- 1Department of Nutrition and Dietetics, College of Nutrition, Kanto Gakuin University, Yokohama, Japan
- 2Department of Health and Nutrition Sciences, Faculty of Human Health, Komazawa Women’s University, Tokyo, Japan
Spontaneous activity in the developing central nervous system occurs before the brain responds to external sensory inputs, and appears in the hindbrain and spinal cord as rhythmic electrical discharges of cranial and spinal nerves. This spontaneous activity recruits a large population of neurons and propagates like a wave over a wide region of the central nervous system. Here, we review spontaneous activity in the chick hindbrain by focusing on this large-scale synchronized activity. Asynchronous activity that is expressed earlier than the above mentioned synchronized activity and activity originating in midline serotonergic neurons are also briefly mentioned.
Introduction
The developing nervous system generates spontaneous activity that is suggested to play a critical role in neural development (Moody and Bosma, 2005; Blankenship and Feller, 2010). Some of the earliest activity is expressed in the hindbrain and spinal cord. This spontaneous activity was originally considered to be a prototype of the rhythmic discharges in the adult central nervous system, such as respiratory and locomotor patterns. This classic view has been challenged by recent studies showing that the embryonic spontaneous activity has different characteristics of spatio-temporal patterns, origin, and pharmacological substrates from those of the adult central pattern generators (e.g., see the discussions of Chub and O’Donovan, 1998; Thoby-Brisson et al., 2005).
In past studies, spontaneous activity has often been analyzed using isolated hindbrains or spinal cords as these structures were considered to produce independent activities, which has caused some confusion. In fact, during early development activity propagates over a wide area of the central nervous system, maximally extending to the lumbosacral cord and forebrain, and thus most regions of the central nervous system are functionally correlated (Momose-Sato et al., 2001b, 2007, 2009, 2012a).
Here, we review spontaneous activity in the chick embryo by focusing on this large-scale synchronized activity. Much of what we learned about the synchronized activity has come from studies on the chick embryo because the embryo in an externally laid egg is readily available and amenable to surgical and pharmacological manipulation. Synchronized activity having characteristics similar to those described here has also been reported in the mammalian embryo, and we refer the reader to related reviews, as well as literatures focusing on the spinal cord (O’Donovan, 1999; Chatonnet et al., 2002; Marder and Rehm, 2005; Moody and Bosma, 2005; Greer et al., 2006; Hanson et al., 2008; O’Donovan et al., 2008; Bosma, 2010; Momose-Sato and Sato, 2013), which would be a great help for better understanding the present topic. In the last sections of this article, we also briefly mention asynchronous activity that is expressed earlier than the above mentioned synchronized activity, and activity originating in midline serotonergic neurons.
Large-Scale Synchronized Activity
Activity Pattern
The earliest studies of spontaneous activity in the chick embryo involve descriptions of embryonic motility observed in ovo. Preyer (1885) described this behavior more than a century ago, and it has been well characterized by Hamburger and Balaban (1963) (for reviews see Bekoff, 2001; Oppenheim and Lauder, 2001). Embryonic motility from stage 21 (E3.5, E: days of incubation in chicks) appears as periodically recurring sequences of slight flexions of the neck, with subsequently two or more S-waves extending from the head to the tail (Hamburger and Balaban, 1963). Direct evidence for the neurogenic basis of this behavior has been obtained from electrophysiological studies of spinal neuronal activity in ovo, which revealed a parallel between the electrical discharges and embryonic motility (Ripley and Provine, 1972; Provine, 1973).
The cranial and spinal nerves show similar rhythmic bursting when the hindbrain and/or spinal cord is isolated in vitro (Landmesser and O’Donovan, 1984; Fortin et al., 1995; O’Donovan et al., 1998), indicating that motor outputs from these nerves produce embryonic motility. The activity is recorded as recurring episodes composed of several bursts, appearing at a low frequency with an inter-episode interval of a few minutes (Figure 1A). Similar spontaneous activity is detected in mouse and rat embryos (Nakayama et al., 1999; Abadie et al., 2000; Hanson and Landmesser, 2003; Ren and Greer, 2003; Momose-Sato et al., 2007, 2012a), indicating that this activity is globally generated across species.
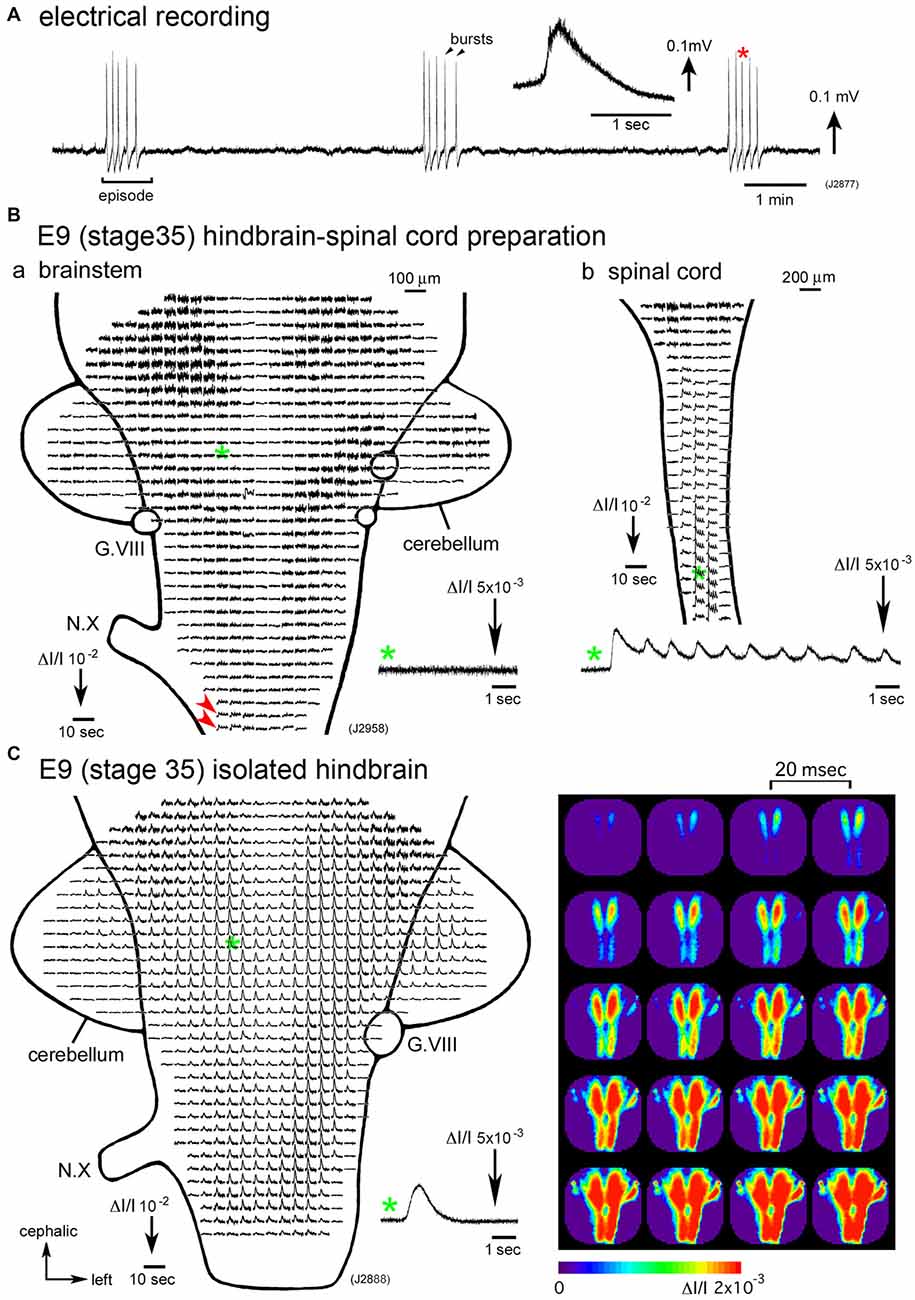
Figure 1. (A) Electrical recording of spontaneous activity in the chick embryo. The signal was recorded from a hindbrain-spinal cord preparation dissected from an E6 (stage 28) embryo with a glass micro-suction electrode applied to the root of the vagus nerve. In the inset, an enlarged trace of a single burst indicated with a red asterisk is presented. (B) Voltage-sensitive dye recording of the spontaneous activity in an E9 (stage 35) hindbrain-spinal cord preparation. Recordings in (Ba,b) were obtained from the hindbrain and cervical cord of the same preparation, respectively. Enlargements in optical signals indicated with green asterisks are presented in the lower side. Oscillatory activity was detected from the lower medulla (Ba, red arrowheads) and spinal cord (Bb), but not in the pons (Ba, a green asterisk and inset), midbrain, or cerebellum. (C) Voltage-sensitive dye recording of the spontaneous activity that appeared in the isolated hindbrain at E9 (stage 35). A pseudo-color image on the right shows the propagation pattern of the spontaneous activity. The frame interval was 20 ms. Data shown in Figures 1B,C, 2 were obtained using a 1020ch optical recording system (Hirota et al., 1995; Momose-Sato et al., 2001a) with a voltage-sensitive dye, NK2761. The scale on the recordings and images indicates the fractional change in transmitted light intensity, ΔI/I. G.VIII, vestibulo-cochlear ganglion; N.X, vagus nerve (Reproduced from Mochida et al., 2009b; and Momose-Sato and Sato, 2014).
Experiments manipulating the rhombomere (segmental structures of the early hindbrain) and rhombomere-specific genes have suggested that the expression of the burst is associated with the odd-numbered rhombomeres (r3 and r5) and their interaction with the adjacent even-numbered rhombomeres (Fortin et al., 1999), with some molecular cues expressed in rhombomere segments, such as Krox20, regulating the bursting pattern (Chatonnet et al., 2002; Borday et al., 2003; Coutinho et al., 2004). Although these experiments were performed in isolated hindbrains, in which the primary rhythm generator was deprived (see “Origin” Section), the results suggest that inter-segmental interactions are important for the generation of recurring bursting patterns.
Synchronization
The spontaneous activity in the hindbrain and spinal cord is associated with the co-activation of different nerves/regions. Synchronization occurs between different rostrocaudal levels, between the left and right sides, and between the nerves innervating the flexor and extensor muscles (Provine, 1973; Fortin et al., 1994, 1995; Milner and Landmesser, 1999). Thus, the early spontaneous activity may be correlated with intersegmental and bilateral interactions between different neuronal subsets (Fortin et al., 1995). This has been visually demonstrated in optical studies using voltage-sensitive dyes, in which spontaneous as well as sensory-evoked waves spread over a wide region of the central nervous system, including the spinal cord, hindbrain, midbrain, cerebellum, and part of the forebrain (Momose-Sato et al., 2001b, 2007, 2009, 2012a). The propagation velocity of the wave was too slow to be attributed to axonal conduction along unmyelinated fibers at the corresponding stage (O’Donovan et al., 1994; Arai et al., 2007; Momose-Sato et al., 2007). The mechanisms underlying the spread of this activity may include the sequential synaptic activation of adjacent regions coupled by short-range synaptic connections (Fortin et al., 1995; O’Donovan et al., 2008), the non-synaptic release of transmitters and paracrine-like intercellular communication (Demarque et al., 2002; Scain et al., 2010), and the coordination of chemical transmitters with gap junctions, as well as electrical interactions between neighboring neurons (Hanson and Landmesser, 2003; Ren et al., 2006).
Development
Correlated spontaneous discharges are recorded from stage 24 (E4) in the hindbrain (Fortin et al., 1994; Momose-Sato et al., 2009) and from stage 22.5–24 (E3.5–E4) in the spinal cord (Milner and Landmesser, 1999; Hanson and Landmesser, 2004). Marked changes occur in the activity patterns during development, such as an increase in the interval between episodes, the number of burst discharges within the episodes (a single burst to multiple bursts), and a decrease in the inter-burst interval within an episode (O’Donovan and Landmesser, 1987; Fortin et al., 1994; Milner and Landmesser, 1999).
Synchronization over the brain and spinal cord is observed during a particular period of development, E4–E8 (Momose-Sato et al., 2009; Momose-Sato and Sato, 2014). From E9 onward, spontaneous activity becomes segregated in the spinal cord, and the signal is very small or undetectable in the hindbrain (Momose-Sato and Sato, 2014; Figure 1B). The results indicate that the activity at E9 and later is no longer “large-scale”, but is specialized to the spinal network. Interestingly, spontaneous activity becomes detectable in the E9 hindbrain when the hindbrain is isolated, although no such activity is observed when the spinal cord is intact (Momose-Sato and Sato, 2014; Figure 1C). This seems due to a change in neural excitability in the hindbrain, which is caused by deprivation of the spinal rhythm generator (also see “Origin” Section).
In the mouse and rat embryos, it has been reported that large-scale synchronized activity is substituted by segregated activity in the caudal spinal cord and rostrolateral medulla, which seem to correspond to the locomotor and respiratory rhythm generator, respectively (Momose-Sato et al., 2012a). The underlying mechanism in the mouse involves the switching of γ-aminobutyric acid (GABA) acid responses from excitatory to inhibitory (Momose-Sato et al., 2012b). Nevertheless, this seems not to be the case in the chick embryo because the application of the GABAA receptor antagonist bicuculline does not restore the activity in the E9 hindbrain (Momose-Sato and Sato, 2014) and even suppresses the activity in the spinal cord until at least E11 (Chub and O’Donovan, 1998). Possible mechanisms include the intrinsic excitability of neurons being decreased with hyperpolarization of the resting membrane potential or other changes in electrical properties, and also weakened functional connections in neurons under the level required to mediate the conduction of the activity. It is also possible that some inhibitory signals are derived from ascending fibers or targets innervated by descending fibers, or the effects of neuromodulators (Whelan, 2003; Marder and Rehm, 2005).
Origin
Which part of the central nervous system functions as a generator of the large-scale synchronized activity? An optical imaging study using hindbrain-whole spinal cord preparations have shown that the origin of the spontaneous wave is in the upper cervical cord/lower medulla at stage 24–29 (E4–E6) with some variations between the activities (Figures 2A,B). As development proceeds to stage 30–34 (E7–E8), the region responsible for generating the wave shifts caudally, and the activity is initiated in any part of the spinal cord (Momose-Sato et al., 2009; Figure 2B).
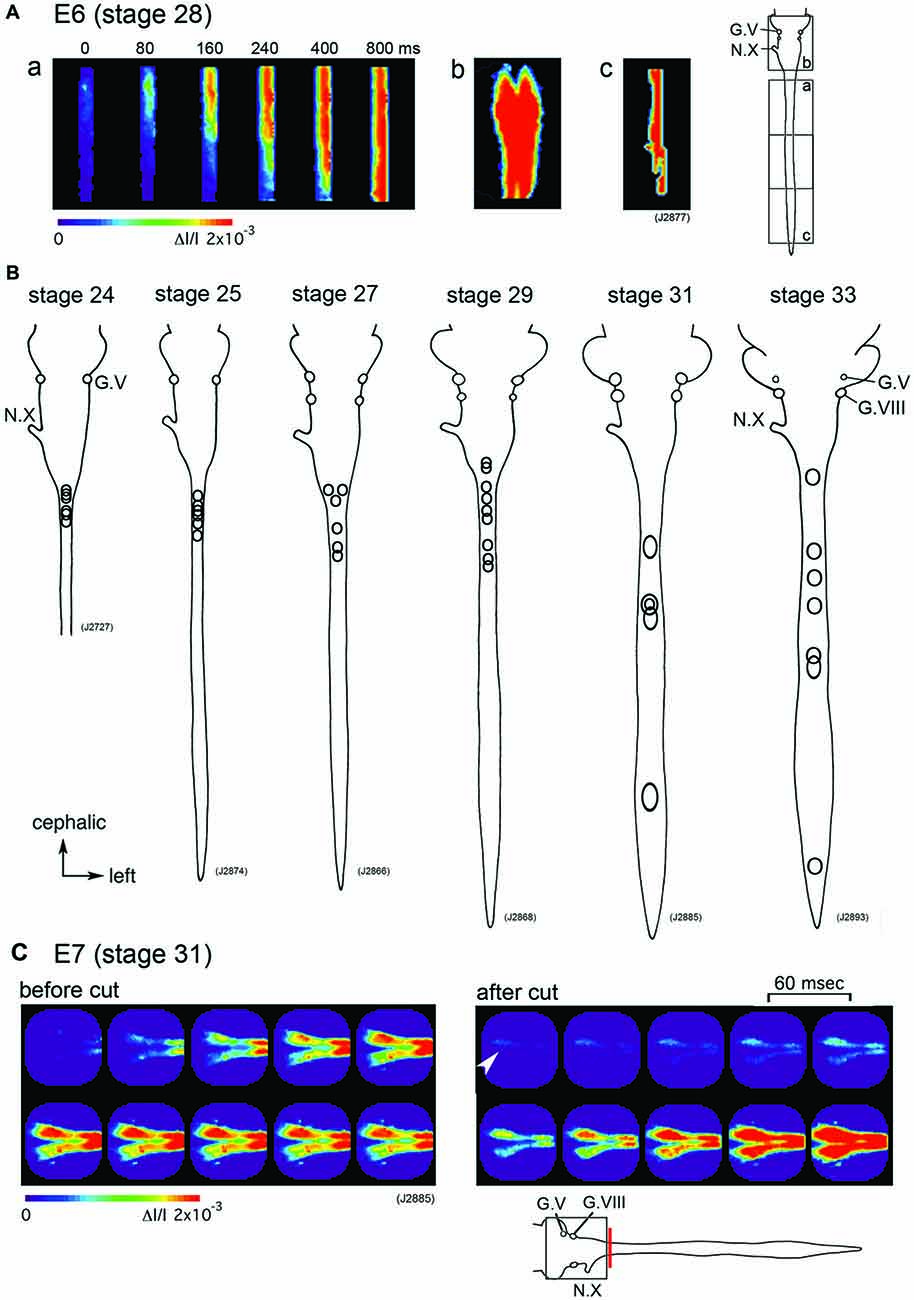
Figure 2. (A) The propagation pattern of the spontaneous activity in an E6 (stage 28) hindbrain-spinal cord preparation. The activity initiated in the upper cervical cord (a). Images (Ab,c) present maximum responses in the hindbrain and caudal cord, respectively. Images were obtained from the hindbrain and spinal cord indicated with squares in the right inset. (B) The origins of the spontaneous activity are indicated with circles for the most typical preparation at stage 24–33. In each preparation, one circle corresponds to one spontaneous wave, and variations in circle locations show variations in the origin of the activity. (C) Pseudo-color images of the spontaneous activity in an E7 (stage 31) hindbrain-spinal cord preparation before (left images) and after (right images) the obex was cut. Images were obtained from the hindbrain region indicated with a square in the lower inset. The red vertical line shows the location where the cut was made. An arrowhead in the right image indicates the origin of the activity. The frame interval was 60 ms. G.V, trigeminal ganglion; G.VIII, vestibulo-cochlear ganglion; N.X, vagus nerve (Reproduced from Momose-Sato et al., 2009; and Momose-Sato and Sato, 2014).
Although spontaneous activity is typically initiated in the spinal cord in intact preparations, activity can be detected in the isolated hindbrain, in which the primary rhythm generator is deprived (Fortin et al., 1995, 1999; Momose-Sato and Sato, 2014). Furthermore, this rhythmic pattern is preserved in transverse sections of the hindbrain (Fortin et al., 1995). Moreover, the spontaneous activity is generated by single rhombomeres when isolated at E2 (stage 10–11; Fortin et al., 1999; Borday et al., 2003). Thus, neurons and/or neuronal networks producing spontaneous activity are widely distributed in the hindbrain and spinal cord, with a specific excitable region probably pacing the activity in intact preparations. When the primary pacing area is removed, as is the case for the isolated hindbrain, other potential generators become initiators of spontaneous activity, and produce activity with a similar pattern to the previous one (Momose-Sato et al., 2007; Momose-Sato and Sato, 2014; Figure 2C). This homeostatic compensation is an active process, which is associated with an increase in excitability and/or the number of neurons recruited to the activity (Momose-Sato and Sato, 2014).
According to the model proposed in the chick spinal cord, the generation of spontaneous activity is dependent on the degree of neuronal connectivity and the number of units recruited to the wave. The activity is not generated in a particular class of cells, but is initiated where the connection is sufficient enough to sustain the propagation of activity throughout the whole network (Chub and O’Donovan, 1998; O’Donovan et al., 1998). A similar model might be applicable to the neural network in the hindbrain.
Pharmacology
Nicotinic acetylcholine receptors, specifically those without the α7-subunit, mediate spontaneous synchronized activity at early developmental stages (stage 25–28: E4.5–E6), and glutamate receptors at later stages (stage 33~: E8~; Chub and O’Donovan, 1998; Milner and Landmesser, 1999; Hanson and Landmesser, 2004; Mochida et al., 2009b). GABA and glycine also act as excitatory mediators, most likely because the Cl− reversal potential (ECl) is more positive than the resting potential, which is caused by the high intracellular Cl− concentration (Chub and O’Donovan, 2001).
In mouse and rat embryos, it has been reported that spontaneous activity undergoes two types of developmental changes in pharmacological substrates. One is a switching of the dominant contributor from nicotinic acetylcholine receptors to glutamate receptors, and the other is the change in GABA/glycinergic responses from depolarizing/excitatory to hyperpolarizing/inhibitory (Nakayama et al., 1999; Ren and Greer, 2003; Myers et al., 2005; Ladle et al., 2007; Momose-Sato et al., 2012b). In the chick embryo, the dominant neuronal response to GABA and glycine is depolarizing/excitatory at least until E8 in the hindbrain (Momose-Sato et al., 1998) and E10–E11 in the spinal cord (Chub and O’Donovan, 1998; Gonzalez-Islas and Wenner, 2006). On the other hand, some neurons in the hindbrain reticular formation receive hyperpolarizing inputs from GABAergic neurons, which seem to regulate the high-frequency bursts of the spontaneous activity (Fortin et al., 1999).
In addition to chemical synaptic antagonists, synchronized activity is inhibited by putative gap junction blockers such as octanol, carbenoxolone, and 18ß-glycyrrhetinic acid (Milner and Landmesser, 1999; Mochida et al., 2009b). Although interpretation of the results gained using these blockers is not forthcoming because of the non-specific effects of the drugs on cell membrane conductance, a recent study in the mouse spinal cord demonstrated that 18ß-glycyrrhetinic acid and meclofenamic acid exhibited specific effects on gap junctions (Czarnecki et al., 2014), suggesting that the correlated activity is mediated by the coordination of chemical neurotransmitter systems and gap junctional communication.
Asynchronous Activity Expressed Earlier than the Synchronized Activity
In addition to the synchronized activity discussed above, asynchronous excitation has been detected in the chick hindbrain using Ca2+-imaging (Mochida et al., 2009a). In this study, both retrogradely labeled reticulospinal and vestibuloocular neurons exhibited asynchronous transients earlier than the emergence of synchronized activity in each population (stage 25 in the reticulospinal neurons and stage 26 in the vestibuloocular neurons). The asynchronous and synchronous activities were considered to be independent phenomena produced by different mechanisms since: (1) the asynchronous activity was not inhibited by tetrodotoxin, which blocks the synchronous activity; and (2) there is no temporal or developmental relationship between the two activities (Mochida et al., 2009a). Thus, in the early stages of development, the activity of individual neurons is independent, but later becomes synchronous. A similar developmental sequence of spontaneous activity occurs in the mouse hindbrain (Abadie et al., 2000; Gust et al., 2003) and other brain regions (for a review see Allene and Cossart, 2010).
Midline Spontaneous Activity
Hughes et al. (2009) reported spontaneous activity that arises in the midregion of the hindbrain and travels in both the rostral and caudal directions along the midline. This activity resembled the midline spontaneous activity reported in the mouse embryo, which is produced by serotonergic neurons (for a review see Bosma, 2010). In the chick, spontaneous waves that originate in the spinal cord usually precede the midline activity (Hughes et al., 2009), suggesting that it occurs secondarily following the synchronized activity.
Future Perspectives
Recent advances in electrophysiology, molecular biology, and optical imaging have shed much light on our understanding of the widely-propagating synchronized activity in the hindbrain and spinal. Despite much knowledge on the global features of this activity, many unanswered questions remain. Perhaps the most important issue is the functional significance of the activity. Primordial activity in the spinal cord has been suggested to play a significant role in developmental processes, including axon pathfinding (Hanson and Landmesser, 2004; Hanson et al., 2008) and establishment of locomotor function (Myers et al., 2005). On the other hand, investigations in the hindbrain are less advanced. Spontaneous synchronized activity is observed in several systems of the developing brain, and it is suggested to play an instructive role in the synaptic network formation (Zhang and Poo, 2001; Kirkby et al., 2013; Andreae and Burrone, 2014). Synchronized activity in the chick hindbrain is only expressed within a restricted period, E4–E8, during which functional synaptic connections are established in the brainstem nuclei (Momose-Sato et al., 2001a; Glover et al., 2008; Momose-Sato and Sato, 2011). One challenge for the future would be to ascertain whether this process is under the control of spontaneous activity in the hindbrain.
Author Contributions
YM-S designed the research. YM-S and KS performed experiments, analyzed data, and prepared the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP26560068 (YM-S) and JP15K06720 (KS) and the Japan Epilepsy Research Foundation.
Abbreviations
E, embryonic day, which shows days of incubation in chicks; GABA, γ-aminobutyric acid.
References
Abadie, V., Champagnat, J., and Fortin, G. (2000). Branchiomotor activities in mouse embryo. Neuroreport 11, 141–145. doi: 10.1097/00001756-200001170-00028
Allene, C., and Cossart, R. (2010). Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J. Physiol. 588, 83–91. doi: 10.1113/jphysiol.2009.178798
Andreae, L. C., and Burrone, J. (2014). The role of neuronal activity and transmitter release on synapse formation. Curr. Opin. Neurobiol. 27, 47–52. doi: 10.1016/j.conb.2014.02.008
Arai, Y., Mentis, G. Z., Wu, J.-Y., and O’Donovan, M. J. (2007). Ventrolateral origin of each cycle of rhythmic activity generated by the spinal cord of the chick embryo. PLoS One 2:e417. doi: 10.1371/journal.pone.0000417
Bekoff, A. (2001). Spontaneous embryonic motility: an enduring legacy. Int. J. Dev. Neurosci. 19, 155–160. doi: 10.1016/s0736-5748(00)00089-7
Blankenship, A. G., and Feller, M. B. (2010). Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 11, 18–29. doi: 10.1038/nrn2759
Borday, C., Abadie, V., Chatonnet, F., Thoby-Brisson, M., Champagnat, J., and Fortin, G. (2003). Developmental molecular switches regulating breathing patterns in CNS. Respir. Physiol. Neurobiol. 135, 121–132. doi: 10.1016/s1569-9048(03)00031-4
Bosma, M. M. (2010). Timing and mechanism of a window of spontaneous activity in embryonic mouse hindbrain development. Ann. N Y Acad. Sci. 1198, 182–191. doi: 10.1111/j.1749-6632.2009.05423.x
Chatonnet, F., Thoby-Brisson, M., Abadie, V., Domínguez del Toro, E., Champagnat, J., and Fortin, G. (2002). Early development of respiratory rhythm generation in mouse and chick. Respir. Physiol. Neurobiol. 131, 5–13. doi: 10.1016/s1569-9048(02)00033-2
Chub, N., and O’Donovan, M. J. (1998). Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J. Neurosci. 18, 294–306.
Chub, N., and O’Donovan, M. J. (2001). Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J. Neurophysiol. 85, 2166–2176.
Coutinho, A. P., Borday, C., Gilthorpe, J., Jungbluth, S., Champagnat, J., Lumsden, A., et al. (2004). Induction of a parafacial rhythm generator by rhombomere 3 in the chick embryo. J. Neurosci. 24, 9383–9390. doi: 10.1523/JNEUROSCI.2408-04.2004
Czarnecki, A., Le Corronc, H., Rigato, C., Le Bras, B., Couraud, F., Scain, A.-L., et al. (2014). Acetylcholine controls GABA-, glutamate- and glycine-dependent giant depolarizing potentials that govern spontaneous motoneuron activity at the onset of synaptogenesis in the mouse embryonic spinal cord. J. Neurosci. 34, 6389–6404. doi: 10.1523/JNEUROSCI.2664-13.2014
Demarque, M., Represa, A., Becq, H., Khalilov, I., Ben-Ari, Y., and Aniksztejn, L. (2002). Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36, 1051–1061. doi: 10.1016/s0896-6273(02)01053-x
Fortin, G., Champagnat, J., and Lumsden, A. (1994). Onset and maturation of branchio-motor activities in the chick hindbrain. Neuroreport 5, 1149–1152. doi: 10.1097/00001756-199405000-00032
Fortin, G., Jungbluth, S., Lumsden, A., and Champagnat, J. (1999). Segmental specification of GABAergic inhibition during development of hindbrain neural networks. Nat. Neurosci. 2, 873–877. doi: 10.1038/13172
Fortin, G., Kato, F., Lumsden, A., and Champagnat, J. (1995). Rhythm generation in the segmented hindbrain of chick embryos. J. Physiol. 486, 735–744. doi: 10.1113/jphysiol.1995.sp020849
Glover, J. C., Sato, K., and Momose-Sato, Y. (2008). Using voltage-sensitive dye recording to image the functional development of neuronal circuits in vertebrate embryos. Dev. Neurobiol. 68, 804–816. doi: 10.1002/dneu.20629
Gonzalez-Islas, C., and Wenner, P. (2006). Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron 49, 563–575. doi: 10.1016/j.neuron.2006.01.017
Greer, J. J., Funk, G. D., and Ballanyi, K. (2006). Preparing for the first breath: prenatal maturation of respiratory neural control. J. Physiol. 570, 437–444. doi: 10.1113/jphysiol.2005.097238
Gust, J., Wright, J. J., Pratt, E. B., and Bosma, M. M. (2003). Development of synchronized activity of cranial motor neurons in the segmented embryonic mouse hindbrain. J. Physiol. 550, 123–133. doi: 10.1113/jphysiol.2002.038737
Hamburger, V., and Balaban, M. (1963). Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev. Biol. 7, 533–545. doi: 10.1016/0012-1606(63)90140-4
Hanson, M. G., and Landmesser, L. T. (2003). Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J. Neurosci. 23, 587–600.
Hanson, M. G., and Landmesser, L. T. (2004). Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 43, 687–701. doi: 10.1016/j.neuron.2004.08.018
Hanson, M. G., Milner, L. D., and Landmesser, L. T. (2008). Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res. Rev. 57, 77–85. doi: 10.1016/j.brainresrev.2007.06.021
Hirota, A., Sato, K., Momose-Sato, Y., Sakai, T., and Kamino, K. (1995). A new simultaneous 1020-site optical recording system for monitoring neural activity using voltage-sensitive dyes. J. Neurosci. Methods 56, 187–194. doi: 10.1016/0165-0270(94)00123-x
Hughes, S. M., Easton, C. R., and Bosma, M. M. (2009). Properties and mechanisms of spontaneous activity in the embryonic chick hindbrain. Dev. Neurobiol. 69, 477–490. doi: 10.1002/dneu.20712
Kirkby, L. A., Sack, G. S., Firl, A., and Feller, M. B. (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. doi: 10.1016/j.neuron.2013.10.030
Ladle, D. R., Pecho-Vrieseling, E., and Arber, S. (2007). Assembly of motor circuits in the spinal cord: driven to function by genetic and experience-dependent mechanisms. Neuron 56, 270–283. doi: 10.1016/j.neuron.2007.09.026
Landmesser, L. T., and O’Donovan, M. J. (1984). Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord prepraration. J. Physiol. 347, 189–204. doi: 10.1113/jphysiol.1984.sp015061
Marder, E., and Rehm, K. J. (2005). Development of central pattern generating circuits. Curr. Opin. Neurobiol. 15, 86–93. doi: 10.1016/j.conb.2005.01.011
Milner, L. D., and Landmesser, L. T. (1999). Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J. Neurosci. 19, 3007–3022.
Mochida, H., Fortin, G., Champagnat, J., and Glover, J. C. (2009a). Differential involvement of projection neurons during emergence of spontaneous activity in the developing avian hindbrain. J. Neurophysiol. 101, 591–602. doi: 10.1152/jn.90835.2008
Mochida, H., Sato, K., and Momose-Sato, Y. (2009b). Switching of the transmitters that mediate hindbrain correlated activity in the chick embryo. Eur. J. Neurosci. 29, 14–30. doi: 10.1111/j.1460-9568.2008.06569.x
Momose-Sato, Y., and Sato, K. (2011). The embryonic brain and development of vagal pathways. Respir. Physiol. Neurobiol. 178, 163–173. doi: 10.1016/j.resp.2011.01.012
Momose-Sato, Y., and Sato, K. (2013). Large-scale synchronized activity in the embryonic brainstem and spinal cord. Front. Cell. Neurosci. 7:36. doi: 10.3389/fncel.2013.00036
Momose-Sato, Y., and Sato, K. (2014). Maintenance of the large-scale depolarization wave in the embryonic chick brain against deprivation of the rhythm generator. Neuroscience 266, 186–196. doi: 10.1016/j.neuroscience.2014.02.014
Momose-Sato, Y., Mochida, H., and Kinoshita, M. (2009). Origin of the earliest correlated neuronal activity in the chick embryo revealed by optical imaging with voltage-sensitive dyes. Eur. J. Neurosci. 29, 1–13. doi: 10.1111/j.1460-9568.2008.06568.x
Momose-Sato, Y., Nakamori, T., and Sato, K. (2012a). Spontaneous depolarization wave in the mouse embryo: origin and large-scale propagation over the CNS identified with voltage-sensitive dye imaging. Eur. J. Neurosci. 35, 1230–1241. doi: 10.1111/j.1460-9568.2012.07997.x
Momose-Sato, Y., Nakamori, T., and Sato, K. (2012b). Pharmacological mechanisms underlying the switching from the large-scale depolarization wave to segregated activity in the mouse CNS. Eur. J. Neurosci. 35, 1242–1252. doi: 10.1111/j.1460-9568.2012.08040.x
Momose-Sato, Y., Sato, K., and Kamino, K. (2001a). Optical approaches to embryonic development of neural functions in the brainstem. Prog. Neurobiol. 63, 151–197. doi: 10.1016/s0301-0082(00)00023-x
Momose-Sato, Y., Sato, K., Mochida, H., Yazawa, I., Sasaki, S., and Kamino, K. (2001b). Spreading depolarization waves triggered by vagal stimulation in the embryonic chick brain: optical evidence for intercellular communication in the developing central nervous system. Neuroscience 102, 245–262. doi: 10.1016/s0306-4522(00)00477-2
Momose-Sato, Y., Sato, K., and Kinoshita, M. (2007). Spontaneous depolarization waves of multiple origins in the embryonic rat CNS. Eur. J. Neurosci. 25, 929–944. doi: 10.1111/j.1460-9568.2007.05352.x
Momose-Sato, Y., Sato, K., Hirota, A., and Kamino, K. (1998). GABA-induced intrinsic light-scattering changes associated with voltage-sensitive dye signals in embryonic brainstem slices: coupling of depolarization and cell shrinkage. J. Neurophysiol. 79, 2208–2217.
Moody, W. J., and Bosma, M. M. (2005). Ion channel development, spontaneous activity and activity-dependent development in nerve and muscle cells. Physiol. Rev. 85, 883–941. doi: 10.1152/physrev.00017.2004
Myers, C. P., Lewcock, J. W., Hanson, M. G., Gosgnach, S., Aimone, J. B., Gage, F. H., et al. (2005). Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron 46, 37–49. doi: 10.1016/j.neuron.2005.02.022
Nakayama, K., Nishimaru, H., Iizuka, M., Ozaki, S., and Kudo, N. (1999). Rostrocaudal progression in the development of periodic spontaneous activity in fetal rat spinal motor circuits in vitro. J. Neurophysiol. 81, 2592–2595.
O’Donovan, M. J. (1999). The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol. 9, 94–104. doi: 10.1016/s0959-4388(99)80012-9
O’Donovan, M. J., Bonnot, A., Mentis, G. Z., Arai, Y., Chub, N., Shneider, N. A., et al. (2008). Imaging the spatiotemporal organization of neural activity in the developing spinal cord. Dev. Neurobiol. 68, 788–803. doi: 10.1002/dneu.20620
O’Donovan, M. J., Chub, N., and Wenner, P. (1998). Mechanisms of spontaneous activity in developing spinal networks. Dev. Neurobiol. 37, 131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h
O’Donovan, M. J., and Landmesser, L. (1987). The development of hindlimb motor activity studied in the isolated spinal cord of the chick embryo. J. Neurosci. 7, 3256–3264.
O’Donovan, M., Ho, S., and Yee, W. (1994). Calcium imaging of rhythmic network activity in the developing spinal cord of the chick embryo. J. Neurosci. 14, 6354–6369.
Oppenheim, R. W., and Lauder, J. M. (2001). Viktor Hamburger at 100: eight decades of neuroembryological research, 1920–2000. Int. J. Dev. Neurosci. 19, 117–122. doi: 10.1016/s0736-5748(00)00072-1
Preyer, W. (1885). Specielle Physiologie des Embryo, Grieben, Leipzig. (cited in Hamburger and Balaban, 1963).
Provine, R. R. (1973). “Neurophysiological aspects of behavior development in the chick embryo,” in Studies on the Development of Behavior and the Nervous System. Vol. 1 Behavioral Embryology, ed. G. Gottlieb (New York, NY: Academic Press), 77–102.
Ren, J., and Greer, J. J. (2003). Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J. Neurophysiol. 89, 1187–1195. doi: 10.1152/jn.00539.2002
Ren, J., Momose-Sato, Y., Sato, K., and Greer, J. J. (2006). Rhythmic neuronal discharge in the medulla and spinal cord of fetal rats in the absence of synaptic transmission. J. Neurophysiol. 95, 527–534. doi: 10.1152/jn.00735.2005
Ripley, K. L., and Provine, R. R. (1972). Neural correlates of embryonic motility in the chick. Brain Res. 45, 127–134. doi: 10.1016/0006-8993(72)90220-x
Scain, A.-L., Le Corronc, H., Allain, A.-E., Muller, E., Rigo, J.-M., Meyrand, P., et al. (2010). Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J. Neurosci. 30, 390–403. doi: 10.1523/jneurosci.2115-09.2010
Thoby-Brisson, M., Trinh, J.-B., Champagnat, J., and Fortin, G. (2005). Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J. Neurosci. 25, 4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005
Whelan, P. J. (2003). Developmental aspects of spinal locomotor function: insights from using the in vitro mouse spinal cord preparation. J. Physiol. 553, 695–706. doi: 10.1113/jphysiol.2003.046219
Keywords: optical recording, spontaneous activity, chick embryo, brainstem, development
Citation: Momose-Sato Y and Sato K (2016) Development of Spontaneous Activity in the Avian Hindbrain. Front. Neural Circuits 10:63. doi: 10.3389/fncir.2016.00063
Received: 05 June 2016; Accepted: 29 July 2016;
Published: 12 August 2016.
Edited by:
Catherine Carr, University of Maryland, College Park, USAReviewed by:
Daniel Llano, University of Illinois at Urbana–Champaign, USAMasaaki Torii, Children’s National Health System, USA
Copyright © 2016 Momose-Sato and Sato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoko Momose-Sato, eW1zQGthbnRvLWdha3Vpbi5hYy5qcA==
 Yoko Momose-Sato
Yoko Momose-Sato Katsushige Sato
Katsushige Sato