- 1Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, United States
- 2Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States
- 3Chan Zuckerberg Biohub, San Francisco, CA, United States
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by altered social interaction and communication, and repetitive, restricted, inflexible behaviors. Approximately 1.5-2% of the general population meet the diagnostic criteria for ASD and several brain regions including the cortex, amygdala, cerebellum and basal ganglia have been implicated in ASD pathophysiology. The midbrain dopamine system is an important modulator of cellular and synaptic function in multiple ASD-implicated brain regions via anatomically and functionally distinct dopaminergic projections. The dopamine hypothesis of ASD postulates that dysregulation of dopaminergic projection pathways could contribute to the behavioral manifestations of ASD, including altered reward value of social stimuli, changes in sensorimotor processing, and motor stereotypies. In this review, we examine the support for the idea that cell-autonomous changes in dopaminergic function are a core component of ASD pathophysiology. We discuss the human literature supporting the involvement of altered dopamine signaling in ASD including genetic, brain imaging and pharmacologic studies. We then focus on genetic mouse models of syndromic neurodevelopmental disorders in which single gene mutations lead to increased risk for ASD. We highlight studies that have directly examined dopamine neuron number, morphology, physiology, or output in these models. Overall, we find considerable support for the idea that the dopamine system may be dysregulated in syndromic ASDs; however, there does not appear to be a consistent signature and some models show increased dopaminergic function, while others have deficient dopamine signaling. We conclude that dopamine dysregulation is common in syndromic forms of ASD but that the specific changes may be unique to each genetic disorder and may not account for the full spectrum of ASD-related manifestations.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder primarily characterized by deficits in social interactions, as well as repetitive, restricted, and inflexible behaviors (American Psychiatric Association, 2013). ASD is typically diagnosed early in life, although the clinical presentation and symptom severity can vary widely between individuals and across the lifespan. The latest CDC estimates suggest that 1 in 54 children are diagnosed with ASD by the age of 8, with ASD diagnosis being over four times more prevalent in boys than girls (Maenner et al., 2020).
The exact neurobiological basis of ASD remains unknown. This is in part due to a heterogenous behavioral presentation accompanied by widely divergent genetic, transcriptomic and epigenomic signatures found in individuals with ASD (De La Torre-Ubieta et al., 2016), although convergent molecular subtypes are being identified (Ramaswami et al., 2020). Studies in animal models and human subjects suggest that different brain regions and circuits contribute to distinct aspects of ASD symptomatology. Several brain regions implicated in ASD include the cortex (Donovan and Basson, 2017; Varghese et al., 2017), amygdala (Zalla and Sperduti, 2013; Donovan and Basson, 2017), cerebellum (Fatemi et al., 2012; Hampson and Blatt, 2015), and basal ganglia (Fuccillo, 2016; Subramanian et al., 2017). In particular, alterations in basal ganglia circuits may be a key driver of the restricted, repetitive behaviors in ASD (Fuccillo, 2016). Consistent with this, human neuroimaging studies have identified structural changes in the basal ganglia that correlate with repetitive behaviors (Asano et al., 2001). Increased striatal volume, the caudate nucleus in particular, has been identified in several studies (Hollander et al., 2005; Langen et al., 2009; Langen et al., 2014), while for other basal ganglia structures both volumetric decreases and increases have been reported (Estes et al., 2011; Schuetze et al., 2016). One study reported a decrease in ventral striatal volume in ASD subjects correlating with the severity of social deficits (Baribeau et al., 2019).
Dopaminergic projections originating from the midbrain exert wide-spread influence over multiple brain regions implicated in ASD pathophysiology including the basal ganglia, cortex and amygdala. Within these regions, dopamine (DA) serves as an important neuromodulator that fine-tunes cellular and synaptic function. Importantly, DA neurons and their projections are anatomically and functionally distinct. Dopaminergic input to the dorsal striatum originates primarily from the substantia nigra pars compacta (SNc; nigrostriatal pathway) and is fundamental to the context-appropriate, flexible selection of actions (Howard et al., 2017). In turn, ventral tegmental area (VTA) dopaminergic projections to the ventral striatum including the nucleus accumbens (mesolimbic pathway) are involved in reward processing (Watabe-Uchida et al., 2017), salience (De Jong et al., 2019), and motivation (Hamid et al., 2016; Mohebi et al., 2019). Cortical dopaminergic projections (mesocortical pathway) are sparser but provide important modulation of cognitive processes, including sensory gating and working memory (Ott and Nieder, 2019). Precisely controlled and appropriately timed release and clearance of DA at its targets is critical for adaptive behavior and learning. As discussed in other articles in this special issue, the dopaminergic system is highly heterogeneous. Recent gene profiling studies have identified several distinct sub-populations of DA neurons defined by their unique transcriptional signature (Poulin et al., 2014; La Manno et al., 2016; Hook et al., 2018; Kramer et al., 2018; Saunders et al., 2018; Tiklová et al., 2019; Poulin et al., 2020). Mouse models are being developed to allow intersectional genetic targeting of these newly defined dopaminergic populations to better understand their anatomical connectivity, functional properties and behavioral roles (Poulin et al., 2018; Kramer et al., 2021). Such approaches will enable a more fine-grained understanding of dopaminergic functions in both health and disease.
Given that DA is a key modulator of neuronal activity in several brain regions associated with ASD, there is compelling reason to ask whether dysfunctional DA signaling could impact brain activity across a range of ASD-implicated brain structures. The formally articulated dopamine hypothesis of ASD (Paval, 2017) postulates that functional dysregulation of DA projection pathways could contribute to the behavioral alterations that lead to ASD symptomology. For example, aberrant mesolimbic DA signaling could diminish the reward value assigned to social stimuli, eventually resulting in failure to acquire socioemotional reciprocity and/or communication skills. Alterations in mesocortical DA neurotransmission could translate to abnormal sensory processing and/or cognitive rigidity. Aberrant nigrostriatal DA signaling could promote stereotyped or repetitive motor movements and/or hyper-reliance on habitual behavioral control. Thus, the dopamine hypothesis of ASD as currently stated, predicts that either hyper- or hypo-dopaminergic signaling within target regions could lead to or exacerbate ASD-related behavioral changes (Paval, 2017). This is consistent with the idea of an “inverted U” shape to describe the optimal level of DA receptor signaling, wherein too much or too little DA are detrimental to cognitive functions (Arnsten, 1997; Cools and D’esposito, 2011). The DA hypothesis of ASD further predicts that aberrations at distinct projection targets could control discrete aspects of ASD symptomatology. Importantly, any changes in dopaminergic neurotransmission would likely lead to complex adaptations in downstream circuitry, involving pre- and post-synaptic alterations and plasticity.
In this review we will first summarize the evidence for dopaminergic involvement in ASD gleaned from human studies and then examine whether cell-autonomous changes in the DA system are commonly observed in animal models of ASD, with a focus on genetic mouse models. A particular question we aim to address is whether the wide diversity of genetic mouse models of ASD, with both constitutive (in which the mutation is present in all cells throughout development) and conditional mutations (in which the gene is altered in DA neurons only), show concordant changes in the function of midbrain DA neurons that may be causal for ASD-related behavioral changes. In other words, is there a consistent dopaminergic signature observable across different genetic causes of ASD?
For the purposes of this review, we will focus on the cell autonomous properties of DA neurons. Several important parameters determine the functionality of midbrain DA neurons at the level of the cell bodies, or somas, and axon terminals, typically examined within the striatum, which has the densest dopaminergic innervation. First, the number of DA neurons determines whether ASD-linked-mutations may cause DA neuron degeneration, or, in contrast, enhance the generation or survival of dopaminergic cells. Second, morphological properties such as cell size and dendritic architecture are examined to study structural aberrations that may impact the intrinsic excitability or connectivity of DA neurons. Third, changes in the firing properties of DA neurons can be assessed using electrophysiology or functional imaging techniques. Fourth, the levels of tyrosine hydroxylase (TH), the rate limiting enzyme in DA synthesis, determine overall DA production capacity and can be examined both in cell bodies and axon terminals by western blot or immunohistochemistry. DA production and storage determine the total tissue DA content in somas and axonal target regions, which is typically examined by high-performance liquid chromatography (HPLC). Finally, functional measures of basal (spontaneous) or evoked DA release by fast-scan cyclic voltammetry (sub-second timescale) or microdialysis (temporal resolution of minutes) are used to examine DA release and re-uptake in the midbrain somatodendritic region or axon terminal target regions.
Evidence for Dopaminergic Involvement in ASD
Brain Imaging and Pharmacological Interventions in Individuals With ASD
Neuroimaging studies in individuals with ASD support the potential involvement of altered DA signaling, especially in striatum and prefrontal cortex, in the behavioral manifestations of ASD. For example, PET imaging of fluorodopa accumulation revealed reduced presynaptic DA levels in the prefrontal cortex of children with ASD (Ernst et al., 1997). In turn, PET imaging of radioligand binding to the dopamine active transporter (DAT) showed increased binding in the orbitofrontal cortex of high-functioning adults with ASD (Nakamura et al., 2010); however, changes in dopaminergic function at rest have not been detected universally (Makkonen et al., 2008). During task performance, a reduction in phasic striatal DA events evoked by social stimuli in children and adolescents with ASD have been reported in several studies (Scott-Van Zeeland et al., 2010; Zürcher et al., 2020). Together, these data support the involvement of functional changes in dopaminergic signaling in ASD. Yet, differences in experimental methodology and the inherent heterogeneity among individuals with ASD, including differences between age groups, also lead to conflicting results across studies and warrant further investigation.
Pharmacological manipulations of DA neurotransmission are currently used to manage some of the symptoms of ASD. Roughly 80% of children with ASD suffer from irritability (Mayes et al., 2011). Several dopamine receptor D2 subtype antagonists, such as risperidone and aripiprazole, have been successfully used to improve irritability, aggression and tantrum behaviors in individuals with ASD (Mccracken et al., 2002; Owen et al., 2009; Aman et al., 2010). However, current guidelines recommend against long-term and wide-spread use of these medications due to significant side effects and modest evidence for clinical efficacy (Leclerc and Easley, 2015; Howes et al., 2018). Nonetheless, meta-analyses of placebo-controlled randomized clinical trials do find significant, albeit modest in size, improvement in irritability and aggression scores in children and adolescents with ASD following short-term (<6 months) risperidone or aripiprazole treatment (Sharma and Shaw, 2012; Hirsch and Pringsheim, 2016). Secondary outcome measures and post hoc analyses further show a significant decrease in stereotypy scores with aripiprazole in children with ASD (Aman et al., 2010; Marcus et al., 2011).
Together, functional neuroimaging and pharmacological interventions in human subjects support dopaminergic involvement in ASD. However, evidence from human studies for a role of DA in core autism symptoms of reduced sociability and restricted, repetitive behaviors is correlational. In contrast, rodent models in which manipulations of DA signaling are possible conclusively demonstrate causal DA involvement in sociability, stereotypies, and other ASD-relevant manifestations (Presti et al., 2003; Gunaydin et al., 2014; Lee et al., 2018).
Genetic Evidence for Dopaminergic Involvement in ASD
ASD is a complex polygenic disorder, with SFARI gene currently listing 1003 genes implicated in autism, with 418 genes classified as high confidence or strong candidate ASD risk genes (1 database accessed 04/22/2021, version 2020 Q4). Strong candidate genes fall into two main categories based on the current SFARI classification. Those assigned ‘score 1’ are high confidence candidates and can be found on the SPARK gene list or on the list of genes reported by Satterstrom et al. (2020). These genes generally have at least three de novo likely gene-disrupting mutations reported in the literature. Genes assigned ‘score 2’ are strong candidates with two likely gene-disrupting mutations reported, implicated by GWAS replicated across different cohorts and supported by functional evidence. Both of these categories are therefore strongly supported by currently available published literature to play a causal role in ASD. However, the large majority of ASD-associated genes fall into the ‘score 3’ category based on a single reported mutation and either unreplicated association studies or rare inherited mutations. These ‘score’ metrics allow for consistent evaluation of the strength of evidence across different genes and have been widely adopted by researchers.
Polymorphisms and mutations in genes encoding proteins that directly control DA neurotransmission have been implicated in ASD (Nguyen et al., 2014). These include the genes encoding the dopamine active transporter (“DAT,” SLC6A3) (Hamilton et al., 2013; Bowton et al., 2014; Campbell et al., 2019; Dicarlo et al., 2019), plasma membrane monoamine transporter (SLC29A4) (Adamsen et al., 2014), several dopamine receptors (DRD1, DRD2, DRD3, DRD4) (Hettinger et al., 2008; De Krom et al., 2009; Reiersen and Todorov, 2011; Staal et al., 2011, 2018; Hettinger et al., 2012), and genes encoding proteins important for dopamine synthesis (DDC) (Toma et al., 2012) and catabolism (MAO, COMT) (Cohen et al., 2003; Yoo et al., 2009, 2013; Cohen et al., 2011; Verma et al., 2014; Wassink et al., 2014). Only SLC6A3 is currently classified as a strong candidate ‘score 2’ gene, while others (SLC29A4, DRD1, DRD2 DRD3, DDC, MAO) fall into the ‘score 3’ category where causal involvement in ASD is supported only by suggestive evidence. Taken together, there is evidence that mutations in genes encoding key dopaminergic proteins may contribute to ASD risk, however, none of these currently rise to the top of the list of high confidence ASD-related genes.
Notably, there is overlap in the dopaminergic genes identified as altered in individuals with ASD with those found in individuals diagnosed with attention-deficit hyperactivity disorder, bipolar disorder, schizophrenia or obsessive-compulsive disorder (Khanzada et al., 2017). This suggests that while these disorders have distinct presentations, they may share some overlapping etiology, which could commonly involve aberrant DA signaling within basal ganglia and cortical circuits.
Dopaminergic Perturbations Can Cause ASD-Related Phenotypes in Mice
Several SLC6A3 mutations identified in individuals with ASD have been modeled in mice. In a mouse model with a rare Slc6a3 coding variant Val559, implicated in ASD in two unrelated male probands (Bowton et al., 2014), researchers observed increased basal striatal DA levels with in vivo microdialysis and a reduction in vertical motor behaviors such as rearing (Mergy et al., 2014). In a different model, with a threonine-to-methionine substitution at the 356 site in DAT (T356M) (Hamilton et al., 2013), researchers found reduced DA clearance rate concomitant with increased striatal DA metabolism and reduced DA synthesis (Dicarlo et al., 2019). Behaviorally, T356M mice showed hyperlocomotion, increased performance in the rotarod test and increased vertical rearing, resembling motor stereotypies. The mice also showed no preference for interacting with a novel mouse over a novel object, indicating reduced social approach behavior (Dicarlo et al., 2019). This study, in particular, demonstrates both construct and face validity of the Slc6a3 T356M mouse for studying ASD-relevant changes in dopaminergic neurotransmission in vivo.
In general, pathogenic SLC6A3 mutations, including those implicated in human ASD, are likely to increase the availability of extracellular DA due to reduced DAT function. This can happen either via impaired DA uptake and/or anomalous efflux leading to augmented DA signaling. In turn, increased DA signaling, either by reducing DAT expression in SNc neurons with siRNA or optogenetic stimulation of the nigrostriatal pathway, is sufficient to cause repetitive behaviors and sociability deficits in wild-type mice (Lee et al., 2018). Further, intra-striatal infusion of the D1 receptor antagonist SCH 23390 reduces naturally occurring motor stereotypies in a deer mouse model of ASD in the absence of general motor suppression (Presti et al., 2003). By contrast, in the ventral striatum, optogenetic suppression of the VTA-to-Nucleus Accumbens (NAc) (mesolimbic) pathway reduces sociability in mice, while activation increases social interactions via NAc D1 receptors (Gunaydin et al., 2014). Together, these studies suggest possible sub-region differences in DA dynamics contributing to distinct aspects of ASD-associated behaviors: hyperdopaminergia in the dorsal striatum could cause persistent and spontaneous stereotypies, while hypodopaminergia in the nucleus accumbens could lead to reduced sociability.
There are several unique features of the DA system that could support such distinct region-specific functional outcomes manifesting in ASD symptomatology. First, as a neuromodulator responsible for fine-tuning circuit function, either too much or too little DA signaling can cause aberrant information processing and behavior. The inverted U relationship between DA signaling and functional outcomes supports this interpretation (Cools and D’esposito, 2011). Second, the inherent heterogeneity of dopaminergic cell types based on projection targets, action potential properties and other parameters (Lammel et al., 2008), can support ‘independent’ functional outcomes within distinct brain regions through a combination of distinct cell intrinsic characteristics and local modulatory mechanisms (Sulzer et al., 2016). Third, the loop architecture of basal ganglia circuits could theoretically lend itself to either selective reinforcement or suppression of one dopaminergic domain by another (e.g., modulation of motor output by limbic domains) (Aoki et al., 2019; Lee et al., 2020). Finally, it should also be noted that compromised uptake or anomalous efflux of DA can augment dopaminergic catabolism thereby reducing DA recycling and increasing the burden of de novo DA synthesis, which over time could lead to reduced DA levels (Lee et al., 2018; Dicarlo et al., 2019). This, in turn, would compromise dopaminergic neurotransmission due to lack of neurotransmitter availability. Thus, an initial hyperdopaminergic state may over time evolve into deficient DA signaling. Consequently, it is possible that aberrations in DA neurotransmission in ASD are dynamic and evolve over time. In this respect, longitudinal studies conducted in humans with ASD and in mouse models would be helpful to our understanding of DA involvement in ASD pathophysiology across the lifespan.
Genetic Mouse Models of ASD
Without explicit biomarkers available for ASD and diagnostic criteria based on complex behaviors, ASD-related phenotypes in mouse models can only be approximated. Typically, studies assess ultrasonic vocalizations, social preference, social recognition and social interaction as a proxy for communication and sociability deficits, and motor learning and motor stereotypies as a proxy for repetitive, restrictive behaviors (Kazdoba et al., 2015). Tasks assessing cognitive performance, cognitive flexibility, sensory perception and learning have also been utilized. Yet, with these measurements of behavioral outcomes it is difficult to distinguish between mouse models of ASD and those modeling other neuropsychiatric conditions, which frequently present with overlapping behavioral phenotypes. Thus, while functional studies indeed provide evidence for ASD-related mutations in ‘dopaminergic’ genes causing changes in DA metabolism, release, re-uptake, or pre/post-synaptic signaling, the specificity of the reported behavioral phenotypes to ASD could be questioned. In other words, observing changes in the DA system with mutations that directly affect DA neurotransmission is expected, but do other genetic causes of ASD also alter DA signaling?
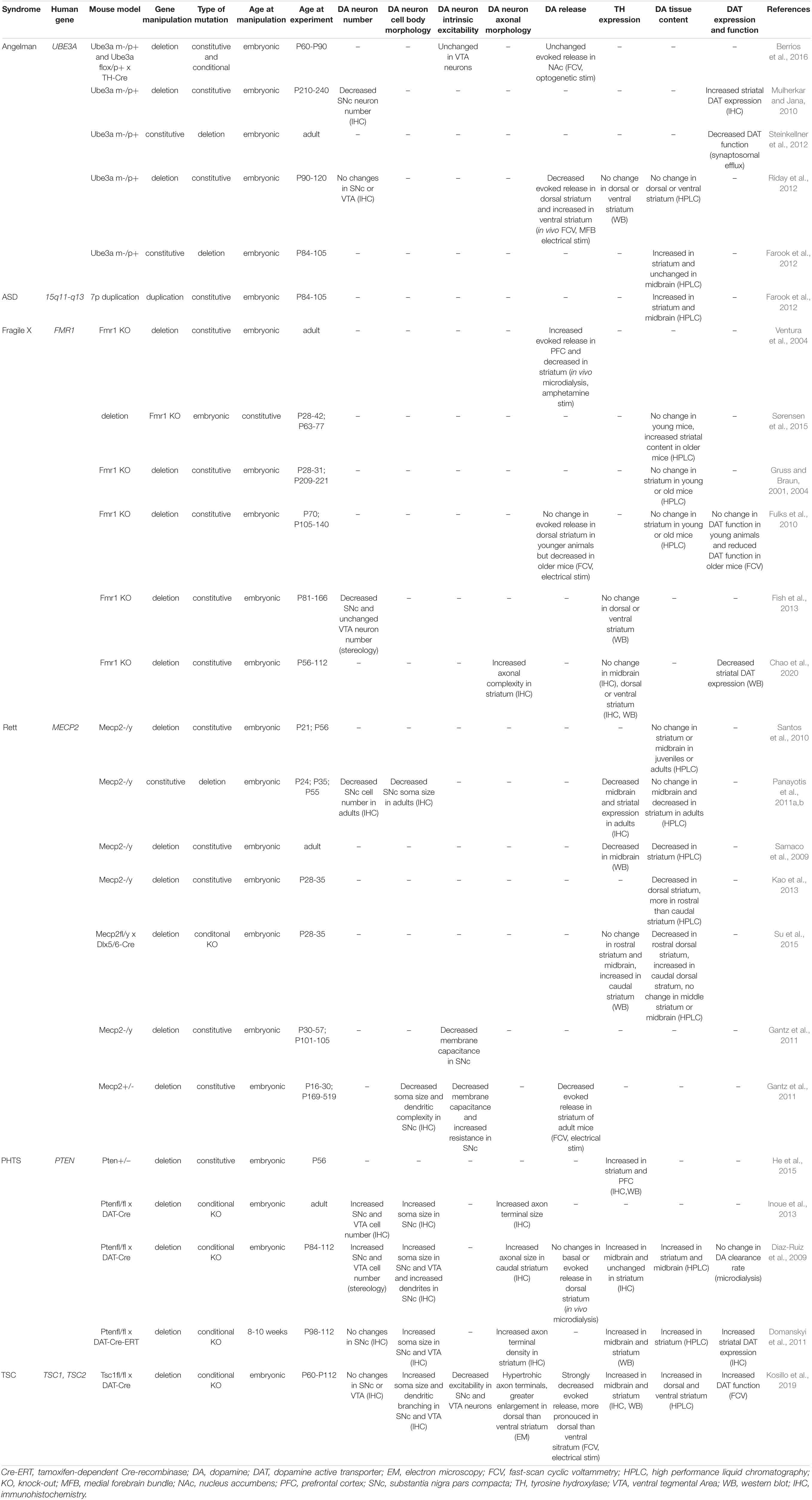
To address whether there is a ‘dopamine signature’ in ASD, it can be useful to examine mouse models with mutations in high confidence ASD-risk genes. Several of these genes are associated with syndromic neurodevelopmental disorders, which also frequently present with epilepsy, intellectual disability and other medical and behavioral conditions. Angelman syndrome, Fragile X syndrome, Rett syndrome, PTEN hamartoma tumor syndrome, and Tuberous Sclerosis Complex are some examples of syndromic neurodevelopmental disorders associated with high rates of ASD. Genetic mouse models of these neurodevelopmental disorders are well-established and show some ASD-relevant behavioral phenotypes. These can include reduced social interaction, impaired cognitive performance, and perseverative or repetitive behaviors. Here we summarize studies that have directly examined dopaminergic function in these models. Table 1 provides a summary of the mouse models discussed and the main findings related to DA neuron structure and function.
UBE3A, Angelman Syndrome and ASD
UBE3A encodes an E3-ubiquitin ligase, which is part of the ubiquitin proteasome pathway that adds ubiquitin molecules to proteins that are destined to be degraded. In neurons, UBE3A is expressed from the maternal allele due to imprinting (Rougeulle et al., 1997; Vu and Hoffman, 1997). Loss-of-function mutations in the maternal UBE3A copy or de novo deletions of chromosomal region 15q11-q13, in which UBE3A resides, cause the severe neurodevelopmental disorder Angelman syndrome (AS), characterized by abnormal motor development, lack of speech, seizures, and a happy demeanor (Kishino et al., 1997; Matsuura et al., 1997). In turn, duplications of UBE3A or the 15q11-q13 locus are strongly associated with ASD, although small deletions have also been reported in ASD probands (Nurmi et al., 2001; Glessner et al., 2009; Hogart et al., 2010). UBE3A is a high confidence ‘score 1’ autism gene with 22 possible genetic variants reported.
There are several indications that UBE3A mutations could cause perturbations to the dopaminergic system. First, Ube3a is highly expressed in midbrain DA neurons in mice (Gustin et al., 2010). Second, the ubiquitin-proteasome degradation pathway is critically important for DA neuron function with aberrations in this system linked to the pathogenesis of both familial and sporadic forms of Parkinson’s disease (Olanow and Mcnaught, 2006). Third, patients with AS present with movement or balance disorders, usually ataxia of gait and/or tremulous movement of limbs, and frequently have rigidity and bradykinesia (Clayton-Smith and Laan, 2003). Reminiscent of Parkinson’s disease, which is caused by the degeneration of DA neurons, case reports suggest that resting tremor and rigidity in AS patients can be alleviated with the drug levodopa, a precursor for DA (Harbord, 2001) (although see also Tan et al., 2017).
Multiple studies have examined the dopaminergic system in a mouse model of AS that lacks the maternal copy of Ube3a (Ube3am–/p+) (Jiang et al., 1998). An initial study reported that 7-8 month old male mice with loss of Ube3a had a reduced number of DA neurons in the SNc, but no change in striatal TH expression (Mulherkar and Jana, 2010). In contrast, using the same mouse model, another study in 3-4 month old male mice found no changes in midbrain DA neuron number or TH levels in dorsal or ventral striatum (Riday et al., 2012). Together, these data suggest a possible age-dependent decline in SNc DA neurons with loss-of-function of the maternal copy of Ube3a.
Electrophysiology recordings of VTA DA neurons in adult 2-3 month old Ube3am−/p+ mice showed no changes in their intrinsic excitability (Berrios et al., 2016); however, SNc neurons were not assessed. Several studies have measured DA release in Ube3am–/p+ mice, with mixed results. Using in vivo fast-scan cyclic voltammetry (FCV), researchers observed increased electrically evoked DA release in the ventral striatum, but decreased release in the dorsal striatum in 3-4 month old male animals (Riday et al., 2012). Based on these results, it is attractive to speculate that AS-associated phenotypes of motor difficulties and happy disposition are generally congruent with reduced DA release in dorsal striatum and enhanced DA release in ventral striatum reported for the Ube3am–/p+ mouse model (Riday et al., 2012). That said, a later study found no changes in optogenetically evoked DA release in the nucleus accumbens in 2-3 month-old Ube3am–/p+ mice or in mice with maternal deletion of Ube3a in TH+ neurons only (Berrios et al., 2016). Despite no changes in DA release, the authors did observe a large decrease in GABA co-release from TH+ VTA neurons with maternal deletion of Ube3a.
In terms of DA production, while the study by Riday et al. did not find any differences in DA levels in the striatum or NAc measured by HPLC, another study reported a different result. In this study the authors compared Ube3am–/p+ mice with mice harboring a 6.3 Mb duplication of the conserved linkage group on mouse chromosome 7, which is equivalent to human chromosome 15q11-q13 (Nakatani et al., 2009). Increased striatal DA levels were detected in animals with maternal deletion of Ube3a, and either paternal or maternal duplication of the 15q11-q13 locus (Farook et al., 2012). Further work will be needed to understand at a mechanistic level how both deletion and duplication of Ube3a could increase striatal DA tissue content.
The maximum rate of DA re-uptake back into the pre-synaptic terminal, which is a key factor that controls extracellular DA levels, is determined by DAT availability and its substrate affinity. Several lines of evidence suggest that Ube3a loss impacts DAT function. For example, superfusion experiments of synaptosomes showed significantly increased basal efflux of [3H]MPP+ in Ube3am–/p+ mice compared to wild-type (Steinkellner et al., 2012). [3H]MPP+ is taken up selectively by DAT, and hence enables assessment of DAT function. Increased basal efflux of [3H]MPP+ suggests that with maternal Ube3a loss, extracellular DA levels could be increased, consistent with the findings of Farook et al. In the same synaptosomal preparation, researchers observed impaired [3H]MPP+ efflux in response to amphetamine (Steinkellner et al., 2012), which augments extracellular DA by reverse transport. Together, these observations indicate that DAT function is altered in Ube3am–/p+ mice. This conclusion is further supported by the observation that Ube3am–/p+ mice have reduced behavioral sensitivity to cocaine (Riday et al., 2012), a drug that enhances extracellular DA availability by blocking DAT function.
Overall, research published to date suggests that maternal loss of Ube3a has a significant impact on several aspects of DA neuron function. Specifically, there is a possibility of an age-dependent decline in SNc DA neuron number, a reduction in evoked DA release in the dorsal striatum, altered striatal DA tissue content, and aberrant DAT function when Ube3a expression is disrupted. The augmented evoked DA release in the ventral striatum of Ube3am–/p+ mice may involve non-cell autonomous factors (Sulzer et al., 2016), as it was observed in vivo but was not replicated with cell type-specific optogenetic stimulation of DA release in slices. It would be interesting for future studies to assess whether restoration of dopaminergic function is sufficient to improve behavioral phenotypes in mice with altered Ube3a expression.
FMR1 and Fragile X Syndrome
Mutations in the X chromosome gene FMR1, which encodes the fragile X, which encodes the fragile X mental retardation protein (FMRP), cause Fragile X Syndrome (FXS), characterized by intellectual disability, physical features, and behavioral conditions. Up to ∼50% of male and ∼20% of female individuals with FXS are diagnosed with ASD (Bailey et al., 2008; Kaufmann et al., 2017). Currently, FXS is the leading monogenetic cause of ASD. FMRP has multiple functions but is most well known as an RNA binding protein that represses the translation of specific mRNAs (Darnell and Klann, 2013; Banerjee et al., 2018). The mRNAs bound by FMRP encode synaptic proteins as well as other proteins implicated in ASD risk (Darnell et al., 2011; Ascano et al., 2012). FMR1 mutations include a trinucleotide CGG repeat expansion in the 5’ untranslated region (UTR) (Farzin et al., 2006; Clifford et al., 2007; Tassone et al., 2012). The full mutation exceeding 200 repeats is thought to result in abnormal methylation, which effectively silences FMR1 expression (Pieretti et al., 1991; Primerano et al., 2002), while the premutation range of 55-200 CGG repeats typically causes increased FMR1 mRNA expression (Kenneson et al., 2001; Peprah et al., 2010). FMR1 is a high confidence ‘score 1’ autism gene with 35 possible genetic variants associated with ASD.
In terms of dopaminergic signaling, FMRP has been shown to be critical for the ability of D1-type DA receptors to exercise their neuromodulatory function in the prefrontal cortex (Wang et al., 2008; Wang et al., 2010; Paul et al., 2013). FMRP also serves as a mediator of cocaine-induced behavioral and synaptic plasticity in the NAc (Smith et al., 2014). Further, there are several lines of evidence suggesting that FMRP directly controls DA neuron function. First, FMRP is highly expressed in SNc neurons (Zorio et al., 2017). Second, there are several reports documenting the occurrence of Fragile X-associated tremor/ataxia syndrome (FXTAS) in adults over 50, particularly men, who carry an expanded FMR1 allele in the premutation range (55-200 CGG repeats) (Leehey et al., 2007; Hall et al., 2009; Hall et al., 2010). These individuals present with a progressive neurodegenerative disorder characterized by kinetic tremor, ataxia and parkinsonism, although they typically do not meet the diagnostic criteria for Parkinson’s disease. Notably, a recent report showed that FMRP protein is lost in the SNc of Parkinson’s disease patients, and is further down-regulated as a result of Parkinson’s-linked α-synuclein overexpression in cultured DA neurons and mouse brain (Tan et al., 2019). Together, these studies indicate that changes in dopaminergic function may occur when FMR1 expression is altered.
There are several animal models of FXS but the most widely used is the Fmr1–/y mouse, which has a disrupted coding sequence resulting in loss of FMRP protein expression (Bakker et al., 1994; Kazdoba et al., 2014). In 12-16-week-old adult male mice, researchers demonstrated a significant reduction in the number of DA neurons in the SNc but not the VTA of Fmr1–/y mice, with no overall change in TH levels in the dorsal or ventral striatum (Fish et al., 2013). The latter observation was replicated in a study showing normal TH expression in the striatum and midbrain in 8-16 week old Fmr1–/y mice (Chao et al., 2020). However, it was observed that TH-positive dopaminergic axons in the striatum appeared more branched (Chao et al., 2020). This may indicate a compensatory change, as experimentally induced death of SNc DA neurons leads to increased axonal sprouting of the remaining dopaminergic cells (Tanguay et al., 2021). Together, these studies indicate that nigrostriatal DA neurons are particularly sensitive to loss of FMRP, with the surviving SNc DA neurons undergoing dynamic remodeling to increase axonal arborization and potentially boost TH expression to augment dopaminergic signaling.
In terms of dopaminergic output, Ventura and colleagues showed using in vivo microdialysis that there were no differences in basal DA levels in Fmr1–/y mice in the prefrontal cortex but there was significantly reduced basal DA efflux within the striatum (Ventura et al., 2004). Reduced striatal DA transmission was also observed by FCV experiments in acute striatal slices, which showed that in Fmr1–/y mice, evoked DA release was significantly diminished at 15-20 weeks of age, but not at 10 weeks of age (Fulks et al., 2010). Together these studies suggest that nigrostriatal-projecting SNc neurons may have reduced output when FMRP expression is disrupted and that this change may be age-dependent.
Changes in DAT function have also been observed in mouse models of FXS. Functional assessment of striatal DA re-uptake by FCV revealed an age-dependent decrease in DAT activity in adult Fmr1–/y mice (Fulks et al., 2010). Another group verified decreased striatal expression of DAT in 8-12 week old Fmr1–/y mice with western blot (Chao et al., 2020). The observed changes in DA release and re-uptake are not likely due to altered DA availability as total striatal tissue DA content does not appear to be consistently altered either in juvenile (4-6 week old) or adult (10-30 week old) Fmr1–/y mice (Gruss and Braun, 2001, 2004; Fulks et al., 2010; Sørensen et al., 2015).
In summary, constitutive Fmr1 deletion causes DA neuron loss that is specific to SNc neurons. The remaining DA neurons upregulate their TH expression such that there is no overall change in total striatal tissue DA content. Nonetheless, there is a significant age-dependent decrease in evoked DA release in the striatum, accompanied by reduced DA re-uptake due to a reduction in DAT expression. Both downregulation of DAT and increased axonal sprouting are likely compensatory changes, which attempt to augment DA signaling by the remaining SNc DA neurons. Thus, loss of FMRP appears to reduce dopamine signaling within the striatum; however, dopaminergic cells projecting to other regions, such as the prefrontal cortex, may be differentially affected.
MECP2 and Rett Syndrome
Mutations in the MECP2 gene, located on the X chromosome, cause the severe neurodevelopmental disorder Rett syndrome (RTT) (Amir et al., 1999), which is characterized by progressive loss of motor and language functions, breathing problems, seizures, intellectual disability and autistic-like behaviors (Hagberg et al., 2002; Jeffrey et al., 2010). RTT affects 1/15,000 girls (Laurvick et al., 2006), although reports of male MECP2 mutation carries have emerged recently (Reichow et al., 2015; Chahil et al., 2018; Pitzianti et al., 2019). MeCP2 protein has several proposed functions including in RNA splicing control and transcriptional regulation via binding to methylated DNA (Nan et al., 1997; Nan et al., 1998; Chahrour et al., 2008; Ip et al., 2018; Qiu, 2018). Importantly, MECP2 mutations which both increase (e.g., duplication) and decrease (e.g., loss-of-function) its function are strongly implicated in neurodevelopmental disorders, including ASD and RTT (Carney et al., 2003; Samaco, 2004; Van Esch et al., 2005; Ramocki et al., 2010; Wang et al., 2016; Wen et al., 2017). There is significant variability in the clinical presentation of MECP2 mutation carriers, with some types of mutations conferring milder phenotypes (Bebbington et al., 2008; Neul et al., 2008). Overall, MECP2 mutations are strongly associated with ASD (Loat et al., 2008), and currently it is a high confidence ‘score 1’ autism gene with 180 possible genetic variants associated with ASD.
The importance of MECP2 for dopaminergic function is supported by observations of low levels of monoaminergic metabolites and monoamine content in the cerebrospinal fluid of RTT patients (Roux and Villard, 2009; Samaco et al., 2009). In girls with RTT syndrome there are also reports of increased D2 receptor density in the brain (Chiron et al., 1993) and reduced DAT expression in the caudate-putamen (Wenk, 2007), both of which indicate reduced DA neurotransmission. Furthermore, regressive changes in fine motor control are associated with hyperkinetic features in young RTT patients, followed by bradykinesia and postural aberrations in RTT individuals in their late 20s (Fitzgerald et al., 1990; Temudo et al., 2008); both phenotypes are suggestive of dopaminergic alterations. In mice, Mecp2 is expressed widely throughout the brain, including in the midbrain (Pelka et al., 2005). Mecp2–/y mice are a widely used mouse model with constitutive loss of Mecp2 (Guy et al., 2001). Similar to human RTT patients, Mecp2–/y mice show deterioration of movement coordination between 4 and 9 weeks of age (Panayotis et al., 2011b; Kao et al., 2013; Liao, 2019). In turn, preservation of MeCP2 function selectively in TH-Cre-expressing catecholaminergic neurons is sufficient to prevent motor disturbances and other phenotypes (Lang et al., 2013).
Starting at 5 weeks of age, Mecp2–/y mice present with a significant reduction in the number of SNc DA neurons and have reduced SNc neuron soma size (Panayotis et al., 2011b). Reductions in SNc neuron soma size and dendritic arborization have also been reported in female Mecp2+/– mice and notably, these changes can be observed in the pre-symptomatic stage at 3-4 weeks of age (Gantz et al., 2011). Consistent with SNc cell loss, decreased TH expression in the midbrain and striatum of Mecp2–/y mice has been observed in several studies (Samaco et al., 2009; Kao et al., 2013). In the midbrain, a significant decrease in the activated form of TH, which is phosphorylated at Ser40 (pSer40-TH), is first detectable at 5 weeks of age, and levels decline further by 8 weeks (Panayotis et al., 2011b). These studies point to a significant reduction in the DA synthesis capacity of the remaining SNc neurons in Mecp2–/y mice. Consistent with this conclusion, several studies report a substantial reduction in total tissue DA content in the striatum (Panayotis et al., 2011a, b; Kao et al., 2013), with some also finding a significant reduction in total tissue DA content in the ventral midbrain (Kao et al., 2013). Given that these changes can be observed prior to symptom onset, this suggests that changes in nigrostriatal DA may be causal for the behavioral changes in Mecp2–/y mice, particularly related to motor function (Liao, 2019). Yet not all studies replicate the reduction in total striatal DA content in this model. One report documents no alterations in DA or its primary metabolite DOPAC at 3 or 8 weeks of age in striatum or cortical regions, yet finds substantial and progressive decline in cerebellar DA content (Santos et al., 2010). The latter finding suggests possible cerebellar contribution to the motor phenotypes in RTT.
On balance, the evidence for reduced DA synthesis and content in mouse models of RTT is strong, and consistent with the finding that evoked DA release from SNc axon terminals in the dorsal striatum is decreased to just under 50% of control levels in Mecp2+/– female mice (Gantz et al., 2011). Impaired dopaminergic transmission in Mecp2+/– animals is also supported by findings of decreased D2 autoreceptor current density in SNc cell bodies (Gantz et al., 2011). Since D2 autoreceptors normally constrain DA synthesis and release, a reduction in the D2R-generated current suggests a potential homeostatic alteration whereby the D2-mediated suppression of DA release is reduced in an attempt to boost DA signaling. Therefore, in Mecp2+/– mice, DA transmission is compromised both at the axon terminals in the striatum and within the somatodendritic compartment in the midbrain. Consistent with this, augmentation of DA signaling with combined administration of levodopa and a Dopa-decarboxylase inhibitor is able to improve motor phenotypes and increase the life-span of Mecp2–/y mice (Szczesna et al., 2014).
The reduction in total tissue DA content in Mecp2+/– mice appears more pronounced in the rostral than caudal striatum (Kao et al., 2013). Emerging evidence suggests that there are regional specializations of striatal function and discrete computational circuits across the rostro-caudal axis, which in turn differentially control behavior (Hintiryan et al., 2016; Liao, 2019; Miyamoto et al., 2019). The observation of a rostral-to-caudal gradient of compromised DA synthesis was also found in Mecp2-conditional KO mice (Su et al., 2015) in which Mecp2 deletion was restricted to forebrain GABAergic neurons. In this mouse model, researchers found that the DA tissue content was reduced in rostral striatum and increased in caudal striatum, with a concurrent reduction in pSer40-TH in rostral striatum and increase in caudal striatum (Su et al., 2015). Hence, GABAergic neurons lacking Mecp2 appear to exert region-specific extrinsic control over local striatal DA production. Interestingly, conditional deletion of Mecp2 from DA neurons using TH-Cre mice (Lindeberg et al., 2004) also leads to reduced TH expression and DA tissue content (Samaco et al., 2009). Therefore, multiple cell types may be involved in altered DA transmission in the context of Mecp2 loss.
Overall, studies from mouse models show that disruption of Mecp2 expression leads to a progressive loss of SNc DA neurons and a reduction in the soma size and dendritic branching of the remaining neurons, which is first observed in juvenile animals. The remaining SNc DA neurons have a diminished DA synthesis capacity and show a substantial reduction in dopaminergic transmission at striatal axon terminals and in the somatodendritic region. Furthermore, both cell autonomous and non-cell autonomous mechanisms result in compromised DA synthesis with Mecp2 loss.
PTEN and PTEN Hamartoma Tumor Syndromes
PTEN encodes the phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (PTEN), which functions as a tumor suppressor by negatively regulating PI3K-AKT signaling (Maehama and Dixon, 1998; Stambolic et al., 1998). Loss-of-function mutations in PTEN cause a group of disorders known as PTEN hamartoma tumor syndrome (PHTS), which are characterized by benign tumors in peripheral organs and brain overgrowth (Hobert and Eng, 2009). PTEN mutations have been found in individuals with ASD and macrocephaly (head circumference >2 SD above the mean), with an estimated prevalence of 5-50% of PTEN mutation carriers meeting ASD diagnostic criteria (Butler et al., 2005; Buxbaum et al., 2007; Varga et al., 2009; Mcbride et al., 2010; Hansen-Kiss et al., 2017; Ciaccio et al., 2019). Several studies also find a strong association between PTEN mutations, including gene-disrupting de novo mutations, and ASD in individuals with typical brain/head growth (O’roak et al., 2012a, b; De Rubeis et al., 2014; Iossifov et al., 2015). Based on the evidence across multiple ASD patient cohorts, PTEN is classified as a high confidence ‘score 1’ autism gene with 129 possible genetic variants associated with ASD.
PTEN is involved in many processes relevant to brain development and neural circuit function via its regulation of PI3K-AKT and mechanistic target of rapamycin (mTOR) signaling (Van Diepen and Eickholt, 2008; Garcia-Junco-Clemente and Golshani, 2014; Skelton et al., 2019). By controlling cell cycle progression and cell migration/adhesion, PTEN is important for the proliferation and differentiation of neural progenitors (Groszer, 2001; Zhou and Parada, 2012; Tilot et al., 2015). In addition, PTEN controls neuronal size, dendritic morphology, and synaptic transmission (Fraser et al., 2008; Jurado et al., 2010; Sperow et al., 2012; Takeuchi et al., 2013). Specific to dopaminergic neurons, researchers have found elevated TH expression in the cortex and striatum of mice with a missense mutation in Pten (He et al., 2015). Additionally, cell type-specific deletion of Pten from DA neurons is sufficient to alter some aspects of social behavior (Clipperton-Allen and Page, 2014). Several studies demonstrated that loss of Pten protects dopaminergic cells from neurotoxic lesions and significantly enhances their survival (Diaz-Ruiz et al., 2009; Domanskyi et al., 2011). Furthermore, Pten suppression facilitates DA neuron integration in striatal grafts transplanted into a Parkinson’s disease mouse model (Zhang et al., 2012). Therefore, concurrent increases in AKT and mTOR signaling caused by Pten deletion enable increased TH expression and enhanced DA neuron survival under conditions of cell stress.
To investigate how cell autonomous loss of Pten affects the DA system, several mouse lines have been generated with either embryonic deletion of Pten from DA neurons using DAT-IRES-Cre mice or postnatal deletion using tamoxifen-inducible DAT-Cre mice. Pten deletion beginning at 8-10 weeks of age (Domanskyi et al., 2011) or embryonic Pten loss (Diaz-Ruiz et al., 2009) both resulted in a substantial increase in the soma size of SNc and VTA DA neurons and increased Th mRNA levels in the midbrain. Furthermore, both embryonic and adult Pten deletion caused increased TH protein expression in midbrain and striatum, with a concurrent increase in striatal DA tissue content (Diaz-Ruiz et al., 2009; Domanskyi et al., 2011). However, increased TH expression and striatal DA content had no effect on basal or evoked DA release or the rate of DA clearance by DAT examined with microdialysis (Diaz-Ruiz et al., 2009).
Some differences in the consequences of adult versus embryonic loss of Pten from DA neurons have been observed. For example, adult, but not embryonic, Pten deletion results in increased density of striatal DA axon terminals (Domanskyi et al., 2011). Mice with embryonic Pten deletion on the other hand, have an increased number of TH+ neurons in the SNc and VTA (Diaz-Ruiz et al., 2009; Inoue et al., 2013). This suggests that depending on the developmental timing, Pten loss promotes dopaminergic function either via increasing the numbers of DA neurons or enhancing axonal branching of DA neurons.
In summary, deletion of Pten leads to a substantial increase in DA neuron soma size in both the SNc and VTA and causes significant upregulation of TH expression and DA tissue content in the midbrain and striatum. This increase in DA synthesis and tissue content, however, does not appear to affect basal or evoked striatal DA release. Adult Pten deletion causes an increase in striatal DA axon density 6 weeks later, while embryonic loss does not appear to alter axonal arbors but increases the total number of TH+ neurons in the midbrain. Together these studies demonstrate that loss of Pten impacts several aspects of DA neuron structure and function, generally leading to DA neuron hypertrophy.
TSC1/2 and Tuberous Sclerosis Complex
TSC1 and TSC2 encode the proteins hamartin and tuberin, respectively. Hamartin and tuberin form a multimeric protein complex, which negatively regulates mTOR complex 1 (mTORC1) signaling (Tee et al., 2002; Huang and Manning, 2008). Loss-of-function mutations in either TSC1 or TSC2 cause Tuberous Sclerosis Complex (TSC), a neurodevelopmental disorder characterized by benign tumors in multiple organs, focal cortical malformations, epilepsy, and psychiatric and behavioral conditions (Krueger et al., 2013; De Vries et al., 2015; Henske et al., 2016). Between 25 and 50% of individuals with TSC are diagnosed with ASD, with greater prevalence in males (Smalley, 1998; Numis et al., 2011; Curatolo et al., 2015; De Vries et al., 2020). Importantly, mutations in TSC1 and TSC2 have also been reported in individuals with ASD independent of TSC (Schaaf et al., 2011; Esteban et al., 2012; O’roak et al., 2012a; Iossifov et al., 2015; Kalsner et al., 2018). Thus, both TSC1 and TSC2 are high confidence ‘score 1’ genes with 34 and 82 possible genetic variants, respectively, associated with ASD.
Several lines of evidence support the importance of mTORC1 signaling for DA neuron function. DA neurons have high metabolic demands to support their extensive axonal processes (Matsuda et al., 2009) and mTORC1 is a central regulator of cellular metabolism via its control of protein and lipid synthesis, mitochondrial biogenesis and autophagy (Saxton and Sabatini, 2017). Related to this, stimulation of mTORC1 signaling by expression of a constitutively active form of Rheb (caRheb), the small GTP-ase that directly promotes mTORC1 activity, protects SNc DA neurons from neurotoxin lesions (Kim et al., 2012). In turn, both acute inhibition of mTORC1 signaling with rapamycin (Hernandez et al., 2012) or 2 week-long mTOR deletion in adult VTA neurons (Liu et al., 2018) reduce evoked DA release in the dorsal and ventral striatum, respectively.
To examine the impact of constitutive mTORC1 activation on the dopaminergic system in the context of TSC, our group generated a DA neuron-specific Tsc1 KO mouse (Tsc1fl/fl; DAT-IRES-Cre). Following embryonic loss of Tsc1 and mTORC1 hyperactivation, no change in DA neuron cell number was observed (Kosillo et al., 2019). However, both SNc and VTA DA neurons had significantly increased soma size, as well as increased total length and number of dendritic arborizations (Kosillo et al., 2019). This morphological restructuring caused a significant reduction in DA neuron intrinsic excitability due to changes in passive membrane properties (Kosillo et al., 2019). TH protein expression in the striatum and midbrain and striatal DA tissue content were significantly increased (Kosillo et al., 2019). Despite increased DA synthesis and storage, evoked DA release assessed with FCV in striatal slices was severely compromised following Tsc1 loss, with more profound deficits in the dorsal striatum compared to ventral regions (Kosillo et al., 2019). Electron microscopy analysis of striatal axon terminals showed significant enlargement of TH+ axon profiles, consistent with somatodendritic hypertrophy, which was most pronounced in the dorsal striatum (Kosillo et al., 2019). Axonal enlargement, in turn, was associated with greater vesicle distance from the plasma membrane and reduced vesicle clustering, which together likely reduces the efficiency of vesicle recruitment to release sites. Thus, the more pronounced axon terminal restructuring in the dorsal striatum is consistent with the larger DA release deficits in the dorsal versus ventral striatum. Notably, loss of Tsc1 from DA neurons did not impact motor or social behaviors but selectively impaired behavioral flexibility in a reversal learning task.
Importantly, some of the phenotypes observed in the Kosillo et al. study were also found in a postnatal model in which mTORC1 hyperactivation was caused by transduction of caRheb into SNc DA neurons at 8 weeks of age (Kim et al., 2012). 4 weeks post-injection, constitutive mTORC1 activation caused a significant increase in DA neuron soma size in the SNc and an increase in striatal TH expression, TH+ axon number, and DA tissue content (Kim et al., 2012), similar to postnatal loss of Pten (Domanskyi et al., 2011).
Overall, Tsc1 loss leads to significant hypertrophy of SNc and VTA DA neurons at the level of soma, dendrites and axon terminals, while the total DA neuron number remains unchanged. Increased cell size, in turn, renders Tsc1 KO dopaminergic cells hypoexcitable. Tsc1 loss also results in significant upregulation of midbrain and striatal TH expression supporting elevated DA synthesis, with total striatal DA tissue content substantially increased. Despite this, evoked striatal DA release is compromised, at least in part due to aberrations in the architecture of axon terminals.
Discussion
Across the five syndromic forms of ASD discussed here, there is clear evidence for alterations in the dopaminergic system that can include changes in DA neuron number, morphology, excitability, DA synthesis, and release. However, there is no consistent pattern of changes across different models. Soma size and DA synthesis capacity are impacted in all five syndromic ASD models, yet these can be changed in opposite ways. Alterations in cell number, axonal and dendritic architecture, membrane excitability and DA release were observed in some of the models; however, these properties were not universally assessed. Overall, findings from both syndromic and non-syndromic ASD mouse models support dopaminergic dysregulation being part of the molecular and cellular signature of autism in the brain (Robinson and Gradinaru, 2018). However, changes in DA function are not universal and are unlikely to be sufficient to drive all aspects of ASD.
The molecular processes controlled by UBE3A, FMRP, MECP2, PTEN and TSC1/TSC2 are important for maintaining balanced protein production and degradation and DA neurons may be particularly sensitive to aberrations in proteostasis. The studies discussed above show that alterations in protein degradation (Ube3a), gene expression (Mecp2), RNA regulation (Fmr1) or protein synthesis (Pten, Tsc1) can impact DA neuron structure and function. The overarching theme from these models is that interference with protein degradation or RNA processing leads to DA neuron death specifically in the SNc, coupled with progressive impairments in motor function. Specifically, gait, fine and gross motor coordination and motor learning are significantly compromised in both Ube3a and Mecp2 mouse models (Jiang et al., 1998; Mulherkar and Jana, 2010; Panayotis et al., 2011b; Kao et al., 2013). Progressive motor decline is also seen in mice with Fmr1 CGG expansion in the pre-mutation range (Van Dam et al., 2005). While not a core diagnostic feature of ASD, motor deficits are common in individuals with ASD (Fournier et al., 2010; Bhat et al., 2011; Esposito et al., 2011), and may even contribute to difficulties with speech acquisition. In turn, chronic increases in cellular anabolic processes do not cause degeneration of DA neurons but are sufficient to impair cognitive flexibility (Tsc1) (Kosillo et al., 2019) or social approach (Pten) (Clipperton-Allen and Page, 2014), which are related to core ASD symptoms. Further, there are indications of reduced sociability (Chao et al., 2020) and social discrimination (Sørensen et al., 2015) in Fmr1 mouse models, while mice overexpressing Ube3a in the VTA exhibit sociability deficits that are precipitated by seizures (Krishnan et al., 2017). Together, these studies suggest that mutations in ASD-risk genes can alter dopaminergic function in a variety of ways, but that dopaminergic changes are not sufficient to recapitulate all aspects of ASD in a given mouse model.
Studies published to date have examined aspects of DA neuron function using a variety of metrics and approaches, and different experimental designs. Factors such as whether the gene disruption occurs embryonically or in adulthood, or constitutively or in a cell type-specific manner, could all contribute to differing results. Consequently, heterogeneity in methodology and outcome measures, such as whether DA release is measured by FCV or microdialysis, makes it challenging to make comparisons across different ASD models and studies. To formally address whether there are convergent changes in dopaminergic function in ASD, the optimal starting point would be assessment of the DA system in mice with a constitutive mutation, similar to patients, that show a wide range of ASD-related behavior phenotypes. Subsequently, the same mutation could be introduced selectively in DA neurons to define which dopaminergic signatures and behavioral phenotypes can be recapitulated with DA-specific manipulations.
Many of the findings discussed in this review come from in vitro and ex vivo assays that have provided fundamental insights into the state of the DA system in syndromic ASD mouse models. Recent developments in DA monitoring, including GRABDA and D-Light GPCR-based DA sensors (Labouesse et al., 2020; Patriarchi et al., 2020; Sun et al., 2020), coupled to spectral- and depth-resolved fiber photometry (Meng et al., 2018; Pisano et al., 2019), now grant unprecedented access to monitoring DA neurotransmission in vivo. The use of these newly developed tools to monitor DA dynamics in awake behaving animals, potentially across multiple brain regions simultaneously, will allow for comprehensive mapping of brain-wide DA neurotransmission dynamics and changes associated ASD. Furthermore, recently developed anatomical tools, which enable whole-brain pathway mapping via viral circuit tracing can be applied together with chemogenetic and optogenetic manipulations to aid in our understanding of ASD pathophysiology, as previously highlighted (Robinson and Gradinaru, 2018). Together, application of novel anatomical, circuit manipulating and DA monitoring tools will provide new insights into dopaminergic aberrations associated with ASD by connecting cellular and circuit-level aberrations with behavioral changes. This approach will facilitate the development of a data-driven theoretical frameworks on the role of DA in ASD pathogenesis.
In summary, as a neuromodulator, DA fulfills many distinct functions, and either too much or too little DA is likely to be disruptive to neural circuits with complex cascading effects. Some of these aspects may manifest early in development and over time exacerbate behavioral abnormalities culminating in ASD. In addition, dopaminergic changes associated with ASD are likely to be dynamic in nature, changing across the lifespan, and distinct between various brain regions and neural circuits. Consequently, further investigations are warranted to build a more complete picture of how DA dysregulation may contribute to ASD symptomology.
Author Contributions
PK drafted the initial manuscript. HB contributed to the review and editing. Both authors devised the topic and scope of the review.
Funding
This work was supported by the SFARI Research Grant #514428 and NIH R01NS105634 (to HB). HB and PK were supported by the NARSAD Young Investigator Grants from the Brain & Behavior Research Foundation (#25073 to HB and #27458 to PK). HB is a Chan Zuckerberg Biohub Investigator.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Adamsen, D., Ramaekers, V., Ho, H. T. B., Britschgi, C., Rüfenacht, V., Meili, D., et al. (2014). Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene. Mol. Autism 5:43. doi: 10.1186/2040-2392-5-43
Aman, M. G., Kasper, W., Manos, G., Mathew, S., Marcus, R., Owen, R., et al. (2010). Line-Item Analysis of the Aberrant Behavior Checklist: Results from Two Studies of Aripiprazole in the Treatment of Irritability Associated with Autistic Disorder. J. Child Adolesc. Psychopharmacol. 20, 415–422. doi: 10.1089/cap.2009.0120
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders : DSM-5, 5th Edn. Washington, DC: American Psychiatric Association.
Amir, R. E., Van Den Veyver, I. B., Wan, M., Tran, C. Q., Francke, U., and Zoghbi, H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188.
Aoki, S., Smith, J. B., Li, H., Yan, X., Igarashi, M., Coulon, P., et al. (2019). An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. eLife 8:e49995
Arnsten, A. F. (1997). Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 11, 151–162. doi: 10.1177/026988119701100208
Asano, E., Chugani, D. C., Muzik, O., Behen, M., Janisse, J., Rothermel, R., et al. (2001). Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology 57, 1269–1277. doi: 10.1212/wnl.57.7.1269
Ascano, M., Mukherjee, N., Bandaru, P., Miller, J. B., Nusbaum, J. D., Corcoran, D. L., et al. (2012). FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386. doi: 10.1038/nature11737
Bailey, D. B., Raspa, M., Olmsted, M., and Holiday, D. B. (2008). Co-occurring conditions associated withFMR1gene variations: Findings from a national parent survey. Am. J. Medical Genet. Part A 146A, 2060–2069. doi: 10.1002/ajmg.a.32439
Bakker, C., Verheij, C., Willemsen, R., Vanderhelm, R., Oerlemans, F., Vermey, M., et al. (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 78, 23–33.
Banerjee, A., Ifrim, M. F., Valdez, A. N., Raj, N., and Bassell, G. J. (2018). Aberrant RNA translation in fragile X syndrome: From FMRP mechanisms to emerging therapeutic strategies. Brain Res. 1693, 24–36. doi: 10.1016/j.brainres.2018.04.008
Baribeau, D. A., Dupuis, A., Paton, T. A., Hammill, C., Scherer, S. W., Schachar, R. J., et al. (2019). Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND Network. Transl. Psychiatry 9:72.
Bebbington, A., Anderson, A., Ravine, D., Fyfe, S., Pineda, M., De Klerk, N., et al. (2008). Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology 70, 868–875. doi: 10.1212/01.wnl.0000304752.50773.ec
Berrios, J., Stamatakis, A. M., Kantak, P. A., Mcelligott, Z. A., Judson, M. C., Aita, M., et al. (2016). Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nat. Communicat. 7:10702.
Bhat, A. N., Landa, R. J., and Galloway, J. C. (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 91, 1116–1129. doi: 10.2522/ptj.20100294
Bowton, E., Saunders, C., Reddy, I. A., Campbell, N. G., Hamilton, P. J., Henry, L. K., et al. (2014). SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Translat. Psychiatry 4, e464–e464.
Butler, M. G., Dasouki, M. J., Zhou, X. P., Talebizadeh, Z., Brown, M., Takahashi, T. N., et al. (2005). Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 42, 318–321. doi: 10.1136/jmg.2004.024646
Buxbaum, J. D., Cai, G., Chaste, P., Nygren, G., Goldsmith, J., Reichert, J., et al. (2007). Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 484–491.
Campbell, N. G., Shekar, A., Aguilar, J. I., Peng, D., Navratna, V., Yang, D., et al. (2019). Structural, functional, and behavioral insights of dopamine dysfunction revealed by a deletion in SLC6A3. Proc. Natl. Acad. Sci. 116, 3853–3862. doi: 10.1073/pnas.1816247116
Carney, R. M., Wolpert, C. M., Ravan, S. A., Shahbazian, M., Ashley-Koch, A., Cuccaro, M. L., et al. (2003). Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. 28, 205–211. doi: 10.1016/s0887-8994(02)00624-0
Chahil, G., Yelam, A., and Bollu, P. C. (2018). Rett Syndrome in Males: A Case Report and Review of Literature. Cureus 10:e3414.
Chahrour, M., Jung, S. Y., Shaw, C., Zhou, X., Wong, S. T., Qin, J., et al. (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229. doi: 10.1126/science.1153252
Chao, O. Y., Pathak, S. S., Zhang, H., Dunaway, N., Li, J.-S., Mattern, C., et al. (2020). Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol. Brain 13:111.
Chiron, C., Bulteau, C., Loc’h, C., Raynaud, C., Garreau, B., Syrota, A., et al. (1993). Dopaminergic D2 receptor SPECT imaging in Rett syndrome: increase of specific binding in striatum. J. Nucl. Med. 34, 1717–1721.
Ciaccio, C., Saletti, V., D’arrigo, S., Esposito, S., Alfei, E., Moroni, I., et al. (2019). Clinical spectrum of PTEN mutation in pediatric patients. A bicenter experience. Eur. J. Med. Genet. 62:103596. doi: 10.1016/j.ejmg.2018.12.001
Clayton-Smith, J., and Laan, L. (2003). Angelman syndrome: a review of the clinical and genetic aspects. J. Med. Genet. 40, 87–95. doi: 10.1136/jmg.40.2.87
Clifford, S., Dissanayake, C., Bui, Q. M., Huggins, R., Taylor, A. K., and Loesch, D. Z. (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J. Autism Dev. Disord. 37, 738–747. doi: 10.1007/s10803-006-0205-z
Clipperton-Allen, A. E., and Page, D. T. (2014). Pten haploinsufficient mice show broad brain overgrowth but selective impairments in autism-relevant behavioral tests. Hum. Mol. Genet. 23, 3490–3505. doi: 10.1093/hmg/ddu057
Cohen, I. L., Liu, X., Lewis, M. E. S., Chudley, A., Forster-Gibson, C., Gonzalez, M., et al. (2011). Autism severity is associated with child and maternal MAOA genotypes. Clin. Genet. 79, 355–362. doi: 10.1111/j.1399-0004.2010.01471.x
Cohen, I. L., Liu, X., Schutz, C., White, B. N., Jenkins, E. C., Brown, W. T., et al. (2003). Association of autism severity with a monoamine oxidase A functional polymorphism. Clin. Genet. 64, 190–197. doi: 10.1034/j.1399-0004.2003.00115.x
Cools, R., and D’esposito, M. (2011). Inverted-U–Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biol. Psychiatr. 69, e113–e125.
Curatolo, P., Moavero, R., and De Vries, P. J. (2015). Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 14, 733–745. doi: 10.1016/s1474-4422(15)00069-1
Darnell, J. C., and Klann, E. (2013). The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci. 16, 1530–1536. doi: 10.1038/nn.3379
Darnell, J. C., Van Driesche, S. J., Zhang, C., Hung, K. Y., Mele, A., Fraser, C. E., et al. (2011). FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell 146, 247–261.
De Jong, J. W., Afjei, S. A., Pollak Dorocic, I., Peck, J. R., Liu, C., Kim, C. K., et al. (2019). A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 101, 133–151e137.
De Krom, M., Staal, W. G., Ophoff, R. A., Hendriks, J., Buitelaar, J., Franke, B., et al. (2009). A Common Variant in DRD3 Receptor Is Associated with Autism Spectrum Disorder. Biol. Psychiat. 65, 625–630. doi: 10.1016/j.biopsych.2008.09.035
De La Torre-Ubieta, L., Won, H., Stein, J. L., and Geschwind, D. H. (2016). Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22, 345–361. doi: 10.1038/nm.4071
De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., Cicek, A. E., et al. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215.
De Vries, P. J., Belousova, E., Benedik, M. P., Carter, T., Cottin, V., Curatolo, P., et al. (2020). Tuberous Sclerosis Complex-Associated Neuropsychiatric Disorders (TAND): New Findings on Age, Sex, and Genotype in Relation to Intellectual Phenotype. Front. Neurol. 11:603. doi: 10.3389/fneur.2020.00603
De Vries, P. J., Whittemore, V. H., Leclezio, L., Byars, A. W., Dunn, D., Ess, K. C., et al. (2015). Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr. Neurol. 52, 25–35.
Diaz-Ruiz, O., Zapata, A., Shan, L., Zhang, Y., Tomac, A. C., Malik, N., et al. (2009). Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PLoS One 4:e7027. doi: 10.1371/journal.pone.0007027
Dicarlo, G. E., Aguilar, J. I., Matthies, H. J. G., Harrison, F. E., Bundschuh, K. E., West, A., et al. (2019). Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviors. J. Clin. Investigat. 129, 3407–3419. doi: 10.1172/jci127411
Domanskyi, A., Geissler, C., Vinnikov, I. A., Alter, H., Schober, A., Vogt, M. A., et al. (2011). Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB J. 25, 2898–2910. doi: 10.1096/fj.11-181958
Donovan, A. P., and Basson, M. A. (2017). The neuroanatomy of autism - a developmental perspective. J. Anat. 230, 4–15. doi: 10.1111/joa.12542
Ernst, M., Zametkin, A. J., Matochik, J. A., Pascualvaca, D., and Cohen, R. M. (1997). Low medial prefrontal dopaminergic activity in autistic children. Lancet 350:638. doi: 10.1016/s0140-6736(05)63326-0
Esposito, G., Venuti, P., Apicella, F., and Muratori, F. (2011). Analysis of unsupported gait in toddlers with autism. Brain Dev. 33, 367–373. doi: 10.1016/j.braindev.2010.07.006
Esteban, F. J., Kelleher, R. J. III, Geigenmüller, U., Hovhannisyan, H., Trautman, E., Pinard, R., et al. (2012). High-Throughput Sequencing of mGluR Signaling Pathway Genes Reveals Enrichment of Rare Variants in Autism. PLoS One 7:e35003. doi: 10.1371/journal.pone.0035003
Estes, A., Shaw, D. W., Sparks, B. F., Friedman, S., Giedd, J. N., Dawson, G., et al. (2011). Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res. 4, 212–220. doi: 10.1002/aur.193
Farook, M. F., Decuypere, M., Hyland, K., Takumi, T., Ledoux, M. S., and Reiter, L. T. (2012). Altered Serotonin, Dopamine and Norepinepherine Levels in 15q Duplication and Angelman Syndrome Mouse Models. PLoS One 7:e43030. doi: 10.1371/journal.pone.0043030
Farzin, F., Perry, H., Hessl, D., Loesch, D., Cohen, J., Bacalman, S., et al. (2006). Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J. Dev. Behav. Pediatr. 27, S137–S144.
Fatemi, S. H., Aldinger, K. A., Ashwood, P., Bauman, M. L., Blaha, C. D., Blatt, G. J., et al. (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11, 777–807. doi: 10.1007/s12311-012-0355-9
Fish, E. W., Krouse, M. C., Stringfield, S. J., Diberto, J. F., Robinson, J. E., and Malanga, C. J. (2013). Changes in sensitivity of reward and motor behavior to dopaminergic, glutamatergic, and cholinergic drugs in a mouse model of fragile X syndrome. PLoS One 8:e77896. doi: 10.1371/journal.pone.0077896
Fitzgerald, P. M., Jankovic, J., and Percy, A. K. (1990). Rett syndrome and associated movement disorders. Movem. Disord. 5, 195–202. doi: 10.1002/mds.870050303
Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N., and Cauraugh, J. H. (2010). Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J. Autism Dev. Disord. 40, 1227–1240. doi: 10.1007/s10803-010-0981-3
Fraser, M. M., Bayazitov, I. T., Zakharenko, S. S., and Baker, S. J. (2008). Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience 151, 476–488. doi: 10.1016/j.neuroscience.2007.10.048
Fuccillo, M. V. (2016). Striatal Circuits as a Common Node for Autism Pathophysiology. Front. Neurosci. 10:27. doi: 10.3389/fnins.2016.00027
Fulks, J. L., O’bryhim, B. E., Wenzel, S. K., Fowler, S. C., Vorontsova, E., Pinkston, J. W., et al. (2010). Dopamine Release and Uptake Impairments and Behavioral Alterations Observed in Mice that Model Fragile X Mental Retardation Syndrome. ACS Chem. Neurosci. 1, 679–690. doi: 10.1021/cn100032f
Gantz, S. C., Ford, C. P., Neve, K. A., and Williams, J. T. (2011). Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J. Neurosci. 31, 12629–12637. doi: 10.1523/jneurosci.0684-11.2011
Garcia-Junco-Clemente, P., and Golshani, P. (2014). PTEN: A master regulator of neuronal structure, function, and plasticity. Communicat. Integrat. Biol. 7:e28358. doi: 10.4161/cib.28358
Glessner, J. T., Wang, K., Cai, G., Korvatska, O., Kim, C. E., Wood, S., et al. (2009). Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459, 569–573.
Groszer, M. (2001). Negative Regulation of Neural Stem/Progenitor Cell Proliferation by the Pten Tumor Suppressor Gene in Vivo. Science 294, 2186–2189. doi: 10.1126/science.1065518
Gruss, M., and Braun, K. (2001). Alterations of Amino Acids and Monoamine Metabolism in Male Fmr1 Knockout Mice: A Putative Animal Model of the Human Fragile X Mental Retardation Syndrome. Neural Plasticity 8, 285–298. doi: 10.1155/np.2001.285
Gruss, M., and Braun, K. (2004). Age- and region-specific imbalances of basal amino acids and monoamine metabolism in limbic regions of female Fmr1 knock-out mice. Neurochem. Int. 45, 81–88. doi: 10.1016/j.neuint.2003.12.001
Gunaydin, L. A., Grosenick, L., Finkelstein, J. C., Kauvar, I. V., Fenno, L. E., Adhikari, A., et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. doi: 10.1016/j.cell.2014.05.017
Gustin, R. M., Bichell, T. J., Bubser, M., Daily, J., Filonova, I., Mrelashvili, D., et al. (2010). Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol. Dis. 39, 283–291. doi: 10.1016/j.nbd.2010.04.012
Guy, J., Hendrich, B., Holmes, M., Martin, J. E., and Bird, A. (2001). A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326. doi: 10.1038/85899
Hagberg, B., Hanefeld, F., Percy, A., and Skjeldal, O. L. A. (2002). An update on clinically applicable diagnostic criteria in Rett syndrome. Eur. J. Paediatr. Neurol. 6, 293–297. doi: 10.1053/ejpn.2002.0612
Hall, D. A., Howard, K., Hagerman, R., and Leehey, M. A. (2009). Parkinsonism in FMR1 premutation carriers may be indistinguishable from Parkinson disease. Parkinson. Related Disord. 15, 156–159. doi: 10.1016/j.parkreldis.2008.04.037
Hall, D., Pickler, L., Riley, K., Tassone, F., and Hagerman, R. (2010). Parkinsonism and cognitive decline in a fragile X mosaic male. Movem. Disord. 25, 1523–1524. doi: 10.1002/mds.23150
Hamid, A. A., Pettibone, J. R., Mabrouk, O. S., Hetrick, V. L., Schmidt, R., Vander Weele, C. M., et al. (2016). Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126. doi: 10.1038/nn.4173
Hamilton, P. J., Campbell, N. G., Sharma, S., Erreger, K., Herborg Hansen, F., Saunders, C., et al. (2013). De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol. Psychiatry 18, 1315–1323. doi: 10.1038/mp.2013.102
Hampson, D. R., and Blatt, G. J. (2015). Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. 9:420. doi: 10.3389/fnins.2015.00420
Hansen-Kiss, E., Beinkampen, S., Adler, B., Frazier, T., Prior, T., Erdman, S., et al. (2017). A retrospective chart review of the features of PTEN hamartoma tumour syndrome in children. J. Med. Genet. 54, 471–478. doi: 10.1136/jmedgenet-2016-104484
Harbord, M. (2001). Levodopa responsive Parkinsonism in adults with Angelman Syndrome. J. Clin. Neurosci. 8, 421–422. doi: 10.1054/jocn.2000.0753
He, X., Thacker, S., Romigh, T., Yu, Q., Frazier, T. W. Jr., and Eng, C. (2015). Cytoplasm-predominant Pten associates with increased region-specific brain tyrosine hydroxylase and dopamine D2 receptors in mouse model with autistic traits. Mol. Autism 6:63.
Henske, E. P., Jóźwiak, S., Kingswood, J. C., Sampson, J. R., and Thiele, E. A. (2016). Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2:16035.
Hernandez, D., Torres, C. A., Setlik, W., Cebrián, C., Mosharov, E. V., Tang, G., et al. (2012). Regulation of Presynaptic Neurotransmission by Macroautophagy. Neuron 74, 277–284. doi: 10.1016/j.neuron.2012.02.020
Hettinger, J. A., Liu, X., Hudson, M. L., Lee, A., Cohen, I. L., Michaelis, R. C., et al. (2012). DRD2 and PPP1R1B (DARPP-32) polymorphisms independently confer increased risk for autism spectrum disorders and additively predict affected status in male-only affected sib-pair families. Behav. Brain Funct. 8:19. doi: 10.1186/1744-9081-8-19
Hettinger, J. A., Liu, X., Schwartz, C. E., Michaelis, R. C., and Holden, J. J. A. (2008). A DRD1 haplotype is associated with risk for autism spectrum disorders in male−only affected sib−pair families. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 147B, 628–636. doi: 10.1002/ajmg.b.30655
Hintiryan, H., Foster, N. N., Bowman, I., Bay, M., Song, M. Y., Gou, L., et al. (2016). The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114. doi: 10.1038/nn.4332
Hirsch, L. E., and Pringsheim, T. (2016). Aripiprazole for autism spectrum disorders (ASD). Cochrane Database Systemat. Rev. 2016:CD009043.
Hobert, J. A., and Eng, C. (2009). PTEN hamartoma tumor syndrome: An overview. Genet. Med. 11, 687–694. doi: 10.1097/gim.0b013e3181ac9aea
Hogart, A., Wu, D., Lasalle, J. M., and Schanen, N. C. (2010). The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol. Dis. 38, 181–191. doi: 10.1016/j.nbd.2008.08.011
Hollander, E., Anagnostou, E., Chaplin, W., Esposito, K., Haznedar, M. M., Licalzi, E., et al. (2005). Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol. Psychiat. 58, 226–232. doi: 10.1016/j.biopsych.2005.03.040
Hook, P. W., Mcclymont, S. A., Cannon, G. H., Law, W. D., Morton, A. J., Goff, L. A., et al. (2018). Single-Cell RNA-Seq of Mouse Dopaminergic Neurons Informs Candidate Gene Selection for Sporadic Parkinson Disease. Am. J. Hum. Genet. 102, 427–446. doi: 10.1016/j.ajhg.2018.02.001
Howard, C. D., Li, H., Geddes, C. E., and Jin, X. (2017). Dynamic Nigrostriatal Dopamine Biases Action Selection. Neuron 93, 1436–1450e1438.
Howes, O. D., Rogdaki, M., Findon, J. L., Wichers, R. H., Charman, T., King, B. H., et al. (2018). Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 32, 3–29. doi: 10.1177/0269881117741766
Huang, J., and Manning, B. D. (2008). The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412, 179–190. doi: 10.1042/bj20080281
Inoue, K., Rispoli, J., Yang, L., Macleod, D., Beal, M. F., Klann, E., et al. (2013). Coordinate regulation of mature dopaminergic axon morphology by macroautophagy and the PTEN signaling pathway. PLoS Genet. 9:e1003845. doi: 10.1371/journal.pgen.1003845
Iossifov, I., Levy, D., Allen, J., Ye, K., Ronemus, M., Lee, Y. H., et al. (2015). Low load for disruptive mutations in autism genes and their biased transmission. Proc. Natl. Acad. Sci. U S A. 112, E5600–E5607.
Ip, J. P. K., Mellios, N., and Sur, M. (2018). Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 19, 368–382. doi: 10.1038/s41583-018-0006-3
Jeffrey, L. N., Kaufmann, W. E., Glaze, D. G., Christodoulou, J., Clarke, A. J., Bahi-Buisson, N., et al. (2010). Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 68, 944–950. doi: 10.1002/ana.22124
Jiang, Y.-H., Armstrong, D., Albrecht, U., Atkins, C. M., Noebels, J. L., Eichele, G., et al. (1998). Mutation of the Angelman Ubiquitin Ligase in Mice Causes Increased Cytoplasmic p53 and Deficits of Contextual Learning and Long-Term Potentiation. Neuron 21, 799–811. doi: 10.1016/s0896-6273(00)80596-6
Jurado, S., Benoist, M., Lario, A., Knafo, S., Petrok, C. N., and Esteban, J. A. (2010). PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 29, 2827–2840. doi: 10.1038/emboj.2010.160
Kalsner, L., Twachtman-Bassett, J., Tokarski, K., Stanley, C., Dumont-Mathieu, T., Cotney, J., et al. (2018). Genetic testing including targeted gene panel in a diverse clinical population of children with autism spectrum disorder: Findings and implications. Mol. Genet. Genomic Med. 6, 171–185. doi: 10.1002/mgg3.354
Kao, F.-C., Su, S.-H., Carlson, G. C., and Liao, W. (2013). MeCP2-mediated alterations of striatal features accompany psychomotor deficits in a mouse model of Rett syndrome. Brain Struct. Funct. 220, 419–434. doi: 10.1007/s00429-013-0664-x
Kaufmann, W. E., Kidd, S. A., Andrews, H. F., Budimirovic, D. B., Esler, A., Haas-Givler, B., et al. (2017). Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 139, S194–S206.
Kazdoba, T. M., Leach, P. T., and Crawley, J. N. (2015). Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 15, 7–26. doi: 10.1111/gbb.12256
Kazdoba, T. M., Leach, P. T., Silverman, J. L., and Crawley, J. N. (2014). Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res. 3, 118–133. doi: 10.5582/irdr.2014.01024
Kenneson, A., Zhang, F., Hagedorn, C. H., and Warren, S. T. (2001). Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 10, 1449–1454. doi: 10.1093/hmg/10.14.1449
Khanzada, N. S., Butler, M. G., and Manzardo, A. M. (2017). GeneAnalytics Pathway Analysis and Genetic Overlap among Autism Spectrum Disorder, Bipolar Disorder and Schizophrenia. Int. J. Mol. Sci. 18:527. doi: 10.3390/ijms18030527
Kim, S. R., Kareva, T., Yarygina, O., Kholodilov, N., and Burke, R. E. (2012). AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol. Ther. 20, 275–286. doi: 10.1038/mt.2011.213
Kishino, T., Lalande, M., and Wagstaff, J. (1997). UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 15, 70–73. doi: 10.1038/ng0197-70
Kosillo, P., Doig, N. M., Ahmed, K. M., Agopyan-Miu, A., Wong, C. D., Conyers, L., et al. (2019). Tsc1-mTORC1 signaling controls striatal dopamine release and cognitive flexibility. Nat. Commun. 10:5426.
Kramer, D. J., Aisenberg, E. E., Kosillo, P., Friedmann, D., Stafford, D. A., Lee, A. Y.-F., et al. (2021). Generation of a DAT-P2A-Flpo mouse line for intersectional genetic targeting of dopamine neuron subpopulations. Cell Rep. 35:109123. doi: 10.1016/j.celrep.2021.109123
Kramer, D. J., Risso, D., Kosillo, P., Ngai, J., and Bateup, H. S. (2018). Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability. eNeuro 5, ENEURO.152–ENEURO.118.
Krishnan, V., Stoppel, D. C., Nong, Y., Johnson, M. A., Nadler, M. J. S., Ozkaynak, E., et al. (2017). Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 543, 507–512. doi: 10.1038/nature21678
Krueger, D. A., Northrup, H., and International Tuberous, Sclerosis Complex, and Consensus, G. (2013). Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 49, 255–265.
La Manno, G., Gyllborg, D., Codeluppi, S., Nishimura, K., Salto, C., Zeisel, A., et al. (2016). Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 167, 566.e–580.e.
Labouesse, M. A., Cola, R. B., and Patriarchi, T. (2020). GPCR-Based Dopamine Sensors—A Detailed Guide to Inform Sensor Choice for In Vivo Imaging. Int. J. Mol. Sci. 21:8048. doi: 10.3390/ijms21218048
Lammel, S., Hetzel, A., Häckel, O., Jones, I., Liss, B., and Roeper, J. (2008). Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System. Neuron 57, 760–773. doi: 10.1016/j.neuron.2008.01.022
Lang, M., Wither, R. G., Brotchie, J. M., Wu, C., Zhang, L., and Eubanks, J. H. (2013). Selective preservation of MeCP2 in catecholaminergic cells is sufficient to improve the behavioral phenotype of male and female Mecp2-deficient mice. Hum. Mol. Genet. 22, 358–371. doi: 10.1093/hmg/dds433
Langen, M., Bos, D., Noordermeer, S. D., Nederveen, H., Van Engeland, H., and Durston, S. (2014). Changes in the development of striatum are involved in repetitive behavior in autism. Biol. Psychiatry 76, 405–411. doi: 10.1016/j.biopsych.2013.08.013
Langen, M., Schnack, H. G., Nederveen, H., Bos, D., Lahuis, B. E., De Jonge, M. V., et al. (2009). Changes in the developmental trajectories of striatum in autism. Biol. Psychiatry 66, 327–333. doi: 10.1016/j.biopsych.2009.03.017
Laurvick, C. L., De Klerk, N., Bower, C., Christodoulou, J., Ravine, D., Ellaway, C., et al. (2006). Rett syndrome in Australia: a review of the epidemiology. J. Pediatr. 148, 347–352.
Leclerc, S., and Easley, D. (2015). Pharmacological therapies for autism spectrum disorder: a review. P T 40, 389–397.
Lee, J., Wang, W., and Sabatini, B. L. (2020). Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nat. Neurosci. 23, 1388–1398. doi: 10.1038/s41593-020-00712-5
Lee, Y., Kim, H., Kim, J. E., Park, J. Y., Choi, J., Lee, J. E., et al. (2018). Excessive D1 Dopamine Receptor Activation in the Dorsal Striatum Promotes Autistic-Like Behaviors. Mol. Neurobiol. 55, 5658–5671. doi: 10.1007/s12035-017-0770-5
Leehey, M. A., Berry-Kravis, E., Goetz, C. G., Zhang, L., Hall, D. A., Li, L., et al. (2007). FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology 70, 1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5
Liao, W. (2019). Psychomotor Dysfunction in Rett Syndrome: Insights into the Neurochemical and Circuit Roots. Dev. Neurobiol. 79, 51–59. doi: 10.1002/dneu.22651
Lindeberg, J., Usoskin, D., Bengtsson, H., Gustafsson, A., Kylberg, A., Söderström, S., et al. (2004). Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73. doi: 10.1002/gene.20065
Liu, X., Li, Y., Yu, L., Vickstrom, C. R., and Liu, Q. S. (2018). VTA mTOR Signaling Regulates Dopamine Dynamics, Cocaine-Induced Synaptic Alterations, and Reward. Neuropsychopharmacology 43, 1066–1077. doi: 10.1038/npp.2017.247
Loat, C. S., Curran, S., Lewis, C. M., Duvall, J., Geschwind, D., Bolton, P., et al. (2008). Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism. Genes Brain Behav. 7, 754–760.
Maehama, T., and Dixon, J. E. (1998). The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 273, 13375–13378. doi: 10.1074/jbc.273.22.13375
Maenner, M. J., Shaw, K. A., Baio, J., Washington, A., Patrick, M., Dirienzo, M., et al. (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Surveill. Summar. 69, 1–12. doi: 10.15585/mmwr.ss6802a1
Makkonen, I., Riikonen, R., Kokki, H., Airaksinen, M. M., and Kuikka, J. T. (2008). Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child Neurol. 50, 593–597. doi: 10.1111/j.1469-8749.2008.03027.x
Marcus, R. N., Owen, R., Manos, G., Mankoski, R., Kamen, L., Mcquade, R. D., et al. (2011). Safety and Tolerability of Aripiprazole for Irritability in Pediatric Patients With Autistic Disorder. J. Clin. Psychiatry 72, 1270–1276. doi: 10.4088/jcp.09m05933
Matsuda, W., Furuta, T., Nakamura, K. C., Hioki, H., Fujiyama, F., Arai, R., et al. (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 29, 444–453. doi: 10.1523/jneurosci.4029-08.2009
Matsuura, T., Sutcliffe, J. S., Fang, P., Galjaard, R.-J., Jiang, Y.-H., Benton, C. S., et al. (1997). De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet. 15, 74–77. doi: 10.1038/ng0197-74
Mayes, S. D., Calhoun, S. L., Murray, M. J., Ahuja, M., and Smith, L. A. (2011). Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Res. Autism Spectr. Disord. 5, 474–485. doi: 10.1016/j.rasd.2010.06.012
Mcbride, K. L., Varga, E. A., Pastore, M. T., Prior, T. W., Manickam, K., Atkin, J. F., et al. (2010). Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 3, 137–141. doi: 10.1002/aur.132
Mccracken, J. T., Mcgough, J., Shah, B., Cronin, P., Hong, D., Aman, M. G., et al. (2002). Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347, 314–321.
Meng, C., Zhou, J., Papaneri, A., Peddada, T., Xu, K., and Cui, G. (2018). Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits. Neuron 98, 707.e–717.e.
Mergy, M. A., Gowrishankar, R., Gresch, P. J., Gantz, S. C., Williams, J., Davis, G. L., et al. (2014). The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc. Natl. Acad. Sci. U S A. 111, E4779–E4788.
Miyamoto, Y., Nagayoshi, I., Nishi, A., and Fukuda, T. (2019). Three divisions of the mouse caudal striatum differ in the proportions of dopamine D1 and D2 receptor-expressing cells, distribution of dopaminergic axons, and composition of cholinergic and GABAergic interneurons. Brain Struct. Funct. 224, 2703–2716. doi: 10.1007/s00429-019-01928-3
Mohebi, A., Pettibone, J. R., Hamid, A. A., Wong, J. T., Vinson, L. T., Patriarchi, T., et al. (2019). Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70. doi: 10.1038/s41586-019-1235-y
Mulherkar, S. A., and Jana, N. R. (2010). Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiol. Dis. 40, 586–592. doi: 10.1016/j.nbd.2010.08.002
Nakamura, K., Sekine, Y., Ouchi, Y., Tsujii, M., Yoshikawa, E., Futatsubashi, M., et al. (2010). Brain Serotonin and Dopamine Transporter Bindings in Adults With High-Functioning Autism. Archi. General Psychiat. 67, 59–68. doi: 10.1001/archgenpsychiatry.2009.137
Nakatani, J., Tamada, K., Hatanaka, F., Ise, S., Ohta, H., Inoue, K., et al. (2009). Abnormal Behavior in a Chromosome- Engineered Mouse Model for Human 15q11-13 Duplication Seen in Autism. Cell 137, 1235–1246. doi: 10.1016/j.cell.2009.04.024
Nan, X., Campoy, F. J., and Bird, A. (1997). MeCP2 Is a Transcriptional Repressor with Abundant Binding Sites in Genomic Chromatin. Cell 88, 471–481. doi: 10.1016/s0092-8674(00)81887-5
Nan, X., Ng, H.-H., Johnson, C. A., Laherty, C. D., Turner, B. M., Eisenman, R. N., et al. (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389. doi: 10.1038/30764
Neul, J. L., Fang, P., Barrish, J., Lane, J., Caeg, E. B., Smith, E. O., et al. (2008). Specific mutations in Methyl-CpG-Binding Protein 2 confer different severity in Rett syndrome. Neurology 70, 1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa
Nguyen, M., Roth, A., Kyzar, E. J., Poudel, M. K., Wong, K., Stewart, A. M., et al. (2014). Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD). Neurochem. Int. 66, 15–26. doi: 10.1016/j.neuint.2014.01.002
Numis, A. L., Major, P., Montenegro, M. A., Muzykewicz, D. A., Pulsifer, M. B., and Thiele, E. A. (2011). Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 76, 981–987. doi: 10.1212/wnl.0b013e3182104347
Nurmi, E. L., Bradford, Y., Chen, Y.-H., Hall, J., Arnone, B., Gardiner, M. B., et al. (2001). Linkage Disequilibrium at the Angelman Syndrome Gene UBE3A in Autism Families. Genomics 77, 105–113. doi: 10.1006/geno.2001.6617
Olanow, C. W., and Mcnaught, K. S. (2006). Ubiquitin-proteasome system and Parkinson’s disease. Mov. Disord. 21, 1806–1823.
O’roak, B. J., Vives, L., Fu, W., Egertson, J. D., Stanaway, I. B., Phelps, I. G., et al. (2012a). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622. doi: 10.1126/science.1227764
O’roak, B. J., Vives, L., Girirajan, S., Karakoc, E., Krumm, N., Coe, B. P., et al. (2012b). Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250. doi: 10.1038/nature10989
Ott, T., and Nieder, A. (2019). Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 23, 213–234. doi: 10.1016/j.tics.2018.12.006
Owen, R., Sikich, L., Marcus, R. N., Corey-Lisle, P., Manos, G., Mcquade, R. D., et al. (2009). Aripiprazole in the Treatment of Irritability in Children and Adolescents With Autistic Disorder. Pediatrics 124, 1533–1540. doi: 10.1542/peds.2008-3782
Panayotis, N., Ghata, A., Villard, L., and Roux, J.-C. (2011a). Biogenic amines and their metabolites are differentially affected in the Mecp2-deficient mouse brain. BMC Neurosci. 12:47. doi: 10.1186/1471-2202-12-47
Panayotis, N., Pratte, M., Borges-Correia, A., Ghata, A., Villard, L., and Roux, J.-C. (2011b). Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse. Neurobiol. Dis. 41, 385–397. doi: 10.1016/j.nbd.2010.10.006
Patriarchi, T., Mohebi, A., Sun, J., Marley, A., Liang, R., Dong, C., et al. (2020). An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods 17, 1147–1155. doi: 10.1038/s41592-020-0936-3
Paul, K., Venkitaramani, D. V., and Cox, C. L. (2013). Dampened dopamine-mediated neuromodulation in prefrontal cortex of fragile X mice. J. Physiol. 591, 1133–1143. doi: 10.1113/jphysiol.2012.241067
Paval, D. (2017). A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 39, 355–360. doi: 10.1159/000478725
Pelka, G. J., Watson, C. M., Christodoulou, J., and Tam, P. P. L. (2005). Distinct expression profiles of Mecp2 transcripts with different lengths of 3’UTR in the brain and visceral organs during mouse development. Genomics 85, 441–452. doi: 10.1016/j.ygeno.2004.12.002
Peprah, E., He, W., Allen, E., Oliver, T., Boyne, A., and Sherman, S. L. (2010). Examination of FMR1 transcript and protein levels among 74 premutation carriers. J. Hum. Genet. 55, 66–68. doi: 10.1038/jhg.2009.121
Pieretti, M., Zhang, F., Fu, Y.-H., Warren, S. T., Oostra, B. A., Caskey, C. T., et al. (1991). Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822. doi: 10.1016/0092-8674(91)90125-i
Pisano, F., Pisanello, M., Lee, S. J., Lee, J., Maglie, E., Balena, A., et al. (2019). Depth-resolved fiber photometry with a single tapered optical fiber implant. Nat. Methods 16, 1185–1192. doi: 10.1038/s41592-019-0581-x
Pitzianti, M. B., Santamaria Palombo, A., Esposito, S., and Pasini, A. (2019). Rett Syndrome in Males: The Different Clinical Course in Two Brothers with the Same Microduplication MECP2 Xq28. Int. J. Environ. Res. Public Health 16:3075. doi: 10.3390/ijerph16173075
Poulin, J. F., Caronia, G., Hofer, C., Cui, Q., Helm, B., Ramakrishnan, C., et al. (2018). Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271. doi: 10.1038/s41593-018-0203-4
Poulin, J.-F., Gaertner, Z., Moreno-Ramos, O. A., and Awatramani, R. (2020). Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 43, 155–169. doi: 10.1016/j.tins.2020.01.004
Poulin, J.-F., Zou, J., Drouin-Ouellet, J., Kim, K.-Y. A., Cicchetti, F., and Awatramani, R. B. (2014). Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell Rep. 9, 930–943. doi: 10.1016/j.celrep.2014.10.008
Presti, M. F., Mikes, H. M., and Lewis, M. H. (2003). Selective blockade of spontaneous motor stereotypy via intrastriatal pharmacological manipulation. Pharmacol. Biochem. Behav. 74, 833–839. doi: 10.1016/s0091-3057(02)01081-x
Primerano, B., Tassone, F., Hagerman, R. J., Hagerman, P., Amaldi, F., and Bagni, C. (2002). Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA 8, 1482–1488.
Qiu, Z. (2018). Deciphering MECP2 -associated disorders: disrupted circuits and the hope for repair. Curr. Opin. Neurobiol. 48, 30–36. doi: 10.1016/j.conb.2017.09.004
Ramaswami, G., Won, H., Gandal, M. J., Haney, J., Wang, J. C., Wong, C. C. Y., et al. (2020). Integrative genomics identifies a convergent molecular subtype that links epigenomic with transcriptomic differences in autism. Nat. Commun. 11:4873.
Ramocki, M. B., Tavyev, Y. J., and Peters, S. U. (2010). TheMECP2duplication syndrome. Am. J. Med. Genet. Part A 152A, 1079–1088. doi: 10.1002/ajmg.a.33184
Reichow, B., George-Puskar, A., Lutz, T., Smith, I. C., and Volkmar, F. R. (2015). Brief Report: Systematic Review of Rett Syndrome in Males. J. Autism Dev. Disord. 45, 3377–3383. doi: 10.1007/s10803-015-2519-1
Reiersen, A. M., and Todorov, A. A. (2011). Association between DRD4 genotype and Autistic Symptoms in DSM-IV ADHD. J. Can. Acad. Child Adolesc. Psychiatry 20, 15–21.
Riday, T. T., Dankoski, E. C., Krouse, M. C., Fish, E. W., Walsh, P. L., Han, J. E., et al. (2012). Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J. Clin. Investigat. 122, 4544–4554. doi: 10.1172/jci61888
Robinson, J. E., and Gradinaru, V. (2018). Dopaminergic dysfunction in neurodevelopmental disorders: recent advances and synergistic technologies to aid basic research. Curr. Opin. Neurobiol. 48, 17–29. doi: 10.1016/j.conb.2017.08.003
Rougeulle, C., Glatt, H., and Lalande, M. (1997). The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 17, 14–15. doi: 10.1038/ng0997-14
Roux, J.-C., and Villard, L. (2009). Biogenic Amines in Rett Syndrome: The Usual Suspects. Behav. Genet. 40, 59–75. doi: 10.1007/s10519-009-9303-y
Samaco, R. C. (2004). Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 13, 629–639. doi: 10.1093/hmg/ddh063
Samaco, R. C., Mandel-Brehm, C., Chao, H. T., Ward, C. S., Fyffe-Maricich, S. L., Ren, J., et al. (2009). Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc. Natl. Acad. Sci. 106, 21966–21971. doi: 10.1073/pnas.0912257106
Santos, M., Summavielle, T., Teixeira-Castro, A., Silva-Fernandes, A., Duarte-Silva, S., Marques, F., et al. (2010). Monoamine deficits in the brain of methyl-CpG binding protein 2 null mice suggest the involvement of the cerebral cortex in early stages of Rett syndrome. Neuroscience 170, 453–467. doi: 10.1016/j.neuroscience.2010.07.010
Satterstrom, F. K., Kosmicki, J. A., Wang, J., Breen, M. S., De Rubeis, S., An, J.-Y., et al. (2020). Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 180, 568.e–584.e.
Saunders, A., Macosko, E. Z., Wysoker, A., Goldman, M., Krienen, F. M., De Rivera, H., et al. (2018). Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015.e–1030.e.
Saxton, R. A., and Sabatini, D. M. (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. doi: 10.1016/j.cell.2017.02.004
Schaaf, C. P., Sabo, A., Sakai, Y., Crosby, J., Muzny, D., Hawes, A., et al. (2011). Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum. Mol. Genet. 20, 3366–3375. doi: 10.1093/hmg/ddr243
Schuetze, M., Park, M. T., Cho, I. Y., Macmaster, F. P., Chakravarty, M. M., and Bray, S. L. (2016). Morphological Alterations in the Thalamus, Striatum, and Pallidum in Autism Spectrum Disorder. Neuropsychopharmacology 41, 2627–2637. doi: 10.1038/npp.2016.64
Scott-Van Zeeland, A. A., Dapretto, M., Ghahremani, D. G., Poldrack, R. A., and Bookheimer, S. Y. (2010). Reward processing in autism. Autism Res. 3, 53–67.
Sharma, A., and Shaw, S. R. (2012). Efficacy of Risperidone in Managing Maladaptive Behaviors for Children With Autistic Spectrum Disorder: A Meta-Analysis. J. Pediatr. Health Care 26, 291–299. doi: 10.1016/j.pedhc.2011.02.008
Skelton, P. D., Stan, R. V., and Luikart, B. W. (2019). The Role of PTEN in Neurodevelopment. Mol. Neuropsychiat. 5, 60–71. doi: 10.1159/000504782
Smith, L. N., Jedynak, J. P., Fontenot, M. R., Hale, C. F., Dietz, K. C., Taniguchi, M., et al. (2014). Fragile X Mental Retardation Protein Regulates Synaptic and Behavioral Plasticity to Repeated Cocaine Administration. Neuron 82, 645–658. doi: 10.1016/j.neuron.2014.03.028
Sørensen, E. M., Bertelsen, F., Weikop, P., Skovborg, M. M., Banke, T., Drasbek, K. R., et al. (2015). Hyperactivity and lack of social discrimination in the adolescent Fmr1 knockout mouse. Behav. Pharmacol. 26, 733–740. doi: 10.1097/fbp.0000000000000152
Sperow, M., Berry, R. B., Bayazitov, I. T., Zhu, G., Baker, S. J., and Zakharenko, S. S. (2012). Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J. Physiol. 590, 777–792. doi: 10.1113/jphysiol.2011.220236
Staal, W. G., De Krom, M., and De Jonge, M. V. (2011). Brief Report: The Dopamine-3-Receptor Gene (DRD3) is Associated with Specific Repetitive Behavior in Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 42, 885–888. doi: 10.1007/s10803-011-1312-z
Staal, W. G., Langen, M., Van Dijk, S., Mensen, V. T., and Durston, S. (2018). DRD3 gene and striatum in autism spectrum disorder. Br. J. Psychiatry 206, 431–432. doi: 10.1192/bjp.bp.114.148973
Stambolic, V., Suzuki, A., De La Pompa, J. L., Brothers, G. M., Mirtsos, C., Sasaki, T., et al. (1998). Negative Regulation of PKB/Akt-Dependent Cell Survival by the Tumor Suppressor PTEN. Cell 95, 29–39. doi: 10.1016/s0092-8674(00)81780-8
Steinkellner, T., Yang, J.-W., Montgomery, T. R., Chen, W.-Q., Winkler, M.-T., Sucic, S., et al. (2012). Ca2+/Calmodulin-dependent Protein Kinase IIα (αCaMKII) Controls the Activity of the Dopamine Transporter. J. Biol. Chem. 287, 29627–29635.
Su, S.-H., Kao, F.-C., Huang, Y.-B., and Liao, W. (2015). MeCP2 in the Rostral Striatum Maintains Local Dopamine Content Critical for Psychomotor Control. J. Neurosci. 35, 6209–6220. doi: 10.1523/jneurosci.4624-14.2015
Subramanian, K., Brandenburg, C., Orsati, F., Soghomonian, J. J., Hussman, J. P., and Blatt, G. J. (2017). Basal ganglia and autism - a translational perspective. Autism Res. 10, 1751–1775. doi: 10.1002/aur.1837
Sulzer, D., Cragg, S. J., and Rice, M. E. (2016). Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 6, 123–148. doi: 10.1016/j.baga.2016.02.001
Sun, F., Zhou, J., Dai, B., Qian, T., Zeng, J., Li, X., et al. (2020). Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166. doi: 10.1038/s41592-020-00981-9
Szczesna, K., De La Caridad, O., Petazzi, P., Soler, M., Roa, L., Saez, M. A., et al. (2014). Improvement of the Rett Syndrome Phenotype in a Mecp2 Mouse Model Upon Treatment with Levodopa and a Dopa-Decarboxylase Inhibitor. Neuropsychopharmacology 39, 2846–2856. doi: 10.1038/npp.2014.136
Takeuchi, K., Gertner, M. J., Zhou, J., Parada, L. F., Bennett, M. V. L., and Zukin, R. S. (2013). Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc. Natl. Acad. Sci. 110, 4738–4743. doi: 10.1073/pnas.1222803110
Tan, W. H., Bird, L. M., Sadhwani, A., Barbieri−Welge, R. L., Skinner, S. A., Horowitz, L. T., et al. (2017). A randomized controlled trial of levodopa in patients with Angelman syndrome. Am. J. Med. Genet. Part A 176, 1099–1107.
Tan, Y., Sgobio, C., Arzberger, T., Machleid, F., Tang, Q., Findeis, E., et al. (2019). Loss of fragile X mental retardation protein precedes Lewy pathology in Parkinson’s disease. Acta Neuropathol. 139, 319–345. doi: 10.1007/s00401-019-02099-5
Tanguay, W., Ducrot, C., Giguère, N., Bourque, M.-J., and Trudeau, L.-E. (2021). Neonatal 6-OHDA lesion of the SNc induces striatal compensatory sprouting from surviving SNc dopaminergic neurons without VTA contribution. [Preprint].
Tassone, F., Choudhary, N. S., Tassone, F., Durbin-Johnson, B., Hansen, R., Hertz-Picciotto, I., et al. (2012). Identification of Expanded Alleles of the FMR1 Gene in the CHildhood Autism Risks from Genes and Environment (CHARGE) Study. J. Autism Dev. Disord. 43, 530–539. doi: 10.1007/s10803-012-1580-2
Tee, A. R., Fingar, D. C., Manning, B. D., Kwiatkowski, D. J., Cantley, L. C., and Blenis, J. (2002). Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. 99, 13571–13576. doi: 10.1073/pnas.202476899
Temudo, T., Ramos, E., Dias, K., Barbot, C., Vieira, J. P., Moreira, A., et al. (2008). Movement disorders in Rett syndrome: An analysis of 60 patients with detected MECP2 mutation and correlation with mutation type. Mov. Disord. 23, 1384–1390. doi: 10.1002/mds.22115
Tiklová, K., Björklund, ÅK., Lahti, L., Fiorenzano, A., Nolbrant, S., Gillberg, L., et al. (2019). Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 10:581.
Tilot, A. K., Frazier, T. W., and Eng, C. (2015). Balancing Proliferation and Connectivity in PTEN-associated Autism Spectrum Disorder. Neurotherapeutics 12, 609–619. doi: 10.1007/s13311-015-0356-8
Toma, C., Hervás, A., Balmaña, N., Salgado, M., Maristany, M., Vilella, E., et al. (2012). Neurotransmitter systems and neurotrophic factors in autism: association study of 37 genes suggests involvement of DDC. World J. Biol. Psychiatry 14, 516–527. doi: 10.3109/15622975.2011.602719
Van Dam, D., Errijgers, V., Kooy, R. F., Willemsen, R., Mientjes, E., Oostra, B. A., et al. (2005). Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS). Behav. Brain Res. 162, 233–239. doi: 10.1016/j.bbr.2005.03.007
Van Diepen, M. T., and Eickholt, B. J. (2008). Function of PTEN during the Formation and Maintenance of Neuronal Circuits in the Brain. Dev. Neurosci. 30, 59–64. doi: 10.1159/000109852
Van Esch, H., Bauters, M., Ignatius, J., Jansen, M., Raynaud, M., Hollanders, K., et al. (2005). Duplication of the MECP2 Region Is a Frequent Cause of Severe Mental Retardation and Progressive Neurological Symptoms in Males. Am. J. Hum. Genet. 77, 442–453. doi: 10.1086/444549
Varga, E. A., Pastore, M., Prior, T., Herman, G. E., and Mcbride, K. L. (2009). The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet. Med. 11, 111–117. doi: 10.1097/gim.0b013e31818fd762
Varghese, M., Keshav, N., Jacot-Descombes, S., Warda, T., Wicinski, B., Dickstein, D. L., et al. (2017). Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 134, 537–566.
Ventura, R., Pascucci, T., Catania, M. V., Musumeci, S. A., and Puglisi-Allegra, S. (2004). Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav. Pharmacol. 15, 433–442. doi: 10.1097/00008877-200409000-00018
Verma, D., Chakraborti, B., Karmakar, A., Bandyopadhyay, T., Singh, A. S., Sinha, S., et al. (2014). Sexual dimorphic effect in the genetic association of monoamine oxidase A (MAOA) markers with autism spectrum disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 50, 11–20. doi: 10.1016/j.pnpbp.2013.11.010
Vu, T. H., and Hoffman, A. R. (1997). Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat. Genet. 17, 12–13. doi: 10.1038/ng0997-12
Wang, H., Kim, S. S., and Zhuo, M. (2010). Roles of Fragile X Mental Retardation Protein in Dopaminergic Stimulation-induced Synapse-associated Protein Synthesis and Subsequent α-Amino-3-hydroxyl-5-methyl-4-isoxazole-4-propionate (AMPA) Receptor Internalization. J. Biol. Chem. 285, 21888–21901. doi: 10.1074/jbc.m110.116293
Wang, H., Wu, L.-J., Kim, S. S., Lee, F. J. S., Gong, B., Toyoda, H., et al. (2008). FMRP Acts as a Key Messenger for Dopamine Modulation in the Forebrain. Neuron 59, 634–647. doi: 10.1016/j.neuron.2008.06.027
Wang, T., Guo, H., Xiong, B., Stessman, H. A., Wu, H., Coe, B. P., et al. (2016). De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat. Commun. 7:13316.
Wassink, T. H., Hazlett, H. C., Davis, L. K., Reiss, A. L., and Piven, J. (2014). Testing for association of the monoamine oxidase A promoter polymorphism with brain structure volumes in both autism and the fragile X syndrome. J. Neurodevelop. Disord. 6:6.
Watabe-Uchida, M., Eshel, N., and Uchida, N. (2017). Neural Circuitry of Reward Prediction Error. Annu. Rev. Neurosci. 40, 373–394. doi: 10.1146/annurev-neuro-072116-031109
Wen, Z., Cheng, T.-L., Li, G.-Z., Sun, S.-B., Yu, S.-Y., Zhang, Y., et al. (2017). Identification of autism-related MECP2 mutations by whole-exome sequencing and functional validation. Mol. Autism 8:43.
Wenk, G. (2007). Alterations in Dopaminergic Function in Rett Syndrome. Neuropediatrics 26, 123–125. doi: 10.1055/s-2007-979741
Yoo, H. J., Cho, I. H., Park, M., Yang, S. Y., and Kim, S. A. (2013). Association of the catechol-o-methyltransferase gene polymorphisms with Korean autism spectrum disorders. J. Korean Med. Sci. 28, 1403–1406. doi: 10.3346/jkms.2013.28.9.1403
Yoo, H. J., Lee, S. K., Park, M., Cho, I. H., Hyun, S. H., Lee, J. C., et al. (2009). Family- and population-based association studies of monoamine oxidase A and autism spectrum disorders in Korean. Neurosci. Res. 63, 172–176. doi: 10.1016/j.neures.2008.11.007
Zalla, T., and Sperduti, M. (2013). The amygdala and the relevance detection theory of autism: an evolutionary perspective. Front. Hum. Neurosci. 7:894. doi: 10.3389/fnhum.2013.00894
Zhang, Y., Granholm, A. C., Huh, K., Shan, L., Diaz-Ruiz, O., Malik, N., et al. (2012). PTEN deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to MitoPark mice. Brain 135, 2736–2749. doi: 10.1093/brain/aws196
Zhou, J., and Parada, L. F. (2012). PTEN signaling in autism spectrum disorders. Curr. Opin. Neurobiol. 22, 873–879. doi: 10.1016/j.conb.2012.05.004
Zorio, D. A., Jackson, C. M., Liu, Y., Rubel, E. W., and Wang, Y. (2017). Cellular distribution of the fragile X mental retardation protein in the mouse brain. J. Comp. Neurol. 525, 818–849. doi: 10.1002/cne.24100
Keywords: dopamine, autism spectrum disorder, Angelman syndrome, Fragile X syndrome, Rett syndrome, PTEN hamartoma tumor syndrome, Tuberous Sclerosis Complex, genetic mouse models
Citation: Kosillo P and Bateup HS (2021) Dopaminergic Dysregulation in Syndromic Autism Spectrum Disorders: Insights From Genetic Mouse Models. Front. Neural Circuits 15:700968. doi: 10.3389/fncir.2021.700968
Received: 27 April 2021; Accepted: 21 June 2021;
Published: 23 July 2021.
Edited by:
Talia Newcombe Lerner, Northwestern University, United StatesReviewed by:
Marc Vincent Fuccillo, University of Pennsylvania, United StatesWei Li, University of Alabama at Birmingham, United States
Copyright © 2021 Kosillo and Bateup. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen S. Bateup, YmF0ZXVwQGJlcmtlbGV5LmVkdQ==
 Polina Kosillo
Polina Kosillo Helen S. Bateup
Helen S. Bateup