- 1Department of Otorhinolaryngology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Maternal and Child Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Ministry of Education Key Laboratory of Environment and Health, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
The link between hearing impairment and air pollution has not been established, and the moderating effect of a healthy diet has never been investigated before. The purpose of this study was to investigate the association between air pollution and hearing impairment in British adults aged 37–73 years, and whether the association was modified by a healthy diet. We performed a cross-sectional population-based study with 158,811 participants who provided data from United Kingdom Biobank. A multivariate logistic regression model was used to investigate the link between air pollution and hearing impairment. Subgroup and effect modification analyses were carried out according to healthy diet scores, gender, and age. In the fully adjusted model, we found that exposure to PM10, NOX, and NO2 was associated with hearing impairment [PM10: odds ratio (OR) = 1.15, 95% confidence interval (95% CI) 1.02–1.30, P = 0.023; NOX: OR = 1.02, 95% CI 1.00–1.03, P = 0.040; NO2: OR = 1.03, 95% CI 1.01–1.06, P = 0.044], while PM2.5 and PM2.5 absorbance did not show similar associations. We discovered an interactive effect of age and air pollution on hearing impairment, but a healthy diet did not. The findings suggested that exposure to PM10, NOX and NO2 was linked to hearing impairment in British adults, whereas PM2.5 and PM2.5 absorbance did not show similar associations. These may help researchers focus more on the impact of air pollution on hearing impairment and provide a basis for developing effective prevention strategies.
Introduction
Hearing impairment is one of the most common age-related chronic health problems (Vos et al., 2016). The rate of clinically significant hearing impairment is doubling approximately every decade (Lin et al., 2011; Goman and Lin, 2016). Hearing impairment has been reported to be the second most prevalent disorder and the dominant cause of years lived with disability among global non-infectious diseases (Vos et al., 2016). In contrast with normal hearing adults of the same age, those with hearing impairment have a greater incidence of hospitalization (Genther et al., 2013), death (Contrera et al., 2015), falls (Lin and Ferrucci, 2012), cardiovascular disorders (McKee et al., 2018), depression (Li et al., 2014), and dementia (Lin et al., 2013). Consequently, hearing impairment causes a huge burden on the emotional and physical wellbeing of individuals (Dawes et al., 2014b). It is predicted that one-fifth of the population of the United Kingdom will suffer from hearing impairment by 2035 (Taylor et al., 2020). Accordingly, the key is to prevent hearing impairment. Hearing impairment is caused by a combination of hereditary and environmental factors (Cunningham and Tucci, 2017). The identification of modifiable risk factors is critical to provide the basis for preventive strategies.
Global trends in urbanization and industrialization have led to a growing problem of air pollution (Landrigan, 2017), which has become the main public health issue across the world (Brunekreef and Holgate, 2002). Of note, growing evidence demonstrates that air pollution exposure is not only connected with respiratory disorders, such as lung cancer (Xing et al., 2019), but also with cardiovascular diseases (Lelieveld et al., 2019; Hayes et al., 2020), inflammatory diseases (Chang et al., 2016), diabetes (Strak et al., 2017), and neurodegenerative diseases (Chen et al., 2017). Besides, the main environmental risk factor for human death is air pollution (Gordon et al., 2014). Lately, there have been reports that air pollution may impact hearing health, but available data is limited. A recent study (Tsai et al., 2020) found that participants exposed to fine particulate matter (PM2.5: particulate matter ≤ 2.5 μm in diameter) and nitrogen dioxide (NO2) had a substantially increased risk of sudden sensorineural hearing loss (SSNHL). Another study (Chang et al., 2020) showed that increased concentrations of NO2 were linked to a higher risk of sensorineural hearing loss, while in a nested case-control study (Choi et al., 2019), SSNHL was associated with NO2 exposure, but particulate matter with a diameter of 10 μm or less (PM10) was not associated with SSNHL. Similarly, another study (Lee et al., 2019) also found no association between PM10 and number of SSNHL patient. Although these studies explored the association of air pollution with sensorineural hearing loss, the results remained controversial.
A healthy diet might preserve hearing (Spankovich and Le Prell, 2013; Curhan et al., 2018, 2020), as described by their role in preventing chronic illnesses (Yevenes-Briones et al., 2021). A healthy diet includes multiple components that support antioxidant function and protect against free radical damage (Curhan et al., 2020), thereby regulating oxidative stress and delaying mitochondrial dysfunction (Yevenes-Briones et al., 2021). In addition, a healthy diet might be beneficial to hearing impairment by protecting microvascular and macrovascular damage to cochlear blood flow (Appel et al., 2006; Fung et al., 2008), providing the essential nutrients for an adequate cochlear blood supply (Yevenes-Briones et al., 2021), and reducing inflammation (Neale et al., 2016). According to previous research, dietary patterns could modify the relationship between air pollution and health-related outcomes, such as cardiovascular disease mortality risk (Lim et al., 2019) and cognitive function (Zhu et al., 2022). However, the moderating effect of a healthy diet on the link between hearing impairment and air pollution has not been investigated before. Therefore, in this cross-sectional study, we aimed to explore the link between air pollution and hearing impairment and to analyze whether a healthy diet has moderating effects on this link.
Materials and Methods
Study Subjects
The United Kingdom Biobank is an international and accessible data resource1 containing data on more than half a million people aged from 37 to 73 years (99.5% were between 40 and 69 years) in England, Scotland, and Wales (Collins, 2012). Adults living within a 25-mile radius of one of 22 Biobank Assessment Centers in the United Kingdom were invited by email to join the United Kingdom Biobank between 2006 and 2010, achieving a response rate of approximately 5.5% (Sudlow et al., 2015). Participants completed a computer touch screen questionnaire (which included questions on topics such as population, health, lifestyle, environment as well as medical history, etc.) and underwent physical measurements, including a hearing test. Written informed consent was signed by all the participants. The research was carried out with the general approval of the National Health Service and the National Research Ethics Service. The subjects of the current study were all those participants for whom data on both air pollution measures and hearing test results were available.
Hearing Test
The speech-in-noise hearing test (i.e., digit triplet test, DTT) of the United Kingdom Biobank provided participants with 15 groups of English monosyllabic numbers to evaluate the listening thresholds (i.e., signal-to-noise ratio) at different sound levels.2 Each ear was examined separately, in the order that the participants were allocated at random. Participants first wore circumaural headphones and selected the most comfortable volume. Then, they started the speech-in-noise hearing test to identify and type the three numbers they had heard by touching the screen interface. The noise level of the subsequent triple would increase if the triplet was correctly recognized; otherwise, it would reduce. The speech reception threshold (SRT) was defined as the signal-to-noise ratio of correctly understanding half of the presented speech. The SRT ranged from −12 to +8 dB, with a lower score representing better performance. Based on the cutoff point established by Dawes et al. (2014b), the better performance ear was chosen for this study, and participants were divided into normal (SRT < −5.5 dB) and hearing impairment (SRT ≥ −5.5 dB) groups.
The DTT shows a very good correlation with the pure tone hearing test (r = 0.77) (Jansen et al., 2010), so it can be considered as a measure of hearing impairment (Dawes et al., 2014b). There are some advantages to the DTT, for example, there is no need for a sound booth and the test can be delivered via the internet (Moore et al., 2014). The most common hearing complaint is difficulty in hearing over background noise (Pienkowski, 2017), so the speech-in-noise hearing test used to evaluate hearing function represents an ecologically effective as well as objective hearing indicator (Couth et al., 2019).
Measures of Air Pollution
The air pollution data recorded in the United Kingdom Biobank were from the Small Area Health Statistics Unit,3 a part of the BioShaRE-EU Environmental Determinants of Health Project.4 The Land Use Regression model was applied to assess air pollution in 2010 by modeling at each residential address of the participants, which was developed as part of the European Study of Cohorts for Air Pollution Effects.5 The Land Use Regression model used to calculate the spatial distribution of air pollutants was based on geographic predictors such as traffic, land use, and topography in the geographical information system. In this study, the air pollutants assessed were PM2.5, PM10, PM2.5 absorbance, NOX, and NO2, of which all were annual average concentrations in μg/m3. More details about the air pollution data used in the United Kingdom Biobank are available elsewhere.6
Assessment of Other Variables
Age, gender, ethnicity, educational background, employment, smoking status, and alcohol intake were utilized as baseline data. The ethnic background of participants was divided into six categories: White, Black, Asian, Chinese, Mixed, and other. The educational background was divided into six categories: higher national diploma (HND), national vocational qualification (NVQ), higher national certificate (HNC), or equivalent; A levels or AS levels (including the higher school certificate), or equivalent; O levels (including the school certificate), general certificate of secondary educations (GCSEs), or equivalent; certificate of secondary educations (CSEs), or equivalent; college or university degree; and other professional qualification. Employment status was divided into seven categories: retired; unable to work because of sickness or disability; looking after home and/or family; unemployed; in paid employment or self-employed; student (full-time or part-time); or doing unpaid or voluntary work. Smoking status (Dawes et al., 2014a) was divided into three categories: never-smokers, current and former smokers. Alcohol consumption frequency was divided into five categories: daily or almost daily; three or four times a week; once or twice a week; occasional drinking; and never. Body mass index (BMI) was categorized as obese (BMI ≥ 30), overweight (25 ≤ BMI < 30), normal weight (18.5 ≤ BMI < 25), and underweight (BMI < 18.5). Evaluation of physical activity was conducted through the questions in the International Physical Activity Questionnaire, which graded activity into three degrees: low, moderate, and high.7 A questionnaire8 containing the usual dietary intake was completed by United Kingdom Biobank participants during the baseline assessment. The intake of fruits (fresh fruit intake and dried fruit intake), vegetables (cooked vegetable intake and salad/raw vegetable intake), fish (oily fish intake and non-oily fish intake), processed meat and unprocessed red meat (beef intake, lamb/mutton intake, and pork intake) from the United Kingdom Biobank food intake questionnaire was used to calculate the health diet scores (Wang et al., 2021): fruit intake ≥ three pieces per day, vegetable intake ≥ four tablespoons per day, fish intake ≥ twice per week, processed meat intake ≤ twice per week, unprocessed red meat intake ≤ twice per week. Each favorable dietary factor gave a point, so the healthy diet scores were 0–5. The serum concentrations of glycosylated hemoglobin and total cholesterol were regarded as continuous variables. Vascular problems included angina, heart attack, stroke, and high blood pressure.
Data Analysis
All analyses were performed using R version 4.0.2. The data are summarized descriptively. Continuous variables are represented as mean (standard deviation) and comparison between the two groups was performed by independent sample t test. The classification variables are represented as percentages (%) and the rate was compared by χ2 test. The link between air pollution and hearing impairment was investigated using a multivariate logistic regression model with and without adjusting for other variables. Model 1 was unadjusted, Model 2 was adjusted for age and gender, and Model 3 was further adjusted for race, educational level, employment, smoking status and alcohol consumption frequency, BMI, physical activity, glycosylated hemoglobin, total cholesterol, and vascular diseases (heart attack, stroke, angina, and hypertension). Moreover, we evaluated the association between subgroups stratified by healthy diet scores (low: 0–2, and high: 3–5), gender (female and male) and age (≤50, 51–60, and >60). The Wald test was used to test interactions among subgroups. P < 0.05 (two-sided test) was considered statistically significant.
Results
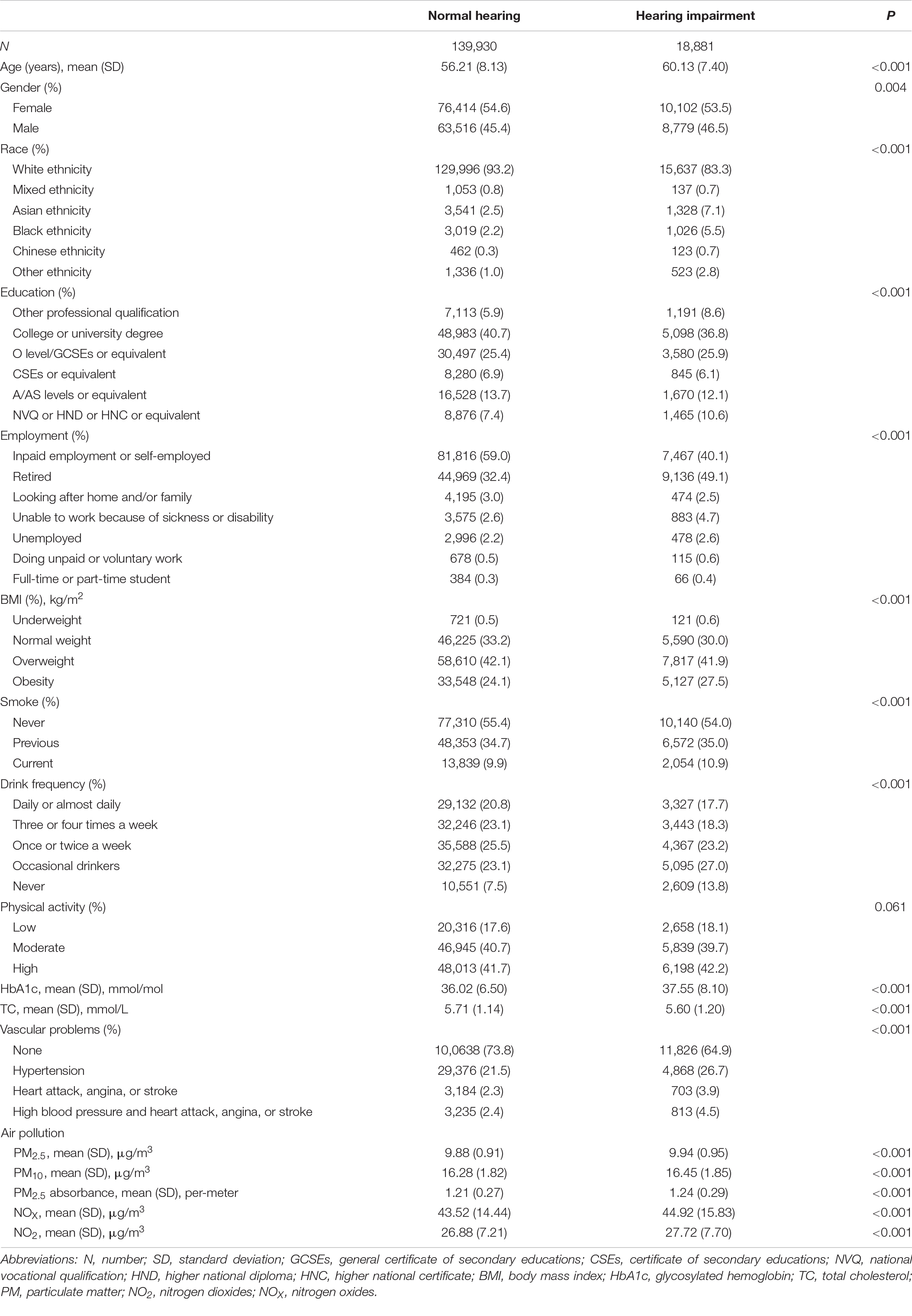
In total, 158,811 subjects were enrolled in this study, including 18,881 (11.9%) with hearing impairment and 139,930 (88.1%) with normal hearing, 54.5% were female (n = 86,516), 91.7% were white (n = 145,633), with the mean (standard deviation) age of 56.68 (8.15) years. The distribution of baseline characteristics and air pollution in the two groups is shown in Table 1. Except for physical activity, other variables were significantly distributed in the two groups (P < 0.05). In comparison to the group of people with normal hearing, the subjects in the hearing impairment group were older on average, non-whites. In addition, they were more likely to be obese and to have cardiovascular problems. Furthermore, the hearing impairment group was exposed to higher mean annual concentrations of air pollutants than the normal hearing group (Table 1).
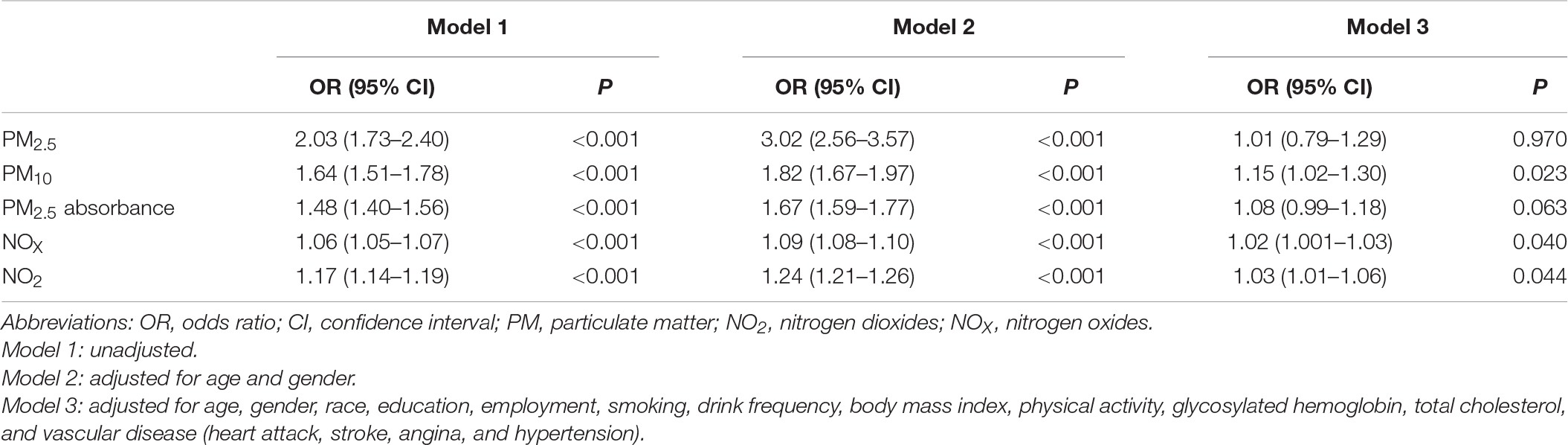
Table 2 shows the risks of several air pollutants and hearing impairment. Model 1 (without adjustment for any confounders) showed significant associations between air pollutants and hearing impairment (P < 0.001) [PM2.5: odds ratio (OR) = 2.03, 95% confidence interval (95% CI) 1.73–2.40; PM10: OR = 1.64, 95% CI 1.51–1.78; PM2.5 absorbance: OR = 1.48, 95% CI 1.40–1.56; NOX: OR = 1.06, 95% CI 1.05–1.07; NO2: OR = 1.17, 95% CI 1.41–1.19]. After adjusting for age and gender, Model 2 showed that air pollutants were still significantly associated with hearing impairment (P < 0.001), and all OR values were larger than Model 1 (PM2.5: OR = 3.02, 95% CI 2.56–3.57; PM10: OR = 1.82, 95% CI 1.67–1.97; PM2.5 absorbance: OR = 1.67, 95% CI 1.59–1.77; NOX: OR = 1.09, 95% CI 1.08–1.10; NO2: OR = 1.24, 95% CI 1.21–1.26). Except for PM2.5 and PM2.5 absorbance, which showed no significant associations with hearing impairment (P = 0.970 and P = 0.063, respectively), we observed that the associations between the other pollutants and hearing impairment remained in Model 3 after further adjusting for other confounders on the basis of Model 2 (PM10: OR = 1.15, 95% CI 1.02–1.30, P = 0.023; NOx: OR = 1.02, 95% CI 1.00–1.03, P = 0.040; NO2: OR = 1.03, 95% CI 1.01–1.06, P = 0.044), even though the estimates were lower than those in Models 1 and 2.
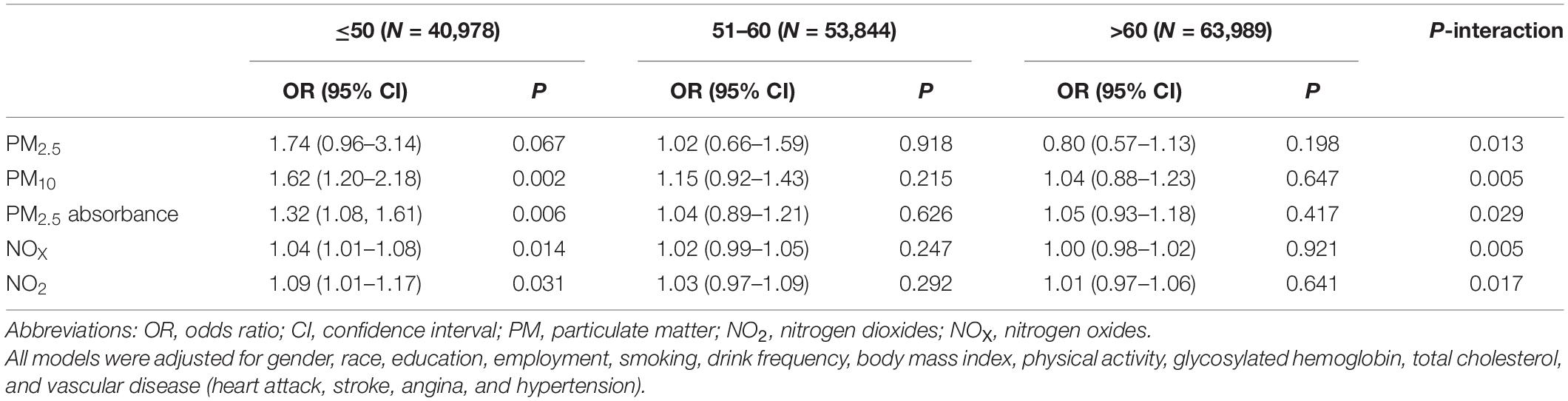
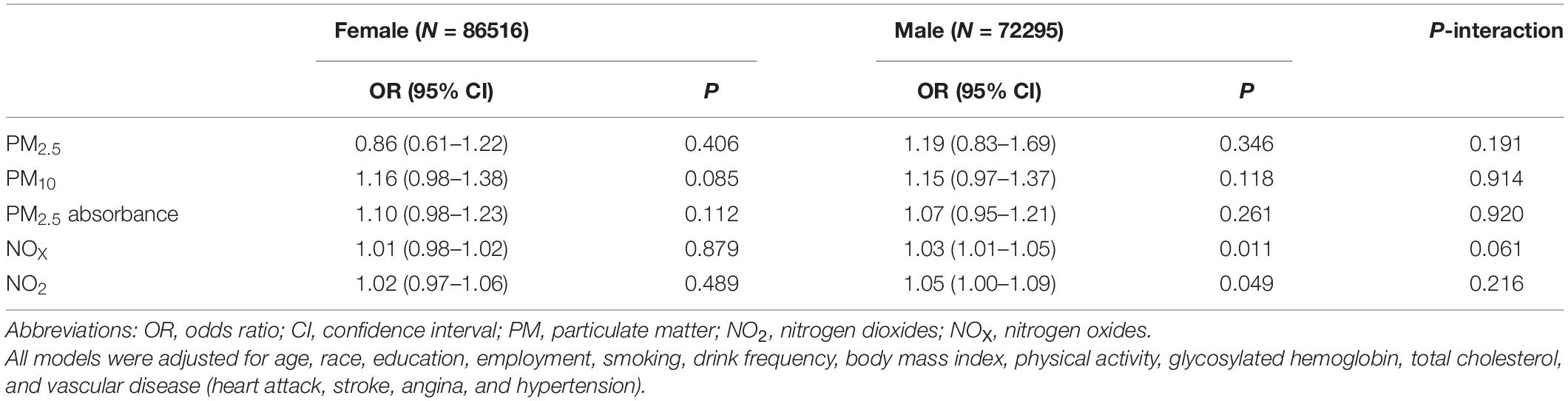
Table 3 shows the associations between several air pollutants and hearing impairment, stratified by healthy diet scores. In this study, no significant associations and moderating effects were observed. After stratification by age (Table 4), we found that PM10, PM2.5 absorbance, NOX, and NO2 were associated with hearing impairment in participants up to and including 50 years of age (PM10: OR = 1.62, 95% CI 1.20–2.18, P = 0.002; PM2.5 absorbance: OR = 1.32, 95% CI 1.08–1.61, P = 0.006; NOX: OR = 1.04, 95% CI 1.01–1.08, P = 0.014; NO2: OR = 1.09, 95% CI 1.01–1.17, P = 0.031). In participants aged 51 to 60 years and above 60, there was no connection between air pollution and hearing impairment. Additionally, there was a statistically significant interaction between age and air pollution with hearing impairment (P < 0.05). Further, after stratifying by gender (Table 5), we found that NOX and NO2 were correlated with hearing impairment in men.

Table 3. Associations of air pollution and hearing impairment in subgroups stratified by healthy diet scores.
Discussion
In this cross-sectional study, we investigated the association between hearing impairment and air pollution (comprising PM2.5, PM10, PM2.5 absorbance, NOX, and NO2) using United Kingdom Biobank data. We found that exposure to PM10, NOX, and NO2 was linked to hearing impairment after adjusting for confounding factors, while PM2.5 and PM2.5 absorbance showed no similar correlations. Furthermore, there was no modification of these associations by a healthy diet. Regarding age, interaction effects were observed.
The relationship between air pollution and hearing impairment has not been fully established yet. Several studies indicated that exposure to NO2 could be related to hearing problems. Chang et al. (2020) found that people exposed to moderate (hazard ratio, HR = 1.40, 95% CI 1.27–1.54) and high levels of NO2 (HR = 1.63, 95% CI 1.48–1.81) were at higher risk of developing sensorineural hearing loss than those exposed to the low level. The results of Tsai et al. (2020) were similar, finding a significantly increased risk of SSNHL in those exposed to high concentrations of NO2 (adjusted HR = 1.02, 95% CI 1.01–1.04). Likewise, Choi et al. (2019) discovered that SSNHL was associated with short-term exposure to NO2 (14 days) (adjusted OR = 3.12, 95% CI 2.16–4.49). Consistent with previous studies, NO2 was associated with hearing impairment in our study. Moreover, NOX, a term that contains several nitrogen compounds but is mainly composed of nitrogen oxide and NO2, showed an association with hearing impairment.
In contrast to our expectations, we found a significant association between PM10 and hearing impairment but not PM2.5. Conversely, previous studies (Choi et al., 2019; Lee et al., 2019) showed no correlation between PM10 and hearing impairment. A study reported (Tsai et al., 2020) a significantly higher risk of developing SSNHL with moderate (adjusted HR = 1.58, 95% CI 1.21–2.06) or high (adjusted HR = 1.32, 95% CI 1.00–1.74) level exposure to PM2.5 compared to those exposed to the low level. And another study discovered a slight negative association between the maximum PM2.5 concentration and the admission rate of SSNHL (Lee et al., 2019). In 2017, a study (Strak et al., 2017) in a large national health survey reported that oxidative potential of PM2.5 rather than PM2.5, was associated with diabetes prevalence, indicating that the impact of particulate matter on diabetes might vary with the compositions. According to a study (Yin and Harrison, 2008) conducted at three sites (urban roadside, central urban background, and rural) in Birmingham, United Kingdom, organics, nitrate, and sulfate accounted for a substantial amount of the overall mass for both PM10 and PM2.5. This research also showed that proportions of these three major parts and other secondary compositions like iron-rich dust and sodium chloride varied in both. Although discrepancies in associations with diseases after PM2.5 and PM10 exposure could be explained by different compositions of particulate matter, the evidence may still be limited. More research is required to clarify this issue in the future.
Oxidative stress and mitochondrial dysfunction play a crucial role in hearing impairment (Yamasoba et al., 2013). Air pollution might be involved in oxidative stress by producing or directly acting as reactive oxygen species (Kelly, 2003), which can then induce mitochondrial damage (Rodríguez-Martínez et al., 2013). Dysfunctional mitochondria increase reactive oxygen species generation and accumulation, reducing the mitochondrial membrane potential, activating the apoptosis pathway, and causing the death of inner ear hair cells (Park et al., 2016). What’s more, air pollution might indirectly be associated with hearing impairment by causing cardiovascular diseases through pro-inflammatory pathways and the production of reactive oxygen species (Simkhovich et al., 2008; Brook et al., 2010). It has been demonstrated that cardiovascular diseases are risk factors for hearing impairment (Oron et al., 2014; Tan et al., 2018). Nonetheless, the link between air pollution and hearing impairment was still evident after adjusting for related vascular problems in Model 3, suggesting that other mechanisms may also be involved in the link between air pollution and hearing impairment.
There was evidence that a healthy diet could protect against hearing impairment by reducing vascular damage, decreasing inflammation, and inhibiting oxidative damage (Curhan et al., 2020; Yevenes-Briones et al., 2021). Based on similar mechanistic pathways, modifying the health effects of air pollution by diet may be possible. But in our study, no effect modification of diet was observed. Studies previously showed an interaction between dietary patterns and air pollution exposure on health-related outcomes. In a birth cohort in Northeast China, animal foods pattern was found to significantly modify the association between exposure to NO2 and carbon monoxide and gestational diabetes mellitus, with higher intake related to a higher rate of gestational diabetes mellitus following exposure to air pollution (Hehua et al., 2021). A Mediterranean diet reduced cardiovascular disease mortality risk related to long-term exposure to air pollutants in a large prospective US cohort (Lim et al., 2019). A prospective cohort study of Chinese older adults reported that a plant-based dietary pattern mitigated the adverse effects of air pollution on cognitive function (Zhu et al., 2022).
It seems to be accepted that hearing impairment becomes more common with increasing age (Díaz et al., 2016). Nevertheless, the association between air pollution and hearing impairment was only found in participants younger under or equal to 50 years of age in this study. An interaction effect between age and air pollution on hearing impairment was also observed. Age is an unmodifiable risk factor for hearing impairment, which could lead to cochlear aging (Yamasoba et al., 2013). However, modifiable risk factors play a significant part in the development of hearing impairment at a relatively young age (i.e., <85 years old), while their effects decrease in the oldest people (i.e., ≥85 years old) (Zhan et al., 2010). Therefore, we speculated that air pollution, a modifiable risk factor, might have a greater impact on people younger than or equal to 50 years old compared to those over 50 years old, even if our study subjects were all under 85 years old.
Our research used data from the United Kingdom Biobank, a national cohort with good quality control. Additionally, the hearing test was based on the DTT data in the United Kingdom Biobank, which represented an ecologically effective and objective hearing indicator. We also adjusted for many confounders (including demographic information, lifestyle, and related diseases affecting hearing) to reduce their potential impact. However, our research also had some limitations. Above all, the cross-sectional design of this study was inadequate to account for the cause and effect between air pollution and hearing impairment, and further longitudinal studies are needed. Second, the sample of participants in United Kingdom Biobank was suggested to be unrepresentative of the general population because of the bias toward recruiting participants who were generally healthier and had a higher socioeconomic status (Fry et al., 2017). Hence, the subsample from United Kingdom Biobank and estimated hearing impairment rate in this study might not be representative of the general population. Third, like other epidemiological studies of air pollution, there might be potential misclassifications of air pollution exposure in this study because air pollution exposure was evaluated at the place of residence. Fourth, in the United Kingdom, where emissions regulations are strict and average pollution level is relatively low, it is not clear to what extent this study can be generalizable to other settings. Finally, in spite of adjusting for many confounders in our study, the potential effects of residual confounds of unmeasured variables could not be excluded, such as the use of ototoxic drugs, which was not considered due to lack of data.
Conclusion
In conclusion, we found that exposure to PM10, NOX, and NO2 was associated with hearing impairment in British adults, while PM2.5 and PM2.5 absorbance did not show similar correlations. Our findings may help researchers pay more attention to the impact of air pollution on hearing impairment and provide a basis for developing effective prevention strategies.
Data Availability Statement
The data supporting the results of this study can be found in the website of UK Biobank (www.ukbiobank.ac.uk) upon application.
Ethics Statement
The study involving human participants was carried out with the ethical approval obtained by United Kingdom Biobank from the National Health Service National Research Ethics Service.
Author Contributions
YS, YT, and LY conceived the overall project and developed the methods as well as procedures throughout the study. DL and LY managed the data collection and data entry and carried out data verification and statistical analyses. LY drafted the first version of the manuscript. All authors oversaw statistical analysis, involved in the interpretation of the results, reviewed, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 82071058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all the participants of United Kingdom Biobank. We also sincerely thank those who participated in data collection and management of United Kingdom Biobank. This study has been carried out with the use of the United Kingdom Biobank resource (application number 69741).
Footnotes
- ^ www.UKbiobank.ac.UK
- ^ https://biobank.ctsu.ox.ac.UK/crystal/label.cgi?id=100049
- ^ http://www.sahsu.org/
- ^ http://www.bioshare.eu/
- ^ http://www.escapeproject.eu/
- ^ https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/EnviroExposEst.pdf
- ^ https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/ipaq_analysis.pdf
- ^ https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100052
References
Appel, L. J., Brands, M. W., Daniels, S. R., Karanja, N., Elmer, P. J., Sacks, F. M., et al. (2006). Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47, 296–308. doi: 10.1161/01.HYP.0000202568.01167.B6
Brook, R. D., Rajagopalan, S., Pope, C. A. III, Brook, J. R., Bhatnagar, A., Diez-Roux, A. V., et al. (2010). Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. doi: 10.1161/CIR.0b013e3181dbece1
Brunekreef, B., and Holgate, S. T. (2002). Air pollution and health. Lancet 360, 1233–1242. doi: 10.1016/s0140-6736(02)11274-8
Chang, K. H., Hsu, C. C., Muo, C. H., Hsu, C. Y., Liu, H. C., Kao, C. H., et al. (2016). Air pollution exposure increases the risk of rheumatoid arthritis: a longitudinal and nationwide study. Environ. Int. 94, 495–499. doi: 10.1016/j.envint.2016.06.008
Chang, K. H., Tsai, S. C., Lee, C. Y., Chou, R. H., Fan, H. C., Lin, F. C., et al. (2020). Increased Risk of Sensorineural Hearing Loss as a Result of Exposure to Air Pollution. Int. J. Environ. Res. Public Health 17:1969. doi: 10.3390/ijerph17061969
Chen, C. Y., Hung, H. J., Chang, K. H., Hsu, C. Y., Muo, C. H., Tsai, C. H., et al. (2017). Long-term exposure to air pollution and the incidence of Parkinson’s disease: a nested case-control study. PLoS One 12:e0182834. doi: 10.1371/journal.pone.0182834
Choi, H. G., Min, C., and Kim, S. Y. (2019). Air pollution increases the risk of SSNHL: a nested case-control study using meteorological data and national sample cohort data. Sci. Rep. 9:8270. doi: 10.1038/s41598-019-44618-0
Collins, R. (2012). What makes UK Biobank special? Lancet 379, 1173–1174. doi: 10.1016/s0140-6736(12)60404-8
Contrera, K. J., Betz, J., Genther, D. J., and Lin, F. R. (2015). Association of hearing impairment and mortality in the National Health and Nutrition Examination Survey. JAMA Otolaryngol. Head Neck Surg. 141, 944–946. doi: 10.1001/jamaoto.2015.1762
Couth, S., Mazlan, N., Moore, D. R., Munro, K. J., and Dawes, P. (2019). Hearing Difficulties and Tinnitus in Construction, Agricultural, Music, and Finance Industries: contributions of Demographic, Health, and Lifestyle Factors. Trends Hear. 23:2331216519885571. doi: 10.1177/2331216519885571
Cunningham, L. L., and Tucci, D. L. (2017). Hearing Loss in Adults. N. Engl. J. Med. 377, 2465–2473. doi: 10.1056/NEJMra1616601
Curhan, S. G., Halpin, C., Wang, M., Eavey, R. D., and Curhan, G. C. (2020). Prospective Study of Dietary Patterns and Hearing Threshold Elevation. Am. J. Epidemiol. 189, 204–214. doi: 10.1093/aje/kwz223
Curhan, S. G., Wang, M., Eavey, R. D., Stampfer, M. J., and Curhan, G. C. (2018). Adherence to Healthful Dietary Patterns Is Associated with Lower Risk of Hearing Loss in Women. J. Nutr. 148, 944–951. doi: 10.1093/jn/nxy058
Dawes, P., Fortnum, H., Moore, D. R., Emsley, R., Norman, P., Cruickshanks, K., et al. (2014b). Hearing in middle age: a population snapshot of 40- to 69-year olds in the United Kingdom. Ear and Hearing 35, e44–e51. doi: 10.1097/AUD.0000000000000010
Dawes, P., Cruickshanks, K. J., Moore, D. R., Edmondson-Jones, M., McCormack, A., Fortnum, H., et al. (2014a). Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J. Assoc. Res. Otolaryngol. 15, 663–674. doi: 10.1007/s10162-014-0461-0
Díaz, C., Goycoolea, M., and Cardemil, F. (2016). Hipoacusia: trascendencia, Incidencia Y Prevalencia. Revista. Médica. Clínica. Las Condes 27, 731–739. doi: 10.1016/j.rmclc.2016.11.003
Fry, A., Littlejohns, T. J., Sudlow, C., Doherty, N., Adamska, L., Sprosen, T., et al. (2017). Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 186, 1026–1034. doi: 10.1093/aje/kwx246
Fung, T. T., Chiuve, S. E., McCullough, M. L., Rexrode, K. M., Logroscino, G., and Hu, F. B. (2008). Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 168, 713–720. doi: 10.1001/archinte.168.7.713
Genther, D. J., Frick, K. D., Chen, D., Betz, J., and Lin, F. R. (2013). Association of hearing loss with hospitalization and burden of disease in older adults. J. Am. Med. Assoc. 309, 2322–2324. doi: 10.1001/jama.2013.5912
Goman, A. M., and Lin, F. R. (2016). Prevalence of Hearing Loss by Severity in the United States. Am. J. Public health 106, 1820–1822. doi: 10.2105/ajph.2016.303299
Gordon, S. B., Bruce, N. G., Grigg, J., Hibberd, P. L., Kurmi, O. P., Lam, K. B., et al. (2014). Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2, 823–860. doi: 10.1016/s2213-2600(14)70168-7
Hayes, R. B., Lim, C., Zhang, Y., Cromar, K., Shao, Y., Reynolds, H. R., et al. (2020). PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 49, 25–35. doi: 10.1093/ije/dyz114
Hehua, Z., Yang, X., Qing, C., Shanyan, G., and Yuhong, Z. (2021). Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ. Int. 147:106347. doi: 10.1016/j.envint.2020.106347
Jansen, S., Luts, H., Wagener, K. C., Frachet, B., and Wouters, J. (2010). The French digit triplet test: a hearing screening tool for speech intelligibility in noise. Int. J. Audiol. 49, 378–387. doi: 10.3109/14992020903431272
Kelly, F. J. (2003). Oxidative stress: its role in air pollution and adverse health effects. Occupat. Environ. Med. 60, 612–616. doi: 10.1136/oem.60.8.612
Landrigan, P. J. (2017). Air pollution and health. Lancet Public health 2, e4–e5. doi: 10.1016/s2468-2667(16)30023-8
Lee, H. M., Kim, M. S., Kim, D. J., Uhm, T. W., Yi, S. B., Han, J. H., et al. (2019). Effects of meteorological factor and air pollution on sudden sensorineural hearing loss using the health claims data in Busan, Republic of Korea. Am. J. Otolaryngol. 40, 393–399. doi: 10.1016/j.amjoto.2019.02.010
Lelieveld, J., Klingmüller, K., Pozzer, A., Pöschl, U., Fnais, M., Daiber, A., et al. (2019). Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 40, 1590–1596. doi: 10.1093/eurheartj/ehz135
Li, C. M., Zhang, X., Hoffman, H. J., Cotch, M. F., Themann, C. L., and Wilson, M. R. (2014). Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. Otolaryngol. Head Neck Surg. 140, 293–302. doi: 10.1001/jamaoto.2014.42
Lim, C. C., Hayes, R. B., Ahn, J., Shao, Y., Silverman, D. T., Jones, R. R., et al. (2019). Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 139, 1766–1775. doi: 10.1161/CIRCULATIONAHA.118.035742
Lin, F. R., and Ferrucci, L. (2012). Hearing loss and falls among older adults in the United States. Arch. Intern. Med. 172, 369–371. doi: 10.1001/archinternmed.2011.728
Lin, F. R., Thorpe, R., Gordon-Salant, S., and Ferrucci, L. (2011). Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. Series Biol. Sci. Med. Sci. 66, 582–590. doi: 10.1093/gerona/glr002
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
McKee, M. M., Stransky, M. L., and Reichard, A. (2018). Hearing loss and associated medical conditions among individuals 65 years and older. Disab. Health J. 11, 122–125. doi: 10.1016/j.dhjo.2017.05.007
Moore, D. R., Edmondson-Jones, M., Dawes, P., Fortnum, H., McCormack, A., Pierzycki, R. H., et al. (2014). Relation between speech-in-noise threshold, hearing loss and cognition from 40-69 years of age. PLoS One 9:e107720. doi: 10.1371/journal.pone.0107720
Neale, E. P., Batterham, M. J., and Tapsell, L. C. (2016). Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr. Res. 36, 391–401. doi: 10.1016/j.nutres.2016.02.009
Oron, Y., Elgart, K., Marom, T., and Roth, Y. (2014). Cardiovascular risk factors as causes for hearing impairment. Audiol. Neuro-otol. 19, 256–260. doi: 10.1159/000363215
Park, Y. H., Shin, S. H., Byun, S. W., and Kim, J. Y. (2016). Age- and Gender-Related Mean Hearing Threshold in a Highly-Screened Population: the Korean National Health and Nutrition Examination Survey 2010–2012. PLoS One 11:e0150783. doi: 10.1371/journal.pone.0150783
Pienkowski, M. (2017). On the Etiology of Listening Difficulties in Noise Despite Clinically Normal Audiograms. Ear Hear. 38, 135–148. doi: 10.1097/aud.0000000000000388
Rodríguez-Martínez, E., Martínez, F., Espinosa-García, M. T., Maldonado, P., and Rivas-Arancibia, S. (2013). Mitochondrial dysfunction in the hippocampus of rats caused by chronic oxidative stress. Neuroscience 252, 384–395. doi: 10.1016/j.neuroscience.2013.08.018
Simkhovich, B. Z., Kleinman, M. T., and Kloner, R. A. (2008). Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J. Am. Coll. Cardiol. 52, 719–726. doi: 10.1016/j.jacc.2008.05.029
Spankovich, C., and Le Prell, C. G. (2013). Healthy diets, healthy hearing: National Health and Nutrition Examination Survey, 1999–2002. Int. J. Audiol. 52, 369–376. doi: 10.3109/14992027.2013.780133
Strak, M., Janssen, N., Beelen, R., Schmitz, O., Vaartjes, I., Karssenberg, D., et al. (2017). Long-term exposure to particulate matter, NO(2) and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ. Int. 108, 228–236. doi: 10.1016/j.envint.2017.08.017
Sudlow, C., Gallacher, J., Allen, N., Beral, V., Burton, P., Danesh, J., et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS medicine 12:e1001779. doi: 10.1371/journal.pmed.1001779
Tan, H. E., Lan, N. S. R., Knuiman, M. W., Divitini, M. L., Swanepoel, D. W., Hunter, M., et al. (2018). Associations between cardiovascular disease and its risk factors with hearing loss-A cross-sectional analysis. Clin. Otolaryngol. 43, 172–181. doi: 10.1111/coa.12936
Taylor, H., Shryane, N., Kapadia, D., Dawes, P., and Norman, P. (2020). Understanding ethnic inequalities in hearing health in the UK: a cross-sectional study of the link between language proficiency and performance on the Digit Triplet Test. BMJ Open 10:e042571. doi: 10.1136/bmjopen-2020-042571
Tsai, S. C.-S., Hsu, Y.-C., Lai, J.-N., Chou, R.-H., Fan, H.-C., Lin, F. C.-F., et al. (2020). Long-Term Exposure to Air Pollution and The Risk of Developing Sudden Sensorineural Hearing Loss. J. Transl. Med. doi: 10.21203/rs.3.rs-72326/v1
Vos, T., Allen, C., Arora, M., Barber, R. M., Bhutta, Z. A., Brown, A., et al. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602. doi: 10.1016/s0140-6736(16)31678-6
Wang, M., Zhou, T., Song, Y., Li, X., Ma, H., Hu, Y., et al. (2021). Joint exposure to various ambient air pollutants and incident heart failure: a prospective analysis in UK Biobank. Eur. Heart J. 42, 1582–1591. doi: 10.1093/eurheartj/ehaa1031
Xing, D. F., Xu, C. D., Liao, X. Y., Xing, T. Y., Cheng, S. P., Hu, M. G., et al. (2019). Spatial association between outdoor air pollution and lung cancer incidence in China. BMC public health 19:1377. doi: 10.1186/s12889-019-7740-y
Yamasoba, T., Lin, F. R., Someya, S., Kashio, A., Sakamoto, T., and Kondo, K. (2013). Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear. Res. 303, 30–38. doi: 10.1016/j.heares.2013.01.021
Yevenes-Briones, H., Caballero, F. F., Struijk, E. A., Machado-Fragua, M. D., Ortola, R., Rodriguez-Artalejo, F., et al. (2021). Diet Quality and the Risk of Impaired Speech Reception Threshold in Noise: the UK Biobank cohort. Ear Hear. doi: 10.1097/AUD.0000000000001108
Yin, J., and Harrison, R. M. (2008). Pragmatic mass closure study for PM1.0, PM2.5 and PM10 at roadside, urban background and rural sites. Atmospher. Environ. 42, 980–988. doi: 10.1016/j.atmosenv.2007.10.005
Zhan, W., Cruickshanks, K. J., Klein, B. E., Klein, R., Huang, G. H., Pankow, J. S., et al. (2010). Generational differences in the prevalence of hearing impairment in older adults. Am. J. Epidemiol. 171, 260–266. doi: 10.1093/aje/kwp370
Keywords: hearing impairment, air pollution, digit triplet test (DTT), United Kingdom Biobank (UKB), healthy diet
Citation: Yuan L, Li D, Tian Y and Sun Y (2022) The Risk of Hearing Impairment From Ambient Air Pollution and the Moderating Effect of a Healthy Diet: Findings From the United Kingdom Biobank. Front. Cell. Neurosci. 16:856124. doi: 10.3389/fncel.2022.856124
Received: 16 January 2022; Accepted: 24 February 2022;
Published: 06 April 2022.
Edited by:
Hai Huang, Tulane University, United StatesReviewed by:
Zhiqiang Yan, Fudan University, ChinaHongzhe Li, VA Loma Linda Healthcare System, United States
Copyright © 2022 Yuan, Li, Tian and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Sun, sunyu@hust.edu.cn
†These authors share first authorship
 Lanlai Yuan
Lanlai Yuan