
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Cell. Neurosci. , 13 October 2023
Sec. Cellular Neuropathology
Volume 17 - 2023 | https://doi.org/10.3389/fncel.2023.1296958
This article is part of the Research Topic Tubulinopathies: Fundamental and Clinical Challenges View all 7 articles
Editorial on the Research Topic
Tubulinopathies: fundamental and clinical challenges
Tubulinopathies are a wide family of neurological disorders caused by mutations in genes encoding tubulins, the structural units of microtubules. They represent a growing class of pathologies for which no therapies are currently available.
More than 255 tubulin mutations have been reported so far (Attard et al., 2022) and the number of tubulin mutations as well as the phenotypic spectrum of tubulinopathies are ever increasing. Our knowledge about the pathogenesis of tubulinopathies remains however fragmentary and the development of therapeutic interventions will be a huge challenge.
Our understanding about the pathogenesis of tubulinopathies is further hampered by our limited knowledge on tubulin and microtubule regulatory mechanisms at the transcriptional, translational and post-translational level (Figures 1A, B). Many of these mechanisms have recently emerged e.g., the modulation of microtubule polymerization by glutathionylation (Chen et al., 2021) but their biological significance is not yet full understood thus making it difficult to faithfully predict the effects of tubulin mutations on microtubule function.
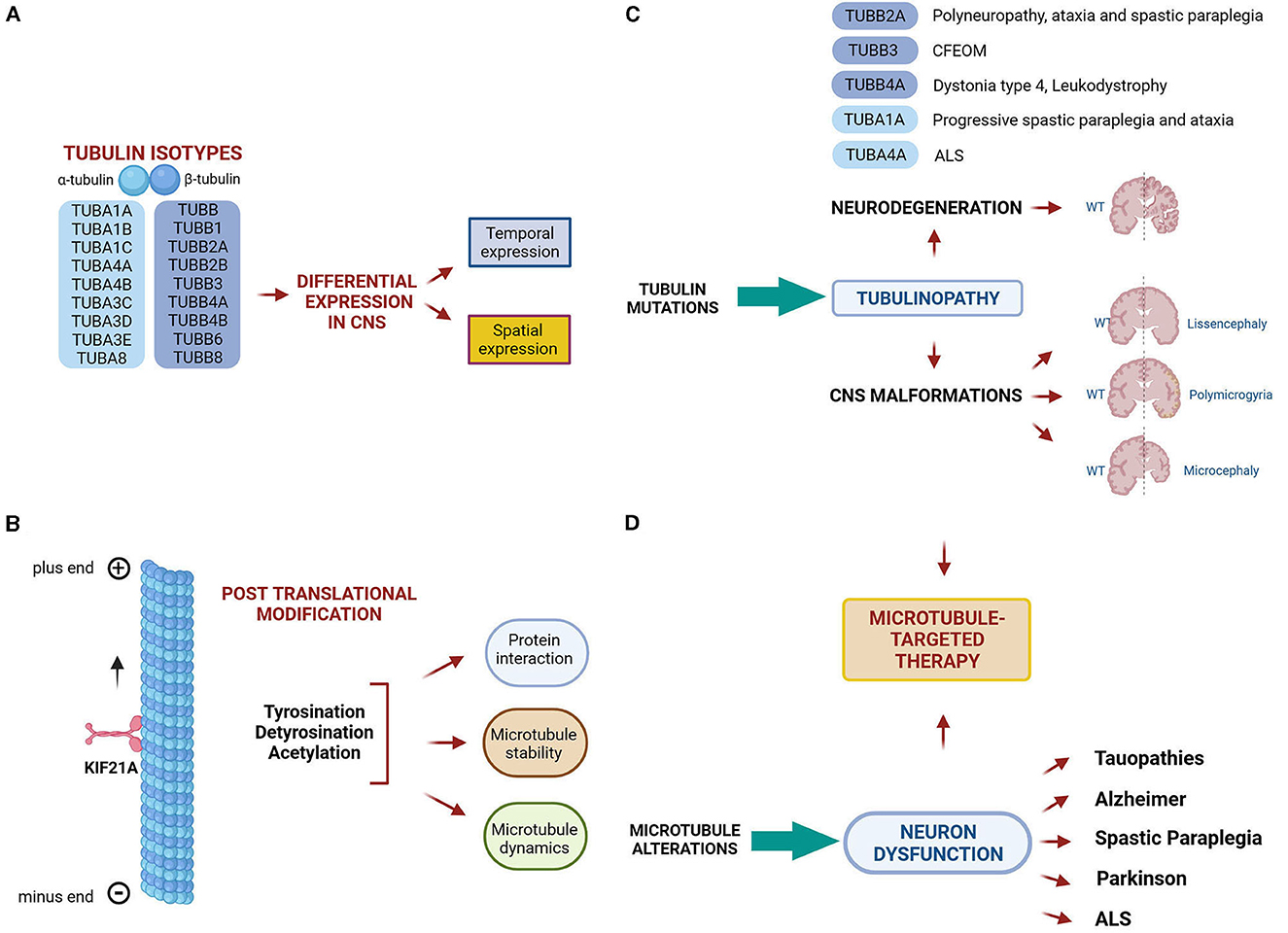
Figure 1. Microtubule comlexity and microtubule related diseases. (A, B) Microtubule complexity is generated by the expression of different α and β-tubulin isotypes and by the addition of post translational modifications, which have the potential to tune microtubule dynamics, stability and interactions with MAPS. (C) Mutations in tubulin genes cause a class of neurological diseases known as tubulinopathies, which include both malformations of the central nervous system and neurodegenerative disorders. (D) Microtubule alteration are also considered a pathomechanism underlying neuron dysfunction in a wide range of neurodegenerative diseases. (C, D) Thus, microtubule- based therapies represent a promising strategy to prevent or rescue microtubule dysfunction occurring in neurological disorders. The figure has been created in “BioRender.com”.
The Research Topic entitled “Tubulinopathies: fundamental and clinical challenges” aims to improve our knowledge about tubulinopathies, and more generally about mechanisms governing microtubule in normal and pathological conditions. The contributions of the Research Topic fall principally into two lines of research.
The first line concerns the clinical and molecular characteristics of tubulinopathies and the pathogenic effects of tubulin mutations. Tubulinopathies classically represent a broad spectrum of neurodevelopmental disorders leading to cortical malformations (Bahi-Buisson et al., 2014) (Figure 1C). Recent discoveries have demonstrated that mutations in several tubulin isotypes can cause various malformations beyond the cerebral cortex (Cederquist et al., 2012; Madrigal et al., 2019) and even trigger neurodegeneration (Smith et al., 2014; Sferra et al., 2018).
Pathogenic mutations in TUBB3 for instance were shown to be associated with isolated or syndromic congenital fibrosis of the extraocular muscles (CFEOM) and with malformations of cortical development (MCDs) (Poirier et al., 2010; Tischfield et al., 2010). In the present Research Topic, Puri et al. provide a comprehensive review of CFEOM- and MCD-related TUBB3 mutations, their associated phenotypes and pathomechanisms. In particular, they report that TUBB3 missense mutations can cause errors in the growth and guidance of cranial and central axons, as well as in cortical neuronal migration and organization (Figure 1C). The effects of some TUBB3 mutations are mimicked by specific mutations in the neuronal kinesin KIF21A, pointing to common pathogenic mechanisms (Figure 1C).
By contrast, the α-tubulin TUBA1A has been mainly associated with lissencephaly and a total of 121 TUBA1A mutations have been identified in patients with neurodevelopmental disorders (Hoff et al., 2022). Zocchi et al. describe the first TUBA1A mutation associated with a neurodegenerative condition characterized by ataxia and progressive spastic paraplegia. They demonstrate that the disease-causing mutation decreases TUBA1A protein stability, proposing a novel disease mechanisms in which the haploinsufficiency of this may be sufficient to drive neurodegeneration (Figure 1C). Earlier studies have shown that TUBA4A mutations can be responsible for rare forms of the neurodegenerative disorder amyotrophic lateral sclerosis (Smith et al., 2014).
The second line of research addresses the structural dynamics of tubulins, microtubules and microtubule-associated proteins (MAPs) as well as post translational modifications (PTMs) of tubulins in normal and pathological conditions (Figure 1B).
Hoff et al. highlight how deepening our knowledge on the interaction between tubulins, microtubules and MAPs can better predict the effects of tubulin mutations. The authors offer a description of tubulin structural dynamics and MAPs, and explain how microtubules and MAPs regulate critical steps of neurodevelopment. They further discuss how tubulin mutations can cause tubulinopathies by altering interactions between microtubules and their regulators or by impairing microtubule dynamics.
By modulating the expression of tubulin tyrosine ligase (TTL), the enzyme that catalyzes the post-translational addition of tyrosine to the detyrosinated end of α-tubulin, Müller, Ringer et al. demonstrate that the balance of tyrosinated and detyrosinated microtubules finely regulates cell shape and adhesion in both D2 epithelial cell cultures and D3 intestinal organoids. The authors also propose that TTL has a central role in regulating the assembly of focal adhesion since its depletion prolonged the persistence of vinculin at these sites and its binding efficiency to different focal adhesion adapters.
In a companion paper, the same research group demonstrates that tyrosinated and detyrosinated microtubules exert opposite effects during cell migration of MDCK cells and MDCK cyst formation. The authors highlight a specific role of detyrosinated tubulin in promoting cell motility and direction and propose EB1, a MAP that tracks microtubule plus ends, as a key mediator during cell migration, connecting detyrosinated microtubule to cell membrane, at adhesion plaques (Müller, Gorek et al.). Dysregulation of tubulin PTMs occur in a wide range of brain disorders (Dompierre et al., 2007; Cartelli et al., 2013; Zhang et al., 2015) and both tubulin PTMs as well as some of their catalyzing enzyme have emerged as promising therapeutic targets for the treatment of neurological conditions (Rogowski et al., 2021) (Figure 1D).
Wali et al. demonstrate that peripheral blood mononuclear cells (PBMCs) of patients affected by Spastin (SPAST)-associated hereditary spastic paraplegia (HSP), exhibit reduced levels of acetylated α-tubulin, thus reflecting the pathological reduction of acetylated α-tubulin previously observed in patient-derived neuronal cells (Wali et al., 2020). They further demonstrate that the tubulin binding agent noscapine increases the level of acetylated tubulin both in patient PBMCs after in vitro treatment and in mouse brain following in vivo administration. The authors thus propose that PBMCs of SPAST-related HSP patients may represent a suitable in vitro model to study the pathology and the measurement of acetylated tubulin a tool to quantify the treatment effects of noscapine. A schematic representation of microtubule complexity and microtubule related diseases is shown in Figure 1.
We hope that this Research Topic will help to better understand the complex network of microtubule functions and regulations, to understand how the latter act at the cellular and sub-cellular levels, and how they are differentially affected by tubulin mutations.
In addition, we hope that the Research Topic will foster further functional and genetic studies on microtubule function and dysfunction in neurons to expand the mutational and phenotypic spectrum of tubulinopathies. These efforts will be key to design innovative microtubule-based interventions for the treatment of tubulinopathies.
AS: Writing—original draft. EB: Writing—review and editing. GH: Writing—review and editing.
This work was supported by grants from Italian Ministry of Health (Ricerca Finalizzata Giovani Ricercatori GR-2019-12370042 to AS, RC2022 to EB), Agence Nationale Pour la Recherche (ANR), INSERM, AFM, and ARSLA to GH. This work was also supported by the Italian Ministry of Health with Current Research funds.
The topic editors would like to express their gratitude to all authors who proposed their work and to all researchers who reviewed the submissions to this Research Topic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Attard, T. J., Welburn, J. P. I., and Marsh, J. A. (2022). Understanding molecular mechanisms and predicting phenotypic effects of pathogenic tubulin mutations. PLoS Comput. Biol. 18, e1010611. doi: 10.1371/journal.pcbi.1010611
Bahi-Buisson, N., Poirier, K., Fourniol, F., Saillour, Y., Valence, S., Lebrun, N., et al. (2014). The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain 137, 1676–1700. doi: 10.1093/brain/awu082
Cartelli, D., Casagrande, F., Busceti, C. L., Bucci, D., Molinaro, G., and Traficante, A. (2013). Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci. Rep. 3, 1837. doi: 10.1038/srep01837
Cederquist, G. Y., Luchniak, A., Tischfield, M. A., Peeva, M., Song, Y., Menezes, M. P., et al. (2012). An inherited TUBB2B mutation alters a kinesin-binding site and causes polymicrogyria, CFEOM and axon dysinnervation. Hum. Mol. Genet. 2, 5484–5499. doi: 10.1093/hmg/dds393
Chen, M., Wang, J., Yang, Y., Zhong, T., Zhou, P., Ma, H., et al. (2021). Redox-dependent regulation of end-binding protein 1 activity by glutathionylation. Sci. China Life Sci. 64, 575–583. doi: 10.1007/s11427-020-1765-6
Dompierre, J. P., Godin, J. D., Charrin, B. C., Cordelières, F. P., King, S. J., Humbert, S., et al. (2007). Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007
Hoff, K. J., Aiken, J. E., Gutierrez, M. A., Franco, S. J., and Moore, J. K. (2022). TUBA1A tubulinopathy mutants disrupt neuron morphogenesis and override XMAP215/Stu2 regulation of microtubule dynamics. Elife 11, e76189. doi: 10.7554/eLife.76189.sa2
Madrigal, I., Rabionet, R., Alvarez-Mora, M. I., Sanchez, A., Rodríguez-Revenga, L., Estivill, X., et al. (2019). Spectrum of clinical heterogeneity of β-tubulin TUBB5 gene mutations. Gene 5, 12–17. doi: 10.1016/j.gene.2019.02.002
Poirier, K., Saillour, Y., Bahi-Buisson, N., Jaglin, X. H., Fallet-Bianco, C., Nabbout, R., et al. (2010). Mutations in the neuronal β-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 19, 4462–4473. doi: 10.1093/hmg/ddq377
Rogowski, K., Hached, K., Crozet, C., and van der Laan, S. (2021). Tubulin modifying enzymes as target for the treatment of tau-related diseases. Pharmacol. Ther. 218, 107681. doi: 10.1016/j.pharmthera.2020.107681
Sferra, A., Fattori, F., Rizza, T., Flex, E., Bellacchio, E., Bruselles, A., et al. (2018). Defective kinesin binding of TUBB2A causes progressive spastic ataxia syndrome resembling sacsinopathy. Hum. Mol. Genet. 27, 1892–1904. doi: 10.1093/hmg/ddy096
Smith, B. N., Ticozzi, N., Fallini, C., Gkazi, A. S., Topp, S., Kenna, K. P., et al. (2014). Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron 84, 324–331. doi: 10.1016/j.neuron.2014.09.027
Tischfield, M. A., Baris, H. N., Wu, C., Rudolph, G., Van Maldergem, L., He, W., et al. (2010). Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140, 74–87. doi: 10.1016/j.cell.2009.12.011
Wali, G., Liyanage, E., Blair, N. F., Sutharsan, R., Park, J. S., Mackay-Sim, A., et al. (2020). Oxidative stress-induced axon fragmentation is a consequence of reduced axonal transport in hereditary spastic paraplegia SPAST patient neurons. Front. Neurosci. 14, 401. doi: 10.3389/fnins.2020.00401
Keywords: tubulinopathies, tubulin (microtubules), neurodegeneration, neurodevelopment, TUBA1A, TUBB3, KIF21A
Citation: Sferra A, Bertini E and Haase G (2023) Editorial: Tubulinopathies: fundamental and clinical challenges. Front. Cell. Neurosci. 17:1296958. doi: 10.3389/fncel.2023.1296958
Received: 19 September 2023; Accepted: 20 September 2023;
Published: 13 October 2023.
Edited and reviewed by: Dirk M. Hermann, University of Duisburg-Essen, Germany
Copyright © 2023 Sferra, Bertini and Haase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Sferra, YW50b25lbGxhLnNmZXJyYUBvcGJnLm5ldA==; Georg Haase, Z2VvcmcuaGFhc2VAdW5pdi1hbXUuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.