- Department of Neurobiology, Hokkaido University Graduate School of Medicine, Sapporo, Japan
Modification of axonal excitability directly impacts information transfer through the neuronal networks in the brain. However, the functional significance of modulation of axonal excitability by the preceding neuronal activity largely remains elusive. One remarkable exception is the activity-dependent broadening of action potential (AP) propagating along the hippocampal mossy fibers. The duration of AP is progressively prolonged during repetitive stimuli and facilitated presynaptic Ca2+ entry and subsequent transmitter release. As an underlying mechanism, accumulated inactivation of axonal K+ channels during AP train has been postulated. As the inactivation of axonal K+ channels proceeds on a timescale of several tens of milliseconds slower than the millisecond scale of AP, the contribution of K+ channel inactivation in AP broadening needs to be tested and evaluated quantitatively. Using the computer simulation approach, this study aimed to explore the effects of the removal of the inactivation process of axonal K+ channels in the simple but sufficiently realistic model of hippocampal mossy fibers and found that the use-dependent AP broadening was completely abolished in the model replaced with non-inactivating K+ channels. The results demonstrated the critical roles of K+ channel inactivation in the activity-dependent regulation of axonal excitability during repetitive action potentials, which critically imparts additional mechanisms for robust use-dependent short-term plasticity characteristics for this particular synapse.
Introduction
Use-dependent modification of the synaptic strength offers the neural basis for encoding temporal information of the activity into the subsequent synaptic strength. The gain and the time course of the use-dependent modification are defined strictly depending on the types of synapses for their roles in neural computation. The hippocampal mossy fiber synapse is well-known for exhibiting characteristic robust short-term synaptic plasticity with large frequency-dependent facilitation over a more than 10-fold range (Salin et al., 1996; Henze et al., 2000; Nicoll and Schmitz, 2005). The underlying mechanisms have been studied extensively using experimental approaches, including direct subcellular recordings from the axon terminals (Bischofberger et al., 2006; Schmidt-Hieber et al., 2008; Ohura and Kamiya, 2018; Vandael et al., 2021) and fluorescent Ca2+ measurement (Kamiya and Ozawa, 1999; Kamiya et al., 2002). In addition to the classical residual calcium mechanism (Katz and Miledi, 1968; Zucker and Regehr, 2002), it has been demonstrated that modification of the duration of axonal action potentials contributes substantially to robust short-term plasticity at this synapse (Geiger and Jonas, 2000). Activity-dependent broadening of axonal action potentials has been attributed to the accumulated inactivation of axonal K+ channels different from those observed in squid giant axons which do not inactivate during prolonged depolarization (Hodgkin and Huxley, 1952). As demonstrated for pituitary nerve terminals (Jackson et al., 1991) and invertebrate neurons (Byrne and Kandel, 1996), the previous study using direct subcellular recording from the mossy fiber terminals showed that the axonal K+ currents display robust inactivation during prolonged depolarization with a time constant of several tens of milliseconds (Geiger and Jonas, 2000; see also Cooper et al., 1998). However, as each action potential during the train lasts for a millisecond timescale, it is less certain whether these axonal K+ channels inactivate during repetitive action potentials with a millisecond time course. To get insight into the roles of inactivating K+ channels at the mossy fiber bouton, we attempted to test for the consequence of replacing the K+ channel model with those without inactivation as a classical Hodgkin–Huxley type model using a computational approach. For quantitative evaluation of the contribution of accumulated inactivation of axonal K+ channels during repetitive action potentials, we compared the simulation results between those obtained by the models containing or lacking inactivation properties. It was found that the activity-dependent broadening of action potential observed in the mossy fiber model with inactivating K+ channels was completely abolished when the K+ channel model was replaced with a non-inactivating one. The calculated K+ current was progressively decreased while the calculated Ca2+ current was increased during the repetitive action potentials, and these use-dependent modulations were completely abolished when the K+ channel model was replaced with a non-inactivating one. Taken together, our simulation data suggested that inactivating profiles of K+ channels impart additional mechanisms for short-term synaptic plasticity of an extremely large wide dynamic range characteristic for the hippocampal mossy fiber synapse.
Materials and methods
Simulation
The simulated membrane potential (Vm) was calculated according to the model suggested by Engel and Jonas (2005) based on the data recorded from mossy fiber boutons (see also Kamiya, 2019). All model and simulation files have been uploaded to the ModelDB database (https://senselab.med.yale.edu/modeldb/ accession no. 267617). The model assumed a Hodgkin–Huxley-type gating model adapted to channels recorded in mossy fiber terminals. For K+ channel, we used either the non-inactivating K+ channel from Schmidt-Hieber and Bischofberger, 2010 (hhmfb.mod, ModelDB accession no. 128079) or the inactivating K+ channel that they constructed by taking the non-inactivating K+ channel and adding an inactivation parameter based on recombinant KV1.4 channels described by Wissmann et al., 2003 (KIn.mod, ModelDB accession no. 128079). Simulations were performed using NEURON 7.8 for Windows (Hines and Carnevale, 1997). The passive electrical properties of the axon were assumed to be uniform, with a specific membrane capacitance Cm of 1 μF cm−2, a specific membrane resistance Rm of 10,000 Ω cm2, and an intracellular resistivity Ri of 70 Ω cm (Engel and Jonas, 2005; Alle and Geiger, 2006). The resting membrane potential was set to −80 mV. The structure of the mossy fiber (Acsády et al., 1998; Henze et al., 2000) was approximated by a soma (diameter, 10 μm), 10 axonal cylinders (diameter, 0.2 μm; length, 100 μm), and 10 en passant boutons (diameter, 4 μm). The number of segments was 1 μm−1 in all simulations. The reversal potential of the leak conductance was set to −81 mV to maintain stability. For all simulations in this study, the time step was set as 0.01 ms to describe the time course of fast action potentials and the underlying currents.
Voltage-gated Na+ channels, K+ channels, and leakage channels were inserted into the soma, axon, and boutons, as in the previous study (Kamiya, 2019). The Na+ conductance density was set to 50 mS cm−2 for the axon and boutons and 10 mS cm−2 for the soma. The K+ conductance density was set to 36 mS cm−2 throughout all parts of the neurons, unless otherwise stated. In some simulations, K+ conductance density was changed to half (18 mS cm−2) or 10 times (360 mS cm−2) from the original value of 36 mS cm−2 to observe the change in action potential broadening by repetitive stimulation. Action potentials were evoked by the injection of depolarizing current into the soma (2 ms, 0.2 nA). The equilibrium potentials for Na+ and K+ ions were assumed to be +50 and −85 mV, respectively. In some simulations, the non-inactivating K+ channel model, which lacks inactivation properties, was used instead of inactivating K+ channel model. The hhmfb.mod, a Hodgkin–Huxley-type model for the set of sodium, potassium, and leakage channels adapted to channels in mossy fiber terminals, was downloaded from ModelDB (accession no. 128079) for a non-inactivating IK model for sodium and potassium conductances. For inactivating IK model, the potassium conductance of hhmfb.mod was omitted and instead inserted Kin.mod, a Hodgkin–Huxley model of inactivating K channels representing kinetics of KV1.4, downloaded from the same ModelDB (accession no. 128079).
The models of presynaptic Ca2+ channels of P/Q type, N type, and R type are reconstructed previously (Kamiya, 2022) using the kinetic parameters obtained experimentally (Li et al., 2007). The gating models of these presynaptic Ca2+ channels assumed six states gating model consisting of five closed states (C0–C4) and a single open state (O) for each subtype. Transitions from C0 to C4 are assumed to be voltage-dependent, while a transition from C4 to O is voltage-independent. The equilibrium potential for Ca2+ ions was assumed to be +60 mV according to the value obtained from the recording from hippocampal mossy fiber terminals (Li et al., 2007) and used for the simulation in their study.
For simulations in Figure 7, the kmb.mod, a model for the KV7 M-type K channels on the mossy fiber boutons was downloaded from ModelDB (accession no. 245417) and implemented evenly to the axons and boutons. The conductance density was set to 5 mS cm−2 according to the simulation in the previous study (Martinello et al., 2019).
Axonal action potentials, as well as K+ and Ca2+ currents at the mossy fiber boutons evoked by the repetitive action potentials of 50 times at 10, 20, and 50 Hz, were calculated for testing the use-dependent modification.
Results
Simulated action potentials with inactivating and non-inactivating K+ channel models
So far, we have reconstructed a simple and sufficiently realistic model of hippocampal mossy fibers with a “pearl chain” structure (Engel and Jonas, 2005) implemented with models of axonal voltage-dependent Na+ and K+ channels (Kamiya, 2019) and presynaptic Ca2+ channels (Kamiya, 2022) based on the kinetic parameters determined experimentally (Geiger and Jonas, 2000; Engel and Jonas, 2005; Li et al., 2007). First, we tested the time course of Na+ (INa), K+ (IK), and Ca2+ (ICa) currents in a single-compartment model with a 7 μm diameter. INa and IK were tested with 100 ms depolarization to 30 mV, and ICa was tested with 20 ms depolarization to 0 mV. K+ current in the original model shows robust inactivation during 100 ms depolarization to 30 mV (Figure 1A) as shown in the black asterisk in the lower trace. In contrast, the K+ current in the non-inactivating IK model does not show inactivation during the same depolarizing command (Figure 1B) as shown in the blue double-asterisk in the lower trace. Using these two model sets of IK with and without inactivation in the multicompartment model of hippocampal mossy fibers (Figure 2A), we compared the waveform of the propagating action potentials (Vm), as well as K+ (IK) and Ca2+ (ICa) currents in the models equipped with the inactivating (Figure 2B) and the non-inactivating IK (Figure 2C). As shown in superimposed traces, the replacement of the K+ channel model with the one lacking inactivation shows a minimal effect on the repolarization phase (Figure 2D).
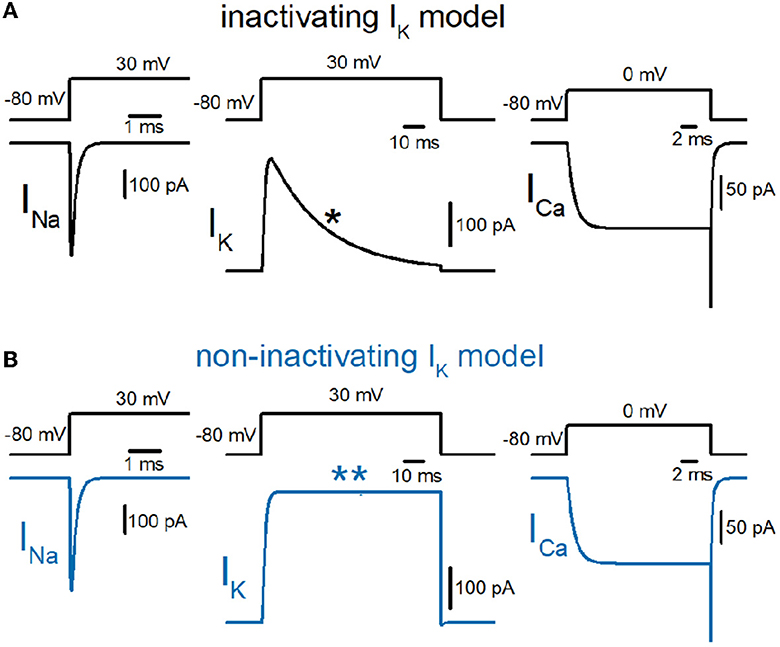
Figure 1. Reconstruction of axonal sodium, potassium, and presynaptic calcium current on the hippocampal mossy fibers. (A) Kinetic properties of sodium (INa), potassium (IK), and calcium (ICa) current of the models reconstructed from the experimental data obtained from hippocampal mossy fiber boutons. Single-compartment models of 7 μm diameter were used for these simulations. The IK currents with fast inactivation (as marked by the asterisk) were used for calculation (black traces). (B) Those of the models whose potassium channel property was replaced with the non-inactivating one (as marked by a double-asterisk, blue traces).
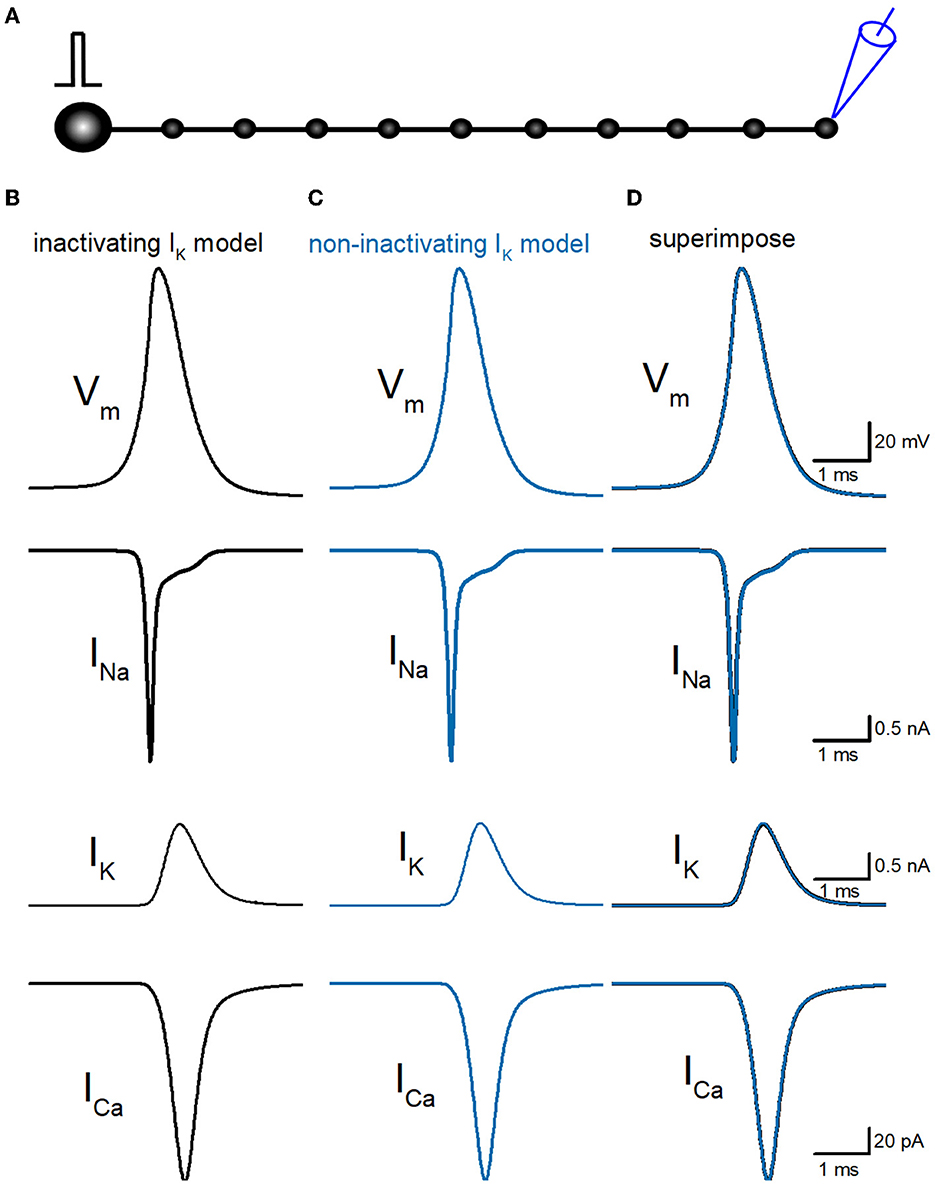
Figure 2. Reconstruction of propagating action potentials with inactivating and non-inactivating axonal K+ channels. (A) Schematic drawing of the multi-compartment model of the granule cell and mossy fiber was reconstructed according to the previous study by Alle and Geiger (2006). (B) Simulated propagating action potentials at the mossy fiber bouton (Vm), axonal Na+ (INa), K+ (IK), and Ca2+ (ICa) currents during the action potentials with the inactivating IK model. (C) Those of non-inactivating IK model (blue traces). (D) Superimposed traces of (B, C).
Use-dependent action potential broadening in hippocampal mossy fiber model
Then, we examined whether our model displays a broadening of action potential in response to repetitive stimuli as reported in the experimental study (Geiger and Jonas, 2000). To examine the use-dependent broadening of action potentials, 50 times stimuli at 10, 20, and 50 Hz were tested to observe the frequency dependency. In these conditions, all action potentials were elicited by the repetitive stimuli faithfully, excluding the possibility of conduction block by inactivation of Na+ channels. Repetitive stimuli of 50 times at 20 Hz progressively slowed the repolarization, thereby causing a broadening of action potentials (Figure 3A). Half-duration of the 50th action potential (1.18 ms) was 152% of the 1st action potential (0.777 ms) for the 20-Hz train. We also calculated K+ (IK) and Ca2+ (ICa) currents during repetitive action potentials, to look for the consequence of action potential broadening. IK during action potential progressively decreased the amplitude (Figure 3B), as expected for cumulative inactivation by the repetitive trains. The peak amplitude of the 50th IK (0.732 nA) was 47.0% of the 1st responses (1.56 nA) for the 20-Hz train. On the other hand, ICa during the action potential is progressively increased by the repetitive train of action potentials (Figure 3C). The peak amplitude of the 50th ICa (174 pA) was 117% of the 1st responses (149 pA) for the 20-Hz train. The Ca2+ charge was also calculated as it was reported to be more correlated with AP broadening than ICa peak amplitude (Geiger and Jonas, 2000). The charge due to the inflow of Ca2+ was enhanced by repetitive stimuli due to the potential activity-dependent changes in the synaptic strength being more related to the physiological states affecting the signaling in the brain. The calculated charge of Ca2+ inflow was enhanced by 165% of control by a 20-Hz train of 50 stimuli.
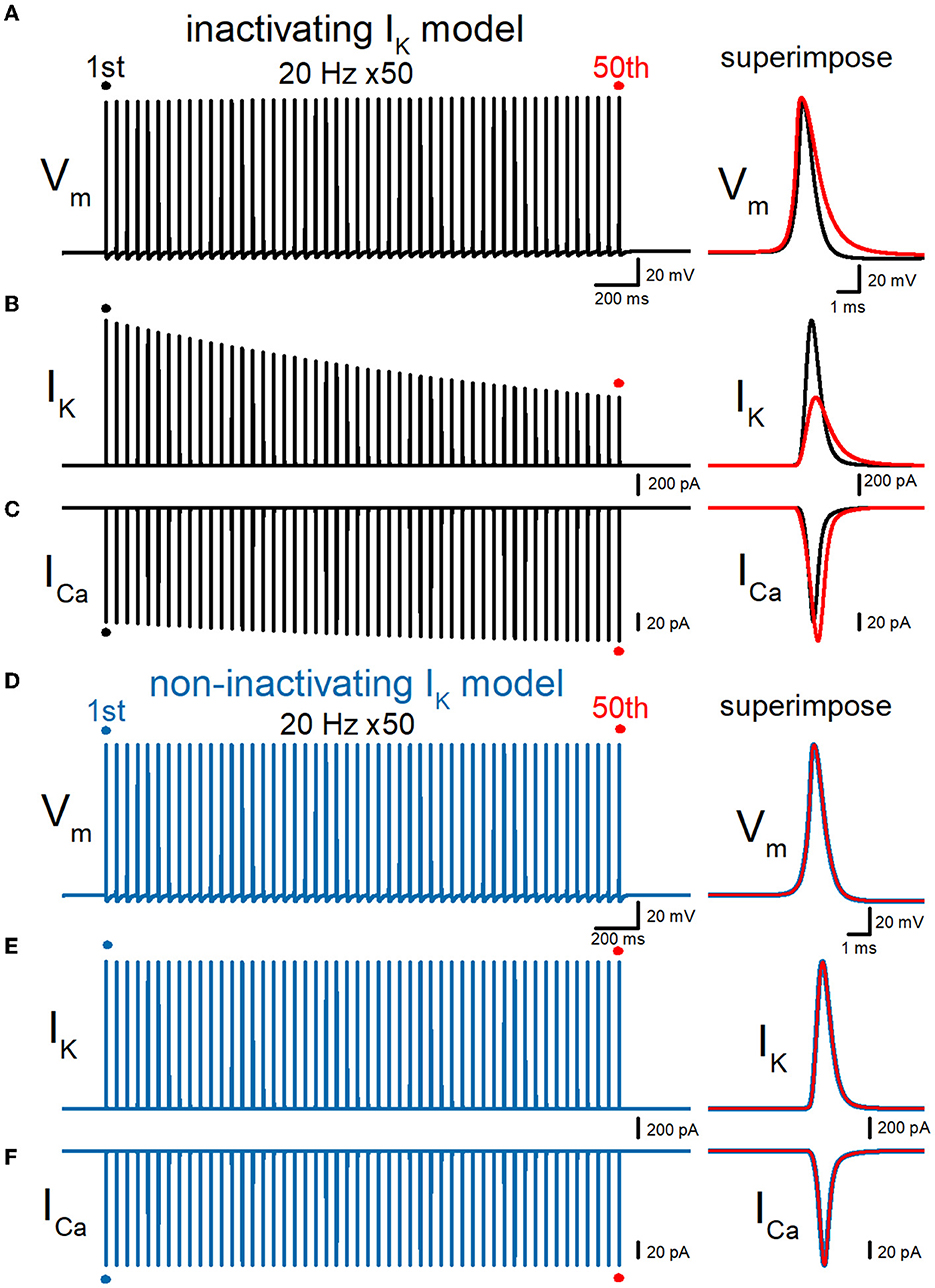
Figure 3. Effects of repetitive stimuli on action potentials, axonal IK, and presynaptic ICa simulated with inactivating and non-inactivating axonal K+ channels. (A) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the inactivating IK model. (B) Axonal K+ current elicited by the repetitive action potentials (IK) in inactivating IK model. (C) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in inactivating the IK model. (D) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the non-inactivating IK model. (E) Axonal K+ current elicited by the repetitive action potentials (IK) in the non-inactivating IK model. (F) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in the non-inactivating IK model. In each right panel, the responses to the 50th stimulus (red traces) were superimposed with that elicited by the 1st stimulus (black or blue traces) for comparison.
Effects of replacement with non-inactivating K+ channels
To quantitatively evaluate the contribution of accumulated inactivation of axonal K+ channels, next we attempted to replace the K+ channel model with those lacking inactivation. The same repetitive stimuli of 50 times at 20 Hz did not induce a broadening of action potentials (Figure 3D). Half-duration of the 50th action potential (0.773 ms) was 100% of the 1st action potential (0.772 ms) for the 20-Hz train. As a consequence, the calculated IK and ICa did not show robust use-dependent changes in the amplitude (Figures 3E, F). The peak amplitude of the 50th IK (1.57 nA) and ICa (149 pA) were 99.6 and 102% of the 1st responses (1.58 nA, 149 pA) for the 20-Hz train. Taking into account all these results, it was supposed that axonal K+ channels on the hippocampal mossy fibers undergo progressive inactivation during repetitive APs, and then cause use-dependent broadening of action potentials, which potentially contributes as an additional mechanism for short-term synaptic plasticity with an extremely wide dynamic range at this synapse.
Frequency dependency of the use-dependent AP broadening
Then, we explored the frequency dependency of the effects of repetitive stimulation. A train of 50 stimuli at 10 Hz caused a broadening of action potentials in the inactivating IK model (Figure 4A). Half-duration of the 50th action potential (1.05 ms) was 136% of the 1st action potential (0.777 ms) for the 10-Hz train (open circles, Figure 4D). Half-duration of the 50th action potential (1.18 ms) was 152% of the 1st action potential (0.777 ms) for the 20-Hz train (Figures 4B, D). Half-duration of the 50th action potential (1.39 ms) was 179% of the 1st action potential (0.777 ms) for the 50-Hz train (Figures 4C, D). IK during action potential progressively decreased the amplitude (Figure 4D, open squares), as expected for cumulative inactivation by the repetitive trains. ICa showed the use-dependent changes in the amplitude in a frequency-dependent manner (Figure 4D, open diamonds). The peak amplitudes of the 50th IK, ICa, and the charge of ICa were 55.5, 114, and 146% of the 1st responses for the 10-Hz train (Figures 4A, D). The peak amplitudes of the 50th IK and ICa were 47.0, 117, and 165% of the 1st responses (1.56 nA, 150 pA) for the 20-Hz train (Figures 4B, D). The peak amplitudes of the 50th IK, ICa, and the charge of ICa were 35.6, 112, and 184% of the 1st responses (1.56 nA, 150 pA) for the 50-Hz train (Figures 4C, D). These effects were exclusively observed in the simulation with the inactivating IK model and never observed in the simulation with the non-inactivating IK model (blue-filled symbols in Figure 5D). All the results indicate that use-dependent broadening of axonal action potentials propagating along mossy fibers, possibly due to cumulative inactivation of axonal K+ channels. Robust changes in the amplitudes of ICa suggest that this mechanism substantially contributes to the large-amplitude short-term synaptic plasticity characteristic for this particular synapse.
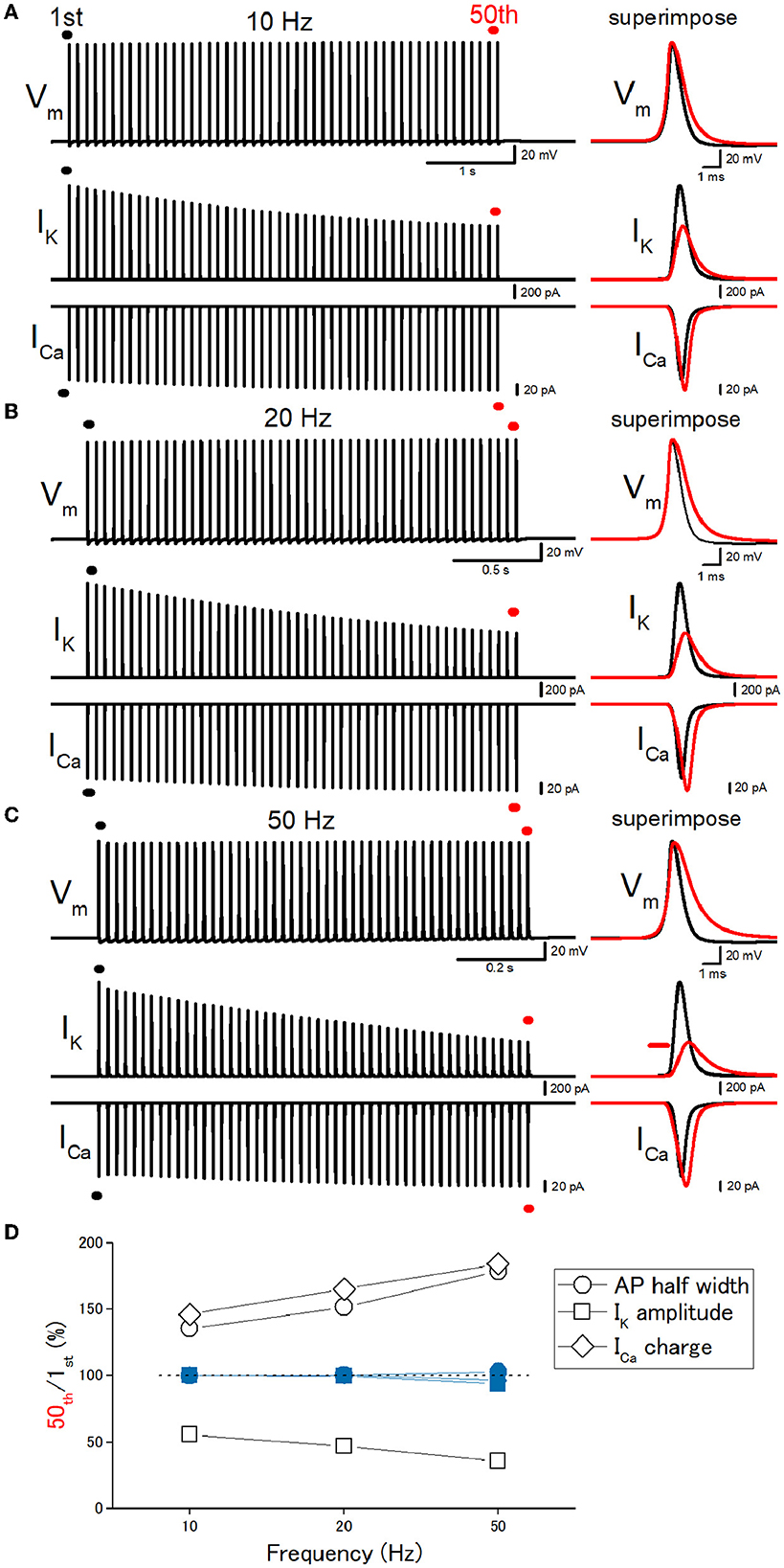
Figure 4. Frequency dependency of the effects of repetitive stimulation. (A) Simulated propagating action potentials at the mossy fiber bouton (Vm), axonal K+ current (IK), and presynaptic Ca2+ currents (ICa) elicited by the repetitive stimuli of 50 times at 10 Hz. (B) Action potentials elicited by the repetitive stimuli of 50 times at 20 Hz. (C) Action potentials elicited by the repetitive stimuli of 50 times at 50 Hz. In each right panel, the responses to the 50th stimulus (red traces) were superimposed with that elicited by the 1st stimulus (black traces) for comparison. (D) Frequency dependency of the effects. The relative values of 50th responses/1st responses of the half-width of AP (open circles), the peak amplitude of IK (open squares), and the charge of ICa (open diamonds). The data of the simulation using the non-inactivating IK models are similarly shown in blue symbols.
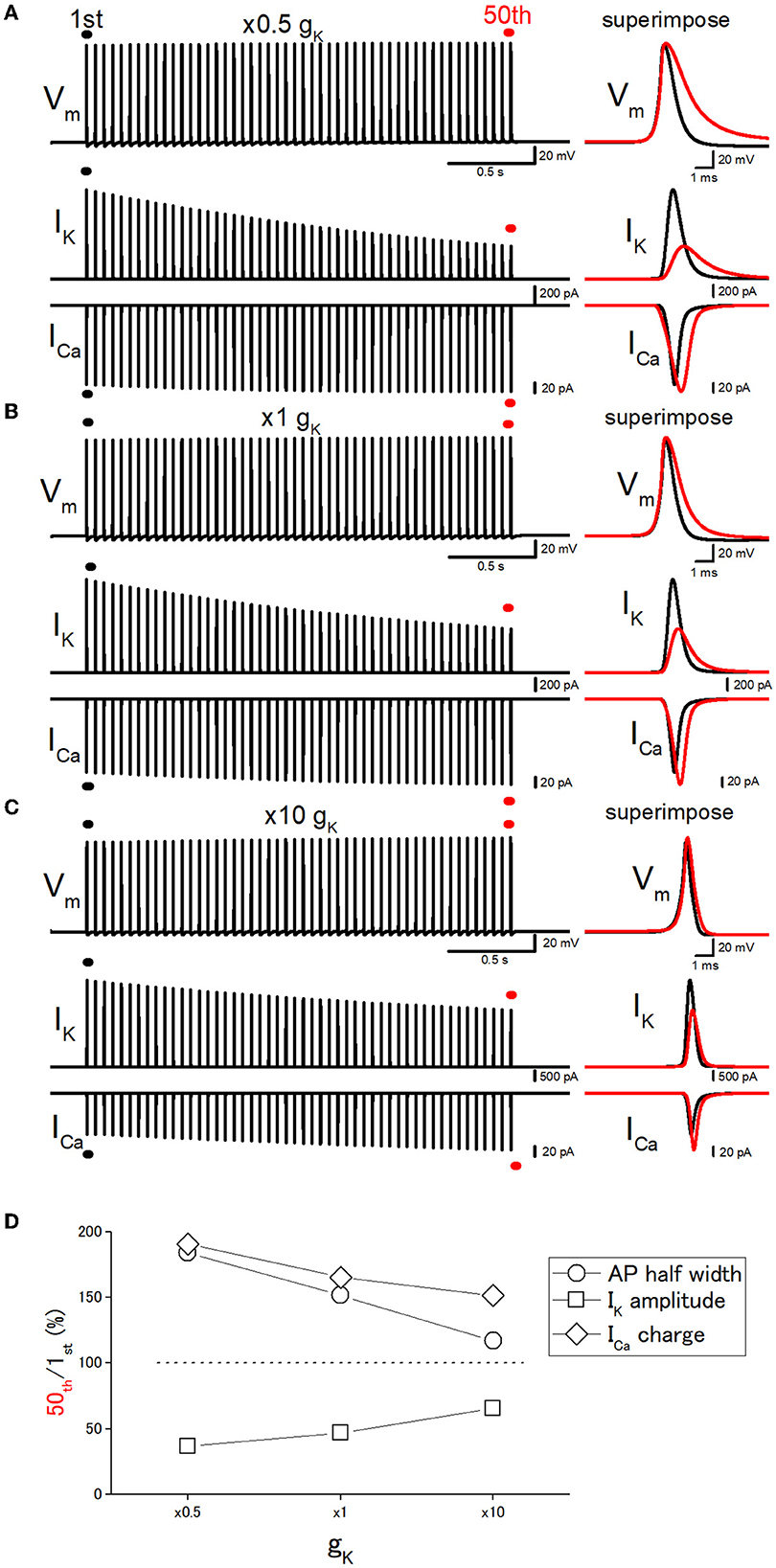
Figure 5. Effects of changes in the potassium conductance (gK) on the use-dependent broadening of axonal action potentials. (A) Simulated propagating action potentials at the mossy fiber bouton (Vm), axonal K+ current (IK), and presynaptic Ca2+ currents (ICa) elicited by the repetitive stimuli of 50 times at 20 Hz with the models in which the potassium conductance (gK) was reduced to half (×0.5) of the original model. (B) Action potentials simulated with the original (×1) gK value. (C) Action potentials simulated with the model in which the gK value was increased to 10 times the original one. In each right panel, the responses to the 50th stimulus (red traces) were superimposed with that elicited by the 1st stimulus (black traces) for comparison. (D) Dose-dependent relationships of the effects of changes in gK values. The relative values of 50th responses/1st responses of the half-width of AP (open circles), the peak amplitude of IK (open squares), and the charge of ICa (open diamonds).
Effect of changes in gK on the use-dependent AP broadening
Next, it was attempted to test whether the changes in the basal duration of the action potential affect the activity-dependent broadening, as expected from the scenario that cumulative inactivation of IK during the repetitive action potentials governs the broadening of action potentials. For this purpose, the gK (potassium conductance) values were changed systematically. The 10 times increase in gK level (from 36 to 360 mS cm−2) shortened the half-duration of action potential from 0.777 ms to 0.523 ms by speeding up the repolarization phase as shown in Figure 5C. It should be noted that repetitive stimuli of 50 times at 20 Hz induced a smaller broadening of action potentials when gK level was increased 10 times. With 10 times gK of 360 mS cm−2, the half-duration of the 50th action potential, the amplitudes of IK and ICa, and the charge of ICa were 116, 65.6, 138, and 151% of the 1st action potential for the 20-Hz train (Figures 5C, D), while the half-duration of the 50th action potential (1.18 ms), the amplitudes of IK and ICa, and the charge of ICa was 152, 47.0, 117 165% of the 1st action potential (0.777 ms) with the original gK of 36 mS cm−2 (Figures 5B, D). On the other hand, when the gK value was decreased to half (18 mS cm−2) of the original value of 36 mS cm−2, the half-duration of the 50th action potential, the amplitudes of IK and ICa, and the charge of ICa were 184, 36.6, 109, and 191% of the 1st action potential (0.904 ms) for the 20-Hz train (Figures 5A, D). All the results are consistent with the notion that the duration of the action potential is critical for the use-dependent broadening of action potentials because cumulative inactivation of IK during the repetitive action potentials is expected to occur strongly, therefore resulting in the larger broadening of action potentials.
Simulation of the responses at the en passant bouton
So far, simulations were made to calculate the responses from the bouton at the end of the axon as illustrated schematically in Figure 2A, for comparison with the experimental findings obtained from giant boutons. This may raise the concern that the recording from the bouton on the end of the axon may have to take into account the sealed-end effects. To address this issue, we repeated the simulations to calculate the responses from the ninth en passant boutons as illustrated in Figure 6A. Similar to the recording from the boutons at the end of the axon in Figure 3, repetitive stimuli of 50 times at 20 Hz progressively slowed the repolarization and thereby caused a broadening of action potentials. Half-duration of the 50th action potential (1.47 ms) was 165% of the 1st action potential (0.890 ms) for the 20-Hz train (Figure 6B). IK during action potential progressively decreased the amplitude (Figure 6C). The peak amplitude of the 50th IK (0.629 nA) was 40.6% of the 1st responses (1.55 nA) for the 20-Hz train. On the other hand, ICa during the action potential is progressively increased by the repetitive train of action potentials (Figure 6D). The peak amplitude of the 50th ICa (165 pA) was 121% of the 1st responses (136 pA) for the 20-Hz train. The calculated Ca2+ charge was enhanced by 192% of control by a 20-Hz train of 50 stimuli. Thus, similar activity-dependent modulation of action potential half-width, IK amplitude, and ICa charges was reproduced at the en passant bouton. Importantly, these activity-dependent changes were almost abolished when the axonal K+ channel model was replaced with a non-inactivating K+ channel model (Figures 6E–G). All the results in the series of simulations support the conclusion that accumulated inactivation of axonal K+ channels underlies activity-dependent broadenings of APs.
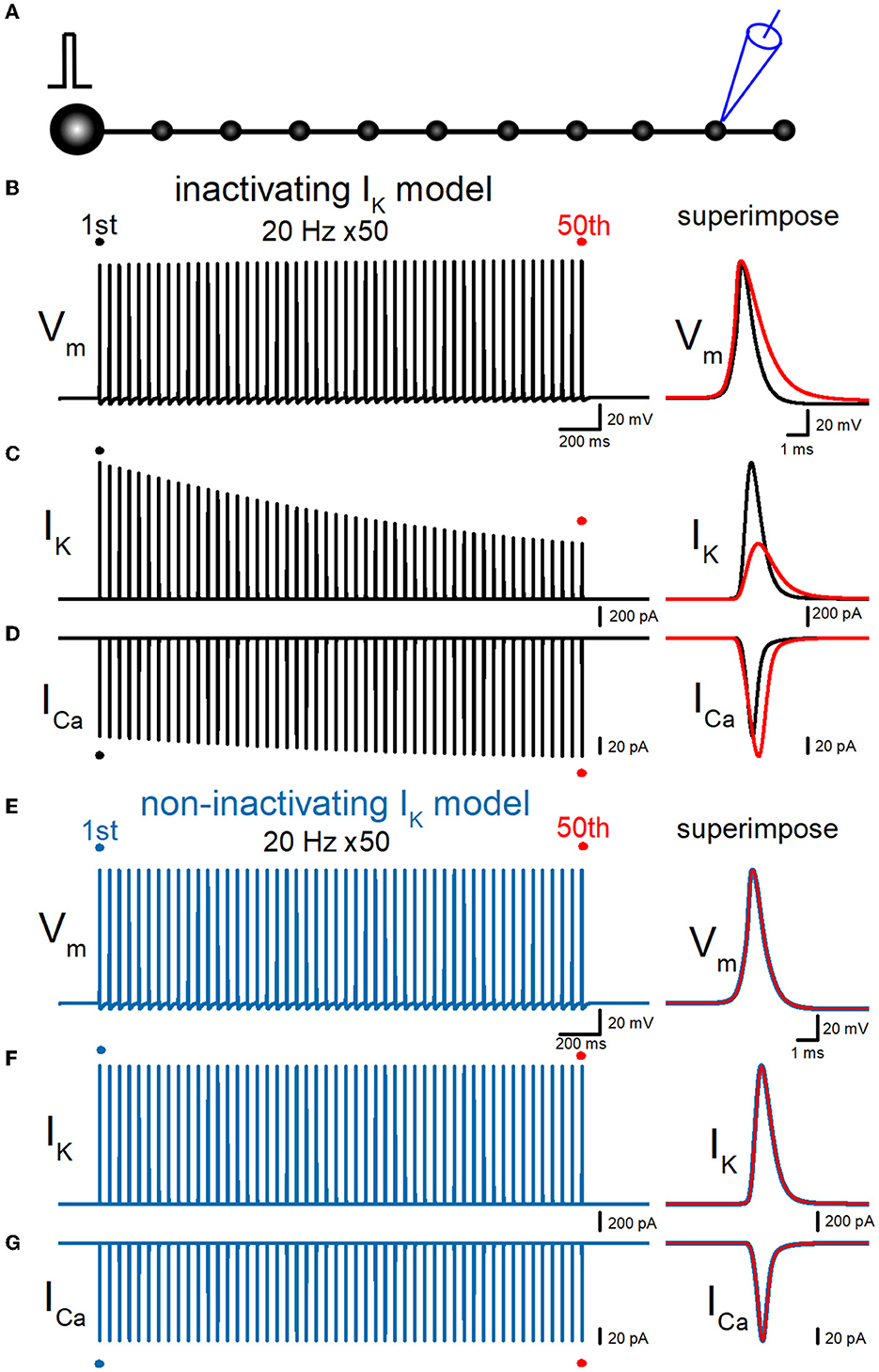
Figure 6. Effects of repetitive stimuli on action potentials, axonal IK, and presynaptic ICa recorded from the en passant boutons. (A) Schematic drawing of the multi-compartment model of the granule cell and mossy fiber and the recording site at the ninth en passant boutons. (B) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the inactivating IK model. (C) Axonal K+ current elicited by the repetitive action potentials (IK) in inactivating IK model. (D) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in inactivating the IK model. (E) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the non-inactivating IK model. (F) Axonal K+ current elicited by the repetitive action potentials (IK) in the non-inactivating IK model. (G) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in the non-inactivating IK model. In each right panel, the responses to the 50th stimulus (red traces) were superimposed with that elicited by the 1st stimulus (black or blue traces) for comparison.
Effect of implementing KV7 M-type K+ channel model
All the previous simulation was tested by the simple model of mossy fibers implemented with voltage-dependent Na+-, K+-, and Ca2+ channels models reconstructed from the experimentally recorded properties of the channels from the mossy fiber boutons. As previous experiments have demonstrated the presence of KV7 (Martinello et al., 2019), KV3, and Ca-dependent K+ channels (Alle et al., 2011) using direct subcellular recording, the information is limited and has not constructed the detailed channel models for quantitative simulation. To examine the effect of KV7 M-type K+ channels, we repeated the simulations with the model implemented with the model used in the previous study (Martinello et al., 2019) and tested the effect of repetitive stimuli 50 times at 20 Hz. It was noted that the peak of action potentials was delayed for the 50th action potentials as shown in the right superimposed traces, expecting from the shunting due to these channels being active at the resting membrane potentials. The repetitive stimuli slowed the repolarization and caused a broadening of action potentials. Half-duration of the 50th action potential (1.08 ms) was 120% of the 1st action potential (0.896 ms) for the 20-Hz train (Figure 7A). IK during action potential progressively decreased the amplitude (Figure 7B). The peak amplitude of the 50th IK (1.48 nA) was 81.5% of the 1st responses (1.81 nA) for the 20-Hz train. On the other hand, ICa during the action potential is progressively increased by the repetitive train of action potentials (Figure 7C). The peak amplitude of the 50th ICa (112 pA) was 102% of the 1st responses (110 pA) for the 20-Hz train. The calculated Ca2+ charge was enhanced by 118% of control by a 20-Hz train of 50 stimuli. Thus, similar activity-dependent modulation of AP half-width, IK amplitude, and ICa charges was reproduced at the en passant bouton. Importantly, these activity-dependent changes were almost abolished when the axonal K+ channel model was replaced with a non-inactivating K+ channel model (Figures 7D–F). It is obvious to need revision of the model to be more realistic by supplementing with the properties and exact localization of these channels on the mossy fiber axons by the precise experimental approach in future studies.
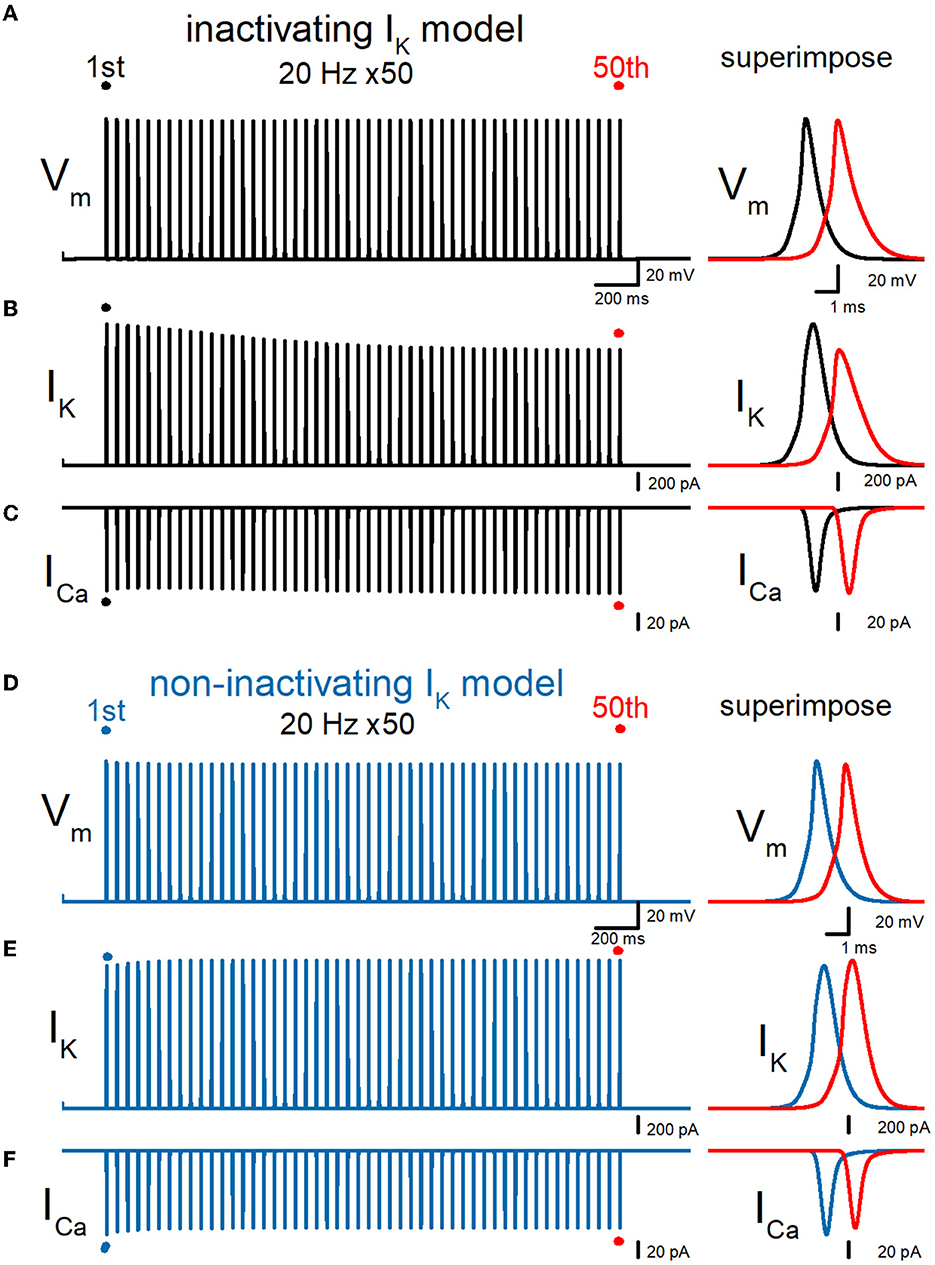
Figure 7. Effects of repetitive stimuli on action potentials, axonal IK, and presynaptic ICa with axonal KV7 M-type K channels. (A) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the inactivating IK model implemented with the KV7 M-type K channel model. (B) Axonal K+ current elicited by the repetitive action potentials (IK) in inactivating IK model. (C) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in inactivating the IK model. (D) Simulated propagating action potentials at the mossy fiber bouton (Vm) elicited by repetitive stimuli of 50 times at 20 Hz in the non-inactivating IK model. (E) Axonal K+ current elicited by the repetitive action potentials (IK) in the non-inactivating IK model. (F) Ca2+ currents (ICa) elicited by axonal action potentials during the repetitive stimuli in the non-inactivating IK model. In each right panel, the responses to the 50th stimulus (red traces) were superimposed with that elicited by the 1st stimulus (black or blue traces) for comparison.
Discussion
In this study, a numerical simulation approach was adopted to examine the contribution of the inactivation of potassium channels in an activity-dependent broadening of action potentials propagating on the hippocampal mossy fibers. Using a simple but sufficiently realistic model of hippocampal mossy fiber supplemented with inactivating and non-inactivating K+ channels on axonal membranes, we compared the duration of action potentials during repetitive stimuli and found that use-dependent broadening of action potentials occurs with the model implemented with inactivating K+ channels, while the model with non-inactivating K+ channels does not show the broadening of action potentials. All the findings are supporting the notion that the inactivation property of the axonal K+ channels imparts use-dependent modification of the synaptic strength in addition to the well-established residual Ca2+ mechanism for short-term synaptic plasticity.
Inactivating K+ channels on the hippocampal mossy fiber axon
Recent advances in subcellular recordings from the axons have updated several classical views on physiological understandings of axons in the central nervous system. One remarkable notion different from those obtained by classical studies is the inactivation property of axonal potassium channels. For instance, K+ channels on the hippocampal mossy fiber terminals were demonstrated to display robust fast inactivation during prolonged depolarization (Geiger and Jonas, 2000; Alle et al., 2011) as expected from the kinetic properties of channels composed of KV1 and KV3 subunits, clearly different from the non-inactivating property of Hodgkin–Huxley-type potassium channels in squid giant axons (Hodgkin and Huxley, 1952). This notion is not specific to this particular type of synapse, as the voltage-dependent K+ current recorded from the calyx of Held, the best-characterized axon terminals in the central nervous system, also displays fast inactivation upon prolonged depolarization (Dodson et al., 2002; Ishikawa et al., 2003; Tong et al., 2010; Choudhury et al., 2020). Direct axonal recording study from cerebellar stellate interneuron revealed that inactivating KV1 and KV3 subunits differently distributed along the course of axons (Rowan et al., 2014, 2016) also supported the notion that inactivating K+ channels critically involved in the fine-tuning of axonal signaling (Johnston et al., 2010).
Inactivating properties are supposed to be involved in “analog-digital facilitation” representing the enhancement of synaptic transmitter release by the prolonged depolarization of axonal membrane potentials (Debanne et al., 2011, 2013; Ohura and Kamiya, 2016). Slowing of action potential repolarization caused by prolonged membrane depolarization modulates transmitter release from the axon terminals of layer V pyramidal neurons in the cerebral cortex (Shu et al., 2006). Similar analog modulation has been demonstrated in the axons of cultured CA3 pyramidal neurons (Sasaki et al., 2011) and the calyx of Held (Richardson et al., 2022). As action potentials propagating along mossy fibers were followed by substantial after depolarization lasting for several tens of ms (Geiger and Jonas, 2000), it may also affect the waveforms of subsequent action potentials during repetitive action potentials.
As another consequence of the inactivation of axonal K+ channels, use-dependent broadening of axonal action potentials due to slowed repolarization has been suggested experimentally (Geiger and Jonas, 2000), although awaiting evaluation of quantitative consistency. As IK critically determines the duration of axonal action potentials at the hippocampal mossy fiber boutons (Alle et al., 2009), a slight change in the axonal IK would impact the subsequent synaptic transmission. This study attempted to test this notion by computer simulation using a model of a hippocampal mossy fiber axon. Consistent with the notion, a model implemented with inactivating K+ channels displayed notable action potential broadening by repetitive stimuli. In contrast, the replacement of the model with non-inactivating K+ channels did not change the duration of action potentials by repetitive action potentials. These results demonstrated the roles of K+ channel inactivation in a use-dependent broadening of action potentials.
In the previous experimental study, the authors tested the cumulative inactivation of K+ channels by using a repetitive voltage steps protocol (Geiger and Jonas, 2000). The K+ current elicited by +20 mv voltage pulses for 3 ms from the −90 mV holding potential repeating at 100 Hz (7 ms interpulse intervals) progressively decreased during the repetitive voltage pulses. However, action potentials recorded at the mossy fiber terminals were much briefer than 3 ms (half-duration 379 ± 8 μs) and therefore need to be evaluated quantitatively using action potential waveform. This study demonstrated that the K+ current elicited by action potentials progressively declines during repetitive trains, supporting the notion of cumulative inactivation of K+ channels by repetitive action potentials.
Inactivating axonal K+ channel imparts use-dependent short-term plasticity
Supposing a brief depolarization during action potentials inactivates a fraction of axonal K+ channels, it is expected that a use-dependent decrease in K+ current occurs in a frequency-dependent manner. Consistent with the prediction, we confirmed the cumulative frequency-dependent effects on action potential-driven K+ current. Likewise, simulated presynaptic Ca2+ current was also enhanced frequency-dependently. In our previous study (Kamiya, 2022), the models of P/Q type, N-type, and R-type Ca2+ channels were reconstructed by adopting kinetic parameters obtained by the voltage-clamp experiments in direct recordings from the mossy fiber terminals (Li et al., 2007). Using these Ca2+ channel models, Ca2+ currents during action potentials were calculated and found to progressively increase the amplitude by the repetitive stimuli, as expected from the broadening of action potentials. This implicates the inactivation of axonal K+ channels imparts use-dependent short-term synaptic plasticity at hippocampal mossy fiber synapse within the range of physiological frequency.
It should be noted that the duration of the simulated action potentials in this study (half-duration of 0.773 ms) was longer than that of experimentally observed action potentials (half-duration of 0.379 ms) as reported previously (Geiger and Jonas, 2000). One of the possible reasons for the slower action potentials in our simulation is the difference in temperature between the simulation and the experiments. Engel and Jonas (2005) measured sodium current at room temperature and described the kinetic parameters of gating of Na+ channels on the mossy fiber terminals, and the model adopted in this study used these parameters. On the other hand, action potentials were recorded at a physiological temperature of 34°C and thus would expect to speed up the action potential time course (Geiger and Jonas). Although the models need to be improved by taking into account the temperature, the simulated action potentials in this study seem similar to the simulated action potentials reported previously (Engel and Jonas, 2005) and therefore used for the analysis of the contribution of K+ channel inactivation in use-dependent action potential broadening.
In this study, a series of numerical simulations using a simple model of hippocampal mossy fiber was performed to test the possible contribution of K+ channel inactivation in activity-dependent modification of axonal excitability. The model implemented with inactivating K+ channels displays robust use-dependent broadening of action potentials by repetitive stimuli, while that with non-inactivating K+ channels does not change the duration. Our simulations also demonstrated the frequency dependency of the effect as expected from the cumulative nature of the inactivation of axonal K+ channels. The enhanced Ca2+ entry by repetitive action potentials was also demonstrated quantitatively. Taking all pieces of evidence, the inactivating property of axonal action potentials provides an important component for activity-dependent short-term synaptic plasticity with an extremely wide dynamic range at the hippocampal mossy fiber synapse.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HK designed the research and wrote the manuscript. HK and FZ performed simulations and analyzed data. Both authors contributed to the article and approved the submitted version.
Funding
This study was supported by Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (21K06434 and 21H05166 to HK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acsády, L., Kamondi, A., Sík, A., Freund, T., and Buzsáki, G. (1998). GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 18, 3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998
Alle, H., and Geiger, J. R. (2006). Combined analog and action potential coding in hippocampal mossy fibers. Science 311, 1290–1293. doi: 10.1126/science.1119055
Alle, H., Kubota, H., and Geiger, J. R. (2011). Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. J. Neurosci. 31, 8001–8012. doi: 10.1523/JNEUROSCI.0972-11.2011
Alle, H., Roth, A., and Geiger, J. R. (2009). Energy-efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408. doi: 10.1126/science.1174331
Bischofberger, J., Engel, D., Li, L., Geiger, J. R., and Jonas, P. (2006). Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 1, 2075–2081. doi: 10.1038/nprot.2006.312
Byrne, J. H., and Kandel, E. R. (1996). Presynaptic facilitation revisited: state and time dependence. J. Neurosci. 16, 425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996
Choudhury, N., Linley, D., Richardson, A., Anderson, M., Robinson, S. W., Marra, V., et al. (2020). Kv3.1 and Kv3.3 subunits differentially contribute to Kv3 channels and action potential repolarization in principal neurons of the auditory brainstem. J. Physiol. 598, 2199–2222. doi: 10.1113/JP279668
Cooper, E. C., Milroy, A., Jan, Y. N., Jan, L. Y., and Lowenstein, D. H. (1998). Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J. Neurosci. 18, 965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998
Debanne, D., Bialowas, A., and Rama, S. (2013). What are the mechanisms for analogue and digital signalling in the brain? Nat. Rev. Neurosci. 14, 63–69. doi: 10.1038/nrn3361
Debanne, D., Campanac, E., Bialowas, A., Carlier, E., and Alcaraz, G. (2011). Axon physiology. Physiol. Rev. 91, 555–602. doi: 10.1152/physrev.00048.2009
Dodson, P. D., Barker, M. C., and Forsythe, I. D. (2002). Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J. Neurosci. 22, 6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002
Engel, D., and Jonas, P. (2005). Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45, 405–417. doi: 10.1016/j.neuron.2004.12.048
Geiger, J. R., and Jonas, P. (2000). Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron 28, 927–939. doi: 10.1016/S0896-6273(00)00164-1
Henze, D. A., Urban, N. N., and Barrionuevo, G. (2000). The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98, 407–427. doi: 10.1016/S0306-4522(00)00146-9
Hines, M. L., and Carnevale, N. T. (1997). The NEURON simulation environment. Neural Comput. 9, 1179–1209. doi: 10.1162/neco.1997.9.6.1179
Hodgkin, A. L., and Huxley, A. F. (1952). Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116, 449–472. doi: 10.1113/jphysiol.1952.sp004717
Ishikawa, T., Nakamura, Y., Saitoh, N., Li, W. B., Iwasaki, S., and Takahashi, T. (2003). Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J. Neurosci. 23, 10445–10453. doi: 10.1523/JNEUROSCI.23-32-10445.2003
Jackson, M. B., Konnerth, A., and Augustine, G. J. (1991). Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc. Natl. Acad. Sci. U. S. A. 88, 380–384. doi: 10.1073/pnas.88.2.380
Johnston, J., Forsythe, I. D., and Kopp-Scheinpflug, C. (2010). Going native: voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 588(Pt 17), 3187–3200. doi: 10.1113/jphysiol.2010.191973
Kamiya, H. (2019). Modeling analysis of axonal after potential at hippocampal mossy fibers. Front. Cell. Neurosci. 13, 210. doi: 10.3389/fncel.2019.00210
Kamiya, H. (2022). Modeling analysis of subthreshold voltage signaling along hippocampal mossy fiber axons. Front. Cell. Neurosci. 16, 966636. doi: 10.3389/fncel.2022.966636
Kamiya, H., and Ozawa, S. (1999). Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J. Physiol. 518(Pt 2), 497–506. doi: 10.1111/j.1469-7793.1999.0497p.x
Kamiya, H., Ozawa, S., and Manabe, T. (2002). Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J. Neurosci. 22, 9237–9243. doi: 10.1523/JNEUROSCI.22-21-09237.2002
Katz, B., and Miledi, R. (1968). The role of calcium in neuromuscular facilitation. J. Physiol. 195, 481–492. doi: 10.1113/jphysiol.1968.sp008469
Li, L., Bischofberger, J., and Jonas, P. (2007). Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J. Neurosci. 27, 13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007
Martinello, K., Giacalone, E., Migliore, M., Brown, D. A., and Shah, M. M. (2019). The subthreshold-active KV7 current regulates neurotransmission by limiting spike-induced Ca2+ influx in hippocampal mossy fiber synaptic terminals. Commun. Biol. 2, 145. doi: 10.1038/s42003-019-0408-4
Nicoll, R. A., and Schmitz, D. (2005). Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876. doi: 10.1038/nrn1786
Ohura, S., and Kamiya, H. (2016). Excitability tuning of axons in the central nervous system. J. Physiol. Sci. 66, 189–196. doi: 10.1007/s12576-015-0415-2
Ohura, S., and Kamiya, H. (2018). Short-term depression of axonal spikes at the mouse hippocampal mossy fibers and sodium channel-dependent modulation. eNeuro 5, ENEURO.0415-17.2018. doi: 10.1523/ENEURO.0415-17.2018
Richardson, A., Ciampani, V., Stancu, M., Bondarenko, K., Newton, S., Steinert, J. R., et al. (2022). Kv3.3 subunits control presynaptic action potential waveform and neurotransmitter release at a central excitatory synapse. Elife 11:e75219. doi: 10.7554/eLife.75219
Rowan, M. J., DelCanto, G., Yu, J. J., Kamasawa, N., and Christie, J. M. (2016). Synapse-level determination of action potential duration by K(+) channel clustering in axons. Neuron 91, 370–383. doi: 10.1016/j.neuron.2016.05.035
Rowan, M. J., Tranquil, E., and Christie, J. M. (2014). Distinct Kv channel subtypes contribute to differences in spike signaling properties in the axon initial segment and presynaptic boutons of cerebellar interneurons. J. Neurosci. 34, 6611–6623. doi: 10.1523/JNEUROSCI.4208-13.2014
Salin, P. A., Scanziani, M., Malenka, R. C., and Nicoll, R. A. (1996). Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 93, 13304–13309. doi: 10.1073/pnas.93.23.13304
Sasaki, T., Matsuki, N., and Ikegaya, Y. (2011). Action-potential modulation during axonal conduction. Science 331, 599–601. doi: 10.1126/science.1197598
Schmidt-Hieber, C., and Bischofberger, J. (2010). Fast sodium channel gating supports localized and efficient axonal action potential initiation. J. Neurosci. 30, 10233–10242. doi: 10.1523/JNEUROSCI.6335-09.2010
Schmidt-Hieber, C., Jonas, P., and Bischofberger, J. (2008). Action potential initiation and propagation in hippocampal mossy fibre axons. J. Physiol. 586, 1849–1857. doi: 10.1113/jphysiol.2007.150151
Shu, Y., Hasenstaub, A., Duque, A., Yu, Y., and McCormick, D. A. (2006). Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441, 761–765. doi: 10.1038/nature04720
Tong, H., Steinert, J. R., Robinson, S. W., Chernova, T., Read, D. J., Oliver, D. L., et al. (2010). Regulation of Kv channel expression and neuronal excitability in rat medial nucleus of the trapezoid body maintained in organotypic culture. J. Physiol. 588(Pt 9), 1451–1468. doi: 10.1113/jphysiol.2009.186676
Vandael, D., Okamoto, Y., Borges-Merjane, C., Vargas-Barroso, V., Suter, B. A., and Jonas, P. (2021). Subcellular patch-clamp techniques for single-bouton stimulation and simultaneous pre- and postsynaptic recording at cortical synapses. Nat. Protoc. 16, 2947–2967. doi: 10.1038/s41596-021-00526-0
Wissmann, R., Bildl, W., Oliver, D., Beyermann, M., Kalbitzer, H. R., Bentrop, D., et al. (2003). Solution structure and function of the “tandem inactivation domain” of the neuronal A-type potassium channel Kv1.4. J. Biol. Chem. 278, 16142–16150. doi: 10.1074/jbc.M210191200
Keywords: axon, hippocampus, inactivation, mossy fiber, potassium channel, simulation
Citation: Zheng F and Kamiya H (2023) Simulation test for impartment of use-dependent plasticity by inactivation of axonal potassium channels on hippocampal mossy fibers. Front. Cell. Neurosci. 17:1154910. doi: 10.3389/fncel.2023.1154910
Received: 31 January 2023; Accepted: 31 March 2023;
Published: 26 April 2023.
Edited by:
Dominique Debanne, INSERM U1072 Neurobiologie des canaux Ioniques et de la Synapse, FranceReviewed by:
Stephanie Ratte, University of Toronto, CanadaMickael Zbili, INSERM U1028 Centre de Recherche en Neurosciences de Lyon, France
Copyright © 2023 Zheng and Kamiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haruyuki Kamiya, a2FtaXlhJiN4MDAwNDA7bWVkLmhva3VkYWkuYWMuanA=
 Fumeng Zheng
Fumeng Zheng Haruyuki Kamiya
Haruyuki Kamiya