
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neural Circuits, 22 November 2017
Volume 11 - 2017 | https://doi.org/10.3389/fncir.2017.00092
This article is part of the Research TopicSleep and Circuit PlasticityView all 10 articles
Sleep plays important roles in sensory and motor memory consolidation. Sleep oscillations, reflecting neural population activity, involve the reactivation of learning-related neurons and regulate synaptic strength and, thereby affect memory consolidation. Among sleep oscillations, slow waves (0.5–4 Hz) are closely associated with memory consolidation. For example, slow-wave power is regulated in an experience-dependent manner and correlates with acquired memory. Furthermore, manipulating slow waves can enhance or impair memory consolidation. During slow wave sleep, inter-areal interactions between the cortex and hippocampus (HC) have been proposed to consolidate declarative memory; however, interactions for non-declarative (HC-independent) memory remain largely uninvestigated. We recently showed that the directional influence in a slow-wave range through a top-down cortical long-range circuit is involved in the consolidation of non-declarative memory. At the synaptic level, the average cortical synaptic strength is known to be potentiated during wakefulness and depressed during sleep. Moreover, learning causes plasticity in a subset of synapses, allocating memory to them. Sleep may help to differentiate synaptic strength between allocated and non-allocated synapses (i.e., improving the signal-to-noise ratio, which may facilitate memory consolidation). Herein, we offer perspectives on inter-areal interactions and synaptic plasticity for memory consolidation during sleep.
Neural oscillations during sleep support memory consolidation, which refers to post-learning processes whereby new memory transitions from a fragile to a stable form. The existence of the memory consolidation process has been verified by the transient susceptibility of memory to amnesic interventions including sleep-deprivation and drugs inactivating neural activity or preventing synaptic plasticity (Dudai et al., 2015). Synchronized neural activity during sleep forms oscillations with distinct frequencies. During non-rapid eye movement (NREM) sleep, three major oscillations are involved in memory consolidation: slow waves that originate from the neocortex; 12–15 Hz spindles that arise from the thalamus and spread to cortical and hippocampal networks (Lustenberger et al., 2016); and 140–200 Hz hippocampal ripples (Girardeau et al., 2009; Ego-Stengel and Wilson, 2010; Maingret et al., 2016). Slow waves reflect spontaneous alterations of depolarized (up) and hyperpolarized (down) states in cortical neurons (Vyazovskiy and Harris, 2013). They typically originate from layer 5 neurons and spread to other cortical layers (Chauvette et al., 2010). Among cortical areas, slow waves can occur locally, but become more global during deep sleep (Nir et al., 2011; Vyazovskiy et al., 2011). Although slow waves can spread over cortical areas as traveling waves that may originate from different sites, they typically originate in the frontal cortex (FC) in humans (Massimini et al., 2004). While slow waves are prominent during deep NREM sleep, spindles are more obvious during light NREM sleep (Steriade, 2003). Spindles are generated through the interplay of GABAergic interneurons of the reticular nucleus of the thalamus and thalamocortical cells (Steriade et al., 1985, 1987; Halassa et al., 2011). However, corticothalamic projections govern the widespread synchronization of spindles among thalamic areas (Contreras and Steriade, 1996; Contreras et al., 1996; Steriade, 2003). Ripples in the hippocampus (HC) are synchronized with cortical up-states and spindles (Siapas and Wilson, 1998; Sirota et al., 2003; Rothschild et al., 2017), and have been proposed to operate in the inter-areal interactions between the HC and cortex in memory consolidation. During rapid eye movement (REM) sleep, memory consolidation is dependent on medial septum gamma-aminobutyric acid (GABA)-releasing neurons, which generates 4–10 Hz theta oscillations in the HC (Boyce et al., 2016). Among these sleep oscillations, slow waves have significant roles in memory consolidation, which has clinical implications for enhancing memory, because triggering slow waves in humans and animals can enhance memory consolidation (Marshall et al., 2006; Binder et al., 2013, 2014; Ngo et al., 2013, 2015; Miyamoto et al., 2016; Rembado et al., 2017).
Sleep also has functional significance in synaptic plasticity, which is a well-known mechanism involved in learning and memory. Synaptic plasticity has several activity-dependent rules. In the Bienenstock-Cooper-Munro (BCM) frequency-dependent curve (Bienenstock et al., 1982; Cooper and Bear, 2012; Jedlicka et al., 2015), pre-synaptic stimulation at low frequency (~1 Hz) causes synaptic depression, while stimulation at high frequency (>10 Hz) causes synaptic potentiation. Thus, slow waves are believed to depress synaptic activity, although recent studies have shown that slow-wave-like stimulation or lower-frequency (0.1 Hz) stimulation can cause synaptic potentiation (Chauvette et al., 2012; Sandler et al., 2016). In the spike-timing-dependent plasticity (STDP) rule (Dan and Poo, 2004), spike or activity order in pre-synapse and post-synapse define the direction of synaptic plasticity. Hierarchical top-down slow-wave flow (Massimini et al., 2004; Nir et al., 2011; Miyamoto et al., 2016) may lead to synaptic plasticity according to the STDP rule. Furthermore, the hypothesis of synaptic memory allocation, the process that determines which synapses work for specific memory traces—suggests that memory is encoded by a specific subset of synapses (Rogerson et al., 2014; Hayashi-Takagi et al., 2015). During up-states of slow waves, reactivation of neurons (Pavlides and Winson, 1989; Miyamoto et al., 2016) or neuronal ensembles (Ji and Wilson, 2007; Rothschild et al., 2017), which are activated during the awake experience, may differentiate synaptic strength between memory-allocated synapses and non-allocated synapses. Herein, we discuss synaptic plasticity with slow waves representing a candidate mechanism of memory consolidation.
Sensory or motor experience regulates the spectral power of slow waves (slow-wave power) in experience-related brain areas. In humans, tactile stimulation for 6 h in the right hand causes a local increase in slow-wave power in the left hemisphere (Kattler et al., 1994). Moreover, motor learning for several tens of minutes also enhances slow-wave power in the motor area, during post-learning NREM sleep, and this increase in slow-wave power correlates with motor performance improvement (Huber et al., 2004). Conversely, arm immobilization for 12 h impairs motor performance and slow-wave power in the sensorimotor area (Huber et al., 2006). Similarly, depriving visual information by means of 3 months dark-rearing reduces slow-wave power in the visual cortex, but not in the somatosensory cortex in cats and mice (Miyamoto et al., 2003). These experience-dependent changes in slow-wave power indicate a correlation between slow waves and memory.
Researchers have manipulated sleep oscillations to demonstrate causal relations to the consolidation of declarative and non-declarative memories (Stickgold, 2005). Non-declarative (HC-independent) memory, which should not be affected by hippocampal ripples, may have different consolidation mechanisms during sleep; however, these mechanisms remain unclear. In humans, boosting slow waves by means of transcranial direct current stimulation (tDCS) in the FC (Marshall et al., 2006) or with closed-loop in-phase auditory stimulation (Ngo et al., 2013) during NREM sleep can enhance declarative memory consolidation in paired-associate (word-pairs) learning tasks. In non-declarative finger-sequence tapping task, boosting spindles enhanced skill memory consolidation (Lustenberger et al., 2016), and closed-loop auditory stimulation, to perturb slow waves in the motor cortex, made motor execution less efficient (Fattinger et al., 2017). Regarding non-declarative (HC-independent) perceptual memory, we have developed a sleep-dependent tactile discrimination task in mice, and applied optogenetic stimulation (Miyamoto and Murayama, 2016) to the task-associated primary somatosensory cortex (S1) and/or secondary motor cortex (M2), which forms a somatosensory perception-related top-down cortical circuit (Manita et al., 2015, 2017). When the stimulation was synchronized with the frequency of slow waves, it resulted in enhanced memory consolidation. Therefore, slow waves can contribute not only to declarative memory but also to non-declarative skill and perceptual memory.
The causal roles of hippocampal ripples in memory consolidation have also been intensively studied. Ripples are believed to mediate transference of reactivated memory information from the HC to the cortex to support systems memory consolidation (i.e., the post-learning, time-dependent reorganization of long-term memory representations over distributed brain areas; Takehara-Nishiuchi et al., 2006; Inostroza and Born, 2013; Dudai et al., 2015; Kitamura et al., 2017). In support of this concept, disruption of ripples with electrical stimulation impaired consolidation of HC-dependent spatial memory (Girardeau et al., 2009; Ego-Stengel and Wilson, 2010). To more directly test the causal effect of the hippocampo-cortical dialog on memory consolidation, Maingret et al. (2016) enhanced the temporal correlation between the HC and medial prefrontal cortex using ripple-triggered electrical stimulation of deep cortical layers (Maingret et al., 2016). Because triggering cortical oscillations after hippocampal ripples enhanced spatial memory performance, information flow from the HC to the cortex is believed to be important for memory consolidation. Conversely, information flow from the prefrontal cortex or sensory cortex (SC) to the HC (Isomura et al., 2006; Rothschild et al., 2017) may also function in memory consolidation (Figure 1A). Whether bidirectional or unidirectional interaction between the HC and cortex is involved in memory consolidation is yet to be determined.
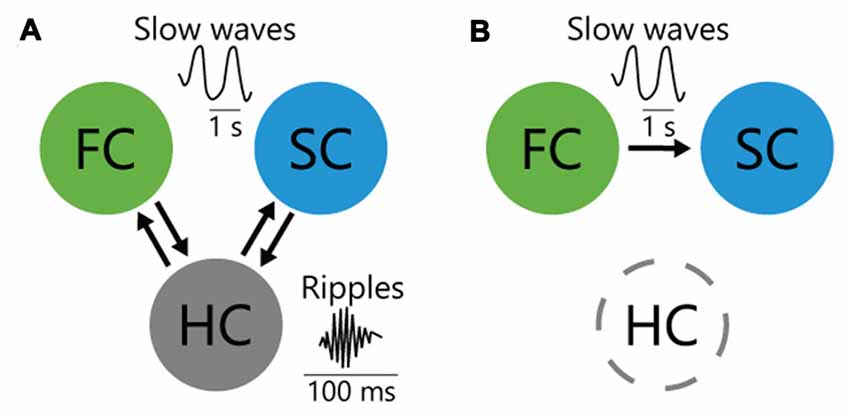
Figure 1. Interareal interactions among cortices and hippocampus (HC) for memory consolidation. (A) HC bidirectionally interacts with frontal cortex (FC) and sensory cortex (SC) through entorhinal cortex during sleep, which may contribute to consolidation of HC-dependent memory. (B) In HC-independent perceptual memory, top-down inputs from the FC to SC during sleep consolidate memory.
Inter-areal interactions have been studied extensively in HC-dependent declarative memory; however, those for HC-independent non-declarative memory remain unclear (Dudai et al., 2015). To elucidate the inter-areal interactions for non-declarative tactile-perceptual memory task, we optogenetically manipulated a bidirectional cortico-cortical pathway between anterior M2 and posterior S1, which supports tactile perception (Manita et al., 2015). While suppressing the posteroanterior S1→M2 (i.e., bottom-up) pathway did not impair memory consolidation, suppressing the anteroposterior M2→S1 (i.e., top-down) slow-wave flow impaired emergence of experience-related reactivated neuron in S1 and impaired memory consolidation, which initially demonstrated the causal contribution of the top-down pathway (Figure 1B). Because similar hierarchical slow-wave flows from the anterior to the posterior cortex during NREM sleep are observed globally in the cortex (Massimini et al., 2004; Nir et al., 2011; Phillips et al., 2012), they may also function in memory reactivation and consolidation in other modalities.
To test the sufficiency of slow waves to elicit memory consolidation, we optogenetically stimulated layer 5 neurons to trigger slow wave production, as has been done previously (Beltramo et al., 2013). In Figure 2, we reproduced from our previous study (Miyamoto et al., 2016) and added new data in Figure 2D and a part of Figure 2F. By using optogenetic stimulation during NREM sleep, we synchronized or anti-synchronized slow waves between M2 and S1 (Miyamoto et al., 2016). Synchronized stimulation enhanced the retention periods of tactile recognition memory, while anti-synchronized stimulation impaired memory consolidation (Figure 2C from Miyamoto et al., 2016). A recent optogenetic study reported that neuronal activity in the visual cortex during NREM sleep was required for visual experience-dependent plasticity (Durkin et al., 2017). In reverse, to reveal the brain area that is sufficient for enhancement of perceptual memory consolidation, we applied optogenetic stimulation to S1 or M2, alone. Stimulation of M2 per se did not enhance tactile memory retention periods (Figure 2D). The stimulation of S1 per se could enhance memory retention periods for at least 4 days, indicating that S1 is sufficient for memory consolidation (Figure 2D). However, mouse performance with the stimulation (Figure 2D) was lower than that with the synchronized stimulation of S1 and M2 (Figure 2C from Miyamoto et al., 2016). These results indicate that coordinated activation of S1 and M2 via top-down slow wave flow is more beneficial for memory consolidation than activation of the sensory area alone. Nonetheless, selective stimulation of a sensory area may have advantages in terms of selective enhancement of memory consolidation in one modality, without affecting systems involving other modalities.
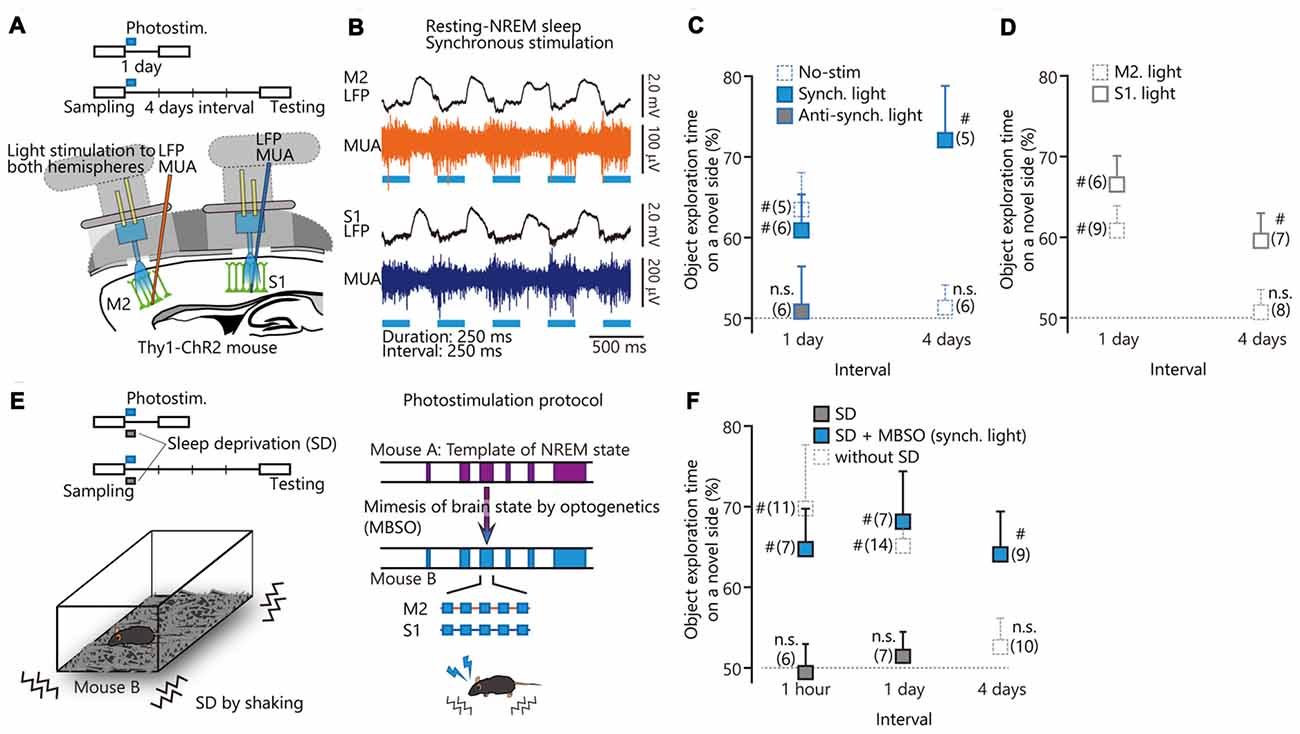
Figure 2. Optogenetic cortical stimulation in slow-wave rhythm for enhancing or impairing memory consolidation. (A) Optogenetic activation of secondary motor cortex (M2) and primary somatosensory cortex (S1) with local field potential (LFP) recordings using single electrodes. See “Methods” in Miyamoto et al. (2016). (B) LFPs and multiunit activity (MUA) recordings from M2 (top) and S1 (bottom) during resting-non-rapid eye movement (NREM) sleep with synchronous photostimulation. (C,D) Task performance after photostimulation during resting-NREM sleep. (E) (Left) Behavioral paradigm. After the sampling period, transgenic mice were deprived of sleep for 1 h with synchronized coactivation of M2 and S1. (Right) Photostimulation protocol. The photostimulation (2 Hz) during the sleep deprivation (SD) experiment was applied according to the NREM sleep pattern from mouse A. (F) Summary of task performance after photostimulation. The cumulative illumination time was 30 min. Statistical significance from 50% chance level (#P < 0.05) was assessed by a 1-sample t test. Panels (A–C,E) and a part of (F) from Miyamoto et al. (2016). Reprinted with permission from AAAS.
To test whether memory consolidation is supported by slow waves per se or by slow waves in the context of global cortical activity and neuromodulation during sleep, we used optogenetic stimulation in sleep-deprived mice. We stimulated M2 and S1 synchronously at 2 Hz to mimic sleep slow waves during sleep-deprived states (Figure 2E from Miyamoto et al., 2016). This synchronous stimulation not only restored memory deficits during sleep deprivation (SD), but prolonged memory retention periods compared to mice with normal sleep (Figure 2F from Miyamoto et al., 2016, excepting data sets of 1 h and 4 days, without SD). Memory consolidation during awake and sleep periods is not only a fundamental question in neuroscience but may also have implications for the treatment of memory deficits in patients with sleep disorders such as insomnia (Backhaus et al., 2006).
We demonstrated that artificial optogenetic stimulation of layer 5 neurons in slow-wave rhythm could restore deficits in memory consolidation caused by SD. This, however, leads to an important question: is it possible to consolidate memory only with spontaneous NREM-like cortical slow waves during REM sleep (Funk et al., 2016) or during active wakefulness (Fisher et al., 2016)? In our perceptual memory task, memory consolidation was dependent on sleep 0–1 h after learning. The 1 h includes the total duration of wakefulness (approximately 43%), NREM (approximately 55%) and REM (approximately 3%; Miyamoto et al., 2016). Because the REM state period was negligible, and spontaneous slow waves are rare and not repetitive during active wakefulness (Vyazovskiy and Harris, 2013; Fisher et al., 2016), neural activity in slow-wave rhythm in the NREM periods, when we applied optogenetic stimulation to sleep-deprived awake mice, would be important for memory consolidation.
The sleep synaptic homeostasis hypothesis (SHY), a leading hypothesis of synaptic plasticity through the sleep/wake cycle, states that the net synaptic strength increases during wakefulness and decreases during sleep (Tononi and Cirelli, 2003, 2006, 2014; Born and Feld, 2012). Slow waves may be related to sleep synaptic depression according to the BCM synaptic modification rule (i.e., low-frequency stimulation induces long-term depression), which was theorized to account for experience-dependent modification of neural activity in the visual cortex (Bienenstock et al., 1982; Nakao et al., 2004; Cooper and Bear, 2012). In fact, the SHY is supported by molecular evidence: protein expression levels of GluA1-containing AMPA receptors, glutamate receptors mediating excitatory inputs, are higher during the active phase in the animal’s circadian rhythm than during the inactive phase, in the cortex and the HC (Vyazovskiy et al., 2008). The SHY is also supported by structural evidence: the net spine number and the size of axon-spine interface (ASI) decreases after sleep (Maret et al., 2011; de Vivo et al., 2017). Moreover, synaptic efficacy measured electrophysiologically in cortico-cortical synapses increases after wake and decreases after sleep (Vyazovskiy et al., 2008). Although these studies have shown synaptic plasticity on average, they support the SHY in the sense that net synaptic potentiation occurs during wakefulness and net synaptic depression occurs during sleep in the cortex.
In contrast, some studies have reported that slow waves may induce synaptic potentiation. Chauvette et al. (2012) have shown that synaptic potentiation occurs through sleep in the thalamo-cortical system in vivo. They have also shown that recapitulation of slow waves in vitro by means of paired presynaptic stimulation and postsynaptic hyperpolarization induces long-term potentiation in layer II/III pyramidal neurons. Surprisingly, unpaired stimulation at even lower frequency (0.1 Hz), which is conventionally used for assessing baseline synaptic strength in many LTP/LTD studies (Urba et al., 1991; Magee and Johnston, 1997; Markram et al., 1997; Sjöström et al., 2001; Gordon et al., 2006), can also induce synaptic potentiation in tuft dendrites (Sandler et al., 2016). The results of these studies are in contradiction to the SHY in the sense that sleep slow wave or even lower-frequency stimulation can induce synaptic potentiation in some pathways.
To summarize the above, some studies are inconsistent with the SHY, which may be due to detection of synaptic potentiation in certain pathways; however, the total synaptic strength assessed by means of the AMPA receptor protein in the cortex is depressed during sleep (Vyazovskiy et al., 2008; Diering et al., 2017).
Learning activates a subset of neurons, which likely contribute to the memory traces or memory engrams, which are a theoretical means by which memory is physically stored in the brain (Han et al., 2007; Reijmers et al., 2007; Liu et al., 2012; Tonegawa et al., 2015; Rashid et al., 2016). Similarly, only a limited number of neurons increase their firing rates during post-learning sleep as compared to pre-learning sleep, which may be important for memory consolidation (Grosmark and Buzsáki, 2016; Miyamoto et al., 2016). Thus, in the context of memory, plasticity should be considered separately for each neuron. While the total firing rates in the cortex decreases during sleep (Vyazovskiy et al., 2009), the plasticity of each neuron during sleep is not clear. Recent studies in the cortex (Watson et al., 2016) and HC (Miyawaki and Diba, 2016) confirmed that the total firing rates is depressed during sleep; however, the direction of the changes in firing rate differed, depending on the initial firing rate of each neuron. Watson et al. (2016) showed that, during sleep, cortical neurons with initially higher firing rates decreased their firing rates, while neurons with initially lower firing rates increased their firing rates. Watson et al. (2016) suggested that sleep helps to equalize neurons with higher and lower firing rates, and hypothesized that “homeostatic downscaling affects mainly the minority high-firing neurons to provide network stability, whereas ‘silent’ and slow-firing neurons comprise a large pool of reserve for learning, development and regeneration-induced specific plasticity”. To reveal the mechanisms of memory consolidation during sleep, how subsets of neurons related to learning and memory change their firing rates during sleep should be an interesting future question.
Memory traces can also be allocated to a subset of synapses. Motor learning activates and potentiates a subset of synapses in the motor cortex (Yang et al., 2014; Cichon and Gan, 2015), and selective shrinkage of those synapses using optogenetics erases motor memory (i.e., motor skills; Hayashi-Takagi et al., 2015). How does sleep rearrange the synaptic strength of potentiated (allocated) and non-potentiated (non-allocated) synapses for memory consolidation? According to the SHY, two candidate patterns of the plasticity have been suggested (Tononi and Cirelli, 2006, 2014). First, in the global downscaling model, all synapses are depressed non-selectively in proportion to their baseline strength. If the relative relationship in synaptic strength between potentiated and non-potentiated synapses is maintained through synaptic depression during sleep, it may help to prevent deterioration of memory information. Global downscaling is supported by in vitro homeostatic plasticity studies in which neural activity level was up- or downregulated globally and markedly (Turrigiano et al., 1998; Davis, 2006; Turrigiano, 2011). Second, the down-selection model implies selectivity, i.e., that some synapses are depressed, while others do not show synaptic depression or show only relatively mild depression. If sleep suppresses non-potentiated, but not potentiated, synapses during learning, the signal-to-noise ratio will increase, which may be good for memory consolidation.
Although these hypotheses have not been tested directly, recent studies (Diering et al., 2017; de Vivo et al., 2017) have shown cortical synaptic depression during sleep, at single-synapse resolution. de Vivo et al. (2017) measured the size of the ASI, the direct area of apposition between the pre- and post-synapse, as an index of synaptic strength, using electron microscopy in mice experiencing sleep or wakefulness (spontaneous wake or SD). At the population level, 80% of the synapses were weaker in the sleep group, while the distribution of the top 20% was similar between the two groups. However, because they could not follow the same synapses through the sleep/wake cycle, it could not be determined how individual synapses change. Diering et al. (2017) used fluorescence-tagged AMPA receptors in dendritic spines and measured their fluorescence intensity to assess synaptic strength at a single-synapse resolution, using in vivo time-lapse 2-photon imaging. They showed that synapses with a higher intensity of fluorescence are depressed, while synapses that are less fluorescent are preserved. Cirelli (2017) stated that: “This finding contrasts with the ASI changes described above, which seem to spare the largest spines, but the two studies are difficult to compare directly: they both focused on superficial layers of motor cortex, but the ASI experiments were performed in young mice in which all synapses within the reconstructed dendritic branches were measured, while the two-photon experiment used mature mice and focused on stable spines of the apical dendrites of layer V neurons”. While recent studies have reported sleep-related synaptic depression at a single-synapse resolution, how potentiated synapses with learning are processed during sleep remains a significant question.
Characteristic oscillations during NREM sleep (slow waves, spindles and ripples) are causally linked to memory consolidation. In humans, visually acquired declarative memory is slow wave-dependent, while non-declarative motor memory is spindle-dependent (Marshall et al., 2006; Ngo et al., 2013; Lustenberger et al., 2016). Similarly, in rodents, visually acquired declarative memory is dependent on slow waves (Binder et al., 2013, 2014) and ripples (Girardeau et al., 2009; Ego-Stengel and Wilson, 2010; Maingret et al., 2016); however, the causal relation between slow waves and non-declarative memory remain unclear. Our optogenetic study in mice demonstrated that slow waves are causally related to non-declarative memory of tactile-perceptual information (Miyamoto et al., 2016). Taken together, while spindles are causally related to motor skill memory, perceptually acquired memory, whether it is HC-dependent or -independent, may be causally related to slow waves.
Interestingly, our study further demonstrated that memory consolidation of somatosensory information can be enhanced by synchronous stimulation to M2 and S1, even during awake periods under SD. This suggests that memory consolidation does not require sleep per se, but rather requires sleep oscillations. Furthermore, this finding may be generalized to declarative and other types of non-declarative memory: if slow waves, spindles, and/or ripples are enhanced by external stimuli during awake periods, it may restore deficits in memory due to SD. In contrast, we showed that anti-synchronous stimulation in slow-wave rhythm to M2 and S1 impaired the tactile-perceptual memory in mice. Such a stimulation pattern to specific brain areas underlying a specific function could be a new strategy for erasing unwanted memories, such as fear memories.
Although slow waves consolidate memory, the neural circuitry mechanism(s) remain unclear. First, the activities of individual neurons or neural ensembles during slow waves that cause synaptic plasticity and memory consolidation remain unclear. Recent advances in all-optical imaging and manipulation of neural activity with cellular resolution (Rickgauer et al., 2014; Szabo et al., 2014; Packer et al., 2015; Miyamoto and Murayama, 2016) may be able to visualize and control reactivation during up-states of slow waves and reveal causal roles in synaptic plasticity and memory consolidation. Second, the direction of synaptic plasticity during sleep is controversial. Artificial low-frequency stimulation has been shown to cause either synaptic depression or potentiation, which may be dependent on the stimulation protocol or pathway. Although the net synaptic strength decreases in the cortex and HC through sleep, all synapses may not be depressed uniformly. Instead, memory reactivation during post-learning sleep may cause synaptic potentiation or prevent synaptic depression in some synapses. How oscillation and memory reactivation during post-leaning sleep rearranges synapses that have been modulated through learning is an intriguing question for future investigations.
This study was carried out in accordance with the recommendations of “the guidelines of RIKEN”. The protocol was approved by “the Animal Investigation Committee of the RIKEN BSI”.
DM wrote the manuscript. DH performed experiments and analyzed data. MM supervised the project.
This work was supported by a Grant-in-Aid for Young Scientists (A) from the Japan Society for the Promotion of Science, the Life Science Foundation of Japan, the Kowa Life Science Foundation and the KAO Corporation to MM.
The authors declare that this study received funding from the KAO corporation. The funder was not involved in the study design or collection, analysis, or interpretation of the data.
Backhaus, J., Junghanns, K., Born, J., Hohaus, K., Faasch, F., and Hohagen, F. (2006). Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol. Psychiatry 60, 1324–1330. doi: 10.1016/j.biopsych.2006.03.051
Beltramo, R., D’Urso, G., Dal Maschio, M., Farisello, P., Bovetti, S., Clovis, Y., et al. (2013). Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat. Neurosci. 16, 227–234. doi: 10.1038/nn.3306
Bienenstock, E. L., Cooper, L. N., and Munro, P. W. (1982). Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48.
Binder, S., Berg, K., Gasca, F., Lafon, B., Parra, L. C., Born, J., et al. (2014). Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul. 7, 508–515. doi: 10.1016/j.brs.2014.03.001
Binder, S., Rawohl, J., Born, J., and Marshall, L. (2013). Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats. Front. Behav. Neurosci. 7:220. doi: 10.3389/fnbeh.2013.00220
Born, J., and Feld, G. B. (2012). Sleep to upscale, sleep to downscale: balancing homeostasis and plasticity. Neuron 75, 933–935. doi: 10.1016/j.neuron.2012.09.007
Boyce, R., Glasgow, S. D., Williams, S., and Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. doi: 10.1126/science.aad5252
Chauvette, S., Seigneur, J., and Timofeev, I. (2012). Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron 75, 1105–1113. doi: 10.1016/j.neuron.2012.08.034
Chauvette, S., Volgushev, M., and Timofeev, I. (2010). Origin of active states in local neocortical networks during slow sleep oscillation. Cereb. Cortex 20, 2660–2674. doi: 10.1093/cercor/bhq009
Cichon, J., and Gan, W. B. (2015). Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature 520, 180–185. doi: 10.1038/nature14251
Cirelli, C. (2017). Sleep, synaptic homeostasis and neuronal firing rates. Curr. Opin. Neurobiol. 44, 72–79. doi: 10.1016/j.conb.2017.03.016
Contreras, D., Destexhe, A., Sejnowski, T. J., and Steriade, M. (1996). Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274, 771–774. doi: 10.1126/science.274.5288.771
Contreras, D., and Steriade, M. (1996). Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J. Physiol. 490, 159–179. doi: 10.1113/jphysiol.1996.sp021133
Cooper, L. N., and Bear, M. F. (2012). The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat. Rev. Neurosci. 13, 798–810. doi: 10.1038/nrn3353
Dan, Y., and Poo, M. M. (2004). Spike timing-dependent plasticity of neural circuits. Neuron 44, 23–30. doi: 10.1016/j.neuron.2004.09.007
Davis, G. W. (2006). Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 29, 307–323. doi: 10.1146/annurev.neuro.28.061604.135751
de Vivo, L., Bellesi, M., Marshall, W., Bushong, E. A., Ellisman, M. H., Tononi, G., et al. (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510. doi: 10.1126/science.aah5982
Diering, G. H., Nirujogi, R. S., Roth, R. H., Worley, P. F., Pandey, A., and Huganir, R. L. (2017). Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515. doi: 10.1126/science.aai8355
Dudai, Y., Karni, A., and Born, J. (2015). The consolidation and transformation of memory. Neuron 88, 20–32. doi: 10.1016/j.neuron.2015.09.004
Durkin, J., Suresh, A. K., Colbath, J., Broussard, C., Wu, J., Zochowski, M., et al. (2017). Cortically coordinated NREM thalamocortical oscillations play an essential, instructive role in visual system plasticity. Proc. Natl. Acad. Sci. U S A 114, 10485–10490. doi: 10.1073/pnas.1710613114
Ego-Stengel, V., and Wilson, M. A. (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10. doi: 10.1002/hipo.20707
Fattinger, S., de Beukelaar, T. T., Ruddy, K. L., Volk, C., Heyse, N. C., Herbst, J. A., et al. (2017). Deep sleep maintains learning efficiency of the human brain. Nat. Commun. 8:15405. doi: 10.1038/ncomms15405
Fisher, S. P., Cui, N., McKillop, L. E., Gemignani, J., Bannerman, D. M., Oliver, P. L., et al. (2016). Stereotypic wheel running decreases cortical activity in mice. Nat. Commun. 7:13138. doi: 10.1038/ncomms13138
Funk, C. M., Honjoh, S., Rodriguez, A. V., Cirelli, C., and Tononi, G. (2016). Local slow waves in superficial layers of primary cortical areas during REM sleep. Curr. Biol. 26, 396–403. doi: 10.1016/j.cub.2015.11.062
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsáki, G., and Zugaro, M. B. (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223. doi: 10.1038/nn.2384
Gordon, U., Polsky, A., and Schiller, J. (2006). Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J. Neurosci. 26, 12717–12726. doi: 10.1523/JNEUROSCI.3502-06.2006
Grosmark, A. D., and Buzsáki, G. (2016). Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science 351, 1440–1443. doi: 10.1126/science.aad1935
Halassa, M. M., Siegle, J. H., Ritt, J. T., Ting, J. T., Feng, G., and Moore, C. I. (2011). Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat. Neurosci. 14, 1118–1120. doi: 10.1038/nn.2880
Han, J. H., Kushner, S. A., Yiu, A. P., Cole, C. J., Matynia, A., Brown, R. A., et al. (2007). Neuronal competition and selection during memory formation. Science 316, 457–460. doi: 10.1126/science.1139438
Hayashi-Takagi, A., Yagishita, S., Nakamura, M., Shirai, F., Wu, Y. I., Loshbaugh, A. L., et al. (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338. doi: 10.1038/nature15257
Huber, R., Ghilardi, M. F., Massimini, M., Ferrarelli, F., Riedner, B. A., Peterson, M. J., et al. (2006). Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9, 1169–1176. doi: 10.1038/nn1758
Huber, R., Ghilardi, M. F., Massimini, M., and Tononi, G. (2004). Local sleep and learning. Nature 430, 78–81. doi: 10.1038/nature02663
Inostroza, M., and Born, J. (2013). Sleep for preserving and transforming episodic memory. Annu. Rev. Neurosci. 36, 79–102. doi: 10.1146/annurev-neuro-062012-170429
Isomura, Y., Sirota, A., Ozen, S., Montgomery, S., Mizuseki, K., Henze, D. A., et al. (2006). Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882. doi: 10.1016/j.neuron.2006.10.023
Jedlicka, P., Benuskova, L., and Abraham, W. C. (2015). A voltage-based stdp rule combined with fast BCM-like metaplasticity accounts for LTP and concurrent “Heterosynaptic” LTD in the dentate gyrus in vivo. PLoS Comput. Biol. 11:e1004588. doi: 10.1371/journal.pcbi.1004588
Ji, D., and Wilson, M. A. (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. doi: 10.1038/nn1825
Kattler, H., Dijk, D. J., and Borbély, A. A. (1994). Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep Res. 3, 159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x
Kitamura, T., Ogawa, S. K., Roy, D. S., Okuyama, T., Morrissey, M. D., Smith, L. M., et al. (2017). Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78. doi: 10.1126/science.aam6808
Liu, X., Ramirez, S., Pang, P. T., Puryear, C. B., Govindarajan, A., Deisseroth, K., et al. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. doi: 10.1038/nature11028
Lustenberger, C., Boyle, M. R., Alagapan, S., Mellin, J. M., Vaughn, B. V., and Fröhlich, F. (2016). Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 26, 2127–2136. doi: 10.1016/j.cub.2016.06.044
Magee, J. C., and Johnston, D. (1997). A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275, 209–213. doi: 10.1126/science.275.5297.209
Maingret, N., Girardeau, G., Todorova, R., Goutierre, M., and Zugaro, M. (2016). Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964. doi: 10.1038/nn.4304
Manita, S., Miyakawa, H., Kitamura, K., and Murayama, M. (2017). Dendritic spikes in sensory perception. Front. Cell. Neurosci. 11:29. doi: 10.3389/fncel.2017.00029
Manita, S., Suzuki, T., Homma, C., Matsumoto, T., Odagawa, M., Yamada, K., et al. (2015). A top-down cortical circuit for accurate sensory perception. Neuron 86, 1304–1316. doi: 10.1016/j.neuron.2015.05.006
Maret, S., Faraguna, U., Nelson, A. B., Cirelli, C., and Tononi, G. (2011). Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 14, 1418–1420. doi: 10.1038/nn.2934
Markram, H., Lübke, J., Frotscher, M., and Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. doi: 10.1126/science.275.5297.213
Marshall, L., Helgadóttir, H., Mölle, M., and Born, J. (2006). Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. doi: 10.1038/nature05278
Massimini, M., Huber, R., Ferrarelli, F., Hill, S., and Tononi, G. (2004). The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870. doi: 10.1523/jneurosci.1318-04.2004
Miyamoto, D., Hirai, D., Fung, C. C., Inutsuka, A., Odagawa, M., Suzuki, T., et al. (2016). Top-down cortical input during NREM sleep consolidates perceptual memory. Science 352, 1315–1318. doi: 10.1126/science.aaf0902
Miyamoto, H., Katagiri, H., and Hensch, T. (2003). Experience-dependent slow-wave sleep development. Nat. Neurosci. 6, 553–554. doi: 10.1038/nn1064
Miyamoto, D., and Murayama, M. (2016). The fiber-optic imaging and manipulation of neural activity during animal behavior. Neurosci. Res. 103, 1–9. doi: 10.1016/j.neures.2015.09.004
Miyawaki, H., and Diba, K. (2016). Regulation of hippocampal firing by network oscillations during sleep. Curr. Biol. 26, 893–902. doi: 10.1016/j.cub.2016.02.024
Nakao, K., Matsuyama, K., Matsuki, N., and Ikegaya, Y. (2004). Amygdala stimulation modulates hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. U S A 101, 14270–14275. doi: 10.1073/pnas.0405709101
Ngo, H. V., Martinetz, T., Born, J., and Mölle, M. (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553. doi: 10.1016/j.neuron.2013.03.006
Ngo, H. V., Miedema, A., Faude, I., Martinetz, T., Mölle, M., and Born, J. (2015). Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process. J. Neurosci. 35, 6630–6638. doi: 10.1523/JNEUROSCI.3133-14.2015
Nir, Y., Staba, R. J., Andrillon, T., Vyazovskiy, V. V., Cirelli, C., Fried, I., et al. (2011). Regional slow waves and spindles in human sleep. Neuron 70, 153–169. doi: 10.1016/j.neuron.2011.02.043
Packer, A. M., Russell, L. E., Dalgleish, H. W., and Häusser, M. (2015). Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods 12, 140–146. doi: 10.1038/nmeth.3217
Pavlides, C., and Winson, J. (1989). Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9, 2907–2918.
Phillips, K. G., Bartsch, U., McCarthy, A. P., Edgar, D. M., Tricklebank, M. D., Wafford, K. A., et al. (2012). Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron 76, 526–533. doi: 10.1016/j.neuron.2012.09.016
Rashid, A. J., Yan, C., Mercaldo, V., Hsiang, H. L., Park, S., Cole, C. J., et al. (2016). Competition between engrams influences fear memory formation and recall. Science 353, 383–387. doi: 10.1126/science.aaf0594
Reijmers, L. G., Perkins, B. L., Matsuo, N., and Mayford, M. (2007). Localization of a stable neural correlate of associative memory. Science 317, 1230–1233. doi: 10.1126/science.1143839
Rembado, I., Zanos, S., and Fetz, E. E. (2017). Cycle-triggered cortical stimulation during slow wave sleep facilitates learning a BMI task: a case report in a non-human primate. Front. Behav. Neurosci. 11:59. doi: 10.3389/fnbeh.2017.00059
Rickgauer, J. P., Deisseroth, K., and Tank, D. W. (2014). Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824. doi: 10.1038/nn.3866
Rogerson, T., Cai, D. J., Frank, A., Sano, Y., Shobe, J., Lopez-Aranda, M. F., et al. (2014). Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 15, 157–169. doi: 10.1038/nrn3667
Rothschild, G., Eban, E., and Frank, L. M. (2017). A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 20, 251–259. doi: 10.1038/nn.4457
Sandler, M., Shulman, Y., and Schiller, J. (2016). A novel form of local plasticity in tuft dendrites of neocortical somatosensory layer 5 pyramidal neurons. Neuron 90, 1028–1042. doi: 10.1016/j.neuron.2016.04.032
Siapas, A. G., and Wilson, M. A. (1998). Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128. doi: 10.1016/s0896-6273(00)80629-7
Sirota, A., Csicsvari, J., Buhl, D., and Buzsáki, G. (2003). Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U S A 100, 2065–2069. doi: 10.1073/pnas.0437938100
Sjöström, P. J., Turrigiano, G. G., and Nelson, S. B. (2001). Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164. doi: 10.1016/s0896-6273(01)00542-6
Steriade, M. (2003). The corticothalamic system in sleep. Front. Biosci. 8, d878–d899. doi: 10.2741/1043
Steriade, M., Deschenes, M., Domich, L., and Mulle, C. (1985). Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J. Neurophysiol. 54, 1473–1497.
Steriade, M., Domich, L., Oakson, G., and Deschenes, M. (1987). The deafferented reticular thalamic nucleus generates spindle rhythmicity. J. Neurophysiol. 57, 260–273.
Stickgold, R. (2005). Sleep-dependent memory consolidation. Nature 437, 1272–1278. doi: 10.1038/nature04286
Szabo, V., Ventalon, C., De Sars, V., Bradley, J., and Emiliani, V. (2014). Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron 84, 1157–1169. doi: 10.1016/j.neuron.2014.11.005
Takehara-Nishiuchi, K., Nakao, K., Kawahara, S., Matsuki, N., and Kirino, Y. (2006). Systems consolidation requires postlearning activation of NMDA receptors in the medial prefrontal cortex in trace eyeblink conditioning. J. Neurosci. 26, 5049–5058. doi: 10.1523/JNEUROSCI.4381-05.2006
Tonegawa, S., Liu, X., Ramirez, S., and Redondo, R. (2015). Memory engram cells have come of age. Neuron 87, 918–931. doi: 10.1016/j.neuron.2015.08.002
Tononi, G., and Cirelli, C. (2003). Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62, 143–150. doi: 10.1016/j.brainresbull.2003.09.004
Tononi, G., and Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62. doi: 10.1016/j.smrv.2005.05.002
Tononi, G., and Cirelli, C. (2014). Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. doi: 10.1016/j.neuron.2013.12.025
Turrigiano, G. (2011). Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 34, 89–103. doi: 10.1146/annurev-neuro-060909-153238
Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C., and Nelson, S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896. doi: 10.1038/36103
Urba, W. J., Kopp, W. C., Clark, J. W., Smith, J. W. II., Steis, R. G., Huber, C., et al. (1991). The in vivo immunomodulatory effects of recombinant interferon γ plus recombinant tumor necrosis factor-alfa. J. Clin. Oncol. 9, 1831–1839. doi: 10.1200/jco.1991.9.10.1831
Vyazovskiy, V. V., Cirelli, C., Pfister-Genskow, M., Faraguna, U., and Tononi, G. (2008). Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 11, 200–208. doi: 10.1038/nn2035
Vyazovskiy, V. V., and Harris, K. D. (2013). Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat. Rev. Neurosci. 14, 443–451. doi: 10.1038/nrn3494
Vyazovskiy, V. V., Olcese, U., Hanlon, E. C., Nir, Y., Cirelli, C., and Tononi, G. (2011). Local sleep in awake rats. Nature 472, 443–447. doi: 10.1038/nature10009
Vyazovskiy, V. V., Olcese, U., Lazimy, Y. M., Faraguna, U., Esser, S. K., Williams, J. C., et al. (2009). Cortical firing and sleep homeostasis. Neuron 63, 865–878. doi: 10.1016/j.neuron.2009.08.024
Watson, B. O., Levenstein, D., Greene, J. P., Gelinas, J. N., and Buzsáki, G. (2016). Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852. doi: 10.1016/j.neuron.2016.03.036
Keywords: cortex, hippocampus, memory, oscillation, sleep, synaptic plasticity
Citation: Miyamoto D, Hirai D and Murayama M (2017) The Roles of Cortical Slow Waves in Synaptic Plasticity and Memory Consolidation. Front. Neural Circuits 11:92. doi: 10.3389/fncir.2017.00092
Received: 10 June 2017; Accepted: 08 November 2017;
Published: 22 November 2017.
Edited by:
Takao K. Hensch, Harvard University, United StatesReviewed by:
Heiko J. Luhmann, Johannes Gutenberg-Universität Mainz, GermanyCopyright © 2017 Miyamoto, Hirai and Murayama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Miyamoto, ZC1taXlhbW90b0BicmFpbi5yaWtlbi5qcA==
Masanori Murayama, bWFzYV9tdXJheWFtYUBicmFpbi5yaWtlbi5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.