
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neural Circuits , 23 August 2022
Volume 16 - 2022 | https://doi.org/10.3389/fncir.2022.983211
This article is part of the Research Topic Forebrain Control of Breathing and Sudden Death in Epilepsy View all 10 articles
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death among patients with refractory epilepsy. While the exact etiology of SUDEP is unknown, mounting evidence implicates respiratory dysfunction as a precipitating factor in cases of seizure-induced death. Dysregulation of breathing can occur in epilepsy patients during and after seizures as well as interictally, with many epilepsy patients exhibiting sleep-disordered breathing (SDB), such as obstructive sleep apnea (OSA). The majority of SUDEP cases occur during the night, with the victim found prone in or near a bed. As breathing is modulated in both a time-of-day and sleep state-dependent manner, it is relevant to examine the added burden of nocturnal seizures on respiratory function. This review explores the current state of understanding of the relationship between respiratory function, sleep state and time of day, and epilepsy. We highlight sleep as a particularly vulnerable period for individuals with epilepsy and press that this topic warrants further investigation in order to develop therapeutic interventions to mitigate the risk of SUDEP.
Epilepsy is one of the most common neurological disorders. One in 26 Americans will develop epilepsy during their lifetime (Kotsopoulos et al., 2002 ; Hesdorffer et al., 2011). Despite its prevalence, approximately 35% of patients will not achieve seizure freedom with medical treatment (Kwan and Brodie, 2000; Chen et al., 2018). Though there has been continued expansion in the availability of anti-seizure medications (ASM), patients who exhibit an inadequate response to initial ASM treatment are likely to have medically refractory epilepsy (Kwan and Brodie, 2000). The leading cause of death among these individuals with poor seizure control is sudden unexpected death in epilepsy or SUDEP (Devinsky et al., 2016). SUDEP is defined as the “sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death in patients with epilepsy, with or without evidence for a seizure and excluding documented status epilepticus, in which postmortem examination does not reveal a toxicologic or anatomic cause of death” (Nashef et al., 1998). While by definition SUDEP does not have to follow a seizure, there is strong evidence to suggest it is a seizure-related phenomenon, with its agonal mechanisms beginning during or in the immediate aftermath of a seizure (Nashef et al., 1998; Nilsson et al., 1999; Surges et al., 2009; Surges and Sander, 2012; Bozorgi and Lhatoo, 2013). There is a slight predominance of SUDEP cases in males compared to females (Tennis et al., 1995; Nilsson et al., 1999; Shankar et al., 2013).
Despite the tremendous burden of SUDEP, its underlying pathological mechanisms are poorly understood. However, evidence is accumulating that implicates seizure-related respiratory failure as a major factor in this deadly phenomenon (Ryvlin et al., 2013; Buchanan et al., 2014; Kim et al., 2018; Dhaibar et al., 2019). In SUDEP cases that were captured in epilepsy monitoring units (EMU), terminal apnea preceded terminal asystole in every case (Ryvlin et al., 2013). Further, mechanical ventilation has been found to greatly reduce seizure-induced mortality, both in human patients and animal models (Tupal and Faingold, 2006; Ryvlin et al., 2013; Buchanan et al., 2014). Thus, further investigation into respiratory dysfunction in epilepsy is critical to untangle the underlying mechanisms of SUDEP, as well as to assist clinicians in developing respiratory-focused interventions.
Another consistent observation is that SUDEP cases predominantly occur during the night (Nobili et al., 2011; Lamberts et al., 2012; Sveinsson et al., 2018). Around 95% of SUDEP cases occur inside the victim’s residence, with the majority of victims found in or near a bed in a prone position (Opeskin and Berkovic, 2003; Zhuo et al., 2012; Ali et al., 2017; Sveinsson et al., 2018). Despite occurring so close to home, the vast majority of these cases are unwitnessed (Lamberts et al., 2012; Zhuo et al., 2012; Rugg-Gunn et al., 2016; Purnell et al., 2018). Patients who die of SUDEP are twice as likely to have a history of nocturnal seizures, and thus the presence of nocturnal seizures are considered a risk factor for SUDEP (Lamberts et al., 2012; Shankar et al., 2013; Sveinsson et al., 2018; Van Der Lende et al., 2018). Seizures and epileptiform discharges occur more frequently during non-rapid eye movement (NREM) sleep in both human patients and animal models (Bazil and Walczak, 1997; Malow et al., 1998). Sleep state can influence the frequency, severity, and duration of seizures (Bazil and Walczak, 1997; Ng and Pavlova, 2013). Seizures occurring during sleep tend to be longer and are more likely to evolve into focal and bilateral tonic-clonic seizures (Bazil and Walczak, 1997).
As humans tend to consolidate their sleep during the night, many investigations of and conclusions about SUDEP risk factors conflate sleep-state and nighttime as one in the same. In reality, sleep and circadian rhythmicity can independently alter physiological processes, including respiratory and cardiac function (Snyder et al., 1964; Browne et al., 1983; Spengler et al., 2000; Mortola, 2004; Buchanan, 2013). The major influence of sleep and circadian timing on respiration makes this a salient point of examination when considering SUDEP pathophysiology. The aim of this review is to examine the distinct influences of sleep and circadian rhythms on respiration both in a healthy brain and in patients with epilepsy (Figure 1). We hope to not only highlight the factors that make nocturnal seizures more deadly, but to better differentiate between sleep-state and time-of-day influences on breathing, so that clinicians can develop specific preventative strategies for fatal seizure-induced respiratory dysfunction.
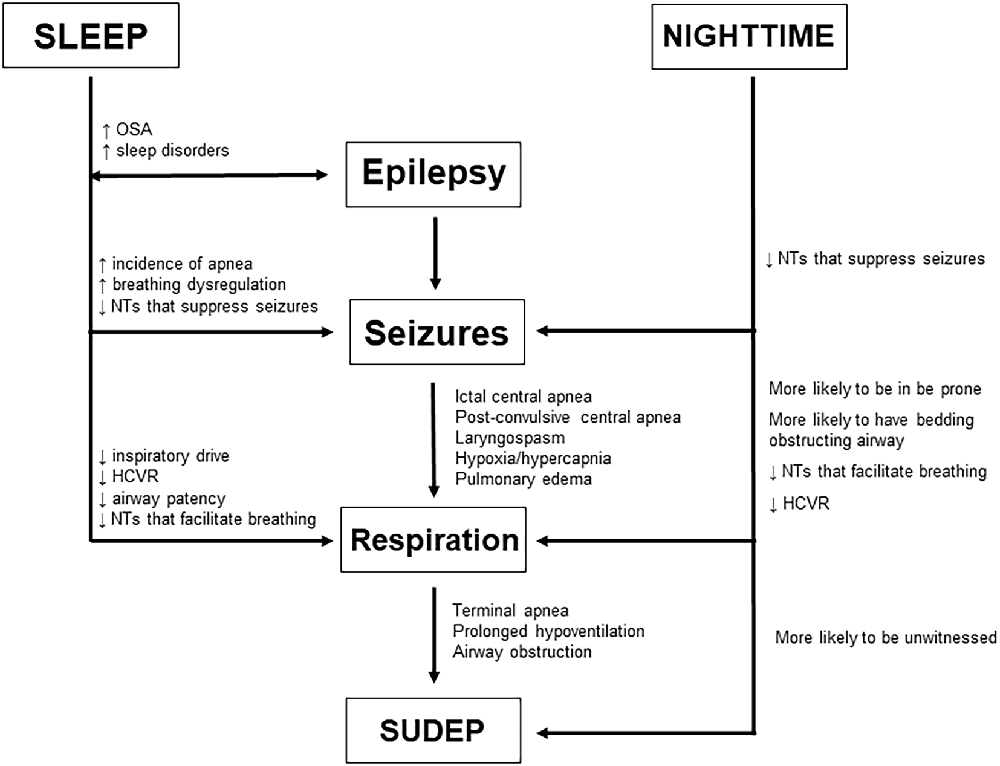
Figure 1. Potential risk factors associated with seizures emerging from sleep vs. nocturnal seizures and how they may facilitate SUDEP by modulating epilepsy, seizures, and respiration as well as seizure-induced death itself.
It has long been appreciated that breathing is regulated in a sleep state-dependent manner (Snyder et al., 1964; Spengler et al., 2000; Haxhiu et al., 2003; Mortola, 2004; Malik et al., 2012; Buchanan, 2013). Inspiratory drive is lower during NREM sleep and lowest during rapid-eye movement (REM) sleep, with tidal volume (VT) being reduced to 73% of its level during wakefulness (Douglas et al., 1982a; Figure 2A). Within NREM sleep, the nadir of minute ventilation (VE) occurs during NREM stage 3 (N3) sleep—although this is likely driven by the reduction in VT. This results in an end-tidal carbon dioxide (ETCO2) concentration that is 1–2 torr higher than waking levels (Krieger, 2005). This drop in VT and VE is likely due to decreased chemosensitivity during the onset of sleep (Bulow, 1963; Douglas et al., 1982b, c). During sleep there is a decrease in the respiratory response to hypercapnia (Reed and Kellogg, 1958; Birchfield et al., 1959; Cherniack, 1981; Douglas et al., 1982c; Berthon-Jones and Sullivan, 1984; Figure 3A) as well as hypoxia (Berthon-Jones and Sullivan, 1982; Douglas et al., 1982b; Malik et al., 2012). Like inspiratory drive, there is an even larger decrease in the hypoxia-induced respiratory drive during REM compared to NREM sleep (Berthon-Jones and Sullivan, 1984; Malik et al., 2012). There are sex-specific differences in the response to hypercapnia, with males exhibiting a 50% decrease in the hypercapnic ventilatory response (HCVR) compared to wakefulness, while females exhibit a reduced HCVR during wakefulness compared to males but have less apparent reductions in the response during sleep (Berthon-Jones and Sullivan, 1984). Progesterone has been found to stimulate breathing during sleep, including increasing hypoxic and hypercapnic respiratory responses (Javaheri and Guerra, 1990; Saaresranta et al., 1999). Progesterone oscillates in a circadian fashion, with its zenith at around midnight (Junkermann et al., 1982; Gharib et al., 2018). No sex-specific differences in respiratory responses to hypoxia have been identified (Malik et al., 2012).
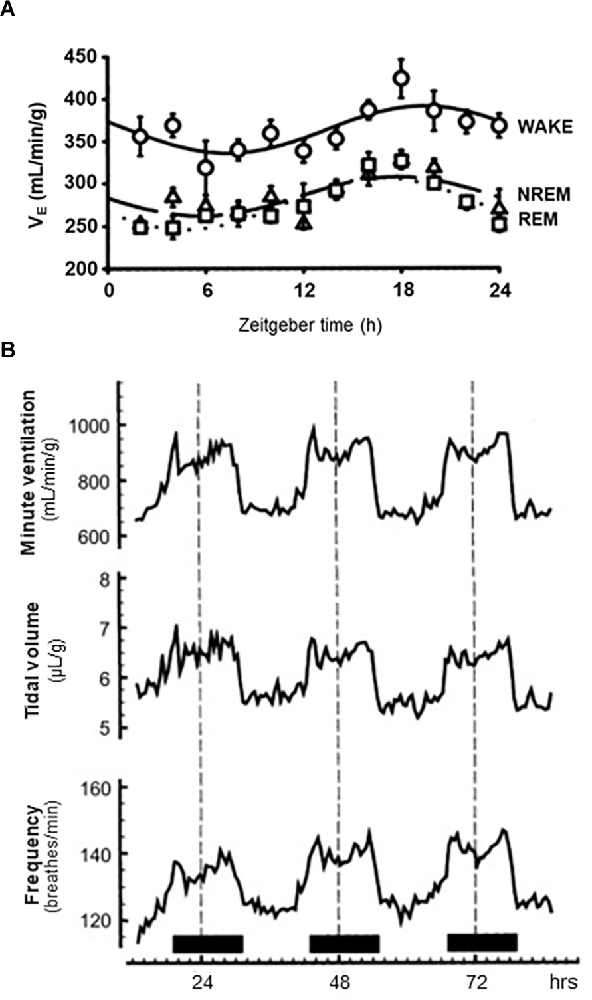
Figure 2. Circadian and sleep state-dependent effects on ventilation. (A) 72-h traces of average minute ventilation (top), tidal volume (middle) and breathing frequency (bottom) in adult male rats housed under a 12:12 h light:dark cycle and receiving room air (21% O2, balance N2). Solid horizontal bars at the bottom indicate periods where lights were off. (B) 24-h trace of average minute ventilation in rats during wake, non-rapid eye movement (NREM) sleep, and rapid-eye movement (REM) sleep as indicated. All animals housed in a 12:12 h light:dark cycle. (A) Redrawn with permission from Seifert and Mortola (2002). (B) Redrawn with permission from Stephenson et al. (2001).
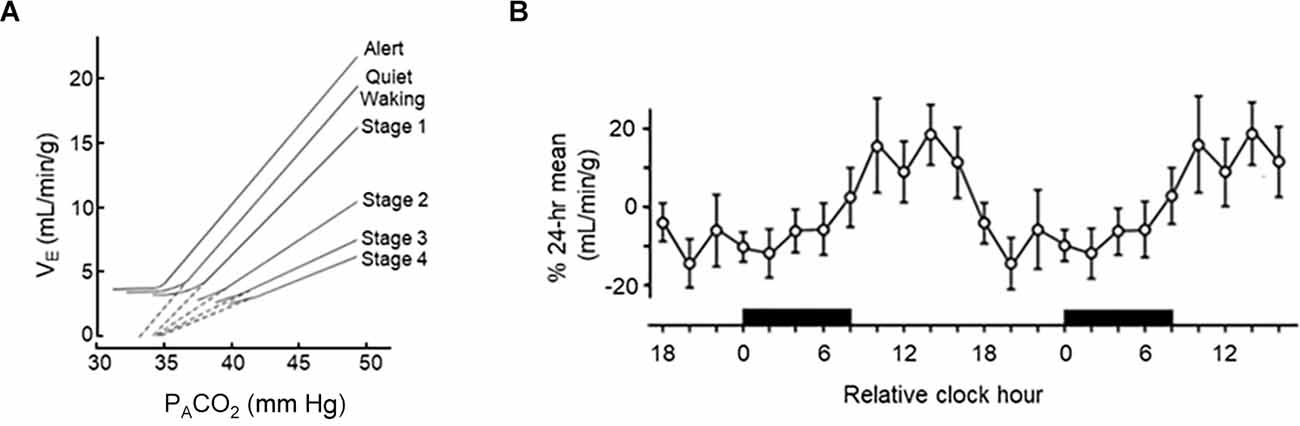
Figure 3. Circadian and sleep state-dependent effects on the hypercapnic ventilatory response (HCVR). (A) 48-h trace of circadian variations in HCVR in adult humans. (B) Sleep state-dependent differences in HCVR in adult males. (A) Redrawn with permission from Spengler et al. (2000). (B) Redrawn with permission from Bulow (1963).
Breathing during NREM sleep has a more regular pattern compared to breathing during wakefulness, without altering mean breathing frequency (Malik et al., 2012). Conversely, during REM sleep there is more variability in respiratory patterns, including increased frequency, decreased regularity, and brief periods of central apnea (Aserinsky and Kleitman, 1953; Cherniack, 1981; Malik et al., 2012). There is some evidence that indicates this irregular breathing is a response to cortical inputs that reflect the content of the individual’s dream (Oudiette et al., 2018). Periodic breathing, which is characterized as clusters of breaths separated by intervals of central apnea or near apnea, also sometimes occurs during sleep. Although previously thought to arise from a severe neurological or cardiovascular condition, it now found that periodic breathing can occur in healthy individuals, especially during hypoxia (Berssenbrugge et al., 1983; Cherniack, 1999; Ainslie et al., 2013). During intervals of periodic breathing, cyclic changes in ventilation as well as the partial pressures of carbon dioxide (CO2) and oxygen (O2) can trigger oscillations in heart rate, blood pressure, autonomic nervous system activity, and upper-airway resistance. This may create a feedback loop whereby these oscillations in turn affect ventilation and increase the length and symmetry of these periodic breathing cycles (Cherniack, 1999). Males tend to exhibit periodic breathing in response to hypoxia more frequently than females (Pramsohler et al., 2019). Breathing patterns are heavily dependent on the pre-Bötzinger complex (pre-BötC; Smith et al., 1991; Buchanan, 2013; Del Negro et al., 2018; Muñoz-Ortiz et al., 2019). When neurons expressing neurokinin-1 receptors (NK1R) in the pre-BötC complex were bilaterally ablated in adult rats there was a progressive and irreversible disruption in breathing stability, which initially occurred only during sleep, but eventually led to ataxic breathing during wakefulness as well (Mckay and Feldman, 2008). When these pre-BötC NK1R-expressing neurons were unilaterally ablated, there was a disruption in respiratory pattern and increase in the frequency of central sleep apnea and hypopneas solely during sleep, particularly during REM sleep, which never developed during wakefulness (Mckay and Feldman, 2008).
During sleep there is a reduction in upper airway patency and an increase in respiratory resistance. This is caused by a preferential reduction in tone of laryngeal and pharyngeal muscles that help to maintain the structure of the upper airway (Cherniack, 1981; Haxhiu et al., 1987; Buchanan, 2013; Kubin, 2016). This reduced patency and can be especially problematic during REM sleep, when breathing is particularly unstable (Cherniack, 1981). Upper airway tone is controlled by inputs from trigeminal (CN V), facial (CN VII), and hypoglossal (CN XII) motor neurons (Buchanan, 2013). The genioglossus muscle, which is innervated by hypoglossal motor neurons, is the largest and most extensively studied of the airway dilator muscles. It has been suggested that decreased serotonergic and noradrenergic inputs to hypoglossal motor neurons during REM sleep causes atonia of the genioglossus (Fenik et al., 2005). The genioglossus and other muscles of the upper airway require both tonic and phasic inspiratory activation in order to protect against collapse (Kubin, 2016). When the tone of these airway-dilating muscles can no longer oppose the negative inspiratory pressure, the result is obstructive sleep apnea (OSA), which features recurrent episodes of hypopneas and apneas (Remmers et al., 1978; Kubin, 2016). While these obstructive apneas only occur during sleep, frequent sleep apnea and hypoventilation can result in breathing abnormalities during wakefulness (Simonds, 1994).
Changes in several non-centrally mediated respiratory mechanisms are also associated with the onset of sleep. During NREM sleep, the activity of the intercostal muscles is increased compared to wakefulness (Malik et al., 2012). This may be indicative of increased contribution of the chest wall to respiration in order to compensate for decreased central inspiratory drive. During REM sleep, there is a loss of tonic activity in the intercostals and diaphragm (Tusiewicz et al., 1977; Bryan and Muller, 1980; Malik et al., 2012). Chest wall compliance is also increased during this time, and, in conjunction with decreased intercostal tone, can cause paradoxical collapse of the chest during inspiration (Malik et al., 2012). Lastly, the pulmonary stretch receptor reflex and irritant receptor reflex are suppressed during sleep—thus, coughing in response to apnea only occurs after arousal (Douglas, 2000). In summary, sleep is a period where many facets of breathing are suppressed—thus rendering it a particularly vulnerable period for further insults to the respiratory system.
Early studies of time-of-day variability in mammalian (adult rat) breathing physiology revealed time-of-day differences in breathing; however, the effect was limited to CO2 production and the mean inspiratory air flow (Peever and Stephenson, 1997). Under hypercapnic conditions, breathing frequency and VE also appeared to be time-of-day dependent. Unfortunately, these studies only involved two time-points, limiting the resolution of a daily rhythm, which may have been masked by higher frequency ultradian variation in breathing (Stupfel and Pletan, 1983; Stupfel et al., 1985).
The first clear evidence that respiratory function demonstrated daily oscillations came from Seifert et al. (2000). Adult rats were housed in 10 L barometric chambers with carefully controlled in-flow and out-flow of gas, allowing for measurement of breathing physiology over the course of several days. Clear time-of-day differences in frequency, VT and VE were observed. The highest levels were observed during the dark phase, coinciding with elevated temperature and activity. These findings were further expanded in a later study, demonstrating that O2 consumption (a measure of metabolic activity), inspiratory time, and expiratory time also varied across the day (Seifert and Mortola, 2002; Figure 2B). Interestingly, controlling for level of activity did not eliminate the effect of time of day on VE, VT, frequency of breathing, or O2 consumption. The authors conclude that the daily variability observed in breathing (specifically ventilation) is likely driven by other physiological variables oscillating throughout the day, such as temperature and oxygen consumption.
Using similar methods to Seifert et al., 2000, long-term respiratory monitoring in non-human primates has also been performed (Iizuka et al., 2010). Whole body plethysmography was performed in 11 unrestrained, unanesthetized male cynomolgus monkeys. Like findings from adult rats, multiple respiratory parameters, including respiratory rate, VT, and VE were shown to vary depending on the time of day. However, recordings were only obtained hourly, and it is unclear if sleep-state was controlled for.
Time-of-day variability in a number of respiratory parameters has also been demonstrated in humans (Spengler and Shea, 2000; Spengler et al., 2000). In a carefully controlled laboratory setting, which included the removal of external time cues (except for lighting), a constant environmental temperature, controlled dietary intake, and carefully controlled sleep schedules. Temporal variation in rectal temperature and plasma cortisol were used as endogenous circadian markers. ETCO2, O2 consumption, and CO2 production were all shown to oscillate throughout the 24 h day, with highest levels in the morning. Interestingly, there was also time-of-day variability in HCVR magnitude, a finding previously demonstrated in awake, adult rats (Peever and Stephenson, 1997; Figure 3B), suggesting respiration-influencing chemosensitivity may also be under circadian regulation. Sensitivity to isocapnic hypoxic challenge has some evidence of time-of-day dependence; however, the effect is far less pronounced (Siekierka et al., 2007).
While the studies described above in rats, monkeys, and humans demonstrate temporal variation in breathing physiology, they did not control for the rhythmic effect of light. Therefore, whether this variability is due to the effect of an external time cue or that of an endogenous circadian rhythm cannot be concluded. The first study of time-of-day dependent on breathing physiology that accounted for the influence of light was performed in garter snakes (Hicks and Riedesel, 1983). Animals were housed in either a 14:10 light-dark cycle or constant darkness environment. Under these conditions, it was revealed that time-of-day variability in oxygen consumption, breathing frequency, VT, and VE persisted in constant darkness, suggesting endogenous regulation of these breathing parameters.
This time-of-day dependent variation in breathing has been shown to be endogenously circadian in mice and mediated by the body’s central circadian pacemaker, the suprachiasmatic nucleus (Purnell and Buchanan, 2020). C57BL/6J mice were housed in either 12:12 light-dark or constant darkness environments, and running wheels were used to assess active phase onset for the determination of individual free-running locomotive rhythms. As sleep-state has been shown to influence breathing (as described in detail earlier above), measurements of breathing were only performed while the animals were awake. Time-of-day variability in the frequency of breathing and VE, but not VT, was observed. Both frequency and VE were highest during the dark phase of the day. This time-of-day rhythm was shown to be circadian, as these two rhythms persisted when animals were housed in constant darkness. Electrolytic lesioning of the suprachiasmatic nucleus eliminated these breathing rhythms, suggesting that the circadian variation in breathing was controlled by the suprachiasmatic nucleus.
Although the suprachiasmatic nucleus is frequently referred to as the master circadian oscillator, nuclei outside of the suprachiasmatic nucleus and peripheral tissues may contain autonomous circadian clocks (Mohawk et al., 2012). Such peripheral clocks have been described in brainstem and spinal cord neurons involved in the coordination and output necessary to maintain normal respiration. Through the measurement of molecular clock gene transcripts, such as Clock, Bmal1, and Per1/2, researchers have identified robust molecular clock gene rhythms in the nucleus tractus solitarius (Kaneko et al., 2009; Chrobok et al., 2020), phrenic motor nucleus (Kelly et al., 2020), and laryngeal, tracheal, bronchial, and lung tissues within the airway (Bando et al., 2007). Bando et al. (2007) demonstrated that the peripheral clock of the airway tissues could be rendered arrhythmic following electrolytic lesioning of the suprachiasmatic nucleus. Similarly, genetically arrhythmic Clock 1/Clock2 knock-out mice did not demonstrate peripheral rhythmicity of clock gene expression in airway tissues. In conclusion, circadian phase exerts its own powerful influences on breathing, irrespective of vigilance state.
Some patients with epilepsy experience breathing abnormalities at baseline which may be further compromised during a seizure. Sainju et al. found a blunted hypercapnic ventilatory response in a subset of patients with epilepsy, placing them at greater risk for peri-ictal hypoventilation (Sainju et al., 2019). Patients with Dravet syndrome (DS) similarly exhibit a decreased ventilatory response to CO2 (Kim et al., 2018). Several animal models of epilepsy present with respiratory dysregulation, even in the absence of a seizure. The Kcna1-null mutant model exhibits progressive respiratory dysfunction with age (Simeone et al., 2018). Like their human counterparts, the Scn1aR1407X/+ human knock-in mouse model of DS has a diminished ventilatory response to CO2, as well as baseline hypoventilation and apnea (Kuo et al., 2019). A similar loss of the hypercapnic ventilatory response has been found in animals that have undergone amygdala kindling (Totola et al., 2019). Hajek and Buchanan (2016) found that mice with increased respiratory rate variability at baseline are more likely to die following a maximal electroshock (MES) seizure. These findings support the idea that interictal respiratory dysfunction may serve as a biomarker for those at greater risk for SUDEP.
Seizures themselves can cause profound alterations in respiration, including coughing, apnea, hyperventilation, bronchial spasms, increased pulmonary vascular pressure, laryngospasm, and pulmonary edema (Bayne and Simon, 1981; Kennedy et al., 2015; Nakase et al., 2016; Rugg-Gunn et al., 2016). Seizures appear to cause varying degrees of respiratory dysregulation depending on seizure type and origin (Bateman et al., 2008; Blum, 2009). Longer duration of seizures is associated with a greater degree of dysfunction, particularly in regard to hypercapnia, pulmonary pressure, and pulmonary edema (Bayne and Simon, 1981; Bateman et al., 2008; Seyal et al., 2010; Kennedy et al., 2015).
Hypoventilation during a seizure may occur due to airway obstruction or dysregulation of the brain’s respiratory centers and usually results in hypercapnia and hypoxemia (Rugg-Gunn et al., 2016). Dravet syndrome patients in particular demonstrate peri-ictal hypoventilation, which precedes the onset of bradycardia (Kim et al., 2018). Hypoventilation can lead to secondary cardiac failure, especially during seizures where oxygen saturation (SaO2) drops below 90% (Seyal et al., 2011). A cause of some ictal hypoventilation is central apnea. Ictal central apnea (ICA) is a relatively frequent occurrence during seizures, especially ones with bihemispheric involvement (Nashef et al., 1996; Rugg-Gunn et al., 2016). ICA occurs exclusively in focal epilepsy, emerging during 33–50% of focal seizures (Lacuey et al., 2018; Vilella et al., 2019; Tio et al., 2020). ICA can precede electrographic seizure activity as well as clinical seizure onset by up to 7–10 s (Nishimura et al., 2015; Tio et al., 2020). These apneas tend to be brief and do not substantially impact O2 saturation (Bateman et al., 2008). A multivariate analysis indicated that contralateral seizure spread and seizure duration mutually contribute to increased ETCO2 that follows ICA (Seyal et al., 2010). Several animal models of epilepsy and SUDEP exhibit ICA, including Scn1aR1407X/+ mice, in which mechanical ventilation can prevent fatal seizure-induced respiratory arrest (Kim et al., 2018). Additionally, a model of status epilepticus induced in sheep features ICA and hypoventilation in 100% of the animals, with some resulting in death (Johnston et al., 1997). Post-convulsive central apnea (PCCA), in contrast, occurs in both focal and generalized epilepsies, suggesting a separate pathophysiology from ICA (Vilella et al., 2019). PCCA is less common than ICA—occurring during only 18% of generalized seizures. However, PCCA may be much more dangerous than ICA. PCCA is associated with a longer recovery time from hypoxemia, and it is considered by some to be a biomarker for SUDEP (Jin et al., 2017; Vilella et al., 2019).
Seizures may impair a person’s ability to autoresuscitate after central apnea. Autoresuscitation is a spontaneous protective cardiorespiratory phenomenon which promotes the recovery of normal breathing and heart rate after primary apnea by initiating a gasping reflex (Adolph, 1969; Guntheroth and Kawabori, 1975). Failure to autoresuscitate has been documented in infant deaths that were eventually classified as sudden infant death syndrome (SIDS; Meny et al., 1994; Sridhar et al., 2003). There are numerous parallels between SIDS and SUDEP, including normal autopsy, prone position, predominance during the nighttime, predicted respiratory mechanism, and evidence of serotonergic system dysfunction (Richerson and Buchanan, 2011; Buchanan, 2019).
Obstructive apnea, or laryngospasm, is another seizure-associated phenomenon that can result in death (Stewart, 2018). DBA/2 mice, which display lethal audiogenic seizures, have a significantly reduced mortality rate following seizures after being implanted with a tracheal T-tube as a surrogate airway (Irizarry et al., 2020). Seizures induced via kainic acid in rats have been documented to cause partial or complete glottic closure and subsequent death (Nakase et al., 2016; Budde et al., 2018; Jefferys et al., 2019). It has been postulated that fatal obstructive apnea is a consequence of bronchial spasms or hypotonia of the muscles involved in respiration (Stöllberger and Finsterer, 2004). Nakase et al. proposed that ictal laryngospasm is caused by the spread of a seizure via autonomic medullary motor regions to the laryngeal branches of the vagus nerve (Nakase et al., 2016).
Spreading depolarization (SD) may be one of the underlying mechanisms behind cardiorespiratory failure in SUDEP. In Cacna1aS218L mutant mice, which carry a gain of function mutation in the Cav2.1 voltage-gated calcium channel, brainstem SD occurs during all spontaneous fatal seizures, as well as a subset of nonfatal seizures (Jansen et al., 2019). Additionally, seizure-related SD in the ventrolateral medulla is correlated with the incidence of respiratory suppression (Jansen et al., 2019). Chemically induced seizures in Kcna1 and Scn1a mutant mice cause a wave of SD in the dorsal medulla, which may temporarily silence the cells that would serve to reoxygenate the brain following a seizure (Aiba and Noebels, 2015). This depolarizing blockade may cause a positive feedback loop in which the brain cannot reoxygenate following a seizure during which oxygen saturation has dropped dramatically, potentially leading to complete cardiorespiratory arrest (Aiba and Noebels, 2015).
Numerous other potential mechanisms underlying ictal respiratory dysfunction and failure have been proposed. A leading hypothesis is that seizures activate inhibitory subcortical projections to brainstem respiratory centers (Dlouhy et al., 2015; Lacuey et al., 2017). It has also been found that central apnea occurs in human patients when seizures spread to the amygdala (Dlouhy et al., 2015; Rhone et al., 2020). Similarly, stimulation of the amygdala as well as the hippocampus produces central apnea that patients are not aware of (Dlouhy et al., 2015; Lacuey et al., 2017; Nobis et al., 2018), and they are able to voluntarily initiate inspiration when prompted (Dlouhy et al., 2015). Further studies revealed that stimulation of the basal amygdala in particular (including the basomedial and basolateral nuclei) was particularly likely to cause apnea, while stimulation of more lateral regions produced fewer apneas (Rhone et al., 2020). In DBA/1 mice, unilateral lesions to the amygdala was sufficient to suppress seizure-induced respiratory arrest (S-IRA) (Marincovich et al., 2021). This suggests that apnea is due to the loss of involuntary ventilatory drive rather than an issue with the respiratory motor output pathways or musculature.
Despite the implications of both respiratory and cardiac dysfunction contributing to SUDEP, recent evidence has surfaced suggesting respiratory failure precedes cardiac failure during instances of seizure-induced death. In 2013, a multi-center MORTality in EMUs Study (MORTEMUS) of SUDEP incidents in EMUs found that all recorded cases of SUDEP featured terminal respiratory arrest prior to terminal asystole (Ryvlin et al., 2013). Similar results have been found in the Kcna1-null mouse model (Dhaibar et al., 2019) and in an MES model (Buchanan et al., 2014). Another indicator of respiratory failure’s pivotal role in SUDEP is that mechanical ventilation, if administered immediately, can greatly reduce mortality in both human patients and animal models (Tupal and Faingold, 2006; Ryvlin et al., 2013; Buchanan et al., 2014). In a similar vein, oxygenation prior to seizure induction can prevent fatal audiogenic seizures in several strains of audiogenic mice, without impacting seizure incidence or severity (Venit et al., 2004). To summarize, seizures cause profound alterations in breathing which may directly contribute to seizure-induced death.
Approximately 10–15% of epilepsy patients have seizures solely or primarily during sleep (Grigg-Damberger and Foldvary-Schaefer, 2021). Seizures occurring during sleep tend to be longer and are more likely to evolve into focal and bilateral tonic-clonic seizures (Bazil and Walczak, 1997). As mentioned above, longer convulsive seizures are associated with an increased degree of respiratory dysfunction (Bayne and Simon, 1981; Bateman et al., 2008; Seyal et al., 2010; Kennedy et al., 2015). Seizures emerging from sleep are also more likely to be associated with the presence of post-ictal generalized EEG suppression (PGES) and greater oxygen desaturation (Latreille et al., 2017). A clinical study in 20 patients with epilepsy found that 44% of nocturnal seizures are associated with ICA, and although the difference did not reach significance, a smaller fraction of wake-related seizures were accompanied by ICA (28%; Latreille et al., 2017). MES seizures in mice that are induced during NREM sleep are also associated with greater respiratory dysfunction than those induced during wakefulness (Hajek and Buchanan, 2016). When factoring in time of day, MES seizures that were induced during the day, the rodent inactive phase, resulted in a greater degree of postictal respiratory and EEG suppression than those induced during the nighttime. This effect was even greater when the seizures were induced during this time while the animal was in NREM sleep (Purnell et al., 2017). When DBA/1 mouse model of audiogenic seizures were exposed to an audiogenic stimulus during the day, the ensuing seizures resulted in death during 21.7% of trials. Conversely, seizures induced during the night resulted in seizure-induced death in 46.7% of trials (Purnell et al., 2021b; Figure 4A). The same study used mice living in constant darkness to access circadian influence on seizure-induced death in the MES mouse model. They found that during the subjective night there was a decrease in postictal ventilation and an increase in the probability of seizure-induced death without altering seizure severity (Purnell et al., 2021b; Figure 4B). Kv1.1 potassium channel knockout (KO) mice and SCN1AR1407X/+ mice, which experience progressive breathing dysregulation (Kim et al., 2018; Kuo et al., 2019; Iyer et al., 2020), also experience seizure-induced death more commonly during the nighttime (Figures 4C,D).
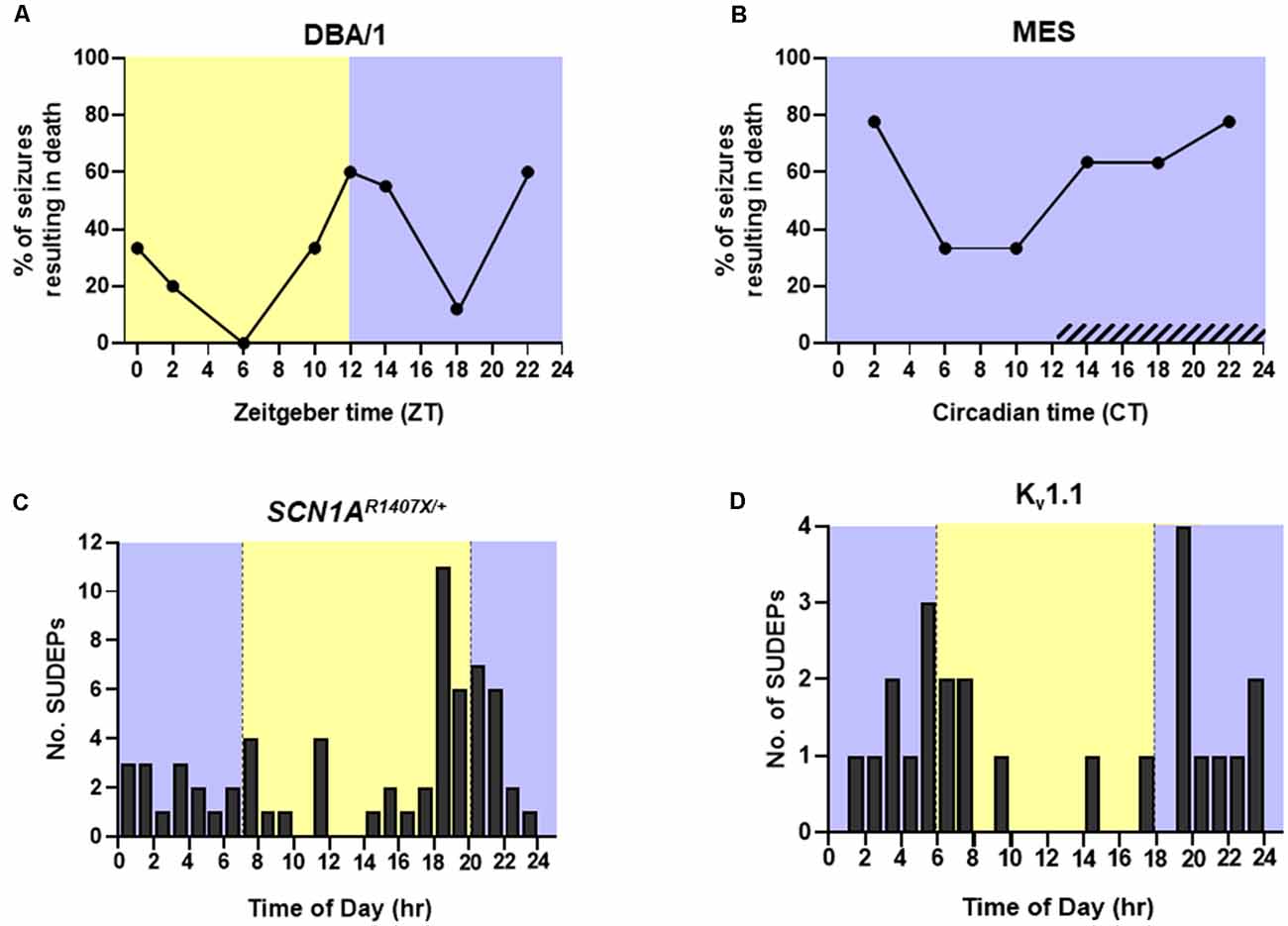
Figure 4. Time-of-day and circadian probability of seizure-induced death in mouse models of epilepsy. Temporal distribution of spontaneous seizure-induced death in (C) SCN1AR1407X/+ and (D) Kv1.1 knockout mice housed in a 12:12 h light:dark cycle. (A) Percentage of audiogenic seizures resulting in death in DBA/1 mice housed in a 12:12 h light:dark cycle. (B) Percentage of maximal electroshock (MES) seizures resulting in death in mice housed in constant darkness. Redrawn with permission from (C) Teran et al. (2019), (D) Moore et al. (2014), (A,B) Purnell et al. (2021b).
Because humans typically sleep during the night, nighttime seizures are often unwitnessed (Lamberts et al., 2012; Zhuo et al., 2012; Rugg-Gunn et al., 2016; Purnell et al., 2018). Lamberts et al. (2012) reported that 86% of SUDEP cases are unwitnessed. It is hypothesized that being unaccompanied during a nocturnal seizure may carry even more risk than the severity of sleep-related respiratory dysfunction or PGES duration (Peng et al., 2017; Sveinsson et al., 2020). The presence of someone who could intervene and administer lifesaving resuscitative measures may mean the difference between a case of near SUDEP and actual SUDEP (Nashef et al., 1998; Langan et al., 2005; Lamberts et al., 2012). Increasing nocturnal supervision through the use of monitoring devices, checkups, or having another person asleep in the same room is associated with decreased SUDEP risk (Langan et al., 2005; Ryvlin et al., 2006; Harden et al., 2017). The majority of SUDEP victims are found prone in or near a bed (Opeskin and Berkovic, 2003; Sowers et al., 2013; Ali et al., 2017; Sveinsson et al., 2018). Many generalized seizures are followed by a period of PGES where the patient is more likely to be immobile, unresponsive, and require resuscitative measures (Semmelroch et al., 2012; Kuo et al., 2016). If a patient is unresponsive after a seizure that renders them prone, their nose and mouth may become obstructed by bedding. This may result in upper airway occlusion or asphyxiation against the surface the patient is positioned on. Outside of total airway occlusion, ending a seizure in the prone position on bedding may impairing postictal breathing by increasing inspiratory resistance and causing the patient to rebreathe trapped air (Kemp et al., 1994; Tao et al., 2010, 2015; Rugg-Gunn et al., 2016). This would cause an acute rise in CO2 in the blood, potentially leading to severe acidosis, which would potentiate the postictal immobility and further prolong the respiratory dysfunction until terminal apnea and asystole develop (Peng et al., 2017; Purnell et al., 2018).
Although seizures are frequently thought to be unpredictable phenomenon, patients often display time-of-day-specific timing of seizure onset. In a recent study of patients implanted with responsive neurostimulators, it was shown that nearly 90% of patients with focal epilepsy had circadian timing of seizure onset (Leguia et al., 2021). Interestingly, circadian risk of seizure onset could be clustered into five general times of day, with seizures more likely to occur during the morning, mid-afternoon, evening, early night, or late night.
The circadian influence on seizures may be due in part to the bi-directional relationship of epilepsy and clock genes. Alterations in clock mechanisms increase the susceptibility for epilepsy, while seizures have the potential to disrupt the internal clock (Re et al., 2020). A significantly higher current is required to induce both maximal and generalized seizures in wild type (WT) mice during the dark phase of their diurnal cycle compared to the light phase. This rhythm is abolished in Bmal1 KO mice, who also exhibit significantly lower seizure thresholds at all times compared to their WT counterparts (Gerstner et al., 2014). Similarly, conditional KO of Bmal1 in neurons in the dentate gyrus increased the susceptibility to pilocarpine-induced seizures in mice (Wu et al., 2021). Hippocampal BMAL1 expression is reduced overtime in pilocarpine-treated rats as they begin to develop spontaneous seizures—suggesting that BMAL1 also plays a role in epileptogenesis (Matos et al., 2018). Levels of BMAL1 protein have been found to be decreased in the dentate gyrus and CA1 of mice with TLE (Wu et al., 2021). Mutations in the RAR related orphan receptor alpha (RORA) gene, which encodes for an activator of Bmal1 transcription, have been linked to intellectual developmental disorder with or without epilepsy or cerebellar ataxia (IDDECA) (Guissart et al., 2018). Deletion of the gene Clock in cortical pyramidal neurons in mice results in epileptiform discharges in excitatory neurons as well as a decreased seizure threshold (Li et al., 2017). Real-time quantitative PCR (qPCR) analysis has revealed a loss in the rhythmic expression of CLOCK and decreased levels of its transcript in a post-status epilepticus rat model (Santos et al., 2015). Clock RNA and protein are similarly downregulated in brain tissue resected from patients with TLE (Li et al., 2017). Another oscillating clock gene, Per1, is upregulated in the hippocampus following electrical and kainic acid-induced seizures in mice (Eun et al., 2011). One study found an alteration in the rhythmic expression of PER1, PER2, and PER3 in a rat model of pilocarpine-induced seizures (Santos et al., 2015). However, a subsequent study found that an increase in PER1 expression and a decrease in PER2 expression prior to the development of spontaneous seizures, while PER3 expression was unaltered (Matos et al., 2018). To conclude, sleep and circadian phase have direct effects on periictal breathing and potentially the development of epilepsy itself.
Apart from nocturnal seizures, patients with epilepsy also have a greater prevalence of sleep disorders compared to healthy individuals (Vaughn and D’cruz, 2004). A myriad of studies over the past 30 years have repeatedly found that adults with epilepsy are 2–3 times more likely to have a sleep/wake disorder compared to the general population (Grigg-Damberger and Foldvary-Schaefer, 2021). Patients with temporal lobe epilepsy exhibit reduced sleep efficiency and more arousals compared to those with frontal lobe epilepsy (Crespel et al., 2000). In addition, amygdala kindling decreases REM sleep in experimental animals, and selective REM sleep deprivation accelerates the kindling process (Cohen and Dement, 1970; Tanaka and Naquet, 1975). The Scn1aR1407X/+ mouse shows impairments in circadian sleep regulation, including a fragmented rhythm of NREM sleep and an elongated circadian period of sleep (Sanchez et al., 2019).
Sleep deprivation caused by sleep disorders of frequent nocturnal seizures can result in sleep deprivation. Sleep deprivation itself can induce seizures and interictal spiking (Mattson et al., 1965; Pratt et al., 1968; Malow et al., 2000b; Konduru et al., 2021). In amygdala kindled cats, acute sleep deprivation reduces seizure and after discharge threshold (Shouse and Sterman, 1982). However, more prolonged sleep deprivation increases their susceptibility to both kindled and penicillin-induced seizures, regardless of sleep state (Shouse, 1988). Additionally, when kindled rats were administered a microinjection of a cholinergic agonist into the pontine reticular formation to enhance REM sleep, the result was a significant increase in the current threshold needed to elicit afterdischarge spiking in the amygdala (Kumar et al., 2007). Sleep deprivation studies in healthy individuals have shown hypertension and increased sympathetic nervous system activity after nights where sleep was less than 5 h (Lusardi et al., 1996; Tochikubo et al., 1996; Gangwisch et al., 2006). Thus, sleep deprivation may not only worsen seizures themselves, but also leave patients more vulnerable to seizure-induced autonomic insults.
Up to 9–11% of adult patients with epilepsy exhibit SDB (Vendrame et al., 2014; Popkirov et al., 2019). This number jumps up to 40% when looking at children with epilepsy (Kaleyias et al., 2008). A recent case study highlighted a male patient with a history of secondary generalized tonic/clonic seizures who displayed paroxysmal nocturnal breathing. The patient experienced periods of breathing arrest in conjunction with an odd expiratory noise—primarily during REM sleep or the transition between REM and NREM—despite being seizure free for a year (Künstler et al., 2022).
OSA is a relatively common form of SDB, in which the upper airway collapses, preventing ventilation. The ensuing apnea provokes an arousal response which allows for re-positioning and recovery of gas exchange (Butler et al., 2015). The precise occurrence of OSA in people with epilepsy has yet to reach a consensus. Popkirov et al. estimates that 7% of epilepsy patients have mild-to-moderate OSA (Popkirov et al., 2019). A separate polysomnography study postulates that one-third of patients with medically refractory epilepsy who were candidates for epilepsy surgery have concomitant OSA (Malow et al., 2000b). This is also closer to an estimate produced from a meta-analysis in 2017, which determined the prevalence of mild-to-severe OSA in patients with epilepsy to be 33.4%—2.4 times more likely than healthy comparisons (Lin et al., 2017). This same meta-analysis found that the prevalence of OSA in patients with refractory epilepsy was not greater than the overall prevalence of OSA in patients with epilepsy (Lin et al., 2017). Patients with generalized epilepsy experience more severe OSA than those with focal epilepsy. Both populations reported similar degrees of abnormal daytime sleepiness. Older age, higher body mass index (BMI), and a history of hypertension are also associated with more severe OSA (Scharf et al., 2020). The incidence of OSA apnea in individuals without epilepsy is higher in males than in females (4% in men, 2% in women) (Block et al., 1979; Young et al., 1993). Men are also much more likely to experience O2 desaturation during apnea compared to women (Block et al., 1979). In patients with epilepsy, males are roughly three times more susceptible to OSA compared to females (Lin et al., 2017).
The length of obstructive apneas tends to increase over the course of a night’s sleep (Montserrat et al., 1996; Butler et al., 2015). It is suggested that this is due to a blunting of the CO2 arousal response over the course of the night, leading to longer periods of hypercapnia before arousal occurs (Montserrat et al., 1996). It is possible that this increase in OSA in epilepsy patients is due to an inherent blunting of chemosensitivity in an epileptic brain. Obese adolescents with OSA have an increased HCVR during wakefulness and a blunted HCVR during sleep (Yuan et al., 2012). There are also endogenous circadian components to the prolongation of respiratory events across the night. At circadian phases that correspond to the early morning, the duration of apnea and hypopneas are typically longer, but apnea/hypopnea index (AHI), a measurement of OSA severity, is low. In contrast, during the late afternoon to early evening, event durations were short and AHI was high. Events during REM sleep also tended to be 14% longer than those emerging from NREM sleep (Butler et al., 2015).
Comorbidity of epilepsy with OSA can increase the incidence of arrhythmias and increase the patient’s risk for sudden cardiac death (Gami and Somers, 2008; Gami et al., 2013). Patients with OSA experience disruption of the autonomic system during sleep (Adlakha and Shepard, 1998), which may be further imbalanced by seizures. While no direct correlation between OSA and SUDEP has been identified, higher revised SUDEP-7 scores [presence of seizures in the past 12 months—especially generalized tonic clonic seizures (GTCS), longer duration of epilepsy, increased number of ASMs, and lower IQ/more cognitive impairment]—are associated with probable SUDEP (Phabphal et al., 2021). OSA decreases the amount of time a person spends asleep each night, potentially leading to further sleep deprivation. Sleep deprivation is particularly dangerous for those with epilepsy as it can have an epileptogenic effect (Nobili et al., 2011; Popkirov et al., 2019). It follows then that when epilepsy patients with OSA were treated with continuous positive airway pressure (CPAP) they exhibited better seizure control than their untreated peers (Lin et al., 2017).
Vagus nerve stimulation (VNS) is a technique used to treat refractory epilepsy via a neurostimulation device. While these devices have been found to lessen seizure frequency and severity, there is a lack of conclusive evidence indicating that VNS lessens SUDEP risk (Annegers et al., 1998; Ryvlin et al., 2018). There is, however; evidence that VNS activation during sleep can induce mild OSA or exacerbate preexisting OSA. VNS activation during sleep is similarly linked to decreased VT and SaO2, increased respiratory rate and AHI, and excessive daytime somnolence (Malow et al., 2000a; Holmes et al., 2003; Marzec et al., 2003; Zambrelli et al., 2016; Somboon et al., 2019; Kim et al., 2022). A recent study has also indicated HCVR slope is attenuated in patients with an active VNS (Sainju et al., 2021). Evidence suggests the exacerbation of OSA after VNS is due to reduction of the glottal space or lack of laryngeal–respiratory coordination (Zambrelli et al., 2016). This is notable as patients with refractory epilepsy are at higher risk for SUDEP and are more likely to opt for VNS as a method of seizure control. To summarize, individuals with epilepsy are more likely to experience sleep disorders and SDB, which may directly influence seizure frequency. Moreover, a common treatment for refractory epilepsy appears to aggravate SDB in these patients.
While the underlying mechanisms behind the sleep and circadian effects on breathing in epilepsy are still not fully understood, numerous neurotransmitters and signaling molecules have been implicated. For instance, the monoaminergic neurotransmitter serotonin (5-HT) plays an important role in sleep-wake regulation and respiration (Jouvet, 1999; Richerson, 2004; Hodges et al., 2009; Ptak et al., 2009; Hodges and Richerson, 2010; Depuy et al., 2011; Buchanan, 2013; Iwasaki et al., 2018; Smith et al., 2018). It is also heavily implicated in epilepsy and SUDEP pathophysiology (Bagdy et al., 2007; Richerson and Buchanan, 2011; Richerson, 2013; Feng and Faingold, 2017; Li and Buchanan, 2019; Petrucci et al., 2020). Serotonergic tone is modulated in both a sleep state and circadian phase-dependent manner, with the nadir occurring during the nighttime and during sleep (Mcginty and Harper, 1976; Rosenwasser et al., 1985; Agren et al., 1986; Rao et al., 1994; Sakai and Crochet, 2001; Mateos et al., 2009; Sakai, 2011; Purnell et al., 2018). 5-HT neurons in both the midbrain and medullary raphe have been demonstrated to be robustly chemosensitive (Larnicol et al., 1994; Richerson, 1995, 2004; Wang et al., 1998; Severson et al., 2003). It is likely that 5-HT neurons in the medulla mediate increased respiration in response to a rise in CO2 whereas midbrain 5-HT neurons mediate non-respiratory responses to CO2, such as arousal (Richerson, 2004; Buchanan and Richerson, 2010; Buchanan et al., 2015; Kaur et al., 2020). Firing of medullary raphe 5-HT neurons is markedly reduced during the ictal and postictal period, coinciding with severe respiratory depression (Zhan et al., 2016). Further, lower postictal serum 5-HT levels have been associated with postictal central apnea (Murugesan et al., 2019). Numerous studies have demonstrated that pre-treatment with serotonergic agents prior to seizure onset can ameliorate this respiratory dysfunction. The incidence of S-IRA in DBA/2 mice can be reduced through the administration of fluoxetine, a selective 5-HT reuptake inhibitor (SSRI), prior to seizure induction (Tupal and Faingold, 2019). A similar finding was discovered in DBA/1 mice, where fluoxetine was also found to reduce S-IRA without increasing basal ventilation or the ventilatory response to 7% CO2 (Zeng et al., 2015; Feng and Faingold, 2017). Other serotonergic agents, including fenfluramine, can selectively block S-IRA without influencing convulsive behavior (Feng and Faingold, 2017; Tupal and Faingold, 2019).
Another monoaminergic signaling molecule with links to sleep/wake regulation, respiration, and epilepsy is norepinephrine (NE; Hobson et al., 1975; Aston-Jones and Bloom, 1981; Foote et al., 1983). Plasma concentrations of NE are significantly lower during nocturnal sleep compared to wakefulness (Linsell et al., 1985). Like, 5-HT, NE also exhibits circadian rhythmicity with the lowest concentrations occurring during the night (Morgan et al., 1973; Agren et al., 1986; Cagampang and Inouye, 1994). The NE reuptake inhibitor (NRI) atomoxetine suppresses seizure-induced respiratory arrest following audiogenic seizures in DBA/1 mice (Zhang et al., 2017; Zhao et al., 2017) as well as MES seizures (Kruse et al., 2019). Another NRI, reboxetine, and the dual 5-HT/NE reuptake inhibitor (SNRI), duloxetine, are also able to suppress respiratory arrest following MES seizures (Kruse et al., 2019). More recently, evidence has indicated that selective activation of the noradrenergic α2 receptor is sufficient to suppress S-IRA in DBA/1 mice (Zhang et al., 2021).
The excitatory neuropeptide orexin is also involved in sleep and arousal and is a wake-promoting substance (Sakurai, 2007; Bonnavion and De Lecea, 2010; Nattie and Li, 2012). Orexin displays a strong diurnal circadian variation. This rhythm has been measured in the cerebral spinal fluid (CSF) and hypothalamus of rats, with an even stronger variation in the CSF of older rats (Yoshida et al., 2001; Desarnaud et al., 2004). This robust circadian rhythm is likely in part due to the dense projections that orexin neurons receive from the suprachiasmatic nucleus (SCN), the brain’s main circadian oscillator (Saper et al., 2005). Orexin neurons also contribute to respiratory function, in part due to orexinergic innervation of serotonergic and noradrenergic nuclei (Kuwaki, 2008; Inutsuka and Yamanaka, 2013). Orexin is thought to play a proconvulsant role in epilepsy, although there is some discrepancy regarding the effects of orexins and their antagonists on seizure activity. In Kcna1-null mutant mice, the dual orexin receptor antagonist (DORA), almorexant, decreases the incidence of severe seizures, improves O2 saturation, and increases overall longevity (Roundtree et al., 2016; Iyer et al., 2020).
The inhibitory neuromodulator, adenosine, is released in large quantities during seizures (During and Spencer, 1992; Berman et al., 2000; Van Gompel et al., 2014). This is likely a mechanism of seizure termination (Shen et al., 2010; Purnell et al., 2021a). Unlike, 5-HT, NE, and orexin, adenosine promotes sleep and suppresses wakefulness (Feldberg and Sherwood, 1954; Buday et al., 1961; Haulicǎ et al., 1973; Huber et al., 2004). As such, adenosine levels increase during wakefulness and are depleted during sleep (Porkka-Heiskanen et al., 2000; Bjorness and Greene, 2009). Adenosine has an inhibitory effect on respiration, predominately causing a reduction in frequency and VT (Eldridge et al., 1984; Lagercrantz et al., 1984; Wessberg et al., 1984). The accumulation and clearance of adenosine is regulated in a circadian manner (Cornélissen et al., 1985; Chagoya De Sánchez et al., 1993; Huston et al., 1996). The adenosine hypothesis of SUDEP was first proposed in 2010, when Shen et al. noted that upregulated adenosine tone in a kainic acid model of epilepsy suppressed seizure activity but paradoxically caused death when seizures did occur (Shen et al., 2010). This hypothesis posits that a surge of adenosine is released during a seizure as a termination mechanism. However, this large increase in extracellular adenosine can result in suppression of breathing which can lead to terminal respiratory failure (Shen et al., 2010; Purnell et al., 2021a).
This is far from a comprehensive list of salient signaling molecules when it comes to respiratory function and SUDEP. However, these neuromodulators are especially of interest in the field of SUDEP and their complex role in sleep-wake regulation, breathing, and seizures makes them excellent candidates for therapeutic intervention.
SUDEP is a complex and devastating phenomenon; the underlying mechanisms of which investigators are just beginning to unravel. The time of day and sleep state in which seizures occur are indisputably factors that can confer further risk to patients with epilepsy. While nighttime and sleep tend to go hand-in-hand, it is crucial that we acknowledge the two are not one in the same and come with their own risk factors from both shared and separate mechanisms. Respiratory failure is a major precipitating factor for seizure-induced death. Monoaminergic neurons, including 5-HT, NE, and orexin, play a crucial role in respiratory function and have seizure-protective properties. Levels of monoaminergic neurons are decreased during the nighttime and even further reduced during sleep. This may explain why seizures emerging from sleep tend to be longer and cause more severe respiratory dysfunction. Other signaling molecules, such as adenosine, may have an even more complex role in SUDEP pathophysiology—contributing to respiratory dysfunction during the process of terminating seizures. Many SUDEP victims are found in a prone position in bed, suggesting that respiratory distress was amplified by airway obstruction. As patients are also more likely to be unaccompanied at night, the chance of successful intervention is low.
Thus, while sleep and nighttime appear to confer their own risk of SUDEP, the fact that the two tend to occur in conjunction contributes greatly to the “perfect storm” of factors that ultimately leads to seizure-induced death. Nevertheless, it is our hope that this review imparts the notion that sleep state and time of day are factors that should be considered independently while developing preventative strategies to mitigate the severity of respiratory dysfunction brought about by seizures.
KJ and BK drafted the initial document. The final manuscript was edited and approved by KJ, BK, and GB. All authors contributed to the article and approved the submitted version.
This published work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants F31NS125955 (to KJ), R01NS095842 (to GB), the Beth L. Tross Epilepsy Professorship from the Carver College of Medicine at the University of Iowa (to GB), and the National Institute of Health/National Institute of General Medicine Sciences T32GM0073367 (to BK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adlakha, A., and Shepard, J. W. (1998). Cardiac arrhythmias during normal sleep and in obstructive sleep apnea syndrome. Sleep Med. Rev. 2, 45–60. doi: 10.1016/s1087-0792(98)90053-3
Adolph, E. F. (1969). Regulations during survival without oxygen in infant mammals. Respir. Physiol. 7, 356–368. doi: 10.1016/0034-5687(69)90019-x
Agren, H., Koulu, M., Saavedra, J. M., Potter, W. Z., and Linnoila, M. (1986). Circadian covariation of norepinephrine and serotonin in the locus coeruleus and dorsal raphe nucleus in the rat. Brain Res. 397, 353–358. doi: 10.1016/0006-8993(86)90638-4
Aiba, I., and Noebels, J. L. (2015). Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci. Transl. Med. 7:282ra46. doi: 10.1126/scitranslmed.aaa4050
Ainslie, P. N., Lucas, S. J. E., and Burgess, K. R. (2013). Breathing and sleep at high altitude. Respir. Physiol. Neurobiol. 188, 233–256. doi: 10.1016/j.resp.2013.05.020
Ali, A., Wu, S., Issa, N. P., Rose, S., Towle, V. L., Warnke, P., et al. (2017). Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav. 76, 1–6. doi: 10.1016/j.yebeh.2017.08.021
Annegers, J. F., Coan, S. P., Hauser, W. A., Leestma, J., Duffell, W., and Tarver, B. (1998). Epilepsy, vagal nerve stimulation by the ncp system, mortality and sudden, unexpected, unexplained death. Epilepsia 39, 206–212. doi: 10.1111/j.1528-1157.1998.tb01360.x
Aserinsky, E., and Kleitman, N. (1953). Regularly occurring periods of eye motility and concomitant phenomena, during sleep. Science 118, 273–274. doi: 10.1126/science.118.3062.273
Aston-Jones, G., and Bloom, F. E. (1981). Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981
Bagdy, G., Kecskemeti, V., Riba, P., and Jakus, R. (2007). Serotonin and epilepsy. J. Neurochem. 100, 857–873. doi: 10.1111/j.1471-4159.2006.04277.x
Bando, H., Nishio, T., Van Der Horst, G. T. J., Masubuchi, S., Hisa, Y., and Okamura, H. (2007). Vagal regulation of respiratory clocks in mice. J. Neurosci. 27, 4359–4365. doi: 10.1523/JNEUROSCI.4131-06.2007
Bateman, L. M., Li, C. S., and Seyal, M. (2008). Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 131, 3239–3245. doi: 10.1093/brain/awn277
Bayne, L. L., and Simon, R. P. (1981). Systemic and pulmonary vascular pressures during generalized seizures in sheep. Ann. Neurol. 10, 566–569. doi: 10.1002/ana.410100613
Bazil, C. W., and Walczak, T. S. (1997). Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia 38, 56–62. doi: 10.1111/j.1528-1157.1997.tb01077.x
Berman, R. F., Fredholm, B. B., Aden, U., and O’connor, W. T. (2000). Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 872, 44–53. doi: 10.1016/s0006-8993(00)02441-0
Berssenbrugge, A., Dempsey, J., Iber, C., Skatrud, J., and Wilson, P. (1983). Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J. Physiol. 343, 507–524. doi: 10.1113/jphysiol.1983.sp014906
Berthon-Jones, M., and Sullivan, C. E. (1982). Ventilatory and arousal responses to hypoxia in sleeping humans. Am. Rev. Respir. Dis. 125, 632–639. doi: 10.1164/arrd.1982.125.6.632
Berthon-Jones, M., and Sullivan, C. E. (1984). Ventilation and arousal responses to hypercapnia in normal sleeping humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 57, 59–67. doi: 10.1152/jappl.1984.57.1.59
Birchfield, R. I., Sieker, H. O., and Heyman, A. (1959). Alterations in respiratory function during natural sleep. J. Lab. Clin. Med. 54, 216–222.
Bjorness, T. E., and Greene, R. W. (2009). Adenosine and sleep. Curr. Neuropharmacol. 7, 238–245. doi: 10.2174/157015909789152182
Block, A. J., Boysen, P. G., Wynne, J. W., and Hunt, L. A. (1979). Sleep apnea, hypopnea and oxygen desaturation in normal subjects. N. Engl. J. Med. 300, 513–517. doi: 10.1056/NEJM197903083001001
Blum, A. S. (2009). Respiratory physiology of seizures. J. Clin. Neurophysiol. 26, 309–315. doi: 10.1097/WNP.0b013e3181b7f14d
Bonnavion, P., and De Lecea, L. (2010). Hypocretins in the control of sleep and wakefulness. Curr. Neurol. Neurosci. Rep. 10, 174–179. doi: 10.1007/s11910-010-0101-y
Bozorgi, A., and Lhatoo, S. D. (2013). Seizures, cerebral shutdown and SUDEP. Epilepsy Curr. 13, 236–240. doi: 10.5698/1535-7597-13.5.236
Browne, K. F., Prystowsky, E., Heger, J. J., Chilson, D. A., and Zipes, D. P. (1983). Prolongation of the q-t interval in man during sleep. Am. J. Cardiol. 52, 55–59. doi: 10.1016/0002-9149(83)90068-1
Bryan, A. C., and Muller, N. L. (1980). Lung mechanics and gas exchange during sleep. Sleep 3, 401–416. doi: 10.1093/sleep/3.3-4.401
Buchanan, G. F. (2013). Timing, sleep and respiration in health and disease. Prog. Mol. Biol. Transl. Sci. 119, 191–219. doi: 10.1016/B978-0-12-396971-2.00008-7
Buchanan, G. F. (2019). Impaired CO2-induced arousal in SIDS and SUDEP. Trends Neurosci. 42, 242–250. doi: 10.1016/j.tins.2019.02.002
Buchanan, G. F., Murray, N. M., Hajek, M. A., and Richerson, G. B. (2014). Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J. Physiol. 592, 4395–4410. doi: 10.1113/jphysiol.2014.277574
Buchanan, G. F., and Richerson, G. B. (2010). Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. U S A 107, 16354–16359. doi: 10.1073/pnas.1004587107
Buchanan, G. F., Smith, H. R., Macaskill, A., and Richerson, G. B. (2015). 5-HT2A receptor activation is necessary for CO2-induced arousal. J. Neurophysiol. 114, 233–243. doi: 10.1152/jn.00213.2015
Buday, P. V., Carr, C. J., and Miya, T. S. (1961). A pharmacologic study of some nucleosides and nucleotides. J. Pharm Pharmacol. 13, 290–299. doi: 10.1111/j.2042-7158.1961.tb11826.x
Budde, R. B., Arafat, M. A., Pederson, D. J., Lovick, T. A., Jefferys, J. G. R., and Irazoqui, P. P. (2018). Acid reflux induced laryngospasm as a potential mechanism of sudden death in epilepsy. Epilepsy Res. 148, 23–31. doi: 10.1016/j.eplepsyres.2018.10.003
Butler, M. P., Smales, C., Wu, H., Hussain, M. V., Mohamed, Y. A., Morimoto, M., et al. (2015). The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep 38, 1793–1801. doi: 10.5665/sleep.5166
Cagampang, F. R. A., and Inouye, S.-I. T. (1994). Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res. 639, 175–179. doi: 10.1016/0006-8993(94)91780-9
Chagoya De Sánchez, V., Múñoz, R. H., Suárez, J., Vidrio, S., Yáñez, L., and Múñoz, M. D. (1993). Day-night variations of adenosine and its metabolizing enzymes in the brain cortex of the rat — possible physiological significance for the energetic homeostasis and the sleep-wake cycle. Brain Res. 612, 115–121. doi: 10.1016/0006-8993(93)91651-8
Chen, Z., Brodie, M. J., Liew, D., and Kwan, P. (2018). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 75, 279–286. doi: 10.1001/jamaneurol.2017.3949
Cherniack, N. S. (1981). Respiratory dysrhythmias during sleep. N. Engl. J. Med. 305, 325–330. doi: 10.1056/NEJM198108063050606
Cherniack, N. S. (1999). Apnea and periodic breathing during sleep. N. Engl. J. Med. 341, 985–987. doi: 10.1056/NEJM199909233411310
Chrobok, L., Northeast, R. C., Myung, J., Cunningham, P. S., Petit, C., and Piggins, H. D. (2020). Timekeeping in the hindbrain: a multi-oscillatory circadian centre in the mouse dorsal vagal complex. Commun. Biol. 3:225. doi: 10.1038/s42003-020-0960-y
Cohen, H. B., and Dement, W. C. (1970). Prolonged tonic convulsions in REM deprived mice. Brain Res. 22, 421–422. doi: 10.1016/0006-8993(70)90487-7
Cornélissen, G., Touitou, Y., Tritsch, G., Bogdan, A., Auzéby, A., Reinberg, A., et al. (1985). Circadian rhythms of adenosine deaminase activity in human erythrocytes: a transverse study on young, elderly and senile demented subjects. La Ricerca in Clin. Lab. 15, 365–374. doi: 10.1007/BF03029152
Crespel, A., Coubes, P., and Baldy-Moulinier, M. (2000). Sleep influence on seizures and epilepsy effects on sleep in partial frontal and temporal lobe epilepsies. Clin. Neurophysiol. 111, S54–S59. doi: 10.1016/s1388-2457(00)00402-8
Del Negro, C. A., Funk, G. D., and Feldman, J. L. (2018). Breathing matters. Nat. Rev. Neurosci. 19, 351–367. doi: 10.1038/s41583-018-0003-6
Depuy, S. D., Kanbar, R., Coates, M. B., Stornetta, R. L., and Guyenet, P. G. (2011). Control of breathing by raphe obscurus serotonergic neurons in mice. J. Neurosci. 31, 1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011
Desarnaud, F., Murillo-Rodriguez, E., Lin, L., Xu, M., Gerashchenko, D., Shiromani, S. N., et al. (2004). The diurnal rhythm of hypocretin in young and old f344 rats. Sleep 27, 851–856. doi: 10.1093/sleep/27.5.851
Devinsky, O., Hesdorffer, D. C., Thurman, D. J., Lhatoo, S., and Richerson, G. (2016). Sudden unexpected death in epilepsy: epidemiology, mechanisms and prevention. Lancet Neurol. 15, 1075–1088. doi: 10.1016/S1474-4422(16)30158-2
Dhaibar, H., Gautier, N. M., Chernyshev, O. Y., Dominic, P., and Glasscock, E. (2019). Cardiorespiratory profiling reveals primary breathing dysfunction in kcna1-null mice: implications for sudden unexpected death in epilepsy. Neurobiol. Dis. 127, 502–511. doi: 10.1016/j.nbd.2019.04.006
Dlouhy, B. J., Gehlbach, B. K., Kreple, C. J., Kawasaki, H., Oya, H., Buzza, C., et al. (2015). Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J. Neurosci. 35, 10281–10289. doi: 10.1523/JNEUROSCI.0888-15.2015
Douglas, N. J., White, D. P., Pickett, C. K., Weil, J. V., and Zwillich, C. W. (1982a). Respiration during sleep in normal man. Thorax 37, 840–844. doi: 10.1136/thx.37.11.840
Douglas, N. J., White, D. P., Weil, J. V., Pickett, C. K., Martin, R. J., Hudgel, D. W., et al. (1982b). Hypoxic ventilatory response decreases during sleep in normal men. Am. Rev. Respir. Dis. 125, 286–289. doi: 10.1164/arrd.1982.125.3.286
Douglas, N. J., White, D. P., Weil, J. V., Pickett, C. K., and Zwillich, C. W. (1982c). Hypercapnic ventilatory response in sleeping adults. Am. Rev. Respir. Dis. 126, 758–762. doi: 10.1164/arrd.1982.126.5.758
During, M. J., and Spencer, D. D. (1992). Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 32, 618–624. doi: 10.1002/ana.410320504
Eldridge, F. L., Millhorn, D. E., and Kiley, J. P. (1984). Respiratory effects of a long-acting analog of adenosine. Brain Res. 301, 273–280. doi: 10.1016/0006-8993(84)91096-5
Eun, B., Kim, H. J., Kim, S. Y., Kim, T. W., Hong, S. T., Choi, K., et al. (2011). Induction of per1 expression following an experimentally induced epilepsy in the mouse hippocampus. Neurosci. Lett. 498, 110–113. doi: 10.1016/j.neulet.2011.03.039
Feldberg, W., and Sherwood, S. L. (1954). Injections of drugs into the lateral ventricle of the cat. J. Physiol. 123, 148–167. doi: 10.1113/jphysiol.1954.sp005040
Feng, H. J., and Faingold, C. L. (2017). Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav. 71, 174–180. doi: 10.1016/j.yebeh.2015.06.008
Fenik, V. B., Davies, R. O., and Kubin, L. (2005). REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 172, 1322–1330. doi: 10.1164/rccm.200412-1750OC
Foote, S. L., Bloom, F. E., and Aston-Jones, G. (1983). Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 63, 844–914. doi: 10.1152/physrev.1983.63.3.844
Gami, A. S., Olson, E. J., Shen, W. K., Wright, R. S., Ballman, K. V., Hodge, D. O., et al. (2013). Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 62, 610–616. doi: 10.1016/j.jacc.2013.04.080
Gami, A. S., and Somers, V. K. (2008). Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J. Cardiovasc. Electrophysiol. 19, 997–1003. doi: 10.1111/j.1540-8167.2008.01136.x
Gangwisch, J. E., Heymsfield, S. B., Boden-Albala, B., Buijs, R. M., Kreier, F., Pickering, T. G., et al. (2006). Short sleep duration as a risk factor for hypertension: analyses of the first national health and nutrition examination survey. Hypertension 47, 833–839. doi: 10.1161/01.HYP.0000217362.34748.e0
Gerstner, J. R., Smith, G. G., Lenz, O., Perron, I. J., Buono, R. J., and Ferraro, T. N. (2014). Bmal1 controls the diurnal rhythm and set point for electrical seizure threshold in mice. Front. Syst. Neurosci. 8:121. doi: 10.3389/fnsys.2014.00121
Gharib, A., Sayyahi, Z., Komaki, A., Barkley, V., Sarihi, A., and Mirnajafi-Zadeh, J. (2018). The role of 5-HT1A receptors of hippocampal CA1 region in anticonvulsant effects of low-frequency stimulation in amygdala kindled rats. Physiol. Behav. 196, 119–125. doi: 10.1016/j.physbeh.2018.08.025
Grigg-Damberger, M., and Foldvary-Schaefer, N. (2021). Bidirectional relationships of sleep and epilepsy in adults with epilepsy. Epilepsy Behav. 116:107735. doi: 10.1016/j.yebeh.2020.107735
Guissart, C., Latypova, X., Rollier, P., Khan, T. N., Stamberger, H., Mcwalter, K., et al. (2018). Dual molecular effects of dominant RORA mutations cause two variants of syndromic intellectual disability with either autism or cerebellar ataxia. Am. J. Hum Genet. 102, 744–759. doi: 10.1016/j.ajhg.2018.02.021
Guntheroth, W. G., and Kawabori, I. (1975). Hypoxic apnea and gasping. J. Clin. Invest. 56, 1371–1377. doi: 10.1172/JCI108217
Hajek, M. A., and Buchanan, G. F. (2016). Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J. Neurophysiol. 115, 2286–2293. doi: 10.1152/jn.00011.2016
Harden, C., Tomson, T., Gloss, D., Buchhalter, J., Cross, J. H., Donner, E., et al. (2017). Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination and implementation subcommittee of the american academy of neurology and the american epilepsy society. Epilepsy Curr. 17, 180–187. doi: 10.5698/1535-7511.17.3.180
Haulicǎ, I., Ababei, L., Brǎnişlteanu, D., and Topoliceanu, F. (1973). Preliminary data on the possible hypnogenic role of adenosine1. J. Neurochem. 21, 1019–1020. doi: 10.1111/j.1471-4159.1973.tb07549.x
Haxhiu, M. A., Mack, S. O., Wilson, C. G., Feng, P., and Strohl, K. P. (2003). Sleep networks and the anatomic and physiologic connections with respiratory control. Front. Biosci. 8, d946–d962. doi: 10.2741/1099
Haxhiu, M. A., Van Lunteren, E., Mitra, J., and Cherniack, N. S. (1987). Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir. Physiol. 70, 183–193. doi: 10.1016/0034-5687(87)90049-1
Hesdorffer, D. C., Tomson, T., Benn, E., Sander, J. W., Nilsson, L., Langan, Y., et al. (2011). Combined analysis of risk factors for SUDEP. Epilepsia 52, 1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x
Hicks, J. W., and Riedesel, M. L. (1983). Diurnal ventilatory patterns in the garter snake, Thamnophis elegans. J. Comp. Physiol. 149, 503–510. doi: 10.1007/BF00690009
Hobson, J. A., Mccarley, R. W., and Wyzinski, P. W. (1975). Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 4, 55–58. doi: 10.1126/science.1094539
Hodges, M. R., and Richerson, G. B. (2010). Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir. Physiol. Neurobiol. 173, 256–263. doi: 10.1016/j.resp.2010.03.006
Hodges, M. R., Wehner, M., Aungst, J., Smith, J. C., and Richerson, G. B. (2009). Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci. 29, 10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009
Holmes, M. D., Chang, M., and Kapur, V. (2003). Sleep apnea and excessive daytime somnolence induced by vagal nerve stimulation. Neurology 61, 1126–1129. doi: 10.1212/01.wnl.0000086812.62554.06
Huber, R., Felice Ghilardi, M., Massimini, M., and Tononi, G. (2004). Local sleep and learning. Nature 430, 78–81. doi: 10.1038/nature02663
Huston, J. P., Haas, H. L., Boix, F., Pfister, M., Decking, U., Schrader, J., et al. (1996). Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience 73, 99–107. doi: 10.1016/0306-4522(96)00021-8
Iizuka, H., Sasaki, K., Odagiri, N., Obo, M., Imaizumi, M., and Atai, H. (2010). Measurement of respiratory function using whole-body plethysmography in unanesthetized and unrestrained nonhuman primates. J. Toxicol. Sci. 35, 863–870. doi: 10.2131/jts.35.863
Inutsuka, A., and Yamanaka, A. (2013). The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. (Lausanne) 4:18. doi: 10.3389/fendo.2013.00018
Irizarry, R., Sukato, D., Kollmar, R., Schild, S., Silverman, J., Sundaram, K., et al. (2020). Seizures induce obstructive apnea in DBA/2J audiogenic seizure-prone mice: lifesaving impact of tracheal implants. Epilepsia 61, e13–e16. doi: 10.1111/epi.16431
Iwasaki, K., Komiya, H., Kakizaki, M., Miyoshi, C., Abe, M., Sakimura, K., et al. (2018). Ablation of central serotonergic neurons decreased rem sleep and attenuated arousal response. Front. Neurosci. 12:535. doi: 10.3389/fnins.2018.00535
Iyer, S. H., Aggarwal, A., Warren, T. J., Hallgren, J., Abel, P. W., Simeone, T. A., et al. (2020). Progressive cardiorespiratory dysfunction in kv1.1 knockout mice may provide temporal biomarkers of pending sudden unexpected death in epilepsy (SUDEP): the contribution of orexin. Epilepsia 61, 572–588. doi: 10.1111/epi.16434
Jansen, N. A., Schenke, M., Voskuyl, R. A., Thijs, R. D., Van Den Maagdenberg, A., and Tolner, E. A. (2019). Apnea associated with brainstem seizures in Cacna1a (s218l) mice is caused by medullary spreading depolarization. J. Neurosci. 39, 9633–9644. doi: 10.1523/JNEUROSCI.1713-19.2019
Javaheri, S., and Guerra, L. F. (1990). Effects of domperidone and medroxyprogesterone acetate on ventilation in man. Respir. Physiol. 81, 359–370. doi: 10.1016/0034-5687(90)90116-g
Jefferys, J. G. R., Arafat, M. A., Irazoqui, P. P., and Lovick, T. A. (2019). Brainstem activity, apnea and death during seizures induced by intrahippocampal kainic acid in anaesthetized rats. Epilepsia 60, 2346–2358. doi: 10.1111/epi.16374
Jin, Z. L., Chen, X. F., Ran, Y. H., Li, X. R., Xiong, J., Zheng, Y. Y., et al. (2017). Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci. Rep. 7:8631. doi: 10.1038/s41598-017-08953-4
Johnston, S. C., Siedenberg, R., Min, J. K., Jerome, E. H., and Laxer, K. D. (1997). Central apnea and acute cardiac ischemia in a sheep model of epileptic sudden death. Ann. Neurol. 42, 588–594. doi: 10.1002/ana.410420409
Jouvet, M. (1999). Sleep and serotonin an unfinished story. Neuropsychopharmacology 21, 24S–27S. doi: 10.1016/S0893-133X(99)00009-3
Junkermann, H., Mangold, H., Vecsei, P., and Runnebaum, B. (1982). Circadian rhythm of serum progesterone levels in human pregnancy and its relation to the rhythm of cortisol. Acta Endocrinol. (Copenh) 101, 98–104. doi: 10.1530/acta.0.1010098
Kaleyias, J., Cruz, M., Goraya, J. S., Valencia, I., Khurana, D. S., Legido, A., et al. (2008). Spectrum of polysomnographic abnormalities in children with epilepsy. Pediatr. Neurol. 39, 170–176. doi: 10.1016/j.pediatrneurol.2008.06.002
Kaneko, K., Yamada, T., Tsukita, S., Takahashi, K., Ishigaki, Y., Oka, Y., et al. (2009). Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 1263, 58–68. doi: 10.1016/j.brainres.2008.12.071
Kaur, S., De Luca, R., Khanday, M. A., Bandaru, S. S., Thomas, R. C., Broadhurst, R. Y., et al. (2020). Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals. Nat. Commun. 11:2769. doi: 10.1038/s41467-020-16518-9
Kelly, M. N., Smith, D. N., Sunshine, M. D., Ross, A., Zhang, X., Gumz, M. L., et al. (2020). Circadian clock genes and respiratory neuroplasticity genes oscillate in the phrenic motor system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R1058–R1067. doi: 10.1152/ajpregu.00010.2020
Kemp, J. S., Nelson, V. E., and Thach, B. T. (1994). Physical properties of bedding that may increase risk of sudden infant death syndrome in prone-sleeping infants. Pediatr. Res. 36, 7–11. doi: 10.1203/00006450-199407001-00002
Kennedy, J. D., Hardin, K. A., Parikh, P., Li, C., and Seyal, M. (2015). Pulmonary edema following generalized tonic clonic seizures is directly associated with seizure duration. Seizure 27, 19–24. doi: 10.1016/j.seizure.2015.02.023
Kim, Y., Bravo, E., Thirnbeck, C. K., Smith-Mellecker, L. A., Kim, S. H., Gehlbach, B. K., et al. (2018). Severe peri-ictal respiratory dysfunction is common in dravet syndrome. J. Clin. Invest. 128, 1141–1153. doi: 10.1172/JCI94999
Kim, J. S., Lee, D. E., Bae, H., Song, J. Y., Yang, K. I., and Hong, S. B. (2022). Effects of vagus nerve stimulation on sleep-disordered breathing, daytime sleepiness and sleep quality in patients with drug-resistant epilepsy. J. Clin. Neurol. 18, 315–322. doi: 10.3988/jcn.2022.18.3.315
Konduru, S. S., Pan, Y.-Z., Wallace, E., Pfammatter, J. A., Jones, M. V., and Maganti, R. K. (2021). Sleep deprivation exacerbates seizures and diminishes GABAergic tonic inhibition. Ann. Neurol. 90, 840–844. doi: 10.1002/ana.26208
Kotsopoulos, I. A., Van Merode, T., Kessels, F. G., De Krom, M. C., and Knottnerus, J. A. (2002). Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 43, 1402–1409. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x
Krieger, J. (2005). Respiratory physiology: breathing in normal subjects. Princ. Pract. Sleep Med. 232, 232–244. doi: 10.1016/B0-72-160797-7/50026-4
Kruse, S. W., Dayton, K. G., Purnell, B. S., Rosner, J. I., and Buchanan, G. F. (2019). Effect of monoamine reuptake inhibition and alpha1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia 60, 495–507. doi: 10.1111/epi.14652
Kubin, L. (2016). Neural control of the upper airway: respiratory and state-dependent mechanisms. Compr. Physiol. 6, 1801–1850. doi: 10.1002/cphy.c160002
Kumar, G., Couper, A., O’brien, T. J., Salzberg, M. R., Jones, N. C., Rees, S. M., et al. (2007). The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology 32, 834–842. doi: 10.1016/j.psyneuen.2007.05.011
Künstler, E. C. S., Schwab, M., and Rupprecht, S. (2022). A 37-year-old man with structural focal epilepsy and paroxysmal nocturnal breathing arrests. Chest 161, e309–e312. doi: 10.1016/j.chest.2021.12.631
Kuo, F. S., Cleary, C. M., Loturco, J. L., Chen, X., and Mulkey, D. K. (2019). Disordered breathing in a mouse model of dravet syndrome. eLife 8:e43387. doi: 10.7554/eLife.43387
Kuo, J., Zhao, W., Li, C. S., Kennedy, J. D., and Seyal, M. (2016). Postictal immobility and generalized EEG suppression are associated with the severity of respiratory dysfunction. Epilepsia 57, 412–417. doi: 10.1111/epi.13312
Kuwaki, T. (2008). Orexinergic modulation of breathing across vigilance states. Respir. Physiol. Neurobiol. 164, 204–212. doi: 10.1016/j.resp.2008.03.011
Kwan, P., and Brodie, M. J. (2000). Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319. doi: 10.1056/NEJM200002033420503
Lacuey, N., Zonjy, B., Hampson, J. P., Rani, M. R. S., Zaremba, A., Sainju, R. K., et al. (2018). The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 59, 573–582. doi: 10.1111/epi.14006
Lacuey, N., Zonjy, B., Londono, L., and Lhatoo, S. D. (2017). Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 88, 701–705. doi: 10.1212/WNL.0000000000003613
Lagercrantz, H., Yamamoto, Y., Fredholm, B. B., Prabhakar, N. R., and Von Euler, C. (1984). Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr. Res. 18, 387–390. doi: 10.1203/00006450-198404000-00018
Lamberts, R. J., Thijs, R. D., Laffan, A., Langan, Y., and Sander, J. W. (2012). Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53, 253–257. doi: 10.1111/j.1528-1167.2011.03360.x
Langan, Y., Nashef, L., and Sander, J. W. (2005). Case-control study of SUDEP. Neurology 64, 1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB
Larnicol, N., Wallois, F., Berquin, P., Gross, F., and Rose, D. (1994). C-fos-like immunoreactivity in the cat’s neuraxis following moderate hypoxia or hypercapnia. J. Physiol. Paris 88, 81–88. doi: 10.3389/fped.2022.884120
Latreille, V., Abdennadher, M., Dworetzky, B. A., Ramel, J., White, D., Katz, E., et al. (2017). Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia 58, e127–e131. doi: 10.1111/epi.13841
Leguia, M. G., Andrzejak, R. G., Rummel, C., Fan, J. M., Mirro, E. A., Tcheng, T. K., et al. (2021). Seizure cycles in focal epilepsy. JAMA Neurol. 78, 454–463. doi: 10.1001/jamaneurol.2020.5370
Li, R., and Buchanan, G. F. (2019). Scurrying to understand sudden expected death in epilepsy: insights from animal models. Epilepsy Curr. 19, 390–396. doi: 10.1177/1535759719874787
Li, Q., Li, Y., Wang, X., Qi, J., Jin, X., Tong, H., et al. (2017). Fbxl4 serves as a clock output molecule that regulates sleep through promotion of rhythmic degradation of the GABAA receptor. Curr. Biol. 27, 3616–3625.e5. doi: 10.1016/j.cub.2017.10.052
Lin, Z., Si, Q., and Xiaoyi, Z. (2017). Obstructive sleep apnoea in patients with epilepsy: a meta-analysis. Sleep Breath. 21, 263–270. doi: 10.1007/s11325-016-1391-3
Linsell, C. R., Lightman, S. L., Mullen, P. E., Brown, M. J., and Causon, R. C. (1985). Circadian rhythms of epinephrine and norepinephrine in man. J. Clin. Endocrinol. Metab. 60, 1210–1215. doi: 10.1210/jcem-60-6-1210
Lusardi, P., Mugellini, A., Preti, P., Zoppi, A., Derosa, G., and Fogari, R. (1996). Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am. J. Hypertens. 9, 503–505. doi: 10.1016/0895-7061(95)00389-4
Malik, V., Smith, D., and Lee-Chiong, T., Jr. (2012). Respiratory physiology during sleep. Sleep Med. Clin. 7, 497–505. doi: 10.1016/j.jsmc.2012.06.011
Malow, B. A., Edwards, J., Marzec, M., Sagher, O., and Fromes, G. (2000a). Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology 55, 1450–1454. doi: 10.1212/wnl.55.10.1450
Malow, B. A., Levy, K., Maturen, K., and Bowes, R. (2000b). Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology 55, 1002–1007. doi: 10.1212/wnl.55.7.1002
Malow, B. A., Lin, X., Kushwaha, R., and Aldrich, M. S. (1998). Interictal spiking increases with sleep depth in temporal lobe epilepsy. Epilepsia 39, 1309–1316. doi: 10.1111/j.1528-1157.1998.tb01329.x
Marincovich, A., Bravo, E., Dlouhy, B., and Richerson, G. B. (2021). Amygdala lesions reduce seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav. 121:106440. doi: 10.1016/j.yebeh.2019.07.041
Marzec, M., Edwards, J., Sagher, O., Fromes, G., and Malow, B. A. (2003). Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia 44, 930–935. doi: 10.1046/j.1528-1157.2003.56202.x
Matos, H. D. C., Koike, B. D. V., Pereira, W. D. S., De Andrade, T. G., Castro, O. W., Duzzioni, M., et al. (2018). Rhythms of core clock genes and spontaneous locomotor activity in post-status epilepticus model of mesial temporal lobe epilepsy. Front. Neurol. 9:632. doi: 10.3389/fneur.2018.00632
Mateos, S. S., Sánchez, C. L., Paredes, S. D., Barriga, C., and Rodríguez, A. B. (2009). Circadian levels of serotonin in plasma and brain after oral administration of tryptophan in rats. Basic Clin. Pharmacol. Toxicol. 104, 52–59. doi: 10.1111/j.1742-7843.2008.00333.x
Mattson, R. H., Pratt, K. L., and Calverley, J. (1965). Electroencephalograms of epileptics following sleep deprivation. Arch. Neurol. 13, 310–315. doi: 10.1001/archneur.1965.00470030090009
Mcginty, D. J., and Harper, R. M. (1976). Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 101, 569–575. doi: 10.1016/0006-8993(76)90480-7
Mckay, L. C., and Feldman, J. L. (2008). Unilateral ablation of pre-bötzinger complex disrupts breathing during sleep but not wakefulness. Am. J. Respir. Crit. Care Med. 178, 89–95. doi: 10.1164/rccm.200712-1901OC
Meny, R., Carroll, J., Carbone, M., and Kelly, D. (1994). Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93, 44–49. doi: 10.1542/peds.93.1.44
Mohawk, J. A., Green, C. B., and Takahashi, J. S. (2012). Central and peripheral circadian clocks in mammals. Ann. Rev. Neurosci. 35, 445–462. doi: 10.1146/annurev-neuro-060909-153128
Montserrat, J. M., Kosmas, E. N., Cosio, M. G., and Kimoff, R. J. (1996). Mechanism of apnea lengthening across the night in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 154, 988–993. doi: 10.1164/ajrccm.154.4.8887596
Moore, B. M., Jerry Jou, C., Tatalovic, M., Kaufman, E. S., Kline, D. D., and Kunze, D. L. (2014). The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP). Epilepsia 55, 1808–1816. doi: 10.1111/epi.12793
Morgan, W. W., Mcfadin, L. S., and Harvey, C. Y. (1973). A daily rhythm in norepinephrine content in regions of the hamster brain. Comp. Gen. Pharmacol. 4, 47–52. doi: 10.1016/0010-4035(73)90020-7
Mortola, J. P. (2004). Breathing around the clock: an overview of the circadian pattern of respiration. Eur. J. Appl. Physiol. 91, 119–129. doi: 10.1007/s00421-003-0978-0
Muñoz-Ortiz, J., Muñoz-Ortiz, E., López-Meraz, L., Beltran-Parrazal, L., and Morgado-Valle, C. (2019). The pre-bötzinger complex: generation and modulation of respiratory rhythm. Neurologia (Engl Ed) 34, 461–468. doi: 10.1088/1361-6560/ac8410
Murugesan, A., Rani, M. R. S., Vilella, L., Lacuey, N., Hampson, J. P., Faingold, C. L., et al. (2019). Postictal serotonin levels are associated with peri-ictal apnea. Neurology 93, e1485–e1494. doi: 10.1212/WNL.0000000000008244
Nakase, K., Kollmar, R., Lazar, J., Arjomandi, H., Sundaram, K., Silverman, J., et al. (2016). Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model. Epilepsy Res. 128, 126–139. doi: 10.1016/j.eplepsyres.2016.08.004
Nashef, L., Garner, S., Sander, J. W., Fish, D. R., and Shorvon, S. D. (1998). Circumstances of death in sudden death in epilepsy: interviews of bereaved relatives. J. Neurol. Neurosurg. Psychiatry 64, 349–352. doi: 10.1136/jnnp.64.3.349
Nashef, L., Walker, F., Allen, P., Sander, J. W., Shorvon, S. D., and Fish, D. R. (1996). Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J. Neurol. Neurosurg. Psychiatry 60, 297–300. doi: 10.1136/jnnp.60.3.297
Nattie, E., and Li, A. (2012). “Chapter 4 - respiration and autonomic regulation and orexin,” in Progress In Brain Research (Vol. 198), ed A. Shekhar (Amsterdam, Netherlands: Elsevier), 25–46. doi: 10.1016/B978-0-444-59489-1.00004-5
Ng, M., and Pavlova, M. (2013). Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res. Treat. 2013:932790. doi: 10.1155/2013/932790
Nilsson, L., Farahmand, B. Y., Persson, P. G., Thiblin, I., and Tomson, T. (1999). Risk factors for sudden unexpected death in epilepsy: a case control study. Lancet 353, 888–893. doi: 10.1016/s0140-6736(98)05114-9
Nishimura, Y., Saito, Y., Kondo, N., Matsuda, E., Fujiyama, M., Morizane, R., et al. (2015). Ictal central apnea and bradycardia in temporal lobe epilepsy complicated by obstructive sleep apnea syndrome. Epilepsy Behav. Case Rep. 4, 41–44. doi: 10.1016/j.ebcr.2015.05.001
Nobili, L., Proserpio, P., Rubboli, G., Montano, N., Didato, G., and Tassinari, C. A. (2011). Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med. Rev. 15, 237–246. doi: 10.1016/j.smrv.2010.07.006
Nobis, W. P., Schuele, S., Templer, J. W., Zhou, G., Lane, G., Rosenow, J. M., et al. (2018). Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann. Neurol. 83, 460–471. doi: 10.1002/ana.25178
Opeskin, K., and Berkovic, S. F. (2003). Risk factors for sudden unexpected death in epilepsy: a controlled prospective study based on coroners cases. Seizure 12, 456–464. doi: 10.1016/s1059-1311(02)00352-7
Oudiette, D., Dodet, P., Ledard, N., Artru, E., Rachidi, I., Similowski, T., et al. (2018). REM sleep respiratory behaviours match mental content in narcoleptic lucid dreamers. Sci. Rep. 8:6128. doi: 10.1038/s41598-018-24179-4
Peever, J. H., and Stephenson, R. (1997). Day-night differences in the respiratory response to hypercapnia in awake adult rats. Respir. Physiol. 109, 241–248. doi: 10.1016/s0034-5687(97)00056-x
Peng, W., Danison, J. L., and Seyal, M. (2017). Postictal generalized EEG suppression and respiratory dysfunction following generalized tonic-clonic seizures in sleep and wakefulness. Epilepsia 58, 1409–1414. doi: 10.1111/epi.13805
Petrucci, A. N., Joyal, K. G., Purnell, B. S., and Buchanan, G. F. (2020). Serotonin and sudden unexpected death in epilepsy. Exp. Neurol. 325:113145. doi: 10.1016/j.expneurol.2019.113145
Phabphal, K., Koonalintip, P., Sithinamsuwan, P., Wongsritrang, K., Amornpojnimman, T., Ekpitakdamrong, N., et al. (2021). Obstructive sleep apnea and sudden unexpected death in epilepsy in unselected patients with epilepsy: are they associated? Sleep Breath. 25, 1919–1924. doi: 10.1007/s11325-021-02307-1
Popkirov, S., Stone, J., and Derry, C. P. (2019). Abnormal sleep in patients with epileptic or dissociative (non-epileptic) seizures: a polysomnography study. Eur. J. Neurol. 26, 255–260. doi: 10.1111/ene.13798
Porkka-Heiskanen, T., Strecker, R. E., and Mccarley, R. W. (2000). Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. doi: 10.1016/s0306-4522(00)00220-7
Pramsohler, S., Schilz, R., Patzak, A., Rausch, L., and Netzer, N. C. (2019). Periodic breathing in healthy young adults in normobaric hypoxia equivalent to 3500 m, 4500 m and 5500 m altitude. Sleep Breath. 23, 703–709. doi: 10.1007/s11325-019-01829-z
Pratt, K. L., Mattson, R. H., Weikers, N. J., and Williams, R. (1968). EEG activation of epileptics following sleep deprivation: a prospective study of 114 cases. Electroencephalogr. Clin. Neurophysiol. 24, 11–15. doi: 10.1016/0013-4694(68)90061-8
Ptak, K., Yamanishi, T., Aungst, J., Milescu, L. S., Zhang, R., Richerson, G. B., et al. (2009). Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci. 29, 3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009
Purnell, B. S., and Buchanan, G. F. (2020). Free-running circadian breathing rhythms are eliminated by suprachiasmatic nucleus lesion. J. Appl. Physiol. (1985) 129, 49–57. doi: 10.1152/japplphysiol.00211.2020
Purnell, B. S., Hajek, M. A., and Buchanan, G. F. (2017). Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J Neurophysiol. 118, 2592–2600. doi: 10.1152/jn.00039.2017
Purnell, B. S., Murugan, M., Jani, R., and Boison, D. (2021a). The good, the bad and the deadly: adenosinergic mechanisms underlying sudden unexpected death in epilepsy. Front. Neurosci. 15:708304. doi: 10.3389/fnins.2021.708304
Purnell, B. S., Petrucci, A. N., Li, R., and Buchanan, G. F. (2021b). The effect of time-of-day and circadian phase on vulnerability to seizure-induced death in two mouse models. J. Physiol. 599, 1885–1899. doi: 10.1113/JP280856
Purnell, B. S., Thijs, R. D., and Buchanan, G. F. (2018). Dead in the night: sleep-wake and time-of-day influences on sudden unexpected death in epilepsy. Front. Neurol. 9:1079. doi: 10.3389/fneur.2018.01079
Rao, M. L., Gross, G., Strebel, B., Halaris, A., Huber, G., Bräunig, P., et al. (1994). Circadian rhythm of tryptophan, serotonin, melatonin and pituitary hormones in schizophrenia. Biol. Psychiatry 35, 151–163. doi: 10.1016/0006-3223(94)91147-9
Re, C. J., Batterman, A. I., Gerstner, J. R., Buono, R. J., and Ferraro, T. N. (2020). The molecular genetic interaction between circadian rhythms and susceptibility to seizures and epilepsy. Front. Neurol. 11:520. doi: 10.3389/fneur.2020.00520
Reed, D. J., and Kellogg, R. H. (1958). Changes in respiratory response to CO2 during natural sleep at sea level and at altitude. J. Appl. Physiol. 13, 325–330. doi: 10.1152/jappl.1958.13.3.325
Remmers, J. E., Degroot, W. J., Sauerland, E. K., and Anch, A. M. (1978). Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 44, 931–938. doi: 10.1152/jappl.1978.44.6.931
Rhone, A. E., Kovach, C. K., Harmata, G. I., Sullivan, A. W., Tranel, D., Ciliberto, M. A., et al. (2020). A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy. JCI Insight 5:e134852. doi: 10.1172/jci.insight.134852
Richerson, G. B. (1995). Response to CO2 of neurons in the rostral ventral medulla in vitro. J. Neurophysiol. 73, 933–944. doi: 10.1152/jn.1995.73.3.933
Richerson, G. B. (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 5, 449–461. doi: 10.1038/nrn1409
Richerson, G. B. (2013). Serotonin: the anti-suddendeathamine? Epilepsy Curr. 13, 241–244. doi: 10.5698/1535-7597-13.5.241
Richerson, G. B., and Buchanan, G. F. (2011). The serotonin axis: shared mechanisms in seizures, depression and SUDEP. Epilepsia 52, 28–38. doi: 10.1111/j.1528-1167.2010.02908.x
Rosenwasser, A. M., Trubowitsch, G., and Adler, N. T. (1985). Circadian rhythm in metabolic activity of suprachiasmatic, supraoptic and raphe nuclei. Neurosci. Lett. 58, 183–187. doi: 10.1016/0304-3940(85)90161-2
Roundtree, H. M., Simeone, T. A., Johnson, C., Matthews, S. A., Samson, K. K., and Simeone, K. A. (2016). Orexin receptor antagonism improves sleep and reduces seizures in kcna1-null mice. Sleep 39, 357–368. doi: 10.5665/sleep.5444
Rugg-Gunn, F., Duncan, J., Hjalgrim, H., Seyal, M., and Bateman, L. (2016). From unwitnessed fatality to witnessed rescue: nonpharmacologic interventions in sudden unexpected death in epilepsy. Epilepsia 57, 26–34. doi: 10.1111/epi.13231
Ryvlin, P., Nashef, L., Lhatoo, S. D., Bateman, L. M., Bird, J., Bleasel, A., et al. (2013). Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 12, 966–977. doi: 10.1016/S1474-4422(13)70214-X
Ryvlin, P., Rheims, S., and Risse, G. (2006). Nocturnal frontal lobe epilepsy. Epilepsia 47, 83–86. doi: 10.1111/j.1528-1167.2006.00698.x
Ryvlin, P., So, E. L., Gordon, C. M., Hesdorffer, D. C., Sperling, M. R., Devinsky, O., et al. (2018). Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 59, 562–572. doi: 10.1111/epi.14002
Saaresranta, T., Polo-Kantola, P. I., Irjala, K., Helenius, H., and Polo, O. (1999). Respiratory insufficiency in postmenopausal women: sustained improvement of gas exchange with short-term medroxyprogesterone acetate. Chest 115, 1581–1587. doi: 10.1378/chest.115.6.1581
Sainju, R. K., Dragon, D. N., Winnike, H. B., Nashelsky, M. B., Granner, M. A., Gehlbach, B. K., et al. (2019). Ventilatory response to CO2 in patients with epilepsy. Epilepsia 60, 508–517. doi: 10.1111/epi.14660
Sainju, R. K., Dragon, D. N., Winnike, H. B., Ten Eyck, P., Granner, M. A., Gehlbach, B. K., et al. (2021). Hypercapnic ventilatory response in epilepsy patients treated with VNS: a case-control study. Epilepsia 62, e140–e146. doi: 10.1111/epi.16997
Sakai, K. (2011). Sleep-waking discharge profiles of dorsal raphe nucleus neurons in mice. Neuroscience 197, 200–224. doi: 10.1016/j.neuroscience.2011.09.024
Sakai, K., and Crochet, S. (2001). Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience 104, 1141–1155. doi: 10.1016/s0306-4522(01)00103-8
Sakurai, T. (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181. doi: 10.1038/nrn2092
Sanchez, R. E. A., Bussi, I. L., Ben-Hamo, M., Caldart, C. S., Catterall, W. A., and De La Iglesia, H. O. (2019). Circadian regulation of sleep in a pre-clinical model of dravet syndrome: dynamics of sleep stage and siesta re-entrainment. Sleep 42:zsz173. doi: 10.1093/sleep/zsz173
Santos, E. A. d. S., Marques, T. E. B. S., Matos, H. D. C., Leite, J. P., Garcia-Cairasco, N., Paçó-Larson, M. L., et al. (2015). Diurnal variation has effect on differential gene expression analysis in the hippocampus of the pilocarpine-induced model of mesial temporal lobe epilepsy. PLoS One 10:e0141121. doi: 10.1371/journal.pone.0141121
Saper, C. B., Scammell, T. E., and Lu, J. (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263. doi: 10.1038/nature04284
Scharf, M. T., Greenberg, P., Wong, S., and Mani, R. (2020). Obstructive sleep apnea risk in patients with focal versus generalized epilepsy. Epilepsy Behav. 111:107190. doi: 10.1016/j.yebeh.2020.107190
Seifert, E. L., Knowles, J., and Mortola, J. P. (2000). Continuous circadian measurements of ventilation in behaving adult rats. Respir. Physiol. 120, 179–183. doi: 10.1016/s0034-5687(00)00108-0
Seifert, E. L., and Mortola, J. P. (2002). The circadian pattern of breathing in conscious adult rats. Respir. Physiol. 129, 297–305. doi: 10.1016/s0034-5687(01)00316-4
Semmelroch, M., Elwes, R. D., Lozsadi, D. A., and Nashef, L. (2012). Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia 53, e21–e24. doi: 10.1111/j.1528-1167.2011.03296.x
Severson, C. A., Wang, W., Pieribone, V. A., Dohle, C. I., and Richerson, G. B. (2003). Midbrain serotonergic neurons are central pH chemoreceptors. Nat. Neurosci. 6, 1139–1140. doi: 10.1038/nn1130
Seyal, M., Bateman, L. M., Albertson, T. E., Lin, T.-C., and Li, C.-S. (2010). Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia 51, 1359–1364. doi: 10.1111/j.1528-1167.2009.02518.x
Seyal, M., Pascual, F., Lee, C. Y., Li, C. S., and Bateman, L. M. (2011). Seizure-related cardiac repolarization abnormalities are associated with ictal hypoxemia. Epilepsia 52, 2105–2111. doi: 10.1111/j.1528-1167.2011.03262.x
Shankar, R., Cox, D., Jalihal, V., Brown, S., Hanna, J., and Mclean, B. (2013). Sudden unexpected death in epilepsy (SUDEP): development of a safety checklist. Seizure 22, 812–817. doi: 10.1016/j.seizure.2013.07.014
Shen, H.-Y., Li, T., and Boison, D. (2010). A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia 51, 465–468. doi: 10.1111/j.1528-1167.2009.02248.x
Shouse, M. N. (1988). Sleep deprivation increases susceptibility to kindled and penicillin seizure events during all waking and sleep states in cats. Sleep 11, 162–171. doi: 10.1093/sleep/11.2.162
Shouse, M. N., and Sterman, M. B. (1982). Acute sleep deprivation reduces amygdala-kindled seizure thresholds in cats. Exp. Neurol. 78, 716–727. doi: 10.1016/0014-4886(82)90086-3
Siekierka, M., Tafil-Klawe, M., Adamczyk, W., Klawe, J. J., and Złomańczuk, P. (2007). Low amplitude daily changes in reflex ventilatory response to progressive isocapnic hypoxia. J. Physiol. Pharmacol. 58, 633–637.
Simeone, K. A., Hallgren, J., Bockman, C. S., Aggarwal, A., Kansal, V., Netzel, L., et al. (2018). Respiratory dysfunction progresses with age in kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia 59, 345–357. doi: 10.1111/epi.13971
Simonds, A. K. (1994). Sleep studies of respiratory function and home respiratory support. BMJ 309, 35–40. doi: 10.1136/bmj.309.6946.35
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W., and Feldman, J. L. (1991). Pre-bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. doi: 10.1126/science.1683005
Smith, H. R., Leibold, N. K., Rappoport, D. A., Ginapp, C. M., Purnell, B. S., Bode, N. M., et al. (2018). Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. J. Neurosci. 38, 1915–1925. doi: 10.1523/JNEUROSCI.2182-17.2018
Snyder, F., Hobson, J. A., Morrison, D. F., and Goldfrank, F. (1964). Changes in respiration, heart rate and systolic blood pressure in human sleep. J. Appl. Physiol. 19, 417–422. doi: 10.1152/jappl.1964.19.3.417
Somboon, T., Grigg-Damberger, M. M., and Foldvary-Schaefer, N. (2019). Epilepsy and sleep-related breathing disturbances. Chest 156, 172–181. doi: 10.1016/j.chest.2019.01.016
Sowers, L. P., Massey, C. A., Gehlbach, B. K., Granner, M. A., and Richerson, G. B. (2013). Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir. Physiol. Neurobiol. 189, 315–323. doi: 10.1016/j.resp.2013.05.010
Spengler, C. M., Czeisler, C. A., and Shea, S. A. (2000). An endogenous circadian rhythm of respiratory control in humans. J. Physiol. 526, 683–694. doi: 10.1111/j.1469-7793.2000.00683.x
Spengler, C. M., and Shea, S. A. (2000). Endogenous circadian rhythm of pulmonary function in healthy humans. Am. J. Respir. Crit. Care Med. 162, 1038–1046. doi: 10.1164/ajrccm.162.3.9911107
Sridhar, R., Thach, B. T., Kelly, D. H., and Henslee, J. A. (2003). Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr. Pulmonol. 36, 113–122. doi: 10.1002/ppul.10287
Stephenson, R., Liao, K. S., Hamrahi, H., and Horner, R. L. (2001). Circadian rhythms and sleep have additive effects on respiration in the rat. J. Physiol. 536, 225–235. doi: 10.1111/j.1469-7793.2001.00225.x
Stewart, M. (2018). An explanation for sudden death in epilepsy (SUDEP). J. Physiol. Sci. 68, 307–320. doi: 10.1007/s12576-018-0602-z
Stöllberger, C., and Finsterer, J. (2004). Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP). Epilepsy Res. 59, 51–60. doi: 10.1016/j.eplepsyres.2004.03.008
Stupfel, M., Damiani, P., Perramon, A., Busnel, M. C., Gourlet, V., and Thierry, H. (1985). Ultradian and circadian respiratory rhythms in grouped small laboratory vertebrate species as a method to assess the effects of environmental challenges. Comp. Biochem. Physiol. Comp. A Physiol. 80, 225–231. doi: 10.1016/0300-9629(85)90545-6
Stupfel, M., and Pletan, Y. (1983). Respiratory ultradian rhythms of mean and low frequencies: a comparative physiological approach. Chronobiologia 10, 283–292.
Surges, R., and Sander, J. W. (2012). Sudden unexpected death in epilepsy: mechanisms, prevalence and prevention. Curr. Opin. Neurol. 25, 201–207. doi: 10.1097/WCO.0b013e3283506714
Surges, R., Thijs, R. D., Tan, H. L., and Sander, J. W. (2009). Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat. Rev. Neurol. 5, 492–504. doi: 10.1038/nrneurol.2009.118
Sveinsson, O., Andersson, T., Carlsson, S., and Tomson, T. (2018). Circumstances of SUDEP: a nationwide population-based case series. Epilepsia 59, 1074–1082. doi: 10.1111/epi.14079
Sveinsson, O., Andersson, T., Mattsson, P., Carlsson, S., and Tomson, T. (2020). Clinical risk factors in SUDEP: a nationwide population-based case-control study. Neurology 94, e419–e429. doi: 10.1212/WNL.0000000000008741
Tanaka, T., and Naquet, R. (1975). Kindling effect and sleep organization in cats. Electroencephalogr. Clin. Neurophysiol. 39, 449–454. doi: 10.1016/0013-4694(75)90045-0
Tao, J. X., Qian, S., Baldwin, M., Chen, X. J., Rose, S., Ebersole, S. H., et al. (2010). SUDEP, suspected positional airway obstruction and hypoventilation in postictal coma. Epilepsia 51, 2344–2347. doi: 10.1111/j.1528-1167.2010.02719.x
Tao, J. X., Sandra, R., Wu, S., and Ebersole, J. S. (2015). Should the “back to sleep” campaign be advocated for SUDEP prevention? Epilepsy Behav. 45, 79–80. doi: 10.1016/j.yebeh.2015.02.020
Tennis, P., Cole, T. B., Annegers, J. F., Leestma, J. E., Mcnutt, M., and Rajput, A. (1995). Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia 36, 29–36. doi: 10.1111/j.1528-1157.1995.tb01661.x
Teran, F. A., Kim, Y., Crotts, M. S., Bravo, E., Emaus, K. J., Richerson, G. B. (2019). Time of day and a ketogenic diet influence susceptibility to SUDEP in Scn1aR1407X/+ mice. Front. Neurol. 10:278. doi: 10.3389/fneur.2019.00278
Tio, E., Culler, G. W., Bachman, E. M., and Schuele, S. (2020). Ictal central apneas in temporal lobe epilepsies. Epilepsy Behav. 112:107434. doi: 10.1016/j.yebeh.2020.107434
Tochikubo, O., Ikeda, A., Miyajima, E., and Ishii, M. (1996). Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 27, 1318–1324. doi: 10.1161/01.hyp.27.6.1318
Totola, L. T., Malheiros-Lima, M. R., Delfino-Pereira, P., Del Vecchio, F., Souza, F. C., Takakura, A. C., et al. (2019). Amygdala rapid kindling impairs breathing in response to chemoreflex activation. Brain Res. 1718, 159–168. doi: 10.1016/j.brainres.2019.05.015
Tupal, S., and Faingold, C. L. (2006). Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 47, 21–26. doi: 10.1111/j.1528-1167.2006.00365.x
Tupal, S., and Faingold, C. L. (2019). Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia 60, 485–494. doi: 10.1111/epi.14658
Tusiewicz, K., Moldofsky, H., Bryan, A. C., and Bryan, M. H. (1977). Mechanics of the rib cage and diaphragm during sleep. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 43, 600–602. doi: 10.1152/jappl.1977.43.4.600
Van Der Lende, M., Hesdorffer, D., Sander, J., and Thijs, R. (2018). Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology 91, e1508–e1518. doi: 10.1212/WNL.0000000000006356
Van Gompel, J. J., Bower, M. R., Worrell, G. A., Stead, M., Chang, S.-Y., Goerss, S. J., et al. (2014). Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia 55, 233–244. doi: 10.1111/epi.12511
Vaughn, B. V., and D’cruz, O. F. (2004). Sleep and epilepsy. Semin. Neurol. 24, 301–313. doi: 10.1055/s-2004-835068
Vendrame, M., Jackson, S., Syed, S., Kothare, S. V., and Auerbach, S. H. (2014). Central sleep apnea and complex sleep apnea in patients with epilepsy. Sleep Breath. 18, 119–124. doi: 10.1007/s11325-013-0858-8
Venit, E. L., Shepard, B. D., and Seyfried, T. N. (2004). Oxygenation prevents sudden death in seizure-prone mice. Epilepsia 45, 993–996. doi: 10.1111/j.0013-9580.2004.02304.x
Vilella, L., Lacuey, N., Hampson, J. P., Rani, M. R. S., Sainju, R. K., Friedman, D., et al. (2019). Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology 92, e171–e182. doi: 10.1212/WNL.0000000000006785
Wang, W., Pizzonia, J. H., and Richerson, G. B. (1998). Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J. Physiol. 511, 433–450. doi: 10.1111/j.1469-7793.1998.433bh.x
Wessberg, P., Hedner, J., Hedner, T., Persson, B., and Jonason, J. (1984). Adenosine mechanisms in the regulation of breathing in the rat. Eur. J. Pharmacol. 106, 59–67. doi: 10.1016/0014-2999(84)90678-2
Wu, H., Liu, Y., Liu, L., Meng, Q., Du, C., Li, K., et al. (2021). Decreased expression of the clock gene bmal1 is involved in the pathogenesis of temporal lobe epilepsy. Mol. Brain 14:113. doi: 10.1186/s13041-021-00824-4
Yoshida, Y., Fujiki, N., Nakajima, T., Ripley, B., Matsumura, H., Yoneda, H., et al. (2001). Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur. J. Neurosci. 14, 1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x
Young, T., Palta, M., Dempsey, J., Skatrud, J., Weber, S., and Badr, S. (1993). The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 328, 1230–1235. doi: 10.1056/NEJM199304293281704
Yuan, H., Pinto, S. J., Huang, J., Mcdonough, J. M., Ward, M. B., Lee, Y. N., et al. (2012). Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep 35, 1257–1267. doi: 10.5665/sleep.2082
Zambrelli, E., Saibene, A. M., Furia, F., Chiesa, V., Vignoli, A., Pipolo, C., et al. (2016). Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia 57, e24–e27. doi: 10.1111/epi.13252
Zeng, C., Long, X., Cotten, J. F., Forman, S. A., Solt, K., Faingold, C. L., et al. (2015). Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav. 45, 1–7. doi: 10.1016/j.yebeh.2015.02.013
Zhan, Q., Buchanan, G. F., Motelow, J. E., Andrews, J., Vitkovs.kiy, P., Chen, W. C., et al. (2016). Impaired serotonergic brainstem function during and after seizures. J. Neurosci. 36, 2711–2722. doi: 10.1523/JNEUROSCI.4331-15.2016
Zhang, R., Tan, Z., Niu, J., and Feng, H.-J. (2021). Adrenergic α2 receptors are implicated in seizure-induced respiratory arrest in DBA/1 mice. Life Sci. 284:119912. doi: 10.1016/j.lfs.2021.119912
Zhang, H., Zhao, H., and Feng, H. J. (2017). Atomoxetine, a norepinephrine reuptake inhibitor, reduces seizure-induced respiratory arrest. Epilepsy Behav. 73, 6–9. doi: 10.1016/j.yebeh.2017.04.046
Zhao, H., Cotten, J. F., Long, X., and Feng, H. J. (2017). The effect of atomoxetine, a selective norepinephrine reuptake inhibitor, on respiratory arrest and cardiorespiratory function in the DBA/1 mouse model of SUDEP. Epilepsy Res. 137, 139–144. doi: 10.1016/j.eplepsyres.2017.08.005
Keywords: epilepsy, SUDEP, sleep, circadian, breathing
Citation: Joyal KG, Kreitlow BL and Buchanan GF (2022) The role of sleep state and time of day in modulating breathing in epilepsy: implications for sudden unexpected death in epilepsy. Front. Neural Circuits 16:983211. doi: 10.3389/fncir.2022.983211
Received: 30 June 2022; Accepted: 25 July 2022;
Published: 23 August 2022.
Edited by:
Christina Gross, Cincinnati Children’s Hospital Medical Center, United StatesReviewed by:
Cameron S. Metcalf, The University of Utah, United StatesCopyright © 2022 Joyal, Kreitlow and Buchanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gordon F. Buchanan, R29yZG9uLWJ1Y2hhbmFuQHVpb3dhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.