- 1Departamento de Física, Universidade Federal de Pernambuco, Recife, Brazil
- 2Department of Physics, University of Ottawa, Ottawa, ON, Canada
- 3Departamento de Física, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil
- 4Departamento de Ciências Fundamentais e Sociais, Universidade Federal da Paraíba, Areia, Brazil
- 5Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal
- 6Life and Health Sciences Research Institute/Biomaterials, Biodegradables and Biomimetics, Braga, Portugal
Recent experimental results on spike avalanches measured in the urethane-anesthetized rat cortex have revealed scaling relations that indicate a phase transition at a specific level of cortical firing rate variability. The scaling relations point to critical exponents whose values differ from those of a branching process, which has been the canonical model employed to understand brain criticality. This suggested that a different model, with a different phase transition, might be required to explain the data. Here we show that this is not necessarily the case. By employing two different models belonging to the same universality class as the branching process (mean-field directed percolation) and treating the simulation data exactly like experimental data, we reproduce most of the experimental results. We find that subsampling the model and adjusting the time bin used to define avalanches (as done with experimental data) are sufficient ingredients to change the apparent exponents of the critical point. Moreover, experimental data is only reproduced within a very narrow range in parameter space around the phase transition.
1. Introduction
In the first results that fueled the critical brain hypothesis, Beggs and Plenz (2003) observed intermittent bursts of local field potentials (LFPs) in in vitro multielectrode recordings of cultured and acute slices of the rat brain. Events occurred with a clear separation of time scales, and were named neuronal avalanches.
A neuronal avalanche can be characterized by its size S, which is the total number of significant voltage deflections recorded by electrodes between periods of silence, and by its duration T, which is the number of consecutive time bins spanned by an avalanche. Beggs and Plenz found power-law distributions for the sizes of avalanches,
with τ ≃ 3/2, and suggested, based on their data, a power-law distribution of avalanche duration,
with τt = 2. These scale-invariant distributions were interpreted as a signature that the brain could be operating at criticality—a second-order phase transition (Beggs and Plenz, 2003; Beggs, 2007; Chialvo, 2010; Shew and Plenz, 2013; Plenz and Niebur, 2014; Tomen et al., 2019).
In particular, these two critical exponents together are compatible with a branching process at its critical point (Harris, 1963), a conclusion that was further strengthened by the experimentally established critical branching parameter of 1 for neuronal avalanches (Beggs and Plenz, 2003). This points to a phase transition between a so-called absorbing phase (zero population firing rate) and an active phase (non-zero stationary population firing rate).
Due to its appeal, simplicity, and familiarity within the statistical physics community, the critical branching process has become a canonical model for understanding criticality in the brain. In fact, these exponents are compatible with a larger class of models, namely, any model belonging to the mean-field directed percolation (MF-DP) universality class (Muñoz et al., 1999). In the theory of critical phenomena, two models which can be different in their details are said to belong to the same universality class when the critical exponents which characterize their phase transition coincide (Binney et al., 1992). In general, probabilistic contagion-like models which have a unique absorbing state (all sites “susceptible” or in the neuroscience context, all neurons quiescent) and no further symmetries tend to belong to the directed-percolation universality class (Janssen, 1981; Grassberger, 1982; Marro and Dickman, 1999). If the network has topological dimension above 4 (such as random or complete graphs), the model usually belongs to the MF-DP universality class.
More recently, these ideas were tested with more advanced experimental techniques, highlighting the prospect of criticality in the awake brain. Two studies have shown that different types of anesthesia strongly affect avalanche statistics. In voltage imaging recordings of the mouse cortex, size distributions were more closely compatible with τ = 1.5 for awake animals than for animals anesthetized with pentobarbital (Scott et al., 2014). A similar trend was observed in two-photon imaging of the rat cortex, where avalanche distributions become increasingly compatible with τ = 1.5 and τt = 2 as the animals recover from isoflurane anesthesia (Bellay et al., 2015).
Other experimental results, however, challenged the MF-DP scenario originally proposed by Beggs and Plenz (2003). For instance, avalanche exponents in ex-vivo recordings of the turtle visual cortex deviated significantly from τ = 3/2 and τt = 2 (Shew et al., 2015). Discrepancies in exponent values were also observed in spike avalanches of rats under ketamine-xylazine anesthesia (Ribeiro et al., 2010) and M/EEG avalanches in resting or behaving humans (Palva et al., 2013; Zhigalov et al., 2015), among others.
Furthermore, Touboul and Destexhe (2017) argued that the power-law signature alone in the distributions of size (Equation 1) and duration (Equation 2) of avalanches is insufficient to claim criticality, since power laws can be observed in non-critical models as well. They suggested that another scaling relation should be tested as a stronger criterion. This was based on the result that at criticality the average avalanche size 〈S〉 for a given duration T must obey
where 1/(σνz) is a combination of critical exponents that at criticality satisfy the so-called crackling noise scaling relation (Muñoz et al., 1999; Sethna et al., 2001; Friedman et al., 2012)
Equation (4) is a stronger criterion for criticality because it is expected not to be satisfied by non-critical models (Touboul and Destexhe, 2017). In the MF-DP case, the avalanche exponents obey (τt − 1)/(τ − 1) = 2 and 1/(σνz) = 2, independently. The absolute difference between the two sides of Equation (4) can even be employed as a metric for the distance to criticality (Ma et al., 2019), or to identify criticality in more general phase transitions of neuronal networks (Girardi-Schappo and Tragtenberg, 2018). Indeed, Ponce-Alvarez et al. (2018) have investigated the crackling noise relation in zebrafish whole-brain activity, obtaining 1/(σνz) ≃ 2 but values of τ and τt incompatible with MF-DP. Miller et al. (2019) have also found 1/(σνz) ≃ 2 in LFP avalanches from awake non-human primates, when the impact of ongoing gamma-oscillations was accurately taken into account.
Recently, cortical spike avalanches of urethane-anesthetized rats were investigated under this methodological lens by Fontenele et al. (2019). This experimental setup is known to yield spiking activity which is highly variable, ranging from very asynchronous to very synchronous population activity (Clement et al., 2008). These regimes can be characterized by different ranges of the coefficient of variation (CV) of the population firing rate (de Vasconcelos et al., 2017), which is thought of as a simple marker of cortical states (Harris and Thiele, 2011). By parsing the data according to levels of spiking variability, Fontenele et al. (2019) found that the scaling relation Equation (4) was satisfied at an intermediate value of CV, suggesting a phase transition away from both the synchronized and desynchronized ends of the spiking variability spectrum. In particular, the values of the avalanche exponents where the scaling relation was satisfied were not all compatible (within error bars) with MF-DP values: 〈τ〉 ≃ 1.52 ± 0.09, 〈τt〉 ≃ 1.7 ± 0.1 and 〈1/(σνz)〉 ≃ 1.28 ± 0.03 (Fontenele et al., 2019). This was interpreted as an incompatibility with the theoretical MF-DP scenario (Fontenele et al., 2019), thus requiring the formulation of models belonging to other universality classes and undergoing other phase transitions.
One hypothesis to explain this controversy is that the study of spike activity is strongly affected by subsampling effects, that is, the measured activity is based on a tiny fraction of the total number of neurons in a given area of the brain. Different groups have shown that subsampling indeed affects the apparent distribution of avalanches (Priesemann et al., 2009, 2014; Ribeiro et al., 2010, 2014; Girardi-Schappo et al., 2013; Levina and Priesemann, 2017; Wilting and Priesemann, 2019). For example, an avalanche evaluated on all elements (full sampling) can be broken into smaller avalanches when recorded in a subset of the network (subsampling). In addition, we highlight that this effect is different from the well-studied phenomenon of finite-size scaling, which is the study of how statistical properties change as the size of the system increases and activity recorded in all sites is analyzed (see e.g., Levina and Priesemann, 2017).
Here, we revisit this issue by studying the data produced by two theoretical models in the MF-DP universality class. We start by showing that the models reproduce well-known analytical results, which however fail to reproduce the experimental data. Then we proceed to treat the model results under the same conditions as those of experimental data. Despite the large number of simulated neurons (~ 105), we intentionally restrict the theoretical analysis to a small subset of cells (~ 102), mimicking the fact that one can only record a few hundred neurons among the millions that comprise the rat's brain (the subsampling issue). Here we show that by combining the subsampling of the model with the analysis pipeline that has been applied to the experimental data (Fontenele et al., 2019), we can reconcile the empirical power-law avalanches with the theoretical MF-DP universality class.
2. Methods
2.1. A Spiking Neuronal Network With Excitation and Inhibition
We used the excitatory/inhibitory network of Girardi-Schappo et al. (2020), where each neuron is a stochastic leaky integrate-and-fire unit with discrete time step equal to 1 ms, connected in an all-to-all graph. A binary variable indicates if the neuron fired [X(t) = 1] or not [X(t) = 0]. The membrane potential of each cell i in either the excitatory (E) or inhibitory (I) population is given by
where J is the synaptic coupling strength, g is the inhibition to excitation (E/I) coupling strength ratio, μ is the leak time constant, and Ie is an external current. The total number of neurons in the network is , where the fractions of excitatory and inhibitory neurons are kept fixed at p = NE/N = 0.8 and q = NI/N = 0.2, respectively, as reported for cortical data (Somogyi et al., 1998). Note that the membrane potential is reset to zero in the time step following a spike.
At any time step, a neuron fires according to a piecewise linear sigmoidal probability Φ(V),
where θ = 1 is the firing threshold, Γ is the firing gain constant, VS = 1/Γ + θ is the saturation potential, and Θ(x > 0) = 1 (zero otherwise) is the step function. For simplicity, the parameter μ = 0 is chosen without lack of generality, since it does not change the phase transition of the model (Girardi-Schappo et al., 2020). The external current Ie > VS is used only to spark a new avalanche in a single excitatory neuron when the network activity dies off (it is kept as Ie = θ otherwise).
This model is known to present a directed percolation critical point (Girardi-Schappo et al., 2020) at gc = p/q − 1/(qΓJ) = 1.5 (for Γ = 0.2 and J = 10), such that g < gc is the active excitation-dominated (supercritical) phase and g > gc corresponds to the inhibition-dominated absorbing state (subcritical). The synapses in the critical point gc are dynamically balanced: fluctuations in excitation are immediately followed by counter fluctuations in inhibition (Girardi-Schappo et al., 2020). The initial condition of the simulations has all neurons quiescent except for a seed neuron to spark activity. This procedure was repeated whenever the system went back to the absorbing state.
2.2. Probabilistic Cellular Automaton Model
The spiking model described in section 2.1 is certainly not the simplest model to present a phase transition in the MF-DP universality class. Therefore, to probe the robustness of our findings, we also simulated a much simpler model: a network of probabilistic excitable cellular automata in a random graph (Kinouchi and Copelli, 2006). This model closely resembles a standard branching process and is known to mimic the changing inhibition-excitation levels of cortical cultures (Shew et al., 2009).
Each site i (i = 1, …, N) has five states: the silent state, si = 0, the active state, si = 1, corresponding to a spike, and the remaining three states, si = 2, 3, 4, in which the site will not respond to incoming stimuli (absolute refractory states). Each site receives input from K presynaptic neighbors which are randomly selected at the start and kept fixed throughout the simulations. A quiescent site i becomes excited [si(t) = 0 → si(t + 1) = 1] with probability pij if a presynaptic neighbor j is active at time t. All presynaptic neighbors are swept and independently considered at each time step, so that
where hi is the probability of unit i spiking due to an external stimulus and is the set of presynaptic neighbors of i. The remaining transitions happen with probability 1, including the transition 4 → 0 that returns the site to its initial quiescent state. The time step of the model corresponds to 1 ms.
We initially chose the random variables {pij} from a uniform distribution in the interval [0, 2λ/K]. The so-called branching ratio λ = K〈pij〉 is the control parameter of the model. This model undergoes a MF-DP phase transition at λ = λc = 1 (Kinouchi and Copelli, 2006). For λ < 1, the system is in the subcritical phase and eventually reaches the absorbing state (si = 0, ∀i). For λ > 1, the system presents self-sustained activity, i.e., a non-zero stationary density of population firings (the supercritical phase). The critical point is not affected by the number of refractory states (Kinouchi and Copelli, 2006).
In our simulations we used K = 10 neighbors for each of the N = 105 sites. Similarly to the spiking neuronal network model, a single random neuron was stimulated (hi = 1) only when the system reached the absorbing state, sparking the network activity and subsequently being set back to hi = 0. The initial condition was set with a single randomly chosen site active and the others in the silent state.
2.3. Experimental Data Acquisition
Urethane is a well-established drug that provides spontaneous changes of brain states that resemble sleep state alternations (Clement et al., 2008). In the last decade, experimental preparations using urethane have helped elucidate questions concerning mechanisms and the functional relevance of state-dependent patterns of brain activity (Curto et al., 2009; Renart et al., 2010; Mochol et al., 2015; de Vasconcelos et al., 2017). The property to promote spontaneous change in the levels of spiking variability cannot be achieved in other anesthesia approaches, such as pentobarbital and isoflurane.
The data used in this paper is original and corroborates the results of Fontenele et al. (2019). We used five rats Long-Evans (Rattus norvegicus) (male, 280–360 g, 2–4 months old). They were obtained from the animal house of the Laboratory of Computational and Systems Neuroscience, Department of Physics, Federal University of Pernambuco (UFPE). The animals were anesthetized with urethane (1.55 g/kg), diluted at 20% in saline, in three intraperitoneal (i.p.) injections, 15 min apart (Sakata and Harris, 2009). Some animals demanded supplement (max 5%) of urethane to reach the proper level of analgesia. In order to ensure that the animals are maintained at the correct depth of anesthesia, responses to painful stimuli (pinching the animal's toes, ears and tail) were always checked throughout the experiment. Once the anesthesia reached its proper level, the rats were placed in a stereotaxic frame and the coordinates to access the primary visual cortex (V1) were marked (Bregma: AP = −7.2, ML = 3.5) (Paxinos and Watson, 2007). A cranial window in the scalp was opened using this coordinate as center, with an area of ~3 mm2. We performed recordings of extra-cellular voltage of neuronal populations by using a 64-channel multielectrode silicon probe (Neuronexus technologies, Buzsaki64spL-A64). This probe has 60 electrodes disposed in six shanks separated by 200 μm, 10 electrodes per shank with impedance of 1–3 MOhm at 1 kHz. Each electrode has 160 μm2 and they are in staggered positions 20 μm apart. We recorded from deeper layers of the rat cortex, similarly to what was previously done in Ribeiro et al. (2010) under ketamine-xylazine and Fontenele et al. (2019) under a setup similar to the one presented here.
Data was sampled at 30 KHz, amplified and digitized in a single head-stage (Intan RHD2164) (Siegle et al., 2017). We recorded spontaneous activity, during long periods (≥3 h). We used the open-source software Klusta to perform the automatic spike sorting on raw electrophysiological data (Rossant et al., 2016). The automatic part is divided in two major steps, spike detection and automatic clustering. The first step detects action potentials and the second one arrange those spikes into clusters according to their similarities (waveforms, PCA, refractory period). After the automatic part, all formed clusters are reanalyzed using the graphic interface phy kwikGUI1. Manual spike sorting allows the identification of each cluster of neuronal activity as single-unit activity (SUA) or multi-unit activity (MUA). We used both SUA and MUA clusters for our study.
2.4. Avalanche Analysis With CV Parsing
To study neuronal avalanches at different levels of spiking variability (Shadlen and Newsome, 1998), we segmented both the neurophysiological and simulated data in non-overlapping windows of width w = 10 s (unless otherwise stated) (Gervasoni et al., 2004). Each of these 10 s epochs was subdivided in non-overlapping intervals {ζj} of duration ΔT = 50 ms (unless otherwise stated) in which we estimated the population spike-count rate Rj. We then calculated the coefficient of variation (CV) for the i-th 10 s window:
where CV is dimensionless, and σi and μi correspond to the standard deviation and the mean of {Rj}, respectively.
For each 10 s window with a particular CV level, we proceeded with the standard avalanche analysis of Beggs and Plenz (2003). The summed population activity was sliced in non-overlapping temporal bins of width Δt = 〈ISI〉 (the average inter-spike interval). Ribeiro et al. (2010) and Yu et al. (2017) have shown that an adaptive bin, evaluated according to the current dynamical state, renders signatures of scale-free dynamics more robust. Following this strategy, we have separately computed Δt = 〈ISI〉 for each 10 s window. Population spikes preceded and followed by silence define a spike avalanche. The number of spikes correspond to the avalanche size S, whereas the number of time bins spanned by the avalanche is its duration T. Following this methodology, we associated each 10 s CVi window with its corresponding set of ni avalanche sizes Si ≡ {Si1, Si2, …, Sini} and durations Ti ≡ {Ti1, Ti2, …, Tini}.
To estimate the avalanche exponents τ and τt, we first ranked the sets {Si} and {Ti} according to their CV values. Next, in order to increase the number of samples while preserving the level of spiking variability, we pooled NB consecutive ranked blocks of similar CV values (NB = 50 unless otherwise stated). For each set of NB blocks we calculated the average coefficient of variation 〈CV〉. The exponents of the size and duration distributions were obtained via a Maximum Likelihood Estimator (MLE) procedure (Deluca and Corral, 2013; Yu et al., 2014; Marshall et al., 2016) on a discrete power-law distribution
The standard choice of fitting parameters, for both experimental and subsampled simulated data, was Smin = 2 and Smax = 100 for size distributions and Tmin = 2 and Tmax = 30 for duration distributions. The exceptions to this choice were for the data shown in Figures 4C,D, due to a change of orders of magnitude in the number of neurons sampled. The specific parameters for these cases are shown in Table 1.
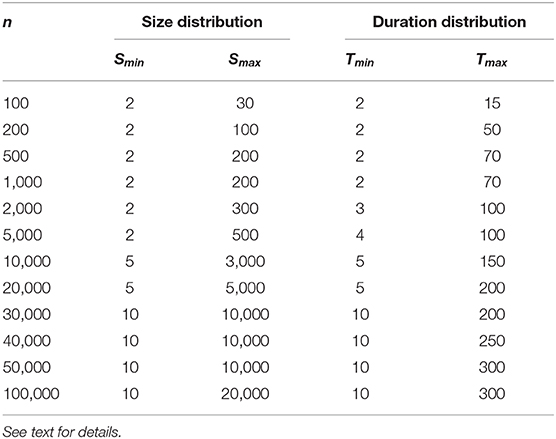
Table 1. Limits chosen for the calculation of the α exponent (Equation 9) via Maximum Likelihood Estimator (MLE) only for the model data shown in Figures 4C,D (Δt = 1 ms).
After the MLE fit we used the Akaike Information Criterion (AIC) as a measure of the relative quality of a given statistical model for a data set:
where is the likelihood at its maximum, k is number of parameters and N the sample size (Akaike, 1975). Starting from the principle that lower AIC indicates a more parsimonious model, we defined Δ ≡ AICln − AICpl, where AICln and AICpl correspond to the AIC of a log-normal and a power-law model, respectively. Therefore, Δ > 0 implies that a power-law model is a better fit to the data than a log-normal. Our scaling relation analyses were restricted to distributions that satisfied Δ > 0.
2.5. Pairwise Correlations
Pairwise spiking correlations were estimated using only the SUA or the simulated data in the following way: first, for each cell k we obtained a spike count time series R(k)(t) at millisecond resolution (ΔT = 1 ms), then each spike count time series R(k) was convolved with a kernel ht1,t2(t) to estimate the k-th mean firing rate n(k)(t):
where ht1,t2(t) is a Mexican-hat kernel obtained by the difference between zero-mean Gaussians with standard deviations t1 = 100 ms and t2 = 400 ms (Renart et al., 2010). The nk(t) were employed to calculate the spiking correlation coefficient between two units k and l:
where Var and Cov are the variance and covariance over t, respectively.
3. Results
3.1. Avalanches in the Fully Sampled Model
We start by illustrating the second order phase transition that the model undergoes at a critical value gc = 1.5 of the inhibition parameter (Girardi-Schappo et al., 2020). As shown in Figure 1A, the stationary density of active sites is positive for g < gc (the supercritical regime) and null for g > gc (the subcritical regime).
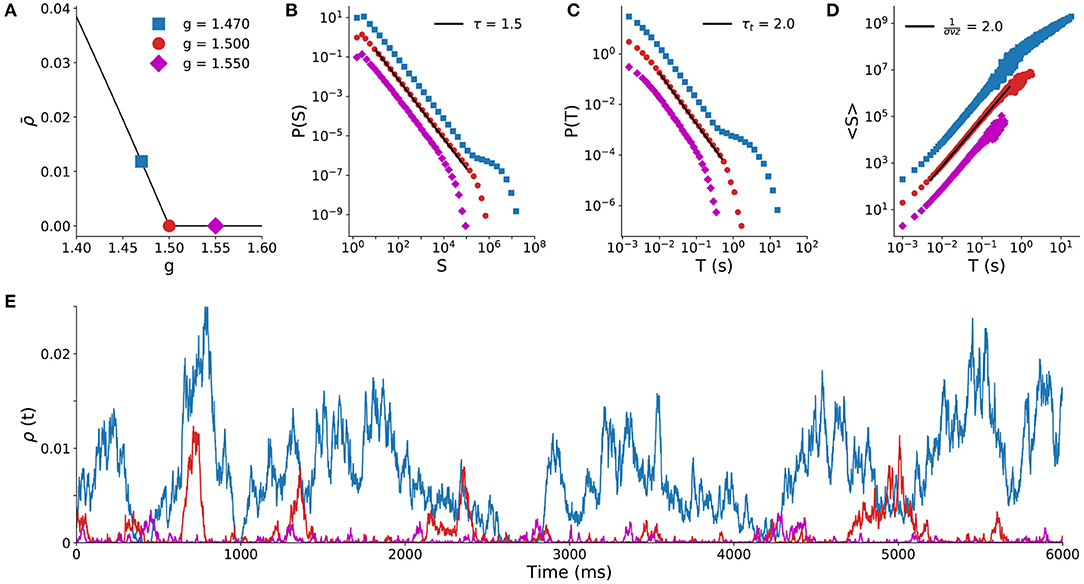
Figure 1. Spiking model results with full sampling. Behavior of the spiking model (N = 105) for different values of the control parameter g. (A) Stationary density of firings as a function of the inhibition strength g (critical point is the red circle at gc = 1.5). Solid line is the mean-field solution (Girardi-Schappo et al., 2020), points are simulation results. Distribution of avalanche sizes (B) and duration (C) for the subcritical (g > gc), critical (g = gc) and supercritical (g < gc) regimes. (D) Average avalanche size 〈S〉 of a given duration (T). (E) Time series of the density of active sites for the three regimes.
At the critical point g = gc, the distribution of avalanche sizes and duration obey the expected power laws (Equations 1 and 2) with exponents τ = 3/2 and τt = 2 (Girardi-Schappo et al., 2020). Subcritical avalanches are exponentially distributed, whereas the supercritical distribution has a trend to display larger and longer avalanches (Figures 1B,C). Both sides of the scaling law in Equation (4) independently agree, since the fit to 〈S〉(T) yields 1/(σνz) = 2 on the critical point (Figure 1D). Figure 1E shows typical time series of firing events for the three regimes. These exponents and dynamic behavior of the model are typical of a system undergoing a MF-DP phase transition.
3.2. Comparison of Subsampled Model and Experiments Stratified by CV
We now revisit the model by subjecting it to the same constraints that apply to experimental datasets (Fontenele et al., 2019) and compare the results between the two. More specifically: (1) data analysis necessarily uses only a tiny fraction of the total neurons in the system and (2) in urethane-anesthetized rats, cortical spiking variability is a proxy for cortical states (Harris and Thiele, 2011) and changes a lot during the hours-long recordings (Clement et al., 2008; de Vasconcelos et al., 2017).
Starting with the experimental results, Figure 2A shows the time series of the coefficient of variation (CV) of the population spiking activity. The lowest CV values correspond to asynchronous spiking activity, whereas the highest values correspond to more synchronized activity (both shown in Figure 2B). When we parsed the data by CV percentiles and evaluated neuronal avalanches for different percentiles, the distributions varied accordingly, with exponents τ, τt, and 1/(σνz) varying continuously across the CV range (Figure 2C) as expected (Fontenele et al., 2019).
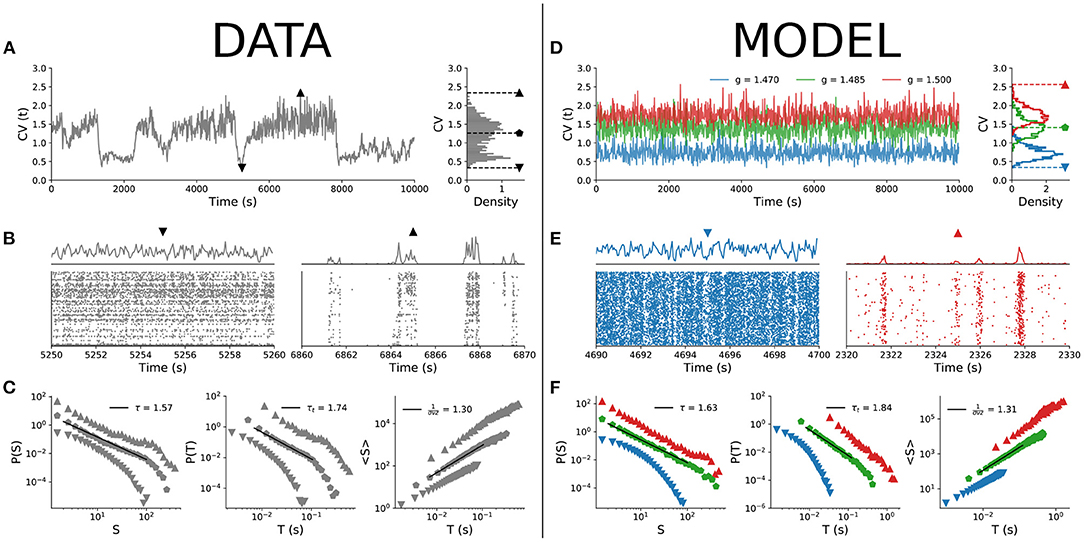
Figure 2. Comparison between empirical data and subsampled spiking model. CV time series and distribution for (A) experimental data (single animal) and (D) model with n = 100. Raster plots and population firing rate in cases of low (▼) and high (▲) values of CV for (B) experimental data and (E) model. Scaling exponents τ, τt, and 1/(σνz) for three different values of CV (denoted by different symbols): (C) experimental data and (F) model. For both experimental data and model, w = 10 s.
Can the MF-DP spiking network model reproduce these experimental results? We found that by sampling only a few neurons out of the entire network, indeed it can. Out of N = 105 simulated neurons, we sampled only n = 100, a number that is of the same order as the amount of neurons captured in our empirical data (Fontenele et al., 2019). Then, we applied to the subsampled simulation data exactly the same analysis pipeline used for experiments (section 2.4).
In the model, we changed the E/I level g to control for the spiking variability level CV. For a fixed value of parameter g, CV is a bell-shaped distribution with finite variance. The CV(t) time series of the model for a single g does not present the dynamical complexity observed experimentally (compare Figures 2A,D). By varying g within a narrow interval around the critical point gc, the CV distribution of the model covers the values observed experimentally (Figure 2D), with less synchronous behavior for low CV and more synchronous activity for high CV (Figure 2E; the full behavior of the CV distribution as a function of parameter g is shown in Supplementary Figure 1A). Parsing the data by CV and running the avalanche statistics for the subsampled model, we obtained scaling exponents that vary continuously, in remarkable similarity to what is observed in the experimental data (Figure 2F).
A critical system with an absorbing-active phase transition which satisfies Equations (1)–(3) is also expected to satisfy the so-called crackling noise scaling relation of Equation (4). Figure 3A shows the independent experimental fits for the left- and right-hand sides of Equation (4) as a function of CV. The crossing at CV* ≃ 1.46±0.08 is consistent with the phase transition reported by Fontenele et al. (2019). In the crossing CV*, we obtain τ* = 1.54 ± 0.12, τt* = 1.73±0.18, and 1/(σνz)* = 1.30 ± 0.02. Plotting τ vs. τt, the experimental data scatter along the line with slope given by 1/(σνz)* for different values of CV (Figure 3B). These results are in agreement with those of Fontenele et al. (2019), again suggesting an incompatibility with the MF-DP universality class.
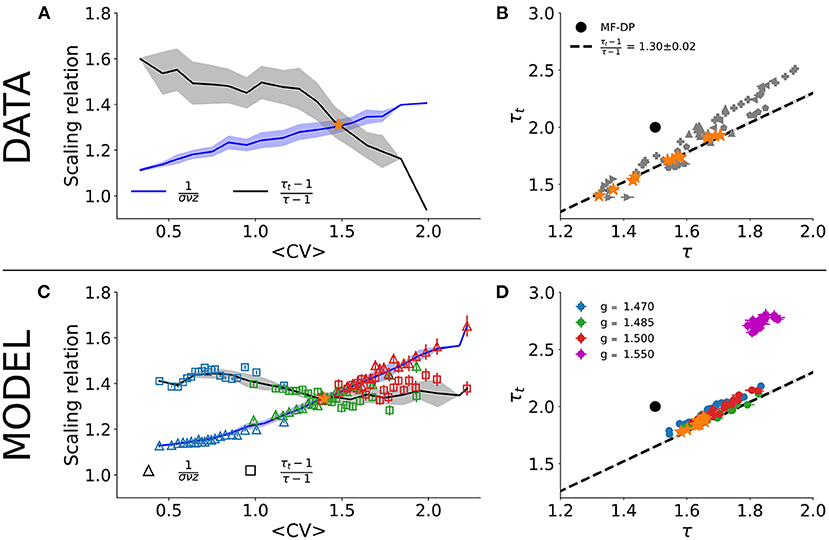
Figure 3. Scaling relation and parametric plot of avalanche exponents. Right- and left-sides of Equation (4) (line and shade are average and standard deviation across the group) as a function of the average CV for (A) experimental data and (C) subsampled model (n = 100; note that color code and values of g are the same as in Figures 2D,F). Scatter plot in the (τ, τt) plane for (B) experimental data and (D) subsampled model. In both cases, Δt = 〈ISI〉 and w = 10 s. The star points in (B,D) indicate the values of τ and τt that satisfied Equation (4) in (A,C). In Supplementary Figure 2, we show the same result in (A) for each rat separately.
The results for the subsampled spiking model, however, suggest otherwise. We did exactly the same procedure with the subsampled model and found a similar CV for the crossing of the critical exponents, , when controlling for the E/I ratio g very close to the critical point gc = 1.5 (Figure 3C). On the crossing , we obtained τ* = 1.65 ± 0.02, τt* = 1.87 ± 0.03, and 1/(σνz)* = 1.34 ± 0.02. Note that these critical exponents are not the true exponents of the model. In fact, they are apparent exponents generated by subsampling the network activity. The true critical exponents are τ = 3/2, τt = 2 and 1/(σνz) = 2 (as shown in Figure 1).
To reproduce the experimental results, the control interval of g was slightly biased toward the supercritical range: gmin ≃ 1.47 ≤ g ≤ gmax ≃ 1.50. Our model predicts, then, that the whole range of experimental results is produced by fluctuations of only about 2% around the critical point (Figure 3D). For instance, for g = 1.55 (3% above the critical point in the subcritical regime), the scaling relation is no longer satisfied and the measured exponents fall far away from the linear relation observed experimentally in the (τ, τt) plane (Figure 3D).
This result shows that the MF-DP phase transition under subsampling conditions is capable of reproducing a whole range of experimentally observed avalanches across a range of CV values. To test the robustness of our findings, we employed exactly the same procedure to a simpler model, a probabilistic cellular automaton (section 2.2). This model is also knowingly of the MF-DP type (Kinouchi and Copelli, 2006), but has a random network topology. All the results were similar (see Supplementary Figure 3), showing that the apparent exponents are a direct consequence of subsampling.
3.3. Dependence on Sampling Fraction and Time Bin Width
How robust are the results of the model at criticality against variation in the sampling size (n) and time bin width (Δt)? First, we considered the time bin width as the population interspike interval Δt = 〈ISI〉. The minimum sampling size we employed was n = 30 so that power laws still satisfied Akaike's Information Criterion. The agreement of both sides of the scaling law enhances with growing sampling fraction (Figures 4A,B). However, 〈ISI〉 decreases with the number of neurons sampled (inset of Figure 4B). When the natural bin decreases below 1 ms (the time step of the model), the analysis no longer makes sense. As n increases, the relation between τ and τt converges to the apparent critical scaling that fits experimental results (Figure 4B).
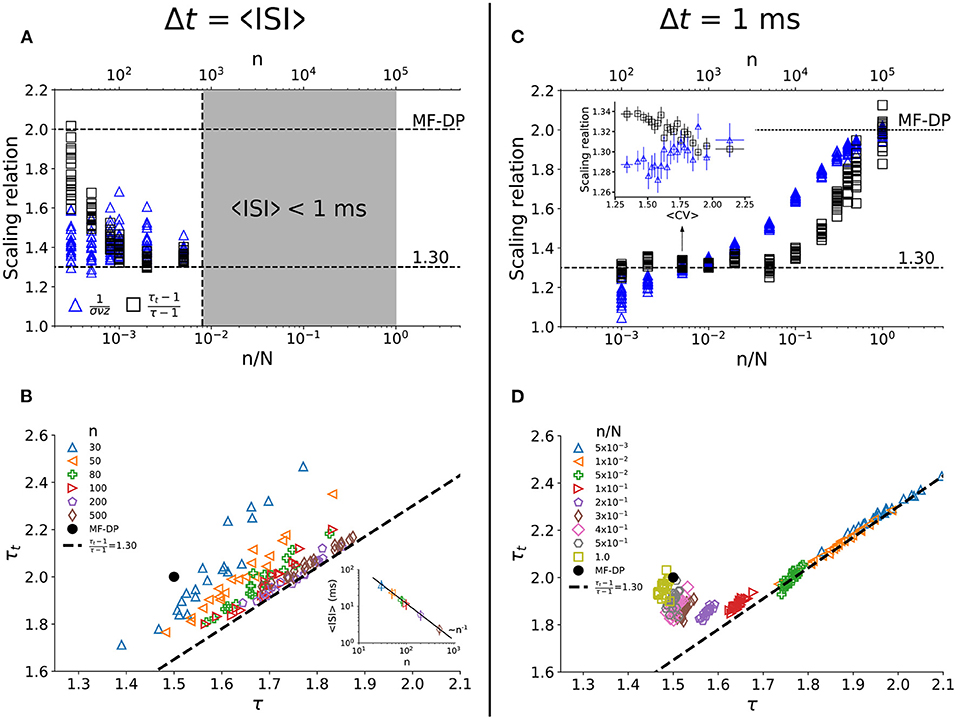
Figure 4. Dependence of the apparent critical exponents on the sampling parameters at criticality. In (A,C), we show both sides of the scaling relation (Equation 4) for all values of CV observed in the simulations. For each value of n/N, one has the equivalent of the projection of Figure 3C onto its vertical axis. For Δt = 〈ISI〉, (A) the scaling relation is satisfied for increasing number of sampled neurons (B) with exponents that agree with experimental data. Since 〈ISI〉 decreases with n [inset of (B)], this analysis breaks down when n is so large that 〈ISI〉 becomes smaller than 1 ms [gray region in (A)], which is the time step of the simulations. For Δt = 1 ms, (C) the scaling relation is satisfied for small n/N, within a relatively wide range of CV values [inset of (C)]. For n/N → 1, results converge to MF-DP values (C,D), as expected. Simulations with N = 105 and gc = 1.5.
To check whether we could recover the MF-DP real exponents from their apparent values as n increases, we chose the smallest time bin possible, Δt = 1 ms. We observed that for a small fraction of sampled units [] the scaling relation (Equation 4) is satisfied (Figure 4C) with apparent critical exponents that match the experimental results (Figure 4D). In fact, the scaling relation in Equation 4 is satisfied for a range of CV values (inset of Figure 4C). Increasing the sampling further [], the scaling relation ceases to be satisfied (Figure 4C) and the avalanche exponents get separated from the experimental scaling relation (Figure 4D). But as n → N, the MF-DP scaling relation is recovered (as it should).
We have further tested the robustness of these findings by varying the time bin width used to defined avalanches (0.75 ≤ Δt/〈ISI〉 ≤ 2). We observed that experiments and model have very similar behavior (Supplementary Figures 4A,B). Furthermore, both model and experiments are virtually insensitive to the width of the CV window w (Supplementary Figures 4C,D). Finally, we also tested whether allowing for small changes of g around gc with n = N would lead to apparent exponents compatible with experimental data. We observed in this case that the exponents and the scaling relation cluster around MF-DP values (Supplementary Figure 5), reinforcing the idea that subsampling is a necessary ingredient for the model to reproduce the experimental results.
3.4. Pairwise Correlation Structure
We also tested the correlation structure of the model and compared it to experimental results. In the literature on cortical states, asynchronous states are associated with pairwise spiking correlations r(k, l) which are distributed around an average close to zero, whereas synchronous states have positive average (Harris and Thiele, 2011). This was quantified in Figure 5A, where is shown to increase monotonically with CV. For the experimental data, reaches zero within the standard deviation of the distribution for sufficiently small CV.
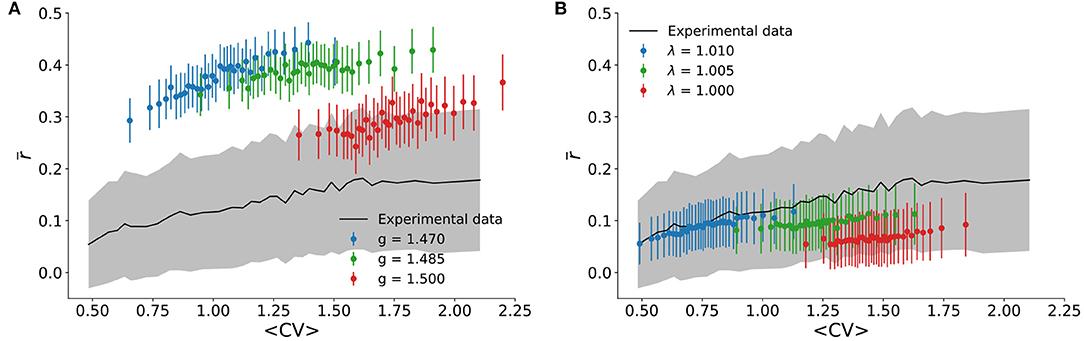
Figure 5. Correlation structure. The experimental pairwise correlation of firing rates is shown as a function of 〈CV〉 (black line is the average , while gray shading is the standard deviation of the distribution). It is compared with theoretical results for (A) the spiking neuronal network with n = 100 sampled neurons, and (B) the cellular automaton model with n = 500 sampled sites.
Compared with the experimental results, the spiking model with inhibition generally overestimates (Figure 5A). This could be due to its all-to-all connectivity. The cellular automaton model on a random graph yields quantitatively better results (Figure 5B). In either case, we observed again that, just like for the scaling relation (Figure 3), the correlation structure of the experimental data was relatively well-reproduced by very small deviations around critical parameter values.
4. Discussion and Conclusions
We revisited the results recently published by Fontenele et al. (2019) by repeating their analyses on new experimental data and two different models. To test the idea that the urethanized cortex hovers around a critical point, we stratified the avalanche analyses across cortical states. For the new experimental data, we verified that the scaling relation combining the three exponents (Equation 4) was indeed satisfied at an intermediate value CV*, away from the synchronous and asynchronous extremes. At this critical value, the three exponents differ from those of the MF-DP universality class, thus confirming previous findings (Fontenele et al., 2019).
We addressed whether the exponents of the MF-DP universality class and those observed experimentally could be reconciled, despite their disagreement. In other words, we returned to the question: if the brain is critical, what is the phase transition? Do the experimental results presented here and in Fontenele et al. (2019) refute branching-process-like models as explanations?
To answer these questions, we relied on two models: an E/I spiking neuronal network in an all-to-all graph; and a probabilistic excitable cellular automaton in a random graph. Despite the simplicity and limitations of these models (which we discuss below), they have a fundamental strength that led us to choose them: they are very well-understood analytically. In both cases, mean-field calculations agree extremely well with simulations, so that we are safe in locating the critical points of these models (Kinouchi and Copelli, 2006; Girardi-Schappo et al., 2020). This is very important for our purposes, because it allows us to test whether the models can reproduce the data, and if so, how close to the critical point they have to be. Besides, their universality class is also well-determined: the exponents shown in Figures 1B–D are those of with MF-DP.
The crucial point is that the results in Figure 1 are based on avalanches which are measured by taking into account all simulated units of the model, a methodological privilege that is not available to an experimentalist measuring spiking activity of a real brain with current technologies. In fact, a considerable amount of work has shown that subsampling can have a drastic effect on the avalanche statistics of models (Priesemann et al., 2009, 2014; Ribeiro et al., 2010, 2014; Girardi-Schappo et al., 2013; Levina and Priesemann, 2017; Wilting and Priesemann, 2019). Therefore, here we set out to test whether MF-DP models could yield results nominally incompatible with that universality class if they were analyzed under the same conditions as the data, i.e., with CV parsing and severe subsampling.
Both subsampled models quantitatively and qualitatively reproduced the central features of the experimental results. The scaling relation (Equation 4) was satisfied at an intermediate value 〈CV〉*, with the correct qualitative behavior of both sides of the equation: 1/(σνz) increasing with CV, while (τt − 1)/(τ − 1) decreasing (see Figures 3A,C and Supplementary Figure 3D). In fact, the values of 〈CV〉*, and those of the apparent exponents of the subsampled MF-DP models, τ*, τt*, and 1/(σνz)*, agreed with the experiments within error bars. Moreover, even away from the point 〈CV〉* where Equation (4) was satisfied, the spread of the exponents τ and τt of the subsampled models followed an almost linear relation (Figure 3D and Supplementary Figure 3E), in good agreement with not only our experimental results (Figure 3B), but also with those of other experimental setups (Fontenele et al., 2019). When we sampled from the whole network, we recovered the true critical exponents of the model (Figures 4C,D), confirming that spatial subsampling and temporal binning are sufficient ingredients to push its critical exponents toward apparent values, hiding its true critical phase transition.
Knowing analytically the critical points of the models, we checked in which parameter range they successfully reproduced the experimental results. As it turns out, the scaling relation and the linear of spread of exponents are reproduced by the subsampled models only if they are tuned within a narrow interval around their critical points. The subsampled model still fits well the urethanized cortex data up to 3% off criticality, slightly biased toward the supercritical state. Note that if the model becomes too subcritical, the size and duration exponents fall very far apart from the experimentally observed linear relation (Figure 3D). If it is too supercritical, there are not enough silent windows to distinguish avalanches in the first place. Whether or not the fluctuations around the critical point of the model could be compatible with a scenario of self-organized-quasi-criticality (Buendía et al., 2020; Kinouchi et al., 2020) remains to be investigated.
Despite the small variation of the model E/I levels controlled by g, the variation of CV is large enough to essentially cover the range of experimentally observed values (Figures 2A,D and Supplementary Figure 3B). This is due in part to the fact that we evaluated CV within finite windows of width w = 10 s. In Supplementary Figure 6 we show that, for the model, the standard deviation of CV is a decreasing function of the time used to estimate, all the way up to w = 500 s. For the data, on the other hand, a better resolution for CV can be obtained by increasing w up to about 20 s, above which the standard deviation no longer decreases. It is important to note, however, that in experiments one needs to reach a good trade-off between a better statistical definition of CV and not mixing different cortical states due to the non-stationarity characteristic of the urethane preparation (as depicted in Figure 2A).
Perhaps even more important than the range of CV values obtained around the critical point of the models is the richness of the experimentally observed temporal evolution of CV (Figure 2A). The model needs to be fine tuned to different values of E/I levels in order to get different average values of CV. This is one of the limitations of the models which would be worth addressing next. One possibility would be to replace static models (i.e., with fixed control parameters) with ones with plasticity, in which coupling parameters are themselves dynamic variables and the critical point is obtained via quasicritical self-organization (Costa et al., 2015, 2017; Brochini et al., 2016; Campos et al., 2017; Kinouchi et al., 2019, 2020; Buendía et al., 2020; Girardi-Schappo et al., 2020).
Moreover, both models failed to capture the steep drop of (τt − 1)/(τ − 1) as a function of CV that is observed in the experimental data above CV* (compare Figure 3A with Figure 3C and Supplementary Figure 3D). This region corresponds to high CV, where the models, which are entering their subcritical regimes, seem unable to quantitatively account for the statistics of the increasingly bursty behavior of the data. Whether different models (or a refinement of the ones presented here) could reproduce these results more accurately remains to be studied.
Another limitation of the models is their simple topology, which in future works could be improved to come closer to cortical circuitry (Potjans and Diesmann, 2014). This would likely come at the cost of foregoing analytical results to start with, thus augmenting the computational efforts involved. But it would certainly allow to probe the robustness of the results presented here against more realistic topologies. On the other hand, there is quantitative agreement between the apparent exponents of both models (each having a different topology) with the experimental exponents. This suggests that at the scale of the present phenomenology, the average topology should play a minor role.
It is also interesting to compare the performance of the two models in reproducing the experimental results. The cellular automaton model has the advantage of simplicity, corresponding essentially to a minimal model in the MF-DP universality class. The E/I balanced network, on the other hand, has the advantage of incorporating inhibition, which is an important ingredient for modeling cortical circuitry. As shown in detail in Supplementary Figure 4, the cellular automaton results generally agreed with experimental results, but those of the E/I balanced network had a consistently better agreement. The only exception in this trend was the correlation structure shown in Figure 5, in which the cellular automaton model fared better than the E/I balanced network. In this sense, the models complement each other.
Our model predicts that, for a fixed bin size, increasing the sampling of the data would eventually lead exponents to coincide with those of MF-DP (Figure 4C). However, below a sufficiently high sampling [see, e.g. in Figure 4C], the scaling relation would not be satisfied for any CV even if the system were critical (as the model is). An experimental verification of these predictions would require the recording of a much larger number of neurons than we have presented here.
The fact that subsampling seems to be a crucial ingredient for explaining the data is a double-edged sword. On the one hand, it allowed us here to reconcile MF-DP models with results for spiking data in the anesthetized rat cortex. On the other hand, note that even measurements which should in principle be less prone to subsampling, such as LFP results in the visual cortex of the turtle (Shew et al., 2015), still fall on the same scaling line of τ vs. τt (Figure 3B) as those of spiking data (Fontenele et al., 2019), both having apparent non-MF-DP critical exponents (note, however, that better controlled LFP results in Miller et al., 2019 are in line with MF-DP). This issue is not addressed by the current model and deserves further investigation. Our results point only to MF-DP models as sufficient, not as necessary, to explain the observed phenomenology. So it is at least conceivable that different models with different phase transitions (di Santo et al., 2018; Dalla Porta and Copelli, 2019; Pinto and Copelli, 2019) could also yield non-trivial true or apparent exponents compatible with the data, even without subsampling (Fontenele et al., 2019).
Finally, our simulation results underscore the methodological vulnerabilities of assessing criticality exclusively via avalanche analysis. Not only are MLE power-law fits sensitive to parameters, but even a more stringent analysis, requiring the crackling noise scaling relation, leads to non-trivial apparent exponents which are an artifact of subsampling, as we have shown. Therefore, the development of additional figures of merit, such as control and order parameters, susceptibilities and others (Tagliazucchi et al., 2012; Yang et al., 2012; Yu et al., 2013; Mora et al., 2015; Tkačik et al., 2015; Girardi-Schappo et al., 2016; Girardi-Schappo and Tragtenberg, 2018; Lotfi et al., 2020), remains a very important line of research to strengthen studies of brain criticality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Housing, surgical and recording procedures were in strict accordance with the CONCEA-MCTI, and was approved by the Federal University of Pernambuco (UFPE) Committee for Ethics in Animal Experimentation (23076.030111/2013-95, 12/2015 and 20/2020).
Author Contributions
TC, MG-S, PC, and MC designed the study, interpreted the results, and wrote the manuscript. TC ran the simulations. TC and AF analyzed the data. TF, LA, TS, and NV obtained the experimental data. All authors read and approved the submitted version.
Funding
The authors acknowledge Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) (grants APQ-0642-1.05/18, APQ-0826-1.05/15, and BFD-0013-1.05/20), Universidade Federal de Pernambuco, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grants 141579/2017-0, 425329/2018-6, and 301744/2018-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and FAPESP (grant 2018/09150-9). This paper was produced as part of the activities of Research, Innovation and Dissemination Center for Neuromathematics (grant No. 2013/07699-0, S. Paulo Research Foundation–FAPESP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Osame Kinouchi for discussions and a critical read of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncir.2020.576727/full#supplementary-material
Footnotes
References
Akaike, H. (1975). A new look at the statistical model identification. IEEE Trans. Autom. Control. 19, 716–723. doi: 10.1109/TAC.1974.1100705
Beggs, J. M. (2007). The criticality hypothesis: how local cortical networks might optimize information processing. Philos. Trans. R. Soc. A 366, 329–343. doi: 10.1098/rsta.2007.2092
Beggs, J. M., and Plenz, D. (2003). Neuronal avalanches in neocortical circuits. J. Neurosci. 23, 11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003
Bellay, T., Klaus, A., Seshadri, S., and Plenz, D. (2015). Irregular spiking of pyramidal neurons organizes as scale-invariant neuronal avalanches in the awake state. eLife 4:e07224. doi: 10.7554/eLife.07224
Binney, J. J., Dowrick, N. J., Fisher, A. J., and Newman, M. E. J. (1992). The Theory of Critical Phenomena: An Introduction to the Renormalization Group. Oxford: Oxford University Press.
Brochini, L., Costa, A. A., Abadi, M., Roque, A. C., Stolfi, J., and Kinouchi, O. (2016). Phase transitions and self-organized criticality in networks of stochastic spiking neurons. Sci. Rep. 6:35831. doi: 10.1038/srep35831
Buendía, V., di Santo, S., Bonachela, J. A., and Muñoz, M. A. (2020). Feedback mechanisms for self-organization to the edge of a phase transition. Front. Phys. 8:333. doi: 10.3389/fphy.2020.00333
Campos, J. G. F., Costa, A. A., Copelli, M., and Kinouchi, O. (2017). Correlations induced by depressing synapses in critically self-organized networks with quenched dynamics. Phys. Rev. E 95:042303. doi: 10.1103/PhysRevE.95.042303
Chialvo, D. R. (2010). Emergent complex neural dynamics. Nat. Phys. 6, 744–750. doi: 10.1038/nphys1803
Clement, E. A., Richard, A., Thwaites, M., Ailon, J., Peters, S., and Dickson, C. T. (2008). Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE 3:e2004. doi: 10.1371/journal.pone.0002004
Costa, A. A., Brochini, L., and Kinouchi, O. (2017). Self-organized supercriticality and oscillations in networks of stochastic spiking neurons. Entropy 19:399. doi: 10.3390/e19080399
Costa, A. A., Copelli, M., and Kinouchi, O. (2015). Can dynamical synapses produce true self-organized criticality? J. Stat. Mech. Theory Exp. 2015:P06004. doi: 10.1088/1742-5468/2015/06/P06004
Curto, C., Sakata, S., Marguet, S., Itskov, V., and Harris, K. D. (2009). A simple model of cortical dynamics explains variability and state dependence of sensory responses in urethane-anesthetized auditory cortex. J. Neurosci. 29, 10600–10612. doi: 10.1523/JNEUROSCI.2053-09.2009
Dalla Porta, L., and Copelli, M. (2019). Modeling neuronal avalanches and long-range temporal correlations at the emergence of collective oscillations: continuously varying exponents mimic M/EEG results. PLoS Comput. Biol. 15:e1006924. doi: 10.1371/journal.pcbi.1006924
de Vasconcelos, N. A. P., Soares-Cunha, C., Rodrigues, A. J., Ribeiro, S., and Sousa, N. (2017). Coupled variability in primary sensory areas and the hippocampus during spontaneous activity. Sci. Rep. 7:46077. doi: 10.1038/srep46077
Deluca, A., and Corral, Á. (2013). Fitting and goodness-of-fit test of non-truncated and truncated power-law distributions. Acta Geophys. 61, 1351–1394. doi: 10.2478/s11600-013-0154-9
di Santo, S., Villegas, P., Burioni, R., and Muñoz, M. A. (2018). Landau-Ginzburg theory of cortex dynamics: scale-free avalanches emerge at the edge of synchronization. Proc. Natl. Acad. Sci. U.S.A. 115, E1356–E1365. doi: 10.1073/pnas.1712989115
Fontenele, A. J., de Vasconcelos, N. A. P., Feliciano, T., Aguiar, L. A. A., Soares-Cunha, C., Coimbra, B., et al. (2019). Criticality between cortical states. Phys. Rev. Lett. 122:208101. doi: 10.1103/PhysRevLett.122.208101
Friedman, N., Ito, S., Brinkman, B. A. W., Shimono, M., DeVille, R. E. L., Dahmen, K. A., et al. (2012). Universal critical dynamics in high resolution neuronal avalanche data. Phys. Rev. Lett. 108:208102. doi: 10.1103/PhysRevLett.108.208102
Gervasoni, D., Lin, S., Ribeiro, S., Soares, E. S., Pantoja, J., and Nicolelis, M. A. L. (2004). Global forebrain dynamics predict rat behavioral states and their transitions. J. Neurosci. 24, 11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004
Girardi-Schappo, M., Bortolotto, G. S., Gonsalves, J. J., Pinto, L. T., and Tragtenberg, M. H. R. (2016). Griffiths phase and long-range correlations in a biologically motivated visual cortex model. Sci. Rep. 6:29561. doi: 10.1038/srep29561
Girardi-Schappo, M., Brochini, L., Costa, A. A., Carvalho, T. T. A., and Kinouchi, O. (2020). Synaptic balance due to homeostatically self-organized quasicritical dynamics. Phys. Rev. Res. 2:012042(R). doi: 10.1103/PhysRevResearch.2.012042
Girardi-Schappo, M., Kinouchi, O., and Tragtenberg, M. H. R. (2013). Critical avalanches and subsampling in map-based neural networks coupled with noisy synapses. Phys. Rev. E 88:024701. doi: 10.1103/PhysRevE.88.024701
Girardi-Schappo, M., and Tragtenberg, M. H. R. (2018). Measuring neuronal avalanches in disordered systems with absorbing states. Phys. Rev. E 97:042415. doi: 10.1103/PhysRevE.97.042415
Grassberger, P. (1982). On phase transitions in schlögl's second model. Z. Phys. B Cond. Matter 47, 365–374. doi: 10.1007/BF01313803
Harris, K. D., and Thiele, A. (2011). Cortical state and attention. Nat. Rev. Neurosci. 12:509. doi: 10.1038/nrn3084
Janssen, H. K. (1981). On the nonequilibrium phase transition in reaction-diffusion systems with an absorbing stationary state. Z. Phys. B Cond. Matter 42, 151–154. doi: 10.1007/BF01319549
Kinouchi, O., Brochini, L., Costa, A. A., Campos, J. G. F., and Copelli, M. (2019). Stochastic oscillations and dragon king avalanches in self-organized quasi-critical systems. Sci. Rep. 9:3874. doi: 10.1038/s41598-019-40473-1
Kinouchi, O., and Copelli, M. (2006). Optimal dynamical range of excitable networks at criticality. Nat. Phys. 2:348. doi: 10.1038/nphys289
Kinouchi, O., Pazzini, R., and Copelli, M. (2020). Mechanisms of self-organized quasicriticality in neuronal networks models. Front. Phys. 8:583212. doi: 10.3389/fphy.2020.583213
Levina, A., and Priesemann, V. (2017). Subsampling scaling. Nat. Commun. 8:15140. doi: 10.1038/ncomms15140
Lotfi, N., Fontenele, A. J., Feliciano, T., Aguiar, L. A., de Vasconcelos, N. A., Soares-Cunha, C., et al. (2020). Signatures of brain criticality unveiled by maximum entropy analysis across cortical states. Phys. Rev. E 102:012408. doi: 10.1103/PhysRevE.102.012408
Ma, Z., Turrigiano, G. G., Wessel, R., and Hengen, K. B. (2019). Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron 104, 655–664. doi: 10.1016/j.neuron.2019.08.031
Marro, J., and Dickman, R. (1999). Nonequilibrium Phase Transition in Lattice Models. Cambridge: Cambridge University Press.
Marshall, N., Timme, N. M., Bennett, N., Ripp, M., Lautzenhiser, E., and Beggs, J. M. (2016). Analysis of power laws, shape collapses, and neural complexity: new techniques and MATLAB support via the NCC toolbox. Front. Physiol. 7:250. doi: 10.3389/fphys.2016.00250
Miller, S. R., Yu, S., and Plenz, D. (2019). The scale-invariant, temporal profile of neuronal avalanches in relation to cortical γ-oscillations. Sci. Rep. 9:16403. doi: 10.1038/s41598-019-52326-y
Mochol, G., Hermoso-Mendizabal, A., Sakata, S., Harris, K. D., and De la Rocha, J. (2015). Stochastic transitions into silence cause noise correlations in cortical circuits. Proc. Natl. Acad. Sci. U.S.A. 112, 3529–3534. doi: 10.1073/pnas.1410509112
Mora, T., Deny, S., and Marre, O. (2015). Dynamical criticality in the collective activity of a population of retinal neurons. Phys. Rev. Lett. 114:078105. doi: 10.1103/PhysRevLett.114.078105
Muñoz, M. A., Dickman, R., Vespignani, A., and Zapperi, S. (1999). Avalanche and spreading exponents in systems with absorbing states. Phys. Rev. E 59, 6175–6179. doi: 10.1103/PhysRevE.59.6175
Palva, J. M., Zhigalov, A., Hirvonen, J., Korhonen, O., Linkenkaer-Hansen, K., and Palva, S. (2013). Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proc. Natl. Acad. Sci. U.S.A. 110, 3585–3590. doi: 10.1073/pnas.1216855110
Paxinos, G., and Watson, C. (2007). The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Amsterdam: Elsevier Science.
Pinto, I. L. D., and Copelli, M. (2019). Oscillations and collective excitability in a model of stochastic neurons under excitatory and inhibitory coupling. Phys. Rev. E 100:062416. doi: 10.1103/PhysRevE.100.062416
Ponce-Alvarez, A., Jouary, A., Privat, M., Deco, G., and Sumbre, G. (2018). Whole-brain neuronal activity displays crackling noise dynamics. Neuron 100, 1446–1459. doi: 10.1016/j.neuron.2018.10.045
Potjans, T. C., and Diesmann, M. (2014). The cell-type specific cortical microcircuit: relating structure and activity in a full-scale spiking network model. Cereb. Cortex 24, 785–806. doi: 10.1093/cercor/bhs358
Priesemann, V., Munk, M. H. J., and Wibral, M. (2009). Subsampling effects in neuronal avalanche distributions recorded in vivo. BMC Neurosci. 10:40. doi: 10.1186/1471-2202-10-40
Priesemann, V., Wibral, M., Valderrama, M., Pröpper, R., Le Van Quyen, M., Geisel, T., et al. (2014). Spike avalanches in vivo suggest a driven, slightly subcritical brain state. Front. Syst. Neurosci. 8:108. doi: 10.3389/fnsys.2014.00108
Renart, A., de la Rocha, J., Bartho, P., Hollender, L., Parga, N., Reyes, A., et al. (2010). The asynchronous state in cortical circuits. Science 327, 587–590. doi: 10.1126/science.1179850
Ribeiro, T. L., Copelli, M., Caixeta, F., Belchior, H., Chialvo, D. R., Nicolelis, M. A. L., et al. (2010). Spike avalanches exhibit universal dynamics across the sleep-wake cycle. PLoS ONE 5:e14129. doi: 10.1371/journal.pone.0014129
Ribeiro, T. L., Ribeiro, S., Belchior, H., Caixeta, F., and Copelli, M. (2014). Undersampled critical branching processes on small-world and random networks fail to reproduce the statistics of spike avalanches. PLoS ONE 9:e94992. doi: 10.1371/journal.pone.0094992
Rossant, C., Kadir, S. N., Goodman, D. F. M., Schulman, J., Hunter, M. L. D., Saleem, A. B., et al. (2016). Spike sorting for large, dense electrode arrays. Nat. Neurosci. 19:634. doi: 10.1038/nn.4268
Sakata, S., and Harris, K. D. (2009). Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64, 404–418. doi: 10.1016/j.neuron.2009.09.020
Scott, G., Fagerholm, E. D., Mutoh, H., Leech, R., Sharp, D. J., Shew, W. L., et al. (2014). Voltage imaging of waking mouse cortex reveals emergence of critical neuronal dynamics. J. Neurosci. 34, 16611–16620. doi: 10.1523/JNEUROSCI.3474-14.2014
Sethna, J. P., Dahmen, K. A., and Myers, C. R. (2001). Crackling noise. Nature 410, 242–250. doi: 10.1038/35065675
Shadlen, M. N., and Newsome, W. T. (1998). The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998
Shew, W. L., Clawson, W. P., Pobst, J., Karimipanah, Y., Wright, N. C., and Wessel, R. (2015). Adaptation to sensory input tunes visual cortex to criticality. Nat. Phys. 11, 659–663. doi: 10.1038/nphys3370
Shew, W. L., and Plenz, D. (2013). The functional benefits of criticality in the cortex. Neuroscientist 19, 88–100. doi: 10.1177/1073858412445487
Shew, W. L., Yang, H., Petermann, T., Roy, R., and Plenz, D. (2009). Neuronal avalanches imply maximum dynamic range in cortical networks at criticality. J. Neurosci. 29, 15595–15600. doi: 10.1523/JNEUROSCI.3864-09.2009
Siegle, J. H., López, A. C., Patel, Y. A., Abramov, K., Ohayon, S., and Voigts, J. (2017). Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 14:045003. doi: 10.1088/1741-2552/aa5eea
Somogyi, P., Tamás, G., Lujan, R., and Buhl, E. H. (1998). Salient features of synaptic organisation in the cerebral cortex. Brain Res. Rev. 26, 113–135. doi: 10.1016/S0165-0173(97)00061-1
Tagliazucchi, E., Balenzuela, P., Fraiman, D., and Chialvo, D. R. (2012). Criticality in large-scale brain fMRI dynamics unveiled by a novel point process analysis. Front. Physiol. 3:15. doi: 10.3389/fphys.2012.00015
Tkačik, G., Mora, T., Marre, O., Amodei, D., Palmer, S. E., Berry, M. J., et al. (2015). Thermodynamics and signatures of criticality in a network of neurons. Proc. Natl. Acad. Sci. U.S.A. 112, 11508–11513. doi: 10.1073/pnas.1514188112
Tomen, N., Herrmann, M. J., and Ernst, U. (2019). The Functional Role of Critical Dynamics in Neural Systems. Cham: Springer.
Touboul, J., and Destexhe, A. (2017). Power-law statistics and universal scaling in the absence of criticality. Phys. Rev. E 95:012413. doi: 10.1103/PhysRevE.95.012413
Wilting, J., and Priesemann, V. (2019). Between perfectly critical and fully irregular: a reverberating model captures and predicts cortical spike propagation. Cereb. Cortex 29, 2759–2770. doi: 10.1093/cercor/bhz049
Yang, H., Shew, W. L., Roy, R., and Plenz, D. (2012). Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J. Neurosci. 32, 1061–1072. doi: 10.1523/JNEUROSCI.2771-11.2012
Yu, S., Klaus, A., Yang, H., and Plenz, D. (2014). Scale-invariant neuronal avalanche dynamics and the cut-off in size distributions. PLoS ONE 9:e99761. doi: 10.1371/journal.pone.0099761
Yu, S., Ribeiro, T. L., Meisel, C., Chou, S., Mitz, A., Saunders, R., et al. (2017). Maintained avalanche dynamics during task-induced changes of neuronal activity in nonhuman primates. eLife 6:e27119. doi: 10.7554/eLife.27119
Yu, S., Yang, H., Shriki, O., and Plenz, D. (2013). Universal organization of resting brain activity at the thermodynamic critical point. Front. Syst. Neurosci. 7:42. doi: 10.3389/fnsys.2013.00042
Keywords: subsampling, neuronal avalanches, brain criticality, scaling relations, cortex, urethane
Citation: Carvalho TTA, Fontenele AJ, Girardi-Schappo M, Feliciano T, Aguiar LAA, Silva TPL, de Vasconcelos NAP, Carelli PV and Copelli M (2021) Subsampled Directed-Percolation Models Explain Scaling Relations Experimentally Observed in the Brain. Front. Neural Circuits 14:576727. doi: 10.3389/fncir.2020.576727
Received: 26 June 2020; Accepted: 30 November 2002;
Published: 15 January 2021.
Edited by:
Axel Sandvig, Norwegian University of Science and Technology, NorwayReviewed by:
Dietmar Plenz, National Institute of Mental Health, National Institutes of Health (NIH), United StatesAfshin Montakhab, Shiraz University, Iran
Copyright © 2021 Carvalho, Fontenele, Girardi-Schappo, Feliciano, Aguiar, Silva, de Vasconcelos, Carelli and Copelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauro Copelli, bWF1cm8uY29wZWxsaUB1ZnBlLmJy
 Tawan T. A. Carvalho
Tawan T. A. Carvalho Antonio J. Fontenele
Antonio J. Fontenele Mauricio Girardi-Schappo
Mauricio Girardi-Schappo Thaís Feliciano
Thaís Feliciano Leandro A. A. Aguiar
Leandro A. A. Aguiar Thais P. L. Silva
Thais P. L. Silva Nivaldo A. P. de Vasconcelos
Nivaldo A. P. de Vasconcelos Pedro V. Carelli
Pedro V. Carelli Mauro Copelli
Mauro Copelli