
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci., 28 April 2022
Sec. Non-Neuronal Cells
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.895871
This article is part of the Research TopicThe Role of Glial Cells in the Autoimmune Diseases of Nervous SystemView all 5 articles
Enteric glial cells (EGCs) are one of the major cell types of neural crest lineage distributed in the gastrointestinal tract. EGCs represent an integral part of the enteric nervous system (ENS) and significantly outnumber ENS neurons. Studies have suggested that EGCs would exert essential roles in supporting the survival and functions of the ENS neurons. Notably, recent evidence has begun to reveal that EGCs could possess multiple immune functions and thereby may participate in the immune homeostasis of the gut. In this review article, we will summarize the current evidence supporting the potential involvement of EGCs in several important immunological disorders, including inflammatory bowel disease, celiac disease, and autoimmune enteropathy. Further, we highlight critical questions on the immunological aspects of EGCs that warrant future research attention.
Enteric glial cells (EGCs) represent an indispensable component of the enteric nervous system (ENS; Gabella, 1972). EGCs and the ENS neurons are both derived from the neural crest during embryonic development (Nagy and Goldstein, 2017; Rao and Gershon, 2018). Notably, EGCs significantly outnumber the ENS neurons in adulthood, i.e., approximately 6:1 in the human myenteric plexus (Hoff et al., 2008). Similar to other glial cells in the central and peripheral nervous systems, e.g., astrocytes and Schwann cells, EGCs have essential roles in maintaining the survival and functions of surrounding neurons (Abdo et al., 2010). Of importance, recent studies have increasingly suggested that EGCs could also exert multiple immune functions and potentially modulate immunological disorders and other disease conditions of the gastrointestinal tract (Ibiza et al., 2016; Kermarrec et al., 2016; Chow et al., 2021; Progatzky et al., 2021). Here, we will first briefly overview the development and classification of EGCs and then focus on their emerging immune functions.
The primary origin of the ENS is vagal neural crest cells that migrate into the gastrointestinal tract from the rostral foregut to the caudal hindgut (Figure 1). In the mouse embryos, vagal neural crest cells adjacent to somites 1–5 form the ENS along the entire length of the gut (Espinosa-Medina et al., 2017). In addition, sacral neural crest cells in the somite 24 are the other origin of the ENS, which only migrate into the hindgut, particularly the post-umbilical gut (Durbec et al., 1996). Once reaching the gastrointestinal tract, neural crest cells would differentiate into EGCs and the ENS neurons. SRY-box transcription factor 10 (SOX10) is one of the specific markers for the progenitors of EGCs and could be detected in the foregut as early as embryonic day 9.5 (E9.5) in the mouse (Anderson et al., 2006; Hoff et al., 2008). Notably, studies have shown that SOX10 is involved in gliogenesis in the gut (Kim et al., 2003). In particular, neural crest cells initially express SOX10 and the tyrosine kinase receptor rearranged during transfection (RET) when migrating into the gut (Schuchardt et al., 1994; Kim et al., 2003), but RET would be downregulated for the generation of EGCs (Young et al., 1999). On the other hand, the SOX10 downregulation and the RET maintenance would designate the differentiation of the ENS neurons (Kim et al., 2003; Lasrado et al., 2017). Brain fatty acid-binding protein (B-FABP) then appears at E11.5 in the progenitors of EGCs (Young et al., 2003), followed by proteolipid protein 1 (PLP1) at E12.5 (Lasrado et al., 2017), S100β at E14.5 (Young et al., 2003), and finally glial fibrillary acidic protein (GFAP) at E16.5 (Rothman et al., 1986). Currently, it is incompletely understood how the specific differentiation of EGCs vs. the ENS neurons is precisely controlled. For additional information on the ENS development, several recent reviews are available for reference (Lake and Heuckeroth, 2013; Rao and Gershon, 2018; Boesmans et al., 2021).
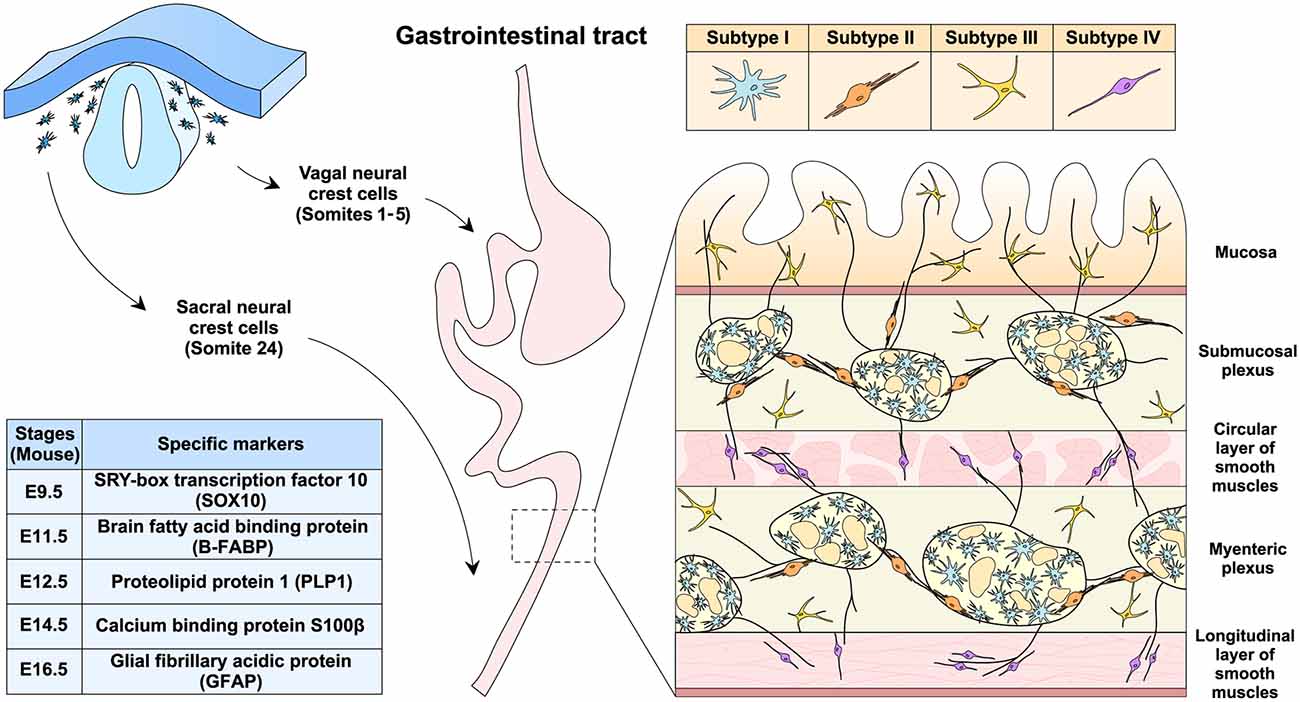
Figure 1. EGCs development, distribution, and classification. EGCs originate from the vagal (somites 1–5) and sacral (somite 24) neural crest cells during embryonic development in the mouse. Progenitor cells of EGCs sequentially express the specific markers, including SOX10 at E9.5, B-FABP at E11.5, PLP-1 at E12.5, S100β at E14.5, and GFAP at E16.5. In the gastrointestinal tract of adult mice, EGCs could be classified into subtypes I to IV based on cellular morphology and distribution within different anatomical layers. As an integral part of the enteric nervous system, EGCs are present both inside and outside the submucosal and myenteric plexus.
EGCs are distinct from other types of glial cells and were initially regarded as astrocyte-like glia in the gut (Gabella, 1972; Jessen and Mirsky, 1983). However, the transcription profile of EGCs shares more similarities with the myelinating glial cells such as Schwann cells and oligodendrocytes than with astrocytes (Rao et al., 2015). Moreover, EGCs have been known for their complex heterogeneity. For example, previous works characterized EGCs in the mouse myenteric plexus into two major subtypes based on cellular morphology, i.e., one with an astrocyte-like appearance and the other with fibrous structures (Hanani and Reichenbach, 1994). Notably, cellular morphology and tissue localization exhibit a strong correlation in the classification of EGCs (Gulbransen and Sharkey, 2012; Seguella and Gulbransen, 2021). Recent studies have further classified the mouse EGCs into four subtypes (Figure 1; Hanani and Reichenbach, 1994; Vanderwinden et al., 2003; Savidge et al., 2007; Badizadegan et al., 2014; Boesmans et al., 2015). Subtype I EGCs are intra-ganglionic star-shaped cells with many irregular branches, resembling astrocytes in the central nervous system. Subtype II EGCs are localized within or at the boundary of the submucosal and myenteric plexus and have long processes projecting along with neuronal axons. Subtype III EGCs are enriched within the lamina propria and also outside the submucosal and myenteric plexus, exhibiting a few processes (e.g., 4–5). Subtype IV EGCs are present within the circular and longitudinal layers of smooth muscles and have a relatively simple bipolar morphology.
Unsurprisingly, the morphological heterogeneity of EGCs could be extended to molecular levels. Studies reported that SOX10, S100β, and GFAP were differentially expressed in different subtypes of EGCs (Boesmans et al., 2015). Also, PLP1 rather than GFAP may represent a universal cellular marker for EGCs in the mouse. In particular, subtype IV EGCs in the mouse colon appear PLP1-positive but GFAP-negative (Rao et al., 2015). Moreover, recent works aided by the single-cell RNA sequencing techniques further elucidated the transcriptional complexity of EGCs (Zeisel et al., 2018; Drokhlyansky et al., 2020). For instance, seven distinct subtypes of EGCs were suggested in the myenteric plexus of the mouse small intestine (Zeisel et al., 2018). On the other hand, EGCs in the adult mouse colon were clustered into three subtypes. In addition, it was reported that EGCs could be categorized into three common and three patient-specific subtypes in the human colon under cancer conditions (Drokhlyansky et al., 2020). However, given the complexity of EGCs at morphological and molecular levels, a consensus on their classification remains to be settled in the research field. In light of this evolving issue, our discussion of the immune functions of EGCs would not try to distinguish specific subtypes.
EGCs could provide metabolic support to the ENS neurons (Abdo et al., 2010). Also, EGCs may act in the structural preservation of the ENS neurons and their axons during gut motility (Gabella, 1972). In addition, it has been implicated that EGCs might transdifferentiate into the ENS neurons under certain pathological conditions (Joseph et al., 2011; Laranjeira et al., 2011; Belkind-Gerson et al., 2015, 2017). Notably, the immunological aspect of EGCs has recently garnered research attention and emerged as a new frontier topic. We will summarize the known immune functions of EGCs and then discuss their potential roles in several immunological diseases of the gut (Figure 2).
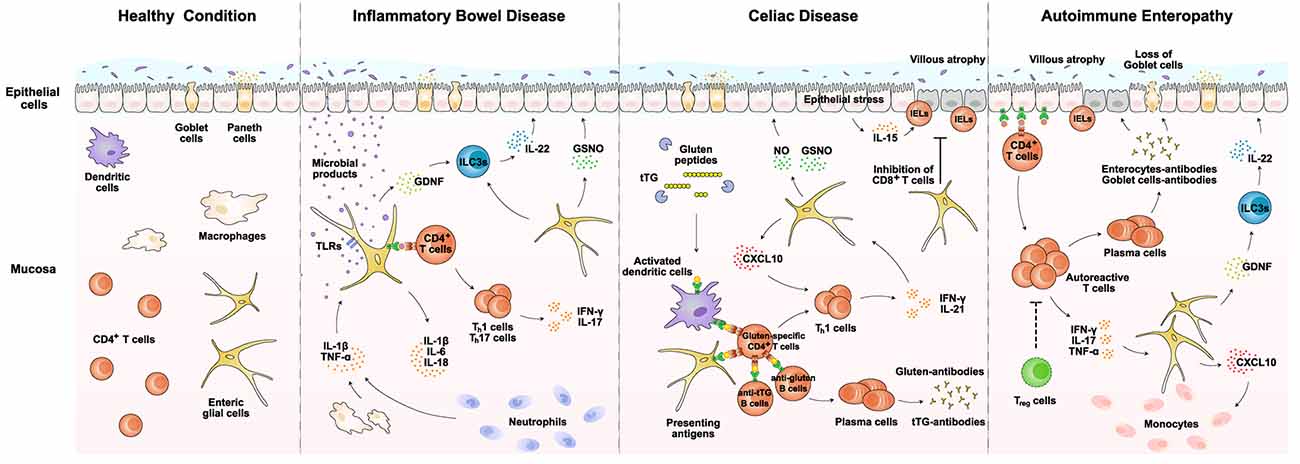
Figure 2. EGCs in immunological disorders of the gut. EGCs exert potential roles in several important immunological disorders of the gut, including inflammatory bowel disease, celiac disease, and autoimmune enteropathy. EGCs could directly sense invading pathogens via specific TLRs and release pro-inflammatory cytokines such as IL-1β and IL-6. Also, EGCs are able to produce CXCL10 and facilitate the recruitment of lymphocytes or monocytes. In addition, EGCs may present disease-related antigens via MHC-II to CD4+ T cells. Moreover, EGCs would produce GDNF and GSNO that indirectly or directly modulate the epithelial integrity. Details of the immune functions of EGCs are described in the main text. Notably, different subtypes of EGCs are not particularly distinguished for immune functions in this diagram. GDNF, glial cell line-derived neurotrophic factor; GSNO, S-nitrosoglutathione; IELs, intraepithelial lymphocytes; ILC3s, type 3 innate lymphoid cells; NO, nitro oxide; TLRs, Toll-like receptors; tTG, tissue transglutaminase.
Though EGCs are non-immune cells, they are capable of producing a variety of cytokines and chemokines in response to pathological stimuli. For example, EGCs express Toll-like receptors (TLRs) such as TLR2, TLR3, and TLR4 (Barajon et al., 2009; Turco et al., 2014). Microbial products such as lipopolysaccharide (LPS) could directly activate EGCs to secrete IFN-γ, IL-1β, IL-6, and CCL2 (Murakami et al., 2009; Rosenbaum et al., 2016). Also, EGCs release the macrophage colony-stimulating factor (M-CSF) that regulates the activation of the muscularis macrophages (Grubisic et al., 2020). Further, EGCs would respond to the pro-inflammatory cytokines derived from immune cells such as macrophages and CD4+ T cells. For instance, IL-1β triggers the upregulation of IL-6 and CCL2 in EGCs (Ruhl et al., 2001; Stoffels et al., 2014). Similarly, IFN-γ and LPS co-stimulation of EGCs causes the release of IL-1β and IL-18 (Cirillo et al., 2011; Yang et al., 2020).
EGCs are the predominant cellular source of glial cell line-derived neurotrophic factor (GDNF) in the gut (Meir et al., 2021). GDNF could strengthen the barrier integrity by preventing the apoptosis of intestinal epithelial cells (Savidge et al., 2007; Zhang et al., 2010). Indeed, genetic ablation of GFAP-positive EGCs in mice resulted in fulminant jejunoileitis (Bush et al., 1998), though such observation has been debated by another approach to removing PLP-1-positive EGCs (Rao et al., 2017). In addition, recent works have suggested that such EGCs-derived GDNF would engage type 3 innate lymphoid cells (ILC3s) and stimulate their release of IL-22. This EGCs-GDNF-ILC3s-IL22 axis participates in controlling mucosal homeostasis (Ibiza et al., 2016). At the same time, EGCs could generate S-nitrosoglutathione (GSNO), which acts on epithelial cells to facilitate the formation of tight junctions (Savidge et al., 2007; Cheadle et al., 2013; Li et al., 2016). Therefore, it has become an emerging theme that EGCs play a critical role in modulating the barrier function of the gut via such signaling factors (Savidge et al., 2007; Bernardini et al., 2012; Cheadle et al., 2013; Li et al., 2016, 2018).
Besides the release of immune factors, EGCs could directly engage T cell-mediated adaptive immune responses. In particular, EGCs express both MHC-I and MHC-II molecules, enabling them to present disease-related antigens to CD4+ T cells (Geboes et al., 1992; da Silveira et al., 2011; Chow et al., 2021). Also, recent studies reported that EGCs would display inhibitory effects on the proliferation and activity of CD8+ T cells (Kermarrec et al., 2016). Though the mechanism underlying such crosstalk between EGCs and CD8+ T cells remains to be fully elucidated, it has been suggested that EGCs may express the checkpoint blockage protein such as PD-L1, similar to that observed in astrocytes of the central nervous system (Gao et al., 2022). In addition, it appears a possibility that EGCs might influence the B cell-mediated immunity, as EGCs are present in Peyer’s patches (Biskou et al., 2022). Further, EGCs express the immunosuppressive checkpoint protein CD200, which could likely influence T cells, B cells, dendritic cells, and other immune cells in the gut (Chang et al., 2011).
Given the accumulating evidence that EGCs exert immune functions in the gut, their involvement in immunological disorders has begun to emerge (Esposito et al., 2007; von Boyen et al., 2011). In particular, EGCs may participate in disease conditions via several mechanisms. First, EGCs could detect invading pathogens and directly elicit the innate immune response (Turco et al., 2014; Rosenbaum et al., 2016). Second, EGCs would respond to pro-inflammatory cytokines derived from other immune cells and augment the local inflammation (Ruhl et al., 2001). Third, EGCs might present disease-related antigens to modulate the gut adaptive immunity (Geboes et al., 1992). Finally, EGCs are able to crosstalk indirectly or directly with the epithelial layer and influence the barrier integrity (Li et al., 2016; Meir et al., 2021). We will focus on the available evidence supporting the roles of EGCs in several gut diseases, i.e., inflammatory bowel disease, celiac disease, and autoimmune enteropathy (Figure 2).
Inflammatory bowel disease (IBD) is one of the most common immunological disorders in the gut. It affects millions of patients globally every year. There are two forms of IBD, i.e., ulcerative colitis and Crohn’s disease (GBD 2017 Inflammatory Bowel Disease Collaborators, 2020). While the underlining causes of IBD vary, it has been recognized that the disease condition is associated with chronic microbial infections, leading to unresolved local inflammation and tissue damage (Halfvarson et al., 2017; Britton et al., 2019). Studies have illustrated that genetic mutations in critical immune signaling components such as nucleotide-binding oligomerization domain containing 2 (NOD2) or IL-18 would predispose the IBD risk (Ogura et al., 2001; Gao et al., 2015). The pathogenic invasion through the epithelial barrier triggers the innate immune responses within the mucosa, resulting in the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Reinecker et al., 1993; Plevy et al., 1997). At the same time, the engagement of CD4+ T cells, particularly Th1 and Th17 cells, would produce IFN-γ and IL-17 (Niessner and Volk, 1995; Fujino et al., 2003). In the normal condition, such a collection of immune actions would eliminate the invading pathogens, enable tissue repair, and restore epithelial integrity. However, in the IBD condition, genetic mutations or other causes would interfere with the effectiveness of immune responses, leading to prolonged inflammation, unresolved infection, and chronic tissue damage (Chang, 2020).
Recent studies have already investigated the role of EGCs in the context of IBD (Bongioanni et al., 1996; Cornet et al., 2001; Cirillo et al., 2009; von Boyen et al., 2011; Bernardini et al., 2012; Li et al., 2018). EGCs could directly detect microbial products such as LPS via TLRs that they expressed, which helps elicit the innate immune response together with macrophages (Turco et al., 2014; Rosenbaum et al., 2016). In the meanwhile, EGCs would respond to the macrophage-derived IL-1β and TNF-α and further enhance their production of IL-1β and IL-6 (Ruhl et al., 2001). In addition, EGCs might present microbial-derived antigens to Th1 and Th17 cells, sustaining their production of pro-inflammatory cytokines IFN-γ and IL-17 (Geboes et al., 1992; Chow et al., 2021). Such immune functions of EGCs represent a critical part of local inflammation under the IBD condition. On the other hand, EGCs are capable of promoting barrier integrity and tissue repair. For example, studies reported that EGCs produce GDNF, which acts on ILC3s to stimulate the IL-22 release (Ibiza et al., 2016). IL-22 has an essential role in epithelial regeneration and repair (Lindemans et al., 2015). EGCs also produce GSNO that strengthens the tight junctions between epithelial cells (Li et al., 2016). Therefore, EGCs could exert both pro-inflammatory and tissue-repairing roles in the process of IBD. Indeed, studies reported that the expression levels of GDNF in EGCs correlated with the disease status of patients. Further, the proliferation of EGCs was observed in the condition of ulcerative colitis, probably reflecting their continuous action to counter tissue damage (von Boyen et al., 2011). More detailed examinations of their spatiotemporal response in patients and mouse models of IBD would illustrate the involvement of EGCs in the onset and progression of this common gut disorder.
Celiac disease is another important immunological disorder occurring in the gut. In contrast to the mechanism of IBD, celiac disease is primarily triggered by extrinsic antigens in foods (Ludvigsson et al., 2013; Lebwohl et al., 2018). It affects approximately 1% of the population globally, mainly in Caucasians but relatively rare in Africans and Asians (Singh et al., 2018). Celiac disease is often encountered by the food intake of gluten-containing products (Lebwohl et al., 2018). Gluten is a protein broadly existing in wheat, barley, and other grains. Because gluten highly enriches proline and glutamine residues, the protein is resistant to digestive enzymes, resulting in large amounts of incompletely digested peptides in the small intestine (Shan et al., 2002). These gluten peptides penetrate through the epithelial barrier and get into the lamina propria. The tissue transglutaminase (tTG) deamidates those gluten peptides, which would then be presented as antigens by dendritic cells (Molberg et al., 1998). Notably, people carrying the HLA-DQ2/DQ8 alleles of MHC-II would have a more effective antigen-presentation of gluten peptides and, as a result, become susceptible to celiac disease (Molberg et al., 1998; Sollid et al., 2012). Upon activation by the gluten peptides, CD4+ T cells could release pro-inflammatory cytokines such as IFN-γ and IL-21, initiating the local inflammation in the gut (Bodd et al., 2010). Moreover, the gluten-specific CD4+ T cells further induce the generation of anti-gluten or anti-tTG B cells (Mesin et al., 2012; du Pré and Sollid, 2015). The antibodies targeting gluten or tTG elicit the humoral immune response, particularly in the small intestine (Zanoni et al., 2006; Cervio et al., 2007). In fact, the anti-tTG autoimmune antibodies are the diagnostic hallmark of celiac disease (Lebwohl et al., 2018). In addition, the ongoing inflammation in the lamina propria induces the phenomenon of epithelial stress. In this pathological event, epithelial cells would produce IL-15, which enhances the recruitment of intraepithelial lymphocytes (IELs; Malamut et al., 2010). IELs could compromise the survival and functions of epithelial cells, resulting in the occurrence of villous atrophy (Meresse et al., 2004). Such aberrant immune responses collectively contribute to the chronic pathology observed in celiac disease. This immunological disorder may even proceed to extra-intestinal manifestations such as type I diabetes (Elfstrom et al., 2014).
Research has begun to explore the functional involvement of EGCs in celiac disease (Esposito et al., 2007). Because EGCs are known to express MHC-II molecules in several other disease conditions (Geboes et al., 1992; da Silveira et al., 2011), it appears plausible that they might present the gluten peptides and help trigger CD4+ T cells in the lamina propria, though this possibility remains to be investigated in detail. In addition, EGCs would respond to pro-inflammatory cytokines such as IFN-γ secreted by Th1 cells and upregulate the expression of CXCL10. CXCL10 may then recruit more T cells into the disease-inflicted regions (Progatzky et al., 2021). At the same time, EGCs could exhibit the disease-alleviating roles in celiac disease. For instance, studies reported that EGCs from patients with Crohn’s disease inhibited CD8+ T cells (Kermarrec et al., 2016). Such immunosuppressive function of EGCs on CD8+ T cells might act to ameliorate the damage to the epithelial barrier in celiac disease. In addition, EGCs produce GDNF that acts via the aforementioned ILC3s-IL22 mechanism to facilitate tissue repair (Ibiza et al., 2016). Further, EGCs could upregulate the expression of inducible nitric oxide synthase (iNOS) in response to S100β, leading to the production of nitric oxide (NO; Esposito et al., 2007). NO and GSNO together function to restore the barrier integrity of the epithelial layer (Li et al., 2016). Future research would document the precise roles of EGCs in celiac disease, which could open up a new dimension in understanding this immunological disorder of the gut.
Similar to celiac disease, autoimmune enteropathy is a gastrointestinal autoimmune disorder. It primarily affects newborns but also occurs in adulthood. The pathological features of this autoimmune disease include severe villous atrophy and the accumulation of intraepithelial CD8+ T cells, resembling those observed in celiac disease (Masia et al., 2014). However, in contrast to celiac disease, autoimmune enteropathy exhibits profound infiltration of monocytes in the lamina propria (Masia et al., 2014). In addition, autoimmune enteropathy has widespread manifestations in extra-intestinal organs, e.g., liver, thyroid, kidney, lung, and skin. The common cause of autoimmune enteropathy is associated with genetic mutations of signaling components involved in the immune tolerance, including forkhead box protein P3 (FoxP3) and the autoimmune regulator (AIRE; Montalto et al., 2009; Chen et al., 2020). Such genetic defects result in the aberrant reaction to self-antigens exposed by epithelial cells or goblet cells. For instance, FoxP3 is an essential transcription factor for the differentiation and functions of regulatory T cells (Treg), which produce the key anti-inflammatory cytokine IL-10 (Hori et al., 2003). Conversely, the loss of FoxP3 abrogates the Treg-mediated immune homeostasis, leading to the appearance of autoimmune CD4+ T cells and B cells in the intestinal tissues and related lymphoid organs (Barzaghi et al., 2012). The autoimmune CD4+ T cells would trigger the recruitment of monocytes (Masia et al., 2014). Moreover, the autoimmune antibodies specific to enterocytes or goblet cells attack the epithelial layer, causing the destruction of barrier integrity and defects in mucus production (Moore et al., 1995; Carroccio et al., 2003).
Though research on the involvement of EGCs in autoimmune enteropathy is still incoming, it is highly possible that EGCs contribute to this disease condition. For example, EGCs release CCL2 or CXCL10 under the LPS or IFN-γ stimulation, which probably facilitates the monocyte recruitment into the lamina propria (Rosenbaum et al., 2016; Progatzky et al., 2021). Further, similar to the scenarios described above, EGCs would maintain the barrier integrity via the indirect GDNF-ILC3s-IL22 axis or the direct production of GSNO (Savidge et al., 2007; Ibiza et al., 2016). Future investigations are warranted to unravel the relevance of EGCs in this specific autoimmune disorder of the gut.
Besides those immunological disorders in the gut, EGCs might also participate in the immune responses under divergent pathological conditions. Studies suggested that GDNF and GSNO derived from EGCs could be critical for the maintenance of barrier integrity by upregulating tight-junction proteins during the rotavirus infection (Hagbom et al., 2020). Similarly, the expression levels of GDNF would be upregulated in the colon tissues infected by Clostridium difficile (von Boyen et al., 2011). Therefore, it appears possible that GDNF released by EGCs may exert a general protective role in various disease conditions of the gastrointestinal tract. In addition, studies reported that the EGCs expression of MHC-II and the co-stimulatory molecules CD80 and CD86 significantly increased in Chagas disease caused by Trypanosoma cruzi, likely contributing to the activation of CD4+ T cells (da Silveira et al., 2011). Therefore, it has become conceivable that EGCs would act in various immunological conditions in the gut, exerting both pro-inflammatory and tissue-repairing functions.
Studies in the past decades have begun to elucidate the critical immune functions of EGCs and their potential involvement in immunological disorders of the gastrointestinal tract. While the research field has witnessed significant advances in the biology of EGCs, we would like to highlight several essential directions that remain to be investigated:
(1) Comprehensive Documentation of EGCs Immune Capacity. Studies have already revealed the immunological aspects of EGCs. However, the comprehensive assessment of cytokines, chemokines, and other immune factors that EGCs could produce is still lacking. In addition, the EGCs expression profile of specific receptors for pattern-associated molecular patterns or damage-associated molecular patterns awaits to be defined. Further, whether EGCs could directly present antigens via MHC-II molecules to activate CD4+ T cells needs to be unequivocally proven. Research into these questions would chart out the immune capacity of EGCs.
(2) Pathological Alterations of EGCs. Notably, the majority of available studies characterized EGCs in normal, healthy conditions. On the other hand, research has begun to reveal that EGCs could undergo significant alterations in the context of different diseases (Linan-Rico et al., 2016; Rosenbaum et al., 2016; Delvalle et al., 2018). For example, EGCs showed a higher expression of interferon-stimulated genes in the conditions of helminth infection or ulcerative colitis (Progatzky et al., 2021). In addition, it was proposed that EGCs could be defined as reactive based on their increased expression of pro-inflammatory pathways (Linan-Rico et al., 2016). Would any new subtype of EGCs emerge in response to specific pathological cues? Would EGCs experience cell death and cell regeneration in the disease-inflicted tissue? These unanswered questions could represent a new dimension in understanding the biology of EGCs.
(3) EGCs Crosstalk With Other Systems. While our current discussion has been centered on the interplay between EGCs and immune cells, EGCs could also interact with other systems within the gut. For example, recent studies reported that the ENS neurons would instruct specific immune responses (Veiga-Fernandes and Mucida, 2016; Wang et al., 2022). As a result, EGCs might indirectly exert immunomodulatory functions by influencing the activity of such ENS neurons (De Giorgio et al., 2012; Gulbransen and Sharkey, 2012). In addition, GSNO and NO have been known to cause vasodilation (Laskin et al., 1994). By producing such chemicals, EGCs may facilitate immune cell infiltration. Detailed studies of the functional interactions between EGCs with the ENS, the vascular system, and the lymphatic system would help uncover the complexity underlying tissue homeostasis in the gut.
(4) EGCs as a Therapeutic Target. Given their critical roles in sustaining the functions of the ENS neurons, EGCs have already been regarded as a novel therapeutic target. In light of the emerging immunological aspects of EGCs, it becomes even more appealing that EGCs could be exploited for their disease-alleviating actions. For instance, enhancing the GDNF production from EGCs could benefit tissue repair in different diseases of the gastrointestinal tract (Steinkamp et al., 2012; Hagbom et al., 2020). Also, EGCs might be manipulated to present tumor-related antigens to facilitate anti-cancer immunity. Exploring such therapeutic potential of EGCs would promote the basic and translational research of this unique glial type in the gut.
In sum, we have reviewed the updated knowledge of EGCs in several immunological disorders of the gastrointestinal tract. While EGCs are emerging as a new frontier in the research field, critical questions have called for future research efforts. Our in-depth understanding of the immune functions of EGCs may provide valuable insight for treating important human diseases.
CL and JY wrote the manuscript. All authors contributed to the article and approved the submitted version.
Research in JY’s lab has been funded by the National Natural Science Foundation of China (#31970974, #32061143007, #32125017, and #32150008) and the National Key Research and Development Program of China (2019YFA0802003). Additional supports for JY’s lab have been from the Center for Life Sciences at Peking University, the Chinese Institute for Brain Research, and the Institute of Molecular Physiology at Shenzhen Bay Laboratory.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdo, H., Derkinderen, P., Gomes, P., Chevalier, J., Aubert, P., Masson, D., et al. (2010). Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 24, 1082–1094. doi: 10.1096/fj.09-139519
Anderson, R. B., Stewart, A. L., and Young, H. M. (2006). Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 323, 11–25. doi: 10.1007/s00441-005-0047-6
Badizadegan, K., Thomas, A. R., Nagy, N., Ndishabandi, D., Miller, S. A., Alessandrini, A., et al. (2014). Presence of intramucosal neuroglial cells in normal and aganglionic human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G1002–1012. doi: 10.1152/ajpgi.00164.2014
Barajon, I., Serrao, G., Arnaboldi, F., Opizzi, E., Ripamonti, G., Balsari, A., et al. (2009). Toll-like receptors 3, 4 and 7 are expressed in the enteric nervous system and dorsal root ganglia. J. Histochem. Cytochem. 57, 1013–1023. doi: 10.1369/jhc.2009.953539
Barzaghi, F., Passerini, L., and Bacchetta, R. (2012). Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front. Immunol. 3:211. doi: 10.3389/fimmu.2012.00211
Belkind-Gerson, J., Graham, H. K., Reynolds, J., Hotta, R., Nagy, N., Cheng, L., et al. (2017). Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 7:2525. doi: 10.1038/s41598-017-02890-y
Belkind-Gerson, J., Hotta, R., Nagy, N., Thomas, A. R., Graham, H., Cheng, L., et al. (2015). Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm. Bowel Dis. 21, 870–878. doi: 10.1097/MIB.0000000000000326
Bernardini, N., Segnani, C., Ippolito, C., De Giorgio, R., Colucci, R., Faussone-Pellegrini, M. S., et al. (2012). Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J. Cell Mol. Med. 16, 318–327. doi: 10.1111/j.1582-4934.2011.01298.x
Biskou, O., Meira de-Faria, F., Walter, S. M., Winberg, M. E., Haapaniemi, S., Myrelid, P., et al. (2022). Increased numbers of enteric glial cells in the peyer’s patches and enhanced intestinal permeability by glial cell mediators in patients with ileal Crohn’s disease. Cells 11:335. doi: 10.3390/cells11030335
Bodd, M., Raki, M., Tollefsen, S., Fallang, L. E., Bergseng, E., Lundin, K. E., et al. (2010). HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 3, 594–601. doi: 10.1038/mi.2010.36
Boesmans, W., Lasrado, R., Vanden Berghe, P., and Pachnis, V. (2015). Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63, 229–241. doi: 10.1002/glia.22746
Boesmans, W., Nash, A., Tasnady, K. R., Yang, W., Stamp, L. A., and Hao, M. M. (2021). Development, diversity and neurogenic capacity of enteric glia. Front. Cell Dev. Biol. 9:775102. doi: 10.3389/fcell.2021.775102
Bongioanni, P., Castagna, M., and Morisi, M. (1996). [Immunohistochemical study of the enteric glia in chronic intestinal inflammatory disease]. Minerva Med. 87, 323–329.
Britton, G. J., Contijoch, E. J., Mogno, I., Vennaro, O. H., Llewellyn, S. R., Ng, R., et al. (2019). Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224.e4. doi: 10.1016/j.immuni.2018.12.015
Bush, T. G., Savidge, T. C., Freeman, T. C., Cox, H. J., Campbell, E. A., Mucke, L., et al. (1998). Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201. doi: 10.1016/s0092-8674(00)81571-8
Carroccio, A., Volta, U., Di Prima, L., Petrolini, N., Florena, A. M., Averna, M. R., et al. (2003). Case Report: Autoimmune enteropathy and colitis in an adult patient. Dig. Dis. Sci. 48, 1600–1606. doi: 10.1023/a:1024705032326
Cervio, E., Volta, U., Verri, M., Boschi, F., Pastoris, O., Granito, A., et al. (2007). Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 133, 195–206. doi: 10.1053/j.gastro.2007.04.070
Chang, C. Y., Lee, Y. H., Jiang-Shieh, Y. F., Chien, H. F., Pai, M. H., Chen, H. M., et al. (2011). Novel distribution of cluster of differentiation 200 adhesion molecule in glial cells of the peripheral nervous system of rats and its modulation after nerve injury. Neuroscience 183, 32–46. doi: 10.1016/j.neuroscience.2011.03.049
Chang, J. T. (2020). Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664. doi: 10.1056/NEJMra2002697
Cheadle, G. A., Costantini, T. W., Lopez, N., Bansal, V., Eliceiri, B. P., and Coimbra, R. (2013). Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One 8:e69042. doi: 10.1371/journal.pone.0069042
Chen, C. B., Tahboub, F., Plesec, T., Kay, M., and Radhakrishnan, K. (2020). A review of autoimmune enteropathy and its associated syndromes. Dig. Dis. Sci. 65, 3079–3090. doi: 10.1007/s10620-020-06540-8
Chow, A. K., Grubisic, V., and Gulbransen, B. D. (2021). Enteric glia regulate lymphocyte ctivation via autophagy-mediated MHC-II expression. Cell Mol. Gastroenterol. Hepatol. 12, 1215–1237. doi: 10.1016/j.jcmgh.2021.06.008
Cirillo, C., Sarnelli, G., Esposito, G., Grosso, M., Petruzzelli, R., Izzo, P., et al. (2009). Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 21, 1209–e112. doi: 10.1111/j.1365-2982.2009.01346.x
Cirillo, C., Sarnelli, G., Turco, F., Mango, A., Grosso, M., Aprea, G., et al. (2011). Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 23, e372–382. doi: 10.1111/j.1365-2982.2011.01748.x
Cornet, A., Savidge, T. C., Cabarrocas, J., Deng, W. L., Colombel, J. F., Lassmann, H., et al. (2001). Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. U S A 98, 13306–13311. doi: 10.1073/pnas.231474098
da Silveira, A. B., de Oliveira, E. C., Neto, S. G., Luquetti, A. O., Fujiwara, R. T., Oliveira, R. C., et al. (2011). Enteroglial cells act as antigen-presenting cells in chagasic megacolon. Hum. Pathol. 42, 522–532. doi: 10.1016/j.humpath.2010.06.016
De Giorgio, R., Giancola, F., Boschetti, E., Abdo, H., Lardeux, B., and Neunlist, M. (2012). Enteric glia and neuroprotection: basic and clinical aspects. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G887–893. doi: 10.1152/ajpgi.00096.2012
Delvalle, N. M., Dharshika, C., Morales-Soto, W., Fried, D. E., Gaudette, L., and Gulbransen, B. D. (2018). Communication between enteric neurons, glia and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol. Gastroenterol. Hepatol. 6, 321–344. doi: 10.1016/j.jcmgh.2018.05.009
Drokhlyansky, E., Smillie, C. S., Van Wittenberghe, N., Ericsson, M., Griffin, G. K., Eraslan, G., et al. (2020). The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622.e23. doi: 10.1016/j.cell.2020.08.003
du Pré, M. F., and Sollid, L. M. (2015). T-cell and B-cell immunity in celiac disease. Best Pract. Res. Clin. Gastroenterol. 29, 413–423. doi: 10.1016/j.bpg.2015.04.001
Durbec, P. L., Larsson-Blomberg, L. B., Schuchardt, A., Costantini, F., and Pachnis, V. (1996). Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358. doi: 10.1242/dev.122.1.349
Elfstrom, P., Sundstrom, J., and Ludvigsson, J. F. (2014). Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 40, 1123–1132. doi: 10.1111/apt.12973
Espinosa-Medina, I., Jevans, B., Boismoreau, F., Chettouh, Z., Enomoto, H., Muller, T., et al. (2017). Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc. Natl. Acad. Sci. U S A 114, 11980–11985. doi: 10.1073/pnas.1710308114
Esposito, G., Cirillo, C., Sarnelli, G., De Filippis, D., D’Armiento, F. P., Rocco, A., et al. (2007). Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 133, 918–925. doi: 10.1053/j.gastro.2007.06.009
Fujino, S., Andoh, A., Bamba, S., Ogawa, A., Hata, K., Araki, Y., et al. (2003). Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70. doi: 10.1136/gut.52.1.65
Gabella, G. (1972). Fine structure of the myenteric plexus in the guinea-pig ileum. J. Anat. 111, 69–97.
Gao, X., Li, W., Syed, F., Yuan, F., Li, P., and Yu, Q. (2022). PD-L1 signaling in reactive astrocytes counteracts neuroinflammation and ameliorates neuronal damage after traumatic brain injury. J. Neuroinflammation 19:43. doi: 10.1186/s12974-022-02398-x
Gao, S. J., Zhang, L., Lu, W., Wang, L., Chen, L., Zhu, Z., et al. (2015). Interleukin-18 genetic polymorphisms contribute differentially to the susceptibility to Crohn’s disease. World J. Gastroenterol. 21, 8711–8722. doi: 10.3748/wjg.v21.i28.8711
GBD 2017 Inflammatory Bowel Disease Collaborators (2020). The global, regional and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30. doi: 10.1016/S2468-1253(19)30333-4
Geboes, K., Rutgeerts, P., Ectors, N., Mebis, J., Penninckx, F., Vantrappen, G., et al. (1992). Major histocompatibility class II expression on the small intestinal nervous system in Crohn’s disease. Gastroenterology 103, 439–447. doi: 10.1016/0016-5085(92)90832-j
Grubisic, V., McClain, J. L., Fried, D. E., Grants, I., Rajasekhar, P., Csizmadia, E., et al. (2020). Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 32:108100. doi: 10.1016/j.celrep.2020.108100
Gulbransen, B. D., and Sharkey, K. A. (2012). Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 9, 625–632. doi: 10.1038/nrgastro.2012.138
Hagbom, M., De Faria, F. M., Winberg, M. E., Westerberg, S., Nordgren, J., Sharma, S., et al. (2020). Neurotrophic factors protect the intestinal barrier from rotavirus insult in mice. mBio 11, e02834–e02919. doi: 10.1128/mBio.02834-19
Halfvarson, J., Brislawn, C. J., Lamendella, R., Vazquez-Baeza, Y., Walters, W. A., Bramer, L. M., et al. (2017). Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2:17004. doi: 10.1038/nmicrobiol.2017.4
Hanani, M., and Reichenbach, A. (1994). Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 278, 153–160. doi: 10.1007/BF00305787
Hoff, S., Zeller, F., von Weyhern, C. W., Wegner, M., Schemann, M., Michel, K., et al. (2008). Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 509, 356–371. doi: 10.1002/cne.21769
Hori, S., Nomura, T., and Sakaguchi, S. (2003). Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061. doi: 10.1126/science.1079490
Ibiza, S., Garcia-Cassani, B., Ribeiro, H., Carvalho, T., Almeida, L., Marques, R., et al. (2016). Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443. doi: 10.1038/nature18644
Jessen, K. R., and Mirsky, R. (1983). Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J. Neurosci. 3, 2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983
Joseph, N. M., He, S., Quintana, E., Kim, Y. G., Nunez, G., and Morrison, S. J. (2011). Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398–3411. doi: 10.1172/JCI58186
Kermarrec, L., Durand, T., Neunlist, M., Naveilhan, P., and Neveu, I. (2016). Enteric glial cells have specific immunosuppressive properties. J. Neuroimmunol. 295–296,79–83. doi: 10.1016/j.jneuroim.2016.04.011
Kim, J., Lo, L., Dormand, E., and Anderson, D. J. (2003). SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17–31. doi: 10.1016/s0896-6273(03)00163-6
Lake, J. I., and Heuckeroth, R. O. (2013). Enteric nervous system development: migration, differentiation and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G1–24. doi: 10.1152/ajpgi.00452.2012
Laranjeira, C., Sandgren, K., Kessaris, N., Richardson, W., Potocnik, A., Vanden Berghe, P., et al. (2011). Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest. 121, 3412–3424. doi: 10.1172/JCI58200
Laskin, J. D., Heck, D. E., and Laskin, D. L. (1994). Multifunctional role of nitric oxide in inflammation. Trends Endocrinol. Metab. 5, 377–382. doi: 10.1016/1043-2760(94)90105-8
Lasrado, R., Boesmans, W., Kleinjung, J., Pin, C., Bell, D., Bhaw, L., et al. (2017). Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722–726. doi: 10.1126/science.aam7511
Lebwohl, B., Sanders, D. S., and Green, P. H. R. (2018). Coeliac disease. Lancet 391, 70–81. doi: 10.1016/S0140-6736(17)31796-8
Li, Y., Ge, Y., Zhu, W., Gong, J., Cao, L., Guo, Z., et al. (2018). Increased enteric glial cells in proximal margin of resection is associated with postoperative recurrence of Crohn’s disease. J. Gastroenterol. Hepatol. 33, 638–644. doi: 10.1111/jgh.13973
Li, Z., Zhang, X., Zhou, H., Liu, W., and Li, J. (2016). Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. J. Trauma Acute Care Surg. 80, 977–984. doi: 10.1097/TA.0000000000001008
Linan-Rico, A., Turco, F., Ochoa-Cortes, F., Harzman, A., Needleman, B. J., Arsenescu, R., et al. (2016). Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility and GI disorders. Inflamm. Bowel Dis. 22, 1812–1834. doi: 10.1097/MIB.0000000000000854
Lindemans, C. A., Calafiore, M., Mertelsmann, A. M., O’Connor, M. H., Dudakov, J. A., Jenq, R. R., et al. (2015). Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564. doi: 10.1038/nature16460
Ludvigsson, J. F., Leffler, D. A., Bai, J. C., Biagi, F., Fasano, A., Green, P. H., et al. (2013). The Oslo definitions for coeliac disease and related terms. Gut 62, 43–52. doi: 10.1136/gutjnl-2011-301346
Malamut, G., El Machhour, R., Montcuquet, N., Martin-Lanneree, S., Dusanter-Fourt, I., Verkarre, V., et al. (2010). IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 120, 2131–2143. doi: 10.1172/JCI41344
Masia, R., Peyton, S., Lauwers, G. Y., and Brown, I. (2014). Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am. J. Surg. Pathol. 38, 1319–1329. doi: 10.1097/PAS.0000000000000317
Meir, M., Kannapin, F., Diefenbacher, M., Ghoreishi, Y., Kollmann, C., Flemming, S., et al. (2021). Intestinal epithelial barrier maturation by enteric glial cells is GDNF-dependent. Int. J. Mol. Sci. 22:1887. doi: 10.3390/ijms22041887
Meresse, B., Chen, Z., Ciszewski, C., Tretiakova, M., Bhagat, G., Krausz, T. N., et al. (2004). Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366. doi: 10.1016/j.immuni.2004.06.020
Mesin, L., Sollid, L. M., and Di Niro, R. (2012). The intestinal B-cell response in celiac disease. Front. Immunol. 3:313. doi: 10.3389/fimmu.2012.00313
Molberg, O., McAdam, S. N., Korner, R., Quarsten, H., Kristiansen, C., Madsen, L., et al. (1998). Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717. doi: 10.1038/nm0698-713
Montalto, M., D’Onofrio, F., Santoro, L., Gallo, A., Gasbarrini, A., and Gasbarrini, G. (2009). Autoimmune enteropathy in children and adults. Scand. J. Gastroenterol. 44, 1029–1036. doi: 10.1080/00365520902783691
Moore, L., Xu, X., Davidson, G., Moore, D., Carli, M., and Ferrante, A. (1995). Autoimmune enteropathy with anti-goblet cell antibodies. Hum. Pathol. 26, 1162–1168. doi: 10.1016/0046-8177(95)90283-x
Murakami, M., Ohta, T., and Ito, S. (2009). Lipopolysaccharides enhance the action of bradykinin in enteric neurons via secretion of interleukin-1β from enteric glial cells. J. Neurosci. Res. 87, 2095–2104. doi: 10.1002/jnr.22036
Nagy, N., and Goldstein, A. M. (2017). Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94–106. doi: 10.1016/j.semcdb.2017.01.006
Niessner, M., and Volk, B. A. (1995). Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin. Exp. Immunol. 101, 428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x
Ogura, Y., Bonen, D. K., Inohara, N., Nicolae, D. L., Chen, F. F., Ramos, R., et al. (2001). A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606. doi: 10.1038/35079114
Plevy, S. E., Landers, C. J., Prehn, J., Carramanzana, N. M., Deem, R. L., Shealy, D., et al. (1997). A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J. Immunol. 159, 6276–6282.
Progatzky, F., Shapiro, M., Chng, S. H., Garcia-Cassani, B., Classon, C. H., Sevgi, S., et al. (2021). Regulation of intestinal immunity and tissue repair by enteric glia. Nature 599, 125–130. doi: 10.1038/s41586-021-04006-z
Rao, M., and Gershon, M. D. (2018). Enteric nervous system development: what could possibly go wrong? Nat. Rev. Neurosci. 19, 552–565. doi: 10.1038/s41583-018-0041-0
Rao, M., Nelms, B. D., Dong, L., Salinas-Rios, V., Rutlin, M., Gershon, M. D., et al. (2015). Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040–2057. doi: 10.1002/glia.22876
Rao, M., Rastelli, D., Dong, L., Chiu, S., Setlik, W., Gershon, M. D., et al. (2017). Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068–1081.e7. doi: 10.1053/j.gastro.2017.07.002
Reinecker, H. C., Steffen, M., Witthoeft, T., Pflueger, I., Schreiber, S., MacDermott, R. P., et al. (1993). Enhanced secretion of tumour necrosis factor-α, IL-6 and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 94, 174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x
Rosenbaum, C., Schick, M. A., Wollborn, J., Heider, A., Scholz, C. J., Cecil, A., et al. (2016). Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS One 11:e0151335. doi: 10.1371/journal.pone.0151335
Rothman, T. P., Tennyson, V. M., and Gershon, M. D. (1986). Colonization of the bowel by the precursors of enteric glia: studies of normal and congenitally aganglionic mutant mice. J. Comp. Neurol. 252, 493–506. doi: 10.1002/cne.902520406
Ruhl, A., Franzke, S., Collins, S. M., and Stremmel, W. (2001). Interleukin-6 expression and regulation in rat enteric glial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G1163–1171. doi: 10.1152/ajpgi.2001.280.6.G1163
Savidge, T. C., Newman, P., Pothoulakis, C., Ruhl, A., Neunlist, M., Bourreille, A., et al. (2007). Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358. doi: 10.1053/j.gastro.2007.01.051
Schuchardt, A., D’Agati, V., Larsson-Blomberg, L., Costantini, F., and Pachnis, V. (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383. doi: 10.1038/367380a0
Seguella, L., and Gulbransen, B. D. (2021). Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 18, 571–587. doi: 10.1038/s41575-021-00423-7
Shan, L., Molberg, O., Parrot, I., Hausch, F., Filiz, F., Gray, G. M., et al. (2002). Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279. doi: 10.1126/science.1074129
Singh, P., Arora, A., Strand, T. A., Leffler, D. A., Catassi, C., Green, P. H., et al. (2018). Global prevalence of celiac disease: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16, 823–836.e2. doi: 10.1016/j.cgh.2017.06.037
Sollid, L. M., Qiao, S. W., Anderson, R. P., Gianfrani, C., and Koning, F. (2012). Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64, 455–460. doi: 10.1007/s00251-012-0599-z
Steinkamp, M., Gundel, H., Schulte, N., Spaniol, U., Pflueger, C., Zizer, E., et al. (2012). GDNF protects enteric glia from apoptosis: evidence for an autocrine loop. BMC Gastroenterol. 12:6. doi: 10.1186/1471-230X-12-6
Stoffels, B., Hupa, K. J., Snoek, S. A., van Bree, S., Stein, K., Schwandt, T., et al. (2014). Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 146, 176–187.e1. doi: 10.1053/j.gastro.2013.09.030
Turco, F., Sarnelli, G., Cirillo, C., Palumbo, I., De Giorgi, F., D’Alessandro, A., et al. (2014). Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 63, 105–115. doi: 10.1136/gutjnl-2012-302090
Vanderwinden, J. M., Timmermans, J. P., and Schiffmann, S. N. (2003). Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 312, 149–154. doi: 10.1007/s00441-003-0716-2
Veiga-Fernandes, H., and Mucida, D. (2016). Neuro-immune interactions at barrier surfaces. Cell 165, 801–811. doi: 10.1016/j.cell.2016.04.041
von Boyen, G. B., Schulte, N., Pfluger, C., Spaniol, U., Hartmann, C., and Steinkamp, M. (2011). Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 11:3. doi: 10.1186/1471-230X-11-3
Wang, H., Foong, J. P. P., Harris, N. L., and Bornstein, J. C. (2022). Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol. 15, 27–39. doi: 10.1038/s41385-021-00443-1
Yang, P. C., Li, X. J., Yang, Y. H., Qian, W., Li, S. Y., Yan, C. H., et al. (2020). The influence of bifidobacterium bifidum and bacteroides fragilis on enteric glial cell-derived neurotrophic factors and inflammasome. Inflammation 43, 2166–2177. doi: 10.1007/s10753-020-01284-z
Young, H. M., Bergner, A. J., and Muller, T. (2003). Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J. Comp. Neurol. 456, 1–11. doi: 10.1002/cne.10448
Young, H. M., Ciampoli, D., Hsuan, J., and Canty, A. J. (1999). Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b- and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev. Dyn. 216, 137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6
Zanoni, G., Navone, R., Lunardi, C., Tridente, G., Bason, C., Sivori, S., et al. (2006). In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 3:e358. doi: 10.1371/journal.pmed.0030358
Zeisel, A., Hochgerner, H., Lonnerberg, P., Johnsson, A., Memic, F., van der Zwan, J., et al. (2018). Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22. doi: 10.1016/j.cell.2018.06.021
Keywords: enteric glial cells, enteric nervous system, inflammatory bowel disease, celiac disease, autoimmune enteropathy
Citation: Liu C and Yang J (2022) Enteric Glial Cells in Immunological Disorders of the Gut. Front. Cell. Neurosci. 16:895871. doi: 10.3389/fncel.2022.895871
Received: 14 March 2022; Accepted: 07 April 2022;
Published: 28 April 2022.
Edited by:
Mingqin Zhu, First Affiliated Hospital of Jilin University, ChinaReviewed by:
Carla Cirillo, INSERM U1214 Centre d’Imagerie Neuro Toulouse (ToNIC), FranceCopyright © 2022 Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, amluZy55YW5nQHBrdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.