- Department of Physiology and Pharmacology, SUNY Downstate Medical Center, Brooklyn, NY, USA
The onset of puberty is associated with alterations in mood as well as changes in cognitive function, which can be more pronounced in females. Puberty onset in female mice is associated with increased expression of α4βδ γ-amino-butyric acid-A (GABAA) receptors (GABARs) in CA1 hippocampus. These receptors, which normally have low expression in this central nervous system (CNS) site, emerge along the apical dendrites as well as on the dendritic spines of pyramidal neurons, adjacent to excitatory synapses where they underlie a tonic inhibition that shunts excitatory current and impairs activation of N-methyl-D-aspartate (NMDA) receptors, the trigger for synaptic plasticity. As would be expected, α4βδ expression at puberty also prevents long-term potentiation (LTP), an in vitro model of learning which is a function of network activity, induced by theta burst stimulation of the Schaffer collaterals to the CA1 hippocampus. The expression of these receptors also impairs spatial learning in a hippocampal-dependent task. These impairments are not seen in δ knock-out (−/−) mice, implicating α4βδ GABARs. α4βδ GABARs are also a sensitive target for steroids such as THP ([allo]pregnanolone or 3α-OH-5α[β]-pregnan-20-one), which are dependent upon the polarity of GABAergic current. It is well-known that THP can increase depolarizing current gated by α4βδ GABARs, but more recent data suggest that THP can reduce hyperpolarizing current by accelerating receptor desensitization. At puberty, THP reduces the hyperpolarizing GABAergic current, which removes the shunting inhibition that impairs synaptic plasticity and learning at this time. However, THP, a stress steroid, also increases anxiety, via its action at α4βδ GABARs because it is not seen in δ−/− mice. These findings will be discussed as well as their relevance to changes in mood and cognition at puberty, which can be a critical period for certain types of learning and when anxiety disorders and mood swings can emerge.
Introduction
Adolescence is a developmental stage when major hormonal and behavioral changes occur. Some reports have characterized adolescence as the end of a critical period for the optimal learning of certain basic tasks, including language acquisition, error detection and spatial memory (Pepin and Dorval, 1986; Johnson and Newport, 1989; Subrahmanyam and Greenfield, 1994; McGivern et al., 2002; Shavalier, 2004). In many cases, the rapid upward trajectory of learning achievement during early development is slowed during the pubertal period (Gur et al., 2012), especially for spatial learning, when gender differences, favoring boys, generally first appear (Kanit et al., 2000; Ardila et al., 2011; Gur et al., 2012). The adolescent period is also known to be a time when emotional changes occur, including mood swings (Buchanan et al., 1992) and increased responses to stress (Susman et al., 1988; Modesti et al., 1994; Lui et al., 2012) as well as the time when anxiety disorders first emerge (Reardon et al., 2009) which, in some cases, continue into adulthood. This review will describe the known changes in populations of extrasynaptic GABAA receptors (GABARs) which occur at puberty and discuss the relevance of these changes in producing behavioral outcomes which limit learning and alter mood during adolescence.
Critical Periods and GABAergic Inhibition
Puberty onset has been described as the end of a critical period for optimal learning of certain basic tasks such as learning a second language (Johnson and Newport, 1989). Although there are many factors contributing to the cognitive changes which characterize adolescence, GABAergic inhibition plays an important role in limiting developmental plasticity at this time. This has been shown earlier in development for the visual cortex where the development of GABAergic input marks the end of the critical period of cortical plasticity for ocular dominance (Fagiolini et al., 2004). Because interneurons mature more slowly than excitatory synapses (Huang et al., 1999; Jiang et al., 2005), GABAergic inhibition arrives at a later stage of development when it can slow or even prevent synaptic plasticity (Guo et al., 2013). In fact, positive GABA modulators such as benzodiazepines (BDZs) can alter the timing of the critical period for the visual system (Iwai et al., 2003). This role of GABA in limiting critical periods is widespread throughout development, and is seen for sound localization, taste and olfaction in addition to vision (Hensch, 2004).
GABA and Puberty
Several lines of evidence suggest that GABAergic inhibition is greater during adolescence due to both pre- and post-synaptic changes. The number of GABA synapses increases at the time of puberty (Jiang et al., 2005), as does expression of GAD65 (Stork et al., 2000) generating an increase in inhibition at this time. Knock-down of GAD65 has been shown to reduce the tonic inhibitory current (Song et al., 2011) due to a reduction in the concentration of ambient GABA and can affect the timing of critical periods (Katagiri et al., 2007) without altering spontaneous synaptic current (Tian et al., 1999). In addition, tonic inhibition increases in the CA1 hippocampus (Shen et al., 2007), which reduces neuronal excitability by decreasing the input resistance of the neuron. This increase in tonic inhibition is due to increased expression of extrasynaptic α4βδ GABARs on the dendrites of CA1 hippocampal pyramidal cells (Figure 1), which emerge at puberty onset in female mice from almost undetectable levels before puberty (Shen et al., 2007).
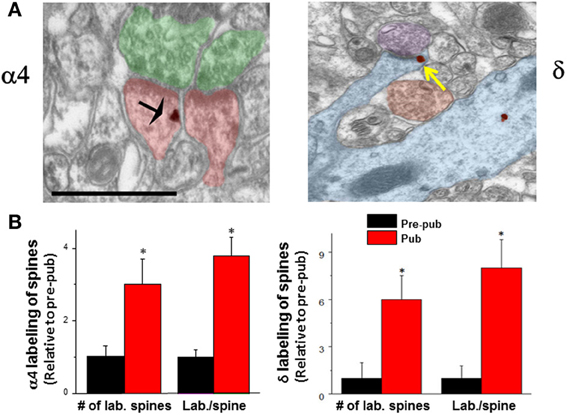
Figure 1. Expression of α4 and δ GABAA receptor subunit increases on dendritic spines of CA1 hippocampal pyramidal cells at puberty. (A) α4 (left) and δ (right) silver-intensified immunogold labeling (SIG) occurs along the plasma membrane of spines forming excitatory synapses. α4, black arrow; δ, yellow arrow. Scale, 100 μm. Shafts also exhibit immunoreactivity. (B) Averaged data. no of labeled spines (α4, *P < 0.018; δ, *P = 0.002) and labeling per spine (α4, *P < 0.005, δ, *P = 0.00091) increase at puberty (Pub) relative to pre-puberty (Pre-pub). n = 50–80 spines [Revised and used with permission (Shen et al., 2010a)].
GABARs
GABARs are membrane, pentameric ligand-gated Cl− channels of diverse composition (Olsen and Sieghart, 2009). Although most GABAs are composed of 2α, 2β, and 1γ (Chang et al., 1990), other subytpes exist from a pool of 6α, 3β, 3γ, δ, ε, θ, π, and ρ. These receptors mediate a Cl− conductance, which is inhibitory in most central nervous system (CNS) sites, including hippocampus, after early development (Rivera et al., 1999). GABA current is outward in most CNS sites due to the K+−Cl− co-transporter KCC2 which maintains a Cl− gradient resulting in hyperpolarizing GABAergic current (Payne, 1997). Because the Cl− reversal potential (EGABA) is close to the membrane potential (Vm) in many CNS sites, GABA generates a shunting inhibition, regardless of the direction of Cl− current, which in some areas such as dentate gyrus (Staley and Mody, 1992), are in the inward direction (i.e., depolarizing). The shunting properties of GABARs result from the fact that, in these cases, the relatively small driving force for generating Cl− current (Vm − EGABA) produces little change in ion flux, but instead primarily reduces the input resistance (R) of the neuron because of the opening of Cl− channels. This reduction in input resistance shunts the incoming current (I) and reduces its impact in producing a change in membrane voltage, as described by Ohm's Law (Vm = I × R). This reduction in input resistance can be independent of the direction of the Cl− current, even though it may be in the depolarizing direction.
Recent reports, however, suggest that the determinants of whether a depolarizing GABAergic tonic current is shunting and inhibitory or excitatory include not only driving force for Cl− current but also the magnitude of the tonic conductance (Song et al., 2011): Smaller GABAergic tonic conductances which are excitatory can be replaced by a shunting inhibition when the conductance is increased by levels of ambient GABA or neurosteroids. Other studies have noted that excitatory versus inhibitory effects of this shunting inhibition also depend upon the precise location of the inhibition as well as the timing of excitatory inputs (Chiang et al., 2012).
Extrasynaptic GABARs
In addition to synaptic expression, some GABAR sub-types express extrasynaptically where they produce a tonic inhibitory current. These include α5β3γ2 GABARs, localized primarily to CA1 hippocampal pyramidal cells (Wisden et al., 1992; Caraiscos et al., 2004), and α4βδ GABARs, localized primarily to dentate gyrus granule cells, thalamic relay nuclei and cortical pyramidal cells (Wisden et al., 1992; Pirker et al., 2000; Stell and Mody, 2002; Stell et al., 2003; Belelli et al., 2005; Chandra et al., 2006) where they generate a tonic inhibitory current (Stell and Mody, 2002). In addition, α1βδ and α1βγ2 express extrasynaptically on hippocampal interneurons (Semyanov et al., 2003; Glykys et al., 2007; Song et al., 2011) while the α3βγ2 does so in basolateral amygdala neurons (Marowsky et al., 2012). The α6βδ GABAR, homologous to α4βδ, has exclusive expression on granule cells in the cerebellum (Nusser et al., 1998). Other recent studies have shown that α1β2 expresses extrasynaptically on hippocampal neurons (Sieghart and Sperk, 2002; Mortensen and Smart, 2006).
α4βδ GABARs
Stoichiometry studies using atomic force microscopy show that subunits within the α4βδ GABAR are arranged α4, β, α4, β, and δ, clock-wise, when viewed from the top (Barrera et al., 2008). This receptor has a high sensitivity to GABA (EC50 = ~0.5 μ M) (Brown et al., 2002; Sundstrom-Poromaa et al., 2002). Thus, it is well-suited for an extrasynaptic location, where ambient GABA is 100 nM–1 μ M (Wu et al., 2003; Wlodarczyk et al., 2013). Although early studies suggested that these receptors exhibit little desensitization (Bianchi et al., 2002), more recent studies show greater desensitization at both physiological temperature (Bright et al., 2011) and room temperature (Mortensen et al., 2010). Desensitization is rapid in response to rapid exposure to GABA; thus, α4βδ GABARs are likely not activated by transmitter spillover (Bright et al., 2011), but instead generate a steady-state current in response to ambient GABA (Stell and Mody, 2002). A recent study has shown that the extrasyaptic α4βδ GABARs which underlie the tonic current in dentate gyrus granule cells are consititutively active (Wlodarczyk et al., 2013), independent of the low concentrations of ambient GABA found in this region, although increases in the GABA concentration can activate these receptors and increase the tonic current. In the granule cells of the cerebellum, tonic current generated by α6βδ provides a necessary reduction in the high input resistance conferred by the small diameter of the soma. In fact, when these receptors are knocked out, a leak K+ channel (TASK) is compensatorily upregulated (Brickley et al., 2001). Similarly, when GABARs containing the α5 subunit are knocked-out, CA1 pyramidal cells compensatorily increase expression of δ-containing GABARs (Glykys and Mody, 2006), while knock-out of δ increases expression of GABARs containing α4 and γ2 subunits in interneurons of the molecular layer of dentate gyrus (Glykys et al., 2008). Taken together, these findings suggest the importance of the tonic inhibitory conductance in neuronal function.
Pharmacology of α4βδ GABARs
BDZs are typically classified as positive allosteric modulators of most GABARs containing α1-3 or α5 and a γ2 subunit (Olsen and Sieghart, 2009). α4βδ GABARs have a unique pharmacological profile because they are insensitive to modulation by BDZs (Wieland et al., 1992; Brown et al., 2002), as are α4βγ2 and α6βδ, due to an arginine to histidine substitution at residue 99 of the α4/6 subunit (Wieland et al., 1992), which prevents BDZ binding. In addition, the inclusion of a δ subunit instead of γ2 also renders these receptors BDZ-insensitive (Brown et al., 2002), because α1 and γ2 form the BDZ binding pocket (Buhr and Sigel, 1997); thus α1βδ GABARs are also BDZ-insensitive. GABA acts as a partial agonist at these receptors (Bianchi and Macdonald, 2003), and instead other compounds including gaboxadol [THIP or 4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3-ol hydrochloride], β-alanine and taurine are full agonists at these receptors (Brown et al., 2002; Bianchi and Macdonald, 2003; Jia et al., 2008), such that the response of neurons to these compounds can be used to verify expression of δ-containing GABARs (Shen et al., 2010a,b). α4βδ GABARs are also sensitive targets of steroids such as THP [(allo)pregnanolone or 3α-OH-5α(β)-pregnan-20-one], and THDOC (3α,21-dihydroxy-5α-pregnan-20-one) (Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002; Bianchi and Macdonald, 2003), which are generally positive modulators of the receptor. These steroids act by increasing receptor efficacy (Bianchi and Macdonald, 2003; Zheleznova et al., 2008). In single channel studies, the steroid THDOC was shown to increase receptor efficacy by adding a third open state of longer duration to the two open states recorded from α4βδ GABARs in the absence of steroid (Wohlfarth et al., 2002). Other studies have shown that, unlike α4β2γ2 GABARs where single channel activity bursts in clusters, recordings from α4βδ GABARs reflect only isolated openings, which have a much lower open probability than other GABARs (Akk et al., 2004; Mortensen et al., 2010). Single channel conductance states of this receptor are similar to α1βγ2, but the mean open time of the highest conductance state is significantly reduced compared to α1βγ2 (Mortensen et al., 2010). In the more commonly expressed α1β2γ2 GABAR, additional studies have been conducted to identify the steroid binding pocket, which extends from the glutamine residue at position 241 in the M1 (transmembrane) segment to asparagine (407) and tyrosine (410) in M4 (Hosie et al., 2006). In this receptor, the steroid THDOC was shown to increase proportion of channels in a long-lived open state (Akk et al., 2010), an effect prevented by mutation of glutamine 241 to serine (Akk et al., 2008), which still permitted steroid potentiation of the receptor. Complete blockade of steroid potentiation of α1β2γ2 was achieved by mutating this glutamine to leucine or tryptophan (Akk et al., 2008).
Neurosteroids and Tonic Current
Neurosteroids can increase the tonic current recorded from dentate gyrus granule cells (Stell et al., 2003), although some studies have not observed this effect, due to the rapid metabolism of steroids such as THP and THDOC in this CNS region (Belelli and Herd, 2003). This effect is mediated by α4βδ GABARs because this effect is reduced in δ−/− mice (Stell et al., 2003).
GABARs containing the δ subunit have been shown to be sensitive to low, behaviorally relevant concentrations of alcohol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), which enhance the GABAergic tonic inhibitory current (Wei et al., 2004; Glykys et al., 2007). However, several other studies have failed to find effects of low concentrations of ethanol on these receptors (Borghese et al., 2006; Yamashita et al., 2007; Baur et al., 2009) or on tonic current in CNS areas with high expression of δ-containing GABARs (Carta et al., 2004; Borghese et al., 2006; Yamashita et al., 2007). The reason for this discrepancy is not clear, but may be related to the phosphorylation state of the cells recorded, as protein kinase C- δ is required for ethanol effects at δ-containing GABARs (Messing et al., 2007; Choi et al., 2008). Developmental regulation of δ expression may also be a factor in neuronal studies, as δ expression changes across development (Laurie et al., 1992).
α4βδ GABARs and Synaptic Plasticity at Puberty
Expression of α4βδ GABARs is typically quite low on CA1 hippocampal pyramidal cells of adult mice compared to the dentate gyrus granule cell (Peng et al., 2002; Wei et al., 2003), where high expression of these receptors yields a tonic current that is 5 to 6-fold greater than that measured in CA1 pyramidal cells assessed by comparing current amplitude in wild-type versus mice which lack δ expression (δ−/−) (Glykys et al., 2008). However, there are marked increases in expression of this receptor on CA1 pyramidal cells at puberty from these nearly undetectable levels noted before puberty (Shen et al., 2007). The increase in α4βδ GABAR expression on CA1 hippocampal pyramidal cells of female mice at puberty is localized both to the dendritic shaft as well as the dendritic spine (Figure 1) adjacent to asymmetric, excitatory synapses (Shen et al., 2010a). This unique location suggests a role for these receptors in regulating cognition during adolescence, because the dendritic spine is the CNS site for induction of synaptic plasticity (Nevian and Sakmann, 2006). Although GABAergic interneurons target dendritic spines in cortical circuits (Kubota et al., 2007), GABAergic input does not do so in CA1 hippocampus, where GABARs, either synaptic or extrasynaptic are not typically found. In adult CA1 hippocampus, GABARs localize to the soma (80%) or the dendritic shaft (20%) (Megias et al., 2001), where they can either be sub-synaptic or extrasynaptic [predominantly α5β3γ2 (Brunig et al., 2002)]. Only during the pubertal period (~post-natal day 35–44) do extrasynaptic α4βδ GABARs increase to significant levels of expression (Shen et al., 2010a,b): The proportion of spines which are immunolabeled with α4 and δ is increased by 3 to 6-fold, respectively, while the immunoreactivity per spine is increased 4 to 8-fold, respectively, as determined with silver-intensified immunogold (SIG) labeling and electron microscopy in the proximal stratum radiatum (Figure 1). Recent estimates suggest that 25% of spines may express α4βδ GABARs (Aoki et al., 2012). Similar increases in immunolabeling are seen on the dendritic shaft. Pubertal expression of α4βδ GABARs in these dendritic compartments persists for a period of about 10 d, and is reduced significantly by about post-natal day 44 (Aoki et al., 2012).
Although it is not possible to know whether this receptor is increased at puberty in humans, there is indirect evidence suggested by the reduced sensitivity of adolescents to the sedative effect of BDZs such as midazolam (Massanari et al., 1997), which would be consistent with increased expression of α4βδ, a BDZ-insensitive GABAR (Wisden et al., 1991; Wafford et al., 1996). There is also an increased incidence of paradoxical anxiety reactions to BDZs in adolescents (Massanari et al., 1997), which is consistent with increased expression of α4βδ on principal neurons (but not on interneurons, where BDZs could preferentially disinhibit the network). Although not definitive, this evidence is at least consistent with the predicted pharmacology if α4βδ GABARs were increased during adolescence in humans.
Physiological Consequences of α4βδ GABAR Expression
Functional expression of α4βδ at puberty was verified by the robust response of CA1 hippocampal pyramidal cells to the GABA agonist gaboxadol at a 100 nM concentration (Shen et al., 2010a), selective for α4βδ GABARs (Brown et al., 2002; Meera et al., 2011). In contrast, gaboxadol produces a negligible response in pre-pubertal CA1 hippocampus. This increase in α4βδ GABAR expression at puberty is associated with a number of predictable outcomes, including a decrease in the input resistance and an increase in the threshold for action potential activation in response to injection of depolarizing current (Figure 2). In addition, activation of NMDA receptors is impaired (Shen et al., 2010a) most likely due to the shunting inhibition produced by these receptors which would reduce the depolarization necessary for Mg+ unblock of the receptor (Herron et al., 1986). Consequently, induction of long-term potentiation (LTP), an in vitro model of learning, produced by stimulation of the Schaffer collaterals to CA1 hippocampus with theta burst stimulation (TBS), is impaired (Figure 3) (Shen et al., 2010a). This deficit in synaptic plasticity is prevented with total blockade of GABARs (120 μ M SR95531) (Stell and Mody, 2002) or with the use of the δ−/− mouse. Thus, these data suggest that α4βδ GABARs which emerge at puberty impair synaptic plasticity during adolescence. In contrast, LTP induction is robust in the hippocampus of pre-pubertal mice. Surprisingly, selective blockade of synaptic GABARs (200 nM SR95531) (Stell and Mody, 2002) does not facilitate LTP induction at puberty, suggesting that the deficit in synaptic plasticity is due to the extrasynaptic GABAR population exclusively (Shen et al., 2010a). Extrasynaptic α5β3γ2 GABARs also play a role in limiting synaptic plasticity induced by low frequency stimulation in adults, where synaptic GABARs are not a factor (Martin et al., 2010). In dentate gyrus, which has high expression of α4βδ GABARs that generate a robust tonic inhibition (Wei et al., 2003; Glykys et al., 2008), tonic inhibition plays a major role in modulating LTP in adult hippocampus, with greater effects than noted in the CA1 hippocampus (Arima-Yoshida et al., 2011). However, high frequency stimulation also differentially increases synaptic inhibition more than synaptic excitation in adult hippocampus, which suggests that synaptic inhibitory current may play a role in altering synaptic plasticity in the adult although this has not been definitively demonstrated (Arima-Yoshida et al., 2011).
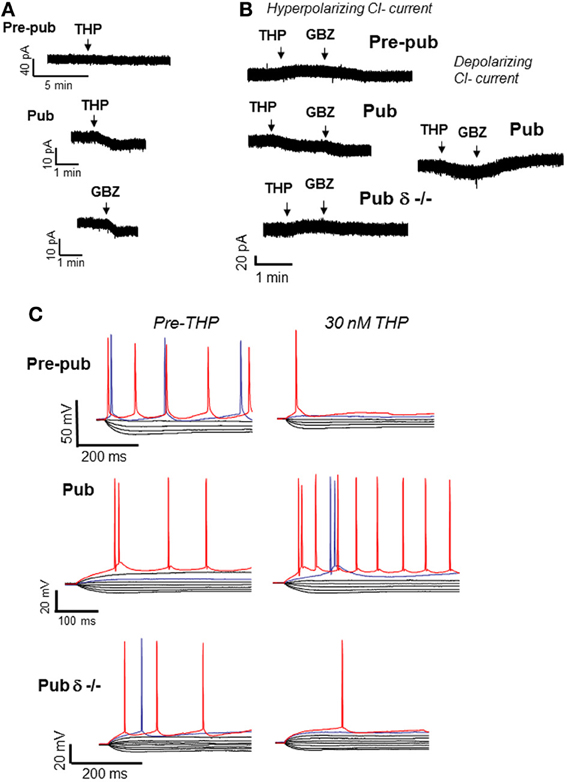
Figure 2. Effects of THP on CA1 hippocampal pyramidal cells at puberty. At the onset of puberty, THP reduces the tonic GABAergic current and increases excitability of CA1 pyramidal cells in the slice. (A) THP (30 nM) effects on the tonic current recorded with gramicidin perforated patch techniques to maintain the internal Cl− milieu. 1 μ M TTX, 1 μ M GABA, and 2 mM kynurenic acid were added to isolate the GABAergic post-synaptic component. L-655, 708, CGP55845, and TEA were also added to block α5 and GABAB receptors and K+ channels, respectively. Pre-pub, pre-pubertal; Pub, pubertal; GBZ, gabazine, a GABA antagonist (presented for comparison). THP reduces the current in Pub slices. (Representative of 5 cells/group) (B) Left panel, Hyperpolarizing current recorded from CA1 hippocampal pyramidal cells in the slice using whole cell patch clamp techniques (ECl = −70 mV, −50 mV holding potential; pipette solution, K-gluconate; bath, 200 nM gabazine to block synaptic current and 2 mM kynurenic acid to block excitatory current). Right panel, effects of THP on the depolarizing tonic current at puberty (ECl = −30 mV, pipet solution, CsCl). THP reduces hyperpolarizing current in wild-type but not δ−/− mice, but potentiates depolarizing current. (Representative of 8–12 cells/group) (C) Whole-cell current-clamp recordings reveal voltage responses recorded in response to increasing 0.3-nA current injection (initial current −1 nA). (The THP trace lacks the 800-pA current trace for ease of comparison.) THP lowers the current threshold for spiking of pyramidal cells at the onset of puberty in wild-type but not δ−/− mice. Red trace, equivalent current injection, threshold for the less excitable state. Blue trace, equivalent current injection, threshold for the more excitable state. (Representative of 7–8 cells/group) [Revised and used with permission (Shen et al., 2007)].
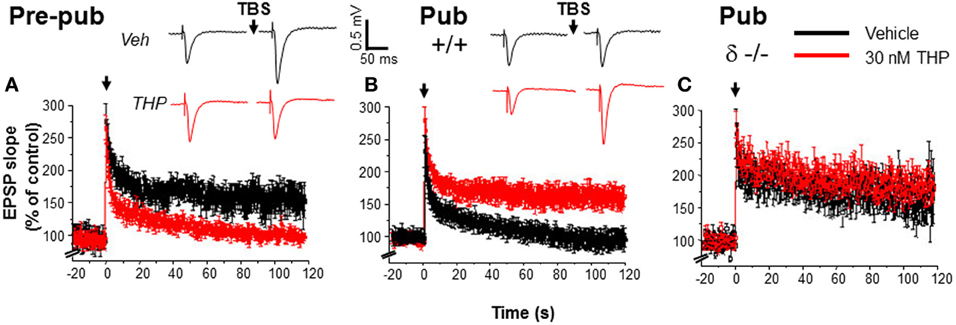
Figure 3. Impaired induction of LTP at puberty: reversal by the stress steroid THP and δ knock-out. Theta burst stimulation (TBS—0 time) induced LTP (black) before puberty Pre-pub, (A) but not in the pubertal Pub, (B) CA1 hippocampus. THP (red, 30 nM) permitted LTP induction at puberty. (Inset, Representative field EPSPs. TBS, arrow. Scale, 0.5 mV, 50 ms.) (C). LTP, Pubertal δ−/−, where TBS induced LTP. n = 6–9/group. [Used with permission (Shen et al., 2010a)].
Earlier studies suggested that LTP induction is impaired in adolescence due to an increase in GABAergic inhibition (Meredith et al., 2003), although puberty onset and α4βδ were not identified in this study. In contrast, the function of NMDARs does not appear to be compromised at puberty because activation of NMDA current is robust in the δ−/− or after total GABAR blockade (Shen et al., 2010a). Therefore, the deficit in synaptic plasticity at puberty appears to be due to increases in shunting inhibition mediated by α4βδ GABARs.
Pubertal Expression of α4βδ GABARs and Spatial Learning
Because α4βδ GABARs localize to dendritic spines of pyramidal cells in CA1 hippocampus, the behavioral outcome has been tested in a spatial learning task. The CA1 hippocampus plays a critical role in spatial learning (Burgess et al., 2002; Bannerman et al., 2004; Pastalkova et al., 2006), where selective deletion of NMDARs in this region impairs the spatial and contextual forms of memory without affecting the temporal aspects of memory formation (Tsien et al., 1996; Place et al., 2012); thus, a hippocampal-dependent task (Cimadevilla et al., 2001) was selected to demonstrate the role of these receptors in spatial learning. To this end, an active place avoidance task has been employed which requires that the animal avoid a mild footshock (<0.2 mA) sub-threshold for release of stress steroids (Friedman et al., 1967), which suggests that this is a relatively unstressful task compared to other animal models of learning (Harrison et al., 2009). For each trial, the latency to enter the avoidance sector during rotation of the platform is a measure of learning. Performance on this task is well-correlated with, and depends upon, successful induction of LTP (Pastalkova et al., 2006). Pubertal mice show impaired spatial learning on this task compared to pre-pubertal mice: they show faster latencies to re-enter the avoidance zone during learning trials and fail to reach criterion (120 s latency to enter the shock sector) after 7 trials (Figure 4). In contrast, pre-pubertal mice reach criterion in 2.5 trials (with a mean latency of 275 s) (Shen et al., 2010b). Potential non-specific effects of other sensorimotor/behavioral outcomes were ruled out in this study because general locomotor activity was not altered (assessed as path length). In addition, the number of shocks/entry was not different between groups suggesting that the shock is equally aversive for all animals and that they are equally able to escape (which would include sensorimotor, motivational and attentional parameters). This learning deficit is not seen in pubertal δ−/− mice, suggesting that it is the increase in α4βδ GABAR expression at puberty which produces these deficits in spatial learning (Shen et al., 2010a). This is most likely due to the shunting inhibition produced by these receptors which impairs activation of hippocampal NMDARs.
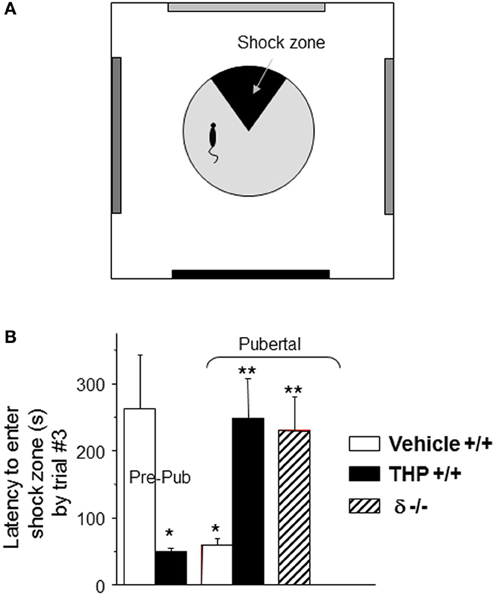
Figure 4. Impaired spatial learning of pubertal mice: reversal by the stress steroid THP and δ knock-out. (A) Spatial learning platform (shock zone, black sector). The platform rotates, but the shock zone is stationary, requiring the animal to move to actively avoid the shock. (B) Latency to first entry of the shock zone, a measure of learning. Pre-pub mice attained the longest entry times. (n = 6–9 mice/group) *P < 0.05 vs. pre-pub; **P < 0.05 vs. pub +/+ vehicle. [Revised and used with permission (Shen et al., 2007)].
α4βδ GABAA Receptors: Effects on Plasticity and Learning in Adult, Male Mice
Because α4βδ GABARs have high expression in the dentate gyrus, recent studies have focused on examining effects of α4 or δ knock-out on learning tasks that are relevant for this area, including trace and conditional fear conditioning, recognition memory and contextual discrimination memory. Although the dentate gyrus circuitry plays a role in the formation of these memories, the specific effect is complex and the outcome of α4βδ knock-out depends on the task examined. Both α4 and δ knock-out enhance trace and conditional fear conditioning (Wiltgen et al., 2005; Moore et al., 2010). However, δ knock-out impairs recognition memory and contextual discrimination memory, suggesting that these receptors facilitate these types of plasticity (Whissell et al., 2013). Intriguingly, this study showed that α4βδ GABARs facilitate neurogenesis in this area (Whissell et al., 2013), which is a likely mechanism for their facilitating effect on learning. As in the previous study, fear memory was enhanced by δ knock-out, but fear memory extinction was impaired, suggesting that the mechanisms for acquisition and extinction of fear memories require different mechanisms.
Another recent study has suggested that α4βδ GABARs may also act presynaptically to regulate neurotransmitter release from mossy fiber afferents to CA3 pyramidal cells (Ruiz et al., 2010). In this case, low concentrations of the neurosteroid THDOC facilitate glutamate release, while the GABA antagonist SR95531 reduces glutamate release and impairs induction of LTP at CA3 synapses. Again, this is an additional example of a case where α4βδ GABARs facilitate synaptic plasticity, suggesting that their effect depends not only on the age of the animal but also on the circuit involved. There are limitations in our understanding of the exact impact of these receptors, however, because it is not possible to localize receptor deletion to specific sites within specific brain areas. Until that becomes possible, our understanding of their impact is restricted to broader regions of the CNS.
The role of the dentate gyrus in mediating the changes in cognition at puberty is not known. Changes in receptor expression have not been quantified in this region across pubertal stages. However, it is likely that effects of THP would enhance recognition memory and contextual discrimination memory at puberty via its ability to potentiate tonic inhibition mediated by α4βδ GABARs expressed on dentate gyrus granule cells, where it would likely facilitate neurogenesis. Thus, this is an important topic for future studies.
Positive Modulators of GABAA Receptors and Synaptic Plasticity
It is well known that positive modulators of the GABAR impair synaptic plasticity and learning, as reported in both rodents and humans. Benzodiazepine tranquilizers are amnestic (Veselis et al., 2009), as are certain anesthetics such as propofol (Veselis et al., 2009) and isoflurane (Saab et al., 2010). Both alcohol and the neurosteroid THP impair spatial learning on the Morris Water Maze (Matthews et al., 2002). In adult CA1 hippocampus, α5β3γ2 GABARs have high expression extrasynaptically where they localize to the dendritic shaft and to the base of the dendritic spine (Brunig et al., 2002). Both knock-out and knock-down of this receptor, with the use of a selective inverse agonist, prevents impairments in learning following administration of the anesthetic etomidate (Cheng et al., 2006). These procedures also enhance fear conditioning (Collinson et al., 2002; Crestani et al., 2002; Chambers et al., 2003). Positive GABA modulators also impair synaptic plasticity in in vitro models, including LTP: The degree of synaptic potentiation is significantly decreased by alcohol, THP and propofol (Izumi et al., 2005; Nagashima et al., 2005; Ma et al., 2005; Tokuda et al., 2011), suggesting that GABA inhibition plays an important role in limiting synaptic plasticity in the hippocampus. In contrast, α5 knock out reduces the threshold for frequency-dependent induction of LTP (Martin et al., 2010).
THP and the Response to Stress
THP is a GABA-modulatory metabolite of progesterone, and is produced both by the ovary and adrenal gland (Mellon and Vaudry, 2001), which increase release of THP before puberty onset (Mannan and O'Shaughnessy, 1988; Fadalti et al., 1999; Shen et al., 2007). Fluctuations in circulating levels of THP occur across the ovarian cycle, reaching peak levels on the afternoon of proestrus and day of diestrus 1, as well as during pregnancy (Palumbo et al., 1995; Concas et al., 1998). In addition, THP can be produced de novo in the brain from cholesterol via side chain cleavage enzyme (Compagnone and Mellon, 2000) in several CNS sites, including the CA1 hippocampal pyramidal cell (Agis-Balboa et al., 2006). Circulating and/or CNS levels of this steroid increase before puberty, but decline to low levels at the onset of puberty, (Fadalti et al., 1999; McCartney et al., 2007; Shen et al., 2007). Unlike most steroids, THP has no known effect at classic nuclear steroid receptors, but instead is a modulator of the GABAR (Smith et al., 2007).
In rodents, circulating levels of THP increase by up to 20-fold after 45 min of restraint stress and other forms of stress, such as CO2 inhalation (Purdy et al., 1991; Higashi et al., 2005; Mukai et al., 2008) when decreases in anxiety are observed (Barbaccia et al., 2001). Similarly, in humans, circulating levels of this steroid increase after sustained stress associated with performance (Droogleever Fortuyn et al., 2004; Girdler et al., 2006). Thus, THP is one factor which is part of the stress response, and because it typically acts as an anxiolytic (Bitran et al., 1999), it would be expected to mitigate the anxiety reaction to stress in adults.
Polarity-Dependent Actions of THP at α4βδ GABARs
The effects of THP at α4βδ GABARs appear to be dependent upon the direction of the Cl− current in the GABA channel. When GABA-generated current is in the depolarizing direction (inward current), THP and related steroids increase current recorded from recombinant α4βδ GABARs (Belelli et al., 2002; Wohlfarth et al., 2002; Bianchi and Macdonald, 2003) (Figures 5A,B). In contrast, when GABA-generated current is in the hyperpolarizing direction (outward current), THP decreases current recorded from recombinant α4βδ GABARs (Shen et al., 2007) (Figures 5D,E). The first outcome is relevant for the dentate gyrus granule cell (Figures 5A–C), where GABA generates a depolarizing, shunting inhibition (Staley and Mody, 1992), and THP increases the tonic, inhibitory current (Staley and Mody, 1992) and reduces neuronal excitability (Stell et al., 2003). This is consistent with the typical effect of THP to reduce anxiety (Bitran et al., 1999). The second outcome is relevant for CA1 hippocampal pyramidal neurons which generate a hyperpolarizing current generated by α4βδ GABARs which express during adolescence (Shen et al., 2007). At this time THP reduces the tonic inhibitory current and increases neuronal excitability (Shen et al., 2007) (Figures 5D–F). Consistent with this novel excitatory effect, THP now increases anxiety, in contrast to its typical anxiety-reducing effect (Shen et al., 2007).
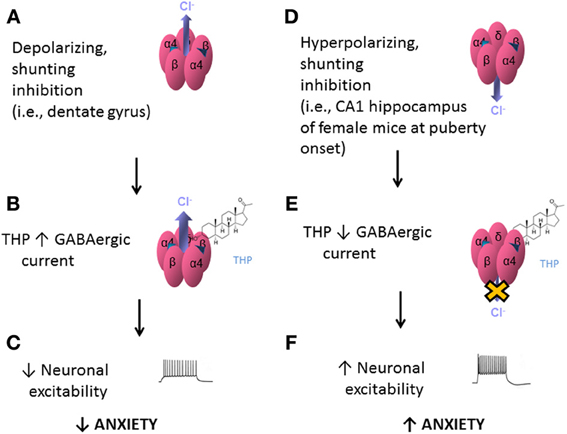
Figure 5. Effects of THP on α4βδ GABARs: summary diagram. Effects of the neurosteroid THP at α4βδ GABARs are dependent upon the polarity of GABAergic current. α4βδ GABARs are depicted with inward, depolarizing current (outward Cl− flux, A) or outward, hyperpolarizing current (inward Cl− flux, D). (A) When the Cl− current generated by these receptors is inward, as would be found in dentate gyrus granule cells, THP potentiates this shunting inhibition (B) However, when the Cl− current generated by these receptors is outward, as is found in CA1 hippocampal pyramidal cells at puberty (D), THP reduces this current (E), via acceleration of the rate and increases in the extent of desensitization (Shen et al., 2007). This differential effect of the steroid, which is dependent upon the direction of Cl− current through the GABA channel, would have different outcomes at the level of the circuit and behavior: When current generated by α4βδ GABARs is a depolarizing, shunting inhibition (A–C), THP would decrease neuronal excitability in limbic structures and decrease anxiety (C). In contrast, when the current generated by α4βδ GABARs is hyperpolarizing (D–F), as observed at puberty, THP increases neuronal excitability and anxiety (F).
THP's novel effect to reduce GABA current generated by α4βδ GABARs are seen in recombinant receptors where either the ion gradient or holding potential are varied, and occur in the absence of alterations in the reversal potential, which suggests that other conductances are not involved (Figure 6) (Shen et al., 2007). THP-induced decreases in hyperpolarizing current recorded from α4βδ GABARs were shown to be due to acceleration in the rate and extent of receptor desensitization (Shen et al., 2007). This conclusion is consistent with findings from Macdonald and colleagues who have shown that α4βδ GABARs desensitize more rapidly when the current is in the hyperpolarizing direction (Haas and Macdonald, 1999; Bianchi et al., 2002) compared to the depolarizing direction, an effect which is accelerated by neurosteroids (Bianchi and Macdonald, 2003). Although a recent report (Bright et al., 2011) has suggested that the extent of rapid desensitization of this receptor, when recorded at physiological temperature, is much greater than initially reported, there still remains substantial current after rapid application of agonist. Thus, THP's effect to reduce hyperpolarizing current at α4βδ GABARs may be due to desensitization of the steady-state current because it is evident even when agonist is not rapidly applied (Shen et al., 2007). THP-induced desensitization of hyperpolarizing current at α4βδ GABARs was shown to be dependent upon the positively charged residue arginine 353 in the TM3-TM4 loop (Shen et al., 2007), which may serve as a modulatory site for Cl−. Modulatory effects of Cl− have been reported for other subtypes of GABARs (Olsen and Snowman, 1982; Houston et al., 2009). In contrast, desensitization of the other major extrasynaptic GABAR, α5β3γ2, is accelerated with depolarizing GABAergic current (Burgard et al., 1996).
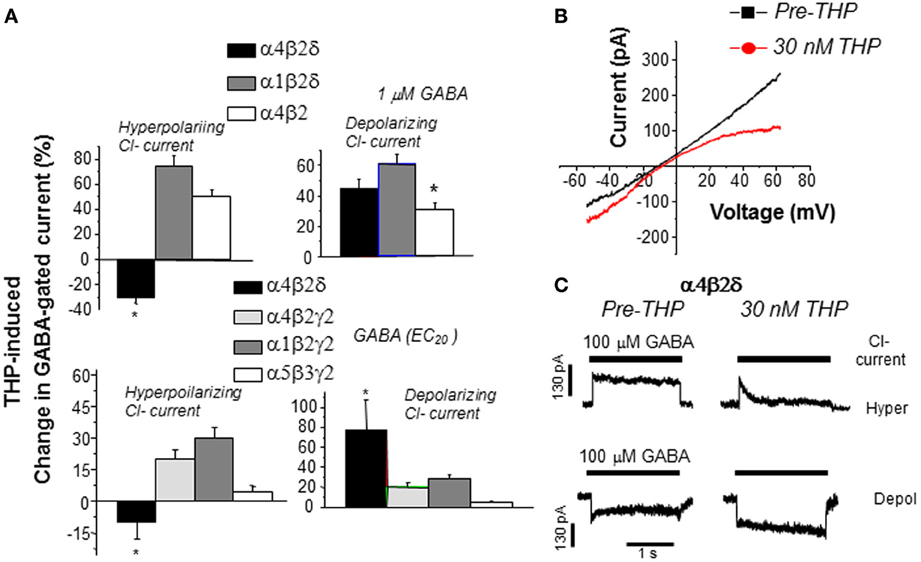
Figure 6. THP exerts polarity-dependent effects at α4βδ GABAA receptors. THP, a neurosteroid, decreases hyperpolarizing current gated by α4βδ GABAA receptors expressed in HEK-293 cells (recorded with whole cell voltage clamp techniques). (A) THP effects on hyperpolarizing and depolarizing currents in response to 1 μ M GABA (upper panel) or the GABA EC20 (lower panel; α4β2δ, 0.1 μ M; α4β2γ2, 5 μ M; α1β2γ2, 10 μ M; α5β3γ2, 5 μ M) from 6–7 cells for each group (Mean ± SEM; *P = 0.05 vs. the other receptor subtypes). n = 6–8 cells/group. (B) Effects of THP (30 nM) on current in response to a voltage ramp over 400 ms where the ECl = 0 mV. THP increases depolarizing current (voltage < 0 mV) but decreases hyperpolarizing current (voltage > 0 mV). (Leak-subtracted current is presented as the average of three traces). n = 5–6 cells/group. (C) Effects of THP on desensitization of hyperpolarizing (upper traces) and depolarizing (lower traces) current at α4βδ receptors (representative of 6 cells per group). THP accelerates desensitization of the hyperpolarizing current. [Used with permission (Shen et al., 2007)].
Paradoxical Excitatory Effects of THP on Hippocampal Function at Puberty
The GABAergic current recorded from CA1 hippocampal pyramidal cells in the slice is hyperpolarizing at puberty (Shen et al., 2007). Thus, as would be expected, THP reduces the tonic inhibitory current at puberty (Figure 2), even when action potential-driven GABA release is blocked by tetrodotoxin (Shen et al., 2007), suggesting that it is acting post-synaptically. However, when the direction of the GABAergic current is artificially reversed to depolarizing by increasing intracellular [Cl−], THP increases the tonic GABAergic current, suggesting that the effect of the steroid is dependent upon the direction of the Cl− current. As expected, THP increases neuronal excitability at this time, assessed both with cell-attached recordings of spontaneous spiking as well as by current clamp recordings (Shen et al., 2007), which reveal that THP reduces the threshold for spiking by increasing the input resistance of the neuron (Figure 2). In addition, THP administration restores NMDA currents, evoked by low frequency stimulation at puberty, to levels similar to those observed before puberty (Shen et al., 2010a). This latter effect may be due to its reduction of the shunting inhibition produced by α4βδ receptors localized to the dendritic spine at puberty. That possibility is supported by the finding that these paradoxical excitatory effects of the steroid at puberty are not seen in the δ−/− mouse (Shen et al., 2010a), implicating α4βδ GABARs. In contrast, THP reduces neuronal excitability before puberty, when α4βδ levels of expression are low.
Effects of the Stress Steroid THP on Synaptic Plasticity and Spatial Learning at Puberty
Synaptic Plasticity
Because THP removes the impediment to activation of NMDA receptors at puberty, additional studies tested whether its administration could also remove the impediment to the induction of LTP at this time, which is dependent upon NMDA receptor activation (Herron et al., 1986). In fact, THP permits robust induction of LTP at puberty, when it is normally not observed (Figure 3), after bath application of the steroid. This outcome is also seen after restricted application of the steroid to the dendrites of the stratum radiatum during theta burst stimulation, suggesting that it facilitates induction (Shen et al., 2010a), rather than maintenance of LTP. This conclusion is confirmed by the finding that THP does not facilitate LTP when it is applied 5 min after theta burst stimulation. THP facilitation of LTP is not seen in the δ−/− hippocampus, suggesting that α4βδ GABARs are responsible for this steroid's effect on synaptic plasticity at puberty (Shen et al., 2010a).
The population of extrasynaptic α5-containing GABARs which are also known to play a role in synaptic plasticity are likely less of a factor in mediating THP's effect. First, they are not reported to localize adjacent to excitatory synapses on spines (Brunig et al., 2002); thus, they would have less of an impact on NMDA receptor activation and the induction of LTP generated by theta burst stimulation. In fact, these receptors have been shown to play a role in frequency-dependent plasticity, such that they lower the frequency necessary to trigger potentiation of CA1 pyramidal cell synaptic responses (Martin et al., 2010). Although it is not yet known whether expression of these receptors increases at the onset of puberty, they are markedly less sensitive to modulation by neurosteroids such as THP compared to α4βδ where THP produces a two-fold increase in potentiation (Belelli et al., 2002). Thus, the small THP-induced potentiation of current at α5-containing GABARs would be expected to only slightly offset the decrease in current at α4βδ GABARs and its effect to trigger robust LTP. The fact that THP completely restores LTP to pre-pubertal levels suggests that any effects of this steroid at α5 GABARs are minor. In fact, the recovery of LTP in the pubertal δ−/− mouse also suggests that the role of α5 GABARs in limiting synaptic plasticity at puberty is also minor.
Spatial Learning
THP also facilitates spatial learning at puberty (Figure 4), assessed using the hippocampal-dependent active avoidance task (Shen et al., 2010a). After THP administration, the pubertal mice reach criterion in less than 3 trials, with a mean latency of 250 s which is more than 3-fold longer than seen in the absence of THP, where mice fail to reach learning criterion in 7 trials. However, THP does not alter the number of shocks delivered per entry, suggesting that pain threshold and other sensorimotor and motivational parameters are not altered by the steroid. Facilitating effects of THP on learning are not seen in the δ−/− mouse, implicating α4βδ GABARs as the target for THP's effect. Because THP can be released by stress (Purdy et al., 1991; Mukai et al., 2008), these results suggest that mild to moderate stress at puberty may have beneficial effects on learning.
Effects of Stress on Learning in Adolescence
A number of other studies have shown that acute stress can enhance learning during adolescence in rodents (Hodes and Shors, 2005; Uysal et al., 2012). Moderate tailshock improves performance on a trace eyeblink conditioning task administered 24 h later (Hodes and Shors, 2005), an effect independent of the estrous cycle and cortisol levels (Hodes and Shors, 2005). This phenomenon is not seen in pre-pubertal or adult, female rats (Wood and Shors, 1998), suggesting that the pubertal period may be a unique period for stress effects on cognition.
In humans, the effect of stress is complex, and is dependent upon whether the stress is acute or chronic, the degree of stress and the cognitive state of the individual. It also depends upon whether the individual perceives themselves as being in control of life situations (“internal locus of control”) as opposed to feeling a helpless victim of external forces (“external locus of control”). When middle school children were assessed in their academic performance during environmental stressors, those students with an internal locus of control exhibited improved performance during stress (Wolk and Bloom, 1978), while those with an external locus of control had diminished performance as stress level increased. This outcome may also depend on the degree of stress, as first described by the Yerkes-Dodson law (Yerkes and Dodson, 1908), which describes an inverted U relationship between stress and the learning of simple tasks (Lupien et al., 2007).
Paradoxical Anxiety-Producing Effects of THP at Puberty
GABARs are known to modulate anxiety responses (Rudolph et al., 1999), and are the targets for most anxiety-reducing drugs, including BDZs, barbiturates and alcohol, as well as for THP, which decreases anxiety in adult rodents. Many CNS areas have been implicated in the control of anxiety, including the dentate gyrus (Kheirbek et al., 2013) and hippocampus (Bitran et al., 1999; Bannerman et al., 2004), where direct, local administration of THP can reduce anxiety in adult rats (Bitran et al., 1999; Bannerman et al., 2004). This is consistent with its typical effect to potentiate inhibition at most GABARs (Olsen and Sieghart, 2009). However, THP increases anxiety in pubertal female mice (Figure 6) (Shen et al., 2007) due to the fact that it reduces GABAergic inhibition in the hippocampus at puberty (Shen et al., 2007) via its effect at α4βδ. This was assessed using the elevated plus maze, an animal model of anxiety following direct administration of THP (10 mg/kg, i.p.) and it was also demonstrated indirectly by using 45 min of restraint stress to increase endogenous levels of THP (Higashi et al., 2005), which increases anxiety unless finasteride or the inactive isomer of THP, 3β-OH-THP, is pre-administered to prevent the formation or the effect of THP, respectively. These anxiety-producing effects of THP and restraint stress at puberty are not seen in the δ−/− mouse (Figure 7), implicating α4βδ GABARs (Shen et al., 2007). Taken together, these findings suggest that stress-induced release of THP produces anxiety at puberty in contrast to its well-established effect to decrease anxiety at other ages. Although not tested during adolescence, there are other GABAR populations which have been implicated in the anxiety response, including α2-containing and α3-containing GABARs (Rudolph et al., 1999; Atack, 2010, 2011; Smith et al., 2012). Reduced expression of these GABAR subtypes, either by puberty onset alone or by stress during adolescence, would be expected to alter anxiety state.
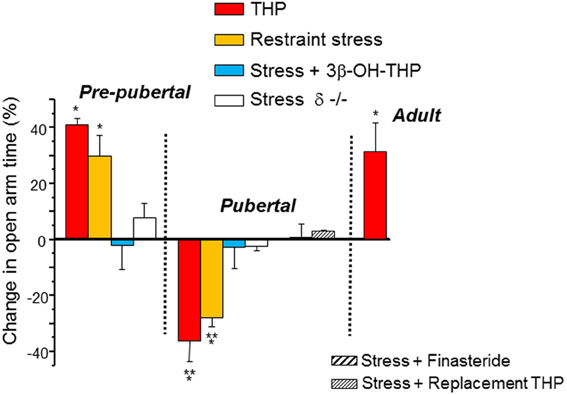
Figure 7. Paradoxical anxiety-producing effects of THP in pubertal mice. Changes in anxiety produced by restraint stress or injection of THP (10 mg/kg, i.p.) are represented as a % change in open arm time in the elevated plus maze (EPM) compared to control values. In some cases the inactive 3β-OH isomer of THP (stress + 3β-OH-THP) or finasteride (a 5α-reductase inhibitor) were preadministered to test the role of THP release in the stress response. Replacement THP (10 mg/kg, i.p., in oil, for 3 d) was administered to prevent the decline in THP at puberty. n = 6–9 mice for each group. *P = 0.05 vs. control, **P = 0.05 vs. Pre-pub. [Used with permission (Shen et al., 2007)].
The paradoxical anxiety-producing effect of THP observed at puberty onset in female mice which is linked with α4βδ GABAR expression (Shen et al., 2007) has also been for observed for THP or progesterone, its parent compound, in women with premenstrual dysphoric disorder (Schmidt et al., 1998; Freeman et al., 2002) and menopausal dysphoria (Andreen et al., 2004), when anxiety-related correlates of THP are concentration-dependent.
Stress and Anxiety During Adolescence
In humans, anxiety responses to performance stress (mental arithmetic, mirror tracing) and social stress are increased at puberty (Susman et al., 1988; Modesti et al., 1994; Sumter et al., 2010), with a greater prevalence in girls (Garber et al., 2002; Leen-Feldner et al., 2007; Ordaz and Luna, 2012). Anxiety disorders are also most likely to emerge at puberty (Hayward et al., 1992; Costello et al., 2003; Kessler et al., 2005). Brain imaging studies in adolescent girls have correlated increased activity of the limbic system, including hippocampus, with these anxiety responses during a psychosocial stress paradigm (Guyer et al., 2009). Thus, the effect of the stress steroid THP, which reverses at puberty, to increase anxiety, may represent one potential mechanism for these enhanced stress responses in females during adolescence.
Regulation of α4βδ GABAR Expression
Recent studies have delineated some of the factors which regulate the expression of α4βδ GABARs. Brain-derived neurotrophic factor (BDNF) plays a role in the activation of the α4 promoter via early growth response factor 3 (Egr3) and the JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathway (Roberts et al., 2006; Lund et al., 2008), as well as in the trafficking of the δ subunit protein to the cell membrane surface (Joshi and Kapur, 2009). Surface expression of α4βδ can be regulated by Ca 2+ and extracellular signal-regulated kinase (ERK) 1/2 (Joshi and Kapur, 2013). Expression of α4 can also be induced by heat shock factor 1, which has been shown following exposure of neurons to alcohol (Pignataro et al., 2007).
Hormonal factors which regulate expression of α4βδ GABARs include the ovarian hormone 17β-estradiol (E2), as well as THP. E2, administered either in vitro or in vivo (Pierson et al., 2005; Shen et al., 2005; Zhou and Smith, 2007) can increase α4 expression in neurons, an effect likely mediated by its ability to increase BDNF (Jezierski and Sohrabji, 2003; Scharfman et al., 2003; Sato et al., 2007). THP, either administered in vivo or in vitro to cultured neurons, can increase expression of these receptors after 0.5–48 h (Shen et al., 2005; Maguire and Mody, 2007; Kuver et al., 2012). The effect of THP to increase receptor trafficking to the membrane surface is related to its ability to increase the efficacy of the receptor (Kuver et al., 2012), where GABA is a partial agonist (Brown et al., 2002; Bianchi and Macdonald, 2003). GABA alone does not increase receptor expression, but other high efficacy agonists, such as gaboxadol (THIP) and β-alanine, are able to increase surface expression of the receptor, an effect mediated by protein kinase C-δ (Kuver et al., 2012), which has high expression in some of the CNS regions where α4βδ is highly expressed (Messing et al., 2007). [However, protein kinase C-δ does not account for the high expression of α4βδ in other regions, such as dorsal striatrum and nucleus accumbens, which do not have high expression of protein kinase C-δ (Choi et al., 2008)]. This effect of THP to increase surface expression of α4βδ is a result of increases in receptor insertion, rather than through decreases in receptor internalization (Kuver et al., 2012).
Non-hormonal factors which regulate α4βδ expression include alcohol, which reduces expression by activating clathrin-mediated endocytosis (Gonzalez et al., 2012), and increased neuronal excitability produced by neuronal depolarization, NMDA receptor activation, traumatic brain injury or stroke (Payne et al., 2008; Mtchedlishvili et al., 2010; Santhakumar et al., 2010). It is likely that δ-containing GABARs play a neuroprotective role in this regard because excitotoxicity levels are increased in brain tissue from δ−/− animals (Santhakumar et al., 2010).
Puberty and THP “Withdrawal”
THP levels decline by 60–70% at the onset of puberty in both the mouse and the human (Mannan and O'Shaughnessy, 1988; Fadalti et al., 1999; Shen et al., 2007). These declining levels of THP (“THP withdrawal”) appear to be responsible for increases in α4βδ GABAR expression at puberty onset of female mice. First, replacement THP (10 mg/kg, i.p. × 3) during the early days of puberty prevents the increase in α4βδ expression (Shen et al., 2007), as well as the ability of THP to reduce the GABAergic tonic current. Replacement THP also prevents the paradoxical excitatory effects and anxiety-producing effects of THP during adolescence (Shen et al., 2007). Second, a THP withdrawal state induced pharmacologically by administration of the 5α-reductase blocker finasteride, also increases α4βδ GABAR expression in hippocampus of pre-pubertal mice (Smith et al., 2006) and results in paradoxical excitatory effects of THP due to its effect to reduce the tonic inhibitory current.
Withdrawal from progesterone (and thus THP) has also been shown to trigger α4βδ expression in interneurons of the periaqueductal grey (Griffiths and Lovick, 2005; Lovick et al., 2005) on the late diestrous stage of the estrous cycle following the peak in circulating levels of progesterone and THP (Griffiths and Lovick, 2005; Lovick et al., 2005). The resultant increase in tonic inhibition of these interneurons leads to increased excitability of the output neurons, which may represent a mechanism for ovarian cycle-induced panic disorder (Lovick, 2000). In contrast, earlier on diestrus, when circulating levels of progesterone and THP are elevated, expression of α4βδ GABARs is increased on dentate gyrus granule cells in association with an increase in the tonic inhibitory current (Maguire et al., 2005) compared to estrus. Animals at this stage show a decrease in seizure susceptibility as well as decreased anxiety.
THP withdrawal may serve as a hormonal model of premenstrual dysphoric disorder (PMDD), which is characterized by adverse mood, including anxiety, during the decline in circulating levels of progesterone and THP at the end of the luteal phase of the menstrual cycle (Cunningham et al., 2009; Rapkin and Winer, 2009). At this time, stress-triggered anxiety and panic attacks have been reported (Vickers and McNally, 2004; Yonkers et al., 2008; Nillni et al., 2011).
The post-partum period can also be considered a time of “THP withdrawal” when THP levels decline precipitously, and α4βδ expression levels in dentate gyrus granule cells are altered (Maguire and Mody, 2009; Maguire et al., 2009; Sanna et al., 2009). These studies report different outcomes which may be dependent upon the rodent species used (mice vs. rats). In mouse studies (Maguire et al., 2009; Maguire and Mody, 2009), α4βδ expression is decreased in dentate gyrus during pregnancy, as well as in striatrum, but unchanged in cerebral cortex. Expression of these receptors in dentate gyrus then increases during the post-partum period. This change in receptor expression may represent a homeostatic response to maintain normal levels of neuronal excitability. In contrast, in studies using rats (Sanna et al., 2009), δ GABAR expression is increased in dentate gyrus during pregnancy, where these receptors contribute to a greater tonic current. The reason for this discrepancy is not clear but may be due to the different ambient levels of THP which are much higher in mouse brain (Porcu et al., 2010) compared to rat, and therefore suggest that control of α4βδ expression is complex.
Conclusions
Hormonally regulated expression of extrasynaptic α4βδ GABARs alters the inhibitory control of neuronal circuits which regulate cognition, seizure threshold and mood (Summary Figure 8). These changes in inhibitory tone are reported across the ovarian cycle, during the post-partum period and at the onset of puberty. Increased expression of α4βδ GABARs during adolescence reduces synaptic plasticity and spatial learning (Summary Figure 8), which may underlie, at least in part, the end of a critical period for optimal learning which has been reported for this time window. In addition, the paradoxical effects of THP at α4βδ GABARs during the pubertal period (Summary Figure 5) may play a role in mood swings and stress-related anxiety which sometimes characterize early adolescence. Genetic aberrations in α4 and/or δ have been reported for certain neuropathologies such as autism, schizophrenia and child-onset mood disorders (Ma et al., 2005; Maldonado-Aviles et al., 2009; Feng et al., 2010). A greater understanding of the role of these extrasynaptic GABARs in behavioral endpoints may help to suggest novel therapeutic strategies for disturbances of mood and cognition.
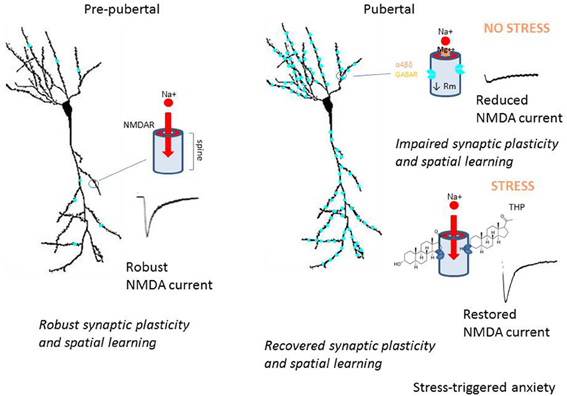
Figure 8. Summary diagram of pubertal expression of α4βδ GABAA receptors on CA1 pyramidal cells: the impact on neuronal function and behavior. This summary diagram indicates low expression of α4βδ GABARs (aqua) on CA1 pyramidal cells pre-pubertally (left). During this period, depolarization of the spine permits Mg2+ unblock of the NMDA receptor (NMDAR), which allows robust activation of NMDAR and NMDAR-dependent events, such as synaptic plasticity and spatial learning. At puberty (right), α4βδ GABARs increase markedly on both the dendritic shaft and spine, where they shunt depolarizing current (upper diagram). As a result, local depolarization fails to unblock the NMDAR, leading to reduced levels of synaptic plasticity and impaired spatial learning. During sustained stress (lower diagram), release of THP selectively reduces the outward GABA-gated current gated by α4βδ GABARs, thus reducing the shunting tonic inhibition they produce. As a result, NMDAR activation is restored to pre-pubertal levels, as are synaptic plasticity and spatial learning. However, the increase in neuronal excitability produced by this stress steroid is also associated with increases in anxiety behavior. These findings suggest that the emergence of extrasynaptic α4βδ GABARs in the hippocampus at puberty may underlie some of the changes in cognition and mood reported during adolescence.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work in this study was supported by grants from the US National Institutes of Health: DA09618, AA12958 and MH100561.
References
Agis-Balboa, R. C., Pinna, G., Zhubi, A., Maloku, E., Veldic, M., Costa, E., et al. (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 14602–14607. doi: 10.1073/pnas.0606544103
Akk, G., Bracamontes, J., and Steinbach, J. H. (2004). Activation of GABA(A) receptors containing the alpha4 subunit by GABA and pentobarbital. J. Physiol. 556, 387–399. doi: 10.1113/jphysiol.2003.058230
Akk, G., Covey, D. F., Evers, A. S., Mennerick, S., Zorumski, C. F., and Steinbach, J. H. (2010). Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Curr. Neuropharmacol. 8, 18–25. doi: 10.2174/157015910790909458
Akk, G., Li, P., Bracamontes, J., Reichert, D. E., Covey, D. F., and Steinbach, J. H. (2008). Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol. Pharmacol. 74, 614–627. doi: 10.1124/mol.108.048520
Andreen, L., Sundstrom-Poromaa, I., Bixo, M., Andersson, A., Nyberg, S., and Backstrom, T. (2004). Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology 30, 212–224. doi: 10.1016/j.psyneuen.2004.07.003
Aoki, C., Sabaliauskas, N., Chowdhury, T., Min, J. Y., Colacino, A. R., Laurino, K., et al. (2012). Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse 66, 391–407. doi: 10.1002/syn.21528
Ardila, A., Rosselli, M., Matute, E., and Inozemtseva, O. (2011). Gender differences in cognitive development. Dev. Psychol. 47, 984–990. doi: 10.1037/a0023819
Arima-Yoshida, F., Watabe, A. M., and Manabe, T. (2011). The mechanisms of the strong inhibitory modulation of long-term potentiation in the rat dentate gyrus. Eur. J. Neurosci. 33, 1637–1646. doi: 10.1111/j.1460-9568.2011.07657.x
Atack, J. R. (2010). GABA-A receptor alpha2/alpha3 subtype-selective modulators as potential nonsedating anxiolytics. Curr. Top Behav. Neurosci. 2, 331–360. doi: 10.1007/7854_2009_30
Atack, J. R. (2011). GABA-A receptor subtype-selective modulators. I. alpha2/alpha3-selective agonists as non-sedating anxiolytics. Curr. Top Med. Chem. 11, 1176–1202. doi: 10.2174/156802611795371350
Bannerman, D. M., Rawlins, J. N. P., McHugh, S. B., Deacon, R. M. J., Yee, B. K., Bast, T., et al. (2004). Regional dissociations within the hippocampus–memory and anxiety. Neurosci. Biobehav. Rev. 28, 273–283. doi: 10.1016/j.neubiorev.2004.03.004
Barbaccia, M. L., Serra, M., Purdy, R. H., and Biggio, G. (2001). Stress and neuroactive steroids. Int. Rev. Neurobiol. 46, 243–272. doi: 10.1016/S0074-7742(01)46065-X
Barrera, N. P. B. J., You, H., Henderson, R. M., Martin, I. L., Dunn, S. M., and Edwardson, J. M. (2008). Atomic force microscopy reveals the stoichiometry and subunit arrangement of the alpha4-beta2-delta GABA-A receptor. Mol. Pharmacol. 73, 960–967. doi: 10.1124/mol.107.042481
Baur, R., Kaur, K. H., and Sigel, E. (2009). Structure of alpha6-beta3-delta GABA(A) receptors and their lack of ethanol sensitivity. J. Neurochem. 111, 1172–1181. doi: 10.1111/j.1471-4159.2009.06387.x
Belelli, D., Casula, A., Ling, A., and Lambert, J. J. (2002). The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology 43, 651–661. doi: 10.1016/S0028-3908(02)00172-7
Belelli, D., and Herd, M. B. (2003). The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J. Neurosci. 23, 10013–10020.
Belelli, D., Peden, D. R., Rosahl, T. W., Wafford, K., and Lambert, J. J. (2005). Extrasynaptic GABA-A receptors for thalamocortical neurons: A molecular target for hypnotics. J. Neurosci. 25, 11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005
Bianchi, M. T., Haas, K. F., and Macdonald, R. L. (2002). Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology 43, 492–502. doi: 10.1016/S0028-3908(02)00163-6
Bianchi, M. T., and Macdonald, R. L. (2003). Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J. Neurosci. 23, 10934–10943.
Bitran, D., Dugan, M., Renda, P., Ellis, R., and Foley, M. (1999). Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 850, 217–224. doi: 10.1016/S0006-8993(99)02150-2
Borghese, C. M., Storustovu, S., Ebert, B., Herd, M. B., Belelli, D., Lambert, J. J., et al. (2006). The delta subunit of GABA-A receptors does not confer sensitivity to low doses of ethanol. J. Pharmacol. Exp. Ther. 316, 1360–1368. doi: 10.1124/jpet.105.092452
Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W., and Farrant, M. (2001). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92. doi: 10.1038/35051086
Bright, D. P., Renzi, M., Bartram, J., McGee, T. P., MacKenzie, G., Hosie, A. M., et al. (2011). Profound desensitization by ambient GABA limits activation of delta-containing GABAA receptors during spillover. J. Neurosci. 31, 753–763. doi: 10.1523/JNEUROSCI.2996-10.2011
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J., and Wafford, K. A. (2002). Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol. 136, 965–974. doi: 10.1038/sj.bjp.0704795
Brunig, I., Scotti, E., Sidler, C., and Fritschy, J. M. (2002). Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55. doi: 10.1002/cne.10102
Buchanan, C. M., Eccles, J. S., and Becker, J. B. (1992). Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol. Bull. 111, 62–107. doi: 10.1037/0033-2909.111.1.62
Buhr, A., and Sigel, E. (1997). A point mutation in the gamma2 subunit of gamma-aminobutyric acid type A receptors results in altered benzodiazepine binding site specificity. Proc. Natl. Acad. Sci. U.S.A. 94, 8824–8829. doi: 10.1073/pnas.94.16.8824
Burgard, E. C., Tietz, E. I., Neelands, T. R., and Macdonald, R. L. (1996). Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol. Pharmacol. 50, 119–127.
Burgess, N., Maguire, E. A., and O'Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. doi: 10.1016/S0896-6273(02)00830-9
Caraiscos, V. B., Elliott, E. M., You-Ten, K. E., Cheng, V. Y., Belelli, D., Newell, J. G., et al. (2004). Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. U.S.A. 101, 3662–3667. doi: 10.1073/pnas.0307231101
Carta, M., Mameli, M., and Valenzuela, C. F. (2004). Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 24, 3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004
Chambers, M. S., Atack, J. R., Broughton, H. B., Collinson, N., Cook, S., Dawson, G. R., et al. (2003). Identification of a novel, selective GABA(A) alpha-5 receptor inverse agonist which enhances cognition. J. Med.Chem. 46, 2227–2240. doi: 10.1021/jm020582q
Chandra, D., Jia, F., Liang, J., Peng, Z., Suryanarayanan, A., Werner, D. F., et al. (2006). GABA-A receptor alpha-4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U.S.A. 103, 15230–15235. doi: 10.1073/pnas.0604304103
Chang, Y., Wang, R., Barot, S., and Weiss, D. S. (1990). Stoichiometry of a recombinant GABA-A receptor. J. Neurosci. 16, 534–541.
Cheng, V. Y., Martin, L. J., Elliott, E. M., Kim, J. H., Mount, H. T., Taverna, F. A., et al. (2006). Alpha5 GABA-A receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J. Neurosci. 26, 3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006
Chiang, P. H., Wu, P. Y., Kuo, T. W., Liu, Y. C., Chan, C. F., Chien, T. C., et al. (2012). GABA is depolarizing in hippocampal dentate granule cells of the adolescent and adult rats. J. Neurosci. 32, 62–67. doi: 10.1523/JNEUROSCI.3393-11.2012
Choi, D. S., Wei, W., Deitchman, J. K., Kharazia, V. N., Lesscher, H. M., McMahon, T., et al. (2008). Protein kinase C delta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 28, 11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008
Cimadevilla, J. M., Wesierska, M., Fenton, A. A., and Bures, J. (2001). Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociaiton of arena cues from room cues by rotation of the arena. Proc. Natl. Acad. Sci. U.S.A. 98, 3532–3536. doi: 10.1073/pnas.051628398
Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., et al. (2002). Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABA-A receptor. J. Neurosci. 22, 5572–5580.
Compagnone, N. A., and Mellon, S. H. (2000). Neurosteroids: biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 21:1–56. doi: 10.1006/frne.1999.0188
Concas, A., Mostallino, M. C., Porcu, P., Follesa, P., Barbaccia, M. L., Trabucchi, M., et al. (1998). Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. U.S.A. 95, 13284–13289. doi: 10.1073/pnas.95.22.13284
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., and Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry 60, 837–844. doi: 10.1001/archpsyc.60.8.837
Crestani, F., Keist, R., Fritschy, J.-M., Benke, D., Vogt, K., Prut, L., et al. (2002). Trace fear conditioning involves hippocampal alpha-5 GABA(A) receptors. Proc. Natl. Acad. Sci. U.S.A. 99, 8980–8985. doi: 10.1073/pnas.142288699
Cunningham, J., Yonkers, K. A., O'Brien, S., and Eriksson, E. (2009). Update on research and treatment of premenstrual dysphoric disorder. Harv. Rev. Psychiatry 17, 120–137. doi: 10.1080/10673220902891836
Droogleever Fortuyn, H. A., van Broekhoven, F., Span, P. N., Backstrom, T., Zitman, F. G., and Verkes, R. J. (2004). Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptors density. Psychoneuroendocrinology 29, 1341–1344. doi: 10.1016/j.psyneuen.2004.02.003
Fadalti, M., Petraglia, F., Luisi, S., Bernardi, F., Casarosa, E., Ferrari, E., et al. (1999). Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr. Res. 46, 323–327. doi: 10.1203/00006450-199909000-00013
Fagiolini, M., Fritschy, J.-M., Low, K., Mohler, H., Rudolph, U., and Hensch, T. K. (2004). Specific GABA-A circuits for visual cortical plasticity. Science 303, 1681–1683. doi: 10.1126/science.1091032
Feng, Y., Kapornai, K., Kiss, E., Tamas, Z., Mayer, L., Baji, I., et al. (2010). Association of the GABRD gene and childhood-onset mood disorders. Genes Brain Behav. 9, 668–672.
Freeman, E. W., Frye, C. A., Rickels, K., Martin, P. A., and Smith, S. S. (2002). Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J. Clin. Psychopharmacol. 22, 516–520. doi: 10.1097/00004714-200210000-00013
Friedman, S. B., Ader, R., Grota, L. J., and Larson, T. (1967). Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosom. Med 29, 323–328.
Garber, J., Keiley, M. K., and Martin, C. (2002). Developmental trajectories of adolescents' depressive symptoms: predictors of change. J. Consult. Clin. Psychol. 70, 79–95. doi: 10.1037/0022-006X.70.1.79
Girdler, S. S., Beth Mechlin, M., Light, K. C., and Morrow, A. L. (2006). Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology 43, 331–336. doi: 10.1111/j.1469-8986.2006.00410.x
Glykys, J., Mann, E. O., and Mody, I. (2008). Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 28, 1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008
Glykys, J., and Mody, I. (2006). Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J. Neurophysiol. 95, 2796–2807. doi: 10.1152/jn.01122.2005
Glykys, J., Peng, Z., Chandra, D., Homanics, G. E., Houser, C. R., and Mody, I. (2007). A new naturally occurring GABA-A receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 10, 40–48. doi: 10.1038/nn1813
Gonzalez, C., Moss, S. J., and Olsen, R. W. (2012). Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of delta-containing GABAA receptors. J. Neurosci. 32, 17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012
Griffiths, J., and Lovick, T. (2005). Withdrawal from progesterone increases expression of alpha4, beta1 and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J. Comp. Neurol. 486, 89–97. doi: 10.1002/cne.20540
Guo, N., Yoshizaki, K., Kimura, R., Suto, F., Yanagawa, Y., and Osumi, N. (2013). A sensitive period for GABAergic interneurons in the dentate gyrus in modulating sensorimotor gating. J. Neurosci. 33, 6691–6704. doi: 10.1523/JNEUROSCI.0032-12.2013
Gur, R. C., Richard, J., Calkins, M. E., Chiavacci, R., Hansen, J. A., Bilker, W. B., et al. (2012). Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 26, 251–265. doi: 10.1037/a0026712
Guyer, A. E., McClure-Tone, E. B., Shiffrin, N. D., Pine, D. S., and Nelson, E. E. (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 80, 1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x
Haas, K. F., and Macdonald, R. L. (1999). GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. 514, 27–45. doi: 10.1111/j.1469-7793.1999.027af.x
Harrison, F. E., Hosseini, A. H., and McDonald, M. P. (2009). Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 198, 247–251. doi: 10.1016/j.bbr.2008.10.015
Hayward, C., Killen, J. D., Hammer, L. D., Litt, I. F., Wilson, D. M., Simmonds, B., et al. (1992). Pubertal stage and panic attack history in sixth- and seventh-grade girls. Am. J. Psychiatry 149, 1239–1243.
Hensch, T. K. (2004). Critical period regulation. Annu. Rev. Neurosci. 27, 549–579. doi: 10.1146/annurev.neuro.27.070203.144327
Herron, C. E., Lester, R. A., Coan, E. J., and Collingridge, G. L. (1986). Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature 322, 265–268. doi: 10.1038/322265a0
Higashi, T., Takido, N., and Shimada, K. (2005). Studies on neurosteroids XVII. Analysis of stress-induced changes in neurosteroid levels in rat brains using liquid chromatography-electron capture atmosperic pressure chemical ionization-mass spectrometry. Steroids 70, 1–11. doi: 10.1016/j.steroids.2004.08.001
Hodes, G. E., and Shors, T. J. (2005). Distinctive stress effects on learning during puberty. Horm. Behav. 48, 163–171. doi: 10.1016/j.yhbeh.2005.02.008
Hosie, A. M., Wilkins, M. E., da Silva, H. M., and Smart, T. G. (2006). Endogenous neurosteroids regulate GABA-A receptors through two discrete transmembrane sites. Nature 444, 486–489. doi: 10.1038/nature05324
Houston, C. M., Bright, D. P., Sivilotti, L. G., Beato, M., and Smart, T. G. (2009). Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J. Neurosci. 29, 10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009
Huang, Z. J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M. F., et al. (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. doi: 10.1016/S0092-8674(00)81509-3
Iwai, Y., Fagiolini, M., Obata, K., and Hensch, T. K. (2003). Rapid critical period induction by tonic inhibition in visual cortex. J. Neurosci. 23, 6695–6702.
Izumi, Y., Nagashima, K., Murayama, K., and Zorumski, CF, (2005). Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136, 509–517. doi: 10.1016/j.neuroscience.2005.08.002
Jezierski, M. K., and Sohrabji, F. (2003). Estrogen enhances retrograde transport of brain-derived neurotrophic factor in the rodent forebrain. Endocrinology 144, 5022–5029. doi: 10.1210/en.2003-0724
Jia, F., Yue, M., Cjandra, D., Keramides, A., Goldstein, P. A., Homanics, G. E., et al. (2008). Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J. Neurosci. 28, 106–115. doi: 10.1523/JNEUROSCI.3996-07.2008
Jiang, B., Huang, Z. J., Morales, B., and Kirkwood, A. (2005). Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res. Brain Res. Rev. 50, 126–133. doi: 10.1016/j.brainresrev.2005.05.007
Johnson, J. S., and Newport, E. L. (1989). Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn. Psychol. 21, 60–99. doi: 10.1016/0010-0285(89)90003-0
Joshi, S., and Kapur, J. (2009). Slow intracellular accumulation of GABA(A) receptor delta subunit is modulated by brain-derived neurotrophic factor. Neuroscience 164, 507–519. doi: 10.1016/j.neuroscience.2009.08.008
Joshi, S., and Kapur, J. (2013). N-Methyl-D-Aspartic acid receptor activation downregulates expression of delta subunit-containing GABAA receptors in cultured hippocampal neurons. Mol. Pharmacol. 84, 1–11. doi: 10.1124/mol.112.084715
Kanit, L., Taskiran, D., Yilmaz, O. A., Balkan, B., Demirgoren, S., Furedy, J. J., et al. (2000). Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res. Bull. 52, 243–248. doi: 10.1016/S0361-9230(00)00232-X
Katagiri, H., Fagiolini, M., and Hensch, T. K. (2007). Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53, 805–812. doi: 10.1016/j.neuron.2007.02.026
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National comorbidity survey replication. Arch. Gen. Psychiatry. 62, 593–602. doi: 10.1001/archpsyc.62.6.593
Kheirbek, M. A., Drew, L. J., Burghardt, N. S., Costantini, D. O., Tannenholz, L., Ahmari, S. E., et al. (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968. doi: 10.1016/j.neuron.2012.12.038
Kubota, Y., Hatada, S., Kondo, S., Karube, F., and Kawaguchi, Y. (2007). Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J. Neurosci. 27, 1139–1150. doi: 10.1523/JNEUROSCI.3846-06.2007
Kuver, A., Shen, H., and Smith, S. S. (2012). Regulation of the surface expression of alpha4-beta2-delta GABA(A) receptors by high efficacy states. Brain Res. 1463, 1–20. doi: 10.1016/j.brainres.2012.04.047
Laurie, D. J., Wisden, W., and Seeburg, P. (1992). The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. III Embryonic and postnatal development. J. Neurosci. 12, 4151–4172.
Leen-Feldner, E. W., Reardon, L. E., and Zvolensky, M. J. (2007). Pubertal status and emotional reactivity to a voluntary hyperventilation challenge predicting panic symptoms and somatic complaints: a laboratory-based multi-informant test. Behav. Modif. 31, 8–31. doi: 10.1177/0145445506295058
Lovick, T. (2000). Panic disorder: a malfunction of multiple transmitter control systems within the midbrain periaqueductal gray matter? Neuroscientist 6, 48–59. doi: 10.1177/107385840000600113
Lovick, T. A., Griffiths, J. L., Dunn, S. M., and Martin, I. L. (2005). Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience 131, 397–405. doi: 10.1016/j.neuroscience.2004.11.010
Lui, P., Padow, V. A., Franco, D., Hall, B. S., Park, B., Klein, Z. A., et al. (2012). Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol. Behav. 107, 104–111. doi: 10.1016/j.physbeh.2012.06.011
Lund, I. V., Hu, Y., Raol, Y. H., Benham, R. S., Faris, R., Russek, S. J., et al. (2008). BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci. Signal 1, ra9. doi: 10.1126/scisignal.1162396
Lupien, S. J., Maheu, F., Tu, M., Fiocco, A., and Schramek, T. E. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 65, 209–237.
Ma, D. Q., Whitehead, P. L., Menold, M. M., Martin, E. R., Ashley-Koch, A. E., Mei, H., et al. (2005). Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am. J. Hum. Genet. 77, 377–388. doi: 10.1086/433195
Maguire, J. L., Ferando, I., Simonsen, C., and Mody, I. (2009). Excitability changes related to GABA-A receptor plasticity during pregnancy. J. Neurosci. 29, 9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009
Maguire, J. L., and Mody, I. (2007). Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 27, 2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007
Maguire, J. L., and Mody, I. (2009). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213. doi: 10.1016/j.neuron.2008.06.019
Maguire, J. L., Stell, B. M., Rafizadeh, M., and Mody, I. (2005). Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804. doi: 10.1038/nn1469
Maldonado-Aviles, J. G., Curley, A. A., Hashimoto, T., Morrow, A. L., Ramsey, A. J., O'Donnell, P., et al. (2009). Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry 166, 450–459. doi: 10.1176/appi.ajp.2008.08101484
Mannan, M. A., and O'Shaughnessy, P. J. (1988). Ovarian steroid metabolism during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J. Reprod. Fertil. 82, 727–734. doi: 10.1530/jrf.0.0820727
Marowsky, A., Rudolph, U., Fritschy, J. M., and Arand, M. (2012). Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABA-A receptors. J. Neurosci. 32, 8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012
Martin, L. J., Zurek, A. A., Macdonald, J. F., Roder, J. C., Jackson, M. F., and Orser, B. A. (2010). Alpha5 GABA-A receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J. Neurosci. 30, 5269–5282. doi: 10.1523/JNEUROSCI.4209-09.2010
Massanari, M., Novitsky, J., and Reinstein, L. J. (1997). Paradoxical reactions in children associated with midazolam use during endoscopy. Clin. Pediatr. (Phila) 36, 681–684. doi: 10.1177/000992289703601202
Matthews, D. B., Morrow, A. L., Tokunaga, S., and McDaniel, J. R. (2002). Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin. Exp. Res. 26, 1747–1751. doi: 10.1111/j.1530-0277.2002.tb02479.x
McCartney, C. R., Blank, S. K., Prendergast, K. A., Chhabra, S., Eagleson, C. A., Helm, K. D., et al. (2007). Obesity and sex steroid changes across puberty: Evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J. Clin. Endocrinol. Metab. 92, 430–436. doi: 10.1210/jc.2006-2002
McGivern, R. F., Andersen, J., Byrd, D., Mutter, K. L., and Reilly, J. (2002). Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain Cogn. 50, 73–89. doi: 10.1016/S0278-2626(02)00012-X
Meera, P., Wallner, M., and Otis, T. (2011). Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J. Neurophysiol. 106, 2057–2011. doi: 10.1152/jn.00450.2011
Megias, M., Emri, Z. S., Freund, M. T. F., and Gulyas, A. I. (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540. doi: 10.1016/S0306-4522(00)00496-6
Mellon, S. H., and Vaudry, H. (2001). Biosynthesis of neurosteroids and regulation of their synthesis. Int. Rev. Neurobiol. 46, 33–78. doi: 10.1016/S0074-7742(01)46058-2
Meredith, R. M., Floyer-Lea, A. M., and Paulsen, O. (2003). Maturation of long-term potentiation induction rules in rodent hippocampus: role of GABAergic inhibition. J. Neurosci. 23, 11142–11146.
Messing, R. O., Choi, D. S., Wei, W., Kharazia, V. N., Deitchman, J. K., Lesscher, H. M. B., et al. (2007). Protein kinase C-delta regulates GABA-mediated tonic inhibition and motor response to ethanol. Alcohol Clin. Exp. Res. 31(Suppl.), 296A.
Modesti, P. A., Pela, I., Cecioni, I., Gensini, G. F., Serneri, G. G., and Bartolozzi, G. (1994). Changes in blood pressure reactivity and 24-hour blood pressure profile occurring at puberty. Angiology 45, 443–450. doi: 10.1177/000331979404500605
Moore, M. D., Cushman, J., Chandra, D., Homanics, G. E., Olsen, R. W., and Fanselow, M. S. (2010). Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA(A) receptor. Neurobiol. Learn Mem. 93, 383–387. doi: 10.1016/j.nlm.2009.12.004
Mortensen, M., and Smart, T. G. (2006). Extrasynaptic alpha-beta subunit GABA-A receptors on rat hippocampal pyramidal neurons. J. Physiol. 577, 841–856. doi: 10.1113/jphysiol.2006.117952
Mortensen, M., Ebert, B., Wafford, K., and Smart, T. G. (2010). Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABA-A receptors. J. Physiol. 588, 1251–1268. doi: 10.1113/jphysiol.2009.182444
Mtchedlishvili, Z., Lepsveridze, E., Xu, H., Kharlamov, E. A., Lu, B., and Kelly, K. M. (2010). Increase of GABA-A receptor-mediated tonic inhibtion in dentate granule cells after traumatic brain injury. Neurobiol. Dis. 38, 464–475. doi: 10.1016/j.nbd.2010.03.012
Mukai, H., Higashi, T., Nagura, Y., and Shimada, K. (2008). Studies on neurosteroids XXV. Influence of 5alpha-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol. Pharm Bull. 31, 1646–1650. doi: 10.1248/bpb.31.1646
Nagashima, K., Zorumski, C. F., and Izumi, Y. (2005). Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology 103, 318–326. doi: 10.1097/00000542-200508000-00015
Nevian, T., and Sakmann, B. (2006). Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 26, 11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006
Nillni, Y. I., Toufexis, D. J., and Rohan, K. J. (2011). Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin. Psychol. Rev. 31, 1183–1191. doi: 10.1016/j.cpr.2011.07.006
Nusser, Z., Sieghart, W., and Somogyi, P. (1998). Segregation of differing GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703.
Olsen, R. W., and Sieghart, W. (2009). GABA-A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56, 141–148. doi: 10.1016/j.neuropharm.2008.07.045
Olsen, R. W., and Snowman, A. (1982). Chloride-dependent enhancment by barbiturates of gamma-aminobutyric acid receptor binding. J. Neurosci. 2, 1812–1823.
Ordaz, S., and Luna, B. (2012). Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology 37, 1135–1157. doi: 10.1016/j.psyneuen.2012.01.002
Palumbo, M. A., Salvestroni, C., Gallo, R., Guo, A. L., Gennazini, A. D., Artini, P. G., et al. (1995). Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J. Endocrinol. Invest. 18, 853–856.
Pastalkova, E., Serrano, P., Pinkhasova, D., Wallace, E., Fenton, A. A., and Sacktor, T. C. (2006). Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144. doi: 10.1126/science.1128657
Payne, H. L., Ives, J. H., Sieghart, W., and Thompson, C. L. (2008). AMPA and kainate receptors mediate mutually exclusive effects on GABA(A) receptor expression in cultured mouse cerebellar granule neurones. J. Neurochem. 104, 173–186.
Payne, J. A. (1997). Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. 273, C1516–C1525.
Peng, Z., Hauer, B., Mihalek, R. M., Homanics, G. E., Sieghart, W., Olsen, R. W., et al. (2002). GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J. Comp. Neurol. 446, 179–197. doi: 10.1002/cne.10210
Pepin, M., and Dorval, M. (1986). Effect of playing a video game on adults' and adolescents' spatial visualization. Edu. Res. Inf. Center ED273266, 2–18.
Pierson, R. C., Lyons, A. M., and Greenfield, L. J. Jr. (2005). Gonadal steroids regulate GABA-A receptor subunit mRNA expression in NT2-N cells. Brain Res. Mol. Brain Res. 138, 105–115. doi: 10.1016/j.molbrainres.2004.10.047
Pignataro, L., Miller, A. N., Ma, L., Midha, S., Protiva, P., Herrera, D. G., et al. (2007). Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J. Neurosci. 27, 12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W., and Sperk, G. (2000). GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. doi: 10.1016/S0306-4522(00)00442-5
Place, R., Lykken, C., Beer, Z., Suh, J., McHugh, T. J., Tonegawa, S., et al. (2012). NMDA signaling in CA1 mediates selectively the spatial component of episodic memory. Learn. Mem. 19, 164–169. doi: 10.1101/lm.025254.111
Porcu, P., O'Buckley, T. K., Alward, S. E., Song, S. C., Grant, K. A., de Wit, H., et al. (2010). Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin. Exp. Res. 34, 432–442. doi: 10.1111/j.1530-0277.2009.01123.x
Purdy, R. H., Morrow, A. L., Moore, P. H., Jr., and Paul, S. M. (1991). Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. Proc. Natl. Acad. Sci. U.S.A. 88, 4553–4557. doi: 10.1073/pnas.88.10.4553
Rapkin, A. J., and Winer, S. A. (2009). Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Exp. Rev. Pharmacoecon. Outcomes Res. 9, 157–170. doi: 10.1586/erp.09.14
Reardon, L. E., Leen-Feldner, E. W., and Hayward, C. (2009). A critical review of the empirical literature on the relation between anxiety and puberty. Clin. Psychol. Rev. 29, 1–23. doi: 10.1016/j.cpr.2008.09.005
Rivera, C., Voipio, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K., et al. (1999). The K-Cl cotransporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. doi: 10.1038/16697
Roberts, D. S., Hu, Y., Lund, I. V., Brooks-Kayal, A. R., and Russek, S. J. (2006). Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha4 subunits in hippocampal neurons. J. Biol. Chem. 281, 29431–29435. doi: 10.1074/jbc.C600167200
Rudolph, U., Crestani, F., Benke, D., Brünig, I., Benson, J. A., Fritschy, J.-M., et al. (1999). Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800. doi: 10.1038/44579
Ruiz, A., Campanac, E., Scott, R. S., Rusakov, D. A., and Kullmann, D. M. (2010). Presynaptic GABA-A receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat. Neurosci. 13, 431–438. doi: 10.1038/nn.2512
Saab, B. J., Maclean, A. J., Kanisek, M., Zurek, A. A., Martin, L. J., Roder, J. C., et al. (2010). Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology 113, 1061–1071. doi: 10.1097/ALN.0b013e3181f56228
Sanna, E., Mostallino, M. C., Murru, L., Carta, M., Talani, G., Zucca, S., et al. (2009). Changes in expression and function of extrasynaptic GABA-A receptors in the rat hippocampus during pregnancy and after delivery. J. Neurosci. 29, 1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009
Santhakumar, V., Jones, R. T., and Mody, I. (2010). Developmental regulation and neuroprotective effects of striatal tonic GABA-A currents. Neuroscience 167, 644–655. doi: 10.1016/j.neuroscience.2010.02.048
Sato, K., Akaishi, T., Matsuki, N., Ohno, Y., and Nakazawa, K. (2007). beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 1150, 108–120. doi: 10.1016/j.brainres.2007.02.093
Scharfman, H. E., Mercurio, T. C., Goodman, J. H., Wilson, M. A., and MacLusky, N. J. (2003). Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J. Neurosci. 23, 11641–11652.
Schmidt, P., Nieman, L., Danaceau, M., Adams, L., and Rubinow, D. (1998). Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. New Engl J. Med. 338, 209–216. doi: 10.1056/NEJM199801223380401
Semyanov, A., Walker, M. C., and Kullmann, D. M. (2003). GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat. Neurosci. 6, 484–490.
Shavalier, M. (2004). The effects of CAD-like software on the spatial ability of middle school students. J. Educat. Comput. Res 31, 37–49.
Shen, H., Gong, Q. H., Aoki, C., Yuan, M., Ruderman, Y., Dattilo, M., et al. (2007). Reversal of neurosteroid effects at alpha4-beta2-delta GABA-A receptors triggers anxiety at puberty. Nat. Neurosci. 10, 469–477.
Shen, H., Gong, Q. H., Yuan, M., and Smith, S. S. (2005). Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49, 573–586. doi: 10.1016/j.neuropharm.2005.04.026
Shen, H., Sabaliauskas, N., Sherpa, A., Fenton, A. A., Stelzer, A., Aoki, C., et al. (2010a). A critical role for a4bd GABA-A receptors in shaping learning deficits at puberty in mice. Science 327, 1515–1518. doi: 10.1126/science.1184245
Shen, H., Sabaliauskas, N., Sherpa, A., Fenton, A. A., Stelzer, A., Aoki, C., et al. (2010b). A critical role for a4bd GABA-A receptors in shaping learning deficits at puberty in mice. Science (Suppl.) 327, 1515–1518. doi: 10.1126/science.1184245
Sieghart, W., and Sperk, G. (2002). Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2, 795–816. doi: 10.2174/1568026023393507
Smith, K. S., Engin, E., Meloni, E. G., and Rudolph, U. (2012). Benzodiazepine-induced anxiolysis and reduction of conditioned fear are mediated by distinct GABA-A receptor subtypes in mice. Neuropharmacology 63, 250–258. doi: 10.1016/j.neuropharm.2012.03.001
Smith, S. S., Ruderman, Y., Frye, C. A., Homanics, G. E., and Yuan, M. (2006). Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5alpha-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology 186, 323–333. doi: 10.1007/s00213-005-0168-3
Smith, S. S., Shen, H., Gong, Q. H., and Zhou, X. (2007). Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol. Ther. 116, 58–76. doi: 10.1016/j.pharmthera.2007.03.008
Song, I., Savtchenko, L., and Semyanov, A. (2011). Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat. Commun. 2, 376. doi: 10.1038/ncomms1377
Staley, K. J., and Mody, I. (1992). Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 68, 197–212.
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., and Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc. Natl. Acad. Sci. U.S.A. 100, 14439–14444. doi: 10.1073/pnas.2435457100
Stell, B. M., and Mody, I. (2002). Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 22, RC223.
Stork, O., Ji, F. Y., Kaneko, K., Stork, S., Yoshinobu, Y., Moriya, T., et al. (2000). Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 865, 45–58. doi: 10.1016/S0006-8993(00)02206-X
Subrahmanyam, K., and Greenfield, P. (1994). Effect of video game practice on spatial skills in girls and boys. J. Appl. Dev. Psychol. 15, 15–32. doi: 10.1016/0193-3973(94)90004-3
Sumter, S. R., Bokhorst, C. L., Miers, A. C., Van, P. J., and Westenberg, P. M. (2010). Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology 35, 1510–1516. doi: 10.1016/j.psyneuen.2010.05.004
Sundstrom-Poromaa, I., Smith, D. H., Gong, Q., Sabado, T. N., Li, X., Light, A., et al. (2002). Hormonally regulated α4β2δ GABA-A receptors are a target for alcohol. Nat. Neurosci. 5, 721–722.
Susman, E. J., Nottelmann, E. D., Dorn, L. D., Inoff-Germain, G., and Chrousos, G. P. (1988). Physiological and behavioral aspects of stress in adolescence. Adv. Exp. Med. Biol. 245, 341–352. doi: 10.1007/978-1-4899-2064-5_27
Tian, N., Petersen, C., Kash, S., Baekkeskov, S., Copenhagen, D., and Nicoll, R. (1999). The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc. Natl. Acad. Sci. U.S.A. 96, 12911–12916. doi: 10.1073/pnas.96.22.12911
Tokuda, K., Izumi, Y., and Zorumski, C. F. (2011). Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J. Neurosci. 31, 9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011
Tsien, J. Z., Huerta, P. T., and Tonegawa, S. (1996). The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338. doi: 10.1016/S0092-8674(00)81827-9
Uysal, N., Sisman, A. R., Dayi, A., Ozbal, S., Cetin, F., Baykara, B., et al. (2012). Acute footshock-stress increases spatial learning-memory and correlates to increased hippocampal BDNF and VEGF and cell numbers in adolescent male and female rats. Neurosci. Lett. 514, 141–146. doi: 10.1016/j.neulet.2012.02.049
Veselis, R. A., Pryor, K. O., Reinsel, R. A., Li, Y., Mehta, M., and Johnson, R., Jr. (2009). Propofol and midazolam inhibit conscious memory processes very soon after encoding: an event-related potential study of familiarity and recollection in volunteers. Anesthesiology 110, 295–312.
Vickers, K., and McNally, R. J. (2004). Is premenstrual dysphoria a variant of panic disorder? A review. Clin. Psychol. Rev. 24, 933–956.
Wafford, K. A., Thompson, S. A., Sikela, J., Wilcox, A. S., and Whiting, P. J. (1996). Functional characterization of human GABA receptors containing the a4 subunit. Mol. Pharmacol. 50, 670–678.
Wallner, M., Hanchar, H. J., and Olsen, R. W. (2003). Ethanol enhances α4β3δ and α6β3δ gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223. doi: 10.1073/pnas.2435171100
Wei, W., Faria, L. C., and Mody, I. (2004). Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABA-A receptros in hippocampal neurons. J. Neurosci. 24, 8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004
Wei, W., Zhang, N., Peng, Z., Houser, C. R., and Mody, I. (2003). Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 23, 10650–10661.
Whissell, P. D., Rosenzweig, S., Lecker, I., Wang, D. S., Wojtowicz, J. M., and Orser, B. A. (2013). deltaGABA receptors promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. doi: 10.1002/ana.23941.
Wieland, H. A., Luddens, H., and Seeburg, P. H. (1992). A single histidine in GABA-A receptors is essential for benzodiazepine agonist binding. J. Biol. Chem. 267, 1426–1429.
Wiltgen, B. J., Sanders, M. J., Ferguson, C., Homanics, G. E., and Fanselow, M. S. (2005). Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABA-A receptor. Learn. Mem. 12, 327–333. doi: 10.1101/lm.89705
Wisden, W., Laurie, D. J., Monyer, H., and Seeburg, P. (1991). Cloning, pharmacological characteristis and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 289, 227–230. doi: 10.1016/0014-5793(91)81076-K
Wisden, W., Laurie, D. J., Monyer, H., and Seeburg, P. (1992). The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 12, 1040–1062.
Wlodarczyk, A. I., Sylantyev, S., Herd, M. B., Kersante, F., Lambert, J. J., Rusakov, D. A., et al. (2013). GABA-independent GABA-A receptor openings maintain tonic currents. J. Neurosci. 33, 3905–3914. doi: 10.1523/JNEUROSCI.4193-12.2013
Wohlfarth, K. M., Bianchi, M. T., and Macdonald, R. L. (2002). Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 22, 1541–1549.
Wolk, S., and Bloom, D. (1978). The interactive effects of locus of control and situational stress upon performance accuracy and time. J. Pers. 46, 279–298. doi: 10.1111/j.1467-6494.1978.tb00180.x
Wood, G. E., and Shors, T. J. (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc. Natl. Acad. Sci. U.S.A. 95, 4066–4071. doi: 10.1073/pnas.95.7.4066
Wu, Y., Wang, W., and Richerson, G. (2003). Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J. Neurophysiol. 89, 2021–2034. doi: 10.1152/jn.00856.2002
Yamashita, M., Marszalec, W., Yeh, J. Z., and Narahashi, T. (2007). Effects of ethanol on tonic GABA current in cerebellar granule cells and mammalian cells recombinantly expressing GABA-A receptors. J. Pharmacol. Exp. Ther. 319, 431–438. doi: 10.1124/jpet.106.106260
Yerkes, R. M., and Dodson, J. D. (1908). The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 18, 459–482. doi: 10.1002/cne.920180503
Yonkers, K. A., O'Brien, P. M., and Eriksson, E. (2008). Premenstrual syndrome. Lancet 371, 1200–1210. doi: 10.1016/S0140-6736(08)60527-9
Zheleznova, N., Sedelnikova, A., and Weiss, D. S. (2008). Alpha1-beta2-delta, a silent GABA-A receptor: recruitment by tracazolate and neurosteroids. Br. J. Pharmacol. 153, 1062–1071. doi: 10.1038/sj.bjp.0707665
Keywords: puberty, GABAA receptor, alpha4, delta, anxiety, cognition, tonic current, synaptic plasticity
Citation: Smith SS (2013) α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity. Front. Neural Circuits 7:135. doi: 10.3389/fncir.2013.00135
Received: 13 June 2013; Accepted: 28 July 2013;
Published online: 03 September 2013.
Edited by:
Alexey Semyanov, RIKEN Brain Science Institute, JapanReviewed by:
Delia Belelli, Ninewells Hospital & Medical School, UKStefano Vicini, Georgetown University School of Medicine, USA
Beverley A. Orser, University of Toronto, Canada
Copyright © 2013 Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheryl S. Smith, Department of Physiology and Pharmacology, SUNY Downstate Medical Center, 450 Clarkson Ave., Brooklyn, NY 11203, USA e-mail:c2hlcnlsLnNtaXRoQGRvd25zdGF0ZS5lZHU=
