
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Cell. Neurosci. , 05 June 2024
Sec. Cellular Neuropathology
Volume 18 - 2024 | https://doi.org/10.3389/fncel.2024.1414704
This article is a correction to:
TAT-HSP27 Peptide Improves Neurologic Deficits via Reducing Apoptosis After Experimental Subarachnoid Hemorrhage
 Xiao-yan Zhou1,2,3,4†
Xiao-yan Zhou1,2,3,4† Jing-yi Sun5†
Jing-yi Sun5† Wei-qi Wang6†
Wei-qi Wang6† Shu-xian Li7†
Shu-xian Li7† Han-xia Li7
Han-xia Li7 Hui-juan Yang7
Hui-juan Yang7 Ming-feng Yang7
Ming-feng Yang7 Hui Yuan7
Hui Yuan7 Zong-yong Zhang6,7*
Zong-yong Zhang6,7* Bao-liang Sun7*
Bao-liang Sun7* Jin-Xiang Han2,3,4*
Jin-Xiang Han2,3,4*A corrigendum on
TAT-HSP27 Peptide Improves Neurologic Deficits via Reducing Apoptosis After Experimental Subarachnoid Hemorrhage
by Zhou, X.-y., Sun, J.-y., Wang, W.-q., Li, S.-x., Li, H.-x., Yang, H.-j., Yang, M.-f., Yuan, H., Zhang, Z.-y., Sun, B.-l., and Han, J.-X. (2022). Front. Cell. Neurosci. 16:878673. doi: 10.3389/fncel.2022.878673
In the published article, there was an error in the images of Figure 7D and Figure 8A as published, where in Figure 7D the incorrect phase contrast image of 31-60 group was used and in Figure 8A the brain picture of vehicle group was incorrect.
The corrected Figure 7D and Figure 8A appear in Figure 7 and Figure 8 below.
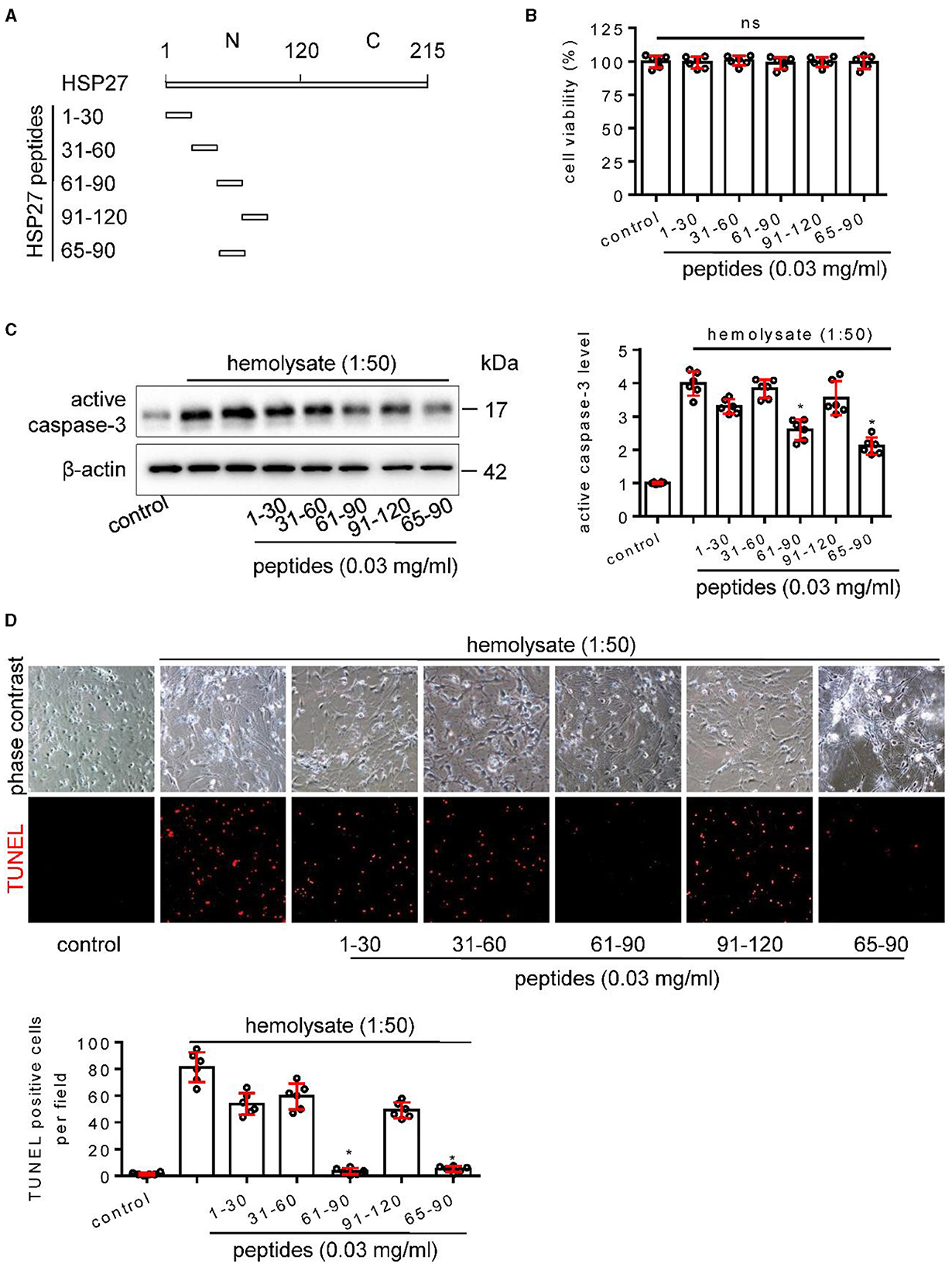
Figure 7. Effect of HSP27 peptides on hemolysate-induced cell apoptosis in primary cortical neurons. (A) Schematic representation of various HSP27 peptides. (B) Hsp27 peptides have no effect on cell viability in primary cortical neurons; Cortical neurons were treated with indicated HSP27 peptides (0.03 mg/ml) in medium (1:50) for 24 h. Cell viability was measured with Cell Counting Kit-8 (CCK-8) and normalized to control. (C, D) Cortical neurons were treated with hemolysate in medium (1:50) or plus indicated HSP27 peptides (0.03 mg/ml) for 24 h. (C) Active caspase-3 levels in each group were detected by Western blot, β-actin serves as a control, and quantification of optical density was normalized to control. (D) Representative images of cortical neurons (phase contrast, ×200) and TUNEL staining (red, ×200), and quantification of TUNEL-positive cells from each group was performed. Data are mean ± SD, n = 6, *p < 0.05 vs. hemolysate treatment, ANOVA with Bonferroni's multiple comparisons test.
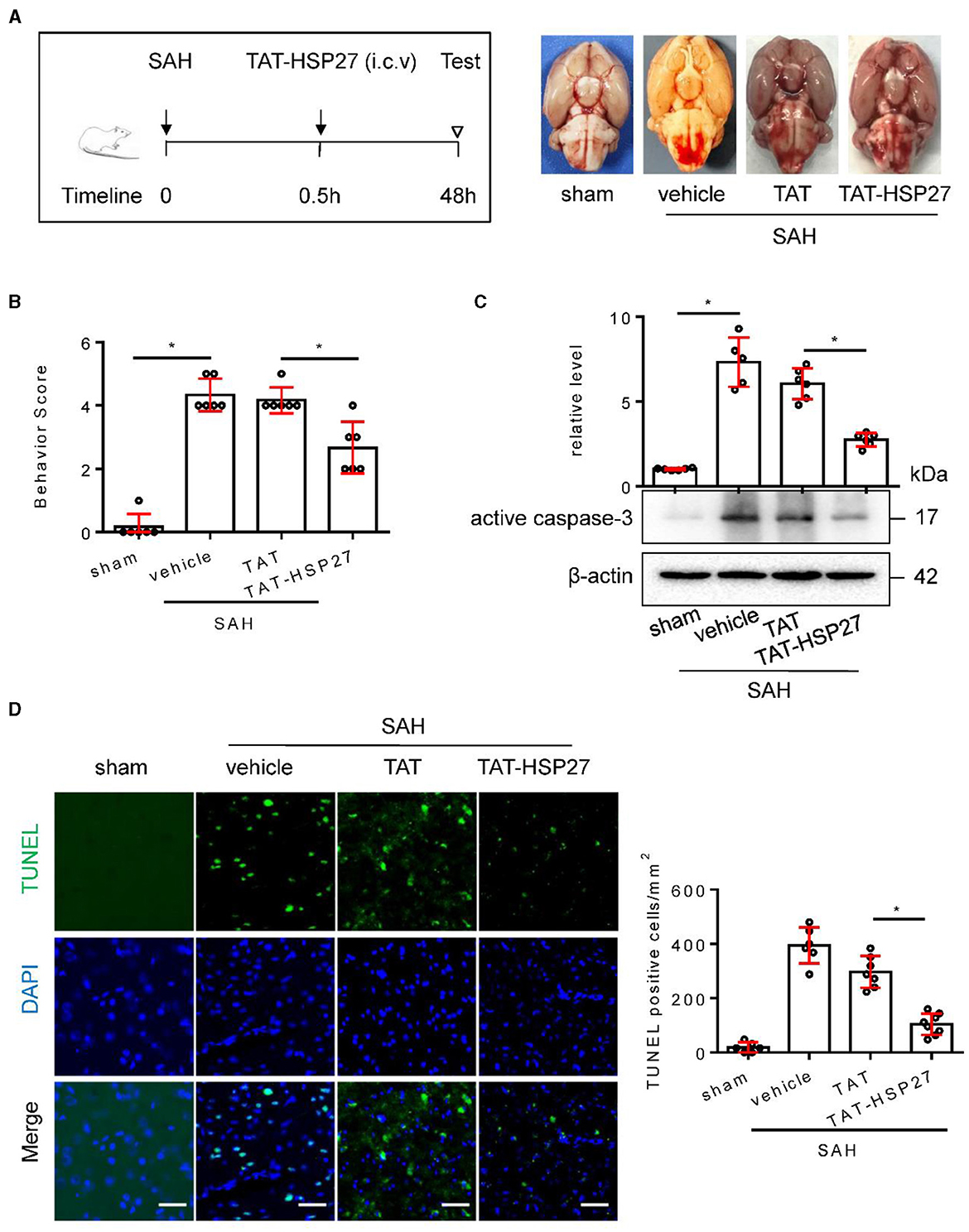
Figure 8. TAT- HSP2765 − 90 peptide attenuated neurological deficits and cell apoptosis after SAH in rats. (A) Experimental design; representative images of rat brain on day 2 after surgery for the sham, SAH + vehicle, SAH + TAT peptide (SAH + TAT), and SAH + TAT-HSP2765 − 90 peptide (SAH + TAT-HSP27) groups. (B) Behavior scores of each group were assessed at 48 h, n = 6. (C) The basal cortex was collected on day 2 following SAH from the sham (n = 6), SAH + vehicle (n = 5), SAH + TAT (n = 6), and SAH + TAT-HSP27 (n = 6) groups; homogenates were blotted with anti-active caspase-3 and anti-β-actin, and quantification of optical density was normalized to sham group. (D) Coronal sections from the sham (n = 6), SAH + vehicle (n = 6), SAH + TAT (n = 7), and SAH + TAT-HSP27 (n = 8) group reperfusion on day 2 after SAH, subjected to immunostaining for the TUNEL (green) in the basal cortex. Quantification was performed by counting the TUNEL positive cells per mm2 region in the basal cortex, scale bar = 50 μm. Data are mean ± SD, *p < 0.05 vs. hemolysate treatment, ANOVA with Bonferroni's multiple comparisons test.
The authors apologize for these errors and state that they do not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: subarachnoid hemorrhage, HSP27, cell apoptosis, neurologic deficits, TAT-HSP2765 − 90 peptide
Citation: Zhou X-y, Sun J-y, Wang W-q, Li S-x, Li H-x, Yang H-j, Yang M-f, Yuan H, Zhang Z-y, Sun B-l and Han J-X (2024) Corrigendum: TAT-HSP27 peptide improves neurologic deficits via reducing apoptosis after experimental subarachnoid hemorrhage. Front. Cell. Neurosci. 18:1414704. doi: 10.3389/fncel.2024.1414704
Received: 09 April 2024; Accepted: 22 May 2024;
Published: 05 June 2024.
Edited and reviewed by: Dirk M. Hermann, University of Duisburg-Essen, Germany
Copyright © 2024 Zhou, Sun, Wang, Li, Li, Yang, Yang, Yuan, Zhang, Sun and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zong-yong Zhang, enl6aGFuZ0BzZGZtdS5lZHUuY24=; Bao-liang Sun, YmxzdW44OEAxNjMuY29t; Jin-Xiang Han, c2Ftc2hqeEBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.