- 1Roche Diagnostics International AG, Rotkreuz, Switzerland
- 2Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
- 3Mental Health Research Centre, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
Introduction
The glutamate hypofunction hypothesis of schizophrenia (Carlsson and Carlsson, 1990; Olney and Farber, 1995; Olney et al., 1999; Coyle, 2006; Moghaddam and Javitt, 2012; Coyle et al., 2020) has been highly influential in the search of novel drugs for the treatment of negative and cognitive schizophrenia symptoms—which current antipsychotic drugs cannot meet. Both metabotropic (e.g., mGluR1, mGluR5) and ionotropic glutamate receptors (namely, NMDARs) have been targeted (Javitt, 2004; Moghaddam, 2004; Maksymetz et al., 2017; Pei et al., 2021; Dogra and Conn, 2022). Reports that schizophrenia negative and cognitive symptoms could be improved by adjunctive treatment of glycine and sarcosine (Javitt et al., 1994; Heresco-Levy et al., 1996a,b, 1999; Tsai et al., 2004; Lane et al., 2010; Lin et al., 2017) had led to the proliferation of synthetic compounds designed to block the reuptake of glycine via glycine transporters (Harvey and Yee, 2013; Singer et al., 2015). This is predicted to boost glutamatergic signaling at NMDARs and thereby alleviate symptoms according to the glutamate hypofunction hypothesis of schizophrenia. The hypothesis is based on: (i) Occupancy of the glycine (strychnine-insensitive) binding site in the NMDA receptor (also known as glycine-B site), by glycine or D-serine, is required for NMDAR channel activation by glutamate, and (ii) Glycine-B site occupancy is normally maintained at sub-saturating levels by removal of extracellular glycine in the vicinity of the synaptic cleft through active glycine reuptake. Thus, elevation of extracellular glycine by blocking its reuptake should effectively enhance impulse-dependent NMDAR currents. To minimize interference of inhibitory neurotransmission at glycinergic synapses mediated by strychnine-sensitive glycine receptors (Gomeza et al., 2003), drug development has primarily focused on inhibitors specific for glycine transporter 1 (GlyT1) to avoid blockade of glycine transporter 2 (GlyT2) (see Harvey and Yee, 2013). Indeed, there is a noticeable absence of published human studies on GlyT2 inhibitors (Schmidt and Thompson, 2016).
Several synthetic selective GlyT1 inhibitors had displayed promising outcomes in preclinical studies during the 1990s and 2000s, but none of them could advance to the bedside due to poor efficacy in subsequent clinical trials (Singer et al., 2015; Cioffi, 2018; Zakowicz and Pawlak, 2022). Bitopertin, developed by Hoffman-La Roche, had reached phase III trials, following highly encouraging phase II results (Umbricht et al., 2014; Bugarski-Kirola et al., 2017; Kantrowitz et al., 2017; Pinard et al., 2018). However, the multi-center trials had ended with disappointment and termination of the drug's development as a potential new generation of adjunctive antipsychotic medication (Singer et al., 2015; Zakowicz and Pawlak, 2022). At the time of writing, Iclepertin (BI 425809), developed by Boehringer Ingelheim (Fleischhacker et al., 2021; Rosenbrock et al., 2023), remains the only other GlyT1 inhibitor currently being evaluated at phase III (NCT04846868, NCT04846881) as an adjuvant treatment to improve cognitive functioning in schizophrenia. The outcomes of these trials are expected in 2025.
Based on behavioral phenotyping of two mouse lines with conditional GlyT1 disruption, we have previously suggested that the behavioral effects of GlyT1 inhibition are critically dependent on cell type and brain region (Möhler et al., 2011; Singer et al., 2015). We predicted that such dependency could pose a major roadblock in drug development. Divergent antipsychotic and pro-cognitive phenotypes have been reported between mutant mice lacking GlyT1 in forebrain (the cerebral cortex and striatum) neurons and mice lacking GlyT1 in both neurons and astrocytes throughout the telencephalon (Möhler et al., 2011; Singer et al., 2015). Critically, NMDAR currents in the hippocampus were enhanced when GlyT1 was restricted to forebrain neurons (Yee et al., 2006), but not when the deletion was extended to astrocytes (Singer et al., 2009). The additional deletion of glial GlyT1 in the hippocampus apparently nullified the pro-NMDAR effects seen after selective neuronal GlyT1 deletion. A similar impression is also apparent when the behavioral phenotypes between the two mutant mouse lines are compared. These outcomes led us to suggest that the therapeutic potential of systemic, brain wide GlyT1 inhibition would likely be limited and inconsistent. The scant clinical data available had also pointed to an impression of “more means less,” of which the developers of bitopertin were certainly aware. They already emphasized the need for careful dose titration after phase 2 trials and proposed that a moderate level of GlyT1 occupancy at around 50% is desirable for achieving the strongest clinical effect (Umbricht et al., 2014). To this end, efforts have been made to develop radio ligands for personalized dose determination. However, we believe that dose titration alone is not sufficient to mimic the critical cell-type and regional specificity of GlyT1 blockade, which we speculate is also a critical determinant for a GlyT1 blocker's antipsychotic potential—efficacy against both positive and negative symptoms.
Here, we attempt to explain some of the roadblocks above and speculate how the neuropharmacological profile of GlyT1-inhibiting drugs may be critically determined by its concomitant regulation of astrocytic GlyT1 activity. The speculative model takes into account evidence for neuron-glial cross talk in the regulation of the synthesis and tracking of glycine as well as D-serine, the two major endogenous obligatory co-agonists at the glycine-B site of NMDARs.
Hypothesis 1: – Disruption of the serine shuttle by astrocytic GlyT1 blockade can impair NMDAR signaling.
The availability of glycine and D-serine at NMDAR-containing glutamatergic synapses is tightly regulated by the surrounding astrocytes (Snyder and Kim, 2000; Betz et al., 2006; Haydon and Carmignoto, 2006; Wolosker, 2011; Shibasaki et al., 2017). One regulatory mechanism depends on the collaborative metabolic interaction between astrocytes and neurons, known as “serine shuttle” (Wolosker and Radzishevsky, 2013). By altering the equilibrium of glycine and serine metabolism in neurons and astrocytes, inhibition of GlyT1 is expected to interfere with the regulatory function of the serine shuttle as depicted in Figure 1.
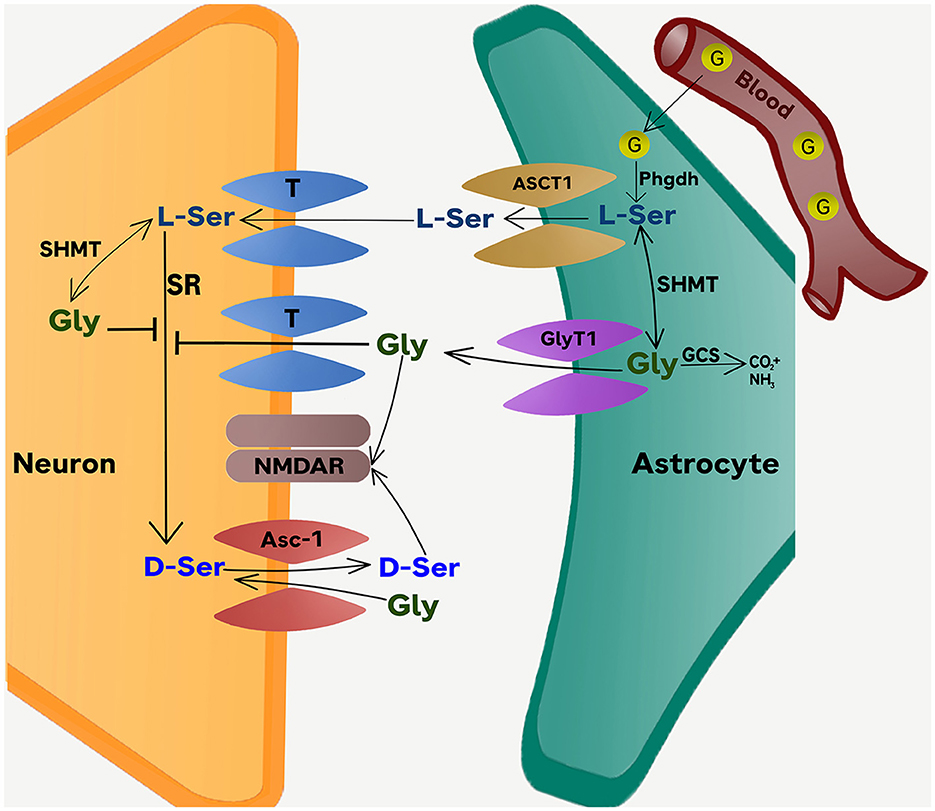
Figure 1. The Phgdh-dependent serine shuttle mechanism. The scheme depicts the conversion of glucose into L-serine in astrocytes and the role of Phgdh-derived L-serine in providing D-serine and glycine to activate synaptic NMDARs. The model also illustrates the dual effect of glycine on D-serine metabolism. The first is the direct inhibition of the neuronal SR by glycine. The second is the transient increase in D-serine release through a Gly/D-Ser exchange catalyzed by the Asc-1 transporter. Serine and glycine are released from astrocytes through ASCT1 and GlyT1 operating in reverse mode (also see Figure 2). Under pharmacological blockade of GlyT1, the primary pool of extracellular glycine is increased as the reuptake of glycine is stopped. In this situation, any additional production of glycine from L-Serine is unlikely to have a significant impact on the already elevated levels of extracellular glycine. At the same time, D-serine which originates from the conversion of L-serine in neurons, becomes a crucial source of D-serine for binding to the glycine-B site. The net effect of GlyT1 inhibition therefore effectively reduces the neuronal production and release of D-serine into the synapse. The disruption in the serine shuttle is expected to undermine, rather than enhance, the excitability of NMDAR at glutamatergic synapses (as determined by brain regions or age), where D-serine acts at the primary co-agonist of NMDAR activation at the glycine-B site. The distribution of NMDAR sites that are more dependent on D-serine than glycine likely varies across brain regions and is modified by other factors such as age and experience. ASCT1, Amino acid transporter (SLC1A4); Asc-1 transporter, alanine-serine-cysteine transporter (SLC7A10); D-Ser, D-serine; G, glucose; Gly, glycine; GCS, glycine cleavage system, a.k.a. the glycine decarboxylase complex or GDC; GlyT1, Glycine transporter 1 (SLC6A9); L-Ser, L-serine; Phgdh, Phosphoglycerate dehydrogenase; SHMT, Serine hydroxymethyltransferase; SR, serine racemase. T, Various types of transporters contributing to the up-take of glycine and L-serine into neurons from the synaptic cleft.
Raising extracellular glycine levels has been shown to reduce extracellular D-serine concentration in vivo indicating that glycine can modify D-serine metabolism (Neame et al., 2019). On the other hand, blocking glycine reuptake into astrocytes via GlyT1 effectively removes a major source of glycine. To compensate for the ensuing fall in intracellular glycine, the conversion of L-serine to glycine catalyzed by serine hydroxymethyltransferase (SHMT) would rise. The resulting astrocytic L-serine deficit would in turn limit the shuttling of L-serine into neighboring neurons (Figure 1), where L-serine is converted to D-serine and glycine. According to this model, curtailing the L-serine shuttle (astrocytes → neurons) is expected to lower the occupancy of glycine-B sites (at NMDARs) due to a fall in D-serine releasable by neurons into the synaptic cleft. The excitability of NMDARs is therefore predicted to diminish rather than enhance. The impact would be the largest where glycine-B site occupancy is critically determined by the synaptic pool of D-serine, which serves as the primary obligatory co-agonist at the NMDARs and thus can effectively regulate NMDAR excitability.
Hypothesis 2 – Extracellular release of glycine via astrocytic GlyT1 can positively modulate NMDAR excitability.
Astrocytes are a major pool of glycine in the brain. Besides glycine re-uptake from extracellular space, another source of astrocytic glycine depends on the conversion of glucose obtained from the blood to L-serine by phosphoglycerate dehydrogenase (Phgdh) and the subsequent conversion of L-serine to glycine by SHMT (which also takes place in neurons) (see Figure 1). In neurons, L-serine is also converted to D-serine by serine racemase (SR). Disruption of this pathway is expected to deprive a major source of releasable D-serine in the synaptic space and therefore reduce glycine-B site occupancy at NMDARs. Yet, NMDAR-mediated signaling appears normal in mice lacking SR with reports of intact NMDAR fast EPSPs and EPSCs (Basu et al., 2009; Li et al., 2013; Rosenberg et al., 2013; Neame et al., 2019). It follows that there is a sufficient baseline level of extracellular glycine supported by alternative mechanisms to maintain near-normal glycine-B site occupancy in the NMDARs of SR-null mice. Moreover, it has been shown that the synthesis of glycine by PHGDH in astrocytes can be critical. Significant impairments in NMDAR-mediated neurotransmission are apparent in SR-null mice when PHGDH activity was suppressed (Neame et al., 2019). It follows that disruption in the release of glycine from astrocytes can influence the excitability of synaptic NMDARs.
It is now known that GlyT1 also mediates the flow of glycine from astrocytes into extracellular space by operating in a reverse mode as opposed to its re-uptake mode of operation (Harsing and Matyus, 2013; Shibasaki et al., 2017). Blockade of astrocytic GlyT1 may undermine the release of glycine synthesized by Phgdh inside astrocytes and consequently the excitability of NMDARs. We hypothesize that the functional significance of this glycine source in SR-null mice may be revealed by the specific deletion of astrocytic GlyT1 in these mice, which is predicted to undermine NMDAR excitability, resembling the effect of Phgdh inactivation in SR-null mice (Neame et al., 2019).
Hypothesis 3 – Inhibiting astrocytic GlyT1 during presynaptic activation can reduce the availability of glycine in the synapse and limit postsynaptic NMDARs excitability.
Harsing and Matyus (2013) were the first to show that GlyT1 in astrocytes operates in a cyclic manner, oscillating between phases of synaptic activation and inactivation, which correspond to the depolarization and repolarization phases of presynaptic glutamatergic axon terminals, respectively (Figure 2). The release of glutamate from the presynaptic terminals triggered by incoming action potentials is capable of activating AMPA/kainite (non-NMDA) receptors expressed in nearby astrocytes. The resulting inward Na+ current would switch the operation of astrocytic GlyT1 from its re-uptake mode to the reverse mode. Hence, during active release of glutamate, astrocytic GlyT1 is releasing glycine into, rather than removing it from, the synaptic cleft and therefore promote NMDAR activation.
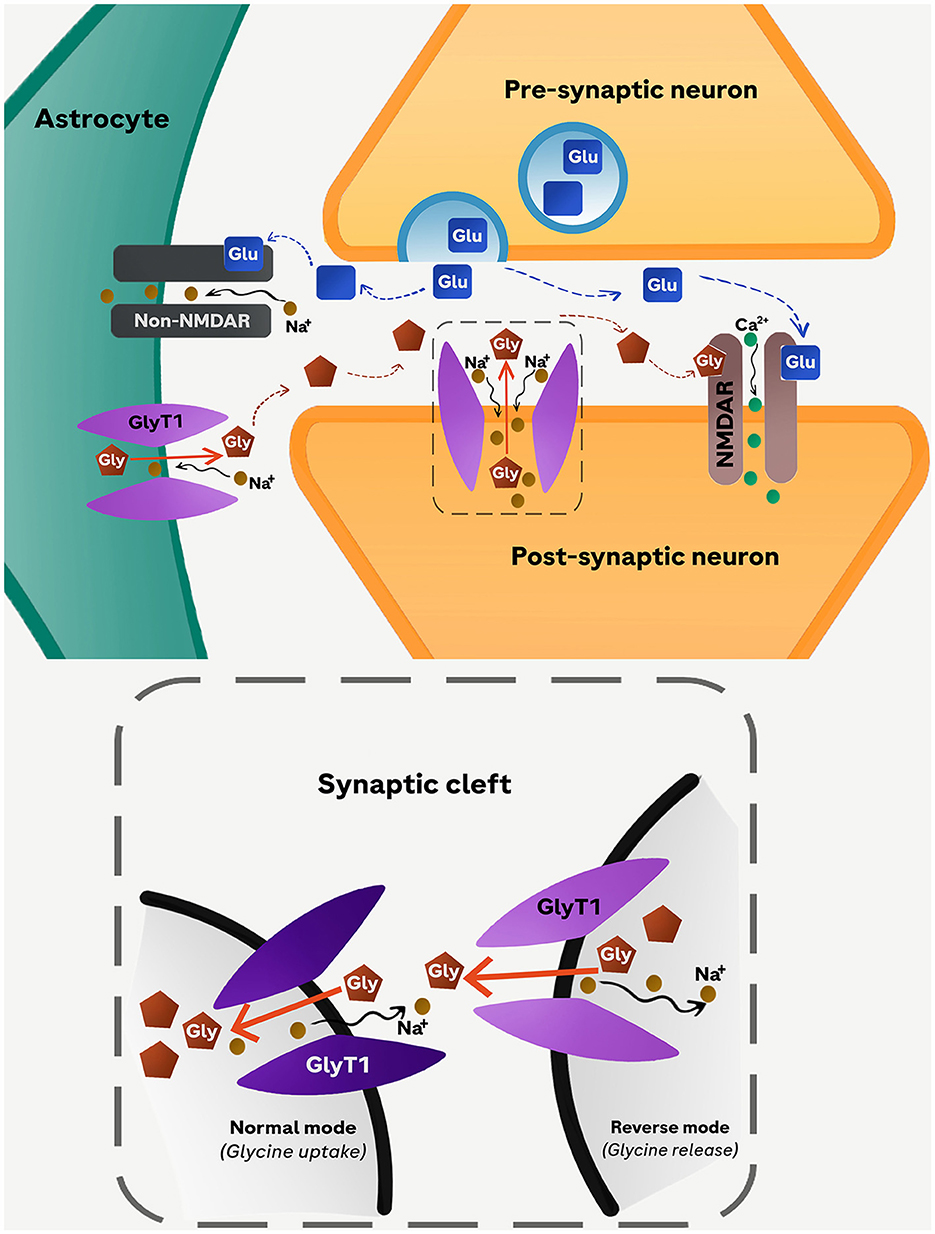
Figure 2. Proposed cyclic operation model of GlyT1. Glutamate released into the synaptic cleft from the presynaptic neuron stimulates non-NMDARs (AMPA/kainate) receptors in astrocytes leading to an influx of Na+. The resulting depolarization of the astrocyte membrane triggers the reverse-mode operation of GlyT1 causing an increased glycine efflux from astrocytes into the synaptic cleft. The simultaneous release of glycine (from astrocytes) and glutamate (from presynaptic neuron) activates NMDARs located in the membrane of the postsynaptic neuron. As the concentration of glycine in the synaptic cleft increases further, the direction of GlyT1 operation switches to normal-mode operation reabsorbing glycine back into astrocytes. Glu, glutamate; Gly, glycine.
According to this cyclic model, blockade of astrocytic GlyT1 during presynaptic activation would curtail the elevation of extracellular glycine from the astrocytic pool, although it is expected to elevate ambient extracellular glycine levels at quiescent axonal terminals. In the former scenario, the NMDARs in the postsynaptic active zones would become less responsive to stimulation by glutamate assuming that the glycine-B site of NMDARs is not saturated. Under a global blockade of GlyT1, therefore, the pro-NMDAR action resulting from the blockade of neuronal GlyT1 would be undermined by the concomitant blockade of astrocytic GlyT1. This may in part explain our observations that restricting GlyT1 deletion to neurons could yield more consistent pro-NMDAR phenotypes than extending its deletion to astrocytes (Möhler et al., 2011; Singer et al., 2015).
The full neurophysiological implication of the cyclic model on individual synaptic connections, as well as at the network level, certainly warrant further exploration. The temporal dynamics and the molecular mechanisms governing the switch between the depolarization (reverse mode) and repolarization (reuptake mode) modes of GlyT1 in astrocytes must be empirically verified to allow the formulation of testable hypotheses to be evaluated at the behavioral levels with suitable preclinical models (Singer and Yee, 2015). Appreciating the bidirectional regulation of glycine trafficking by this population of GlyT1 could revitalize research into GlyT1- blockers capable of acting selectively on one or the other mode of operation. Unraveling the molecular switch between GlyT1's two modes of operation may pave the way for their functional distinction permitting a more precise enhancement of NMDAR function in the schizophrenic brain, thereby overcoming a significant roadblock to drug development. It may be conceivable that synthetic drugs and biologics that may slow down or speed up this switching process could be identified. Finally, the possibility that dysregulation of astrocytic GlyT1 may be linked to negative and cognitive symptoms attributed to underactivity of cortical dopamine D1 receptors is further highlighted by the report that dopamine could modulate the release of glycine from cortical astrocytes via GlyT1 (Shibasaki et al., 2017). This may lead to novel GlyT1-based therapeutic strategies to address imbalances in dopaminergic and glutamatergic signaling in schizophrenia.
In conclusion, we contend that any pharmacological strategies aimed at enhancing NMDAR function by increasing synaptic glycine or D-serine levels must accommodate likely concomitant impact on the serine shuttle, which underlines the complex metabolic interplay between glycine and D-serine in terms of synthesis, clearance and trafficking within and across neurons and astrocytes. This in turn critically determines the cyclical operation of GlyT1 in astrocytes and consequently the excitability of NMDAR, as summarized in the three hypotheses presented here. Suffice to say, they have not exhaustively incorporated the full complexity of glycine regulation in the brain, which also depends on a myriad of amino acid transporters and metabolic pathways omitted here. All of which, however, deserve consideration even when a single player, such as GlyT1, is targeted specifically, not to mention the likely adaptive changes that long-term exposure to such drugs inevitably will induce.
Author contributions
PS: Writing—original draft, Writing—review & editing. BY: Writing—original draft, Writing—review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. BY wishes to thank the generous supports provided by the Mental Health Research Center (P0040606/1-BBCG) of The Hong Kong Polytechnic University in the preparation of this review.
Acknowledgments
The professional rendition of the illustrations by Ms. Jessica Singer-Yahm's consummate craftsmanship is duly acknowledged with gratitude.
Conflict of interest
PS was employed by the Roche Diagnostics International AG.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Basu, A. C., Tsai, G. E., Ma, C. L., Ehmsen, J. T., Mustafa, A. K., Han, L., et al. (2009). Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 14, 719–727. doi: 10.1038/mp.2008.130
Betz, H., Gomeza, J., Armsen, W., Scholze, P., and Eulenburg, V. (2006). Glycine transporters: essential regulators of synaptic transmission. Biochem. Soc. Trans. 34, 55–58. doi: 10.1042/BST0340055
Bugarski-Kirola, D., Blaettler, T., Arango, C., Fleischhacker, W. W., Garibaldi, G., Wang, A., et al. (2017). Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol. Psychiatry 82, 8–16. doi: 10.1016/j.biopsych.2016.11.014
Carlsson, M., and Carlsson, A. (1990). Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr. Bull. 16, 425–432. doi: 10.1093/schbul/16.3.425
Cioffi, C. L. (2018). Glycine transporter-1 inhibitors: a patent review (2011-2016). Expert Opin. Ther. Pat. 28, 197–210. doi: 10.1080/13543776.2018.1429408
Coyle, J. T. (2006). Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol. Neurobiol. 26, 365–384. doi: 10.1007/s10571-006-9062-8
Coyle, J. T., Ruzicka, W. B., and Balu, D. T. (2020). Fifty years of research on schizophrenia: the ascendance of the glutamatergic Synapse. Am. J. Psychiatry 177, 1119–1128. doi: 10.1176/appi.ajp.2020.20101481
Dogra, S., and Conn, P. J. (2022). Metabotropic glutamate receptors as emerging targets for the treatment of schizophrenia. Mol. Pharmacol. 101, 275–285. doi: 10.1124/molpharm.121.000460
Fleischhacker, W. W., Podhorna, J., Gröschl, M., Hake, S., Zhao, Y., Huang, S., et al. (2021). Efficacy and safety of the novel glycine transporter inhibitor BI 425809 once daily in patients with schizophrenia: double-blind, randomised, placebo-controlled phase 2 study. Lancet Psychiatry 8, 191–201. doi: 10.1016/S2215-0366(20)30513-7
Gomeza, J., Ohno, K., Hülsmann, S., Armsen, W., Eulenburg, V., Laube, B., et al. (2003). Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40, 797–806. doi: 10.1016/S0896-6273(03)00673-1
Harsing, L. G., and Matyus, P. (2013). Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and nonsynaptic glycine transporters. Brain Res. Bull. 93, 110–119. doi: 10.1016/j.brainresbull.2012.12.002
Harvey, R. J., and Yee, B. K. (2013). Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence, and pain. Nat. Rev. Drug Discov. 12, 866–885. doi: 10.1038/nrd3893
Haydon, P. G., and Carmignoto, G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031. doi: 10.1152/physrev.00049.2005
Heresco-Levy, U., Javitt, D. C., Ermilov, M., Mordel, C., Horowitz, A., and Kelly, D. (1996a). Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br. J. Psychiatry 169, 610–617. doi: 10.1192/bjp.169.5.610
Heresco-Levy, U., Javitt, D. C., Ermilov, M., Mordel, C., Silipo, G., and Lichtenstein, M. (1999). Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch. Gen. Psychiatry 56, 29–36. doi: 10.1001/archpsyc.56.1.29
Heresco-Levy, U., Silipo, G., and Javitt, D. C. (1996b). Glycinergic augmentation of NMDA receptor-mediated neurotransmission in the treatment of schizophrenia. Psychopharmacol. Bull. 32, 731–740.
Javitt, D. C. (2004). Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 9, 984–997. doi: 10.1038/sj.mp.4001551
Javitt, D. C., Zylberman, I., Zukin, S. R., Heresco-Levy, U., and Lindenmayer, J. P. (1994). Amelioration of negative symptoms in schizophrenia by glycine. Am. J. Psychiatry 151, 1234–1236. doi: 10.1176/ajp.151.8.1234
Kantrowitz, J. T., Nolan, K. A., Epstein, M. L., Lehrfeld, N., Shope, C., Petkova, E., et al. (2017). Neurophysiological effects of bitopertin in schizophrenia. J. Clin. Psychopharmacol. 37, 447–451. doi: 10.1097/JCP.0000000000000722
Lane, H. Y., Lin, C. H., Huang, Y. J., Liao, C. H., Chang, Y. C., and Tsai, G. E. (2010). A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int. J. Neuropsychopharmacol. 13, 451–460. doi: 10.1017/S1461145709990939
Li, Y., Sacchi, S., Pollegioni, L., Basu, A. C., Coyle, J. T., and Bolshakov, V. Y. (2013). Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat. Commun. 4:1760. doi: 10.1038/ncomms2779
Lin, C. Y., Liang, S. Y., Chang, Y. C., Ting, S. Y., Kao, C. L., Wu, Y. H., et al. (2017). Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: a randomised, double-blind, placebo-controlled trial. World J. Biol. Psychiatry 18, 357–368. doi: 10.3109/15622975.2015.1117654
Maksymetz, J., Moran, S. P., and Conn, P. J. (2017). Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol. Brain 10:15. doi: 10.1186/s13041-017-0293-z
Moghaddam, B. (2004). Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology 174, 39–44. doi: 10.1007/s00213-004-1792-z
Moghaddam, B., and Javitt, D. (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15. doi: 10.1038/npp.2011.181
Möhler, H., Boison, D., Singer, P., Feldon, J., Pauly-Evers, M., and Yee, B. K. (2011). Glycine transporter 1 as a potential therapeutic target for schizophrenia-related symptoms: evidence from genetically modified mouse models and pharmacological inhibition. Biochem. Pharmacol. 81, 1065–1077. doi: 10.1016/j.bcp.2011.02.003
Neame, S., Safory, H., Radzishevsky, I., Touitou, A., Marchesani, F., Marchetti, M., et al. (2019). The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc. Natl. Acad. Sci. U. S. A. 116, 20736–20742. doi: 10.1073/pnas.1909458116
Olney, J. W., and Farber, N. B. (1995). Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry 52, 998–1007. doi: 10.1001/archpsyc.1995.03950240016004
Olney, J. W., Newcomer, J. W., and Farber, N. B. (1999). NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 33, 523–533. doi: 10.1016/S0022-3956(99)00029-1
Pei, J. C., Luo, D. Z., Gau, S. S., Chang, C. Y., and Lai, W. S. (2021). Directly and indirectly targeting the glycine modulatory site to modulate NMDA receptor function to address unmet medical needs of patients with schizophrenia. Front. Psychiatry 12:742058. doi: 10.3389/fpsyt.2021.742058
Pinard, E., Borroni, E., Koerner, A., Umbricht, D., and Alberati, D. (2018). Glycine transporter type I (GlyT1) inhibitor, bitopertin: a journey from lab to patient. Chimia 72, 477–484. doi: 10.2533/chimia.2018.477
Rosenberg, D., Artoul, S., Segal, A. C., Kolodney, G., Radzishevsky, I., Dikopoltsev, E., et al. (2013). Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013
Rosenbrock, H., Desch, M., and Wunderlich, G. (2023). Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 273, 1557–1566. doi: 10.1007/s00406-023-01576-z
Schmidt, R. W., and Thompson, M. L. (2016). Glycinergic signaling in the human nervous system: an overview of therapeutic drug targets and clinical effects. Ment. Health Clin. 6, 266–276. doi: 10.9740/mhc.2016.11.266
Shibasaki, K., Hosoi, N., Kaneko, R., Tominaga, M., and Yamada, K. (2017). Glycine release from astrocytes via functional reversal of GlyT1. J. Neurochem. 140, 395–403. doi: 10.1111/jnc.13741
Singer, P., Dubroqua, S., and Yee, B. K. (2015). Inhibition of glycine transporter 1: The yellow brick road to new schizophrenia therapy? Curr. Pharm. Des. 21, 3771–3787. doi: 10.2174/1381612821666150724100952
Singer, P., and Yee, B. K. (2015). A conceptual and practical guide to the behavioural evaluation of animal models of the symptomatology and therapy of schizophrenia. Cell Tissue Res. 2013 Oct;354(1):221-46. doi: 10.1007/s00441-013-1611-0
Singer, P., Yee, B. K., Feldon, J., Iwasato, T., Itohara, S., Grampp, T., et al. (2009). Altered mnemonic functions and resistance to N-METHYL-d-Aspartate receptor antagonism by forebrain conditional knockout of glycine transporter 1. Neuroscience 161, 635–654. doi: 10.1016/j.neuroscience.2009.03.056
Snyder, S. H., and Kim, P. M. (2000). d-amino acids as putative neurotransmitters; focus on d-serine. Neurochem. Res. 25, 553–560. doi: 10.1023/A:1007586314648
Tsai, G., Lane, H. Y., Yang, P., Chong, M. Y., and Lange, N. (2004). Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 55, 452–456. doi: 10.1016/j.biopsych.2003.09.012
Umbricht, D., Alberati, D., Martin-Facklam, M., Borroni, E., Youssef, E. A., Ostland, M., et al. (2014). Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry 71, 637–646. doi: 10.1001/jamapsychiatry.2014.163
Wolosker, H. (2011). Serine racemase and the serine shuttle between neurons and astrocytes. Biochim. Biophys. Acta. 1814, 1558–1566. doi: 10.1016/j.bbapap.2011.01.001
Wolosker, H., and Radzishevsky, I. (2013). The serine shuttle between glia and neurons: implications for neurotransmission and neurodegeneration. Biochem. Soc. Trans. 41, 1546–1550. doi: 10.1042/BST20130220
Yee, B. K., Balic, E., Singer, P., Schwerdel, C., Grampp, T., Gabernet, L., et al. (2006). Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J. Neurosci. 26, 3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006
Keywords: antipsychotics, glycine reuptake, glycine-B site, neuron-glial interaction, NMDA receptors, schizophrenia
Citation: Singer P and Yee BK (2024) Inhibition of astrocytic glycine transporter-1: friend or foe for ameliorating NMDA receptor hypofunction? Front. Cell. Neurosci. 18:1389718. doi: 10.3389/fncel.2024.1389718
Received: 22 February 2024; Accepted: 09 May 2024;
Published: 24 May 2024.
Edited by:
Hai-Ying Shen, Legacy Research Institute, United StatesReviewed by:
Austin W.T. Chiang, Augusta University, United StatesCopyright © 2024 Singer and Yee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Singer, cGhpbGlwcC5zaW5nZXJAcm9jaGUuY29t; Benjamin K. Yee, YmVuamFtaW4ueWVlQHBvbHl1LmVkdS5oaw==
 Philipp Singer
Philipp Singer Benjamin K. Yee
Benjamin K. Yee