, J. (2013). Why movement is captured by music, but less by speech: role of temporal regularity. PLoS ONE 8:e71945. doi: 10.1371/journal.pone.0071945
Demers, M. M., McNevin, N., and Azar, N. R. (2013). ADHD and motor control: a review of the motor control deficiencies associated with attention deficit/hyperactivity disorder and current treatment options. Crit. Rev. Phys. Rehabil. Med. 25, 3–4. doi: 10.1615/CritRevPhysRehabilMed.2013009763
Desain, P., and Honing, H. (1999). Computational models of beat induction: the rule-based approach. J. New Music Res. 28, 29–42. doi: 10.1076/jnmr.28.1.29.3123
DiMaio, S., Grizenko, N., and Joober, R. (2003). Dopamine genes and attention-deficit hyperactivity disorder: a review. J. Psychiatry Neurosci. 28, 27–38.
Drew, M. R., Simpson, E. H., Kellendonk, C., Herzberg, W. G., Lipatova, O., Fairhurst, S., et al. (2007). Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 27, 7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007
Durston, S., van Belle, J., and de Zeeuw, P. (2011). Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol. Psychiatry 69, 1178–1184. doi: 10.1016/j.biopsych.2010.07.037
Emanuele, E., Boso, M., Cassola, F., Broglia, D., Bonoldi, I., Mancini, L., et al. (2010). Increased dopamine DRD4 receptor mRNA expression in lymphocytes of musicians and autistic individuals: bridging the music-autism connection. Neuro Endocrinol. Lett. 31, 122–125.
Engel, A. K., Fries, P., and Singer, W. (2001). Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716. doi: 10.1038/35094565
Fair, D. A., Bathula, D., Nikolas, M. A., and Nigg, J. T. (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. U.S.A. 109, 6769–6774. doi: 10.1073/pnas.1115365109
Fujioka, T., Trainor, L. J., Large, E. W., and Ross, B. (2009). Beta and gamma rhythms in human auditory cortex during musical beat processing. Ann. N. Y. Acad. Sci. 1169, 89–92. doi: 10.1111/j.1749-6632.2009.04779.x
Giedd, J. N., Lenroot, R. K., Shaw, P., Lalonde, F., Celano, M., White, S., et al. (2008). Trajectories of anatomic brain development as a phenotype. Novartis Found. Symp. 289, 101–112; discussion: 112–108; 193–105.
Grahn, J. A. (2012). Neural mechanisms of rhythm perception: current findings and future perspectives. Top. Cogn. Sci. 4, 585–606. doi: 10.1111/j.1756-8765.2012.01213.x
Grahn, J. A., and Brett, M. (2007). Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893–906. doi: 10.1162/jocn.2007.19.5.893
Grahn, J. A., and Brett, M. (2009). Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex 45, 54–61. doi: 10.1016/j.cortex.2008.01.005
Grahn, J. A., and Rowe, J. B. (2009). Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 29, 7540–7548. doi: 10.1523/JNEUROSCI.2018-08.2009
Graybiel, A. M. (1997). The basal ganglia and cognitive pattern generators. Schizophr. Bull. 23, 459–469. doi: 10.1093/schbul/23.3.459
Grube, M., Lee, K.-H., Griffiths, T. D., Barker, A. T., and Woodruff, P. W. (2010). Frontiers: transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Front. Auditory Cogn. Neurosci. 1:171. doi: 10.3389/fpsyg.2010.00171
Hove, M. J., Gravel, N., Spencer, R. M. C., and Valera, E. M. (2017). Finger tapping and pre-attentive sensorimotor timing in adults with ADHD. Exp. Brain Res. 235, 3663–3672. doi: 10.1007/s00221-017-5089-y
Huron, D. B. (2006). Sweet Anticipation: Music and the Psychology of Expectation. Cambridge, MA: MIT press.
Iversen, J. R., Repp, B. H., and Patel, A. D. (2009). Top-down control of rhythm perception modulates early auditory responses. Ann. N. Y. Acad. Sci. 1169, 58–73. doi: 10.1111/j.1749-6632.2009.04579.x
Kaiser, M.-L., Schoemaker, M., Albaret, J.-M., and Geuze, R. (2015). What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Res. Dev. Disabil. 36, 338–357. doi: 10.1016/j.ridd.2014.09.023
Kebir, O., and Joober, R. (2011). Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies. Eur. Arch. Psychiatry Clin. Neurosci. 261, 583–594. doi: 10.1007/s00406-011-0207-5
Keller, P. E., and Rieger, M. (2009). Special issue—Musical movement and synchronization. Music Percept. 26, 397–400. doi: 10.1525/mp.2009.26.5.397
Krause, V., Schnitzler, A., and Pollok, B. (2010). Functional network interactions during sensorimotor synchronization in musicians and non-musicians. Neuroimage 52, 245–251. doi: 10.1016/j.neuroimage.2010.03.081
Large, E. W., Herrera, J. A., and Velasco, M. J. (2015). Neural networks for beat perception in musical rhythm. Front. Syst. Neurosci. 9:159. doi: 10.3389/fnsys.2015.00159
Large, E. W., and Kolen, J. F. (1994). Resonance and the perception of musical meter. Conn. Sci. 6, 177–208. doi: 10.1080/09540099408915723
Large, E. W., and Palmer, C. (2002). Perceiving temporal regularity in music. Cogn. Sci. 26, 1–37. doi: 10.1207/s15516709cog2601_1
Large, E. W., and Snyder, J. S. (2009). Pulse and meter as neural resonance. Ann. N. Y. Acad. Sci. 1169, 46–57. doi: 10.1111/j.1749-6632.2009.04550.x
Leisman, G., and Melillo, R. (2010). Effects of motor sequence training on attentional performance in ADHD children. Int. J. Disabil. Hum. Dev. 9, 275–282. doi: 10.1515/IJDHD.2010.043
Levitin, D. J., Chordia, P., and Menon, V. (2012). Musical rhythm spectra from Bach to Joplin obey a 1/f power law. Proc. Natl. Acad. Sci. U.S.A. 109, 3716–3720. doi: 10.1073/pnas.1113828109
Linkenkaer-Hansen, K., Nikouline, V. V., Palva, J. M., and Ilmoniemi, R. J. (2001). Long-range temporal correlations and scaling behavior in human brain oscillations. J. Neurosci. 21, 1370–1377. doi: 10.1523/JNEUROSCI.21-04-01370.2001
Longuet-Higgins, H. C., and Lee, C. S. (1982). The perception of musical rhythms. Perception 11, 115–128. doi: 10.1068/p110115
Loo, S. K., McGough, J. J., McCracken, J. T., and Smalley, S. L. (2017). Parsing heterogeneity in attention-deficit hyperactivity disorder using EEG-based subgroups. J. Child Psychol. Psychiatry 59, 223–231. doi: 10.1111/jcpp.12814
Mazaheri, A., Fassbender, C., Coffey-Corina, S., Hartanto, T. A., Schweitzer, J. B., and Mangun, G. R. (2014). Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry 76, 422–429. doi: 10.1016/j.biopsych.2013.08.023
McAuley, J. D. (2010). “Tempo and rhythm,” in Music Perception. Springer Handbook of Auditory Research, Vol. 36, eds M. R. Jones, R. R. Fay, and A. N. Popper (New York, NY: Springer Science + Business Media), 165–199.
Merchant, H., Grahn, J. A., Trainor, L., Rohrmeier, M., and Fitch, W. T. (2015). Finding the beat: a neural perspective across humans and non-human primates. Philos. Trans. R. Soc. B Biol. Sci. 370:20140093. doi: 10.1098/rstb.2014.0093
Moreno, S., Bialystok, E., Barac, R., Schellenberg, E. G., Cepeda, N. J., and Chau, T. (2011). Short-term music training enhances verbal intelligence and executive function. Psychol. Sci. 22, 1425–1433. doi: 10.1177/0956797611416999
Mueller, A., Hong, D. S., Shepard, S., and Moore, T. (2017). Linking ADHD to the neural circuitry of attention. Trends Cogn. Sci. 21, 474–488. doi: 10.1016/j.tics.2017.03.009
Müller, V., Sänger, J., and Lindenberger, U. (2013). Intra- and Inter-Brain Synchronization during Musical Improvisation on the Guitar. PLoS ONE 8:e73852. doi: 10.1371/journal.pone.0073852
Nigg, J. T., Willcutt, E. G., Doyle, A. E., and Sonuga-Barke, E. J. (2005). Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry 57, 1224–1230. doi: 10.1016/j.biopsych.2004.08.025
Nikolas, M. A., and Nigg, J. T. (2015). Moderators of neuropsychological mechanism in attention-deficit hyperactivity disorder. J. Abnorm. Child Psychol. 43, 271–281. doi: 10.1007/s10802-014-9904-7
Noreika, V., Falter, C. M., and Rubia, K. (2013). Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia 51, 235–266. doi: 10.1016/j.neuropsychologia.2012.09.036
Nozaradan, S. (2014). Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos. Trans. R. Soc. B Biol. Sci. 369:20130393. doi: 10.1098/rstb.2013.0393
Nozaradan, S., Peretz, I., and Mouraux, A. (2012). Selective neuronal entrainment to the beat and meter embedded in a musical rhythm. J. Neurosci. 32, 17572–17581. doi: 10.1523/JNEUROSCI.3203-12.2012
Nozaradan, S., Schwartze, M., Obermeier, C., and Kotz, S. A. (2017). Specific contributions of basal ganglia and cerebellum to the neural tracking of rhythm. Cortex 95, 156–168. doi: 10.1016/j.cortex.2017.08.015
Palmer, C. (1997). Music performance. Annu. Rev. Psychol. 48, 115–138. doi: 10.1146/annurev.psych.48.1.115
Patel, A. D., and Iversen, J. R. (2014). The evolutionary neuroscience of musical beat perception: the Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 8:57. doi: 10.3389/fnsys.2014.00057
Patel, A. D., Iversen, J. R., Bregman, M. R., and Schulz, I. (2009). Studying synchronization to a musical beat in nonhuman animals. Ann. N. Y. Acad. Sci. 1169, 459–469. doi: 10.1111/j.1749-6632.2009.04581.x
Phillips-Silver, J., and Trainor, L. J. (2005). Feeling the beat: movement influences infant rhythm perception. Science 308, 1430–1430. doi: 10.1126/science.1110922
Phillips-Silver, J., and Trainor, L. J. (2007). Hearing what the body feels: auditory encoding of rhythmic movement. Cognition 105, 533–546. doi: 10.1016/j.cognition.2006.11.006
Povel, D.-J., and Essens, P. (1985). Perception of temporal patterns. Music Percept. 2, 411–440. doi: 10.2307/40285311
Puyjarinet, F., Bégel, V., Lopez, R., Dellacherie, D., and Dalla Bella, S. (2017). Children and adults with Attention-Deficit/Hyperactivity Disorder cannot move to the beat. Sci. Rep. 7, 11550. doi: 10.1038/s41598-017-11295-w
Quintana, J., and Fuster, J. M. (1999). From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cerebral Cortex 9, 213–221. doi: 10.1093/cercor/9.3.213
Raichle, M. E. (2010). Two views of brain function. Trends Cogn. Sci. 14, 180–190. doi: 10.1016/j.tics.2010.01.008
Rammsayer, T., and Altenmüller, E. (2006). Temporal information processing in musicians and nonmusicians. Music Percept. 24, 37–48. doi: 10.1525/mp.2006.24.1.37
Rankin, S. K., Fink, P. W., and Large, E. W. (2014). Fractal structure enables temporal prediction in music. J. Acoust. Soc. Am. 136, EL256–EL262. doi: 10.1121/1.4890198
Rankin, S. K., Large, E. W., and Fink, P. W. (2009). Fractal tempo fluctuation and pulse prediction. Music Percept. 26, 401–413. doi: 10.1525/mp.2009.26.5.401
Repp, B. H. (1995). Expressive timing in Schumann's “Träumerei:”An analysis of performances by graduate student pianists. J. Acoust. Soc. Am. 98, 2413–2427.
Repp, B. H., and Su, Y. -H. (2013). Sensorimotor synchronization: a review of recent research (2006–2012). Psychon. Bull. Rev. 20, 403–452. doi: 10.3758/s13423-012-0371-2
Roden, I., Grube, D., Bongard, S., and Kreutz, G. (2014). Does music training enhance working memory performance? Findings from a quasi-experimental longitudinal study. Psychol. Music 42, 284–298. doi: 10.1177/0305735612471239
Rommelse, N. N., Altink, M. E., Oosterlaan, J., Beem, L., Buschgens, C. J., Buitelaar, J., et al. (2008). Speed, variability, and timing of motor output in ADHD: which measures are useful for endophenotypic research? Behav. Genet. 38, 121–132. doi: 10.1007/s10519-007-9186-8
Rubia, K. (2016). Can functional decoding elucidate meta-analytic brain dysfunctions in adult attention-deficit/hyperactivity disorder? Biol. Psychiatry 80, 890–892. doi: 10.1016/j.biopsych.2016.10.003
Salimpoor, V. N., and Zatorre, R. J. (2013). Neural interactions that give rise to musical pleasure. Psychol. Aesthet. Creat. Arts 7, 62. doi: 10.1037/a0031819
Schlaug, G. (2015). “Musicians and music making as a model for the study of brain plasticity,” in Music, Neurology, and Neuroscience: Evolution, the Musical Brain, Medical Conditions, and Therapies, Progress in Brain Research, Vol. 217, eds E. Altenmüller, S. Finger, and F. Boller (Amsterdam: Elsevier) 37–55. doi: 10.1016/bs.pbr.2014.11.020
Schroeder, C. E., Wilson, D. A., Radman, T., Scharfman, H., and Lakatos, P. (2010). Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol. 20, 172–176. doi: 10.1016/j.conb.2010.02.010
Schubotz, R. I. (2007). Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 11, 211–218. doi: 10.1016/j.tics.2007.02.006
Schultz, W. (1998). Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27. doi: 10.1152/jn.1998.80.1.1
Schultz, W. (2007). Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 30, 259–288. doi: 10.1146/annurev.neuro.28.061604.135722
Schwartze, M., and Kotz, S. A. (2013). A dual-pathway neural architecture for specific temporal prediction. Neurosci. Biobehav. Rev. 37, 2587–2596. doi: 10.1016/j.neubiorev.2013.08.005
Shaffer, R. J., Jacokes, L. E., Cassily, J. F., Greenspan, S. I., Tuchman, R. F., and Stemmer, P. J. (2001). Effect of Interactive Metronome® training on children with ADHD. Am. J. Occupat. Ther. 55, 155–162. doi: 10.5014/ajot.55.2.155
Shaw, P., Gornick, M., Lerch, J., Addington, A., Seal, J., Greenstein, D., et al. (2007). Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 64, 921–931. doi: 10.1001/archpsyc.64.8.921
Silberstein, R. B., Pipingas, A., Farrow, M., Levy, F., Stough, C. K., and Camfield, D. A. (2016). Brain functional connectivity abnormalities in attention-deficit hyperactivity disorder. Brain Behav. 6:e00583. doi: 10.1002/brb3.583
Silk, T. J., Vance, A., Rinehart, N., Bradshaw, J. L., and Cunnington, R. (2009). White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum. Brain Mapp. 30, 2757–2765. doi: 10.1002/hbm.20703
Slater, J., Ashley, R., Tierney, A., and Kraus, N. (2018). Got rhythm? Better inhibitory control is linked with more consistent drumming and enhanced neural tracking of the musical beat in adult percussionists and nonpercussionists. J. Cogn. Neurosci. 30, 14–24. doi: 10.1162/jocn_a_01189
Slater, J., Azem, A., Nicol, T., Swedenborg, B., and Kraus, N. (2017). Variations on the theme of musical expertise: cognitive and sensory processing in percussionists, vocalists and non-musicians. Eur. J. Neurosci. 45, 952–963. doi: 10.1111/ejn.13535
Smit, D. J., Linkenkaer-Hansen, K., and de Geus, E. J. (2013). Long-range temporal correlations in resting-state alpha oscillations predict human timing-error dynamics. J. Neurosci. 33, 11212–11220. doi: 10.1523/jneurosci.2816-12.2013
Smit, D. J. A., and Anokhin, A. P. (2017). Development and genetics of brain temporal stability related to attention problems in adolescent twins. Int. J. Psychophysiol. 115, 86–97. doi: 10.1016/j.ijpsycho.2016.07.498
Smith, A., Taylor, E., Newman, S., and Rubia, K. (2002). Evidence for a pure time perception deficit in children with ADHD. J. Child Psychol. Psychiatry 43, 529–542. doi: 10.1111/1469-7610.00043
Snyder, J. S., and Large, E. W. (2005). Gamma-band activity reflects the metric structure of rhythmic tone sequences. Cogn. Brain Res. 24, 117–126. doi: 10.1016/j.cogbrainres.2004.12.014
Sumbre, G., Muto, A., Baier, H., and Poo, M. M. (2008). Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature 456, 102. doi: 10.1038/nature07351
Swanson, J., Flodman, P., Kennedy, J., Spence, M. A., Moyzis, R., Schuck, S., et al. (2000). Dopamine genes and ADHD. Neurosci. Biobehav. Rev. 24, 21–25. doi: 10.1016/S0149-7634(99)00062-7
Tarr, B., Launay, J., and Dunbar, R. I. (2014). Music and social bonding:“self-other” merging and neurohormonal mechanisms. Front. Psychol. 5:1096. doi: 10.3389/fpsyg.2014.01096
Tierney, A., and Kraus, N. (2013). Neural responses to sounds presented on and off the beat of ecologically valid music. Front. Syst. Neurosci. 7:14. doi: 10.3389/fnsys.2013.00014
Tierney, A., and Kraus, N. (2015). Neural entrainment to the rhythmic structure of music. J. Cogn. Neurosci. 27, 400–408. doi: 10.1162/jocn_a_00704
Toplak, M. E., Dockstader, C., and Tannock, R. (2006). Temporal information processing in ADHD: findings to date and new methods. J. Neurosci. Methods 151, 15–29. doi: 10.1016/j.jneumeth.2005.09.018
Trainor, L. J., Shahin, A. J., and Roberts, L. E. (2009). Understanding the benefits of musical training: effects on oscillatory brain activity. Ann. N. Y. Acad. Sci. 1169, 133–142. doi: 10.1111/j.1749-6632.2009.04589.x
Tye, C., McLoughlin, G., Kuntsi, J., and Asherson, P. (2011). Electrophysiological markers of genetic risk for attention deficit hyperactivity disorder. Expert Rev. Mol. Med. 13, e9–e9. doi: 10.1017/S1462399411001797
Uhlhaas, P. J., Roux, F., Rodriguez, E., Rotarska-Jagiela, A., and Singer, W. (2010). Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 14, 72–80. doi: 10.1016/j.tics.2009.12.002
USDHHS (2013). US Department of Health and Human Services. FDA approval letter for NEBA System. Available online at: http://www.accessdata.fda.gov/cdrh_docs/pdf11/K112711.pdf
Valera, E. M., Spencer, R. M., Zeffiro, T. A., Makris, N., Spencer, T. J., Faraone, S. V., et al. (2010). Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 68, 359–367. doi: 10.1016/j.biopsych.2010.05.012
Wallentin, M., Nielsen, A. H., Friis-Olivarius, M., Vuust, C., and Vuust, P. (2010). The musical ear test, a new reliable test for measuring musical competence. Learn. Individ. Differ. 20, 188–196. doi: 10.1016/j.lindif.2010.02.004
Wiener, M., Lohoff, F. W., and Coslett, H. B. (2011). Double dissociation of dopamine genes and timing in humans. J. Cogn. Neurosci. 23, 2811–2821. doi: 10.1162/jocn.2011.21626
Zatorre, R. J., Chen, J. L., and Penhune, V. B. (2007). When the brain plays music: auditory–motor interactions in music perception and production. Nat. Rev. Neurosci. 8, 547–558. doi: 10.1038/nrn2152
Keywords: music, rhythm, attention deficit hyperactivity disorder, ADHD, cognitive control, motor timing, neuroplasticity, musical expertise
Citation: Slater JL and Tate MC (2018) Timing Deficits in ADHD: Insights From the Neuroscience of Musical Rhythm. Front. Comput. Neurosci. 12:51. doi: 10.3389/fncom.2018.00051
Received: 02 April 2018; Accepted: 18 June 2018;
Published: 06 July 2018.
Edited by:
Daya Shankar Gupta, Camden County College, United StatesReviewed by:
Joachim Hass, Zentralinstitut für Seelische Gesundheit (ZI), GermanyMichael H. Thaut, University of Toronto, Canada
Maurizio Mattia, Istituto Superiore di Sanità, Italy
Copyright © 2018 Slater and Tate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica L. Slater, ai1zbGF0ZXJAbm9ydGh3ZXN0ZXJuLmVkdQ==
 Jessica L. Slater
Jessica L. Slater Matthew C. Tate1,2
Matthew C. Tate1,2