
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci., 06 May 2022
Sec. Non-Neuronal Cells
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.865266
This article is part of the Research TopicThe Function of Schwann Cells in Peripheral Nervous SystemView all 11 articles
 Qisong Su1,2,3†
Qisong Su1,2,3† Moussa Ide Nasser1,2†
Moussa Ide Nasser1,2† Jiaming He4†
Jiaming He4† Gang Deng2,5
Gang Deng2,5 Qing Ouyang2,5
Qing Ouyang2,5 Donglin Zhuang1,6
Donglin Zhuang1,6 Yuzhi Deng2,7
Yuzhi Deng2,7 Haoyun Hu2,7
Haoyun Hu2,7 Nanbo Liu1,3
Nanbo Liu1,3 Zhetao Li1,8
Zhetao Li1,8 Ping Zhu1,2,3,5,7,8,9*
Ping Zhu1,2,3,5,7,8,9* Ge Li1,2,3,9*
Ge Li1,2,3,9*Compared with the central nervous system, the adult peripheral nervous system possesses a remarkable regenerative capacity, which is due to the strong plasticity of Schwann cells (SCs) in peripheral nerves. After peripheral nervous injury, SCs de-differentiate and transform into repair phenotypes, and play a critical role in axonal regeneration, myelin formation, and clearance of axonal and myelin debris. In view of the limited self-repair capability of SCs for long segment defects of peripheral nerve defects, it is of great clinical value to supplement SCs in necrotic areas through gene modification or stem cell transplantation or to construct tissue-engineered nerve combined with bioactive scaffolds to repair such tissue defects. Based on the developmental lineage of SCs and the gene regulation network after peripheral nerve injury (PNI), this review summarizes the possibility of using SCs constructed by the latest gene modification technology to repair PNI. The therapeutic effects of tissue-engineered nerve constructed by materials combined with Schwann cells resembles autologous transplantation, which is the gold standard for PNI repair. Therefore, this review generalizes the research progress of biomaterials combined with Schwann cells for PNI repair. Based on the difficulty of donor sources, this review also discusses the potential of “unlimited” provision of pluripotent stem cells capable of directing differentiation or transforming existing somatic cells into induced SCs. The summary of these concepts and therapeutic strategies makes it possible for SCs to be used more effectively in the repair of PNI.
Mechanical trauma, for example, as sustained in an industrial or traffic accident, is the most frequent cause of peripheral nervous system injury (Faroni et al., 2015). Approximately five million peripheral nerve injuries occur each year with 500,000 surgical operations performed in the United States alone, generating $1.5 billion for the nerve repair industry (Meena et al., 2021). Peripheral nerve injury (PNI) may result in permanent motor and sensory disabilities, and most individuals cannot return to regular employment immediately after injury. Additionally, recovery is poor because of the limited therapeutic impact, which affects patients’ quality of life and imposes a significant economic burden on society and families (Slavin et al., 2021). In contrast to the central nervous system (CNS), the adult peripheral nervous system (PNS) maintains considerable regenerative capacity even after serious injury (Chen et al., 2007). The considerable regeneration capability of the PNS is demonstrated by the capability of injured peripheral axons to regenerate and target their destinations, a process facilitated by the high plasticity of Schwann cells (SCs) in the PNS (Kim et al., 2013; Jessen et al., 2015).
SCs are a critical component of the PNS. Nerves in the periphery are composed of myelinated and unmyelinated fibers. Myelin sheaths, axons, and nerve membranes comprise nerve fibers. The larger axons establish a 1:1 connection with myelinated SCs, whereas the non-myelinated SCs bundle the smaller axons to form Remak bundles (Napoli et al., 2012). Peripheral nerve fibroblasts subsequently facilitate the formation of axon group bundles enclosed by SCs, and larger nerves consist of many bundles wrapped in the epineurium. Primary injury to these neural structures is often induced by direct forces, such as abrupt extension, tears, or compression, and the subsequent vascular ischemia exacerbates the primary injury, resulting in secondary injury (Sullivan et al., 2016). Within 12–24 h of PNS injury, calcium influx activates proteases, resulting in cytoskeleton rupture, axon membrane breakdown, and myelin degradation within 2 days (Schlaepfer and Bunge, 1973; Stoll et al., 1989). SCs dedifferentiate into a repair phenotype as a result of axonal breakdown and myelinolysis (Hall, 2005; Arthur-Farraj et al., 2017). Not only can repair SCs contribute to the clearance of axon and myelin-derived debris but they may also multiply to fill the empty endoneurial tube and create a strip-like column in the basal layer, called Büngner bands (Li et al., 2005; Murinson et al., 2005; Gomez-Sanchez et al., 2015). SCs in Büngner bands release neurotrophic factors that nourish injured neurons and promote axonal regeneration and myelination (Madduri and Gander, 2010; Jessen and Mirsky, 2016). The healing of PNS injury by SCs is outlined in Figure 1. To summarize, SCs are essential for the healing process after PNI. It is critical to study the many identities of SCs that rely on various important chemicals throughout the development, injury, and repair of the PNS to achieve more effective treatment methods for preventing or removing micro-environmental problems in myelination injury.
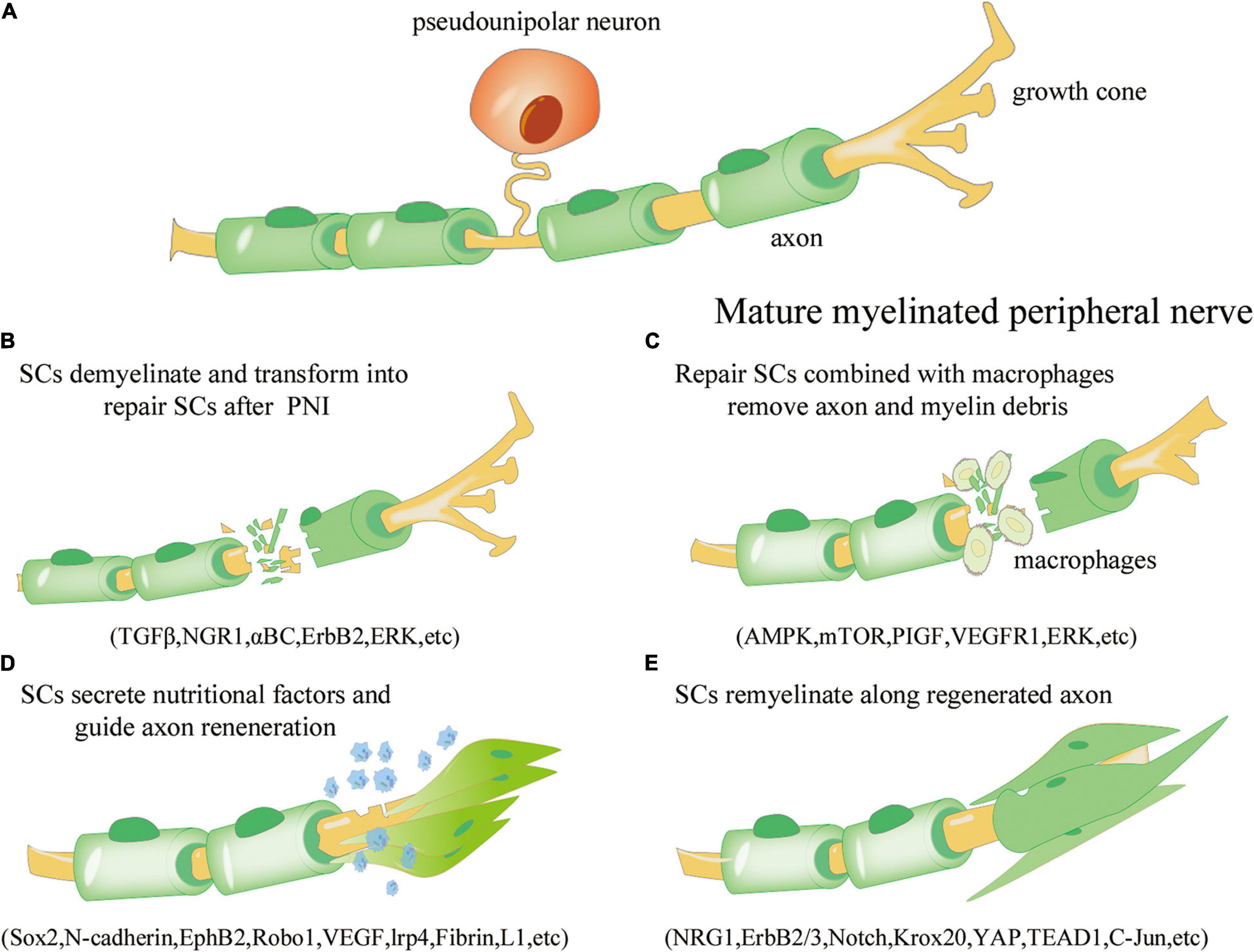
Figure 1. Overview of the repair process of Schwann cells (SCs) after peripheral nerve injury. (A) Under normal physiological conditions, mature myelinated peripheral nerve processes (yellow) are surrounded by the myelin sheath formed by SCs (green). (B) Axonal rupture and myelin disintegration due to peripheral nerve injury. SCs are activated, dedifferentiate, and transform into the repair phenotype. The involved signaling pathways include transforming growth factor beta (TGF-β), neuroregulin 1 (NGR1), αBC transporter, ErbB2, and extracellular signal receptor-kinase (ERK). (C) Repair SCs proliferate and fill the empty endoneurial canal, working with macrophages to remove axonal and myelin-derived debris; the involved signaling pathways include AMP activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), placental growth factor (PIGF), vascular endothelial growth factor receptor 1 (VEGFR1), and ERK. (D) SCs are arranged along axons to form Büngner bands and secrete neurotrophic factors to improve the microenvironment and guide axon regeneration; the involved regulators include Sox1, N-cadherin, EphB2, Robo1, VEGF, lrp4, Fibrin, and L1. (E) SCs are re-myelinated along the regenerated axons to promote neural information transmission; the involved regulators include NRG1, ErbB2/3, Notch, Krox20, yes-associated protein (YAP), transcriptional enhance factor domain transcription factor 4 (TEAD1), and c-Jun.
Given the critical role of SCs in the healing process of PNI, this article presents a comprehensive analysis of the major molecular regulatory networks of SCs involved in the treatment of PNI over the last decade to identify significant targets for increasing SC repair efficiency. Using existing genetic modification of SCs and induced Schwann-like cells (iSCs), their applications in the repair of PNI are summarized, and gene editing using cutting-edge technology to enhance the effect of SC repair possibilities is explored. An “unlimited” supply potential of pluripotent stem cell directional differentiation or conversion of available class cells to iSCs can occur and is explained in this review. The comprehensive review of these studies has enabled researchers to make new advancements from conventional SC transplantation treatment to SC engineering and manufacture and to provide new ideas for stimulating related research.
SCs are derived from neural crest cells (NCCs), which ultimately create myelin sheaths through two intermediate stages: (1) Schwann cell precursors (SCPs) and (2) immature SCs, which provide nutritional support for the development of peripheral nerve axons (Figure 2A). NCCs develop throughout the PNS growth process by forming new peripheral axons with strong proliferative capabilities and high pluripotency (Muppirala et al., 2021). The first stable branch separates the sensory lineage from the common progenitor cells of the autonomic and mesenchymal branches during the migration of NCCs. The second stable branch divides the production of autonomic neurons, and the remaining branches can be attributed to glial differentiation owing to their expression of the transcription markers for early glial cells (Mpz, Fabp7, Zfp488, Plp1, Sox10) and transcriptional markers of SCPs (Soldatov et al., 2019). SCP migration starts with the formation of a basal layer (BL) and the process of wrapping the axons during the juvenile SC stage. When immature SCs are wrapped in a 1:1 manner throughout the radial separation phase, proliferation of the immature SCS slows, and definitive differentiation begins. This phase of differentiation is controlled by the BL, which facilitates an increase in the expression of myelination genes. After differentiation, the juvenile SC skeleton progressively grows until its membrane wraps around the axon and produces a myelin sheath. Following the completion of the package, myelin is compressed to create a mature myelin sheath. Stable and mature SCs are then formed through active signaling pathways.
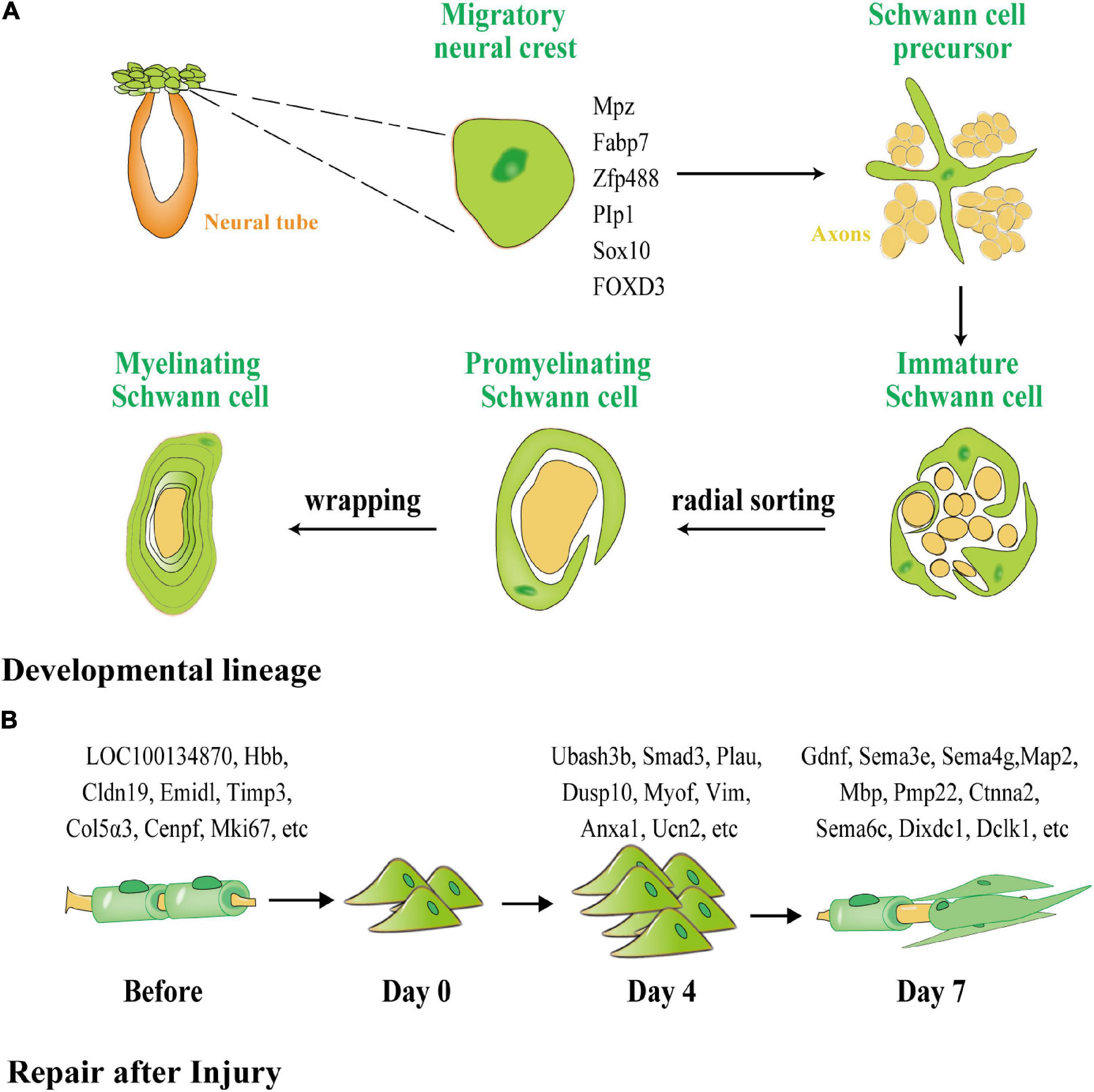
Figure 2. The different processes of myelination of SCs during development and after peripheral nerve injury (PNI). (A) SCs generated from neural crests create myelin sheaths in the PNS. Green represents glial cells and precursors, and orange represents the neural tube and yellow represents neurons and their axons. SCPs are neural crest cells that detach from the neural tube and migrate along the nascent peripheral axons, expressing the early glial markers Mpz, Fabp7, Zfp488, Plp1, Sox10, and forkhead box D3 FOXD3. SCPs differentiate into immature SCs, which, via cytoplasmic extension, enclose the axon bundle. Finally, radial sorting is used to choose a single axon for wrapping. SCs that have been myelinated enclosed axon segments, forming myelin sheaths. (B) Dedifferentiation and remyelination of SCs after PNI. Normal SCs form a myelin sheath around axons with one of four phenotypes: subtype 1 expressing LOC100134871 and Hbb, subtype 2 expressing Cldn19 and Emid1, subtype 3 expressing Timp3 and Col5a3, and subtype 4 expressing Cenpf and Mki67. After PNI, SCs transform into a homogenous phenotype and participate in proliferation and phagocytosis. On the fourth day after injury, 776 lncRNAs were unique to the proliferation of SCs. After day 7, 317 lncRNAs were unique to the remyelination of SCs.
SCs exhibit distinct gene regulatory networks after PNI when compared with SC development (Figure 2B). According to Chen et al. (2021), the cells in normal and injured peripheral nerves may be functionally classified as SCs, neurofibroblasts, immune cells, and vascular-related cells. SCs and neurofibroblasts are critical for the regeneration of peripheral nerves. Zhang et al. (2021) discovered four distinct phenotypes in SCs in newborn rats using single-cell transcriptome analysis, namely, SC subtype 1 resembling connective tissue cells expressing LOC100134871 and Hbb, and highly mature SC subtype 2, expressing Cldn19 and Emid1. Timp3 and Col5a3 were found to be expressed in subtype 3, which is involved in tissue development and differentiation into other SC subtypes, whereas Cenpf and Mki67 were found to be expressed in subtype 4, which is related to cell division, peripheral nerve development, and regeneration. Zhang et al. (2021) used second-generation sequencing to perform deep sequencing in rats on days 4 and 7 (D4 and D7) respectively after sciatic nerve injury, and discovered that 776 long non-coding RNAs (lncRNAs), such as Ubash3b, Smad3, Plau, and others, are unique to D4. Most of these RNAs are related to wound healing, phosphatase binding, and the mitogen-activated protein kinase (MAPK) signaling cascade. Gdnf, Sema3e, Sema4g, and others were found on D7 and appear to be associated with axon regeneration and cell cycle. Throughout development and repair, these diverse expression regulatory networks indicate that separate neural regulation methods for differentiation and induction of iSCs and the mobilization of repair following injury can be used.
Following PNI, mature SCs may undergo de-differentiation through various pathways, migrate to the wounded area, stimulate nerve remyelination, and play a role in directing axon regeneration. Wang et al. (2012) investigated the intrinsic migration characteristics of SCs using single-cell migration experiments and discovered that de-differentiated SCs first develop long protuberances, during which the nucleus was shown to move forward to the front of the cell and then retract to the back of the protuberances after which the protuberances extended again. Numerous studies have shown links between transforming growth factor beta (TGF-β), Slit-Robo, ErbB2 receptor, Hippo and Notch, extracellular signal -related kinase (ERK), and vascular endothelial growth factor (VEGF) signaling pathways and migration, proliferation, and axon guidance of SCs after PNI (Table 1).
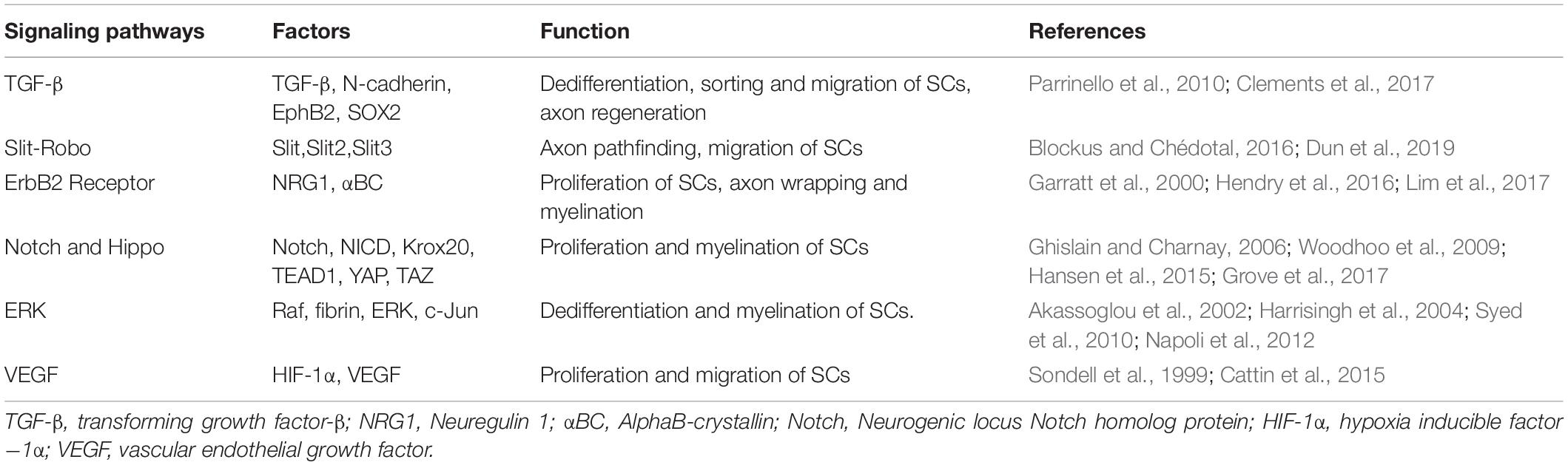
Table 1. Key signaling pathways involved in migration, proliferation and axon guidance of SCs after PNI.
Figure 3 depicts a schematic representation of the many signaling pathways involved in the migration of SCs after PNS injury.
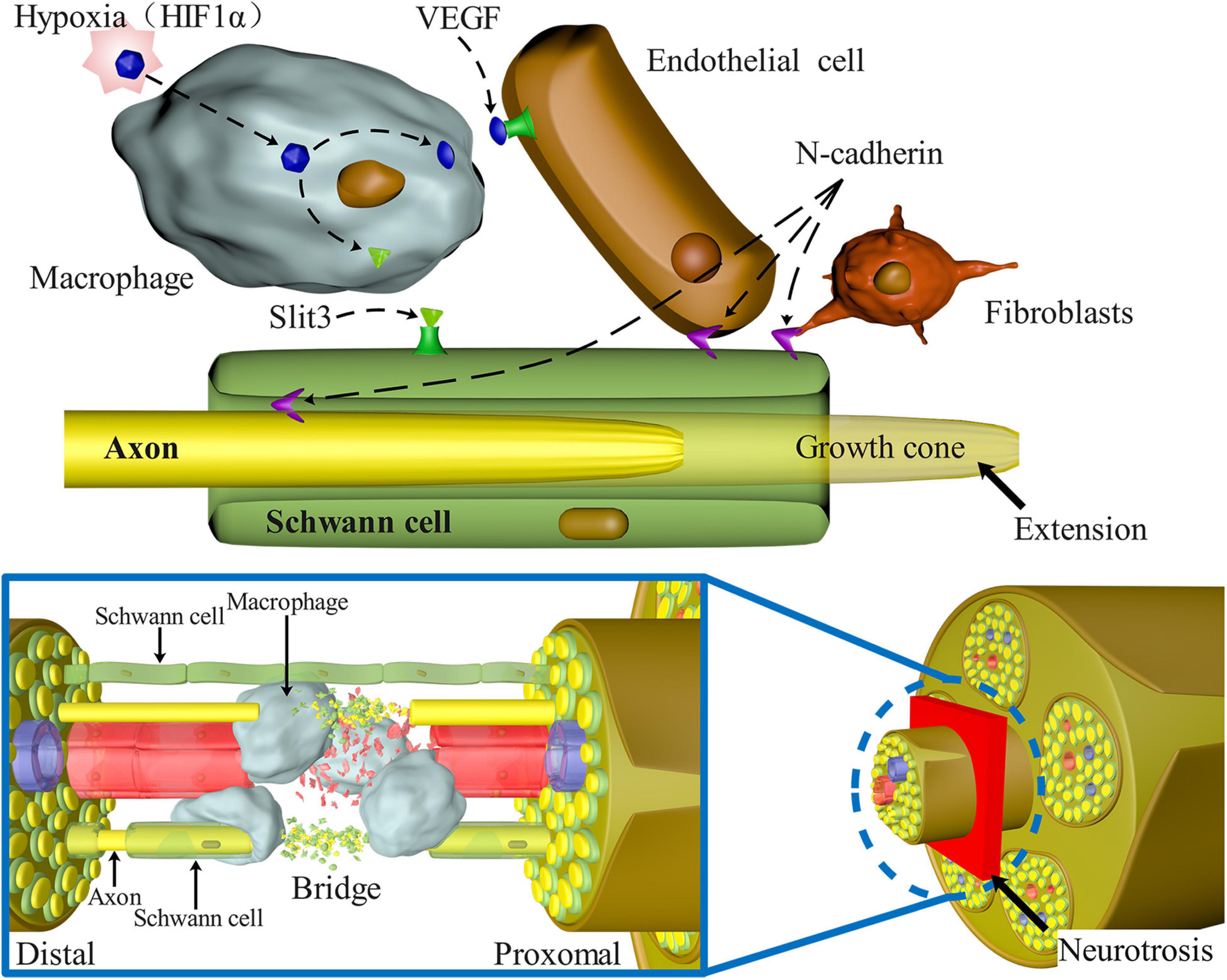
Figure 3. Signaling pathways involved in SC migration, proliferation, and axon guidance in nerve bridges after peripheral nerve injury. N-Cadherin increases the adhesion between SCs, fibroblasts, and endothelial cells, causing SCs to migrate to the outside of the nerve stump and guiding axons to reorient growth at the injury site. Macrophages enter the nerve space and secrete Slit3, which binds with SCs expressing Robo1 to control the formation of correct nerve bridges and promote SC migration and axon guidance. Macrophages in the nerve bridges detect hypoxia and enhance the amount of VEGF-induced blood vessel regeneration, alleviating hypoxia, and acting as an important medium to guide axon regeneration.
Numerous studies have shown that SCs are capable of successfully facilitating PNI repair. Through genetic engineering, the native genes of SCs may be up- or down-regulated, and the natural products required for peripheral nerve regeneration can be generated in a controlled manner. SCs may be genetically modified using various tools and methods that introduce specific genes or sequences into the genome, such as viral vectors, non-viral transfection systems, and clustered regularly spaced short palindromic repeats (CRISPR)/Cas9 gene-editing approaches.
Histone deacetylase (HDAC) is a significant epigenetic regulator of the Notch–Hey2 signaling pathway, which is required for myelination in the peripheral nervous system. Two groups of HDACs, HDAC1 and 2, both of which are involved in the myelination of SCs and HDAC3, which is involved in maintaining myelin homeostasis have been found. Jacob et al. (2011) used lentiviral transfection to selectively knockdown HDAC1 and 2 in SCs, a process leading to a substantial decrease in the expression of SOX10 and Krox20, the main transcriptional regulators of myelination in SCs. HDAC1 and 2 have been shown to have distinct main roles in SCs, with HDAC2 cooperating with Sox10 to initiate the myelination transcriptional process. Concurrently, HDAC1 regulates SC survival by controlling the amount of active β-catenin.
Likewise, Chen et al. (2011) discovered that the absence of HDAC1 and 2 prevented the NF-κB protein complex from binding to p300 and HDAC1/2, resulting in significant acetylation of NF-κB p65, reduction in the positive regulators of myelin development, and activation of differentiation inhibitors. In addition, development of SCs stalled during the immature stage. Interestingly, Brügger et al. (2017) discovered that early treatment with HDAC1/2 inhibitors caused an increase in the production of repair SCs and improvement in axonal regeneration and functional recovery but also led to impaired myelin regeneration. This process raises the possibility of adjusting the expression period of HDAC1 and 2 in SCs after injury during clinical transformation to improve peripheral nerve regeneration. Rosenberg et al. (2018) demonstrated the unique effects of HDAC3, another class of protein deacetylases involved in the Notch-Hey2 signaling pathway, on SCs via gene knockout. These authors demonstrated that although HDAC3 was not found to be necessary for the formation of myelinated SCs, once myelinated SCs are formed, HDAC3 appears to be required for their stability. He et al. (2018) discovered that HDAC3 caused suppression of the neuroregulin/phosphoinositide 3-kinase/protein kinase B (NRG1/PI3K/Akt) signaling pathway and its downstream myelination process, ensuring correct myelination and preventing peripheral myelin expansion in vivo.
Additionally, HDAC3 may bind the p300 histone acetyltransferase and control the expression of the transcriptional enhance factor domain transcription factor 4 (TEAD4) gene thus activating the inhibitory network of myelin development. Zinc-finger e-box-binding Homeobox 2 (Zeb2) is another important regulator of the Notch–Hey2 signaling cascade. Wu et al. (2016) generated Zeb2-defective SCs by non-viral transfection and discovered that Zeb2 regulated the start of SC development by recruiting the co-inhibitory histone deacetylases, HDAC1 and 2, and nucleosome remodeling and histone deacetylation (HDAC1/2-NuRD). Hey2 appears to limit differentiation of SCs by preventing differentiation and promoting their proliferation, whereas Zeb2 seems to promote the maturation and myelination of SCs by directly inhibiting Hey2 and regulating the Notch–Hey2 signaling pathway.
Moesin-ezrin-radixin-like protein (Merlin) tumor suppressors contribute to cell signaling, contact-mediated proliferation, and tumorigenesis. Loss of Merlin was found to disrupt the MAPK and Hippo signaling pathways, leading to enhanced activity of the Hippo effectors, yes-associated protein 1 and (YAP) and related protein, TAZ (Ammoun et al., 2008; Cooper and Giancotti, 2014). Merlin deficiency results in a significant increase in SC proliferation, macrophage infiltration, severe injury to axons and myelin regeneration, decreased induction of c-Jun, dysregulation of the MAPK signaling pathway, and activation of the Hippo signaling pathway following injury (Mindos et al., 2017). Previous studies have demonstrated that silencing YAP in SCs could functionally restore both axon and myelin sheath regeneration caused by Merlin loss and restore the expression of c-Jun and neurotrophic factors in addition to axon regeneration and functional recovery following injury. Deng and coworkers (Deng et al., 2017) showed that activating the Hippo signaling pathway led to an increase in SC proliferation, while TAZ inhibited the Gnas gene, which produces the GαS protein. Gnas deficiency resulted in a dramatic increase in the proliferation of SCs. Consequently, it was hypothesized that Gnas control may aid in limiting SC growth. However, Grove et al. (2020) showed that knock down of YAP/TAZ does not impair the proliferation of SCs or enhance transition of SCs into repair SCs. However, axon sorting and myelin degeneration are impaired in the absence of YAP/TAZ (Poitelon et al., 2016; Grove et al., 2017, 2020). This process occurs because growing SCs need YAP/TAZ to enter the S phase and generate sufficient SCs for correct axon sorting. Concurrently, YAP/TAZ is needed for TEAD1 to activate Krox20, which regulates myelination (Grove et al., 2017).
Activation of the transcription factor c-Jun in SCs acts as a global regulator of Waller’s degeneration and regulates the expression of nutrient factors, adhesion molecules, regeneration locus formation, and myelin clearance. In addition to activating repair programs in SCs, this factor contributes to generating cells specifically designed for regeneration, thereby regulating the regeneration potential of peripheral nerves (Arthur-Farraj et al., 2012). c-Jun deficiency results in the development of defective repair SCs, impairs functional recovery, and results in neuronal death. c-Jun overexpression can effectively lead to an increase in expression and secretion of a variety of neurotrophic factors (NFs), including glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), artemin, leukemia inhibitory factor (LIF), and nerve growth factor (NGF), thereby promoting the migration of SCs. In addition, c-Jun overexpression enhances local proliferation of SCs and neurite development in the presence of axons (Huang L. et al., 2015). Lackington and colleagues (Lackington et al., 2018) created plasmid-carrying nanoparticles and transfected SCs to overexpress nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), and c-Jun. A comparative study revealed that all three types of cells could promote neurite development; however, SCs overexpressing c-Jun had the highest capacity for cell regeneration.
SCs produced an increase in the expression of several axon-regenerating NFs in response to PNI (Hammarberg et al., 1996; Höke et al., 2000; Eggers et al., 2010). By targeting NFs expression with advanced genetic engineering methods, SC repair efficiency after injury could be substantially increased. Shakhbazau et al. (2012) transfected SCs with either NGF or GDNF and discovered that SCs overexpressing NGF enhanced axonal regeneration to a much greater degree. However, NGF and GDNF overexpression may have various consequences on sensory and motor regenerating neurons. The increase in NGF and GDNF levels did not affect the number of regenerated sensory neurons at 1 cm from the distal end of the injury at 4 weeks after injury. GDNF overexpression was found to promote long-distance axon development and nerve re-innervation of the target muscle in motor neurons, whereas NGF had little impact on these parameters (Tannemaat et al., 2008). However, axon regeneration is sluggish and requires considerable time to complete. Marquardt et al. (2015) developed a system for controlling GDNF release over time and in space via lentiviral transfection of SCs and combining this system with biomaterials. They discovered that GDNF overexpression at 4–8 weeks promoted axon regeneration and muscle mass recovery, but that too short a delivery time (4 weeks) prevented axon extension and that too long a delivery time (> 8 weeks) could also result in axon regeneration failure.
Fibroblast growth factor 2 (FGF-2) is a neurotrophic factor produced by fibroblasts and SCs. This factor plays a critical role in SC proliferation and migration (Chen et al., 2010). FGF-2 subtype differences may have a profound impact on nerve regeneration. By comparing SCs overexpressing 18 kDa FGF-2 to SCs overexpressing 21/23 kDa FGF-2, Haastert et al. (2006) discovered that 18 kDa FGF-2 hinders the myelination of regenerated axons. However, 21/23 kDa FGF-2 enhanced the early recovery of sensory function and the myelination of long-distance axons in regenerated axons. Allodi et al. (2014) transplanted SCs overexpressing 18 kDa FGF-2 into a rat model of sciatic nerve injury and observed that nerve reinnervation of the hind limb muscles was accelerated and more obvious following transplantation. The number of motor and sensory neurons reaching the distal nerve increases at the end of follow-up, a process that is conducive to injury repair.
Studies have shown that neurotrophin-3 (NT-3) is a neurotrophic factor that helps reduce inflammatory responses and promotes cell survival and migration in poor microenvironments after injury (Randolph et al., 2007; Li et al., 2016, 2021a). Woolley et al. (2008) discovered that loss of NT-3 led to enhancement of caspase-3 protein production in SCs, suggesting that NT-3 is required for proper SC survival and differentiation. Additionally, NT-3 released by SCs at the axon–glial interface may act on axons and promote release of neuroregulatory proteins, which subsequently signal SCs to accelerate myelination. Zong et al. (2013) implanted SCs into a rat model of sciatic nerve injury. They found that SCs overexpressing NT-3 caused a decrease in motor neuron apoptosis in the injured sciatic nerve, accelerated nerve and axon regeneration, and enhanced the function of the injured nerve.
Repair of PNI with a long-distance nerve deficit present a significant challenge. The gold standard for PNI healing has always been autologous nerve tissue transplantation. Autologous transplantation at the donor site may result in pathological alterations, such as sensory abnormalities, neuroma, infection, and other potential complications. Additionally, autografts are only available in a limited quantity (Lee and Wolfe, 2000). As a result, it is critical to create efficient nerve healing methods to circumvent autologous transplantation restrictions. Similarly, various biomaterials have been used to treat PNI. These biomaterials can provide favorable conditions for peripheral nerve regeneration by simulating the microenvironment through matrix components, secreted factors, cell adhesion, substrate stiffness, and topographical cues and gradients (Figure 4). These biomaterials may be roughly classified into natural and synthetic biomaterials, each with clear benefits. Synthetic biomaterials can adjust material properties to meet various repair needs, such as enhancement of cell adhesion and regulation of mechanical characteristics (Li et al., 2015; Mobasseri et al., 2015; Shahriari et al., 2017). In contrast to synthetic biomaterials, natural materials (such as autografts, allografts, and acellular grafts) generate non-toxic degradation products, possess intrinsic cell-binding domains, and can efficiently activate natural tissue remodeling and repair pathways (Bonnans et al., 2014; Nicolas et al., 2020).
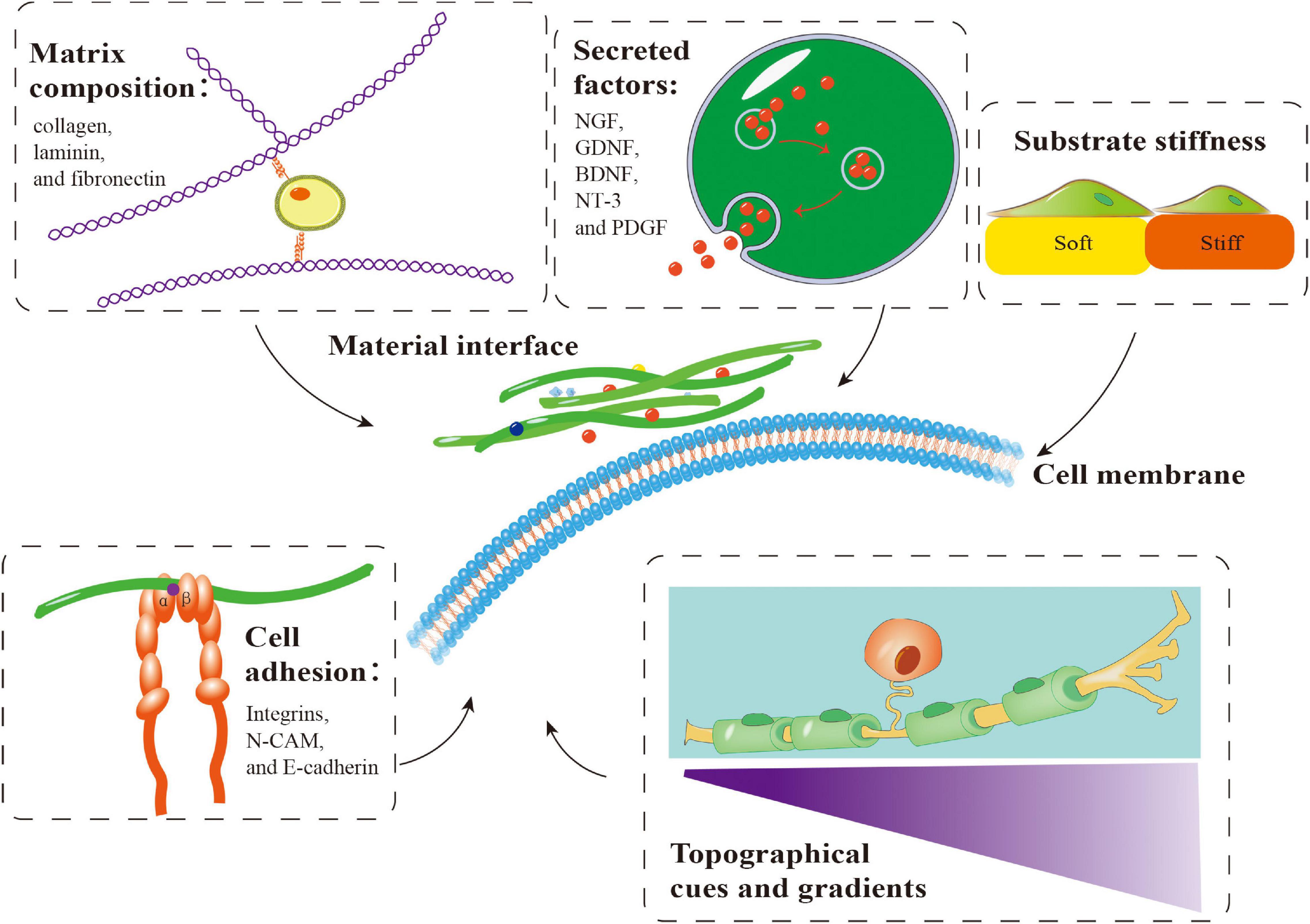
Figure 4. Important factors involved in the application of biomaterials in combination with Schwann cells (SCs) in peripheral nerve regeneration. Matrix components, secreted factors, cell adhesion, substrate stiffness and topographical cues and gradients influence the behavior of SCs on biomaterials, which is crucial for peripheral nerve regeneration. NGF, nerve growth factor; GDNF, glial-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; NT-3, neurotrophin-3; PDGF, platelet-derived growth factor.
He et al. (2015) created a human acellular nerve graft (ANG) to replace autologous nerves and showed that ANG was safe and efficient for nerve deficits ranging in size from 1 to 5 cm. By obliterating antigenic biological components, ANGs, in comparison to allografts, can lead to a decrease in the immune responses. The impact of ANGs on long and massive nerve deficits, in contrast, is far from acceptable. As a result, Qiu et al. (2015) modified ANGs using the cartilage oligomeric matrix protein (COMP)-angiopoietin-1 (Ang1). COMP-Ang1 was found to enhance early neovascularization followed by rapid nerve regeneration, thus significantly increasing the effectiveness of ANGs in healing peripheral nerve lesions in clinical studies. Zhu et al. (2015) used Ginkgo biloba extract 761 (EGb 761) after ANG transplantation in rats with sciatic nerve injury. They discovered that ANG repair treated with EGb 761 showed the same effects on peripheral nerve regeneration and vascularization as autograft repair and was superior to ANG repair alone. However, ANGs are always deficient in the SCs required for nerve regeneration, limiting nerve regeneration in pure ANGs and rendering them inferior to autografts or allografts (Bunge, 1994; Whitlock et al., 2009). By transplanting SCs into ANGs, Jesuraj et al. (2014) discovered that SCs could enhance growth factor expression in ANGs and exhibit equivalent muscular strength and nerve fiber regeneration to allografts, thus boosting peripheral nerve regeneration and functional recovery.
In conclusion, ANG may facilitate more complete removal of cells, myelin sheaths, and other components associated with immune rejection from the grafts, thus lowering the risk of immunological rejection and increasing histocompatibility. In addition, ANGs preserve the nerve’s original structure and efficiently direct the regenerated axons to their target organs. Sun et al. (2020) showed that decellularization selectively removes axon-suppressing molecules, such as myelin associated glycoproteins and chondroitin sulfate proteoglycans present in normal nerves and retains axon-promoted extracellular matrix (ECM) proteins, including collagen IV and laminin. The preserved extracellular matrix components create a milieu similar to peripheral nerve tissue for SC adhesion and proliferation (Carriel et al., 2014). In their latest study, Wang et al. (2022) found that components and spatial organization of the extracellular matrix secreted by bone marrow MSCs closely resemble the acellular nerve and have stronger expression of factors related to nerve regeneration and lower immune responses; therefore, MSCs may be more likely to provide potential alternatives for clinical repair of peripheral nerve defects.
The synthetic nerve-guided catheter (NGC) is another method of replacing autologous nerves. In contrast to allogeneic acellular nerve, NGCs have no donor restriction and are simpler to manufacture on a large scale. The tubular structure of the NGCs bridges the nerve stump and prevents the regenerated nerve from being influenced by the surrounding tissue while concurrently directing the regenerated axon to the distal stump properly. By creating a polyglycolic acid (PGA) multi-channel guidance scaffold, Hu et al. (2008) recruited and directed endogenous SCs to move along the PGA wire and form a cell column comparable with Büngner bands involved in PNI repair. However, nerve regeneration involves cell interactions, the extracellular matrix, and growth factors. Consequently, nerve injury repair methods have evolved from simple nerve catheters to sophisticated tissue engineering approaches that closely resemble the complex milieu seen in autografts. Chitosan has excellent biocompatibility, permeability, plasticity, and biodegradability, making it a suitable candidate material for the construction of artificial nerve grafts (Nawrotek et al., 2016). Chitosan-based nerve grafts mediate a novel molecular mechanism of nerve regeneration in that chitosan degrades into chitosan oligosaccharides following transplantation. This process was found to lead to a decrease in the expression of miR-327 in SCs and increase in CCL2 expression, thereby inducing macrophage infiltration, rebuilding of the microenvironment at the site of injury, and promotion of nerve regeneration (Zhao et al., 2017). Graphene oxide (GO) is a nanomaterial with extraordinary physical and chemical characteristics. Polycaprolactone (PCL) is a biocompatible polymer scaffold with an appropriate hardness. The nano-scaffolds prepared using GO and PCL are beneficial for the proliferation, survival, adhesion, and maintenance of neural properties in SCs. They can effectively promote functional and morphological recovery of peripheral nerves, which is promising for tissue engineering (Qian et al., 2018). Zhang et al. (2020) demonstrated that micropatterning on the inner wall of NGCs constructed with GO was conducive for promoting SCs to secrete neurotrophic factors, such as NGF and BDNF, guiding the direction of axons, inducing macrophage M2 differentiation, and promoting nerve regeneration and the recovery of normal functions. Fadia et al. (2020) created PCL nerve conduits implanted with GDNF microspheres and discovered that they could cause a substantial increase in SC proliferation and nerve conduction when compared with autografts but had no meaningful effect on functional function recovery.
Manipulating the microstructure and mechanical characteristics of biological scaffold materials can alter the differentiation efficiency of Schwann cell-like cells. Electrospinning fibers can be arranged, stacked, or folded to form organized arrays or layered structure and effectively direct axon regeneration (Xie et al., 2010; Huang C. et al., 2015; Xue et al., 2017a). Furthermore, they can efficiently stimulate the development of mesenchymal stem cells (MSCs) into SCs and control the morphology and arrangement of source cells (Xue et al., 2017b). Hu et al. (2020) showed that the combination of amine-functionalized multi-walled carbon nanotubes (MWCNT) with PCL and gelatin and preparation of directional or random conductive nanofibers by electrostatic spinning could lead to a significant improvement in the differentiation efficiency of BMSCs into SCs. This process could also promote axon regrowth in the peripheral nervous system. This research indicates a good solution to address the difficulties associated with the source of SCs and required improvements in the differentiation and functionalization of stem cells to SCs. Thermally-induced phase separation (TIPS) technology has the potential to influence the efficiency of MSCs to transdifferentiate into SC-like phenotypes by causing alterations in the microstructure and mechanical properties of the nanofibers and macroporous and ladder-like structures found in gelatin-based three-dimensional (3D) tubes (Ma and Zhang, 1999; Zeng et al., 2014). Uz et al. (2017) demonstrated that the conduit structure with macroporous and ladder 3D structures could enhance the attachment, proliferation, and diffusion of MSCs to create an interconnected cell network with many living cells and achieve an optimal microenvironment for the transformation of MSCs into SC-like phenotypes. Wu et al. (2020) synthesized composite nanofiber yarns (NYs) from poly(p-dioxanone) (PPDO) and carbon nanotubes (CNT). CNTs are added to NYs to improve their mechanical characteristics and electrical conductivity. Further electrical stimulation (ES) can lead to a substantial increase in expression levels of S100B, GFAP, NGFR, MBP, and MPZ, which are associated with myelination in SCs and an increase in NGF production, EGF, and hepatocyte growth factor (HGF). This process can also promote differentiation of human adipose-derived mesenchymal stem cells (hADMSCs) into SC-like cells. By constructing a biological scaffold to promote stem cell differentiation into SC-like cells and establishing a milieu favorable to SC-like cell differentiation, the risk to the host from viral transfection or drug induction may be significantly minimized. Li et al. (2021b) demonstrated that using electrostatic spinning, micro-nano processing, and biomaterial surface biologization techniques, the constructed neural regeneration micro-environment scaffold with anisotropic micro-nano composite topology could effectively induce directed SC growth. The Wnt/-catenin, ERK2/MAP, and TGF-pathways upregulate the expression of myelination-related genes and proteins, offering an essential approach to the development of a new generation of functional artificial neural implants.
The idea of neural tissue engineering focuses on repair of PNI that is different than a single bridging mechanism and leans toward a more biomimetic and bioactive environment that promotes nerve regeneration and axon development. NGCs manufactured using SCs have been demonstrated to stimulate peripheral nerve regeneration. However, as SCs are inaccessible, and cells placed on NGCs are unable to fully cover the catheter surface, long-distance nerve regeneration fails, and motor function recovery becomes compromised. NGCs implanted into bone marrow stromal cells demonstrate more uniform cell dispersion with the assistance of NGF and rotary cell culture systems, which is favorable for establishing a stable 3D bionic environment, enhancing proliferation and differentiation of bone marrow stromal cells into SC-like cells in a substantial manner, and stimulating long-distance peripheral nerve regeneration (Zhou et al., 2020). Georgiou et al. (2015) converted adipose-derived stem cells into an SC cell-like phenotype in vitro and then implanted the differentiated cells into type 1 collagen gel, which had been stabilized to produce artificial neural tissue. When the designed nerve tissue generated from ASCs was transplanted into a rat model of sciatic nerve injury, it was shown that this engineered nerve tissue possessed a high capacity for nerve regeneration in vivo. Adipose stem cells (ASCs) are a potential cellular vectors because they have the capability of creating cell sheets consisting of cells, intercellular junctions, and extracellular matrix (Yeh et al., 2014). Hsu et al. (2019) prepared functionally enhanced engineered nerves by using ASCs overexpressing BDNF, GDNF, and NGF. This cell plates were shown to be capable of stimulating SC migration, neuron proliferation, and axon development in vitro and enhancing the functional recovery, nerves, axons, and myelin sheath regeneration after sciatic nerve injury.
The combination of engineered peripheral nerve tissue with SCs and biomaterials closely mimics the cell composition, extracellular matrix microenvironment, and three-dimensional space structure of the peripheral nerve, resulting in a graft that is closely resembles autologous nerves and has a broad market application potential. Concurrently, as neurological organ technology advances, peripheral nerve tissue engineered from the combination of SCs and biological materials, will also undergo continuous iteration. Not only will this process improve the effect of PNI replacement, but also will help develop a type of peripheral nerve tissue in vitro for neurogenesis, repair, and drug screening prior to basic and clinical research. Thibodeau et al. (2022) prepared a new type of tissue-engineered neural catheter. The neural conduit consists of a deactivated hollow tube on the outside and a living fibroblast sheet, which is infused with endothelial cells, rolled into concentric layers on the inside to facilitate the formation of a network containing capillary-like structures for rapid binding to the host neuromicrovascular system after transplantation. When this system in combination with SCs was transplanted into rats, this combination was found to have a higher rates of nerve regeneration than the material-only group.
SCs play a critical role in the PNI healing process, but their usage is limited by the difficulty of cell acquisition and its amplification. In addition, isolating, cultivating, and purifying fiber cells, which often multiply quickly in cultured cells, is very challenging (Rutkowski et al., 1995; Andersen et al., 2016; Stratton et al., 2017). To address these obstacles, scientists are investigating safer and more reliable methods for reprogramming various cell sources into induced Schwann-like cells (iSCs) as shown in Table 2.
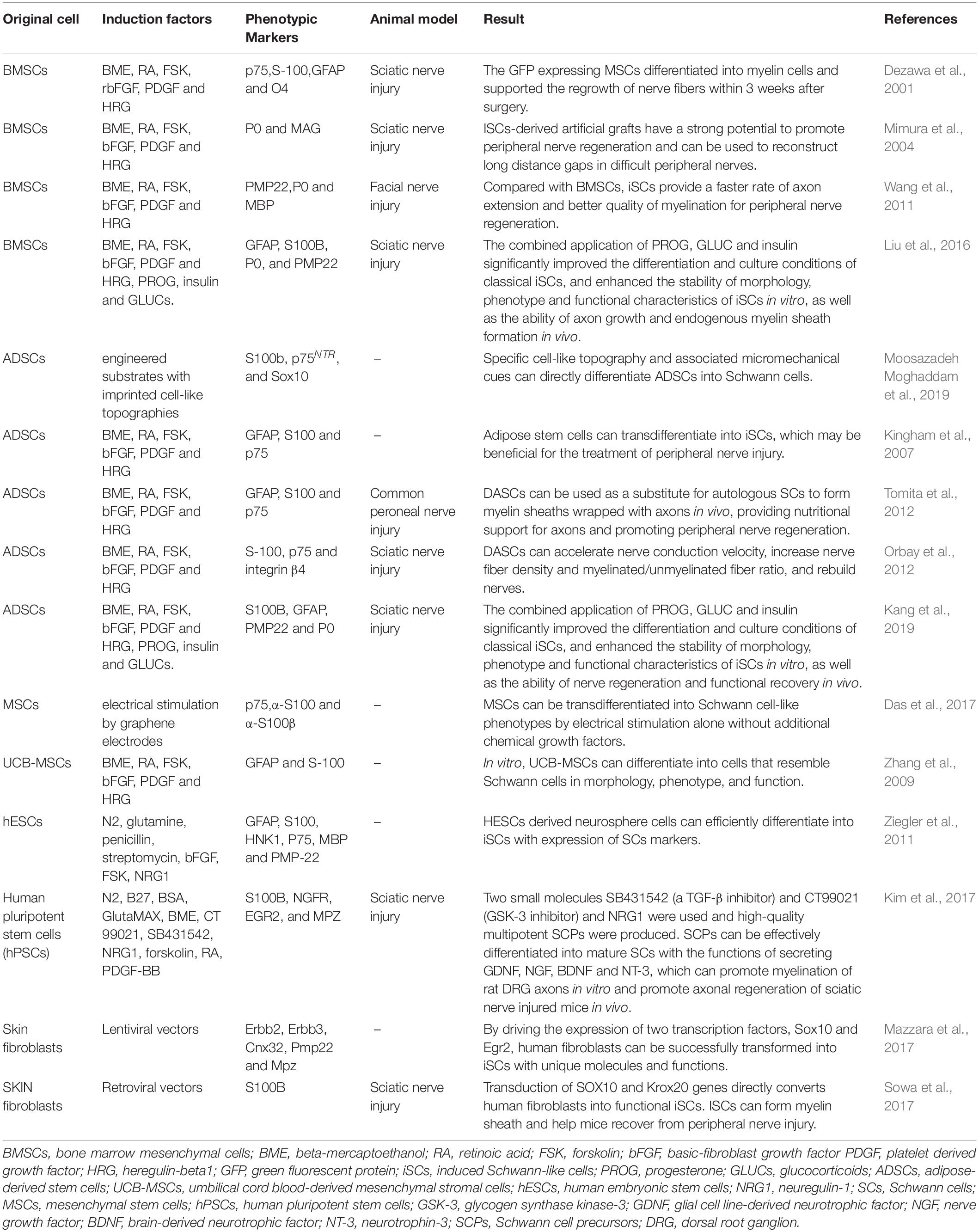
Table 2. The methods used in recent years to reprogram cells from different sources into iSCs are summarized.
Numerous studies have shown that embryonic stem cells (ESCs) and adult stem cells, such as those found in bone marrow, fat, and the umbilical cord, may develop into iSCs (McKenzie et al., 2006; Kingham et al., 2007; Peng et al., 2011; Ziegler et al., 2011; Cai et al., 2017). Although embryonic stem cells proliferate rapidly, their use is controversial and raises ethical questions (Biswas and Hutchins, 2007; Zakrzewski et al., 2019). When ESCs are transplanted into the body, the chance of developing teratomas, localized overgrowth, or cancer increases (Martin et al., 2020). Adult stem cells grow slowly and are often obtained invasively, which limits their therapeutic use (Huang Z. et al., 2020). The development of induced pluripotent stem cells (iPSCs) addresses several constraints associated with stem cells as potential sources of SCs. iPSCs may be isolated from fibroblasts in the skin, peripheral blood mononuclear cells, or even umbilical cord blood cells (Huang et al., 2019). iPSCs have a high capacity for self-renewal and a high rate of proliferation, and they can develop into any type of cell in the endoderm, mesoderm, or ectoderm (Takahashi et al., 2007; Martin et al., 2020). Khuong et al. (2014) utilized lentivirus to promote iSC transformation by driving the expression of two transcription factors, Sox10 and Egr2, in skin fibroblasts. Immunofluorescence research revealed that iSCs, as with primary SCs, exhibited high expression of SC markers, glial fibrillary acidic protein and myelin basic proteins (GFAP and MBP, respectively). In vitro, these iSCs form tight myelin sheaths with regular node patterns around axons. Fibroblast growth factors (FGFs) control brain development and cell fate determination during embryonic development by directing cells to differentiate into neurons or glial cells (Mertens et al., 2016). Huang C.W. et al. (2020) seeded adipose-derived stem cells (ADSCs) onto chitosan-coated culture plates, causing the ADSCs to differentiate into a mixed population of neural lineage-like cells (NLCs) after which they then induced NLCs to differentiate into S100β- and GFAP-positive iSCs using FGF9. Wong et al. (2020) compared the effects of different induction times on the proliferation and secretion capacity of iSCs induced by ADSCs and discovered that while the proliferation capacity negatively correlated with induction time, the expression of SC marker proteins S100, MBP, p75, and GFAP positively correlated with induction time. MBP expression resemble normal SCs on day 19 after induction and substantially enhanced axon development compared with early induction. As a result, iSCs appear to be safe to use for 19 days following induction. Dezawa et al. (2001) used beta-mercaptoethanol, retinoic acid, forskolin, basic-FGF (bFGF), platform-derived growth factor (PDGF), and heregulin to induce BMSCs to differentiate into iSCs. The iSCs obtained presented an SC morphology and expressed p75, S100, GFAP, and O4. When retroviruses expressing green fluorescent protein (GFP) were genetically modified and implanted into a sciatic nerve injury model, extensive nerve fiber regeneration and myelination were found. Matsuse et al. (2010) showed that this technique was equally applicable to MSCs from the human umbilical cord. Liu et al. (2012) used conditioned medium to differentiate human hESCs and iPSCs into NCCs and subsequently into SCs from NCCs, establishing the first report on the myelination of human hESCs or iPSC-derived SCs. Subsequently, Kim et al. (2017) converted human iPSCs to self-renewing SCPs by utilizing inhibitors (SB431542 and CT99021) of the TGF-β and GSK-3 signaling pathways, respectively, in addition to using NRG1. Within 1 week, SCPs may differentiate into mature SCs capable of myelination and secreting neurotrophic factors, such as GDNF, NGF, BDNF, and NT-3. Pan and colleagues (Pan et al., 2017) discovered that peripheral blood-derived mesenchymal stem cells (PBMSCs) may generate iSCs that express SC-specific markers (S100, p75NTR, and CNPase) and functional factors (NGF, NT-3, c-fos, and Krox20). This finding solves the problem of the difficult source of tissue engineering seed cells (Schwann cells) after PNI. SCs encapsulating axons in injured sciatic nerves may function well after transplantation. In conclusion, focused modulation of critical molecules in the developmental gene regulatory network lineage of SCs may more effectively stimulate the creation of these various sources of iSCs, indicating a potential therapeutic application.
By describing the process through which SCs repair PNI, our ability to utilize viruses, non-viral vectors, or gene-editing technologies to alter the SC gene may be improved, enabling us to perform additional tasks favorable to peripheral nerve repair. As the safety and control of these methods are enhanced, genetically engineered SCs will have a broader potential for clinical application in peripheral nerve regeneration. The critical element of PNI is the rapid and precise targeting of axons, a process that may be accomplished by developing specialized biological scaffolding for neural bridging. Consequently, cell and biological scaffolding materials may be combined. At this stage, it is critical to develop a biological scaffolding material capable of more accurately simulating the natural milieu of SCs in vivo. The source of human SCs severely limits their therapeutic use; however, iSCs offer an excellent solution to this problem. However, an important issue to address throughout the iSC creation process is how to help the iSCs exhibit as many SCs characteristics as feasible while exhibiting as few properties of other cells as possible.
Gene modification can purposefully change the expression of certain genes in SCs thus making it easier to achieve therapeutic goals. However, the corresponding genetic modification also raises more unknown safety issues. After transplantation, SC or iSC survival is low due to the poor microenvironment. Through combinations of biological materials, the internal microenvironment of peripheral nerves was simulated to improve cell survival rate. However, some problems, such as uneven distribution, poor penetration and difficulty in stable release of transplanted cells in biological scaffold materials, exist. Therefore, it is urgent to develop biological scaffold materials that can overcome these shortcomings and achieve industrial mass production, which is of great significance for clinical applications. SC products obtained by tissue and/or genetic engineering and stem cell reprogramming have their own advantages and disadvantages, which can meet the needs of PNI treatment to a certain extent. With the advancement in tissue and genetic engineering, stem cell reprogramming, and other technologies, the purpose of SCs has been extended from simple transplantation to multi-functional complex engineered organogenesis therapeutics thus providing a new perspective on the treatment of PNI and organoid construction.
QS, MN, JH, GD, QO, DZ, YD, HH, NL, ZL, PZ, and GL performed the bibliographic research and drafted the manuscript. QS and YD created the table. GD, DZ, and GL prepared the figures. All authors read, approved the final manuscript, analyzed, discussed the literature, commenting on, and approving the manuscript.
This work was supported by the National Natural Science Foundation of China (82001301 and 81974019) to GL and PZ, the National Key Research and Development Program of China (2018YFA0108700 and 2017YFA0105602), NSFC Projects of International Cooperation and Exchanges (81720108004) to PZ, the Key Program of Guangzhou Science Research Plan (201904020047) to PZ, the Co-innovation Foundation of Guangzhou City (201704020221) to GL, and the Special Project of Dengfeng Program of Guangdong Provincial People’s Hospital (KY0120220133, DFJHBF202111, KJ012020630, DFJH201812, KJ012019119, and KJ012019423) to GL and PZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PNI, Peripheral nerve injury; PNS, peripheral nervous system; SCs, Schwann cells; iSCs, induced Schwann-like cells; NCCs, neural crest cells; SCPs, Schwann cell precursors; BL, basal layer; D4, day 4; D7, day 7; lncRNAs, long non-coding RNAs; TGF-β, transforming growth factor-β; NRG1, Neuregulin 1; α BC, AlphaB-crystallin; Notch, Neurogenic locus Notch homolog protein; DRG, dorsal root ganglions; HIF-1α, hypoxia inducible factor − 1 α; VEGF, vascular endothelial growth factor; WD, Wallerian degeneration; NGF, nerve growth factor; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; PIGF, placental growth factor; VEGFR1, vascular endothelial growth factor receptor 1; CNTF, Ciliary neurotrophic factor; STAT3, signal transducer and activator of transcription 3; IL-6, interleukin 6; CRISPR, clustered regularly spaced short palindromic repeats; HDAC, Histone deacetylase; Zeb2, Zinc-finger e-box -binding Homeobox 2; HDAC1/2-NuRD, HDAC1 and 2 and nucleosome remodeling and histone deacetylation; NFs, neurotrophic factors; GDNF, glial cell line-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; LIF, leukemia inhibitory factor; FGF-2, Fibroblast growth factor-2; NT-3, Neurotrophin-3; ANG, acellular nerve graft; COMP, cartilage oligomeric matrix protein; Ang1, angiopoietin1; EGb 761, extract 761; NGC, nerve-guided catheter; PGA, polyglycolic acid; GO, Graphene oxide; MWCNT, multi-walled carbon nanotubes; MSCs, mesenchymal stem cells; TIPS, thermally-induced phase separation; NYs, nanofiber yarns; PPDO, poly(p-dioxanone); CNT, carbon nanotubes; ES, electrical stimulation; EGF, epidermal growth factor; HGF, hepatocyte growth factor; ADMSCs, adipose-derived mesenchymal stem cells; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; FGFs, Fibroblast growth factors; NLCs, neural lineage-like cells; bFGF, basic-FGF; PDGF, platform-derived growth factor; GFP, green fluorescent protein; PBMSCs, peripheral blood-derived mesenchymal stem cells.
Akassoglou, K., Yu, W. M., Akpinar, P., and Strickland, S. (2002). Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33, 861–875. doi: 10.1016/s0896-6273(02)00617-7
Allodi, I., Mecollari, V., González-Pérez, F., Eggers, R., Hoyng, S., Verhaagen, J., et al. (2014). Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regeneration following sciatic nerve injury. Glia 62, 1736–1746. doi: 10.1002/glia.22712
Ammoun, S., Flaiz, C., Ristic, N., Schuldt, J., and Hanemann, C. O. (2008). Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 68, 5236–5245. doi: 10.1158/0008-5472.Can-07-5849
Andersen, N. D., Srinivas, S., Piñero, G., and Monje, P. V. (2016). A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 6:31781. doi: 10.1038/srep31781
Arthur-Farraj, P. J., Latouche, M., Wilton, D. K., Quintes, S., Chabrol, E., Banerjee, A., et al. (2012). c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75, 633–647. doi: 10.1016/j.neuron.2012.06.021
Arthur-Farraj, P. J., Morgan, C. C., Adamowicz, M., Gomez-Sanchez, J. A., Fazal, S. V., Beucher, A., et al. (2017). Changes in the coding and non-coding transcriptome and DNA methylome that define the schwann cell repair phenotype after nerve injury. Cell Rep. 20, 2719–2734. doi: 10.1016/j.celrep.2017.08.064
Biswas, A., and Hutchins, R. (2007). Embryonic stem cells. Stem Cells Dev. 16, 213–222. doi: 10.1089/scd.2006.0081
Blockus, H., and Chédotal, A. (2016). Slit-robo signaling. Development 143, 3037–3044. doi: 10.1242/dev.132829
Bonnans, C., Chou, J., and Werb, Z. (2014). Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801. doi: 10.1038/nrm3904
Brügger, V., Duman, M., Bochud, M., Münger, E., Heller, M., Ruff, S., et al. (2017). Delaying histone deacetylase response to injury accelerates conversion into repair Schwann cells and nerve regeneration. Nat. Commun. 8:14272. doi: 10.1038/ncomms14272
Bunge, R. P. (1994). The role of the Schwann cell in trophic support and regeneration. J. Neurol. 242, S19–S21. doi: 10.1007/bf00939235
Cai, S., Tsui, Y. P., Tam, K. W., Shea, G. K., Chang, R. S., Ao, Q., et al. (2017). Directed differentiation of human bone marrow stromal cells to fate-committed schwann cells. Stem Cell Rep. 9, 1097–1108. doi: 10.1016/j.stemcr.2017.08.004
Carriel, V., Alaminos, M., Garzón, I., Campos, A., and Cornelissen, M. (2014). Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 14, 301–318. doi: 10.1586/14737175.2014.887444
Cattin, A. L., Burden, J. J., Van Emmenis, L., Mackenzie, F. E., Hoving, J. J., Garcia Calavia, N., et al. (2015). Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162, 1127–1139. doi: 10.1016/j.cell.2015.07.021
Chen, B., Banton, M. C., Singh, L., Parkinson, D. B., and Dun, X. P. (2021). Single cell transcriptome data analysis defines the heterogeneity of peripheral nerve cells in homeostasis and regeneration. Front. Cell. Neurosci. 15:624826. doi: 10.3389/fncel.2021.624826
Chen, H. T., Tsai, Y. L., Chen, Y. S., Jong, G. P., Chen, W. K., Wang, H. L., et al. (2010). Dangshen (Codonopsis pilosula) activates IGF-I and FGF-2 pathways to induce proliferation and migration effects in RSC96 Schwann cells. Am. J. Chin. Med. 38, 359–372. doi: 10.1142/s0192415x10007907
Chen, Y., Wang, H., Yoon, S. O., Xu, X., Hottiger, M. O., Svaren, J., et al. (2011). HDAC-mediated deacetylation of NF-κB is critical for Schwann cell myelination. Nat. Neurosci. 14, 437–441. doi: 10.1038/nn.2780
Chen, Z. L., Yu, W. M., and Strickland, S. (2007). Peripheral regeneration. Annu. Rev. Neurosci. 30, 209–233. doi: 10.1146/annurev.neuro.30.051606.094337
Clements, M. P., Byrne, E., Camarillo Guerrero, L. F., Cattin, A. L., Zakka, L., Ashraf, A., et al. (2017). The wound microenvironment reprograms schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96, 98–114.e7. doi: 10.1016/j.neuron.2017.09.008
Cooper, J., and Giancotti, F. G. (2014). Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 588, 2743–2752. doi: 10.1016/j.febslet.2014.04.001
Das, S. R., Uz, M., Ding, S., Lentner, M. T., Hondred, J. A., Cargill, A. A., et al. (2017). Electrical differentiation of mesenchymal stem cells into schwann-cell-like phenotypes using inkjet-printed graphene circuits. Adv. Healthc. Mater. 6:1601087. doi: 10.1002/adhm.201601087
Deng, Y., Wu, L. M. N., Bai, S., Zhao, C., Wang, H., Wang, J., et al. (2017). A reciprocal regulatory loop between TAZ/YAP and G-protein Gαs regulates Schwann cell proliferation and myelination. Nat. Commun. 8:15161. doi: 10.1038/ncomms15161
Dezawa, M., Takahashi, I., Esaki, M., Takano, M., and Sawada, H. (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 14, 1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x
Dun, X. P., Carr, L., Woodley, P. K., Barry, R. W., Drake, L. K., Mindos, T., et al. (2019). Macrophage-derived slit3 controls cell migration and axon pathfinding in the peripheral nerve bridge. Cell Rep. 26, 1458–1472.e4. doi: 10.1016/j.celrep.2018.12.081
Eggers, R., Tannemaat, M. R., Ehlert, E. M., and Verhaagen, J. (2010). A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp. Neurol. 223, 207–220. doi: 10.1016/j.expneurol.2009.07.021
Fadia, N. B., Bliley, J. M., DiBernardo, G. A., Crammond, D. J., Schilling, B. K., Sivak, W. N., et al. (2020). Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci. Transl. Med. 12:eaav7753. doi: 10.1126/scitranslmed.aav7753
Faroni, A., Mobasseri, S. A., Kingham, P. J., and Reid, A. J. (2015). Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 8, 160–167. doi: 10.1016/j.addr.2014.11.010
Garratt, A. N., Britsch, S., and Birchmeier, C. (2000). Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays 22, 987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5
Georgiou, M., Golding, J. P., Loughlin, A. J., Kingham, P. J., and Phillips, J. B. (2015). Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 37, 242–251. doi: 10.1016/j.biomaterials.2014.10.009
Ghislain, J., and Charnay, P. (2006). Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 7, 52–58. doi: 10.1038/sj.embor.7400573
Gomez-Sanchez, J. A., Carty, L., Iruarrizaga-Lejarreta, M., Palomo-Irigoyen, M., Varela-Rey, M., Griffith, M., et al. (2015). Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell. Biol. 210, 153–168. doi: 10.1083/jcb.201503019
Grove, M., Kim, H., Santerre, M., Krupka, A. J., Han, S. B., Zhai, J., et al. (2017). YAP/TAZ initiate and maintain Schwann cell myelination. Elife 6:e20982. doi: 10.7554/eLife.20982
Grove, M., Lee, H., Zhao, H., and Son, Y. J. (2020). Axon-dependent expression of YAP/TAZ mediates Schwann cell remyelination but not proliferation after nerve injury. Elife 9:e50138. doi: 10.7554/eLife.50138
Haastert, K., Lipokatic, E., Fischer, M., Timmer, M., and Grothe, C. (2006). Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms. Neurobiol. Dis. 21, 138–153. doi: 10.1016/j.nbd.2005.06.020
Hall, S. (2005). The response to injury in the peripheral nervous system. J. Bone Joint Surg. Br. 87, 1309–1319. doi: 10.1302/0301-620x.87b10.16700
Hammarberg, H., Piehl, F., Cullheim, S., Fjell, J., Hökfelt, T., and Fried, K. (1996). GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport 7, 857–860. doi: 10.1097/00001756-199603220-00004
Hansen, C. G., Moroishi, T., and Guan, K. L. (2015). YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513. doi: 10.1016/j.tcb.2015.05.002
Harrisingh, M. C., Perez-Nadales, E., Parkinson, D. B., Malcolm, D. S., Mudge, A. W., and Lloyd, A. C. (2004). The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 23, 3061–3071. doi: 10.1038/sj.emboj.7600309
He, B., Zhu, Q., Chai, Y., Ding, X., Tang, J., Gu, L., et al. (2015). Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J. Tissue Eng. Regen. Med. 9, 286–295. doi: 10.1002/term.1707
He, X., Zhang, L., Queme, L. F., Liu, X., Lu, A., Waclaw, R. R., et al. (2018). A histone deacetylase 3-dependent pathway delimits peripheral myelin growth and functional regeneration. Nat. Med. 24, 338–351. doi: 10.1038/nm.4483
Hendry, J. M., Alvarez-Veronesi, M. C., Placheta, E., Zhang, J. J., Gordon, T., and Borschel, G. H. (2016). ErbB2 blockade with Herceptin (trastuzumab) enhances peripheral nerve regeneration after repair of acute or chronic peripheral nerve injury. Ann. Neurol. 80, 112–126. doi: 10.1002/ana.24688
Höke, A., Cheng, C., and Zochodne, D. W. (2000). Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport 11, 1651–1654. doi: 10.1097/00001756-200006050-00011
Hsu, M. N., Liao, H. T., Truong, V. A., Huang, K. L., Yu, F. J., Chen, H. H., et al. (2019). CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics 9, 6099–6111. doi: 10.7150/thno.36790
Hu, W., Gu, J., Deng, A., and Gu, X. (2008). Polyglycolic acid filaments guide Schwann cell migration in vitro and in vivo. Biotechnol Lett. 30, 1937–1942. doi: 10.1007/s10529-008-9795-1
Hu, X., Wang, X., Xu, Y., Li, L., Liu, J., He, Y., et al. (2020). Electric conductivity on aligned nanofibers facilitates the transdifferentiation of mesenchymal stem cells into schwann cells and regeneration of injured peripheral nerve. Adv. Healthc. Mater. 9:e1901570. doi: 10.1002/adhm.201901570
Huang, C., Ouyang, Y., Niu, H., He, N., Ke, Q., Jin, X., et al. (2015). Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl. Mater. Interfaces 7, 7189–7196. doi: 10.1021/am509227t
Huang, L., Quan, X., Liu, Z., Ma, T., Wu, Y., Ge, J., et al. (2015). c-Jun gene-modified Schwann cells: upregulating multiple neurotrophic factors and promoting neurite outgrowth. Tissue Eng. Part A 21, 1409–1421. doi: 10.1089/ten.TEA.2014.0416
Huang, C. W., Lu, S. Y., Huang, T. C., Huang, B. M., Sun, H. S., Yang, S. H., et al. (2020). FGF9 induces functional differentiation to Schwann cells from human adipose derived stem cells. Theranostics 10, 2817–2831. doi: 10.7150/thno.38553
Huang, Z., Powell, R., Phillips, J. B., and Haastert-Talini, K. (2020). Perspective on Schwann cells derived from induced pluripotent stem cells in peripheral nerve tissue engineering. Cells 9:2497. doi: 10.3390/cells9112497
Huang, C. Y., Liu, C. L., Ting, C. Y., Chiu, Y. T., Cheng, Y. C., Nicholson, M. W., et al. (2019). Human iPSC banking: barriers and opportunities. J. Biomed. Sci. 26:87. doi: 10.1186/s12929-019-0578-x
Jacob, C., Christen, C. N., Pereira, J. A., Somandin, C., Baggiolini, A., Lötscher, P., et al. (2011). HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat. Neurosci. 14, 429–436. doi: 10.1038/nn.2762
Jessen, K. R., and Mirsky, R. (2016). The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531. doi: 10.1113/jp270874
Jessen, K. R., Mirsky, R., and Lloyd, A. C. (2015). Schwann cells: development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 7:a020487. doi: 10.1101/cshperspect.a020487
Jesuraj, N. J., Santosa, K. B., Macewan, M. R., Moore, A. M., Kasukurthi, R., Ray, W. Z., et al. (2014). Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve 49, 267–276. doi: 10.1002/mus.23885
Kang, Y., Liu, Y., Liu, Z., Ren, S., Xiong, H., Chen, J., et al. (2019). Differentiated human adipose-derived stromal cells exhibit the phenotypic and functional characteristics of mature Schwann cells through a modified approach. Cytotherapy 21, 987–1003. doi: 10.1016/j.jcyt.2019.04.061
Khuong, H. T., Kumar, R., Senjaya, F., Grochmal, J., Ivanovic, A., Shakhbazau, A., et al. (2014). Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 254, 168–179. doi: 10.1016/j.expneurol.2014.01.002
Kim, H. A., Mindos, T., and Parkinson, D. B. (2013). Plastic fantastic: Schwann cells and repair of the peripheral nervous system. Stem Cells Transl. Med. 2, 553–557. doi: 10.5966/sctm.2013-0011
Kim, H. S., Lee, J., Lee, D. Y., Kim, Y. D., Kim, J. Y., Lim, H. J., et al. (2017). Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Rep. 8, 1714–1726. doi: 10.1016/j.stemcr.2017.04.011
Kingham, P. J., Kalbermatten, D. F., Mahay, D., Armstrong, S. J., Wiberg, M., and Terenghi, G. (2007). Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 207, 267–274. doi: 10.1016/j.expneurol.2007.06.029
Lackington, W. A., Raftery, R. M., and O’Brien, F. J. (2018). In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun. Acta Biomater. 75, 115–128. doi: 10.1016/j.actbio.2018.06.014
Lee, S. K., and Wolfe, S. W. (2000). Peripheral nerve injury and repair. J. Am. Acad. Orthop. Surg. 8, 243–252. doi: 10.5435/00124635-200007000-00005
Li, B., Qiu, T., Iyer, K. S., Yan, Q., Yin, Y., Xie, L., et al. (2015). PRGD/PDLLA conduit potentiates rat sciatic nerve regeneration and the underlying molecular mechanism. Biomaterials 55, 44–53. doi: 10.1016/j.biomaterials.2015.03.028
Li, G., Che, M. T., Zhang, K., Qin, L. N., Zhang, Y. T., Chen, R. Q., et al. (2016). Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 83, 233–248. doi: 10.1016/j.biomaterials.2015.11.059
Li, G., Zhang, B., Sun, J. H., Shi, L. Y., Huang, M. Y., Huang, L. J., et al. (2021a). An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact. Mater. 6, 3766–3781. doi: 10.1016/j.bioactmat.2021.03.036
Li, G., Zheng, T., Wu, L., Han, Q., Lei, Y., Xue, L., et al. (2021b). Bionic microenvironment-inspired synergistic effect of anisotropic micro-nanocomposite topology and biology cues on peripheral nerve regeneration. Sci. Adv. 7:eabi5812. doi: 10.1126/sciadv.abi5812
Li, X., Gonias, S. L., and Campana, W. M. (2005). Schwann cells express erythropoietin receptor and represent a major target for Epo in peripheral nerve injury. Glia 51, 254–265. doi: 10.1002/glia.20202
Lim, E. F., Nakanishi, S. T., Hoghooghi, V., Eaton, S. E., Palmer, A. L., Frederick, A., et al. (2017). AlphaB-crystallin regulates remyelination after peripheral nerve injury. Proc. Natl. Acad. Sci. U. S. A. 114, E1707–E1716. doi: 10.1073/pnas.1612136114
Liu, Q., Spusta, S. C., Mi, R., Lassiter, R. N., Stark, M. R., Höke, A., et al. (2012). Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl. Med. 1, 266–278. doi: 10.5966/sctm.2011-0042
Liu, Y., Chen, J., Liu, W., Lu, X., Liu, Z., Zhao, X., et al. (2016). A modified approach to inducing bone marrow stromal cells to differentiate into cells with mature schwann cell phenotypes. Stem Cells Dev. 25, 347–359. doi: 10.1089/scd.2015.0295
Ma, P. X., and Zhang, R. (1999). Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 46, 60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h
Madduri, S., and Gander, B. (2010). Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 15, 93–103. doi: 10.1111/j.1529-8027.2010.00257.x
Marquardt, L. M., Ee, X., Iyer, N., Hunter, D., Mackinnon, S. E., Wood, M. D., et al. (2015). Finely tuned temporal and spatial delivery of GDNF promotes enhanced nerve regeneration in a long nerve defect model. Tissue Eng. Part A 21, 2852–2864. doi: 10.1089/ten.TEA.2015.0311
Martin, R. M., Fowler, J. L., Cromer, M. K., Lesch, B. J., Ponce, E., Uchida, N., et al. (2020). Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat. Commun. 11:2713. doi: 10.1038/s41467-020-16455-7
Matsuse, D., Kitada, M., Kohama, M., Nishikawa, K., Makinoshima, H., Wakao, S., et al. (2010). Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 69, 973–985. doi: 10.1097/NEN.0b013e3181eff6dc
Mazzara, P. G., Massimino, L., Pellegatta, M., Ronchi, G., Ricca, A., Iannielli, A., et al. (2017). Two factor-based reprogramming of rodent and human fibroblasts into Schwann cells. Nat. Commun. 8:14088. doi: 10.1038/ncomms14088
McKenzie, I. A., Biernaskie, J., Toma, J. G., Midha, R., and Miller, F. D. (2006). Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 26, 6651–6660. doi: 10.1523/jneurosci.1007-06.2006
Meena, P., Kakkar, A., Kumar, M., Khatri, N., Nagar, R. K., Singh, A., et al. (2021). Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 383, 617–644. doi: 10.1007/s00441-020-03301-x
Mertens, J., Marchetto, M. C., Bardy, C., and Gage, F. H. (2016). Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424–437. doi: 10.1038/nrn.2016.46
Mimura, T., Dezawa, M., Kanno, H., Sawada, H., and Yamamoto, I. (2004). Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J. Neurosurg. 101, 806–812. doi: 10.3171/jns.2004.101.5.0806
Mindos, T., Dun, X. P., North, K., Doddrell, R. D., Schulz, A., Edwards, P., et al. (2017). Merlin controls the repair capacity of Schwann cells after injury by regulating Hippo/YAP activity. J. Cell Biol. 216, 495–510. doi: 10.1083/jcb.201606052
Mobasseri, A., Faroni, A., Minogue, B. M., Downes, S., Terenghi, G., and Reid, A. J. (2015). Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng. Part A 21, 1152–1162. doi: 10.1089/ten.TEA.2014.0266
Moosazadeh Moghaddam, M., Bonakdar, S., Shokrgozar, M. A., Zaminy, A., Vali, H., and Faghihi, S. (2019). Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artif Cells Nanomed. Biotechnol. 47, 1022–1035. doi: 10.1080/21691401.2019.1586718
Muppirala, A. N., Limbach, L. E., Bradford, E. F., and Petersen, S. C. (2021). Schwann cell development: from neural crest to myelin sheath. Wiley Interdiscip. Rev. Dev. Biol. 10:e398. doi: 10.1002/wdev.398
Murinson, B. B., Archer, D. R., Li, Y., and Griffin, J. W. (2005). Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J. Neurosci. 25, 1179–1187. doi: 10.1523/jneurosci.1372-04.2005
Napoli, I., Noon, L. A., Ribeiro, S., Kerai, A. P., Parrinello, S., Rosenberg, L. H., et al. (2012). A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73, 729–742. doi: 10.1016/j.neuron.2011.11.031
Nawrotek, K., Tylman, M., Rudnicka, K., Gatkowska, J., and Wieczorek, M. (2016). Epineurium-mimicking chitosan conduits for peripheral nervous tissue engineering. Carbohydr. Polym. 152, 119–128. doi: 10.1016/j.carbpol.2016.07.002
Nicolas, J., Magli, S., Rabbachin, L., Sampaolesi, S., Nicotra, F., and Russo, L. (2020). 3D extracellular matrix mimics: fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 21, 1968–1994. doi: 10.1021/acs.biomac.0c00045
Orbay, H., Uysal, A. C., Hyakusoku, H., and Mizuno, H. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthet. Surg. 65, 657–664. doi: 10.1016/j.bjps.2011.11.035
Pan, M., Wang, X., Chen, Y., Cao, S., Wen, J., Wu, G., et al. (2017). Tissue engineering with peripheral blood-derived mesenchymal stem cells promotes the regeneration of injured peripheral nerves. Exp. Neurol. 292, 92–101. doi: 10.1016/j.expneurol.2017.03.005
Parrinello, S., Napoli, I., Ribeiro, S., Wingfield Digby, P., Fedorova, M., Parkinson, D. B., et al. (2010). EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143, 145–155. doi: 10.1016/j.cell.2010.08.039
Peng, J., Wang, Y., Zhang, L., Zhao, B., Zhao, Z., Chen, J., et al. (2011). Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res. Bull. 84, 235–243. doi: 10.1016/j.brainresbull.2010.12.013
Poitelon, Y., Lopez-Anido, C., Catignas, K., Berti, C., Palmisano, M., Williamson, C., et al. (2016). YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 19, 879–887. doi: 10.1038/nn.4316
Qian, Y., Song, J., Zhao, X., Chen, W., Ouyang, Y., Yuan, W., et al. (2018). 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv. Sci. 5:1700499. doi: 10.1002/advs.201700499
Qiu, L., He, B., Hu, J., Zhu, Z., Liu, X., and Zhu, J. (2015). Cartilage oligomeric matrix protein angiopoeitin-1 provides benefits during nerve regeneration in vivo and in vitro. Ann. Biomed. Eng. 43, 2924–2940. doi: 10.1007/s10439-015-1342-3
Randolph, C. L., Bierl, M. A., and Isaacson, L. G. (2007). Regulation of NGF and NT-3 protein expression in peripheral targets by sympathetic input. Brain Res. 1144, 59–69. doi: 10.1016/j.brainres.2007.01.099
Rosenberg, L. H., Cattin, A. L., Fontana, X., Harford-Wright, E., Burden, J. J., White, I. J., et al. (2018). HDAC3 regulates the transition to the homeostatic myelinating schwann cell state. Cell Rep. 25, 2755–2765.e5. doi: 10.1016/j.celrep.2018.11.045
Rutkowski, J. L., Kirk, C. J., Lerner, M. A., and Tennekoon, G. I. (1995). Purification and expansion of human Schwann cells in vitro. Nat. Med. 1, 80–83. doi: 10.1038/nm0195-80
Schlaepfer, W. W., and Bunge, R. P. (1973). Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 59, 456–470. doi: 10.1083/jcb.59.2.456
Shahriari, D., Shibayama, M., Lynam, D. A., Wolf, K. J., Kubota, G., Koffler, J. Y., et al. (2017). Peripheral nerve growth within a hydrogel microchannel scaffold supported by a kink-resistant conduit. J. Biomed. Mater. Res. A 105, 3392–3399. doi: 10.1002/jbm.a.36186
Shakhbazau, A., Kawasoe, J., Hoyng, S. A., Kumar, R., van Minnen, J., Verhaagen, J., et al. (2012). Early regenerative effects of NGF-transduced Schwann cells in peripheral nerve repair. Mol. Cell. Neurosci. 50, 103–112. doi: 10.1016/j.mcn.2012.04.004
Slavin, B. R., Sarhane, K. A., von Guionneau, N., Hanwright, P. J., Qiu, C., Mao, H. Q., et al. (2021). Insulin-like growth factor-1: a promising therapeutic target for peripheral nerve injury. Front. Bioeng. Biotechnol. 9:695850. doi: 10.3389/fbioe.2021.695850
Soldatov, R., Kaucka, M., Kastriti, M. E., Petersen, J., Chontorotzea, T., Englmaier, L., et al. (2019). Spatiotemporal structure of cell fate decisions in murine neural crest. Science 364:eaas9536. doi: 10.1126/science.aas9536
Sondell, M., Lundborg, G., and Kanje, M. (1999). Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 19, 5731–5740. doi: 10.1523/jneurosci.19-14-05731.1999
Sowa, Y., Kishida, T., Tomita, K., Yamamoto, K., Numajiri, T., and Mazda, O. (2017). Direct conversion of human fibroblasts into schwann cells that facilitate regeneration of injured peripheral nerve in vivo. Stem Cells Transl. Med. 6, 1207–1216. doi: 10.1002/sctm.16-0122
Stoll, G., Griffin, J. W., Li, C. Y., and Trapp, B. D. (1989). Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 18, 671–683. doi: 10.1007/bf01187086
Stratton, J. A., Kumar, R., Sinha, S., Shah, P., Stykel, M., Shapira, Y., et al. (2017). Purification and characterization of schwann cells from adult human skin and nerve. eNeuro 4:ENEURO.0307-16.2017. doi: 10.1523/eneuro.0307-16.2017
Sullivan, R., Dailey, T., Duncan, K., Abel, N., and Borlongan, C. V. (2016). Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int. J. Mol. Sci. 17:2101. doi: 10.3390/ijms17122101
Sun, J. H., Li, G., Wu, T. T., Lin, Z. J., Zou, J. L., Huang, L. J., et al. (2020). Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials 258:120289. doi: 10.1016/j.biomaterials.2020.120289
Syed, N., Reddy, K., Yang, D. P., Taveggia, C., Salzer, J. L., Maurel, P., et al. (2010). Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 30, 6122–6131. doi: 10.1523/jneurosci.1681-09.2010
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. doi: 10.1016/j.cell.2007.11.019
Tannemaat, M. R., Eggers, R., Hendriks, W. T., de Ruiter, G. C., van Heerikhuize, J. J., Pool, C. W., et al. (2008). Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur. J. Neurosci. 28, 1467–1479. doi: 10.1111/j.1460-9568.2008.06452.x
Thibodeau, A., Galbraith, T., Fauvel, C. M., Khuong, H. T., and Berthod, F. (2022). Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials 280:121269. doi: 10.1016/j.biomaterials.2021.121269
Tomita, K., Madura, T., Mantovani, C., and Terenghi, G. (2012). Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J. Neurosci. Res. 90, 1392–1402. doi: 10.1002/jnr.23002
Uz, M., Büyüköz, M., Sharma, A. D., Sakaguchi, D. S., Altinkaya, S. A., and Mallapragada, S. K. (2017). Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 53, 293–306. doi: 10.1016/j.actbio.2017.02.018
Wang, S., Zhu, C., Zhang, B., Hu, J., Xu, J., Xue, C., et al. (2022). BMSC-derived extracellular matrix better optimizes the microenvironment to support nerve regeneration. Biomaterials 280:121251. doi: 10.1016/j.biomaterials.2021.121251
Wang, X., Luo, E., Li, Y., and Hu, J. (2011). Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 1383, 71–80. doi: 10.1016/j.brainres.2011.01.098
Wang, Y., Teng, H. L., and Huang, Z. H. (2012). Intrinsic migratory properties of cultured Schwann cells based on single-cell migration assay. PLoS One 7:e51824. doi: 10.1371/journal.pone.0051824
Whitlock, E. L., Tuffaha, S. H., Luciano, J. P., Yan, Y., Hunter, D. A., Magill, C. K., et al. (2009). Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 39, 787–799. doi: 10.1002/mus.21220
Wong, C. W., Xu, Y., Liu, X., Xu, S., Zhang, Y., Zhu, Z., et al. (2020). Effect of induction time on the proliferation and differentiation of induced schwann-like cells from adipose-derived stem cells. Cell Mol. Neurobiol. 40, 1105–1116. doi: 10.1007/s10571-020-00795-5
Woodhoo, A., Alonso, M. B., Droggiti, A., Turmaine, M., D’Antonio, M., Parkinson, D. B., et al. (2009). Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 12, 839–847. doi: 10.1038/nn.2323
Woolley, A. G., Tait, K. J., Hurren, B. J., Fisher, L., Sheard, P. W., and Duxson, M. J. (2008). Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia 56, 306–317. doi: 10.1002/glia.20614
Wu, L. M., Wang, J., Conidi, A., Zhao, C., Wang, H., Ford, Z., et al. (2016). Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat. Neurosci. 19, 1060–1072. doi: 10.1038/nn.4322
Wu, S., Qi, Y., Shi, W., Kuss, M., Chen, S., and Duan, B. (2020). Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 139, 91–104. doi: 10.1016/j.actbio.2020.11.042
Xie, J., MacEwan, M. R., Schwartz, A. G., and Xia, Y. (2010). Electrospun nanofibers for neural tissue engineering. Nanoscale 2, 35–44. doi: 10.1039/b9nr00243j
Xue, J., Xie, J., Liu, W., and Xia, Y. (2017a). Electrospun nanofibers: new concepts, materials, and applications. Acc. Chem. Res. 50, 1976–1987. doi: 10.1021/acs.accounts.7b00218
Xue, J., Yang, J., O’Connor, D. M., Zhu, C., Huo, D., Boulis, N. M., et al. (2017b). Differentiation of bone marrow stem cells into schwann cells for the promotion of neurite outgrowth on electrospun fibers. ACS Appl. Mater. Interfaces 9, 12299–12310. doi: 10.1021/acsami.7b00882
Yeh, T. S., Fang, Y. H., Lu, C. H., Chiu, S. C., Yeh, C. L., Yen, T. C., et al. (2014). Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials 35, 174–184. doi: 10.1016/j.biomaterials.2013.09.080
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., and Rybak, Z. (2019). Stem cells: past, present, and future. Stem Cell Res. Ther. 10:68. doi: 10.1186/s13287-019-1165-5
Zeng, W., Rong, M., Hu, X., Xiao, W., Qi, F., Huang, J., et al. (2014). Incorporation of chitosan microspheres into collagen-chitosan scaffolds for the controlled release of nerve growth factor. PLoS One 9:e101300. doi: 10.1371/journal.pone.0101300
Zhang, D., Yao, Y., Duan, Y., Yu, X., Shi, H., Nakkala, J. R., et al. (2020). Surface-anchored graphene oxide nanosheets on cell-scale micropatterned Poly(d,l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces 12, 7915–7930. doi: 10.1021/acsami.9b20321
Zhang, H. T., Cheng, H. Y., Zhang, L., Fan, J., Chen, Y. Z., Jiang, X. D., et al. (2009). Umbilical cord blood cell-derived neurospheres differentiate into Schwann-like cells. Neuroreport 20, 354–359. doi: 10.1097/WNR.0b013e328323d74c
Zhang, Y., Zhu, Z., Liu, X., Xu, S., Zhang, Y., Xu, Y., et al. (2021). Integrated analysis of long noncoding RNAs and mRNA expression profiles reveals the potential role of lncRNAs in early stage of post-peripheral nerve injury in Sprague-Dawley rats. Aging 13, 13909–13925. doi: 10.18632/aging.202989
Zhao, Y., Wang, Y., Gong, J., Yang, L., Niu, C., Ni, X., et al. (2017). Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 134, 64–77. doi: 10.1016/j.biomaterials.2017.02.026
Zhou, G., Chang, W., Zhou, X., Chen, Y., Dai, F., Anwar, A., et al. (2020). Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl. Mater. Interfaces 12, 16168–16177. doi: 10.1021/acsami.0c04191
Zhu, Z., Zhou, X., He, B., Dai, T., Zheng, C., Yang, C., et al. (2015). Ginkgo biloba extract (EGb 761) promotes peripheral nerve regeneration and neovascularization after acellular nerve allografts in a rat model. Cell Mol. Neurobiol. 35, 273–282. doi: 10.1007/s10571-014-0122-1
Ziegler, L., Grigoryan, S., Yang, I. H., Thakor, N. V., and Goldstein, R. S. (2011). Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. Rep. 7, 394–403. doi: 10.1007/s12015-010-9198-2
Keywords: Schwann cells, peripheral nervous system, transcriptional regulators, tissue-engineered nerve graft, directed reprogramming
Citation: Su Q, Nasser MI, He J, Deng G, Ouyang Q, Zhuang D, Deng Y, Hu H, Liu N, Li Z, Zhu P and Li G (2022) Engineered Schwann Cell-Based Therapies for Injury Peripheral Nerve Reconstruction. Front. Cell. Neurosci. 16:865266. doi: 10.3389/fncel.2022.865266
Received: 29 January 2022; Accepted: 04 April 2022;
Published: 06 May 2022.
Edited by:
Ji-Fan Hu, Jilin University, ChinaReviewed by:
Yunxiang Luo, The First Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2022 Su, Nasser, He, Deng, Ouyang, Zhuang, Deng, Hu, Liu, Li, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zhu, emh1cGluZ0BnZHBoLm9yZy5jbg==; Ge Li, bGlnZUBnZHBoLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.