
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Neurosci. , 05 April 2023
Sec. Cellular Neuropathology
Volume 17 - 2023 | https://doi.org/10.3389/fncel.2023.1157577
This article is part of the Research Topic Emerging Roles of Extracellular Matrix in the Physiology and Pathophysiology of the Central Nervous System View all 5 articles
In mammals, the accessory olfactory bulb (AOB) receives input from vomeronasal sensory neurons (VSN) which detect pheromones, chemical cues released by animals to regulate the physiology or behaviors of other animals of the same species. Cytoarchitecturally, cells within the AOB are segregated into a glomerular layer (GL), mitral cell layer (MCL), and granule cell layer (GCL). While the cells and circuitry of these layers has been well studied, the molecular mechanism underlying the assembly of such circuitry in the mouse AOB remains unclear. With the goal of identifying synaptogenic mechanisms in AOB, our attention was drawn to Collagen XIX, a non-fibrillar collagen generated by neurons in the mammalian telencephalon that has previously been shown to regulate the assembly of synapses. Here, we used both a targeted mouse mutant that lacks Collagen XIX globally and a conditional allele allowing for cell-specific deletion of this collagen to test if the loss of Collagen XIX causes impaired synaptogenesis in the mouse AOB. These analyses not only revealed defects in excitatory synapse distribution in these Collagen XIX-deficient mutants, but also showed that these mutant mice exhibit altered behavioral responses to pheromones. Although this collagen has been demonstrated to play synaptogenic roles in the telencephalon, those roles are at perisomatic inhibitory synapses, results here are the first to demonstrate the function of this unconventional collagen in glutamatergic synapse formation.
In mammals, the olfactory system plays an essential function in detecting odors and inducing responses to social behaviors, attraction, aversion, and fear. Rodents possess a number of odor sensing subsystems, including the main olfactory system (MOS) which is capable of detecting a broad range of odorants, the accessory olfactory system (AOS) which detects pheromone, septal organ and Grueneberg ganglion systems which have only recently been discovered and remain poorly understood (Ma, 2007; Sakano, 2020; Mori and Sakano, 2021). As the second largest of olfactory subsystem, the AOS consists of the vomeronasal organ (VNO), the accessory olfactory bulb (AOB), and the vomeronasal amygdala, which projects to hypothalamic neuroendocrine centers. Together this chemosensory system processes signals of non-volatile odorants emitted by animals, such as urine and feces, as well as fluids emanating from skin (Ma, 2007; Mohrhardt et al., 2018). Based on this, it was realized that AOS mainly functions in detecting and responding to pheromones, chemical cues which regulate the physiology or behaviors of other members of the same species (Liberles, 2014). Therefore, the AOB plays an important function in guiding physiological and behavioral responses to social and reproductive interactions.
The connectivity and cytoarchitecture of AOB are well characterized (Holy, 2018; Mohrhardt et al., 2018). Specifically, the AOB receives input from vomeronasal sensory neurons (VSN) whose axons extend via the cribriform plate, transverse along the medial aspect of the olfactory bulb, and terminal in the glomerular layer (GL) of AOB (Meredith, 1991; Belluscio et al., 1999; Rodriguez et al., 1999; Holy, 2018; Mohrhardt et al., 2018). Contrary to the MOS, individual VSN axons can divide to terminate in multiple glomeruli in the AOB rather than targeting single glomerulus in the MOB (Larriva-Sahd, 2008; Mohrhardt et al., 2018).
The AOB is situated at the posterior dorsal aspect of the rodent olfactory bulb. It is composed of a GL, mitral cell layer (MCL), granule cell layer (GCL), and narrow lateral olfactory tract (LOT) which is flanked by the MCL and GCL (Figure 1A; Yokosuka, 2012; Mohrhardt et al., 2018). The GL includes clustered glomeruli which are surrounded by periglomerular cells. The MCL contains AOB mitral cells (AMC), juxtaglomerular neurons (JGNs), and some external granule cells (eGCs). It is the dendrites of these AMCs that receive excitatory input from VSN axons in the AOB glomeruli (Brignall et al., 2018). The GCL is mainly occupied by axonless GABAergic internal granule cells (iGCs). The most prominent feature in this layer are the reciprocal dendrite to dendrite synapse between mitral and granule cell dendrites: mitral-to-granule cell synapses are glutamatergic, whereas granule-to-mitral cell synapses are GABAergic (Hayashi et al., 1993; Jia et al., 1999; Taniguchi and Kaba, 2001).
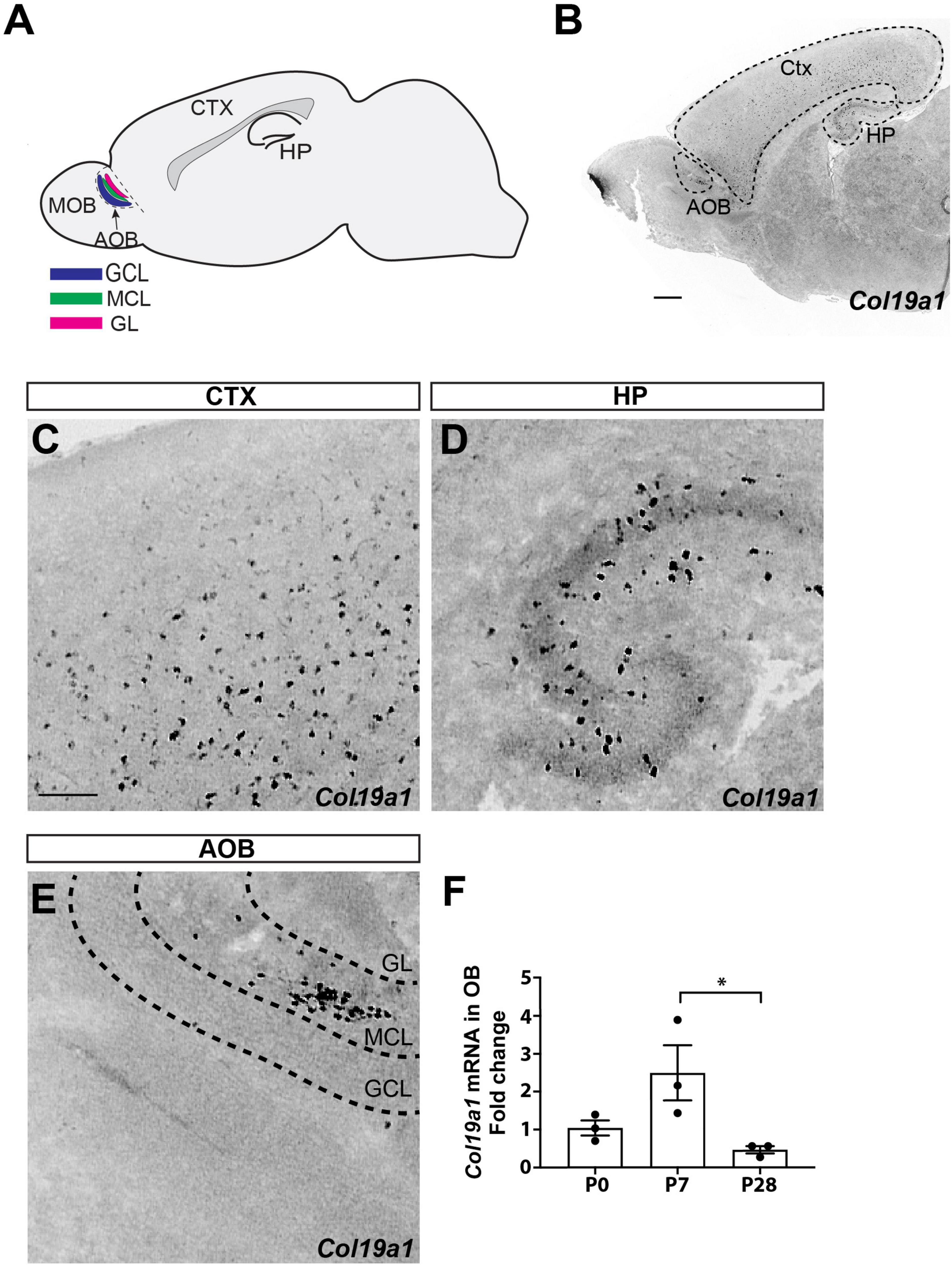
Figure 1. Spatial and temporal expression of Col19a1. (A) Schematic representation of the accessory olfactory bulb (AOB) in a sagittal view of mouse brain. Granule cell layer (GCL), mitral cell layer (MCL), and glomerular cell layer (GL) are each labeled. Cortex (Ctx) and hippocampus (HP) are also indicated. (B) Col19a1 ISH performed on a sagittal wildtype mouse brain (P8). (C–E) Magnified views are shown from (B), with expression of Col19a1 in the Ctx (C), HP (D), and MCL of the AOB (E). (F) Col19a1 mRNA measured with qPCR of cDNAs generated from olfactory bulb RNA collected at birth (P0), P7, and P28. *Indicates p < 0.05 with multiple comparisons ANOVA/Tukey’s post hoc. Scalebars indicate 200 μm.
Although the cells and circuitry of mouse AOB has been well studied, the molecular mechanism underlying the assembly of such circuitry remains unclear. With the goal of identifying synaptogenic mechanisms in AOB, our attention was drawn to Collagen XIX, a neuronally produced non-fibrillar collagen that has previously been shown to regulate the assembly of synapses in the mouse telencephalon. Collagen XIX is necessary for inhibitory synapse formation in mouse hippocampus and neocortex. A short C-terminal fragment containing the non-collagenous domain 1 (NC1 domain) fragment of Collagen XIX, termed NC1[XIX] is sufficient to trigger inhibitory synapse formation in cultured cortical neurons (Sumiyoshi et al., 1997; Su et al., 2010, 2016, 2020). Our transcriptional analysis revealed that Col19a1, the gene that encodes Collagen XIX, is generated in the MCL of AOB. Here we tested whether the loss of Collagen XIX impaired synaptogenesis and the response to AOB-related pheromones using several genetic tools, including a targeted mouse mutant that lacks Collagen XIX globally and a conditional allele allowing for cell-specific deletion of this collagen (Sumiyoshi et al., 2004; Su et al., 2020). Our data show that both the global loss Collagen XIX mice and the cell-specific loss of Collagen XIX mice exhibit pheromones sensing and excitatory synapse formation defects.
C57BL/6 (Charles River Laboratories) and previously generated Collagen XIX-null mice (Sumiyoshi et al., 2004; Su et al., 2010) were used in this study. Additionally, Col19a1fl/fl, Nes-cre::Col19a1fl/fl, and Sst-cre::Col19a1fl/fl mice were generated as previously described (Su et al., 2020) from The Jackson Laboratory Nes-cre (#003771), Vglut2-cre (#016963) and Sst-cre (#013044) mice. Mice were housed in a 12 h dark/light cycle and had ad libitum access to food and water. All experiments were performed in compliance with National Institutes of Health (NIH) guidelines and protocols, and were approved by the Virginia Polytechnic Institute and State University Institutional Animal Care and Use Committee (IACUC).
The following materials were obtained, from Roche (Basel, Switzerland): Fluorescein RNA Labeling Mix, digoxigenin (DIG) RNA Labeling Mix, blocking reagent, and Yeast RNA; from EMS (Hatfield, PA): Paraformaldehyde (PFA, EM-grade) and Tissue Freezing Medium; from Vector Laboratories (Newark, CA): VECTASHIELD; from Bio-Rad (Hercules, CA): Aurum Total RNA Fatty and Fibrous Tissue Kit; from Promega (Madison, WI): pGEM-T Easy Vector Systems; from PerkinElmer (Waltham, MA): tyramide signal amplification (TSA) systems. All other chemicals and reagents were obtained from Thermo Fisher Scientific or Sigma-Aldrich, and all DNA primers from Integrated DNA Technologies.
Riboprobes against Col19a1, Gad1, Syt1, Syt2, Vglut1 and Sst mRNAs were generated as described previously (Su et al., 2010, 2016, 2020; Carrillo et al., 2023), where digoxigenin-labeled NTP (Roche, Basel, Switzerland) and the MAXIscript In Vitro Transcription Kit (Ambion) were used to synthesize riboprobes, which were hydrolyzed to 500 nt. Prior to addition of the riboprobes, tissue sections were prepared for ISH by fixation with 4% paraformaldehyde (PFA) for 10 min, incubation with proteinase K (1 ug/mL proteinase K, 50 mM 7.5 pH Tris, 5 mM EDTA) for 10 min, additional fixation with 4% PFA for 5 min, acetylation (1.33% triethanolamine, 20 mM HCl, 0.25% acetic anhydride) for 10 min, and permeabilization (1% Triton X-100) for 30 min, where each step listed was followed by DEPC-PBS washes. After fixation and permeabilization, sections were blocked with 0.3% H2O2 for 30 min, incubated with a hybridization buffer (1X prehybridization, 0.1 mg/mL yeast tRNA, 0.05 mg/mL heparin, 50% formamide) for 1 h, and incubated overnight with riboprobes at 65°C, concluded by a 65°C wash with 0.2x SSC. Horseradish peroxidase (POD)-conjugated anti-DIG (1:2,000, Roche, Basel, Switzerland) or anti-FL (1:2,000, Roche, Basel, Switzerland) antibodies were used with TSA systems (PerkinElmer, Waltham, MA) fluorescent staining. VectaShield-mounted sections were imaged with a Zeiss LSM 700 microscope (20x air Plan-Apochromat Objective, NA 0.8).
To prepare slides for IHC, mice were first anesthetized with 12.5 ug/mL tribromoethanol (Avertin). Blood was cleared with transcardial perfusion of phosphate-buffered saline (PBS) and subsequently 4% PFA to preserve the tissues. The brain was removed, incubated with 4% PFA for 16 h at 4°C for further fixation, and cryopreserved in 30% sucrose for ≥ 48 h at 4°C. After embedding the fixed tissue in tissue freezing medium (EMS), a Leica CM1850 cryostat was used to prepare 20 μm cryosections. Air-dried sections were then blocked with 2.5% BSA, 5% NGS, and 0.1% Triton-X in PBS for 1 h. Tissue sections were stained with primary antibodies in blocking buffer for 16 h at 4°C: mouse anti-Syt2 (diluted 1:200, Zebrafish International Resource Center), rabbit anti-VGluT1 (diluted 1:500, Synaptic Systems #135303), mouse anti-GAD67 (diluted 1:100, Millipore #MAB5406), rabbit anti-VGLUT2 (diluted 1:500, Synaptic Systems #135402), rabbit anti-IBA1 (diluted 1:1,000, Wako #019-19741), NeuN (diluted 1:250, Millipore), GFAP (diluted 1:1,000, DakoCytomation #Z 0334). After three washes with PBS, sections were incubated with fluorescent secondary antibodies (1:1,000, Invitrogen Life Technologies, Waltham, MA) for 1 h at 22°C. Following three additional washes with PBS, nuclei were stained with DAPI (1:5,000 dilution in water), and sections were subsequently mounted with Vectashield (Vector Laboratories). The sections were imaged with a Zeiss LSM 700 confocal microscope or a Zeiss AxioImager A1 fluorescent microscope. A minimum of three animals for each group were prepared in all IHC experiments.
After isolation of RNA with BioRad Total RNA Extraction from Fibrous and Fatty Tissue kit (BioRad), cDNAs were generated from 200 ng RNA with the Superscript II Reverse Transcription First Strand cDNA Synthesis kit (Invitrogen). 10 ng RNA was combined with primers [5′-ATTGGACATAAGGGCGACAA-3′ and 5′-AGTCTCCTTTGGCTCCTGGT-3′ for Col19a1 (Su et al., 2016) or 5′-GGACCAGAGCGAAAGCATTTG-3′ and 5′- GCCAGTCGGCATCGTTTATG-3′ for 18S] and the iQ SYBRGreen Supermix (BioRad). Quantitative real-time PCR (qPCR) was performed on a Chromo 4 Four Color Real-Time system (BioRad) with 1 cycle of 95°C for 30 s and 40 cycles of amplification (95°C for 5 s, 60°C for 30 s, 55°C for 60 s, read plate) and a melting curve analysis. Relative quantities of RNA were determined using the ΔΔ-CT method (Livak and Schmittgen, 2001). At least three samples (each in triplicate) were examined for each age.
Previously reported olfactory preference tests (Kobayakawa et al., 2007; Zou et al., 2015) were modified for usage with a three-chamber box, where a middle chamber openly connects to two flanking chambers (Stoelting, Wood Dale, IL). Removable doors initially blocked access to the flanking chambers from the empty middle chamber. A 2 cm×2 cm filter paper was introduced to each of the two flanking chambers. Male mice were separated with female mice at least 3 weeks before experiments. A male test mouse (3–5 months old) was habituated in the closed middle chamber for 5 min. Mouse urine was collected in 1.5 ml tubes. 20 μL of previously collected mouse urine was then pipetted onto each of the two filter papers as a source of pheromones, with male urine on one filter paper and female urine on the other filter paper. The testing period lasted for 3 min total, beginning with the simultaneous removal of the two doors blocking the flanking chambers. During the 3 min test period, the time of the testing mouse’s visit to each flanking chamber (male urine chamber, female urine chamber) was recorded to quantify olfactory preference of male versus female pheromones. A minimum of n = 9 mice was included in each group tested.
The intensity of presynaptic terminals in sections of accessary olfactory bulbs was measures by ImageJ. At least 3 mice were analyzed per genotype, and the mean values were compared between groups. Student’s t-test were used to determine the significance between control and mutant groups.
Results are presented as bars indicating mean ± standard error of mean (SEM), with the datapoint of each mouse shown, unless otherwise indicated. Statistical analysis and preparation of graphs was carried out with GraphPad Prism 7. Comparisons of expression between different groups in Figure 1F was determined by performing a two-way analysis of variance (ANOVA test) followed by a Tukey post-hoc test. All other statistical analyses presented were performed by either a paired or unpaired Student’s t-test as indicated. No outlier tests or assessments of normality were conducted.
In our previous studies on Col19a1 expression in developing mouse neocortex we observed cellular expression of this ECM molecule in the olfactory bulb (Su et al., 2016; Figure 1B). To determine which type of cell generates Col19a1 and in what region of the olfactory bulb, we performed in situ hybridization (ISH) using Col19a1 riboprobes on sagittal section of mouse brains. As we reported before, robust Col19a1 expression cells was observed throughout the cerebral cortex and hippocampus (Su et al., 2010, 2016; Figures 1B–D). In contrast to the dispersed cellular expression in telencephalon, we found that Col19a1 expression in the olfactory bulb (OB) was both regionally and lamina specific: Col19a1-expressing cells were specifically distributed in the MCL of the AOB (Figures 1B, E) and anterior olfactory nucleus (Figure 1B and Supplementary Figure 1). We further examined the developmental regulation of Col19a1 mRNA in mouse OB by qPCR. Our results showed that Col19a1 expression was developmentally regulated. Col19a1 expression peaked at 7 days postnatal, after which its expression was significantly decreased (Figure 1F).
Our previous studies showed that Col19a1 mRNA was specifically generated by GABAergic neurons in the cortex and hippocampus (Su et al., 2010, 2016). We therefore hypothesized that Col19a1 would similarly be expressed by GABAergic neurons in the OB. For this we performed in situ hybridization coupled to immunohistochemistry with antibodies against IBA1 (which labels microglia), GFAP (which labels astrocytes) and NeuN (which labels most neurons), respectively. We found that Col19a1 was expressed by neurons but not by glia in mouse AOB (Figures 2A–C). We further performed double in situ hybridization for Col19a1 mRNA and Gad1 mRNA, which was generated by GABAergic neurons. In contrast to neocortex where Col19a1 was exclusively expressed by GABAergic neurons only a fraction [68 ± 3% (n = 3)] of Col19a1+ cells co-expressed Gad1 in the AOB (Figure 2D). More interestingly, When we tested Vglut1 and Vglut2, which were generated by excitatory neurons, we found that a small part [23 ± 3% (n = 3)] of Col19a1 was expressed by Vglut1 positive neurons (Figure 2E), but there is no Col19a1 was expressed by Vglut2 positive neurons (Figure 2F). Furthermore, while Col19a1 expression was largely restricted to Synaptotagmin 1 (Syt1) positive neurons in neocortex (Figures 2J, K), it was co-expressed by Synaptotagmin 2 (Syt2) positive neurons in the mouse OB (Su et al., 2010, 2016), (Figures 2H, I). Taken together, these data suggested Col19a1 was generated by neurons in the developing AOB, but this appears to be a different type of neuron than in neocortex.
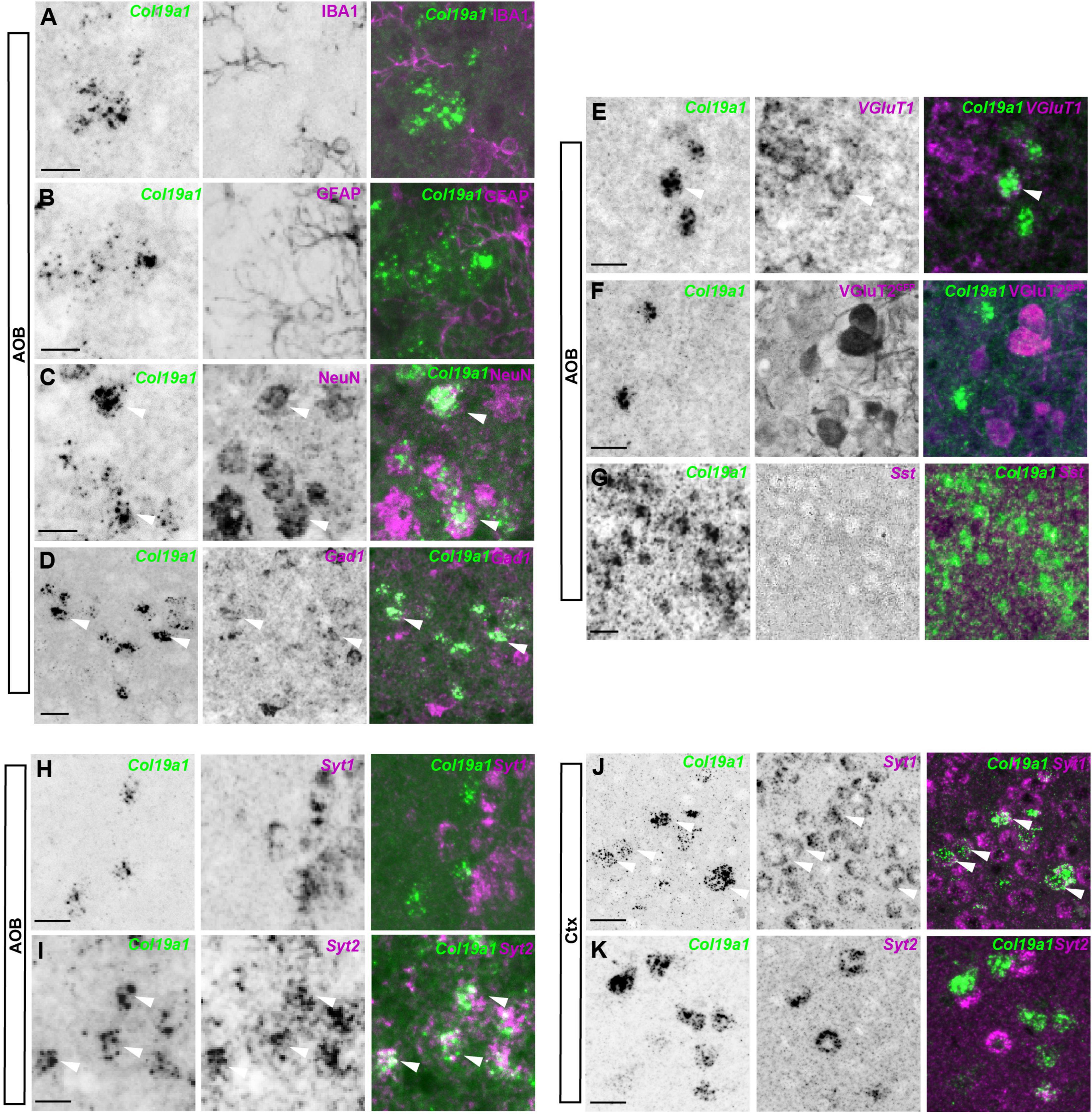
Figure 2. Col19a1 is expressed by distinct subsets of inhibitory neurons in the accessory olfactory bulb (AOB) and cortex. (A–G) ISH with Col19a1 anti-sense riboprobes was used to determine cell type expression in the AOB, Co-staining was performed with IBA1 (microglia) (A), GFAP (astrocytes) (B), NeuN (neurons) (C) and GFP (VGlut2-GFP, Excitatory neurons) (F) antibodies. Double in situ hybridization was performed with riboprobes against Gad1 (inhibitory neurons) (D), Vglut1 (excitatory neurons) (E) or Sst (interneurons) (G), where arrow heads indicate the co-expression of Col19a1 by NeuN+, Gad1+, and Vglut1+cells. (H–K) Comparison of Syt1 and Syt2 co-expression by Col19a1+ Cells in the AOB (H,I) and Ctx (J,K). Arrow heads indicate Syt2+ Col19a1+ cells in the AOB and examples of Syt1+ Col19a1+ and Syt2+ Col19a1+ in the Ctx. All images were acquired from sagittal tissue sections from P11-P14 wildtype mice. Scalebars indicate 20 μm.
Previous studies reported that the MCL of the AOB was critical for pheromone recognition preference (Pankevich et al., 2006), which led us to hypothesize that Col19a1 may be involved in pheromone recognition. We measured male mice’s olfactory preference for female urine using a three-chamber box (Figure 3A). Male mice have a significant preference to investigate the chamber containing filter paper saturated with female urine, rather than a room containing filter paper soaked with male urine (Figure 3B). We first tested how mutant mice globally lacking Collagen XIX (Col19a1–/–) [Mice have no motor activity and anxiety-related phenotypes (Su et al., 2016, 2020)] performed in this pheromone preference assay. We found that Col19a1–/– male mice spent similar time investigating the filter paper containing female or male urine, suggested that the loss of Collagen XIX led to impaired pheromone recognition (Figure 3C).
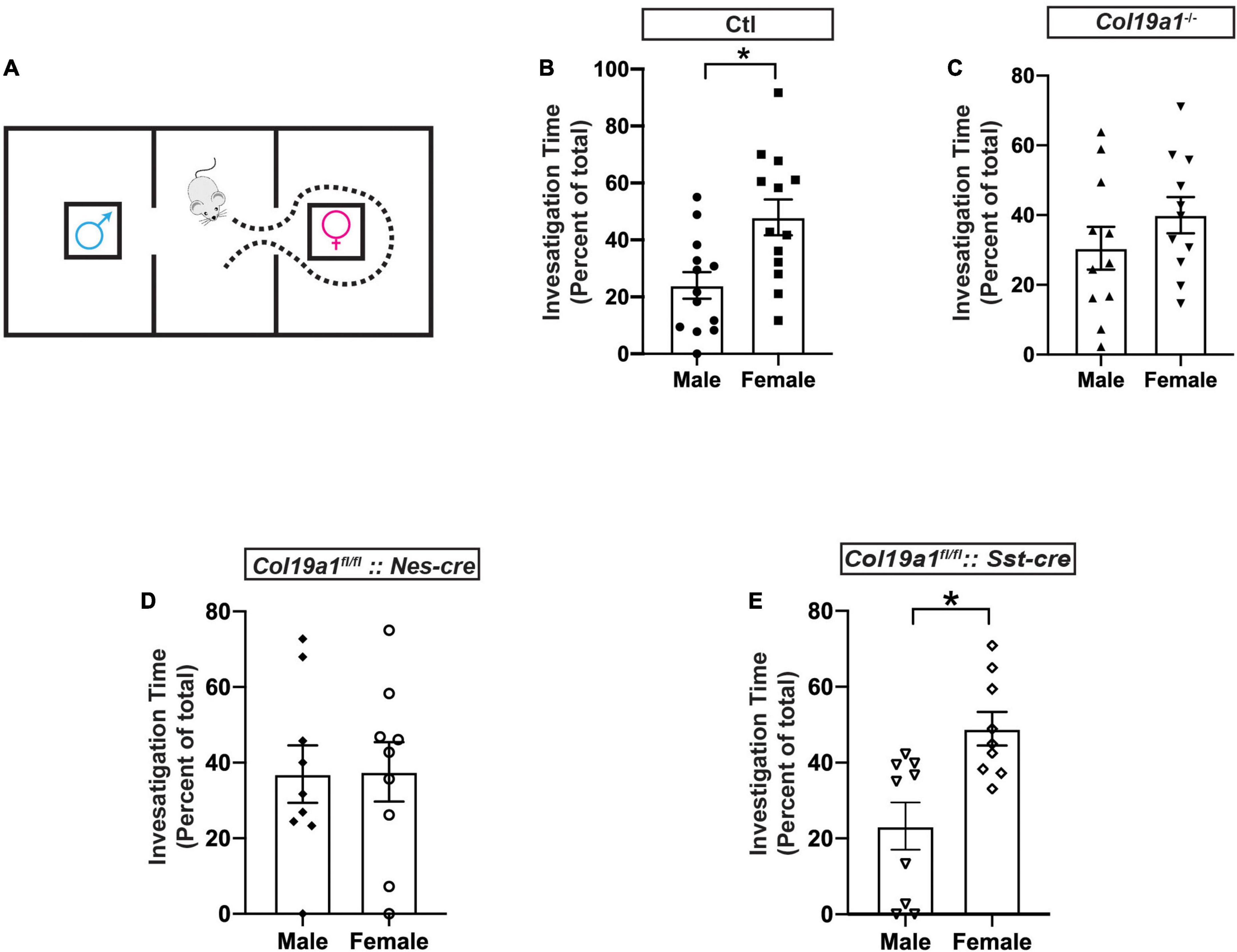
Figure 3. Olfactory function is disrupted in the absence of Col19a1. (A) Schematic for the male olfactory preference for female urine. A male mouse is placed in a three-room chamber, with female urine on filter paper in one chamber and male urine on filter paper in the other. (B–E) Comparison of male mouse preference for female urine over male urine between control (n = 13) (B), Col19a1– /– (n = 11) (C), Col19a1fl/fl: Nestin-cre (n = 9) (D), and Col19a1fl/fl: Sst-cre (n = 9) (E). *Indicates p < 0.05 with a paired Student’s t-test.
To assess whether brain-derived Collagen XIX was specifically necessary for pheromone preference, we crossed a conditional allele of Col19a1 (Col19a1fl/fl) mice with Nestin-cre (Nes-cre) mice to conditionally delete Collagen XIX from mouse brain. We found that the deletion of brain-derived Collagen XIX caused mice to lose pheromone preference as well (Figure 3D).
Since we found that somatostatin (Sst) was not expressed in the AOB (Figure 2G), we then probed whether Col19a1fl/fl:: Sst-cre mice would have changes in pheromone preference to rule out non-AOB contributions of Collagen XIX to this behavior. Similarly to wildtype mice, Col19a1fl/fl:: Sst-cre male mice spent significant more time in a chamber with female urine (Figure 3E). We interpreted this to suggest that the loss of Collagen XIX from the AOB (and not neocortex or anterior olfactory nucleus) impaired pheromone recognition in mice.
In order to uncover the mechanism by which Collagen XIX affects pheromone preference, we sought to test whether synapse formation was impaired in the AOB of these mutant mice, since previous studies showed that this collagen triggered inhibitory synapse formation in other brain regions (Su et al., 2010, 2016, 2020). To test this, we co-stained tissue sections from control and Col19a1–/– mice with antibodies that label inhibitory synapses (i.e., GAD67) and a subset of excitatory synapses (i.e., VGluT2). The data showed that the intensity of GAD67 or VGluT2 immunolabeling was not significantly different between mutants and controls in each layer of the AOB (Figures 4A–H). We next labeled another subset of excitatory synapses with antibodies against VGluT1. Surprisingly, VGluT1 fluorescence intensity was significantly reduced in both the GCL and MCL of Col19a1–/– mutants. VGluT1-immunolabeling in other brain regions appeared unaffected by the loss of Collagen XIX, such as in the GL of the AOB (Figures 4I–M).
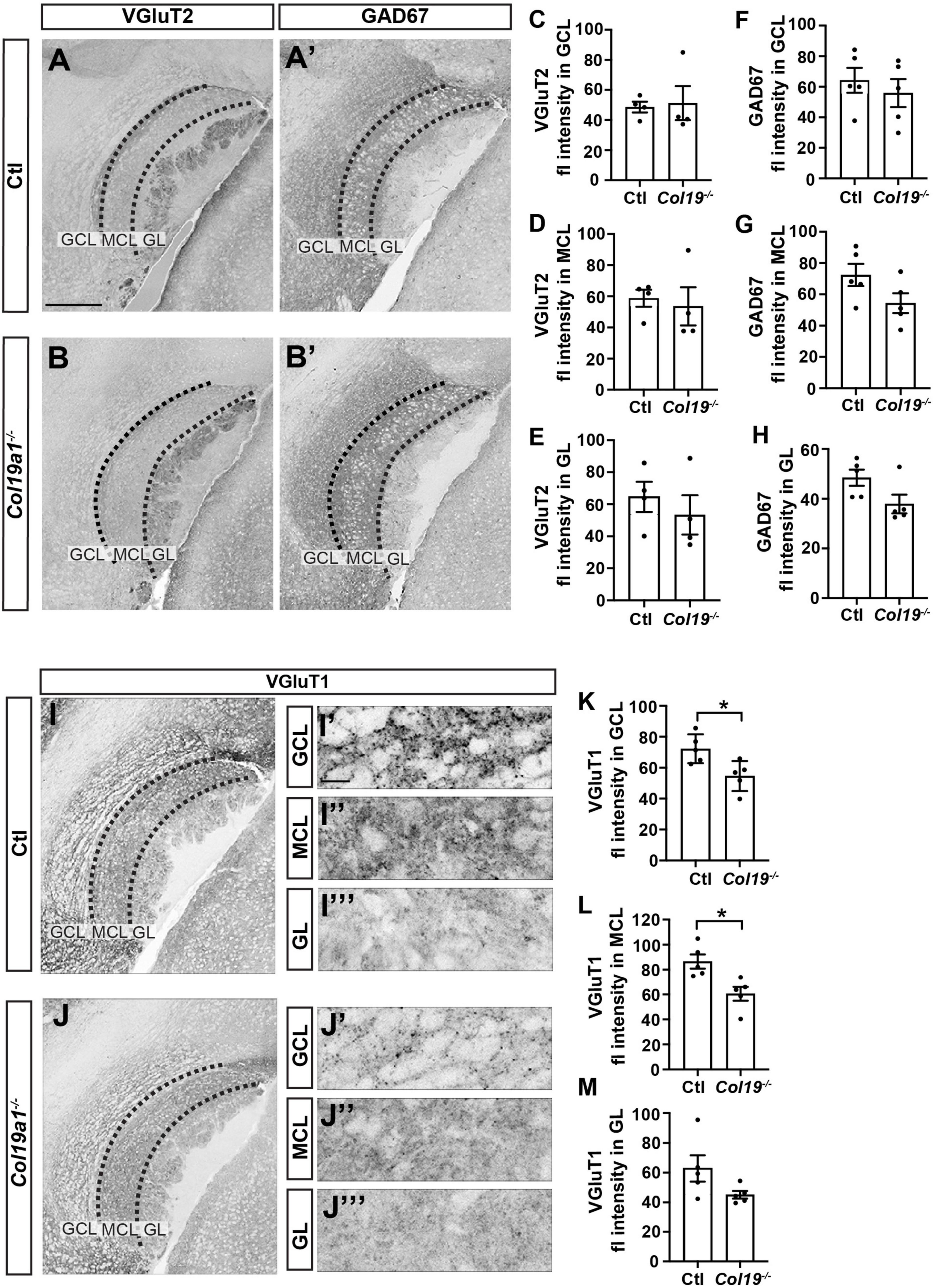
Figure 4. Excitatory synapses in the accessory olfactory bulb (AOB) are disrupted in Col19a1– /– mice. (A,B) Sagittal brain tissue from control and Col19a1– /– mice was stained with antibodies against VGluT2 and GAD67. (C–H) Quantification of VGluT2 and GAD67 immunoreactivity measured in the granule cell layer (GCL), mitral cell layer (MCL), and glomerular layer (GL) regions of the AOB. (I,J) Sagittal tissue from control and Col19a1– /– mice was stained with antibody against VGluT1, with magnifications of the GCL, MCL, and GL of the AOB shown to the side, (K–M) quantifications of VGluT1 immunoreactivity in the MCL, GCL, and GL. Age P14. *Indicates p < 0.05 with unpaired Student’s t-test. Scalebars indicate 200 μm in panels (A–J) and 20 μm in the insets (I’-I”’) and (J’-J”’). (n ≥ 3).
We next tested whether neuronal Collagen XIX was specifically essential to excitatory synapse formation in the AOB. We immuno-stained Col19a1fl/fl::Nes-cre mouse tissues with antibodies against GAD67, VGluT2 and VGluT1. Similar to Col19a1–/– mutants, loss of brain-derived Collagen XIX did not impact VGluT2 or GAD67 immunoreactivity in the AOB, but VGluT1 immunoreactivity was significantly decreased in MCL and GCL of the AOB (Figure 5). We observed synaptagmin 2 (Syt2) which mainly labels inhibitory synapses in mouse cortex. There was no significant difference between mutants and controls (Supplementary Figure 2).
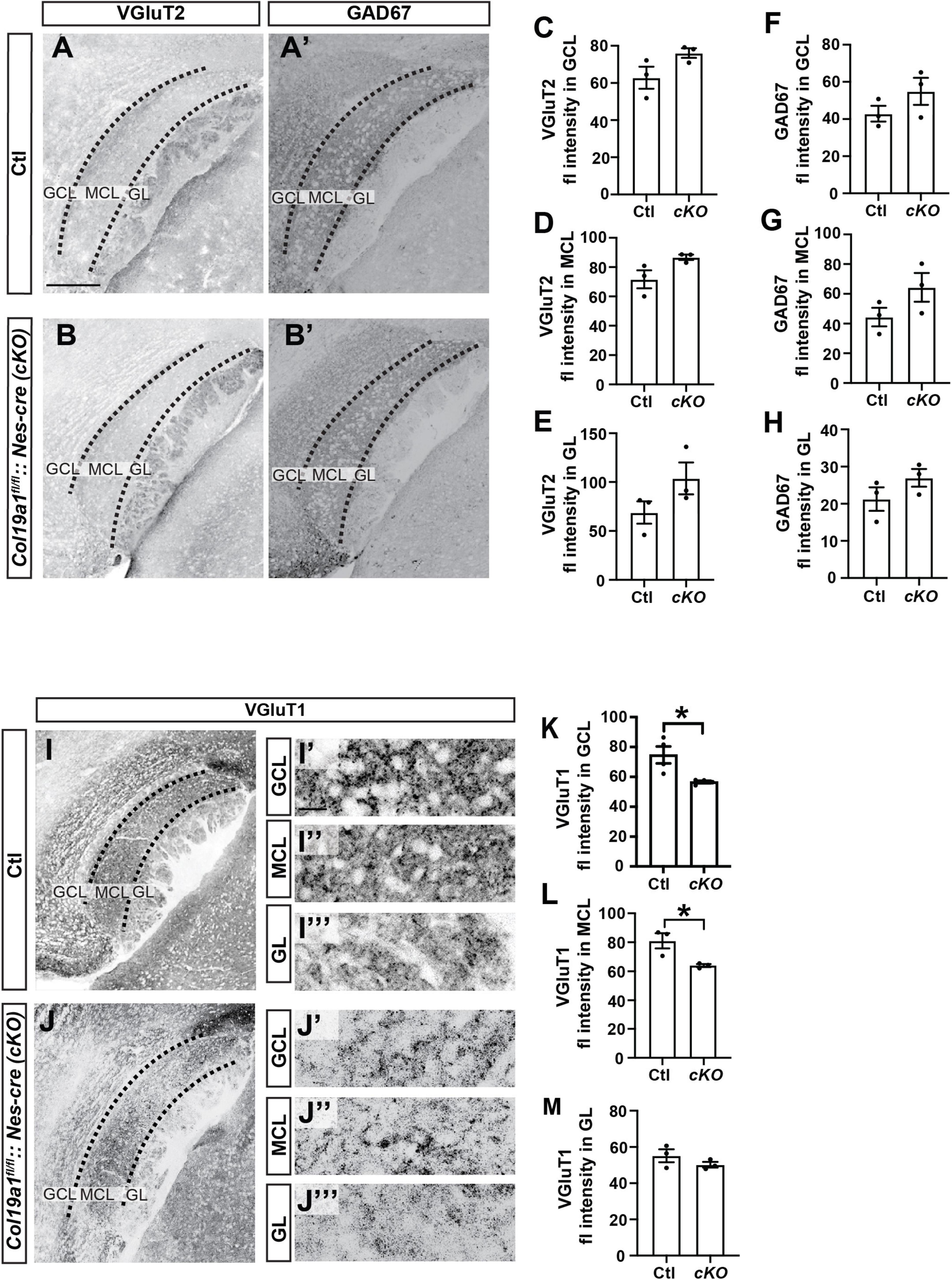
Figure 5. Excitatory synapses in the accessory olfactory bulb (AOB) are disrupted in conditional Col19a1 mutants. (A,B) Sagittal brains from control and Col19a1fl/fl: Nestin-cre (Col19a1fl/fl: Nes-cre) mice were stained with antibodies against VGluT2 and GAD67. (C–H) Quantification of VGluT2 and GAD67 immunoreactivity measured in the granule cell layer (GCL), mitral cell layer (MCL), and glomerular layer (GL) regions of the AOB. (I,J) Sagittal tissue from control and Col19a1fl/fl:Nes-cre mice stained with antibody against VGluT1, with magnifications of the GCL, MCL, and GL of the AOB shown to the side, (K–M). Quantifications of VGluT1 immunoreactivity in the MCL, GCL, and GL. Age P14. *Indicates p < 0.05 with unpaired Student’s t-test. Scalebars indicate 200 μm in panels (A–J) and 20 μm in the insets (I’-I”’) and (J’-J”’). (n ≥ 3).
When we immuno-stained Col19a1fl/fl::Sst-cre mouse tissues with antibodies against GAD67, Syt2, VGluT2, and VGluT1, we did not find significant changes in their immunoreactivity in AOB (Supplementary Figure 3). This further supports that pheromone recognition defects are the result of synaptogenesis defects in Collagen XIX global and neuronal deletion mice.
Collagen XIX has previously been shown to be both necessary and sufficient to trigger inhibitory synapse formation in the neocortex and hippocampus of mice (Su et al., 2010, 2016, 2020). Here, we added to our knowledge of this brain-derived ECM molecule by showing another specific population of neurons that generated Col19a1 mRNA in the AOB. In contrast to cell-specific Col19a1 expression by Syt1+ GABAergic neurons in mouse telencephalon, we found here that only 2/3 the Col19a1+ neurons in the AOB were GABAergic. By ablating the expression of Col19a1 (either globally or in neural cells), we demonstrated its key role in the assembly of VGluT1+ excitatory presynaptic terminal machinery in the MCL and GCL of the AOB, the first description of a developmental function for Col19a1 in excitatory synapse formation. This starkly contrasts Collagen XIX’s previously reported contribution to inhibitory synapse formation in the neocortex and hippocampus (Su et al., 2010, 2016, 2020), suggesting a regional specificity of Collagen XIX in synapse formation. Additionally, behavioral studies showed that Collagen XIX was necessary for male mice to have an olfactory preference for female scents (Figures 3C, D).
Collagen XIX is part of a family of non-fibrillar collagen termed unconventional collagens. Many such collagens (and other ECM proteins) contain protein domains that can be enzymatically released to exert unique bio-activities, distinct from the function of the uncleaved matrix protein. These released fragments are termed matricryptins (Ramont et al., 2007; Su et al., 2016). Previous studies showed that Collagen XIX triggers inhibitory nerve assembly by releasing such a matricryptin, a short C-terminal fragment containing the non-collagenous domain 1 (NC1 domain) fragment of Collagen XIX, termed NC1[XIX] (Su et al., 2016). NC1[XIX] binds integrin receptors, leading to the assembly of presynaptic nerve terminals in a subset of GABAergic interneurons in telencephalon. As such, we interpreted the decrease in VGluT1 immunoreactivity in the Col19a1–/– AOB shown here (Figures 4I–M) to suggest an impaired assembly of excitatory nerve terminals due to the loss of NC1[XIX] signaling.
In many cases, synaptogenic cues are target derived molecules, which means that they are produced from the postsynaptic neurons (Fox and Umemori, 2006), such as target derived adhesion molecules, growth factors, and matrix molecules (Scheiffele et al., 2000; Umemori et al., 2004; Fox et al., 2007; Terauchi et al., 2010; Su et al., 2012). We previously reported that Collagen XIX is not a target derived synaptogenic molecule in mouse neocortex (Su et al., 2016, 2020). Based on the results collected here, we think that Collagen XIX is likely not a target derived synaptogenetic molecule in mouse OB. This is due to the fact that Col19a1 was specifically expressed in the MCL, but it impacts VGluT1+ terminals in both the MCL and GCL. It more likely acts in a paracrine fashion, similar to a number of glial-derived synaptogenic cues (Ullian et al., 2004; Christopherson et al., 2005; Kucukdereli et al., 2011).
We found that wildtype adult male mice have a significant preference for female urine (Figure 3B). This preference for female urine was consistent with the well-characterized finding that male mice prefer investigating female pheromones and urine (Mandiyan et al., 2005; Pankevich et al., 2006; Martel and Baum, 2009; Zou et al., 2013). When Collagen XIX was removed globally (Col19a1–/–) or from all neural cells (Col19a1fl/fl:: Nes-cre), male mice no longer exhibited a preference for female urine (Figures 3C, D). We interpreted this to be due to its presence or function in the AOB, however, it could have been a secondary sequence of the loss of this collagen elsewhere in the brain. We found that Sst+ cells are not present in the AOB (Figure 2G) but are present in the anterior olfactory nucleus (Supplementary Figure 1) and cortex (Su et al., 2020), and are the main source of Collagen XIX in this region. Loss of Collagen XIX from Sst+ cells are sufficient to lead to impair GABAergic synapse formation and behavioral abnormalities in mice (Su et al., 2020). Thus Col19a1fl/fl::Sst-cre mice became a powerful control to address the regional specificity of Col19a1 in pheromone function. In Col19a1fl/fl::Sst-cre mice, the male preference for female urine was similar to wildtype (Figure 3E). We interpreted this to mean that telencephalic (or Sst+ cell) expression of Col19a1 was not necessary for olfactory preference and that Col19a1 expression in the AOB is likely essential to pheromone sensing.
The dependence of olfactory preference on the MCL in the AOB is consistent with previous findings in which ablation of the AOB led to impaired olfactory preference for female pheromones (Jakupovic et al., 2008). The AOB is a key part of the mouse limbic system (Maksimova et al., 2019) and responsible for directing input from sensory neurons in the VNO, which are responsible for sensing pheromones (Pankevich et al., 2004, 2006). The mitral cells in the AOB project to the medial amygdala (MeA) and the posteromedial cortical nucleus of the amygdala (PMCo) and subsequently to multiple regions of the hypothalamus in order to direct reproductive behavior (Kang et al., 2009; Martel and Baum, 2009; Maksimova et al., 2019). Other studies report that when the VNO was removed from mice, less granule cell and MCL activity (less Fos-immunoreactive cells) was observed in the AOB, and the olfactory preference of male mice for female urine was eliminated (Pankevich et al., 2004). The strong dependence of olfactory preference on MCL activity and connectivity supports that Col19a1 is critical to the assembly and function of the VNO pathway (VNO to AOB to MeA to hypothalamus) in processing pheromones.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Virginia Polytechnic Institute and State University Institutional Animal Care and Use Committee (IACUC).
CA performed research and wrote the manuscript. MF acquired resources and funding and edited the manuscript. JS designed and performed research and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the I. D. Wilson Chair in the Virginia Tech College of Science (MF) and through grants from the National Institutes of Health (NIH) (EY-021222, AI-124677, and EY-030568) (MF) and the Brain and Behavior Research Foundation (MF). CA was supported by a fellowship provided by a Fralin Biomedical Research Institute at VTC Translational Neurobiology Summer Undergraduate Research Fellowship (neuroSURF) program supported by NIH Grant NS-105141 (MF).
We thank all other colleagues at Fox Lab for their support and help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor BT declared a past co-authorship [10.1002/cne.25261] with the author MF.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2023.1157577/full#supplementary-material
Supplementary Figure 1 | Most of Col19a1 is expressed by Sst positive neurons in anterior olfactory nucleus. Images were acquired from sagittal tissue sections from P14 Sst-cre-tdT mice. Scalebars indicate 20 μm.
Supplementary Figure 2 | Syt2 synapses are not disrupted in Col19a1fl/fl: Nes-cre conditional mutant AOB. (A,B) Sagittal brains from control and Col19a1fl/fl: Nestin-cre (Col19a1fl/fl: Nes-cre) mice were stained with antibodies against Syt2. (C–E) Quantification of Syt2 immunoreactivity measured in the GCL (C), MCL (D), and GL (E) regions of the AOB. Scalebars indicate 200 μm in panels (A,B). (n = 3).
Supplementary Figure 3 | Synaptogenesis of AOB region is not affected in Col19a1fl/fl: Ssts-cre mouse tissue. (A,B) Sagittal brain tissue from control and Col19a1fl/fl: Sst-cre mice was stained with antibodies against VGluT2 and GAD67. (C–H) Quantification of VGluT2 and GAD67 immunoreactivity measured in the GCL, MCL, and GL regions of the AOB. (I,J) Sagittal tissue from control and Col19a1fl/fl: Sst-cre mice was stained with antibody against VGluT1 and Syt2. (K–P) Quantification of VGluT1 and Syt2 immunoreactivity measured in the GCL, MCL, and GL regions of the AOB. Scalebars indicate 200 μm in panels (A,B) and (I,J). (n = 3).
Belluscio, L., Koentges, G., Axel, R., and Dulac, C. (1999). A map of pheromone receptor activation in the mammalian brain. Cell 97, 209–220. doi: 10.1016/s0092-8674(00)80731-x
Brignall, A. C., Raja, R., Phen, A., Prince, J. E. A., Dumontier, E., and Cloutier, J. F. (2018). Loss of Kirrel family members alters glomerular structure and synapse numbers in the accessory olfactory bulb. Brain Struct. Funct. 223, 307–319. doi: 10.1007/s00429-017-1485-0
Carrillo, G. L., Su, J., Cawley, M. L., Wei, D., Gill, S. K., Blader, I. J., et al. (2023). Complement-dependent loss of inhibitory synapses on pyramidal neurons following Toxoplasma gondii infection. J. Neurochem. [Epub ahead of print]. doi: 10.1111/jnc.15770
Christopherson, K. S., Ullian, E. M., Stokes, C. C., Mullowney, C. E., Hell, J. W., Agah, A., et al. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. doi: 10.1016/j.cell.2004.12.020
Fox, M. A., Sanes, J. R., Borza, D. B., Eswarakumar, V. P., Fassler, R., Hudson, B. G., et al. (2007). Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129, 179–193. doi: 10.1016/j.cell.2007.02.035
Fox, M. A., and Umemori, H. (2006). Seeking long-term relationship: Axon and target communicate to organize synaptic differentiation. J. Neurochem. 97, 1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x
Hayashi, Y., Momiyama, A., Takahashi, T., Ohishi, H., Ogawa-Meguro, R., Shigemoto, R., et al. (1993). Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature 366, 687–690. doi: 10.1038/366687a0
Holy, T. E. (2018). The accessory olfactory system: Innately specialized or microcosm of mammalian circuitry? Annu. Rev. Neurosci. 41, 501–525. doi: 10.1146/annurev-neuro-080317-061916
Jakupovic, J., Kang, N., and Baum, M. J. (2008). Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol. Behav. 93, 467–473. doi: 10.1016/j.physbeh.2007.10.005
Jia, C., Chen, W. R., and Shepherd, G. M. (1999). Synaptic organization and neurotransmitters in the rat accessory olfactory bulb. J. Neurophysiol. 81, 345–355. doi: 10.1152/jn.1999.81.1.345
Kang, N., Baum, M. J., and Cherry, J. A. (2009). A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 29, 624–634. doi: 10.1111/j.1460-9568.2009.06638.x
Kobayakawa, K., Kobayakawa, R., Matsumoto, H., Oka, Y., Imai, T., Ikawa, M., et al. (2007). Innate versus learned odour processing in the mouse olfactory bulb. Nature 450, 503–508. doi: 10.1038/nature06281
Kucukdereli, H., Allen, N. J., Lee, A. T., Feng, A., Ozlu, M. I., Conatser, L. M., et al. (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U.S.A. 108, E440–E449. doi: 10.1073/pnas.1104977108
Larriva-Sahd, J. (2008). The accessory olfactory bulb in the adult rat: A cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J. Comp. Neurol. 510, 309–350. doi: 10.1002/cne.21790
Liberles, S. D. (2014). Mammalian pheromones. Annu. Rev. Physiol. 76, 151–175. doi: 10.1146/annurev-physiol-021113-170334
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, M. (2007). Encoding olfactory signals via multiple chemosensory systems. Crit. Rev. Biochem. Mol. Biol. 42, 463–480. doi: 10.1080/10409230701693359
Maksimova, M. A., Cansler, H. L., Zuk, K. E., Torres, J. M., Roberts, D. J., and Meeks, J. P. (2019). Interneuron functional diversity in the mouse accessory olfactory bulb. eNeuro 6:ENEURO.0058–19.2019. doi: 10.1523/ENEURO.0058-19.2019
Mandiyan, V. S., Coats, J. K., and Shah, N. M. (2005). Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat. Neurosci. 8, 1660–1662. doi: 10.1038/nn1589
Martel, K. L., and Baum, M. J. (2009). A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 29, 368–376. doi: 10.1111/j.1460-9568.2008.06564.x
Meredith, M. (1991). Sensory processing in the main and accessory olfactory systems: Comparisons and contrasts. J. Steroid Biochem. Mol. Biol. 39, 601–614. doi: 10.1016/0960-0760(91)90258-7
Mohrhardt, J., Nagel, M., Fleck, D., Ben-Shaul, Y., and Spehr, M. (2018). Signal detection and coding in the accessory olfactory system. Chem. Senses 43, 667–695. doi: 10.1093/chemse/bjy061
Mori, K., and Sakano, H. (2021). Olfactory circuitry and behavioral decisions. Annu. Rev. Physiol. 83, 231–256. doi: 10.1146/annurev-physiol-031820-092824
Pankevich, D. E., Baum, M. J., and Cherry, J. A. (2004). Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J. Neurosci. 24, 9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004
Pankevich, D. E., Cherry, J. A., and Baum, M. J. (2006). Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav. Neurosci. 120, 925–936. doi: 10.1037/0735-7044.120.4.925
Ramont, L., Brassart-Pasco, S., Thevenard, J., Deshorgue, A., Venteo, L., Laronze, J. Y., et al. (2007). The NC1 domain of type XIX collagen inhibits in vivo melanoma growth. Mol. Cancer Ther. 6, 506–514. doi: 10.1158/1535-7163.MCT-06-0207
Rodriguez, I., Feinstein, P., and Mombaerts, P. (1999). Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell 97, 199–208. doi: 10.1016/s0092-8674(00)80730-8
Sakano, H. (2020). Developmental regulation of olfactory circuit formation in mice. Dev. Growth Differ. 62, 199–213. doi: 10.1111/dgd.12657
Scheiffele, P., Fan, J., Choih, J., Fetter, R., and Serafini, T. (2000). Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669. doi: 10.1016/s0092-8674(00)80877-6
Su, J., Basso, D., Iyer, S., Su, K., Wei, J., and Fox, M. A. (2020). Paracrine role for somatostatin interneurons in the assembly of perisomatic inhibitory synapses. J. Neurosci. 40, 7421–7435. doi: 10.1523/JNEUROSCI.0613-20.2020
Su, J., Chen, J., Lippold, K., Monavarfeshani, A., Carrillo, G. L., Jenkins, R., et al. (2016). Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex. J. Cell Biol. 212, 721–736. doi: 10.1083/jcb.201509085
Su, J., Gorse, K., Ramirez, F., and Fox, M. A. (2010). Collagen XIX is expressed by interneurons and contributes to the formation of hippocampal synapses. J. Comp. Neurol. 518, 229–253. doi: 10.1002/cne.22228
Su, J., Stenbjorn, R. S., Gorse, K., Su, K., Hauser, K. F., Ricard-Blum, S., et al. (2012). Target-derived matricryptins organize cerebellar synapse formation through alpha3beta1 integrins. Cell Rep. 2, 223–230. doi: 10.1016/j.celrep.2012.07.001
Sumiyoshi, H., Inoguchi, K., Khaleduzzaman, M., Ninomiya, Y., and Yoshioka, H. (1997). Ubiquitous expression of the alpha1(XIX) collagen gene (Col19a1) during mouse embryogenesis becomes restricted to a few tissues in the adult organism. J. Biol. Chem. 272, 17104–17111. doi: 10.1074/jbc.272.27.17104
Sumiyoshi, H., Mor, N., Lee, S. Y., Doty, S., Henderson, S., Tanaka, S., et al. (2004). Esophageal muscle physiology and morphogenesis require assembly of a collagen XIX-rich basement membrane zone. J. Cell Biol. 166, 591–600. doi: 10.1083/jcb.200402054
Taniguchi, M., and Kaba, H. (2001). Properties of reciprocal synapses in the mouse accessory olfactory bulb. Neuroscience 108, 365–370. doi: 10.1016/s0306-4522(01)00427-4
Terauchi, A., Johnson-Venkatesh, E. M., Toth, A. B., Javed, D., Sutton, M. A., and Umemori, H. (2010). Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 465, 783–787. doi: 10.1038/nature09041
Ullian, E. M., Christopherson, K. S., and Barres, B. A. (2004). Role for glia in synaptogenesis. Glia 47, 209–216. doi: 10.1002/glia.20082
Umemori, H., Linhoff, M. W., Ornitz, D. M., and Sanes, J. R. (2004). FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270. doi: 10.1016/j.cell.2004.06.025
Yokosuka, M. (2012). Histological properties of the glomerular layer in the mouse accessory olfactory bulb. Exp. Anim. 61, 13–24. doi: 10.1538/expanim.61.13
Zou, J., Storm, D. R., and Xia, Z. (2013). Conditional deletion of ERK5 MAP kinase in the nervous system impairs pheromone information processing and pheromone-evoked behaviors. PLoS One 8:e76901. doi: 10.1371/journal.pone.0076901
Keywords: accessory olfactory bulb, Collagen XIX, glutamatergic synapse, synaptogenesis, pheromone recognition, development
Citation: Amos C, Fox MA and Su J (2023) Collagen XIX is required for pheromone recognition and glutamatergic synapse formation in mouse accessory olfactory bulb. Front. Cell. Neurosci. 17:1157577. doi: 10.3389/fncel.2023.1157577
Received: 02 February 2023; Accepted: 21 March 2023;
Published: 05 April 2023.
Edited by:
Bhanu P. Tewari, University of Virginia, United StatesReviewed by:
Zhonghua Lu, Shenzhen Institute of Advanced Technology (CAS), ChinaCopyright © 2023 Amos, Fox and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmin Su, c3VqbUB2dGMudnQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.