
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Neurosci. , 24 February 2025
Sec. Cellular Neuropathology
Volume 19 - 2025 | https://doi.org/10.3389/fncel.2025.1550139
This article is part of the Research Topic Oligodendroglia Biology and Pathology: Myelination and Beyond View all 4 articles
 Sonia E. Nanescu1
Sonia E. Nanescu1 Natacha M. Wathieu1
Natacha M. Wathieu1 Lauren Rosko1,2
Lauren Rosko1,2 David S. Cha1
David S. Cha1 Mahesh N. Kumar1
Mahesh N. Kumar1 Rafal T. Olszewski1
Rafal T. Olszewski1 Joan Reger1
Joan Reger1 Maryna Baydyuk1
Maryna Baydyuk1 Alisha N. Dua1
Alisha N. Dua1 Wojciech Krezel3
Wojciech Krezel3 Violetta Zujovic4
Violetta Zujovic4 Jeffrey K. Huang1,2*
Jeffrey K. Huang1,2*Myelin regeneration (remyelination) in the CNS depends on the recruitment, proliferation and differentiation of oligodendrocyte precursor cells (OPCs) at demyelinated lesions. However, despite the presence of OPCs, very few oligodendrocytes and myelin are regenerated in chronic multiple sclerosis (MS) lesions for reasons that remain poorly understood. Here, using a spontaneous remyelination model in mice, we found that retinaldehyde dehydrogenase 2 (Raldh2), a rate-limiting enzyme for retinoic acid (RA) synthesis, is upregulated in OPCs and in a subpopulation of microglia/macrophages during remyelination. Tamoxifen induced deletion of Raldh2 globally, or conditionally in OPCs, resulted in significantly fewer proliferating OPCs in lesions, leading to decreased oligodendrocyte numbers and myelin density. Moreover, induced deletion of Raldh2 globally also resulted in increased microglia/macrophage density in lesions. Further, exogenous RA delivery into lesions significantly increased oligodendrocyte lineage cells, while also decreasing proinflammatory microglia/macrophages, with no significant effect on anti-inflammatory microglia/macrophages. Postmortem MS brain sections revealed Raldh2 was absent in the majority of OPCs in chronic inactive lesions compared to the other lesion types. These results suggest that Raldh2 upregulation in lesions is critical for OPC proliferation during remyelination, and reveal that the failure to regenerate sufficient oligodendrocytes and myelin in chronic MS lesions may arise from impaired OPC expansion due to the failure to intrinsically synthesize RA.
Multiple sclerosis (MS) is an immune-driven neurodegenerative disease characterized by chronic inflammation, demyelination and axonal injury in the central nervous system (CNS) (Compston and Coles, 2008; Trapp and Nave, 2008; Reich et al., 2018). Myelin regeneration (remyelination) becomes increasingly difficult at the later stage of the disease, in which chronic CNS inflammation coupled with regenerative failure results in irreparable axonal dystrophy, and widespread neurodegeneration (Franklin, 2002; Lassmann et al., 2012; Dutta and Trapp, 2014). Exactly why regenerative failure occurs in MS remains unclear (Wolswijk, 2000; Franklin, 2002; Hagemeier et al., 2012; Boyd et al., 2013). One possibility is that oligodendrocyte precursor cells (OPCs), once migrated into lesions, fail to expand, leading to insufficient mature oligodendrocytes in lesions for remyelination (Wolswijk, 1998, 2000). However, the mechanisms driving OPC proliferation within the injury microenvironment remains poorly understood.
Retinoic acid (RA) is a highly conserved signaling molecule that stimulates stem/progenitor cell proliferation and differentiation during the development and regeneration of certain tissues and organs (Chambon, 1996; Ogura et al., 1996; Maden and Hind, 2003; Maden, 2007; Duester, 2013). RA promotes signaling by binding to retinoic acid receptors (RARα, RARβ and RARγ) and their heterodimeric partners retinoid X receptors (RXRα, RXRβ and RXRγ) to activate or suppress the transcription of a suite of target genes by binding to promoters containing the retinoic acid response element (RARE) (Rossant et al., 1991; Chambon, 1996; Kumar and Duester, 2011). RXRs, particularly RXRγ, are highly expressed in oligodendrocyte lineage cells and macrophages during remyelination in rodent CNS lesions, and in MS plaques (Huang et al., 2011). The loss of RXRγ expression in mice prevents efficient oligodendrocyte differentiation and remyelination, suggesting that RA signaling plays a critical role in CNS remyelination. However, for RA signaling to occur, RA must be available in demyelinated lesions to drive oligodendrocyte lineage cell progression. During development, RA is widely distributed in the embryonic CNS, and regulates tissue patterning and corticogenesis (Siegenthaler et al., 2009; Duester, 2013). In the adult CNS, RA level is significantly reduced and produced primarily by the meninges, and this is thought to influence homeostatic function through a paracrine mechanism (Zhang et al., 2003; Siegenthaler et al., 2009). However, the cellular source of RA involved in remyelination remains unknown. Since RA signaling is upregulated in oligodendrocyte lineage cells during remyelination, we hypothesize that RA synthesis occurs locally within lesions to stimulate RA signaling in oligodendrocyte lineage cells during remyelination.
RA is generated from retinaldehyde through retinaldehyde dehydrogenase activity, following a series of oxidation reactions from vitamin A. The mammalian CNS expresses at least three types of retinaldehyde dehydrogenase—Raldh1, Raldh2 and Raldh3 (Li et al., 2000; Wagner et al., 2002). Raldh2 is predominantly expressed in the central nervous system (CNS), with its presence in meningeal cells playing a crucial role in brain development (Zhang et al., 2003; Siegenthaler et al., 2009; Haushalter et al., 2017). Additionally, our recent findings indicate that Raldh2 expression in the meninges supports the production and differentiation of oligodendrocyte precursor cells (OPCs) in the corpus callosum (Morrison et al., 2020). Following spinal cord injury, Raldh2 has also been detected in NG2+ cells, and suggested to promote a permissive environment for axon outgrowth (Mey et al., 2005; Goncalves et al., 2018). However, the precise identity of these NG2+ cells in injury remains unclear, and whether they are involved in remyelination remains unknown. Here, we examine the role of Raldh2 in remyelination following focal demyelinating injury in mice. We found that Raldh2 is upregulated in oligodendrocyte lineage cells and a subpopulation of microglia/macrophages in remyelinating lesions and required for OPC proliferation and subsequent oligodendrocyte differentiation after demyelination. Moreover, administration of RA into demyelinated lesions stimulated OPC proliferation, leading to increased mature oligodendrocytes in lesions. We also found that Raldh2 was absent in the majority of OPCs in chronic inactive lesions in postmortem MS brain sections. Our results suggest that intrinsic RA synthesis in adult OPCs is necessary to drive oligodendrocyte lineage cell expansion in demyelinated lesions, and that the loss of RA synthesis in OPCs may result in the failure to regenerate sufficient oligodendrocytes and myelin in chronic MS lesions.
C57BL/6 and CMV-CreERT2, NG2-Cre and Pdgfra-cre/ERT2 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Raldh2 floxed mice were obtained from IGBMC, Strasbourg, France. Raldh2loxP/loxP;CMVCre-ERT were bred by crossing Raldh2 floxed mice with B6.129-Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J (Jackson Laboratory). To induce conditional deletion of Raldh2, 1 mg of 4-hydroxytamoxifen (4OHT) was administered daily by i.p., injections for 4 consecutive days. Animals were allowed to recover for 4 more days before inducing the demyelination lesion. The retinoic acid reporter mouse line Tg(RARE-Hspa1b/lacZ)12Jrt/, which expresses beta-galactosidase (lacZ) gene under the control of the retinoic acid responsive element (RARE), was purchased from Jackson Laboratory (Bar Harbor, ME, USA). Both males and females were used for experiments, with genders in the experimental groups distributed as equally as possible. Experiments were performed according to approved Institutional Animal Care and Use Committee (IACUC) protocols of Georgetown University.
4OHT was purchased from Sigma. 500 μl of 2 mg/ml emulsion were injected daily using peanut oil as vehicle. RA (all trans) was obtained from Sigma and dissolved in dimethyl sulfoxide, then stored at −80°C in the dark. All subsequent dilutions were also kept shielded from light.
Injection of lysolecithin into the spinal cord ventral funiculus was performed between T10 and T11 thoracic vertebrae to create focal demyelination in mice (Jeffery and Blakemore, 1995; Baydyuk et al., 2019). To this end, incision of back skin and muscle was followed by removal of muscles and tendons between adjacent thoracic vertebrae with forceps to expose the spinal cord. The dura was then punctured with a fine needle before injection. A pulled glass needle glued to the tip of a 10 ul Hamilton syringe attached to a micromanipulator was used to deliver 0.5 μl 1.0% lysolecithin (Sigma) into the ventral funiculus of the mouse spinal cord to induce a focal demyelinated lesion. After injection, the muscles overlying the lesion were sutured, which will later help identify the site of lesions, followed by interrupted sutures of the overlying skin with a non-absorbable suture (Ethilon 5.0). For pain management, the analgesic Carprofen was delivered subcutaneously for 3 consecutive days. Extended release buprenorphine was also delivered. The mice were monitored for at least 72 h after surgery for signs of distress and pain before perfusion with 4.0% paraformaldehyde at 7, 14 and 21 days postlesion.
After 4.0% paraformaldehyde (PFA) perfusion, spinal cords were dissected and post fixed for 30 min at room temperature, followed by overnight immersion in 20% sucrose in PBS before embedding in OCT compound and frozen in dry ice. 12 μm sections were generated on Superfrost plus glass slides, and focal demyelination was identified by toluidine blue staining before tissue collection and then storage at −80° until immunostaining. Prior to staining, tissues were allowed to dry for 30 min and incubated for 1 h in the blocking buffer containing 5% normal goat serum and 0.3% TritonTm X-100 made in PBS. Primary antibodies: rabbit Raldh2 (1:200, Abcam) rat anti-CD11b (1:100; AbD Serotec), mouse anti-iNOS/NOS Type II (1:100; BD Pharmingen), chicken Arg1 (1:1000, Millipore), mouse Nkx2.2 (1:100, DSHB) mouse APC/CC1 (1:100, Millipore), mouse and rabbit Olig2 (1:100 and 1:300, respectively, Millipore) and mouse Olig2 (1:100, Millipore), rat MBP4 (1:400, AbD Serotec), rabbit Raldh2 (1:200, Abcam), rabbit Ki67 (1:1000, Thermo Fisher), rat PDGFRα (1:400, BD Pharminogen), rabbit NF 200 (1:100, Sigma), Smi32 (1;1000, Calbiochem), GFAP (1:500, Sigma), rabbit anti-cleaved caspase-3 (1:100; Cell Signaling Technology) were incubated overnight at 4% Celsius in same blocking buffer. Antigen retrieval was used for Olig2, Raldh2, and mouse on mouse antigen retrieval with M.O.M.™ kit (Vector Laboratories) for Nkx2.2 and CC1. For double labeling of Raldh2 and Cd11b, iNOS, or Arginase1, tissue was permeabilized by immersion at −20°C for 10 min in a 1:1 mixture of Acetone and Methanol. Next day, slides were washed 3 times with TBS-tween solution (0.05% Tween) for 10 min, and the following secondary antibodies added for 1 h: Alexa Fluor® 488 IgG (1:500), Alexa Fluor® 594 Goat Anti-Mouse IgG (1:500), Texas Red Goat Anti-Mouse IgG (1:500). After 3 more washes in TBS-tween of 10 min each, slides were mounted with Fluoromount-G (SouthernBiotech). For quantification of immunohistochemical staining, cells were manually counted from low magnification, non-overlapping images. The lesioned area was defined by hyper nuclear (DAPI) staining. Corrected fluorescence for SMI-32 and NF200 was determined by measuring the integrated intensity, subtracting the product of the selected region’s area and the mean background fluorescence as described previously (Psachoulia et al., 2016). A minimum of three sections from n = 3–5 mice were analyzed. The proportion or density of cells was determined per mouse, and the average and standard error were calculated for each group.
Tissues were perfused and collected in the same manner as described above. The primers used for cloning the Raldh2 in situ probe had the sequence AGC TCC TGC CGT CGC CCA CG (forward) and CA CTC CAA TGG GCT CGT GTC (reverse) according to a sequence published previously (Zhao et al., 1996). The protocol used was according to the in situ hybridization procedure previously described (Pringle et al., 1992; Huang et al., 2011).
Retinoic acid reporter mice were perfused with 0.2% glutaraldehyde in PBS and spinal cords postfixed for 10 min on ice, followed by 3 consecutive washes in LacZ wash solution (2 mM MgCl2, 0.01Na Deoxycholate and 0.02% Nonidet P-40 in PBS). Whole mount spinal cords were then incubated for two hours in LacZ wash with 5 mg/ml X-Gal, 5 mM ferrocyanide and 5 mM ferricyanide added to the wash solution to allow enzymatic reaction at 37°C in the dark. Spinal cords were then immersed in sucrose, frozen and sectioned.
Human brain tissue was provided by the UK multiple sclerosis tissue bank (Richard Reynolds, Imperial College, London). Donor informed consent was obtained via a prospective donor scheme following ethical approval by the London Multicenter research ethics committee (MREC 02/2/39). Snap frozen sections of MS patient postmortem tissue was processed at the cryostat with 12 μm sections and rehydrated with PBS prior to any staining. Luxol fast blue immunostaining was performed in conjunction with MHC class II peroxidase conjugated staining to identify and classify the different types of lesions. In order to perform immunohistochemistry with anti-Kp1 (1:100, DAKO), anti-Sox10 (1:100, R&D Systems) and anti-Raldh2 (1:50, Abcam) antibodies, brain sections were microwaved in Low pH unmasking solution (Vector Laboratories) then pre-incubated 1 h with a blocking solution buffer (10% normal goat serum, 0.1% Triton in PBS) at room temperature before the incubation overnight with the primary antibodies. After overnight incubation, slides were extensively washed in 0.1% Triton in PBS and incubated with appropriate secondary antibodies.
Data were analyzed for statistical significance with GraphPad Prism 6 by one-way ANOVA followed by Bonferroni post-hoc for multiple comparisons. For each comparison, averages from 2 to 5 lesions from a group of 3–6 mice were analyzed. Statistical significance is reported as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001.
To track RA signaling during remyelination, lysolecithin-induced spinal cord demyelination (Figure 1A) was performed on the RARE-LacZ transgenic RA reporter mouse line (Rossant et al., 1991), in which the beta-galactosidase (LacZ) gene is driven under control of the retinoic acid response element (RARE) promoter, followed by enzymatic detection of LacZ expression at 14 days postlesion (dpl). We found that beta-galactosidase staining was primarily observed within demyelinated lesions and rarely detected in non-lesioned white matter (Figure 1B). This result suggested that the activation of RA signaling is restricted to lesions after CNS injury and raised the question whether RA synthesis is induced locally in response to injury to stimulate RA signaling. Since retinaldehyde dehydrogenases are critical for RA synthesis, and retinaldehyde dehydrogenase 2 (Raldh2) has previously been shown to regulate CNS function in development and injury (Aoto et al., 2008; Siegenthaler et al., 2009; Goncalves et al., 2018), we next determined if Raldh2 has a role in CNS remyelination. To examine Raldh2 expression, in situ hybridization was performed on lesioned spinal cord sections at 7 dpl. We found that the antisense RNA against Raldh2 was strongly detected in demyelinated lesions compared to sense control (Figure 1C), suggesting that RA synthesis was induced locally in demyelinated lesions.
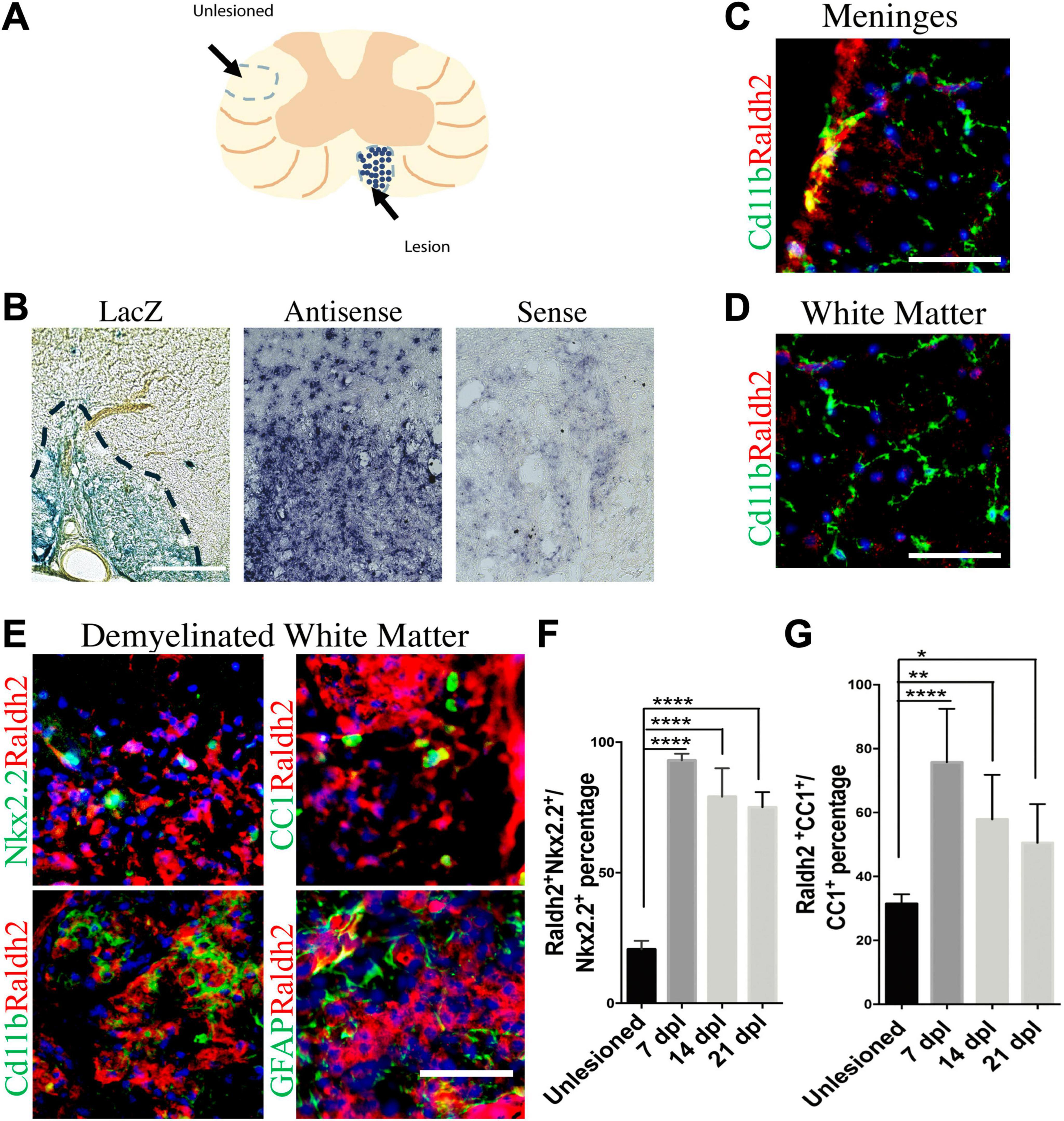
Figure 1. Induction of Raldh2 expression during remyelination. (A) Diagram displaying lesioned and unlesioned white matter analyzed. (B) β-gal staining in lesioned spinal cord of RARE-LacZ reporter mouse at 14 dpl. Lesioned outlined in black. Scale bar 30 μm. (C) In situ hybridization of mouse lesioned spinal cord with antisense or sense probe against Raldh2 at 14 dpl. Scale bar, 50 μm. (D) Representative images for Raldh2 (red) and Cd11b labeled resting microglia (green) at meninges and non-lesioned white matter. Scale bar, 50 μm. (E) Immunodetection of Raldh2 co-labeling with Nkx2.2 for OPCs, CC1 for oligodendrocytes, Cd11b for activated microglia/macrophages, and GFAP for reactive astrocytes at 14 dpl. Scale bar, 100 μm. (F) Percentage of Raldh2 labeled OPCs and (G) oligodendrocytes in unlesioned and lesioned tissue at 7, 14, 21 dpl. One-way ANOVA with Tukey post-hoc for multiple comparisons. Data presented as mean + s.d. ****P < 0.0001, **P < 0.01, * P < 0.05.
To determine which cell populations in lesions expressed Raldh2 during remyelination, co-immunostaining analysis of demyelinated spinal cord tissues for Raldh2 and Nkx2.2+ OPCs, CC1+ oligodendrocytes, Cd11b+ microglia/macrophages, or GFAP+ astrocytes was performed at 7, 14 and 21 dpl, corresponding to OPC recruitment/proliferation, oligodendrocyte differentiation, and remyelination, respectively (Huang et al., 2011). In non-lesioned tissue, we found Raldh2 was most strongly expressed in the meninges and weakly expressed in the white matter (Figure 1D). However, at the lesion site, strong Raldh2 expression was detected in Nkx2.2+ OPCs, CC1+ oligodendrocytes, and Cd11b+ microglia/macrophages, but not GFAP+ reactive astrocytes (Figure 1E). Quantification revealed Raldh2 was expressed in the majority of OPCs and oligodendrocytes in lesions compared to non-lesioned white matter in all three postlesion timepoints analyzed (Figures 1F, G). Moreover, Raldh2 was detected in approximately 25% of total Cd11b+ microglia/macrophages in lesions at 7 dpl, but the percentage was reduced at subsequent timepoints (Figure 2A). To determine if Raldh2 expression in microglia/macrophage corresponded with their activation states, co-immunostaining analysis for Raldh2 and induced nitric oxide (iNOS) corresponding to pro-inflammatory microglia/macrophages, or arginase 1 (Arg1) corresponding to anti-inflammatory microglia/macrophages (Miron et al., 2013; Psachoulia et al., 2016) was performed. We found that Raldh2 was detected in about 50% of pro-inflammatory macrophages at 7 dpl, and that its expression in these cells was significantly reduced by 21 dpl (Figures 2B, D). Moreover, we found that Raldh2 was detected in nearly 100% of anti-inflammatory macrophages across all three postlesioned timepoints (Figures 2C, E). These results demonstrate that Raldh2 is induced in lesions following demyelination and primarily expressed in oligodendrocyte lineage cells during remyelination.
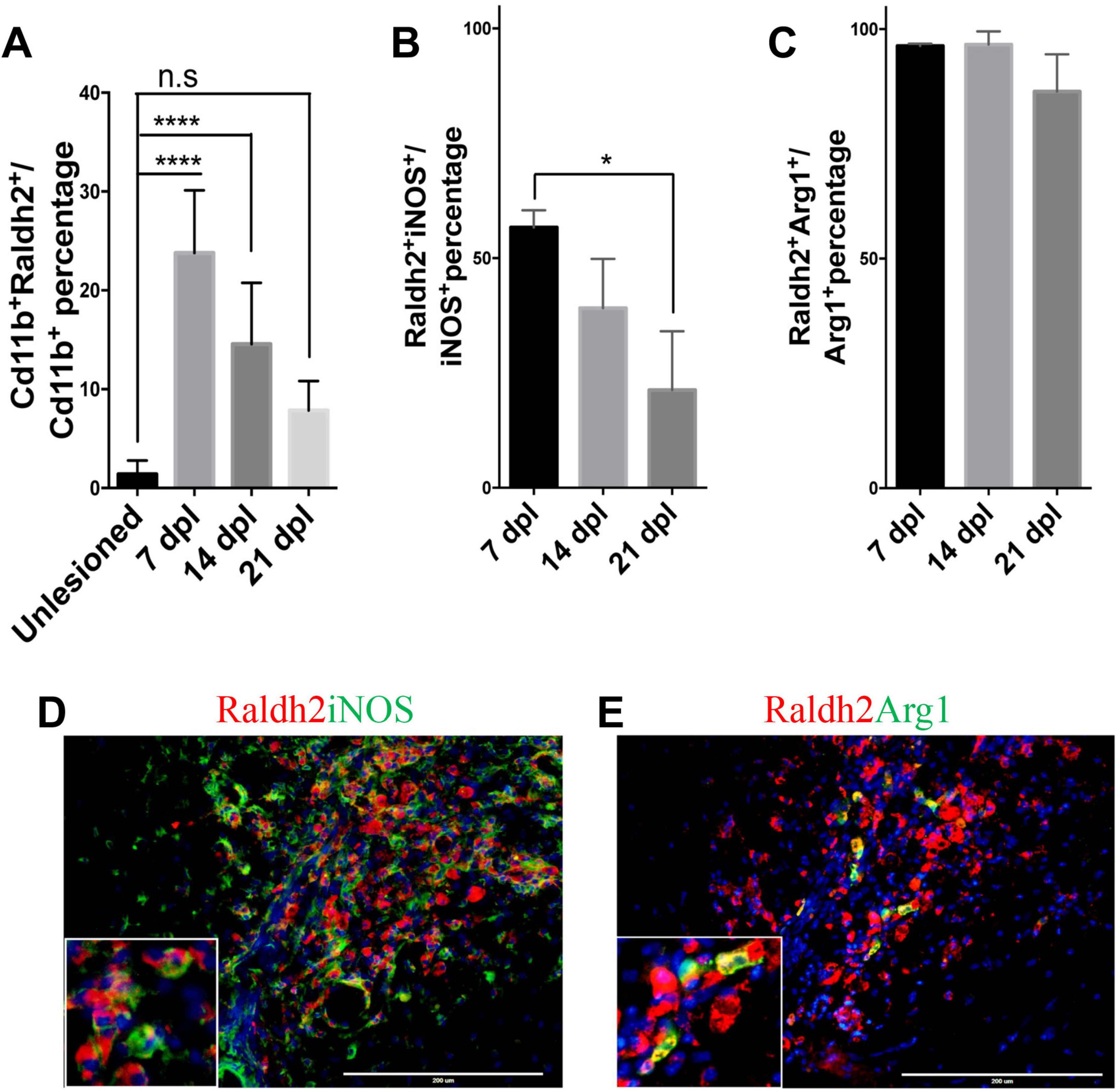
Figure 2. Raldh2 expression in microglia subtypes during remyelination. (A) Percentage of Raldh2+ microglia/macrophages in unlesioned white matter, and at 7, 14, and 21 dpl. Percentage of (B) Raldh2 labeled iNOS+ microglia/macrophages and (C) Raldh2 labeled Arg1+ microglia/macrophages in lesions at 7, 14, and 21 dpl. Representative image of (D) Raldh2+iNOS+ and (E) Raldh2+Arg1+ microglia/macrophages at 7 dpl. Scale bar, 200 μm. One way ANOVA with Tukey post-hoc for multiple comparisons. Data presented as mean + s.d. ****P < 0.0001, *P < 0.05, n.s., not significant.
We next determined if Raldh2 expression is required for oligodendrocyte lineage cell progression following demyelinating injury. Since Raldh2 knockout in mice is embryonically lethal (Niederreither et al., 1999; Mic et al., 2002) and therefore precludes the analysis of its function in the adult CNS, we generated an inducible CMV-Cre-ERT2;Raldh2loxP/loxP mouse line (Raldh2-iKO) that allowed for tamoxifen-induced deletion of the loxP-flanked Raldh2 in adult tissues prior to demyelination. The Raldh2-iKO mice expressed at least one copy of the CMV-cre-ERT2 allele and both copies of the loxP site flanked Raldh2 allele. To induce Raldh2 deletion in adult tissues, intraperitoneal (i.p.) injections of 4-hydroxy-tamoxifen (4OHT) into mice was performed for four consecutive days, followed by four days of rest to allow sufficient time for 4OHT to trigger Raldh2 excision before lysolecithin-induced spinal cord demyelination (Figure 3A). As controls, CMV-Cre-ERT2;Raldh2loxP/loxP mice injected with peanut oil (Ctr+Veh), and CMV-Cre-ERT2 injected with 4OHT (Ctr+Tx) were lesioned. Following demyelination, all mice were sacrificed at either 7, 14, or 21 dpl for immunohistochemistry analysis. To assess the efficiency of 4OHT induced deletion, immunostaining analysis of Raldh2 expression was performed on demyelinated lesions. We found that the Raldh2-iKO mice exhibited a 48–67% reduction of Raldh2 expression in the lesioned area at 7 dpl compared to both controls (Figures 3B–E), suggesting that 4OHT induced the loss of Raldh2 expression in about half of the adult cell population in demyelinated lesions.
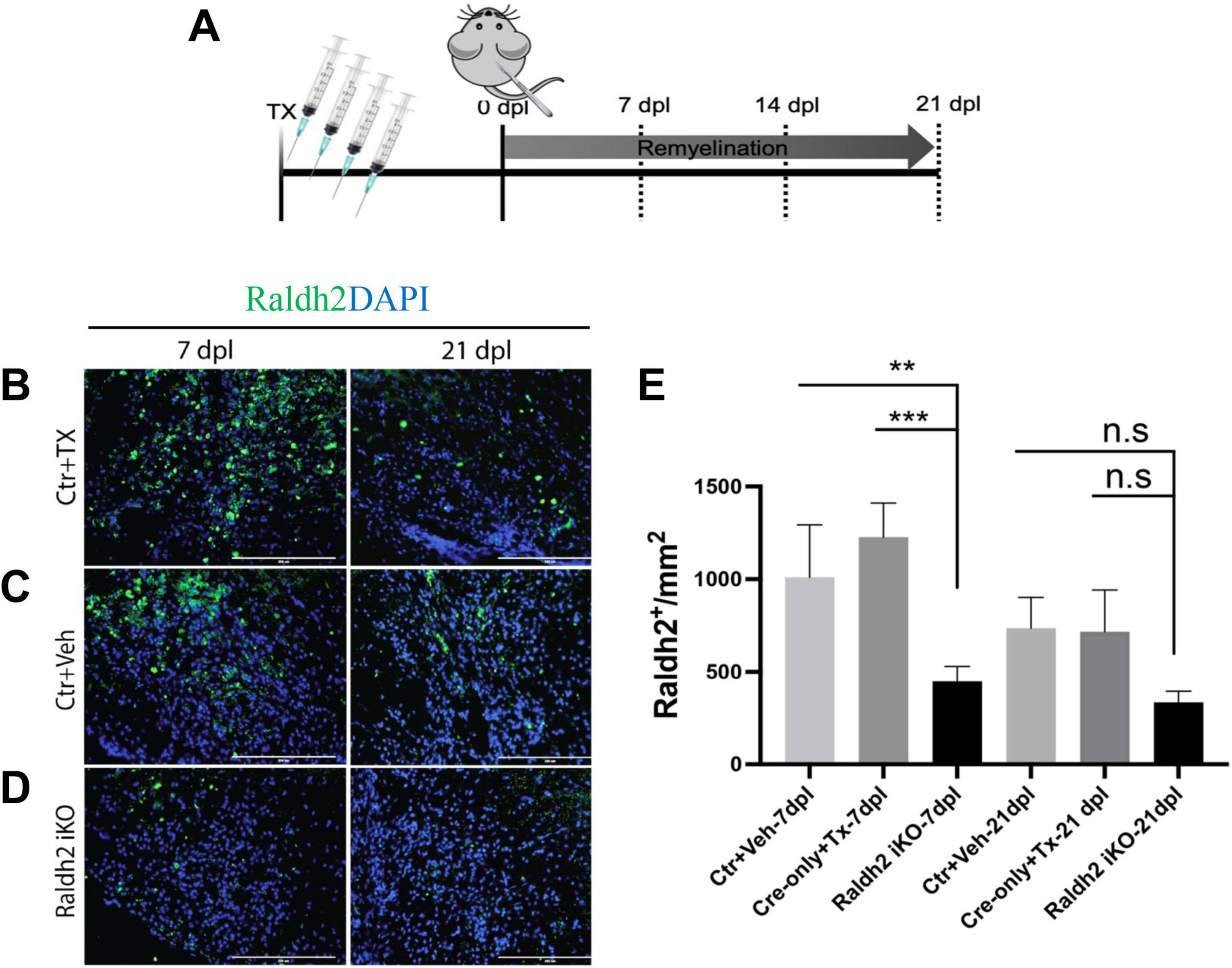
Figure 3. Assessment of 4-hydroxy tamoxifen (4OHT) efficiency in demyelinated lesion of Raldh2-iKO mouse. (A) Diagrams illustrating the inducible Raldh2 deletion strategy. 4OHT was injected for four consecutive days into CMV-Cre-ERT2;Raldh2loxP/loxP (Raldh2-iKO), and Cre-ERT2 (Ctr+Tx) control mice. Additionally, peanut oil instead of 4OHT was injected into Cre-ERT2;Raldh2loxP/loxP mice as a second control (Ctr+Veh). Immunostaining for Raldh2 expression in lesions of (B) Ctr+TX, (C) Ctr+Veh, and (D) Raldh2 iKO at 7 and 21 dpl. (E) Quantification of Raldh2+ cells in lesions per mm2 shows statistically significant reduction of Raldh2 expressed cells at 7 dpl. Scale bar, 200 μm. ANOVA with Tukey post-hoc for multiple comparisons. Data presented as mean + s.d. ***P < 0.001, **P < 0.01.
To examine the impact of Raldh2 loss-of-function on oligodendrocyte lineage cells in demyelinated lesions, co-immunostaining analysis was performed. We found that the Raldh2-iKO mice displayed a significant reduction in the number of Nkx2.2+Olig2+ OPCs in lesions at 7 and 14 dpl compared to the control groups (Figures 4A, B). At 21 dpl, the relative number of OPCs in lesions was low in all three mouse groups compared to the previous timepoints, and not significantly different from each other. This observation was expected, as most OPCs would normally have differentiated into mature oligodendrocytes by 21 dpl. To determine if impaired OPC proliferation contributed to the reduction of OPCs in lesions of the Raldh2-iKO mice, co-immunostaining analysis for Ki67 and PDGFRα (another OPC marker) was performed. We found that the relative number of Ki67+ OPCs in lesions was significantly reduced in the Raldh2-iKO group by greater than 50% at 7 dpl compared to the control groups (Figure 4C). Furthermore, their density remained relatively constant across all three postlesion timepoints, suggesting the OPCs that migrated to the lesion were unable to proliferate. Moreover, we did not detect a significant difference in the number of Caspase3+Nkx2.2+ OPCs in lesions between the Raldh2-iKO group and the control groups in any of the postlesion timepoints analyzed (Figure 4D), suggesting that Raldh2 loss-of-function did not affect apoptosis. These results indicated that Raldh2 expression is necessary for OPC proliferation during remyelination. Next, to determine if the failure of OPCs to proliferate in lesions affected subsequent oligodendrocyte differentiation or remyelination, CC1+Olig2+ co-immunolabeling of oligodendrocytes was performed. We found that the relative density of oligodendrocytes was significantly reduced in the Raldh2-iKO compared to controls (Figures 4E, F). Moreover, the density of oligodendrocytes in lesions remained constant across all three timepoints, suggesting that impaired OPC proliferation resulted in fewer mature oligodendrocytes in lesions. Indeed, quantification of Olig2 immunolabeling in lesions revealed a significant reduction of total oligodendrocyte lineage cells in Raldh2-iKO mice compared to controls in all three postlesion timepoints (Figure 4G). This reduction also resulted in decreased MBP immunostaining in lesions (Figures 4H, I), suggesting that OPC proliferation through RA signaling is necessary for the expansion of oligodendrocyte lineage cell numbers in lesions during remyelination. Although the relative number of oligodendrocyte lineage cells in lesions was reduced in Raldh2-iKO mice, we did not observe an increase in axonal dystrophy in lesions at 21 dpl, as shown by the relative distribution of SMI32+NF200+ axons in lesions (Figures 4J, K).

Figure 4. Induced deletion of Raldh2 globally in adult mice reduces oligodendrogenesis after demyelination. Lesions of Ctr+TX and Ctr+Veh controls, and Raldh2-iKO mice were analyzed at 7, 14, 21 dpl. (A) Representative images of Nkx2.2+Olig2+ OPCs at 14 dpl. Quantification of (B) Nkx2.2+Olig2+ OPCs, (C) PDGFRα+Ki67+ proliferating OPCs, and (D) cleaved Caspase-3+Nkx2.2+ apoptotic OPCs in lesions at 7, 14, 21 dpl. (E) Representative images of CC1+Olig2+ oligodendrocytes at 14 dpl. Quantification of (F) CC1+Olig2+ oligodendrocytes, and (G) Olig2+ oligodendrocyte lineage cells in lesions at 7, 14, 21 dpl. (H) Representative images of myelin basic protein (MBP) staining at 21 dpl, corresponding to remyelination, and (I) quantification of MBP area coverage in lesions at 21 dpl. (J) Representative images of SMI32+NF200+ labeling at 14 dpl, corresponding to dystrophic axons, and (K) quantification of SMI32 staining normalized to NF200 in lesions at 14 dpl. Scale bar, 100 μm. One-way ANOVA with Tukey post-hoc for multiple comparisons except for unpaired two-tailed Student’s t-test in (I). Data presented as mean + s.d. ****P < 0.0001, ***P < 0.001, **P < 0.01, n.s., not significant.
Since Raldh2 is also expressed in a subpopulation of microglia/macrophages during remyelination, we next investigated the effect of Raldh2 loss-of-function on microglia/macrophage activation after demyelination. We found that following demyelination, the Raldh2-iKO group displayed increased density of iNOS+Cd11b+ macrophages in lesions compared to controls. Quantification showed that the number of iNOS+Cd11b+ macrophages in lesions of KO mice was 2-fold greater at 7 dpl, and 10-fold greater at 21 dpl than controls, suggesting that inflammation remained elevated in lesions in the absence of Raldh2 expression (Figures 5A, B). Moreover, we found that the relative density of anti-inflammatory macrophages, which display Arg1+Cd11b+ expression, was initially greater in the Raldh2-iKO group compared to controls at 7 dpl, but their density in lesions was reduced to similar levels as controls at 14 and 21 dpl (Figures 5C, D). These results suggest that RA synthesis in microglia/macrophages may be necessary to regulate their activation state during remyelination.
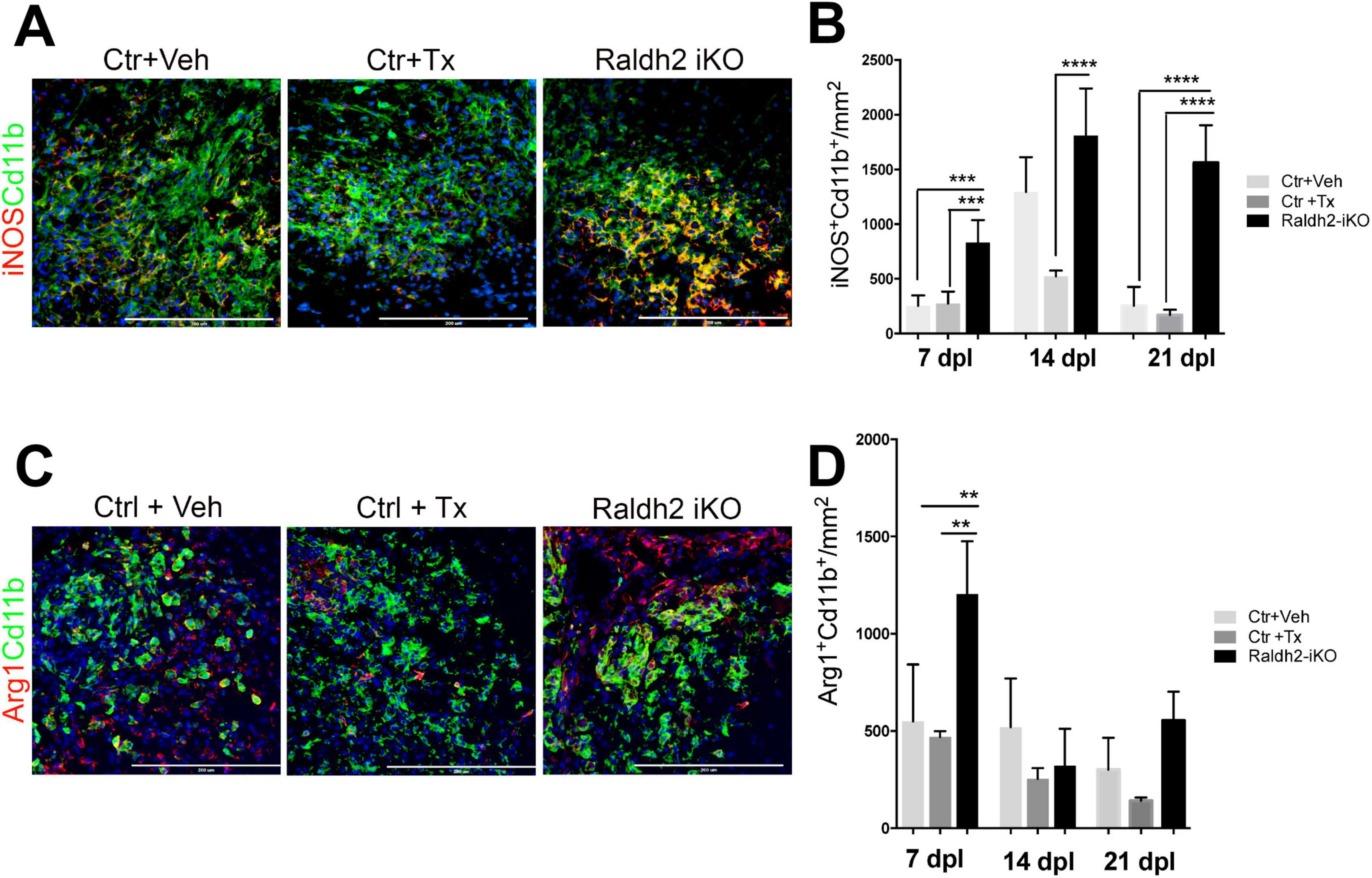
Figure 5. Induced deletion of Raldh2 globally in adult mice increases inflammation after demyelination. Lesions of Ctr+TX and Ctr+Veh controls, and Raldh2-iKO mice were analyzed at 7, 14, 21 dpl. (A) Representative images of iNOS+Cd11b+ microglia/macrophages at 14 dpl, and (B) quantification of iNOS+Cd11b+ microglia/macrophages in lesions at 7, 14, 21 dpl. (C) Representative images of iNOS+Arg1+ microglia/macrophages at 14 dpl, and (D) quantification of Arg1+Cd11b+ microglia/macrophages in lesions at 7, 14, 21 dpl. Scale bar, 200 μm. One-way ANOVA with Tukey post-hoc for multiple comparisons. Data presented as mean + s.d. ****P < 0.0001, ***P < 0.001, **P < 0.01, n.s., not significant.
Since 4OHT induced deletion of Raldh2 in Raldh2-iKO likely occurred in both oligodendrocyte lineage cells and microglia/macrophages, it was not possible to determine if the observed decrease of oligodendrocyte numbers in lesions resulted from Raldh2 loss-of-function in OPCs, or microglia/macrophages. To address this issue, Raldh2 floxed mice were crossed with NG2-Cre mice to generate a NG2-Cre;Raldh2loxP/loxP mouse line (NG2-cKO), in which Raldh2 was conditionally deleted in OPCs during development. The NG2-cKO mice grew to adulthood and did not appear to display obvious gross abnormalities. To determine if conditional deletion of Raldh2 in OPCs affects oligodendrocyte lineage cell progression in demyelinated lesions, lysolecithin-induced demyelination was performed on NG2-cKO, and NG2-Cre mice (control), followed by immunostaining analysis of lesions at 14 dpl. We found that the NG2-cKO group exhibited a significant reduction in number of Nkx2.2+Olig2+ OPCs, by approximately 25%, in demyelinated lesions compared to control (Figure 6A). Moreover, we found that the relative density of OPCs in unlesioned white matter tissue was not significantly different between the NG2-cKO group and control, suggesting that the conditional deletion of Raldh2 in OPCs only affected OPCs that have migrated to the lesion. To determine if OPC proliferation is dependent on Raldh2 expression in OPCs, co-immunostaining analysis for Ki67 and PDGFRα expression was performed. We found that the number of proliferating OPCs was significantly reduced, by approximately 50%, in the lesions of the NG2-cKO group compared to control (Figure 6B). Moreover, the number of CC1+Olig2+ labeled oligodendrocytes was also reduced by approximately 50%, in lesions (Figure 6C). These results suggest the deletion of Raldh2 in NG2+ cells decreased OPC proliferation, leading to reduced mature oligodendrocytes in demyelinated lesions.

Figure 6. Conditional deletion of Raldh2 in OPCs reduces oligodendrogenesis after demyelination. Quantification of (A) Nkx2.2+Olig2+ OPCs, (B) PDGFRα+Ki67+ proliferating OPCs, and (C) CC1+Olig2+ oligodendrocytes in lesioned and adjacent unlesioned white matter of NG2-Cre;Raldh2loxP/loxP (NG2-cKO) and NG2-Cre (control) mice at 14 dpl after focal demyelination. Quantification of (D) Olig2+ oligodendrocyte lineage cells, and (E) Nkx2.2+Olig2+ OPCs in lesions in Pdgfra-CreERT2;Raldh2fl/fl (Pdgfra-iKO), and Pdgfra-CreERT2 (control) mice at 14 dpl following 4OHT injection and focal demyelination. One-way ANOVA with Tukey post-hoc for multiple comparisons except for unpaired two-tailed Student’s t-test in (B,D,E). Data presented as mean + s.d. **P < 0.01, *P < 0.05, n.s., not significant.
However, since NG2 may be transiently expressed by a subpopulation of activated macrophages under excitotoxic CNS lesions (Bu et al., 2001), it remained possible that NG2-Cre mediated excision of Raldh2 during development might affect macrophage activity in lesions. To confirm that Raldh2 expression in OPCs and not NG2+ macrophages is required for OPC proliferation, Raldh2loxP/loxP mice were crossed with Pdgfra-creERT2 mice to generate a Pdgfra-cre/ERT2;Raldh2loxP/loxP (Pdgfra-iKO) mouse line, which allowed the conditional deletion of Raldh2 in adult OPCs by 4OHT administration prior to demyelinating injury. Cre-mediated excision of Raldh2 is not expected to occur in macrophages because they do not express PDGFRα (Bu et al., 2001). As control, 4OHT was injected into Pdgfra-CreERT2 mice. We found that, after 4OHT administration and lysolecithin-induced demyelination, the relative numbers of Olig2+ oligodendrocyte lineage cells (Figure 6D), as well as Nkx2+Olig2+ OPCs (Figure 6E) in lesions were significantly reduced, by approximately 50%, in Pdgfra-iKO mice compared to control. These results, together with our results from the analysis of NG2-cKO mice confirmed that Raldh2 expression in adult OPCs is required for OPC proliferation during remyelination. Moreover, our results suggest that RA is synthesized in OPCs intrinsically in response to injury and activates RA signaling.
Since RA is a diffusible ligand that activates RA receptor signaling, we next asked if exogenously applied RA could promote oligodendrocyte lineage cell progression in demyelinated lesions of mice lacking Raldh2 expression. To test this, we locally injected RA into demyelinated lesions of 4OHT-treated Raldh2-iKO mice and compared them to those without RA injection as controls. We found that a single dose of RA (10–5 M) significantly increased the number of Olig2+ oligodendrocyte lineage cells in lesions of Raldh2-iKO compared to un-injected mice (Figure 7A). Additionally, RA injection led to a two-fold increase in Nkx2.2+Olig2+ OPCs and CC1+Olig2+ oligodendrocytes in lesions (Figures 7B, C). We also found that OPC proliferation, as indicated by co-localized Ki67 and PDGFRα expression, appeared to be increased following RA injection. However, no statistical significance was observed between RA injected Raldh2-iKO and control lesions at 14 dpl (Figure 7D). This result may be expected, as OPC proliferation in lesions typically peaks at earlier postlesion timepoints, and Ki67 expression may already be reduced by 14 dpl. Further, the relative number of apoptotic OPCs in lesions at 14 dpl, based on Caspase3+Nkx2.2+ co-labeling, did not differ significantly between RA-injected and un-injected Raldh2-iKO mice (Figure 7E), indicating that RA injection did not influence OPC survival. These results suggest that exogenous RA rescues the deficit of oligodendrocyte lineage cell numbers in Raldh2-deficient mice.
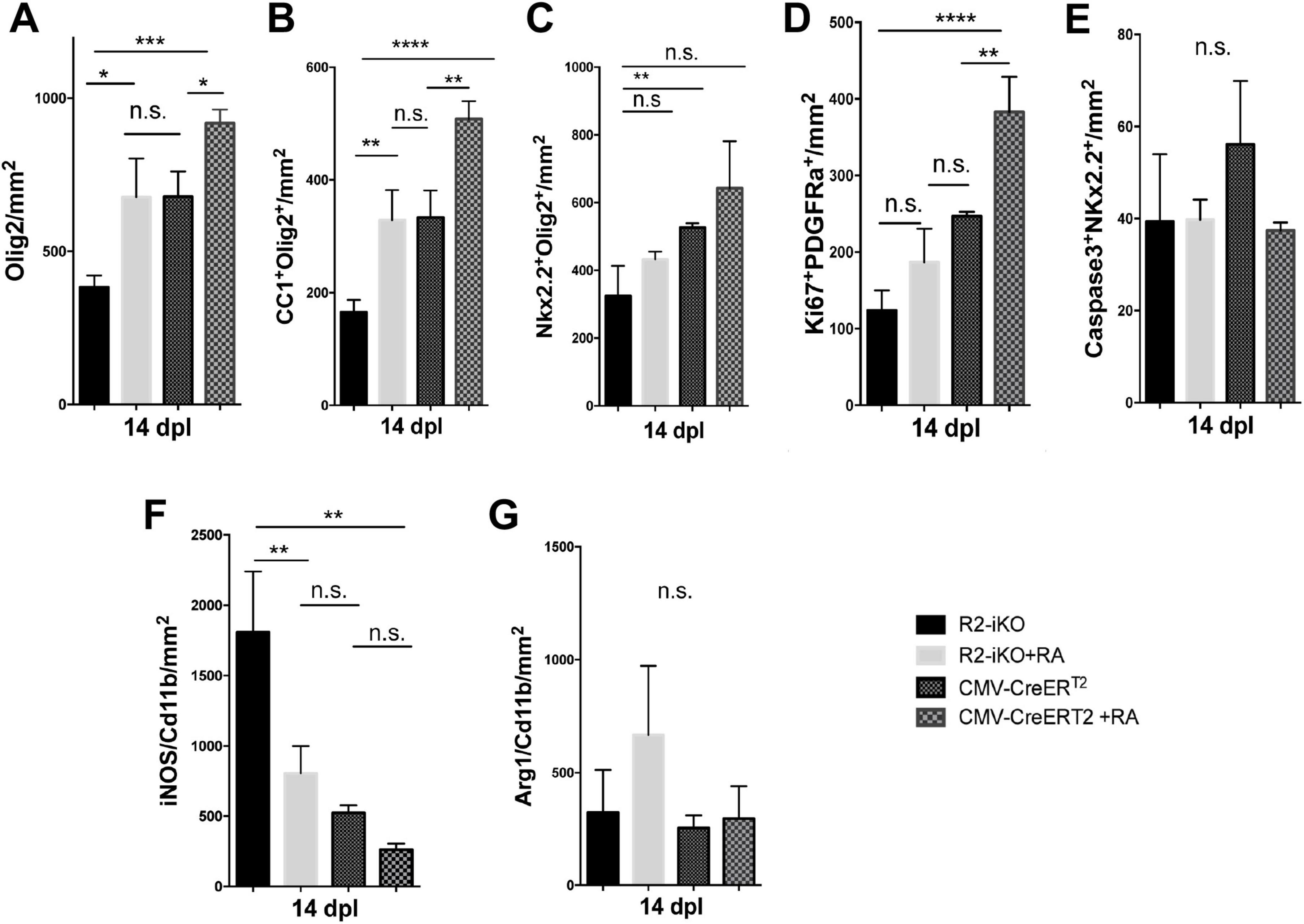
Figure 7. RA injection into demyelinated lesions increases oligodendrogenesis. Quantifications of (A) Olig2+ oligodendrocyte lineage cells, (B) CC1+Olig2+ oligodendrocytes, (C) Nkx2.2+Olig2+ OPCs, (D) Ki67+PDGFRα+ proliferating OPCs, (E) Caspase3+Nkx2.2+ apoptotic OPCs, (F) iNOS+Cd11b+ microglia/macrophages, and (G) Arg1+Cd11b+ microglia/macrophages in lesions of Raldh2-iKO (R2-iKO) and CMV-CreERT2 mice with or without RA injection at 14 dpl. One-way ANOVA with Tukey post-hoc for multiple comparisons. Data presented as mean+s.d. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, n.s., not significant.
We next examined whether RA injection could also enhance oligodendrocyte cell numbers in mice with normal Raldh2 expression. To this end, RA was injected locally into the spinal cord of CMV-CreERT2 mice, which retain both copies of Raldh2, and analyzed the effects at 14 dpl. We found that the number of Olig2+ oligodendrocyte lineage cells in lesions increased by more than 25% compared to CMV-CreERT2 mice without RA injection (Figure 7A). Moreover, RA injection significantly increased both Nkx2.2+Olig2+ OPCs and CC1+Olig2+ oligodendrocytes in lesions (Figures 7B, C). Analysis of Ki67 and PDGFRα co-labeling in lesions revealed that RA injection significantly increased the number of proliferating OPCs, even at 14 dpl (Figure 7D). However, there was no statistically significant difference in apoptotic OPCs between RA-injected and un-injected mice (Figure 7E), suggesting that exogenous RA did not enhance OPC survival. Together, these results suggest that increasing RA concentration in lesions has an additive effect on oligodendrocyte lineage cell numbers. Further, a single dose of RA in demyelinated lesions was sufficient to promote the expansion of the entire oligodendrocyte lineage cell population.
Since Raldh2 loss-of-function in adult CNS also led to increased pro-inflammatory microglia/macrophages in lesions, we next determined if RA injection into demyelinated lesions influences microglia/macrophage activation in Raldh2-iKO mice. We found that the co-injection of RA with lysolecithin into 4OHT treated Raldh2-iKO mice significantly reduced the number of iNOS+Cd11b+ microglia/macrophages in lesions compared to those without RA treatment (Figure 7F). Additionally, RA injection did not significantly affect the number of Arg1+Cd11b+ microglia/macrophages in lesions (Figure 7G). These results suggest that exogenous RA administration suppresses pro-inflammatory macrophage activation in Raldh2-deficient mice. To further assess whether increased RA concentrations influence macrophage activation, we injected RA into lesions of control CMV-CreERT2 mice. However, we found that elevated RA levels did not significantly impact the relative densities of pro-inflammatory or anti-inflammatory microglia/macrophages in lesions (Figures 7F, G). These findings suggest that while microglia/macrophages in lesions remained unresponsive to increased RA, elevated RA levels primarily promoted OPC proliferation, thereby accelerating myelin repair.
The presence of reduced but viable OPCs, and decreased oligodendrocytes in the lesions of Raldh2 deficient mice resembled the pathology of chronic MS lesions, which often display variable number of OPCs, and a severe deficiency in oligodendrocytes and remyelinated axons (Chang et al., 2000; Wolswijk, 2000). Although the cause of this pathology remains unclear, the failure to regenerate oligodendrocytes in lesions has been postulated to contribute to remyelination failure in MS (Franklin, 2002; Franklin and Ffrench-Constant, 2008). This prompted us to determine if Raldh2 expression is altered in the brain of patients with multiple sclerosis (MS). To this end, we characterized the expression of Raldh2 in microglia/macrophages and OPCs in active lesions (site of ongoing demyelination and early-stage repair), perilesions (site of later stage repair), chronic inactive lesions (site of failed repair), and normal appearing white matter (NAWM) from postmortem MS brain sections (Figure 8A). We found that Raldh2 appeared to be expressed in all KP1+ macrophages in all the lesion types examined (Figures 8B, C), suggesting that RA synthesis in microglia/macrophages was not impaired in MS. Moreover, we detected Raldh2 expression in approximately 70% of Sox10+ OPCs in NAWM and perilesion (Figures 8D, E). However, Raldh2 was detected in only approximately 35% of Sox10+ OPCs in chronic lesions, suggesting that a significant portion of OPCs in chronic lesions no longer synthesized RA. Furthermore, the total numbers of Sox10+ OPCs in chronic lesions were significantly smaller than those in NAWM and perilesions (Figures 8D, F), suggesting that the expansion of oligodendrocyte lineage cells was not achieved to the same extent as in normal tissue or active lesions. Interestingly, we also found a greater number of Sox10+ OPCs in perilesions compared to NAWM (Figure 8F). These OPCs displayed stronger Raldh2 staining intensity, and more branched morphology compared to those in the other lesion types (Figure 8G), suggesting that OPCs in perilesions might be more activated, and undergoing proliferation or differentiation. Together, these results reveal the deficiency of oligodendrocytes and remyelinated axons in chronic MS lesions may arise from failed OPC expansion, due to the loss of intrinsic Raldh2 expression.
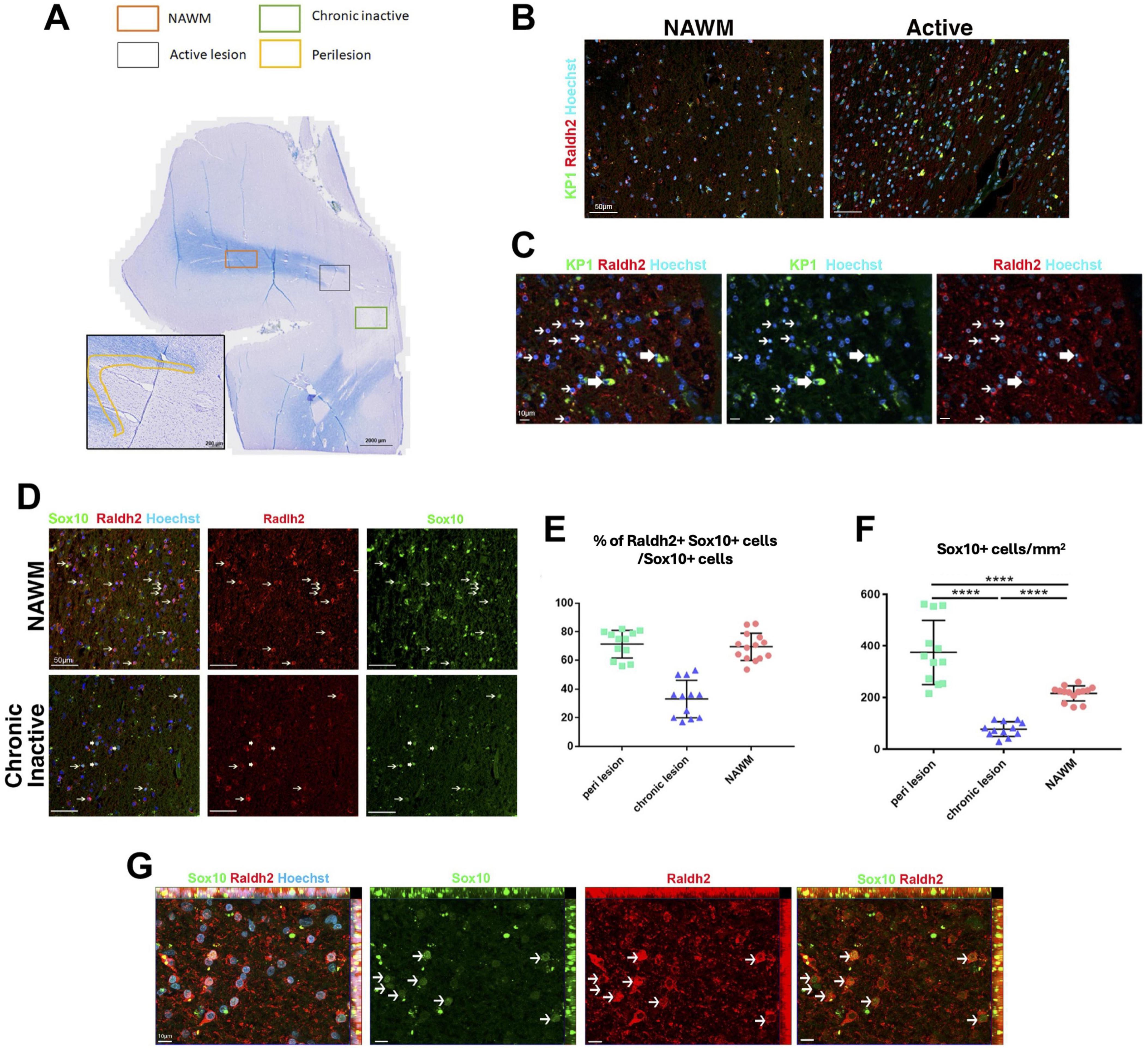
Figure 8. Raldh2 expression in MS patient brain sections. (A) Luxol fast blue staining combined with major histocompatibility complex II peroxidase immunolabeling showing chronic inactive lesion (green square), an active lesion (gray square), perilesion area (yellow highlight) and NAWM (orange square) where quantifications were performed. (B) Representative images of human samples for KP1+Raldh2+ microglia/macrophages in normal appearing white matter (NAWM) and active lesion. Scale bar, 50 μm. (C) Higher magnification of active lesions, in which thin arrows indicate Raldh2+Kp1– cells, and thick arrows indicate Raldh2+KP1+ cells. Scale bar, 10 μm. (D) Representative images of Raldh2+Sox10+ OPCs in NAWM and chronic inactive lesions. Thin arrows indicate Raldh2+Sox10+ cells, and thick arrows indicate Raldh2+Sox10– cells. Scale bar, 50 μm. (E) Percentages of Raldh2+Sox10+ OPCs among total Sox10+ cells in perilesions, chronic lesion, and NAWM. (F) Quantification of Sox10+ OPCs per mm2 within perilesions, chronic lesions, and NAWM. (G) Representative image of Raldh2+Sox10+ OPCs in perilesions. Arrows point to Raldh2+Sox10+ OPCs. Scale bar, 10 μm. One-way ANOVA and Bonferroni post-hoc test. Data presented as mean ± s.e.m. ****P < 0.0001.
We found that Raldh2 is significantly upregulated in OPCs and in a subpopulation of microglia/macrophages during remyelination. Our results suggest that local RA synthesis within lesions is crucial for driving OPC proliferation for oligodendrocyte regeneration and remyelination. Previous in vitro studies have shown that exogenous RA application to OPC or spinal cord cultures regulates oligodendrocyte lineage cell function (Barres et al., 1994; Noll and Miller, 1994), but its role in vivo remained unclear. Moreover, it was unknown if RA regulates oligodendrocyte lineage cells via a paracrine or intracrine mechanism. Our observation that Raldh2 expression in OPCs stimulates their proliferation within demyelinated lesions indicates that RA signaling in OPCs is achieved through an intracrine mechanism under a CNS injury environment. This differs from the homeostatic role of RA on the CNS, in which neural cell function is regulated through a paracrine mechanism (Aoto et al., 2008; Chen and Napoli, 2008). Since Raldh2 is expressed in oligodendrocyte lineage cells and a subset of microglia/macrophages in demyelinated lesions, this intracrine mechanism may serve as an injury response essential for promoting RA signaling within OPCs and microglia/macrophages in the lesions, while avoiding effects on cells outside the lesion. Further, given RA is a lipid soluble metabolite, additional mechanisms may be in place to prevent the RA synthesized in OPCs or microglia/macrophages from diffusing out of the cells and into non-injured tissues. It may be possible that cellular retinoic acid binding proteins or RA catabolizing enzymes are present in the OPC and microglia/macrophage cytoplasm to ensure that RA is sequestered or degraded in the cytoplasm and only active inside the nucleus, thus limiting the diffusion of RA out of cells.
The mechanisms driving Raldh2 expression in OPCs and microglia/macrophages following injury remain unclear. Several growth factors, including PDGF-A, FGF2 and LIF, are known to regulate OPC proliferation during remyelination (Hinks and Franklin, 1999; Armstrong, 2007; Deverman and Patterson, 2012). Moreover, recent studies have shown that factors released from microglia/macrophages or regulatory T-cells can influence oligodendrocyte lineage cell progression during remyelination (Miron et al., 2013; Psachoulia et al., 2016; Dombrowski et al., 2017; Baydyuk et al., 2020). Given the abundance of inflammatory cells in CNS lesions during the early stages of remyelination, inflammatory cytokines may induce Raldh2 expression within demyelinated lesions. For example, TNF-alpha has been shown to stimulate OPC proliferation during remyelination, indicating a dual role in promoting both inflammation and OPC proliferation (Arnett et al., 2001). Additionally, IL-4, which is associated with anti-inflammatory activity, has been shown to facilitate oligodendrocyte differentiation and remyelination (Psachoulia et al., 2016; Zhang et al., 2019). IL-4 is known to work synergistically with RA in macrophages to stimulate Raldh2 expression through a feedforward mechanism (Broadhurst et al., 2012; Lee et al., 2016). Whether a similar mechanism occurs in oligodendrocyte lineage cells during remyelination is still unclear.
How Raldh2 regulates oligodendrocyte remyelination remains to be determined. During vertebrate development, RA is known to interact with Sonic Hedgehog (Shh) to coordinate cell proliferation and patterning (Ogura et al., 1996; Ribes et al., 2006). In adults, Shh maintains neural stem cells and regulates oligodendrocyte development during postnatal myelination (Ahn and Joyner, 2005; Tong et al., 2015). Our recent findings indicate that Raldh2 plays a role in Shh signaling during OPC development in the mouse corpus callosum (Morrison et al., 2020). Since Shh signaling in oligodendrocyte lineage cells is critical for remyelination (Ferent et al., 2013; Samanta et al., 2015; Tong et al., 2015; Sanchez et al., 2018; Russo et al., 2024), potentially mediated through Shh co-receptors like Boc and modulation of Wnt pathways (Zakaria et al., 2019; Ming et al., 2020), it is plausible that Raldh2 influences remyelination via the Shh pathway. In support of this, a recent study demonstrated that Shh activation, through genetic activation of Smoothened (Smo) promotes OPC proliferation (Nocera et al., 2024). Additionally, Megalin (LRP2), a multifunctional endocytic receptor, is known to play a critical role in RA metabolism by influencing Shh signaling (Liu et al., 1998; Ortega et al., 2012; Brożyna et al., 2022). It has been shown that Megalin regulates Shh-induced OPC proliferation and migration during development, and this may involve transient expression of Megalin in astrocytes that facilitates Shh presentation to OPCs or regulating its gradient during development (Ortega et al., 2012). Collectively, these findings suggest the crosstalk between RA, Shh, and Megalin may be critical in regulating oligodendrocyte myelination and repair.
Additionally, we found that most Arg1+ microglia/macrophages in lesions also expressed Raldh2 during remyelination. It is well established that iNOS+ microglia/macrophages, associated with a pro-inflammatory state, decrease, while Arg1+ microglia/macrophages, linked to an anti-inflammatory state, increase during remyelination (Miron et al., 2013; Psachoulia et al., 2016; Hu et al., 2024). The role of Raldh2 in anti-inflammatory microglia/macrophages remains unclear. RA has been shown to modulate macrophage activity by suppressing inflammatory mediators, including the release of TNF-α and nitric oxide (NO) (Mathew and Sharma, 2000; Oliveira et al., 2018). Moreover, RA has been demonstrated to synergize with IL4 to activate anti-inflammatory pathways and facilitate the phenotypic transition of macrophages from a pro-inflammatory to an anti-inflammatory state (Vellozo et al., 2017; Pinos et al., 2023). The presence of Raldh2 in anti-inflammatory microglia/macrophages suggest intrinsic RA synthesis may contribute to this transition during remyelination. Further studies are needed to explore how inflammatory cytokines regulate RA synthesis and signaling in OPCs and microglia/macrophages within demyelinated lesions, which may provide deeper insight into the mechanisms of myelin repair.
Our results indicate that RA signaling is crucial for both OPC proliferation and inflammation resolution. Injection of RA into demyelinated lesions significantly increased OPC proliferation and reduced pro-inflammatory macrophage density, leading to enhanced oligodendrocyte differentiation and remyelination. Interestingly, while RA application promotes OPC proliferation, 9-cis-retinoic acid (9cRA), an RA isomer, does not affect OPCs but instead enhances oligodendrocyte differentiation (Huang et al., 2011). This suggests different RA receptors may be necessary at distinct stages of oligodendrocyte lineage cell progression. Since RA binds to RARs, and 9cRA has a preference for RXRs, it is possible that OPC proliferation in lesions is dependent on RAR signaling (Laeng et al., 1994; Kim et al., 2017), whereas oligodendrocyte differentiation is dependent on RXR signaling. Indeed, 9cRA mediated RXRγ signaling has been demonstrated to promote oligodendrocyte differentiation during remyelination (Huang et al., 2011), and this effect has been shown to be mediated through the heterodimerization of RXRγ with vitamin D receptor (VDR) (de la Fuente et al., 2015).
Interestingly, we detected Raldh2 expression in OPCs and microglia/macrophages within normal-appearing white matter (NAWM) of MS patients, in contrast to its expression pattern in the mouse CNS, where Raldh2 is mostly absent in non-lesioned tissues except in the meninges (Morrison et al., 2020). This discrepancy may be attributed to the smaller size of the mouse brain, where meningeal-derived RA can sufficiently diffuse into the parenchyma and regulate CNS homeostasis via paracrine signaling. In contrast, the larger human brain may require intrinsic RA synthesis by parenchymal cells to maintain homeostasis. However, in response to injury, local upregulation of RA within lesions may be necessary to expand oligodendrocyte lineage cells to facilitate CNS repair in a timely manner. Notably, our observation that Raldh2 expression is absent in most OPCs within chronic MS lesions, compared to its presence in active lesions and NAWM, suggests that the chronic inactive lesion environment may affect Raldh2 expression in OPCs. A failure to expand OPC populations within lesions likely results in fewer mature oligodendrocytes and insufficient remyelination, contributing to progressive neurodegeneration and disease severity. Indeed, MS patients exhibit reduced plasma retinol levels and altered RA receptor expression, linking RA availability to clinical disease activity (Royal et al., 2002).
In summary, we found that the loss of Raldh2 expression in OPCs significantly impairs their proliferation following demyelinating injury, and this defect leads to a reduction in mature oligodendrocytes in lesions. These results highlight the critical role of RA signaling in myelin repair. Therapeutic strategies targeting OPC expansion or preventing Raldh2 loss may improve remyelination in MS and slow disease progression.
We report that the induction of retinoic acid (RA) synthesis through Raldh2 in adult oligodendrocyte precursor cells (OPCs) occurs locally at the injury site and is necessary to stimulate the expansion of OPCs and mature oligodendrocytes during remyelination. Moreover, Raldh2 expression is absent in the majority of OPCs in chronic inactive lesions of multiple sclerosis (MS) patients. This work suggests that the deficiency of mature oligodendrocytes and myelinated axons in chronic MS lesions might arise from the failure to expand OPCs in lesions, and that therapeutic approaches to promote OPC proliferation by increasing RA levels in chronic demyelinated lesions might overcome myelin regenerative failure in MS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by Georgetown University: Institutional Animal Care and Use Committee (IACUC) Office. The study was conducted in accordance with the local legislation and institutional requirements.
SN: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. NW: Data curation, Writing – review and editing. LR: Data curation, Writing – review and editing. DC: Data curation, Writing – review and editing. MK: Data curation, Writing – review and editing. RO: Data curation, Writing – review and editing. JR: Data curation, Writing – review and editing. MB: Data curation, Writing – review and editing. AD: Data curation, Writing – review and editing. WK: Resources, Writing – review and editing. VZ: Data curation, Formal analysis, Investigation, Resources, Writing – review and editing. JH: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This project was supported by grants from National Multiple Sclerosis Society (G-1508-05906 and JF-1806-31381), and National Institutes of Health (R21NS091890, 5R01NS107523, and 2R56NS107523) to JH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahn, S., and Joyner, A. L. (2005). In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 437, 894–897. doi: 10.1038/nature03994
Aoto, J., Nam, C. I., Poon, M. M., Ting, P., and Chen, L. (2008). Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 60, 308–320. doi: 10.1016/j.neuron.2008.08.012
Armstrong, R. C. (2007). Growth factor regulation of remyelination: Behind the growing interest in endogenous cell repair of the CNS. Future Neurol. 2, 689–697. doi: 10.2217/14796708.2.6.689
Arnett, H. A., Mason, J., Marino, M., Suzuki, K., Matsushima, G. K., and Ting, J. P. (2001). TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4, 1116–1122. doi: 10.1038/nn738
Barres, B. A., Lazar, M. A., and Raff, M. C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108.
Baydyuk, M., Cha, D. S., Hu, J., Yamazaki, R., Miller, E. M., Smith, V. N., et al. (2019). Tracking the evolution of CNS remyelinating lesion in mice with neutral red dye. Proc. Natl. Acad. Sci. U.S.A. 116, 14290–14299. doi: 10.1073/pnas.1819343116
Baydyuk, M., Morrison, V. E., Gross, P. S., and Huang, J. K. (2020). Extrinsic factors driving oligodendrocyte lineage cell progression in CNS development and injury. Neurochem. Res. 45, 630–642. doi: 10.1007/s11064-020-02967-7
Boyd, A., Zhang, H., and Williams, A. (2013). Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 125, 841–859. doi: 10.1007/s00401-013-1112-y
Broadhurst, M. J., Leung, J. M., Lim, K. C., Girgis, N. M., Gundra, U. M., Fallon, P. G., et al. (2012). Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog. 8:e1002883. doi: 10.1371/journal.ppat.1002883
Brożyna, A. A., Żmijewski, M. A., Linowiecka, K., Kim, T.-K., Slominski, R. M., and Slominski, A. T. (2022). Disturbed expression of vitamin D and retinoic acid-related orphan receptors α and γ and of megalin in inflammatory skin diseases. Exp. Dermatol. 31, 781–788. doi: 10.1111/exd.14521
Bu, J., Akhtar, N., and Nishiyama, A. (2001). Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia 34, 296–310.
Chang, A., Nishiyama, A., Peterson, J., Prineas, J., and Trapp, B. D. (2000). NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20, 6404–6412.
Chen, N., and Napoli, J. L. (2008). All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 22, 236–245. doi: 10.1096/fj.07-8739com
Compston, A., and Coles, A. (2008). Multiple sclerosis. Lancet 372, 1502–1517. doi: 10.1016/S0140-6736(08)61620-7
de la Fuente, A. G., Errea, O., van Wijngaarden, P., Gonzalez, G. A., Kerninon, C., Jarjour, A. A., et al. (2015). Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol. 211, 975–985. doi: 10.1083/jcb.201505119
Deverman, B. E., and Patterson, P. H. (2012). Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J. Neurosci. 32, 2100–2109. doi: 10.1523/JNEUROSCI.3803-11.2012
Dombrowski, Y., O’Hagan, T., Dittmer, M., Penalva, R., Mayoral, S. R., Bankhead, P., et al. (2017). Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680. doi: 10.1038/nn.4528
Duester, G. (2013). Retinoid signaling in control of progenitor cell differentiation during mouse development. Semin. Cell Dev. Biol. 24, 694–700. doi: 10.1016/j.semcdb.2013.08.001
Dutta, R., and Trapp, B. D. (2014). Relapsing and progressive forms of multiple sclerosis: Insights from pathology. Curr. Opin. Neurol. 27, 271–278. doi: 10.1097/WCO.0000000000000094
Ferent, J., Zimmer, C., Durbec, P., Ruat, M., and Traiffort, E. (2013). Sonic hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J. Neurosci. 33, 1759–1772. doi: 10.1523/JNEUROSCI.3334-12.2013
Franklin, R. J. M. (2002). Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3, 705–714. doi: 10.1038/nrn917
Franklin, R. J. M., and Ffrench-Constant, C. (2008). Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 9, 839–855. doi: 10.1038/nrn2480
Goncalves, M. B., Wu, Y., Trigo, D., Clarke, E., Malmqvist, T., Grist, J., et al. (2018). Retinoic acid synthesis by NG2 expressing cells promotes a permissive environment for axonal outgrowth. Neurobiol. Dis. 111, 70–79. doi: 10.1016/j.nbd.2017.12.016
Hagemeier, K., Brück, W., and Kuhlmann, T. (2012). Multiple sclerosis - Remyelination failure as a cause of disease progression. Histol. Histopathol. 27, 277–287.
Haushalter, C., Schuhbaur, B., Dollé, P., and Rhinn, M. (2017). Meningeal retinoic acid contributes to neocortical lamination and radial migration during mouse brain development. Biol. Open 6, 148–160. doi: 10.1242/bio.021063
Hinks, G. L., and Franklin, R. J. (1999). Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol. Cell. Neurosci. 14, 153–168. doi: 10.1006/mcne.1999.0771
Hu, J., Melchor, G. S., Ladakis, D., Reger, J., Kim, H. W., Chamberlain, K. A., et al. (2024). Myeloid cell-associated aromatic amino acid metabolism facilitates CNS myelin regeneration. NPJ Regen Med 9:1. doi: 10.1038/s41536-023-00345-9
Huang, J. K., Jarjour, A. A., Nait Oumesmar, B., Kerninon, C., Williams, A., Krezel, W., et al. (2011). Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53. doi: 10.1038/nn.2702
Jeffery, N. D., and Blakemore, W. F. (1995). Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J. Neurocytol. 24, 775–781.
Kim, S. Y., Kelland, E. E., Kim, J. H., Lund, B. T., Chang, X., Wang, K., et al. (2017). The influence of retinoic acid on the human oligodendrocyte precursor cells by RNA-sequencing. Biochem. Biophys. Rep. 9, 166–172. doi: 10.1016/j.bbrep.2016.12.004
Kumar, S., and Duester, G. (2011). SnapShot: Retinoic acid signaling. Cell 147, 1422–1422.e1. doi: 10.1016/j.cell.2011.11.034
Laeng, P., Décimo, D., Pettmann, B., Janet, T., and Labourdette, G. (1994). Retinoic acid regulates the development of oligodendrocyte precursor cells in vitro. J. Neurosci. Res. 39, 613–633. doi: 10.1002/jnr.490390602
Lassmann, H., van Horssen, J., and Mahad, D. (2012). Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 8, 647–656. doi: 10.1038/nrneurol.2012.168
Lee, B., Wu, C.-Y., Lin, Y.-W., Park, S. W., and Wei, L.-N. (2016). Synergistic activation of Arg1 gene by retinoic acid and IL-4 involves chromatin remodeling for transcription initiation and elongation coupling. Nucleic Acids Res. 44, 7568–7579. doi: 10.1093/nar/gkw392
Li, H., Wagner, E., McCaffery, P., Smith, D., Andreadis, A., and Dräger, U. C. (2000). A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech. Dev. 95, 283–289.
Liu, W., Yu, W. R., Carling, T., Juhlin, C., Rastad, J., Ridefelt, P., et al. (1998). Regulation of gp330/megalin expression by vitamins A and D. Eur. J. Clin. Invest. 28, 100–107. doi: 10.1046/j.1365-2362.1998.00253.x
Maden, M. (2007). Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 8, 755–765. doi: 10.1038/nrn2212
Maden, M., and Hind, M. (2003). Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 226, 237–244. doi: 10.1002/dvdy.10222
Mathew, J. S., and Sharma, R. P. (2000). Effect of all-trans-retinoic acid on cytokine production in a murine macrophage cell line. Int. J. Immunopharmacol. 22, 693–706. doi: 10.1016/S0192-0561(00)00032-1
Mey, J., Morassutti, D., Brook, G., Liu, R.-H., Zhang, Y.-P., Koopmans, G., et al. (2005). Retinoic acid synthesis by a population of NG2-positive cells in the injured spinal cord. Eur. J. Neurosci. 21, 1555–1568. doi: 10.1111/j.1460-9568.2005.03928.x
Mic, F. A., Haselbeck, R. J., Cuenca, A. E., and Duester, G. (2002). Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129, 2271–2282.
Ming, X., Dupree, J. L., Gallo, V., and Chew, L.-J. (2020). Sox17 promotes oligodendrocyte regeneration by dual modulation of hedgehog and Wnt signaling. iScience 23:101592. doi: 10.1016/j.isci.2020.101592
Miron, V. E., Boyd, A., Zhao, J.-W., Yuen, T. J., Ruckh, J. M., Shadrach, J. L., et al. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218. doi: 10.1038/nn.3469
Morrison, V. E., Smith, V. N., and Huang, J. K. (2020). Retinoic acid is required for oligodendrocyte precursor cell production and differentiation in the postnatal mouse corpus callosum. eNeuro 7, doi: 10.1523/ENEURO.0270-19.2019
Niederreither, K., Subbarayan, V., Dollé, P., and Chambon, P. (1999). Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448. doi: 10.1038/7788
Nocera, S., Marchena, M. A., Fernández-Gómez, B., Gómez-Martín, P., Sánchez-Jiménez, E., Macías-Castellano, A., et al. (2024). Activation of Shh/Smo is sufficient to maintain oligodendrocyte precursor cells in an undifferentiated state and is not necessary for myelin formation and (re)myelination. Glia 72, 1469–1483. doi: 10.1002/glia.24540
Noll, E., and Miller, R. H. (1994). Regulation of oligodendrocyte differentiation: A role for retinoic acid in the spinal cord. Development 120, 649–660.
Ogura, T., Alvarez, I. S., Vogel, A., Rodríguez, C., Evans, R. M., and Izpisúa Belmonte, J. C. (1996). Evidence that Shh cooperates with a retinoic acid inducible co-factor to establish ZPA-like activity. Development 122, 537–542. doi: 10.1242/dev.122.2.537
Oliveira, L. M., Teixeira, F. M. E., and Sato, M. N. (2018). Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflammation 2018:3067126. doi: 10.1155/2018/3067126
Ortega, M. C., Cases, O., Merchán, P., Kozyraki, R., Clemente, D., and de Castro, F. (2012). Megalin mediates the influence of sonic hedgehog on oligodendrocyte precursor cell migration and proliferation during development. Glia 60, 851–866. doi: 10.1002/glia.22316
Pinos, I., Yu, J., Pilli, N., Kane, M. A., and Amengual, J. (2023). Functional characterization of interleukin 4 and retinoic acid signaling crosstalk during alternative macrophage activation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1868:159291. doi: 10.1016/j.bbalip.2023.159291
Pringle, N. P., Mudhar, H. S., Collarini, E. J., and Richardson, W. D. (1992). PDGF receptors in the rat CNS: During late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115, 535–551.
Psachoulia, K., Chamberlain, K. A., Heo, D., Davis, S. E., Paskus, J. D., Nanescu, S. E., et al. (2016). IL4I1 augments CNS remyelination and axonal protection by modulating T cell driven inflammation. Brain 139, 3121–3136. doi: 10.1093/brain/aww254
Reich, D. S., Lucchinetti, C. F., and Calabresi, P. A. (2018). Multiple sclerosis. N. Engl. J. Med. 378, 169–180. doi: 10.1056/NEJMra1401483
Ribes, V., Wang, Z., Dollé, P., and Niederreither, K. (2006). Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development 133, 351–361. doi: 10.1242/dev.02204
Rossant, J., Zirngibl, R., Cado, D., Shago, M., and Giguère, V. (1991). Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 5, 1333–1344.
Royal, W., Gartner, S., and Gajewski, C. D. (2002). Retinol measurements and retinoid receptor gene expression in patients with multiple sclerosis. Mult. Scler. 8, 452–458. doi: 10.1191/1352458502ms858oa
Russo, M., Zahaf, A., Kassoussi, A., Sharif, A., Faure, H., Traiffort, E., et al. (2024). Sonic hedgehog is an early oligodendrocyte marker during remyelination. Cells 13:1808. doi: 10.3390/cells13211808
Samanta, J., Grund, E. M., Silva, H. M., Lafaille, J. J., Fishell, G., and Salzer, J. L. (2015). Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 526, 448–452. doi: 10.1038/nature14957
Sanchez, M. A., Sullivan, G. M., and Armstrong, R. C. (2018). Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination. Neurobiol. Dis. 115, 145–156. doi: 10.1016/j.nbd.2018.04.003
Siegenthaler, J. A., Ashique, A. M., Zarbalis, K., Patterson, K. P., Hecht, J. H., Kane, M. A., et al. (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597–609. doi: 10.1016/j.cell.2009.10.004
Tong, C. K., Fuentealba, L. C., Shah, J. K., Lindquist, R. A., Ihrie, R. A., Guinto, C. D., et al. (2015). A Dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Rep. 5, 461–470. doi: 10.1016/j.stemcr.2015.08.013
Trapp, B. D., and Nave, K.-A. (2008). Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269. doi: 10.1146/annurev.neuro.30.051606.094313
Vellozo, N. S., Pereira-Marques, S. T., Cabral-Piccin, M. P., Filardy, A. A., Ribeiro-Gomes, F. L., Rigoni, T. S., et al. (2017). All-trans retinoic acid promotes an M1- to M2-phenotype shift and inhibits macrophage-mediated immunity to Leishmania major. Front. Immunol. 8:1560. doi: 10.3389/fimmu.2017.01560
Wagner, E., Luo, T., and Dräger, U. C. (2002). Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb. Cortex 12, 1244–1253. doi: 10.1093/cercor/12.12.1244
Wolswijk, G. (1998). Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609.
Wolswijk, G. (2000). Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain 123, 105–115.
Zakaria, M., Ferent, J., Hristovska, I., Laouarem, Y., Zahaf, A., Kassoussi, A., et al. (2019). The Shh receptor Boc is important for myelin formation and repair. Development 146:dev172502. doi: 10.1242/dev.172502
Zhang, J., Smith, D., Yamamoto, M., Ma, L., and McCaffery, P. (2003). The meninges is a source of retinoic acid for the late-developing hindbrain. J. Neurosci. 23, 7610–7620.
Zhang, Q., Zhu, W., Xu, F., Dai, X., Shi, L., Cai, W., et al. (2019). The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 17:e3000330. doi: 10.1371/journal.pbio.3000330
Keywords: remyelination, oligodendrocyte progenitor cell (OPCs), microglia, multiple sclerosis, retinoic acid, Raldh2 (Aldh1a2)
Citation: Nanescu SE, Wathieu NM, Rosko L, Cha DS, Kumar MN, Olszewski RT, Reger J, Baydyuk M, Dua AN, Krezel W, Zujovic V and Huang JK (2025) Intrinsic retinoic acid synthesis is required for oligodendrocyte progenitor expansion during CNS remyelination. Front. Cell. Neurosci. 19:1550139. doi: 10.3389/fncel.2025.1550139
Received: 22 December 2024; Accepted: 06 February 2025;
Published: 24 February 2025.
Edited by:
Wensheng Lin, University of Minnesota Twin Cities, United StatesReviewed by:
Fernando de Castro, Spanish National Research Council (CSIC), SpainCopyright © 2025 Nanescu, Wathieu, Rosko, Cha, Kumar, Olszewski, Reger, Baydyuk, Dua, Krezel, Zujovic and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey K. Huang, amVmZnJleS5odWFuZ0BnZW9yZ2V0b3duLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.