
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Neural Circuits, 04 May 2023
Volume 17 - 2023 | https://doi.org/10.3389/fncir.2023.1172464
This article is part of the Research TopicFate, Maturation and Function of Cortical InhibitionView all 4 articles
 Joram Keijser1,2*
Joram Keijser1,2* Henning Sprekeler1,3*
Henning Sprekeler1,3*Cortical inhibitory interneurons form a broad spectrum of subtypes. This diversity suggests a division of labor, in which each cell type supports a distinct function. In the present era of optimisation-based algorithms, it is tempting to speculate that these functions were the evolutionary or developmental driving force for the spectrum of interneurons we see in the mature mammalian brain. In this study, we evaluated this hypothesis using the two most common interneuron types, parvalbumin (PV) and somatostatin (SST) expressing cells, as examples. PV and SST interneurons control the activity in the cell bodies and the apical dendrites of excitatory pyramidal cells, respectively, due to a combination of anatomical and synaptic properties. But was this compartment-specific inhibition indeed the function for which PV and SST cells originally evolved? Does the compartmental structure of pyramidal cells shape the diversification of PV and SST interneurons over development? To address these questions, we reviewed and reanalyzed publicly available data on the development and evolution of PV and SST interneurons on one hand, and pyramidal cell morphology on the other. These data speak against the idea that the compartment structure of pyramidal cells drove the diversification into PV and SST interneurons. In particular, pyramidal cells mature late, while interneurons are likely committed to a particular fate (PV vs. SST) during early development. Moreover, comparative anatomy and single cell RNA-sequencing data indicate that PV and SST cells, but not the compartment structure of pyramidal cells, existed in the last common ancestor of mammals and reptiles. Specifically, turtle and songbird SST cells also express the Elfn1 and Cbln4 genes that are thought to play a role in compartment-specific inhibition in mammals. PV and SST cells therefore evolved and developed the properties that allow them to provide compartment-specific inhibition before there was selective pressure for this function. This suggest that interneuron diversity originally resulted from a different evolutionary driving force and was only later co-opted for the compartment-specific inhibition it seems to serve in mammals today. Future experiments could further test this idea using our computational reconstruction of ancestral Elfn1 protein sequences.
Cortical inhibitory interneurons are a highly diverse group, differing in their morphology, connectivity, and electrophysiology (Tremblay et al., 2016). Decades of experimental and theoretical work have suggested a role for interneurons in many functions (Kepecs and Fishell, 2014; Tremblay et al., 2016; Sadeh and Clopath, 2021), including the regulation of neural activity (Vogels et al., 2011; Wu et al., 2022), control of synaptic plasticity (Letzkus et al., 2015; Williams and Holtmaat, 2019), increasing temporal precision (Wehr and Zador, 2003; Bhatia et al., 2019), predictive coding (Keller and Mrsic-Flogel, 2018; Hertäg and Clopath, 2022), and gain modulation (Fu et al., 2014; Ferguson and Cardin, 2020). Many of these functions come down to the control of excitation.
Why would the control of excitation require a diversity of interneurons? A key reason could lie in the complexity of excitatory cells (Fishell and Kepecs, 2020; Keijser and Sprekeler, 2022). Pyramidal cells (PCs) consist of several cellular compartments that have different physiological properties [e.g., sodium vs. calcium spikes (Larkum et al., 1999)], receive different inputs [e.g., top-down vs. bottom up (Petreanu et al., 2007; Larkum, 2013), although see Ledderose et al., 2022] and follow distinct synaptic plasticity rules (Letzkus et al., 2006; Sjostrom et al., 2008; Udakis et al., 2020). The control of different pyramidal cell compartments might therefore require inhibition from designated types of interneurons. Indeed, the two most common interneuron types—parvalbumin (PV)- and somatostatin (SST)-expressing cells—are classically distinguished by their connectivity with pyramidal cells: whereas PV-expressing basket cells mainly target the somata of PCs, SST-expressing Martinotti cells mainly target their apical dendrites (Tremblay et al., 2016). The cellular and synaptic properties of these interneurons also seem adapted to this purpose. SST interneurons receive facilitating synapses from PCs (Reyes et al., 1998; Silberberg and Markram, 2007), rendering them sensitive to bursts of action potentials (Goldberg et al., 2004; Murayama et al., 2009; Berger et al., 2010) triggered by plateau potentials in the apical dendrite of PCs (Larkum et al., 1999; Williams and Stuart, 1999). Indeed, SST interneurons control dendritic excitability and bursting of PCs (Murayama et al., 2009; Gentet et al., 2012; Lovett-Barron et al., 2012). PV interneurons, on the other hand, receive depressing synapses (Reyes et al., 1998; Caillard et al., 2000), rendering them less sensitive to these signals (Pouille and Scanziani, 2004). The presynaptic dynamics of PV and SST interneurons therefore seem particularly well-matched to the physiology of pyramidal cells, although both types also inhibit non-pyramidal cells and other interneurons (see e.g., Jiang et al., 2015; Campagnola et al., 2022). These and similar observations have led to the view that interneuron diversity can be understood from a functional perspective, in which the morphology and synaptic and cellular properties of different interneurons are fit to specific functions (Figure 1A) (Kepecs and Fishell, 2014; Fishell and Kepecs, 2020). Consistent with this idea that interneurons are adapted to control different pyramidal cell compartments, we recently showed that properties (connectivity and short-term plasticity) of PV and SST interneurons emerge when optimizing a network model for compartment-specific inhibition (Figure 1B) (Keijser and Sprekeler, 2022).
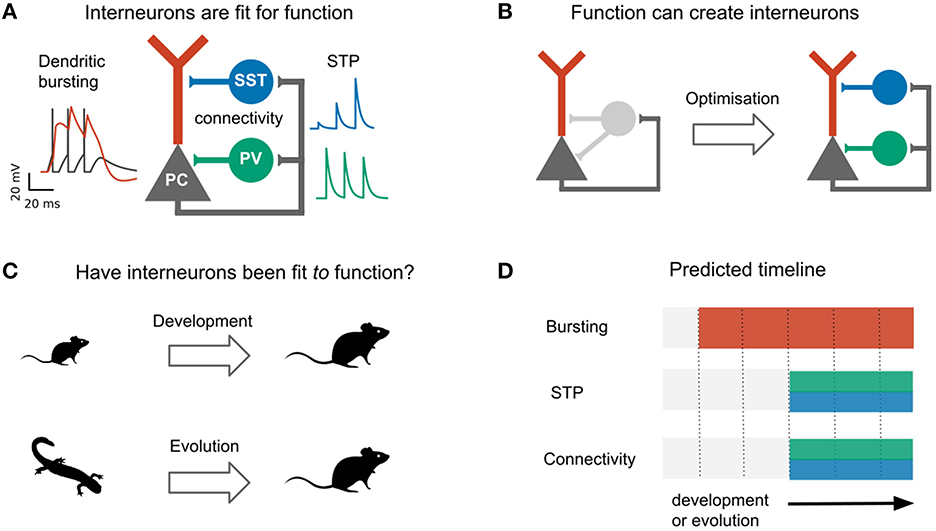
Figure 1. Do development and evolution fit interneurons to function? (A) The connectivity and short-term plasticity (STP) of PV and SST positive interneurons seem adapted to the morphology and electrophysiology of pyramidal cells, as highlighted by an optimization-based model (B). In this model, optimizing interneuron parameters to provide compartment- (soma/dendrite) specific inhibition causes interneurons to diversify into two groups that resemble PV and SST interneurons in their connectivity and short-term plasticity (Keijser and Sprekeler, 2022). (C) The existence of PV and SST subtypes might therefore result from an developmental or evolutionary tuning of interneurons based on pyramidal properties. (D) This predicts that immature (or ancestral) circuits contain bursting pyramidal neurons and undiversified interneurons. Development (or evolution) then diversifies the interneurons into PV and SST subtypes. PC activity and short-term plasticity simulated with models from Tsodyks et al. (1998), Naud et al. (2013), and Naud and Sprekeler (2018), respectively. Animal silhouettes from https://beta.phylopic.org/.
The specialization of PV and SST interneurons to pyramidal soma and dendrites, respectively, makes it tempting to speculate that the diversification of these interneuron subtypes was driven by pyramidal cell properties, either during evolution or during development (Figure 1C). This hypothesis predicts a specific temporal order: during evolution or development, the compartmentalization of pyramidal cells should predate interneuron diversification (Figure 1D).
Here, we evaluate this idea, with a focus on PC and interneuron properties that seem particularly well-adapted to each other: the active dendrites of pyramidal cells, and the connectivity and short-term plasticity of interneurons. Reviewing and reanalyzing recent evolutionary and developmental data, we reconstruct the developmental and evolutionary history of these three properties. We find no support for the idea that interneurons develop or evolved to control preexisting compartments of pyramidal cells. Instead, the central properties of PV and SST interneurons that led to this idea emerge before the PC properties they seem adapted to, in both development and evolution. Rather than pyramidal physiology driving interneuron diversification, this suggests a model in which existing interneuron properties enabled new pyramidal cell functions.
We first discuss the developmental trajectory of pyramidal cells and PV and SST interneurons in the mammalian cortex, to assess whether the diversification of PV and SST interneurons during development is driven by pyramidal cell properties. We mostly consider data from rodents, but many of the findings seem to be conserved among mammals (Hansen et al., 2013; Ma et al., 2013; Shi et al., 2021; Schmitz et al., 2022).
In contrast to pyramidal cells, interneurons are not born in the developing cortex, but subcortically (Anderson et al., 1997; Flames et al., 2007) (Box 1). It is only upon migrating to the cortex that interneurons acquire their mature morphology and physiology. The long period between interneuron birth and maturation has led to different models of interneuron development (Kepecs and Fishell, 2014; Wamsley and Fishell, 2017). One model attributes the late maturation of interneurons to a late specification of their cellular identity, possibly based on external cues within the circuit they embed themselves in Kepecs and Fishell (2014), Wamsley and Fishell (2017). Alternatively, the late emergence of characteristic features could be due to the slow unfolding of a predetermined genetic program that happens independently of the surrounding circuit (Lim et al., 2018a).
Box 1. Birth and migration of cortical interneurons.
Cortical GABAergic interneurons are born in a transient region of the developing brain known as the ganglionic eminence (Anderson et al., 1997; Wamsley and Fishell, 2017; Lim et al., 2018a), from where they tangentially migrate to the cortex (Maŕın and Rubenstein, 2001). The ganglionic eminence can be divided into multiple subregions patterned by unique combinations of transcription factors (Flames et al., 2007; Hansen et al., 2013; Ma et al., 2013; Hu et al., 2017), that activate distinct genetic programs. Since each genetic program corresponds to a different cell type, the majority of the cells born in the medial ganglionic eminence (MGE) will become PV and SST interneurons, whereas the caudal ganglionic eminence (CGE) generates, among others, vasoactive intestinal peptide (VIP)-expressing interneurons (Wichterle et al., 2001; Nery et al., 2002; Xu et al., 2004; Butt et al., 2005; Miyoshi et al., 2010). A key example for a patterning transcription factor that shapes interneuron identity is Nkx2-1, which is expressed within the MGE but not CGE (Sussel et al., 1999; Butt et al., 2005). Nkx2-1 knockout leads MGE-derived interneurons to adopt the fate of CGE-derived interneurons (Butt et al., 2008). Molecular gradients have also been shown to contribute to interneuron diversity within the same eminence: the dorsal-caudal and rostral-ventral MGE preferentially generate SST and PV neurons, respectively 0maturation, franceschetti1(Fogarty et al., 2007; Wonders et al., 2008; Inan et al., 2012; He et al., 2016; Hu et al., 2017; McKenzie et al., 2019).
After birth, interneurons migrate to the developing cortex via two different routes: The superficial marginal zone (the MZ, which will develop into cortical layer 1) and the deeper subventricular zone (SVZ). These different migration routes are used by distinct layer 2–3 (L2–3) SST subtypes (Lim et al., 2018a). Whereas L2-3 SST Martinotti cells migrate via the marginal zone from where they descend to their future location, non-Martinotti cells migrate via the subventricular zone (Lim et al., 2018b). This indicates that SST subtypes (at least in L2–3) are predetermined before their arrival in cortex, possibly even before they “choose” one migratory route over the other. Future L2–3 Martinotti cells forced to migrate via the wrong route (the SVZ) still become Martinotti cells in terms of their transcriptional profile and electrophysiology, but they lack a fully developed layer 1 axon (Lim et al., 2018b). This suggests that developing L2/3 Martinotti cells cannot send their developing axon from deeper to upper layers, but have to leave it there while their cell body descends. Translaminar axons of other neurons such as a less studied PV subtype (Lim et al., 2018b), and cerebellar granule cells (Rakic, 1971) are established via a similar mechanism, suggesting it might be the only reliable way for neurons to develop translaminar projections.
The malleability of interneuron properties during development is therefore currently an open question: Which properties are adapted to the surrounding circuit, and which are predetermined? Whatever properties are adapted, cellular identity (e.g., PV vs. SST) is probably not one of them (Wamsley and Fishell, 2017; Lim et al., 2018a). Interneuron types—at least on a coarse level—are determined by their time and place of birth. Future PV and SST interneurons, for example, are preferentially generated within different parts of the same embryonic structure (Box 1) (Wonders and Anderson, 2006; Lim et al., 2018a).
Recent data suggests that not just interneuron types (e.g., PV vs. SST), but also interneuron subtypes (e.g., SST Martinotti vs. SST non-Martinotti) are specified early in development. Lim et al. (2018a) showed that Martinotti and non-Martinotti cells migrate to the developing cortex via different routes (Box 1). In addition, a developing interneuron's transcriptional profile can be used to predict its future fate (Mayer et al., 2018; Mi et al., 2018; Bandler et al., 2021; Shi et al., 2021).
Although interneurons are therefore likely hardwired to become a certain subtype, it is still possible that interneuron properties such as short-term plasticity or connectivity are subject to activity-dependent fine-tuning. For example, the development of short-term facilitation or a layer 1 axon of SST Martinotti cells might emerge in dependence on pyramidal neuron bursting. In this case, bursting should develop ahead of these SST features.
When do developing pyramidal cells first show dendrite-dependent bursting? Their electrophysiology matures relatively late: dendritic plateau potentials emerge only in the third postnatal week (Franceschetti et al., 1998; Zhu, 2000). This is consistent with the late maturation of their dendritic morphology. PCs develop their intricate apical arborization and tuft dendrites after the second postnatal week (Zhu, 2000; Romand et al., 2011). For example, the tuft length increases almost twofold during the third postnatal week (Romand et al., 2011), and dendritic spikes fail to reach the soma on postnatal day 14 and 28 (Zhu, 2000).
When does short-term facilitation (STF) of PC → SST synapses arise during development? Could its development be driven by bursting in pyramidal cells? Some of the early experiments showed such STF in rat cortex during the third postnatal week (Reyes et al., 1998; Beierlein and Connors, 2002; Silberberg and Markram, 2007). Evidence for an even earlier presence of STF in these synapses comes from molecular studies. The Gosh laboratory has shown that the short-term facilitation in hippocampal (Sylwestrak and Ghosh, 2012) and cortical (Stachniak et al., 2019) PC → SST synapses is due to the transmembrane protein Elfn1, which is expressed by SST mouse and human interneurons (Box 2, Figure 2). In these experiments, STF was measured in the second postnatal week, and the expression of Elfn1 was detected already one week after birth (Tomioka et al., 2014; Favuzzi et al., 2019), providing an early molecular signature of short-term facilitation in SST cells. Short-term facilitation in PC → SST synapses is therefore present before dendrite-dependent bursting in PCs.
Box 2. Genetic basis of short-term facilitation.
Pyramidal cells form short-term depressing synapses onto PV neurons, but short-term facilitating synapses onto SST neurons. This difference is partly attributed to the postsynaptic expression of Elfn1 by SST neurons (Sylwestrak and Ghosh, 2012; Tomioka et al., 2014; Stachniak et al., 2019). Elfn1 is a synaptic protein that contacts the presynaptic boutons of pyramidal cells and controls their release properties. Specifically, Elfn1 induces presynaptic localization of metabotropic glutamate receptor 7 (mGluR7) (Tomioka et al., 2014). Grm7, the gene coding for mGluR7, is near-ubiquitously expressed in mouse (and human) neurons (data from Tasic et al., 2018; Bakken et al., 2021). mGluR7 has a low affinity for glutamate: only high glutamate levels caused by repeated presynaptic stimulation will lead mGluR7 to activate calcium channels, which increase synaptic release and thereby mediate synaptic facilitation. Elfn1 causes facilitation of PC → SST synapses in the hippocampus and different cortical layers (Stachniak et al., 2019). As expected from their expression of Elfn1, human SST (and VIP) interneurons receive facilitating inputs (Campagnola et al., 2022). However, in the mouse brain the correlation between the short-term facilitation and the expression of Elfn1 is very high, but not perfect (Stachniak et al., 2021).
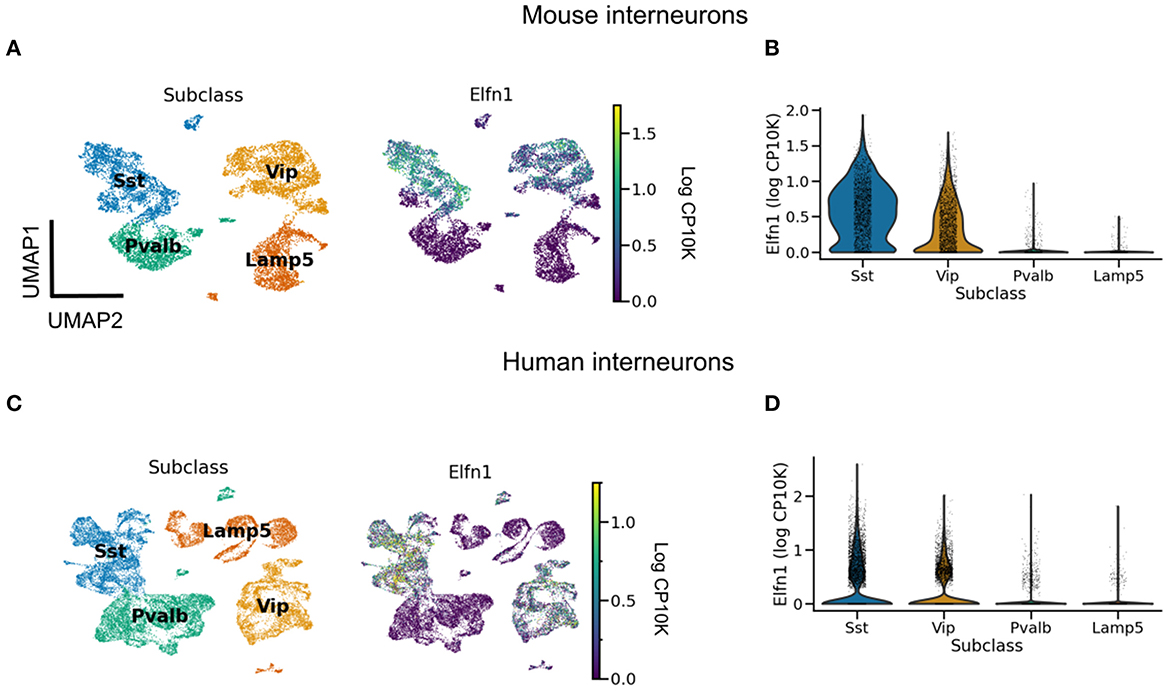
Figure 2. Elfn1 expression correlates with short-term facilitation in mammals. (A) UMAP (McInnes et al., 2018) plot of mouse interneurons colored by subclass (left) and Elfn1 expression (right). The two interneuron types—SST and VIP interneurons—known to receive facilitating synapses both express Elfn1. (B) Violin plot of Elfn1 expression by subclass. CP10K: counts per 10 thousand. (C, D) As (A, B), but for human interneurons. Data from Tasic et al. (2018) (A, B), and Bakken et al. (2021) (C, D).
What about the second difference between PV and SST neurons, their compartment-specific output synapses? SST and PV cells form compartment-specific synapses in visual cortical organotypic cultures that lack external inputs (Cristo et al., 2004). This strongly suggests a role for genetic encoding rather than experience-dependent activity. Indeed, recent work identified important molecular players in the establishment of compartment-specific synapses (Favuzzi et al., 2019). Several genes are involved in the formation of compartment-specific synapses. For example, suppressing Cbln4 in SST interneurons decreased inhibition onto PC dendrites. An over-expression of the same gene in PV interneurons, on the other hand, increased inhibition onto PC dendrites (Favuzzi et al., 2019). Other genes contribute to somatic inhibition in a seemingly analogous way (Favuzzi et al., 2019). Both loss and gain of function were shown around P14. Similarly, somatic inhibition in CA1 abruptly emerges at the end of the second postnatal week (Dard et al., 2022). It is therefore by the second postnatal week that PV and SST interneurons are committed as to where to direct their output synapses.
Intriguingly, Cbln4 is only expressed in a subset of neurons (Figure 3). Clustering revealed that these Cbln4+ neurons correspond to previously identified subtypes. The Tac1 cluster labels non-Martinotti cells that target the dendrites of L4 cells (Nigro et al., 2018; Scala et al., 2019; Gouwens et al., 2020), and the Calb2 and Etv1 clusters correspond to fanning-out Martinotti cells (Gouwens et al., 2020; Wu et al., 2022), consistent with a role of Cbln4 in establishing dendritic synapses. But only a subset of the Myh8 cluster—corresponding to T-shaped Martinotti cells (Wu et al., 2022)— expressed Cbln4, suggesting diverse mechanisms for dendritic targeting.
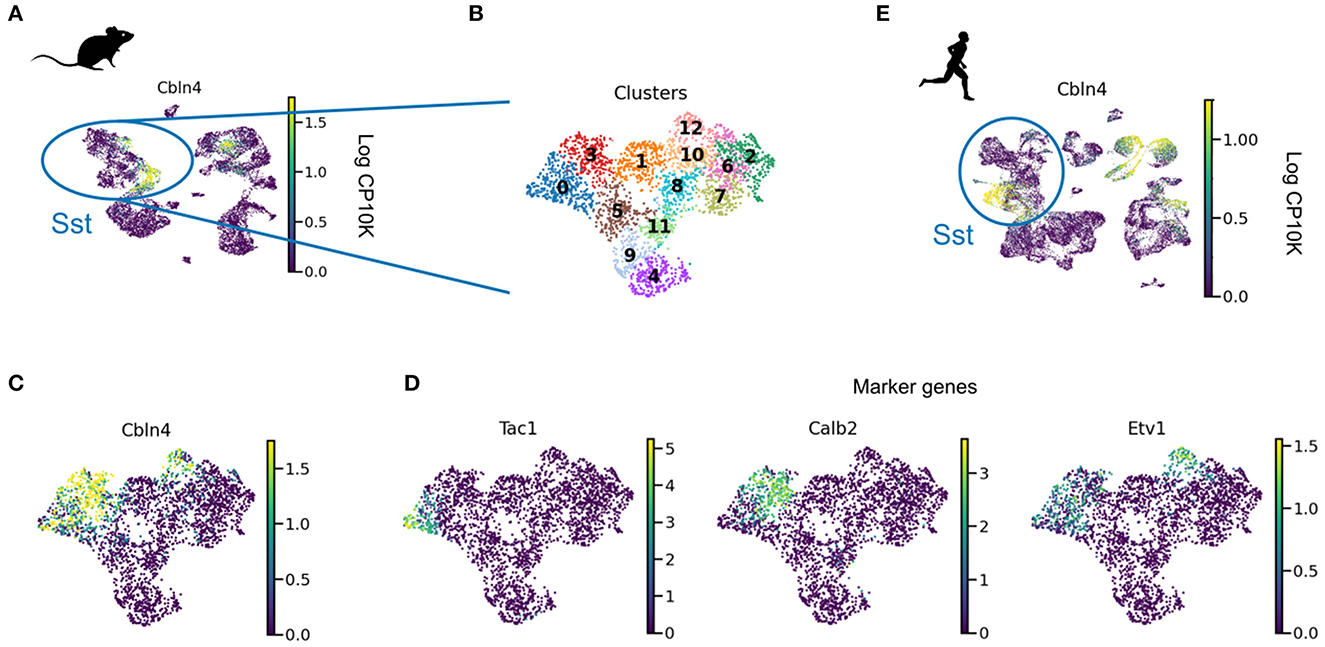
Figure 3. Cbln4 is expressed in a subset of mammalian SST interneurons. (A) UMAP plot of mouse and human interneurons, colored by their expression of Cbln4, a gene that instructs synapse formation onto pyramidal dendrites in mice (Favuzzi et al., 2019). Cbln4 is expressed in certain mouse and human interneuron subtypes, including SST cells. (B) UMAP of SST cells, clustered into subgroups. (C) Cbln4 is expressed in clusters 0, 3, and 12, which also express marker genes Tac1, Calb2, and Etv1 (D), respectively. (E) A subset of human Sst cells also express Cbln4. Data from Tasic et al. (2018) (A–D) and Bakken et al. (2021) (E).
An interneuron's cell type, the plasticity of their input synapses from PCs, and the PC compartments they target are therefore determined before interneurons are fully embedded within cortical circuits, and before pyramidal neurons develop their characteristic morphology and electrophysiology. This suggest that while PV and SST interneurons are fit for the function of compartment-specific inhibition of PCs, some of their characteristic properties are probably not developmentally driven by PC activity.
On a much longer timescale than development, evolution also changes the properties of cell types. This raises the question whether the differentiation of PV and SST interneurons preceded the evolution of the compartmental complexity of pyramidal neurons.
If natural selection tuned PV and SST neurons to pyramidal cell properties, the brains of mammalian ancestors must have contained pyramidal cells with elaborate dendrites, while interneurons were still undifferentiated (Figures 1C, D). This hypothesis cannot be tested directly since our mammalian ancestors are no longer alive, and their fossils provide no information regarding cell types. We therefore have to infer the evolutionary history of cell types by comparing data from modern-day species (Figure 4A) (Arendt et al., 2016; Tosches, 2021b). Although many cell type-specific properties such as short-term plasticity have not been measured in non-standard model organisms (see refs. Gidon et al., 2020; Beaulieu-Laroche et al., 2021; Campagnola et al., 2022 for recent exceptions), transcriptomic correlates can be studied using single cell RNA sequencing (scRNA-seq) (Tang et al., 2009), offering a means for defining and comparing cell types across species (Tanay and Sebé-Pedrós, 2021; Tosches, 2021b).
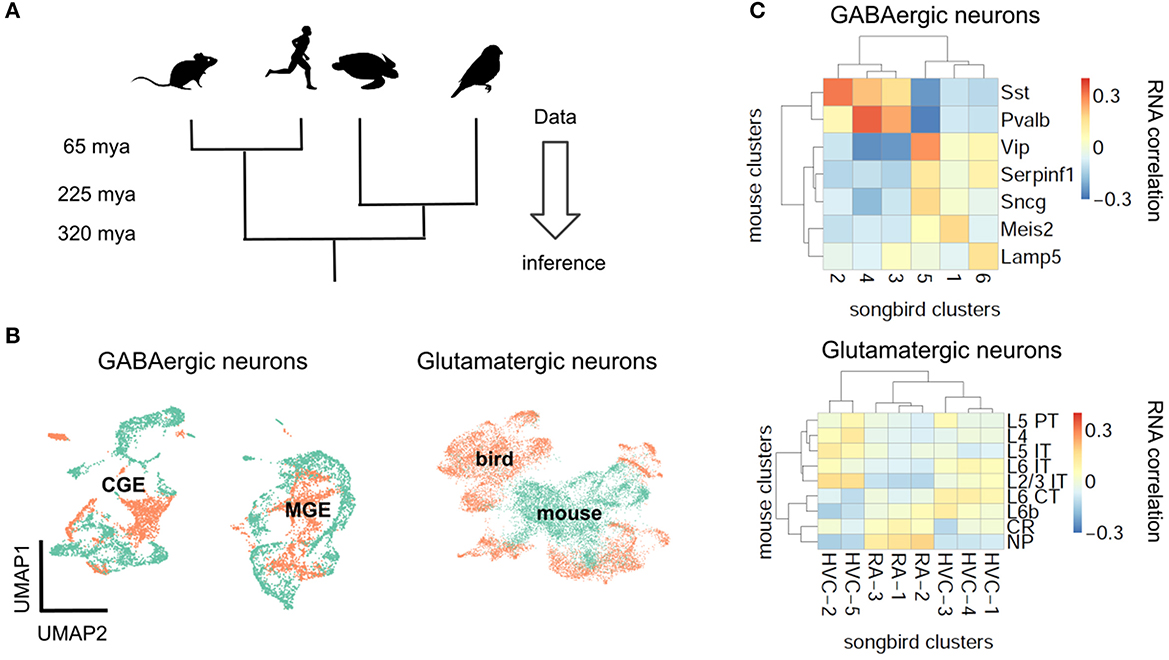
Figure 4. Evolutionary conservation of GABAergic cell types. (A) Phylogenetic approach. (B) Pearson correlation between average RNA expression in clusters of songbird and mouse interneurons. Correlations between GABAergic neurons are typically larger. (C) UMAP plots of integrated gene expression data for GABAergic and glutamatergic neurons. GABAergic neurons first cluster by developmental origin (MGE vs. CGE, see Box 1) and then by species. Mouse data from Tasic et al. (2018), songbird data and correlation analysis from Colquitt et al. (2021).
The first applications of scRNA-seq in neuroscience profiled cell types in mice (Zeisel et al., 2015; Tasic et al., 2016). More recently, scRNA-seq was used to classify neuron types also in reptiles (Tosches et al., 2018) and songbirds (Colquitt et al., 2021). The evolutionary relationships of reptiles, birds and mammals suggest that a feature found in all three lineages predates their divergence, while a feature found exclusively in the mammalian lineage is, in fact, a mammalian invention. This idea enables inferring the evolutionary history of interneurons and pyramidal cells (Figure 4A).
Let us first consider the general evolutionary trajectory of excitatory and inhibitory cell types. Tosches et al. (2018) used scRNA-seq to analyse cells from the turtle and lizard forebrain and compare them with previously published mammalian data (Tasic et al., 2016). They found that reptilian inhibitory neurons cluster into groups that roughly correspond to mammalian interneuron types (Tosches et al., 2018). These results extend earlier findings that found similarities between turtle and mammalian interneurons based on marker genes and morphology (Blanton et al., 1987; Reiner, 1993). Colquitt et al. (2021) recently made analogous observations regarding the similarity of songbird and mouse interneurons (Figures 4B, C). The most parsimonious explanation of this sharing of interneuron types is that similar types already existed in a common ancestor of the three lineages, rather than convergent evolution in three lineages. This homology is likely due to shared developmental origins: the inhibitory interneurons of birds and reptiles are born within the conserved ganglionic eminences. The fact that interneurons of different lineages are homologous does not mean they are identical. For example, the correlation between mouse PV and SST cells and the best matching songbird clusters is 0.37 and 0.31, respectively (Figure 4B). This is higher than the correlation between the best-matching glutamatergic types (0.19, see below), but lower than between some of the different cell types within the same species (mouse PV and SST cells: 0.58). Mammalian and non-mammalian interneurons, while homologous, therefore have likely undergone lineage-specific adaptations.
In contrast to inhibitory interneurons, excitatory neurons are probably not homologous between reptiles, songbirds, and mammals (Figures 4B, C) (Tosches et al., 2018; Colquitt et al., 2021). Excitatory cell types in different species are defined by different combinations of transcription factors. A clear example is given by the Fezf2 and Satb2 genes that specify subcortical (Lodato et al., 2014) and callosal (Alcamo et al., 2008) projections, respectively, of mammalian pyramidal cells. Strikingly, these genes are mutually repressive in the mammalian neurons, but co-expressed in reptilian neurons (Nomura et al., 2018; Tosches et al., 2018). Comparing excitatory neurons in the songbird and the mammalian brain revealed an analogous pattern: Although excitatory neurons in the songbird forebrain express similar genes as their counterparts in mammalian neocortex, these genes are regulated by different transcription factors (Colquitt et al., 2021). Instead, the transcription factors expressed by songbird glutamatergic neurons are similar to those in e.g. the mouse olfactory bulb and olfactory cortex. Since transcription factors specify cellular identity (Hobert, 2008; Arendt et al., 2016) this suggests that excitatory neurons are not conserved across mammals, birds and reptiles (Tosches et al., 2018; Colquitt et al., 2021; Tosches, 2021a).
Inhibitory cell types therefore seem more conserved than excitatory cell types, which appears broadly inconsistent with an evolutionary adaptation of interneurons to pyramidal cells. This is further confirmed when considering the evolutionary history of specific features of excitatory and inhibitory interneurons, in particular, elaborate dendrites and dendrite-dependent bursting and short-term plasticity.
We are not aware of direct measurements of short-term facilitation in non-mammalian species and therefore aimed to infer its presence from the expression of Elfn1 (Box 2). To this end, we reanalyzed publicly available gene expression data for reptilian and songbird interneuron types (Tosches et al., 2018; Colquitt et al., 2021). We found that Elfn1 is also expressed in the types corresponding to mammalian SST (and VIP) interneurons (Figure 5), but not in the type corresponding to PV interneurons. This suggests that SST-like interneurons expressing Elfn1—and potentially faciliating glutamatergic input synapses—were already present in the last common ancestor of reptiles, songbirds and mammals. In terms of potential transcriptomic correlates of synaptic specificity, we find that Cbln4 (Figure 3) is expressed in certain subtypes of turtle SST neurons (Figure 6A). Songbird SST neurons, on the other hand, do not express Cbln4 (Figure 6B). The expression of Cbln4 by Sst interneurons therefore correlates with the presence of apical dendrites in pyramidal cells (Figure 7, see next). The most parsimonious explanation is that Cbln4 expression was lost in the songbird lineage. Alternatively, it could have evolved independently in the mammals and reptiles.
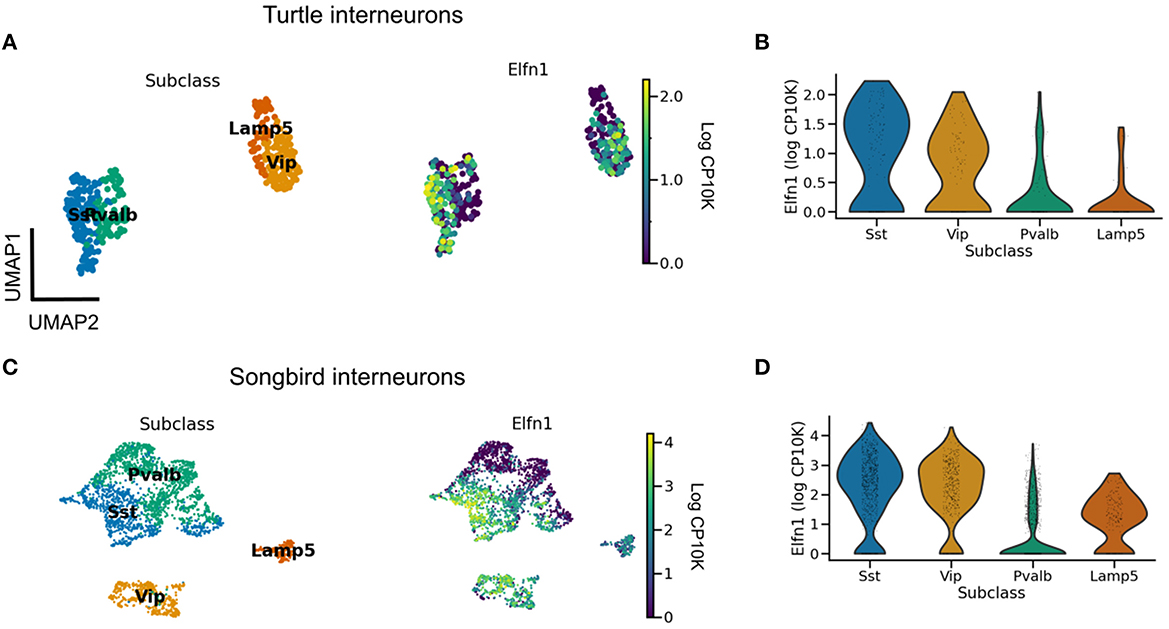
Figure 5. Evolutionary conservation of Elfn1 expression. (A) UMAP plot showing overexpression of Elfn1 in SST-like and VIP-like interneurons in the turtle forebrain. Data from Tosches et al. (2018). (B) Violin plots of Elfn1 expression for each of the clusters. (C, D) As (A, B), but for zebra finch neurons. Data from Colquitt et al. (2021).
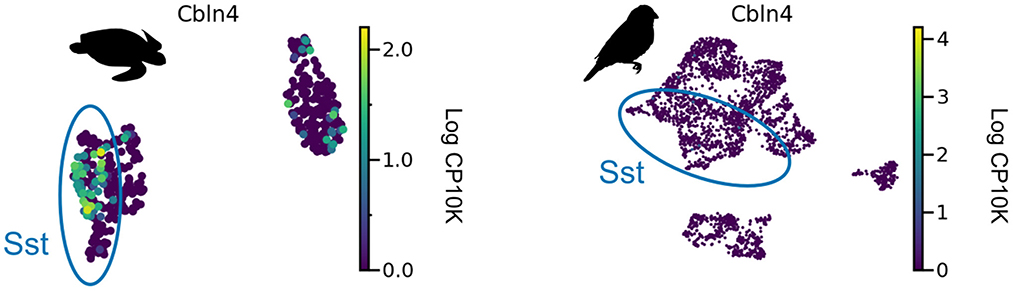
Figure 6. Cbln4 expression in non-mammalian species. Cbln4 is expressed in certain subtypes of turtle SST neurons, but not in songbird SST neurons. Data from Tosches et al. (2018) and Colquitt et al. (2021).
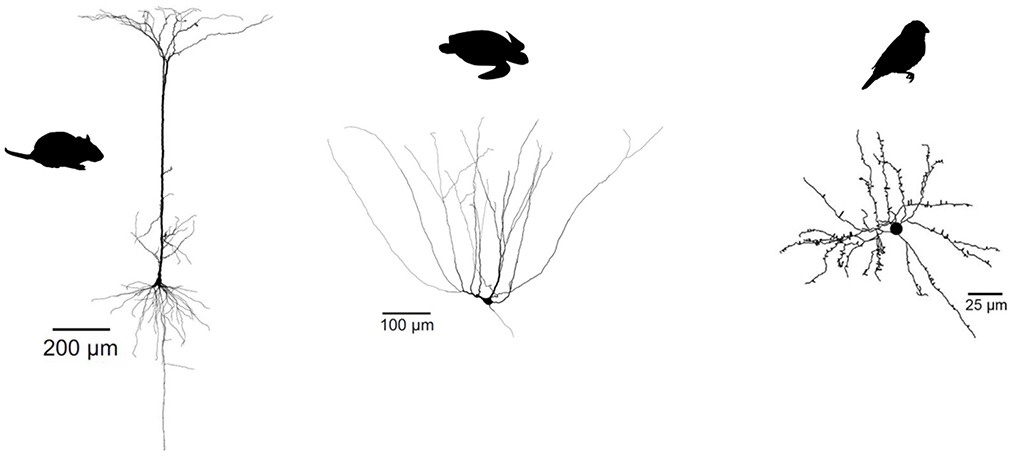
Figure 7. Evolutionary divergence of projection neuron morphology. Both turtle and mammalian projection neurons have a pyramidal morphology, but only mammalian pyramidal neurons have a single apical dendrite. Songbird projection neurons have a stellate, not pyramidal morphology. Turtle and mammalian neurons adapted from Larkum et al. (2008) (published under a Creative Commons License https://creativecommons.org/licenses/by-nc-sa/3.0/). Songbird neuron adapted from Kornfeld et al. (2017) (published under a Creative Commons License https://creativecommons.org/licenses/by/4.0/).
So not just interneuron subtypes, but also some of their specific properties seem evolutionarily conserved. In contrast, glutamatergic cell types in reptiles and birds show a very different dendritic morphology and physiology from their mammalian pyramidal counterparts. Turtle pyramidal cells have multiple apical dendrites, but no basal dendrites (Figure 7) (Connors and Kriegstein, 1986). This clear morphological difference suggests that turtle pyramidal neurons are also electrophysiogically distinct. Larkum et al. (Larkum et al., 2008) showed that, in vitro, turtle pyramidal neurons lack dendritic calcium spikes and dendrite-dependent bursting. Morphologically similar pyramidal cells in rodent piriform cortex also lack active dendrites [(Bathellier et al., 2009; Johenning et al., 2009), but see Kumar et al., 2018]. Interestingly, this is probably not due to an absence of calcium channels, but rather to the presence of A-type potassium channels (Johenning et al., 2009). Songbird excitatory cells have a stellate morphology, and differ therefore even more from mammalian pyramidal cells (Figure 7, see e.g., Devoogd and Nottebohm, 1981; Benezra et al., 2018).
The lack of dendrite-dependent bursting in reptiles and songbirds is consistent with comparative electrophysiology within the mammalian brain. Pyramidal neurons in the piriform cortex are homologous to certain types of glutamatergic turtle and songbird neurons (Colquitt et al., 2021), and also lack dendritic plateau potentials (Bathellier et al., 2009). Pyramidal neurons with mammalian electrophysiological properties therefore evolved after interneurons differentiated into PV and SST cell types (Figure 8).
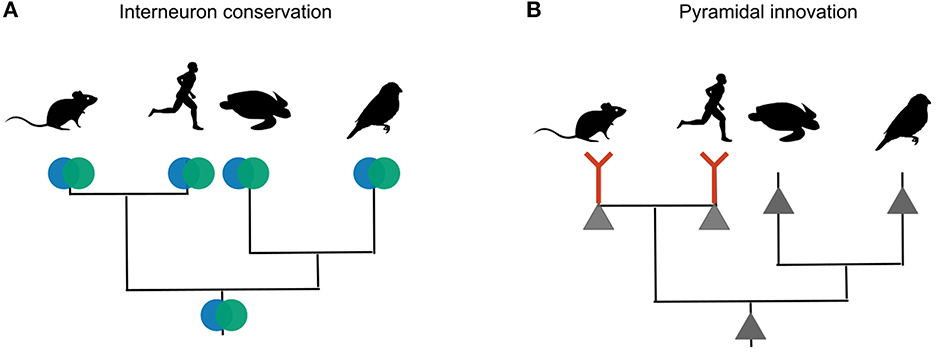
Figure 8. Phylogenetic inference of interneuron and pyramidal evolution. (A) Mice, humans, songbirds and turtles all have PV and SST interneurons. The most likely explanation for these similarities is that the interneuron types were already present in the last common ancestor of these lineages. (B) Only mammalian glutamateric neurons are known to exhibit dendritic plateau potentials that can elicit burst firing. Other lineages probably lack this trait. The most likely explanation is that dendritic bursting evolved only once, in the mammalian lineage.
The expression of Elfn1 by zebra finch and turtle SST-like neurons suggests these cells—and therefore the ancestral SST-like cells— receive(d) facilitating inputs. But it is also possible that the ancestral Elfn1 protein had different functional properties. Previous work has used Elfn1 knockout (Sylwestrak and Ghosh, 2012; Dolan and Mitchell, 2013; Tomioka et al., 2014) and targeted deletions (Dunn et al., 2018) to discover functionally important domains of the mouse variant (Figure 9B). To determine the evolution of Elfn1 at the resolution of individual sites, we computationally reconstructed its ancestral state (Hochberg and Thornton, 2017; Orlandi et al., 2023) (see Methods), starting from the protein sequences of extant species (Figure 9A). Alignment of the extant sequences revealed that on average across species 74.6% of the Elfn1 sites was identical to that of the mouse protein (Figure 9C). Combining the sequence alignment with a probabilistic model of sequence evolution (Jones et al., 1992) and a phylogenetic species-tree allowed us to reconstruct the ancestral protein (Figure 9D). The amount of conservation varied between protein domains and extant species: the zebra finch and turtle sequences were more similar to the ancestral sequence than the mammalian sequences (Figure 9E). Future work could use the reconstructed sequences to determine the evolutionary history and the molecular mechanisms of short-term facilitation.
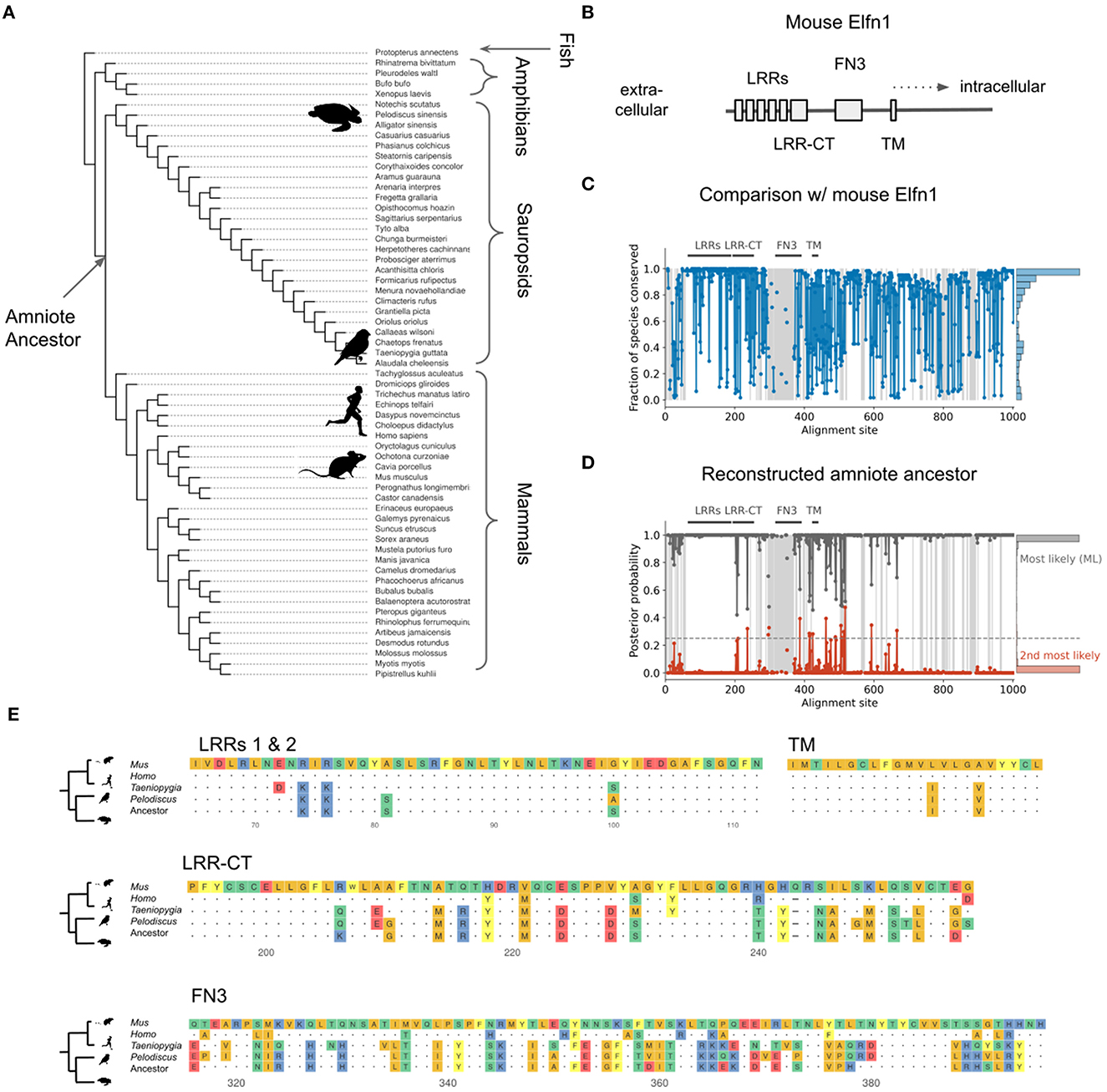
Figure 9. Reconstruction of ancestral Elfn1 protein. (A) Species-tree showing the phylogenetic relationships of the species whose Elfn1 homologs were used to reconstruct the Elfn1 protein of the amniote ancestor. (B) Domain structure of mouse Elfn1 (Dolan et al., 2007; Dunn et al., 2018). LRR, leucine-rich repeat; CT, C-terminal domain; FN3, fibronectin type 3 domain; TM, transmembrane domain. (C) Per-site conservation across the tree shown in (A), computed as the fraction of extant species that share the mouse amino acid at a given site. Dashed lines correspond to gaps. Mean conservation: 0.746. (D) Posterior probability of ancestral protein. Gray: most likely (ML) sequence, red: 2nd most likely. Dashed line: cutoff for using the 2nd most likely base in “altAll” sequence. Mean posterior: 0.986. (E) Multiple sequence alignment of protein domains shown in (A). Only the first two LRRs are shown for space reasons. Dots indicate identity to mouse site, dashes indicate gaps.
The suspicious match between the synaptic properties of PV and SST interneurons and the postsynaptic pyramidal cell compartments suggests that these interneuron properties could be the result of an adaptation to pyramidal cells. Here, we evaluated this idea of interneurons being “fit to function" from an evolutionary and developmental perspective, and showed that the relevant interneuron properties predate those of pyramidal cells both during development and in evolutionary history.
Two lines of evidence indicate that the development of PV and SST interneurons is not induced by mature pyramidal cell activity. First, interneurons become committed to a particular cell type (e.g., PV or SST) before reaching the developing cortex. Interneuron fate therefore cannot be influenced by the activity of pyramidal cells. Second, at least some of the properties of PV and SST interneurons that strongly shape their control of pyramidal cells—short-term plasticity and output connectivity—emerge before the maturation of pyramidal cell morphology and dendritic activity (dendrite-dependent bursting). It should be noted that other interneuron properties clearly are influenced by pyramidal cell activity. Excitatory activity regulates both the survival of interneurons (Denaxa et al., 2018), and the formation of inhibitory synapses (Garćıa et al., 2015; Marques-Smith et al., 2016). Specific types of excitatory neurons determine the laminar allocation of interneurons (Lodato et al., 2011; Wester et al., 2019), and their activity can even change the intrinsic properties of mature interneurons (Dehorter et al., 2015). Cell-extrinsic cues therefore play a role in the normal development of interneurons, but are unlikely to determine their identity and the properties we focused on here.
Analogous arguments suggest that the evolution of PV and SST interneurons also cannot be driven by the dendritic physiology of pyramidal cells. The lineages of birds, reptiles and mammals diverged over 300 million years ago, yet they all contain roughly similar interneuron types—evidence that these types were already present in a common ancestor of the three lineages. In contrast to interneurons, excitatory neurons are not conserved, and therefore probably evolved later. The second line of evolutionary evidence relates to two specific aspects of interneuron diversity: short-term plasticity and output connectivity. Recent scRNA-seq data (Tosches et al., 2018; Colquitt et al., 2021) show that reptilian and songbird SST interneurons express Elfn1, the gene that in mouse SST neurons is necessary and sufficient for short-term facilitation. Certain reptilian, but not songbird, SST subtypes also express Cbln4 that plays a role in the synaptic specificity of mammalian SST cells (Favuzzi et al., 2019).
These data suggest that ancestral interneurons already comprised PV- and SST-like cell types characterized by some of the genes for cell type-specific phenotypes in mammalian interneurons. It does not, however, imply that these phenotypes were actually present in ancestral cells. The expression of Elfn1, for example, is not sufficient for facilitating inputs, as shown in the case of VIP subtypes: Multipolar and bipolar VIP neurons both express Elfn1, but only the multipolar subtype receives facilitating excitation (Stachniak et al., 2019). It will therefore be interesting to directly test the presence of PV- and SST-specific phenotypes in reptiles and birds. If neither the reptile nor the songbird homologue of SST interneurons receives facilitating excitatory inputs, Elfn1 was likely reused for short-term facilitation in mammals. The emergence of short-term facilitation in SST neurons would then be an adaptation to pyramidal bursting, co-opting pre-existing interneuron diversity for “pyramidal cell purposes." The anatomical connectivity of interneurons might similarly have been reused to control pyramidal cells. In the mammalian brain, PV and SST interneurons inhibit not just the somata and dendrites, respectively, of pyramidal cells but also of non-pyramidal cells. Ancestral PV and SST interneurons might therefore have specialized in compartment-specific inhibition, but not of pyramidal cells for which their presynaptic dynamics are so well-matched.
Although our results show that pyramidal cell bursting is unlikely the driver of the differentiation of PV and SST interneurons, this is not in conflict with the functional interpretation of these cell types. In fact, an evolution of active pyramidal cell dendrites before the presence of specialized interneurons would have resulted in aberrant excitation, as seen, e.g., in Elfn1 mutants (Dolan and Mitchell, 2013; Tomioka et al., 2014). This suggests an alternative picture, in which excitatory neurons can only evolve in a way that still allows the existing interneurons to regulate their activity. This still leaves open the question why interneuron diversity evolved in the first place, if it was not for compartment-specific inhibition. Although it is possible that the initial separation between PV and SST cell types was selectively neutral, this is unlikely given their evolutionary conservation. Instead, the existence of PV and SST cells presumably offers advantages to mammalian and non-mammalian brains alike. An important example of an conserved function could be the temporal coordination of inputs and outputs of pyramidal cells based on oscillations (Bartos et al., 2002; Klausberger et al., 2003).
Our findings have potential implications for the neuroscientific interpretation of optimisation-based models of neural networks, which have recently seen a renaissance (Mante et al., 2013; Yamins et al., 2014; Richards et al., 2019; Saxe et al., 2019; Driscoll et al., 2022). Most of these models describe neural data at the relatively abstract level of dynamics and representations (Kriegeskorte and Diedrichsen, 2019; Vyas et al., 2020). Recently, such deep network models have also started to include circuit-level structure such as separate excitatory and inhibitory populations (Song et al., 2016; Naumann et al., 2022), different neuronal timescales (Kim et al., 2019; Perez-Nieves et al., 2021), and short-term plasticity (Masse et al., 2019; Keijser and Sprekeler, 2022). Deep learning is therefore gradually making its way down from the level of dynamical systems to that of circuits, potentially revealing functional roles for circuit elements. Our findings highlight a challenge to achieving this goal: Multiple circuit-level features—such as the properties of interneuron and pyramidal cells—are interdependent. The function of one feature might depend on that of another and vice versa, raising the question which features should be optimized (e.g., interneurons), and which should be assumed as pre-exising constraints or opportunities (e.g., nonlinear PC dendrites). In other words, optimization-based models face the challenge of modeling processes such as co-evolution. Merging the functional and evolutionary/developmental perspectives will therefore be an important challenge for future work.
Code was written in Python [version (v) 3.10.8 (vanRossum, 1995)] and R [v4.2.1 (R Core Team, 2021)], based on practices outlined in the Good Research Codebook (Mineault and Nozawa, 2021). Code for the transcriptomic analyses can be found at https://github.com/JoramKeijser/interneuron_evolution (JoramKeijser, 2023a). Code for the protein reconstruction can be found at https://github.com/JoramKeijser/elfn1_reconstruction (JoramKeijser, 2023b).
We analyzed the following publicly available single cell RNA sequencing data sets: mouse data from Tasic et al. (2018) (downloaded from https://portal.brain-map.org/atlases-and-data/rnaseq/mouse-v1-and-alm-smart-seq), human data from Bakken et al. (2021) (downloaded from https://portal.brain-map.org/atlases-and-data/rnaseq/human-m1-10x), zebra finch data (downloaded from https://cloud.biohpc.swmed.edu/index.php/s/nLicEtkmjGGmRF8?path=%2FHVC_RA), and turtle data from Tosches et al. (2018) (downloaded from https://public.brain.mpg.de/Laurent/ReptilePallium2018/). The paper's code repository contains a script for automatically downloading the corresponding files.
For each data set, the starting point of our analysis was a matrix of gene counts per cell, together with the clustering of cells from the original publications. We converted each of the datasets to Seurat [v4 (Hao et al., 2021)] and AnnData [v0.8 (Virshup et al., 2021)] objects for downstream analysis in Python and R, respectively. For visual comparison, we labeled songbird and turtle cell clusters according to the most similar mammalian interneuron subclass, as determined in the original publications. This involved the merging of fine-level clusters that presumably capture within-subclass differences. For each dataset, we only visualized cells part of, or corresponding to, cortical interneurons. In particular, we did not visualize the correlation of the songbird GABAergic clusters 7, 8, and Pre, since these seem homologous to mouse olfactory bulb interneurons (Colquitt et al., 2021).
We used AnnData and Scanpy [v1.9.1 (Wolf et al., 2018)] to visualize the expression of the Elfn1 and Cbln4 genes. This was done separately for each dataset. We first scaled the counts from each cell to counts per 10 thousand (CP10K) to account for differences in sequencing depth. We then used log plus one pseudo count (log1p) as variance-stabilizing transformation. Finally, we reduced the dimensionality of each dataset, by first finding highly variable genes, performed PCA followed by UMAP (McInnes et al., 2018). We used scanpy's default parameters for each of these steps. To investigate Cbln4 expression within the SST population, we performed dimensionality reduction on all SST cells except long-range projecting Chodl cells. Clustering was done using the Leiden algorithm (Traag et al., 2019) with resolution 1.
We quantified the overall similarity of species-specific cell clusters by replicating the correlation analysis from Tosches et al. (2018) and Colquitt et al. (2021). We separately performed the following analysis on GABAergic and glutamatergic cells, and only compared zebra finch and mice. Specifically, we performed the following steps.
1. Select genes to compare across species. For each species, determine subclass-specific marker genes using Seurat's findAllMarkers (t-test, min.pct = 0.2, max.cells.per.ident = 200) and retain genes with Bonferroni adjusted p-value below 0.05.
2. Intersect the two species-specific lists to find genes that are differentially expressed in both species. This resulted in ~500 genes, depending on the cell type.
3. Average counts within each cluster and transform to log scale for variance-stabilization. Specifically, compute: log(1+x)+0.1, with x the average count.
4. Divide each gene's value by its average across clusters to obtain a “specificity score" invariant to a genes' overall expression (Tosches et al., 2018).
5. Compute the Pearson correlation between all pairs of mouse and songbird clusters.
We visualized the result using the R package pheatmap [v1.10.12 (Kolde, 2012)].
We used Seurat's anchor-based integration (Stuart et al., 2019) to integrate the zebra finch and mouse data. We did this for GABAergic and glutamatergic neurons separately. First, we jointly performed normalization and variance stabilization for each dataset using Seurat's scTransform (Hafemeister and Satija, 2019), with the percentage of mitochondrial counts as covariate. Next, we found the top 3,000 most variable features across datasets, and used these to identify a set of anchors. These were then used to integrate the datasets. Finally, we jointly analyzed the integrated datasets using Seurat's standard visualization pipeline: scaling and centering, PCA, and UMAP.
We used the Topiary pipeline (Orlandi et al., 2023) to reconstruct the amino acid sequences of the ancestral Elfn1 protein based on sequences of extant species. To this end, we first constructed a source dataset consisting of the Elfn1 sequences from Mus musculus (mouse), Homo sapiens (human), Taeniopygia guttata (zebra finch), and Pelodiscus sinensis (Chinese softshell turtle). Next, we used Topiary's seed-to-alignment to find sequence homologs, perform reciprocal BLAST (Altschul et al., 1990) to predict their orthology, reduce sequence redundancy, and align the remaining sequences using Muscle5 (Edgar, 2022). This resulted in 62 aligned sequences that were used as input to Topiary's alignment-to-ancestors. This infers the maximum likelihood (ML) gene tree, the ML substitution model, and the ML ancestral sequences using RAxML-NG (Kozlov et al., 2019). The posterior probability of an ancestral amino acid was computed using the amino acid's likelihood weighted by its prior probability, normalized by the sum over all amino acids. Topiary generates bootstrap replicates of the ML gene tree, and uses GeneRax (Morel et al., 2020) to reconcile the gene tree with the species tree. The number of bootstrap replicates was 700, as automatically determined by the software. This number—but not the reconstructed ML sequence—varied slightly between runs. Finally, we used topiary's bootstrap-reconcile that estimates the branch support for the reconciled tree. The ancestral sequence contained 16 ambiguous sites (based on a posterior probability cutoff of 0.25). Besides the ML sequence, we also report a worst case “altAll" sequence in which these ambiguous sites have been replaced by the next most-likely amino acid. Branch support for the amniote ancestor was 100/100, indicating very high confidence in the existence of this ancestor, as expected. We aligned extant and ancestral sequences using Muscle5, and visualized the resulting alignment using the R package Ggmsa (Zhou et al., 2022).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Ethical review and approval was not required for the animal study because the data was previously collected by different authors, after approval of Animal Ethics Committees.
JK conceived of the project and analyzed the data. HS supervised the project. JK and HS discussed the interpretation of the data and wrote the manuscript. Both authors contributed to the article and approved the submitted version.
We thank Simon J. B. Butt, Loreen Hertäg, and members of the Sprekeler Lab for comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alcamo, E. A., Chirivella, L., Dautzenberg, M., Dobreva, G., Fariñas, I., Grosschedl, R., et al. (2008). Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377. doi: 10.1016/j.neuron.2007.12.012
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Anderson, S., Eisenstat, D., Shi, L., and Rubenstein, J. (1997). Interneuron migration from basal forebrain to neocortex: dependence on dlx genes. Science 278, 474–476. doi: 10.1126/science.278.5337.474
Arendt, D., Musser, J. M., Baker, C. V., Bergman, A., Cepko, C., Erwin, D. H., et al. (2016). The origin and evolution of cell types. Nat. Rev. Genet. 17, 744–757. doi: 10.1038/nrg.2016.127
Bakken, T. E., Jorstad, N. L., Hu, Q., Lake, B. B., Tian, W., Kalmbach, B. E., et al. (2021). Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119. doi: 10.1038/s41586-021-03465-8
Bandler, R. C., Vitali, I., Delgado, R. N., Ho, M. C., Dvoretskova, E., Ibarra Molinas, J. S., et al. (2021). Single-cell delineation of lineage and genetic identity in the mouse brain. Nature 601, 404–409. doi: 10.1038/s41586-021-04237-0
Bartos, M., Vida, I., Frotscher, M., Meyer, A., Monyer, H., Geiger, J. R., et al. (2002). Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc. Nat. Acad. Sci. 99, 13222–13227. doi: 10.1073/pnas.192233099
Bathellier, B., Margrie, T. W., and Larkum, M. E. (2009). Properties of piriform cortex pyramidal cell dendrites: implications for olfactory circuit design. J. Neurosci. 29, 12641–12652. doi: 10.1523/JNEUROSCI.1124-09.2009
Beaulieu-Laroche, L., Brown, N. J., Hansen, M., Toloza, E. H., Sharma, J., Williams, Z. M., et al. (2021). Allometric rules for mammalian cortical layer 5 neuron biophysics. Nature 600, 274–278. doi: 10.1038/s41586-021-04072-3
Beierlein, M., and Connors, B. W. (2002). Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J. Neurophysiol. 88, 1924–1932. doi: 10.1152/jn.2002.88.4.1924
Benezra, S. E., Narayanan, R. T., Egger, R., Oberlaender, M., and Long, M. A. (2018). Morphological characterization of hvc projection neurons in the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 526, 1673–1689. doi: 10.1002/cne.24437
Berger, T. K., Silberberg, G., Perin, R., and Markram, H. (2010). Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol. 8, e1000473. doi: 10.1371/journal.pbio.1000473
Bhatia, A., Moza, S., and Bhalla, U. S. (2019). Precise excitation-inhibition balance controls gain and timing in the hippocampus. Elife 8, e43415. doi: 10.7554/eLife.43415
Blanton, M. G., Shen, J. M., and Kriegstein, A. R. (1987). Evidence for the inhibitory neurotransmitter γ-aminobutyric acid in aspiny and sparsely spiny nonpyramidal neurons of the turtle dorsal cortex. J. Comp. Neurol. 259, 277–297. doi: 10.1002/cne.902590208
Butt, S. J., Fuccillo, M., Nery, S., Noctor, S., Kriegstein, A., Corbin, J. G., et al. (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48, 591–604. doi: 10.1016/j.neuron.2005.09.034
Butt, S. J., Sousa, V. H., Fuccillo, M. V., Hjerling-Leffler, J., Miyoshi, G., Kimura, S., et al. (2008). The requirement of nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron 59, 722–732. doi: 10.1016/j.neuron.2008.07.031
Caillard, O., Moreno, H., Schwaller, B., Llano, I., Celio, M. R., Marty, A., et al. (2000). Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc. Nat. Acad. Sci. 97, 13372–13377. doi: 10.1073/pnas.230362997
Campagnola, L., Seeman, S. C., Chartrand, T., Kim, L., Hoggarth, A., Gamlin, C., et al. (2022). Local connectivity and synaptic dynamics in mouse and human neocortex. Science 375, eabj5861. doi: 10.1126/science.abj5861
Colquitt, B. M., Merullo, D. P., Konopka, G., Roberts, T. F., and Brainard, M. S. (2021). Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits. Science 371, eabd9704. doi: 10.1126/science.abd9704
Connors, B. W., and Kriegstein, A. (1986). Cellular physiology of the turtle visual cortex: distinctive properties of pyramidal and stellate neurons. J. Neurosci. 6, 164–177. doi: 10.1523/JNEUROSCI.06-01-00164.1986
Cristo, G. D., Wu, C., Chattopadhyaya, B., Ango, F., Knott, G., Welker, E., et al. (2004). Subcellular domain-restricted gabaergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat. Neurosci. 7, 1184–1186. doi: 10.1038/nn1334
Dard, R. F., Leprince, E., Denis, J., Balappa, S. R., Suchkov, D., Boyce, R., et al. (2022). The rapid developmental rise of somatic inhibition disengages hippocampal dynamics from self-motion. Elife 11, e78116. doi: 10.7554/eLife.78116.sa2
Dehorter, N., Ciceri, G., Bartolini, G., Lim, L., del Pino, I., and Marín, O. (2015). Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 349, 1216–1220. doi: 10.1126/science.aab3415
Denaxa, M., Neves, G., Rabinowitz, A., Kemlo, S., Liodis, P., Burrone, J., et al. (2018). Modulation of apoptosis controls inhibitory interneuron number in the cortex. Cell Rep. 22, 1710–1721. doi: 10.1016/j.celrep.2018.01.064
Devoogd, T. J., and Nottebohm, F. (1981). Sex differences in dendritic morphology of a song control nucleus in the canary: a quantitative golgi study. J. Comp. Neurol. 196, 309–316. doi: 10.1002/cne.901960209
Dolan, J., and Mitchell, K. J. (2013). Mutation of elfn1 in mice causes seizures and hyperactivity. PLoS ONE 8, e80491. doi: 10.1371/journal.pone.0080491
Dolan, J., Walshe, K., Alsbury, S., Hokamp, K., O'Keeffe, S., Okafuji, T., et al. (2007). The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8, 1–24. doi: 10.1186/1471-2164-8-320
Driscoll, L., Shenoy, K., and Sussillo, D. (2022). Flexible multitask computation in recurrent networks utilizes shared dynamical motifs. bioRxiv. doi: 10.1101/2022.08.15.503870
Dunn, H. A., Patil, D. N., Cao, Y., Orlandi, C., and Martemyanov, K. A. (2018). Synaptic adhesion protein elfn1 is a selective allosteric modulator of group iii metabotropic glutamate receptors in trans. Proc. Nat. Acad. Sci. 115, 5022–5027. doi: 10.1073/pnas.1722498115
Edgar, R. C. (2022). Muscle5: high-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 13, 6968. doi: 10.1038/s41467-022-34630-w
Favuzzi, E., Deogracias, R., Marques-Smith, A., Maeso, P., Jezequel, J., Exposito-Alonso, D., et al. (2019). Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science 363, 413–417. doi: 10.1126/science.aau8977
Ferguson, K. A., and Cardin, J. A. (2020). Mechanisms underlying gain modulation in the cortex. Nat. Rev. Neurosci. 21, 80–92. doi: 10.1038/s41583-019-0253-y
Fishell, G., and Kepecs, A. (2020). Interneuron types as attractors and controllers. Annu. Rev. Neurosci. 43, 1–30. doi: 10.1146/annurev-neuro-070918-050421
Flames, N., Pla, R., Gelman, D. M., Rubenstein, J. L., Puelles, L., and Marín, O. (2007). Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007
Fogarty, M., Grist, M., Gelman, D., Marín, O., Pachnis, V., and Kessaris, N. (2007). Spatial genetic patterning of the embryonic neuroepithelium generates gabaergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007
Franceschetti, S., Sancini, G., Panzica, F., Radici, C., and Avanzini, G. (1998). Postnatal differentiation of firing properties and morphological characteristics in layer v pyramidal neurons of the sensorimotor cortex. Neuroscience 83, 1013–1024. doi: 10.1016/S0306-4522(97)00463-6
Fu, Y., Tucciarone, J. M., Espinosa, J. S., Sheng, N., Darcy, D. P., Nicoll, R. A., et al. (2014). A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152. doi: 10.1016/j.cell.2014.01.050
García, N. V. D. M., Priya, R., Tuncdemir, S. N., Fishell, G., and Karayannis, T. (2015). Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat. Neurosci. 18, 393–401. doi: 10.1038/nn.3946
Gentet, L. J., Kremer, Y., Taniguchi, H., Huang, Z. J., Staiger, J. F., Petersen, C. C., et al. (2012). Unique functional properties of somatostatin-expressing gabaergic neurons in mouse barrel cortex. Nat. Neurosci. 15, 607–612. doi: 10.1038/nn.3051
Gidon, A., Zolnik, T. A., Fidzinski, P., Bolduan, F., Papoutsi, A., Poirazi, P., et al. (2020). Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science 367, 83–87. doi: 10.1126/science.aax6239
Goldberg, J. H., Lacefield, C. O., and Yuste, R. (2004). Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J. Physiol. 558, 465–478. doi: 10.1113/jphysiol.2004.064519
Gouwens, N. W., Sorensen, S. A., Baftizadeh, F., Budzillo, A., Lee, B. R., Jarsky, T., et al. (2020). Integrated morphoelectric and transcriptomic classification of cortical gabaergic cells. Cell, 183, 935–953. doi: 10.1016/j.cell.2020.09.057
Hafemeister, C., and Satija, R. (2019). Normalization and variance stabilization of single-cell rna-seq data using regularized negative binomial regression. Genome Biol. 20, 1–15. doi: 10.1186/s13059-019-1874-1
Hansen, D. V., Lui, J. H., Flandin, P., Yoshikawa, K., Rubenstein, J. L., Alvarez-Buylla, A., et al. (2013). Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat. Neurosci. 16, 1576–1587. doi: 10.1038/nn.3541
Hao, Y., Hao, S., Andersen-Nissen, E., Mauck, I. I. I., Zheng, W. M., Butler, S., et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587. doi: 10.1016/j.cell.2021.04.048
He, M., Tucciarone, J., Lee, S., Nigro, M. J., Kim, Y., Levine, J. M., et al. (2016). Strategies and tools for combinatorial targeting of gabaergic neurons in mouse cerebral cortex. Neuron 91, 1228–1243. doi: 10.1016/j.neuron.2016.08.021
Hertäg, L., and Clopath, C. (2022). Prediction-error neurons in circuits with multiple neuron types: formation, refinement, and functional implications. Proc. Nat. Acad. Sci. 119, e2115699119. doi: 10.1073/pnas.2115699119
Hobert, O. (2008). Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc. Nat. Acad. Sci. 105, 20067–20071. doi: 10.1073/pnas.0806070105
Hochberg, G. K., and Thornton, J. W. (2017). Reconstructing ancient proteins to understand the causes of structure and function. Annu. Rev. Biophys. 46, 247–269. doi: 10.1146/annurev-biophys-070816-033631
Hu, J. S., Vogt, D., Lindtner, S., Sandberg, M., Silberberg, S. N., Rubenstein, J. L., et al. (2017). Coup-tf1 and coup-tf2 control subtype and laminar identity of mge-derived neocortical interneurons. Development 144, 2837–2851. doi: 10.1242/dev.150664
Inan, M., Welagen, J., and Anderson, S. A. (2012). Spatial and temporal bias in the mitotic origins of somatostatin-and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb. Cortex 22, 820–827. doi: 10.1093/cercor/bhr148
Jiang, X., Shen, S., Cadwell, C. R., Berens, P., Sinz, F., Ecker, A. S., et al. (2015). Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350, aac9462. doi: 10.1126/science.aac9462
Johenning, F. W., Beed, P. S., Trimbuch, T., Bendels, M. H., Winterer, J., Schmitz, D., et al. (2009). Dendritic compartment and neuronal output mode determine pathway-specific long-term potentiation in the piriform cortex. J. Neurosci. 29, 13649–13661. doi: 10.1523/JNEUROSCI.2672-09.2009
Jones, D. T., Taylor, W. R., and Thornton, J. M. (1992). The rapid generation of mutation data matrices from protein sequences. Bioinformatics 8, 275–282. doi: 10.1093/bioinformatics/8.3.275
Keijser, J., and Sprekeler, H. (2022). Optimizing interneuron circuits for compartment-specific feedback inhibition. PLoS Comput. Biol. 18, e1009933. doi: 10.1371/journal.pcbi.1009933
Keller, G. B., and Mrsic-Flogel, T. D. (2018). Predictive processing: a canonical cortical computation. Neuron 100, 424–435. doi: 10.1016/j.neuron.2018.10.003
Kepecs, A., and Fishell, G. (2014). Interneuron cell types are fit to function. Nature 505, 318–326. doi: 10.1038/nature12983
Kim, R., Li, Y., and Sejnowski, T. J. (2019). Simple framework for constructing functional spiking recurrent neural networks. Proc. Nat. Acad. Sci. 116, 22811–22820. doi: 10.1073/pnas.1905926116
Klausberger, T., Magill, P. J., Márton, L. F., Roberts, J. D. B., Cobden, P. M., Buzsáki, G., et al. (2003). Brain-state-and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. doi: 10.1038/nature01374
Kornfeld, J., Benezra, S. E., Narayanan, R. T., Svara, F., Egger, R., Oberlaender, M., et al. (2017). Em connectomics reveals axonal target variation in a sequence-generating network. Elife 6, e24364. doi: 10.7554/eLife.24364.020
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B., and Stamatakis, A. (2019). Raxml-ng: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455. doi: 10.1093/bioinformatics/btz305
Kriegeskorte, N., and Diedrichsen, J. (2019). Peeling the onion of brain representations. Annu. Rev. Neurosci. 42, 407–432. doi: 10.1146/annurev-neuro-080317-061906
Kumar, A., Schiff, O., Barkai, E., Mel, B. W., Poleg-Polsky, A., Schiller, J., et al. (2018). Nmda spikes mediate amplification of inputs in the rat piriform cortex. Elife 7, e38446. doi: 10.7554/eLife.38446.018
Larkum, M. (2013). A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 36, 141–151. doi: 10.1016/j.tins.2012.11.006
Larkum, M. E., Watanabe, S., Lasser-Ross, N., Rhodes, P., and Ross, W. N. (2008). Dendritic properties of turtle pyramidal neurons. J. Neurophysiol. 99, 683–694. doi: 10.1152/jn.01076.2007
Larkum, M. E., Zhu, J. J., and Sakmann, B. (1999). A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398, 338–341. doi: 10.1038/18686
Ledderose, J. M., Zolnik, T. A., Toumazou, M., Trimbuch, T., Rosenmund, C., Eickholt, B. J., et al. (2022). Input to cortical layer 1 of somatosensory cortex. bioRxiv. [preprint]. doi: 10.1101/2021.11.26.469979v3
Letzkus, J. J., Kampa, B. M., and Stuart, G. J. (2006). Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J. Neurosci. 26, 10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006
Letzkus, J. J., Wolff, S. B., and Lüthi, A. (2015). Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276. doi: 10.1016/j.neuron.2015.09.024
Lim, L., Mi, D., Llorca, A., and Marín, O. (2018a). Development and functional diversification of cortical interneurons. Neuron 100, 294–313. doi: 10.1016/j.neuron.2018.10.009
Lim, L., Pakan, J. M., Selten, M. M., Marques-Smith, A., Llorca, A., Bae, S. E., et al. (2018b). Optimization of interneuron function by direct coupling of cell migration and axonal targeting. Nat. Neurosci. 21, 920–931. doi: 10.1038/s41593-018-0162-9
Lodato, S., Molyneaux, B. J., Zuccaro, E., Goff, L. A., Chen, H.-H., Yuan, W., et al. (2014). Gene co-regulation by fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nat. Neurosci. 17, 1046–1054. doi: 10.1038/nn.3757
Lodato, S., Rouaux, C., Quast, K. B., Jantrachotechatchawan, C., Studer, M., Hensch, T. K., et al. (2011). Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 69, 763–779. doi: 10.1016/j.neuron.2011.01.015
Lovett-Barron, M., Turi, G. F., Kaifosh, P., Lee, P. H., Bolze, F., Sun, X.-H., et al. (2012). Regulation of neuronal input transformations by tunable dendritic inhibition. Nat. Neurosci. 15, 423–430. doi: 10.1038/nn.3024
Ma, T., Wang, C., Wang, L., Zhou, X., Tian, M., Zhang, Q., et al. (2013). Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci. 16, 1588–1597. doi: 10.1038/nn.3536
Mante, V., Sussillo, D., Shenoy, K. V., and Newsome, W. T. (2013). Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84. doi: 10.1038/nature12742
Marín, O., and Rubenstein, J. L. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790. doi: 10.1038/35097509
Marques-Smith, A., Lyngholm, D., Kaufmann, A.-K., Stacey, J. A., Hoerder-Suabedissen, A., Becker, E. B., et al. (2016). A transient translaminar gabaergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron 89, 536–549. doi: 10.1016/j.neuron.2016.01.015
Masse, N. Y., Yang, G. R., Song, H. F., Wang, X.-J., and Freedman, D. J. (2019). Circuit mechanisms for the maintenance and manipulation of information in working memory. Nat. Neurosci. 22, 1159–1167. doi: 10.1038/s41593-019-0414-3
Mayer, C., Hafemeister, C., Bandler, R. C., Machold, R., Brito, R. B., Jaglin, X., et al. (2018). Developmental diversification of cortical inhibitory interneurons. Nature 555, 457–462. doi: 10.1038/nature25999
McInnes, L., Healy, J., and Melville, J. (2018). Umap: uniform manifold approximation and projection for dimension reduction. arXiv. [preprint]. doi: 10.21105/joss.00861
McKenzie, M. G., Cobbs, L. V., Dummer, P. D., Petros, T. J., Halford, M. M., Stacker, S. A., et al. (2019). Non-canonical wnt signaling through ryk regulates the generation of somatostatin-and parvalbumin-expressing cortical interneurons. Neuron 103, 853–864. doi: 10.1016/j.neuron.2019.06.003
Mi, D., Li, Z., Lim, L., Li, M., Moissidis, M., Yang, Y., et al. (2018). Early emergence of cortical interneuron diversity in the mouse embryo. Science 360, 81–85. doi: 10.1126/science.aar6821
Miyoshi, G., Hjerling-Leffler, J., Karayannis, T., Sousa, V. H., Butt, S. J., Battiste, J., et al. (2010). Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 30, 1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010
Morel, B., Kozlov, A. M., Stamatakis, A., and Szöllősi, G. J. (2020). Generax: a tool for species-tree-aware maximum likelihood-based gene family tree inference under gene duplication, transfer, and loss. Mol. Biol. Evol. 37, 2763–2774. doi: 10.1093/molbev/msaa141
Murayama, M. Pérez-Garci, E., Nevian, T., Bock, T., Senn, W., and Larkum, M. E. (2009). Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141. doi: 10.1038/nature07663
Naud, R., Bathellier, B., and Gerstner, W. (2013). Spike timing prediction with active dendrites. arXiv. [preprint]. doi: 10.48550/arXiv.1311.3586
Naud, R., and Sprekeler, H. (2018). Sparse bursts optimize information transmission in a multiplexed neural code. Proc. Nat. Acad. Sci. 115, E6329–E6338. doi: 10.1073/pnas.1720995115
Naumann, L. B., Keijser, J., and Sprekeler, H. (2022). Invariant neural subspaces maintained by feedback modulation. Elife 11, e76096. doi: 10.7554/eLife.76096.sa2
Nery, S., Fishell, G., and Corbin, J. G. (2002). The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 5, 1279–1287. doi: 10.1038/nn971
Nigro, M. J., Hashikawa-Yamasaki, Y., and Rudy, B. (2018). Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J. Neurosci. 38, 1622–1633. doi: 10.1523/JNEUROSCI.2415-17.2017
Nomura, T., Yamashita, W., Gotoh, H., and Ono, K. (2018). Species-specific mechanisms of neuron subtype specification reveal evolutionary plasticity of amniote brain development. Cell Rep. 22, 3142–3151. doi: 10.1016/j.celrep.2018.02.086
Orlandi, K. N., Phillips, S. R., Sailer, Z. R., Harman, J. L., and Harms, M. J. (2023). Topiary: pruning the manual labor from ancestral sequence reconstruction. Protein Sci. 32, e4551. doi: 10.1002/pro.4551
Perez-Nieves, N., Leung, V. C., Dragotti, P. L., and Goodman, D. F. (2021). Neural heterogeneity promotes robust learning. Nat. Commun. 12, 1–9. doi: 10.1038/s41467-021-26022-3
Petreanu, L., Huber, D., Sobczyk, A., and Svoboda, K. (2007). Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668. doi: 10.1038/nn1891
Pouille, F., and Scanziani, M. (2004). Routing of spike series by dynamic circuits in the hippocampus. Nature 429, 717–723. doi: 10.1038/nature02615
R Core Team. (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation For Statistical Computing.
Rakic, P. (1971). Neuron-glia relationship during granule cell migration in developing cerebellar cortex. a golgi and electonmicroscopic study in macacus rhesus. J. Comp. Neurol. 141, 283–312. doi: 10.1002/cne.901410303
Reiner, A. (1993). Neurotransmitter organization and connections of turtle cortex: implications for the evolution of mammalian isocortex. Comp. Biochem. Physiol. A Physiol. 104, 735–748. doi: 10.1016/0300-9629(93)90149-X
Reyes, A., Lujan, R., Rozov, A., Burnashev, N., Somogyi, P., Sakmann, B., et al. (1998). Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1, 279–285. doi: 10.1038/1092
Richards, B. A., Lillicrap, T. P., Beaudoin, P., Bengio, Y., Bogacz, R., Christensen, A., et al. (2019). A deep learning framework for neuroscience. Nat. Neurosci. 22, 1761–1770. doi: 10.1038/s41593-019-0520-2
Romand, S., Wang, Y., Toledo-Rodriguez, M., and Markram, H. (2011). Morphological development of thick-tufted layer v pyramidal cells in the rat somatosensory cortex. Front. Neuroanat. 5, 5. doi: 10.3389/fnana.2011.00005
Sadeh, S., and Clopath, C. (2021). Inhibitory stabilization and cortical computation. Nat. Rev. Neurosci. 22, 21–37. doi: 10.1038/s41583-020-00390-z
Saxe, A. M., McClelland, J. L., and Ganguli, S. (2019). A mathematical theory of semantic development in deep neural networks. Proc. Nat. Acad. Sci. 116, 11537–11546. doi: 10.1073/pnas.1820226116
Scala, F., Kobak, D., Shan, S., Bernaerts, Y., Laturnus, S., Cadwell, C. R., et al. (2019). Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat. Commun. 10, 4174. doi: 10.1038/s41467-019-12058-z
Schmitz, M. T., Sandoval, K., Chen, C. P., Mostajo-Radji, M. A., Seeley, W. W., Nowakowski, T. J., et al. (2022). The development and evolution of inhibitory neurons in primate cerebrum. Nature 603, 871–877. doi: 10.1038/s41586-022-04510-w
Shi, Y., Wang, M., Mi, D., Lu, T., Wang, B., Dong, H., et al. (2021). Mouse and human share conserved transcriptional programs for interneuron development. Science 374, eabj6641. doi: 10.1126/science.abj6641
Silberberg, G., and Markram, H. (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by martinotti cells. Neuron 53, 735–746. doi: 10.1016/j.neuron.2007.02.012
Sjostrom, P. J., Rancz, E. A., Roth, A., and Hausser, M. (2008). Dendritic excitability and synaptic plasticity. Physiol. Rev. 88, 769–840. doi: 10.1152/physrev.00016.2007
Song, H. F., Yang, G. R., and Wang, X.-J. (2016). Training excitatory-inhibitory recurrent neural networks for cognitive tasks: a simple and flexible framework. PLoS Comput. Biol. 12, e1004792. doi: 10.1371/journal.pcbi.1004792
Stachniak, T. J., Kastli, R., Hanley, O., Argunsah, A. Ö., van der Valk, E. G. T., Kanatouris, G., et al. (2021). Postmitotic prox1 expression controls the final specification of cortical vip interneuron subtypes. J. Neurosci. 41, 8150–8162. doi: 10.1523/JNEUROSCI.1021-21.2021
Stachniak, T. J., Sylwestrak, E. L., Scheiffele, P., Hall, B. J., and Ghosh, A. (2019). Elfn1-induced constitutive activation of mglur7 determines frequency-dependent recruitment of somatostatin interneurons. J. Neurosci. 39, 4461–4474. doi: 10.1523/JNEUROSCI.2276-18.2019
Stuart, T., Butler, A., Hoffman, P., Hafemeister, C., Papalexi, E., Mauck, I. I. I. R., et al. (2019). Comprehensive integration of single-cell data. Cell 177, 1888–1902. doi: 10.1016/j.cell.2019.05.031
Sussel, L., Marin, O., Kimura, S., and Rubenstein, J. (1999). Loss of nkx2, 1. homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370. doi: 10.1242/dev.126.15.3359
Sylwestrak, E. L., and Ghosh, A. (2012). Elfn1 regulates target-specific release probability at ca1-interneuron synapses. Science 338, 536–540. doi: 10.1126/science.1222482
Tanay, A., and Sebé-Pedrós, A. (2021). Evolutionary cell type mapping with single-cell genomics. Trends Genet. 37, 919–932. doi: 10.1016/j.tig.2021.04.008
Tang, F., Barbacioru, C., Wang, Y., Nordman, E., Lee, C., Xu, N., et al. (2009). mrna-seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382. doi: 10.1038/nmeth.1315
Tasic, B., Menon, V., Nguyen, T. N., Kim, T. K., Jarsky, T., Yao, Z., et al. (2016). Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346. doi: 10.1038/nn.4216
Tasic, B., Yao, Z., Graybuck, L. T., Smith, K. A., Nguyen, T. N., Bertagnolli, D., et al. (2018). Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78. doi: 10.1038/s41586-018-0654-5
Tomioka, N. H., Yasuda, H., Miyamoto, H., Hatayama, M., Morimura, N., Matsumoto, Y., et al. (2014). Elfn1 recruits presynaptic mglur7 in trans and its loss results in seizures. Nat. Commun. 5, 1–16. doi: 10.1038/ncomms5501
Tosches, M. A. (2021a). Different origins for similar brain circuits. Science 371, 676–677. doi: 10.1126/science.abf9551
Tosches, M. A. (2021b). From cell types to an integrated understanding of brain evolution: the case of the cerebral cortex. Annu. Rev. Cell Dev. Biol. 37, 495–517. doi: 10.1146/annurev-cellbio-120319-112654
Tosches, M. A., Yamawaki, T. M., Naumann, R. K., Jacobi, A. A., Tushev, G., Laurent, G., et al. (2018). Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888. doi: 10.1126/science.aar4237
Traag, V. A., Waltman, L., and Van Eck, N. J. (2019). From louvain to leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233. doi: 10.1038/s41598-019-41695-z
Tremblay, R., Lee, S., and Rudy, B. (2016). Gabaergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. doi: 10.1016/j.neuron.2016.06.033
Tsodyks, M., Pawelzik, K., and Markram, H. (1998). Neural networks with dynamic synapses. Neural Comput. 10, 821–835. doi: 10.1162/089976698300017502
Udakis, M., Pedrosa, V., Chamberlain, S. E., Clopath, C., and Mellor, J. R. (2020). Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto ca1 pyramidal neurons shapes hippocampal output. Nat. Commun. 11, 1–17. doi: 10.1038/s41467-020-18074-8
vanRossum, G. (1995). Python Reference Manual. Ashburn, VA: Department of Computer Science [CS], (R 9525).
Virshup, I., Rybakov, S., Theis, F. J., Angerer, P., and Wolf, F. A. (2021). anndata: Annotated Data. bioRxiv. doi: 10.1101/2021.12.16.473007
Vogels, T. P., Sprekeler, H., Zenke, F., Clopath, C., and Gerstner, W. (2011). Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573. doi: 10.1126/science.1211095
Vyas, S., Golub, M. D., Sussillo, D., and Shenoy, K. V. (2020). Computation through neural population dynamics. Annu. Rev. Neurosci. 43, 249. doi: 10.1146/annurev-neuro-092619-094115
Wamsley, B., and Fishell, G. (2017). Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat. Rev. Neurosci. 18, 299–309. doi: 10.1038/nrn.2017.30
Wehr, M., and Zador, A. M. (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446. doi: 10.1038/nature02116
Wester, J. C., Mahadevan, V., Rhodes, C. T., Calvigioni, D., Venkatesh, S., Maric, D., et al. (2019). Neocortical projection neurons instruct inhibitory interneuron circuit development in a lineage-dependent manner. Neuron 102, 960–975. doi: 10.1016/j.neuron.2019.03.036
Wichterle, H., Turnbull, D. H., Nery, S., Fishell, G., and Alvarez-Buylla, A. (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759–3771. doi: 10.1242/dev.128.19.3759
Williams, L. E., and Holtmaat, A. (2019). Higher-order thalamocortical inputs gate synaptic long-term potentiation via disinhibition. Neuron 101, 91–102. doi: 10.1016/j.neuron.2018.10.049
Williams, S. R., and Stuart, G. J. (1999). Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J. Physiol. 521(Pt 2), 467. doi: 10.1111/j.1469-7793.1999.00467.x
Wolf, F. A., Angerer, P., and Theis, F. J. (2018). Scanpy: large-scale single-cell gene expression data analysis. Genome Biol. 19, 1–5. doi: 10.1186/s13059-017-1382-0
Wonders, C. P., and Anderson, S. A. (2006). The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696. doi: 10.1038/nrn1954
Wonders, C. P., Taylor, L., Welagen, J., Mbata, I. C., Xiang, J. Z., Anderson, S. A., et al. (2008). A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Biol. 314, 127–136. doi: 10.1016/j.ydbio.2007.11.018
Wu, Y. K., Miehl, C., and Gjorgjieva, J. (2022). Regulation of circuit organization and function through inhibitory synaptic plasticity. Trends Neurosci. 45, 884–898. doi: 10.1016/j.tins.2022.10.006
Xu, Q., Cobos, I., De La Cruz, E., Rubenstein, J. L., and Anderson, S. A. (2004). Origins of cortical interneuron subtypes. J. Neurosci. 24, 2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004
Yamins, D. L., Hong, H., Cadieu, C. F., Solomon, E. A., Seibert, D., DiCarlo, J. J., et al. (2014). Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc. Nat. Acad. Sci. 111, 8619–8624. doi: 10.1073/pnas.1403112111
Zeisel, A., Muñoz-Manchado, A. B., Codeluppi, S., Lönnerberg, P., La Manno, G., Juréus, A., et al. (2015). Cell types in the mouse cortex and hippocampus revealed by single-cell rna-seq. Science 347, 1138–1142. doi: 10.1126/science.aaa1934
Zhou, L., Feng, T., Xu, S., Gao, F., Lam, T. T., Wang, Q., et al. (2022). ggmsa: a visual exploration tool for multiple sequence alignment and associated data. Brief. Bioinform. 23, bbac222. doi: 10.1093/bib/bbac222
Keywords: inhibition, interneuron, evolution, development, microcircuits, single cell RNA seq, neural morphology, pyramidal cell dendrites
Citation: Keijser J and Sprekeler H (2023) Cortical interneurons: fit for function and fit to function? Evidence from development and evolution. Front. Neural Circuits 17:1172464. doi: 10.3389/fncir.2023.1172464
Received: 23 February 2023; Accepted: 30 March 2023;
Published: 04 May 2023.
Edited by:
Gabrielle Pouchelon, Cold Spring Harbor Laboratory, United StatesReviewed by:
Daniel Vogt, Michigan State University, United StatesCopyright © 2023 Keijser and Sprekeler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joram Keijser, am9yYW1rZWlqc2VyQGdtYWlsLmNvbQ==; Henning Sprekeler, aC5zcHJla2VsZXJAdHUtYmVybGluLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.