
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 08 March 2022
Sec. Non-Neuronal Cells
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.836931
This article is part of the Research Topic The Function of Schwann Cells in Peripheral Nervous System View all 11 articles
Peripheral neuropathy is a common neurological issue that leads to sensory and motor disorders. Over time, the treatment for peripheral neuropathy has primarily focused on medications for specific symptoms and surgical techniques. Despite the different advantages of these treatments, functional recovery remains less than ideal. Schwann cells, as the primary glial cells in the peripheral nervous system, play crucial roles in physiological and pathological conditions by maintaining nerve structure and functions and secreting various signaling molecules and neurotrophic factors to support both axonal growth and myelination. In addition, stem cells, including mesenchymal stromal cells, skin precursor cells and neural stem cells, have the potential to differentiate into Schwann-like cells to perform similar functions as Schwann cells. Therefore, accumulating evidence indicates that Schwann cell transplantation plays a crucial role in the resolution of peripheral neuropathy. In this review, we summarize the literature regarding the use of Schwann cell/Schwann cell-like cell transplantation for different peripheral neuropathies and the potential role of promoting nerve repair and functional recovery. Finally, we discuss the limitations and challenges of Schwann cell/Schwann cell-like cell transplantation in future clinical applications. Together, these studies provide insights into the effect of Schwann cells/Schwann cell-like cells on cell therapy and uncover prospective therapeutic strategies for peripheral neuropathy.
Peripheral neuropathies are commonly encountered disorders that result from a great number of etiologies, including trauma and side effects of diseases and treatments (Hughes, 2002). Although there is no standard method to diagnose peripheral neuropathy, the development of imaging and laboratory tests has aided in primary diagnosis, and electromyography and nerve conduction tests are especially beneficial for allowing doctors to narrow down the category and the management of peripheral neuropathies (Barrell and Smith, 2019). The categories used to be mononeuropathies, multifocal neuropathies and polyneuropathies. However, these categories are frequently further divided into axonal, demyelinating, or mixed according to a systematic approach, which is vital for treatment (Hanewinckel et al., 2016). The symptoms often include sensory and motor dysfunctions, including numbness, pain, weakness and paresthesia due to damage to sensory, motor and autonomic fibers. Treatments for peripheral neuropathy are primarily dependent on the subtype and cause of underlying disease, such as grafts for traumatic nerve injury (Baradaran et al., 2021) and metabolic control for diabetic neuropathy (Cernea and Raz, 2021; Holmes and Hastings, 2021). Recently, with insights into cell-based therapy for diseases, emerging evidence has revealed the benefits of cell transplantation in peripheral neuropathic conditions (Hopf et al., 2020; Monje, 2020).
Peripheral neuropathies are affected by disorders of peripheral nerve fibers and cells (Hughes, 2002; Hanewinckel et al., 2016; Barrell and Smith, 2019; Hammi and Yeung, 2021). Schwann cells, which are the primary glial cells in the peripheral nervous system, are predominantly subdivided into myelinating and non-myelinating Schwann cells, both of which are associated with axons through physical support and the release of a variety of neurotrophins and many other signaling molecules during development (Kidd et al., 2013). Relatively large-diameter axons from most motor axons, some sensory axons and are enwrapped by Schwann cells, resulting in the establishment of compact myelin at a ratio of 1:1, which is needed for fast nerve conduction. Other small-diameter axons from autonomous and many sensory neurons, which are known as Remak bundles, are wrapped only by Schwann cells and are not myelinated (Griffin and Thompson, 2008). Schwann cells are recognized as flexible cells due to their capability for rapid transformation after injury (Jessen and Mirsky, 2016). In the injured microenvironment, myelinating Schwann cells and non-myelinating Remak Schwann cells coordinate to repair Schwann cells, resembling the developmental stage through the self-renewal and release of a variety of neurotrophic factors and signaling molecules involved in motor and sensory functional recovery (Stassart and Woodhoo, 2021). Therefore, the role of Schwann cells is pivotal for axonal functions both in physiological and pathological conditions, which leads to increasing attempts to prevent malfunction in Schwann cells or the supply Schwann cells/Schwann cell-like cells for the treatment of peripheral neuropathies (Brewer et al., 2016; Sayad Fathi and Zaminy, 2017; Wing et al., 2017; Al-Massri et al., 2020; Hopf et al., 2020; Monje, 2020). Schwann cells are known to originate from neural crest cells, which can be found in other tissues, such as the epidermis and hair follicle, and have great potential to generate Schwann cell-like cells (McKenzie et al., 2006; Lin et al., 2011). Moreover, with technical innovations in related stem cells, many types of stem cells can differentiate into Schwann cell-like cells or target the regulation of Schwann cells for motor and sensory functional recovery (Caddick et al., 2006; Park et al., 2010; Ma et al., 2015; Cai et al., 2017; Hopf et al., 2020). Thus, in this review, we will primarily discuss the potential applications of Schwann cells/Schwann cell-like cells in peripheral neuropathies induced by common disorders, including peripheral nerve injury, diabetes and chemotherapy, and the challenges for future clinical treatments (Figure 1).
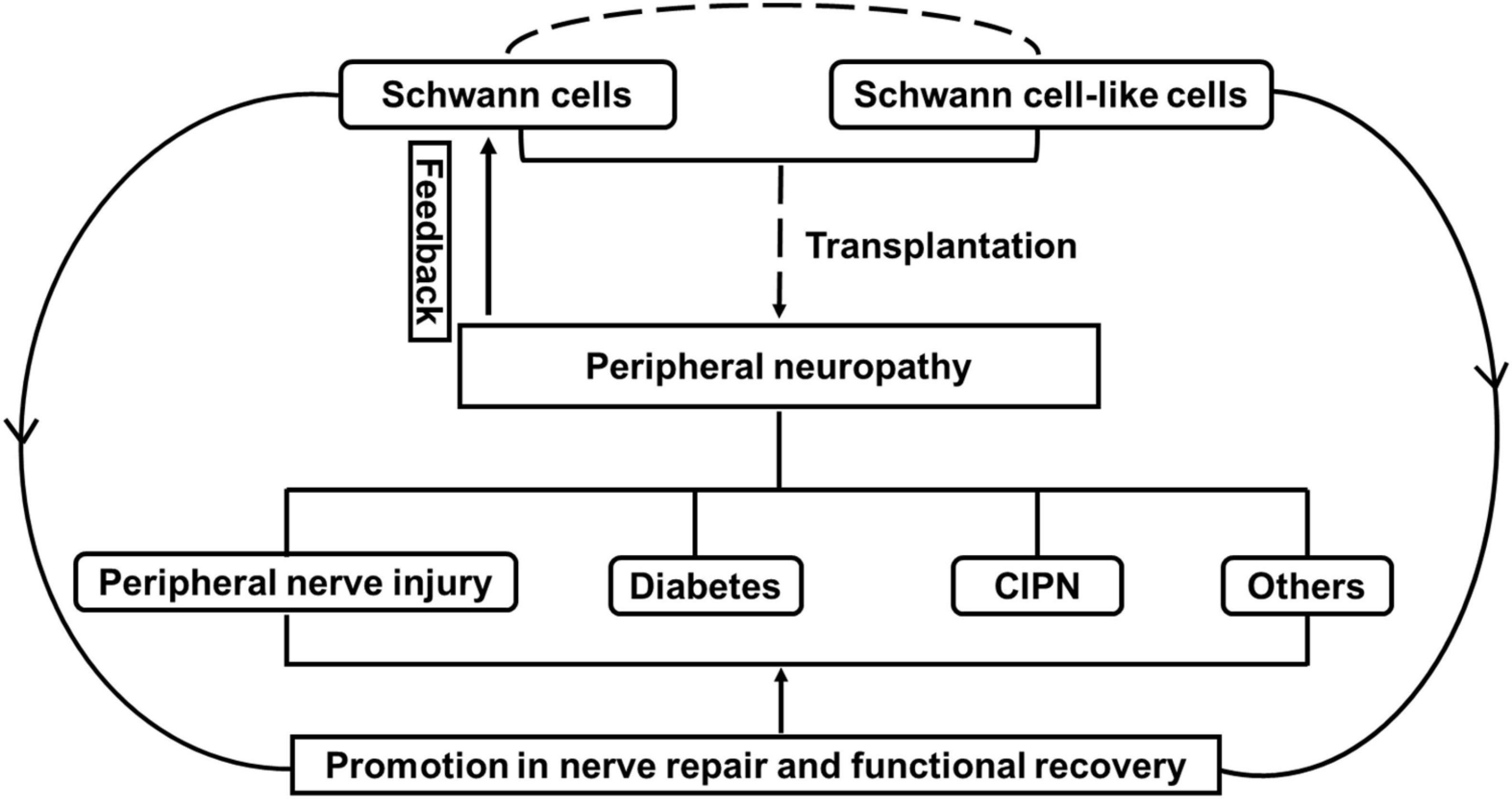
Figure 1. The effect of Schwann cells and Schwann cell-like cells on cell therapy for peripheral neuropathy. Note that peripheral neuropathies induced by peripheral nerve injury, diabetes and chemotherapy-induced peripheral neuropathy (CIPN) often leads to the malfunctional change in Schwann cells. Transplantation with Schwann cells or Schwann cell-like cells (from different sources) attempts to promote nerve repair and functional recovery through the effect of Schwann cells for the treatment of peripheral neuropathies.
Peripheral nerve injury is a common disease that results from trauma or disease and leads to damage to motor and sensor functions. Although the peripheral nervous system has the potential to self-repair nerve injury, peripheral nerve injury-induced neuropathy and lifelong disabilities for patients are common (Menorca et al., 2013). Insights into cellular and molecular mechanisms have revealed that modulating axons and Schwann cells are effective strategies for peripheral nerve injury-induced neuropathy. After injury, injured axons break and form debris in the distal stump, which is called Wallerian degeneration. This debris is segmented and incorporated by Schwann cells, and then phagocytized with the aid with the recruited macrophages (Nazareth et al., 2021). Once this debris is cleaned, the proximal stump will begin to outgrow. During this process, Schwann cells play an important role in the repair of peripheral nerve injury-induced neuropathy. Once axonal injury occurs, activated Schwann cells transform into a dedifferentiated state by expressing developmental genes, releasing various neurotrophic factors to create a reparative environment, and forming Büngner bands, which are a longitudinal column for guiding axonal regrowth through proliferation in the distal stump (Stassart and Woodhoo, 2021). However, this self-repair method is unable to guide axonal outgrowth and target innerved muscles due to a lack of an advantageous environment, which includes the dysfunction of Schwann cells (Lehmann and Hoke, 2016). Therefore, emerging evidence is focused on cell transplantation to supply Schwann cells or repair Schwann cells to promote axonal growth and motor and sensory restoration (Lehmann and Hoke, 2016; Hopf et al., 2020). Among these strategies, cell transplantation in combination with nerve scaffolds is a promising treatment for peripheral nerve injury-induced neuropathy (Rodriguez et al., 2000; Kornfeld et al., 2019). In the case of peripheral nerve injury, the gold standard treatment is end-to-end suturing of the proximal and distal parts by neurosurgical methods. However, this method is only useful for short gaps (<3 mm), and for longer gaps, a nerve or conduit graft is required to bridge the gap (Hopf et al., 2020). Thus, autologous Schwann cell transplantation is the best choice for treatment. However, these cells must be collected from healthy peripheral nerves and harvested in a time-consuming manner, and all of these limitations constrain their wide applications (Sullivan et al., 2016; Baradaran et al., 2021). Therefore, attention has moved toward the use of allogeneic Schwann cells and Schwann cell-like cells from stem cells to promote axonal regeneration and repair peripheral nerve injury-induced neuropathy (Sayad Fathi and Zaminy, 2017; Hopf et al., 2020; Kubiak et al., 2020). Here, we review current developments in Schwann cell or Schwann cell-like cell transplantations for the repair of peripheral nerve injury-induced neuropathy (Tables 1, 2).
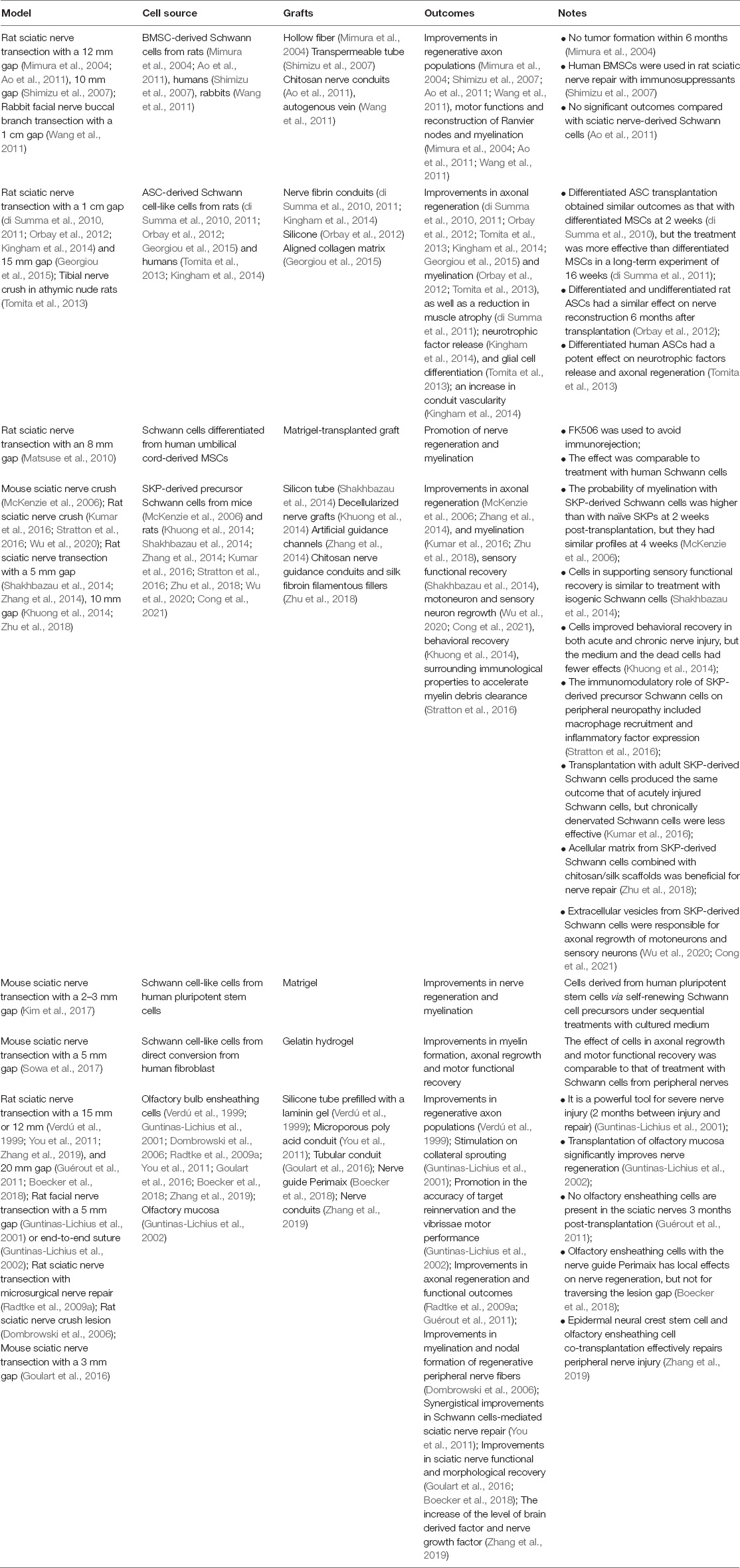
Table 2. The effect of Schwann cell-like cells on cell therapy for peripheral nerve injury-induced neuropathy.
In 1992, a study of the transplantation of autologous Schwann cells derived from adult nerves in permselective guidance channels to repair 8 mm nerve gaps in transected rat sciatic nerves indicated that this combination supported extensive regeneration and myelination. In contrast, a strong immune reaction occurred when heterologous Schwann cells were seeded, resulting in the prevention of nerve regeneration (Guenard et al., 1992). To avoid immune reactions, an immune-deficient rat was used, and the functional capacity of human Schwann cells in an 8 mm gap of transected sciatic nerves was evaluated. The outcomes showed that human Schwann cells could survive and effectively promote axonal regrowth and myelination but were less successful than allogeneic Schwann cells (Levi et al., 1994). A study aimed to evaluate the effect of allogeneic Schwann cell transplantation following rat sciatic nerve injury with a 10 mm gap and showed that compared with syngenetic Schwann cells, allogeneic Schwann cells also promoted axonal regeneration and myelination, but the effect was less than that of syngenetic Schwann cells, and an immune response occurred at 6 weeks post-transplantation when there was no use of immunosuppressive therapy (Mosahebi et al., 2002). In addition, a decellularizing approach has been developed to prevent rejection when allogeneic nerve grafts are applied to injured nerve repair (Hudson et al., 2004). However, due to the loss of Schwann cells, this method is less effective for nerve repair than contact nerves (Hoben et al., 2015). Of note, decellularized nerve conduits combined with Schwann cells to repair peripheral nerve injury obtained good results in non-human primate 6 cm ulnar nerve defects (Hess et al., 2007) and rat sciatic nerve defects (Aszmann et al., 2008; Sun et al., 2009; Hoben et al., 2015) and were demonstrated to be a better therapy than the addition of vascular endothelial growth factor to improve axonal regrowth (Hoben et al., 2015). In human studies (Levi et al., 2016; Gersey et al., 2017), Schwann cells were isolated from sural nerve biopsies and traumatized sciatic nerve stumps. After purification and proliferation, the cells were combined with sural nerve grafts to repair two cases of a 7.5 cm defect (case 1 with complete transection of sciatic nerves by a boat propeller injury) and a 5 cm defect (case two with partial damage of the tibial division of sciatic nerves by a gun wound of the leg). Follow-up was 36 months for the patient in case 1, and the patient regained proximal sensory recovery, including neuropathic pain, and motor function recovery in the common peroneal and tibial distribution (Levi et al., 2016). Twelve months post-operation, the patient in case 2 exhibited recovery of complete motor function and partial sensation in the tibial distribution (Gersey et al., 2017). Despite the fact that after injury, neurotrophic factor release from activated Schwann cells is beneficial for nerve regeneration and functional recovery, a study of allogeneic nerve grafts with Schwann cells overexpressing glial cell line-derived neurotrophic factors resulted in limited axonal regeneration and poor functional recovery (Santosa et al., 2013). Indeed, the timing, volume and distribution of these neurotrophic factors associated with postinjury Schwann cell behavior are critical for the rate of axonal regrowth and functional recovery (Kidd et al., 2013; Jessen and Mirsky, 2016). Due to the difficulty of harvesting human nerve-derived Schwann cells, skin-derived Schwann cells from patients were collected, and gene expression was characterized in human nerve-derived Schwann cells, and the feasibility of transplantation into injured mouse sciatic nerves was evaluated. The results demonstrated that adult human skin-derived Schwann cells were similar to human nerve-derived Schwann cells genetically and phenotypically, which indicates that a highly accessible source of autologous skin-derived Schwann cells may be a substitute for nerve-derived Schwann cells for injured nerve repair (Stratton et al., 2017). Both autogenous and allogenous nerve transplantation require nerve supply from the donor, which leads to donor-site morbidity resulting from the loss of nerves (Kim et al., 2020). A variety of conduits have been developed, including veins and synthetic grafts. Several studies (Chiu et al., 1982; Strauch et al., 1996) have used autogenous venous nerve conduits to successfully support axonal regeneration for short distances (less than a 3 cm gap). Moreover, conduit supplementation with autologous Schwann cells rapidly grew 6 cm peroneal nerve defect-injured nerves compared with treatment alone (Strauch et al., 2001). In addition, Schwann cells in a polyhydroxybutyrate conduit (Mosahebi et al., 2002; Tohill et al., 2004), fibrin conduit (di Summa et al., 2011) and poly (lactic-co-glycolic) acid conduit (Bryan et al., 2000) display more improvements in axonal regrowth and fiber myelination than the use of conduits alone. Based on a study comparing green fluorescent protein-labeled Schwann cells with non-transduced Schwann cells in bioengineered nerve conduits, the outcomes showed that both of treatments had similar growth characteristics (Tohill et al., 2004). Fluorescently labeled Schwann cells will be beneficial for monitoring Schwann cell behaviors and interactions with axons in bioengineered systems.
Schwann cells are primary glial cells in the peripheral nervous system, and autologous and allogeneic Schwann cells are thought to be good choices for the repair of injured nerves. However, because acquiring these Schwann cells from nerves is time-consuming and has secondary morbidity at the donor site, it is desirable to explore cell sources with similar potential to produce Schwann cells. Stem cells with wide distribution, multilineage potential and self-renewal capacity are highly suitable as alternative cell sources for Schwann cells (Sayad Fathi and Zaminy, 2017). Here, we emphasize the effect of stem cell-derived Schwann cell-like cells on cell therapy for peripheral neuropathy.
A major source of Schwann cell-like cells is mesenchymal stem/stromal cells (MSCs), which are readily isolated from a variety of tissues, including bone marrow, skin, adipose tissue and umbilical cord tissue (Sayad Fathi and Zaminy, 2017; Hopf et al., 2020). Bone marrow stromal cell (BMSC)-derived Schwann cells from rats (Mimura et al., 2004; Ao et al., 2011), humans (Shimizu et al., 2007) and rabbits (Wang et al., 2011) may mediate improvements in regenerative axon populations, motor functions and the reconstruction of Ranvier nodes and myelination in rat sciatic nerve transection with a 12 mm gap and a 10 mm gap, as well as in rabbit facial nerve buccal branch transection within a 1 cm gap. Moreover, tumor formation was not detected within 6 months (Mimura et al., 2004), and no significant outcomes occurred compared with treatment with sciatic nerve-derived Schwann cells (Ao et al., 2011). Differentiated adipose-derived stem cells (ASCs) are primarily derived from rats (di Summa et al., 2010, 2011; Orbay et al., 2012; Georgiou et al., 2015) and humans (Tomita et al., 2013; Kingham et al., 2014). Transplantation with these cells in nerve fibrin conduits, silicone or aligned collagen matrix has a potential role in the repair of peripheral neuropathy to improve neurotrophic factor release, axonal regrowth (di Summa et al., 2010, 2011; Orbay et al., 2012; Tomita et al., 2013; Kingham et al., 2014; Georgiou et al., 2015), myelination (Orbay et al., 2012; Tomita et al., 2013) and vascularity (Kingham et al., 2014), as well as reduce muscle atrophy (di Summa et al., 2011). Notably, differentiated and undifferentiated rat ASCs combined with silicone in rat sciatic nerve transection with a 1 cm gap had a similar effect on nerve reconstruction within 6 months (Orbay et al., 2012). However, transplantation with differentiated human ASCs in a nude rat tibial nerve crush model obtained a better outcome than the use of undifferentiated cells (Tomita et al., 2013). In contrast to the effect of undifferentiated ASCs, differentiated ASCs had a similar effect at 2 weeks post-transplantation but were more effective in a long-term experiment of 16 weeks (di Summa et al., 2010, 2011). Although there is no direct evidence of human umbilical cord blood-MSC-derived Schwann cell-like cells for treating peripheral neuropathy (Weiss and Troyer, 2006), Schwann cell-like cells obtained from the mesenchymal tissue surrounding umbilical cord vessels (Wharton jelly) were combined with Matrigel-transplanted grafts to repair rat sciatic nerve transection with an 8 mm gap with the immunosuppressor FK506. The effect was comparable to that of using human Schwann cells to promote nerve regeneration and myelination (Matsuse et al., 2010). Compared with other stem cells, skin-derived precursor cells (SKPs) are more accessible and readily differentiate into Schwann cells, and much more attention has been given to investigating their potential role in cell therapy in peripheral nerve injury-induced neuropathy. These cells are widely used to repair rodent sciatic nerve crush or transection with 5 or 10 mm gaps combined with different kinds of grafts, including silicon tubes (Shakhbazau et al., 2014), decellularized nerve grafts (Khuong et al., 2014), artificial guidance channels (Zhang et al., 2014), and chitosan/silk scaffolds (Zhu et al., 2018). After transplantation, these cells improve sensory functional and behavioral recovery in both acute (4 weeks) and chronic (17 weeks) nerve injury (Khuong et al., 2014; Shakhbazau et al., 2014), axonal regeneration and myelination in vivo (McKenzie et al., 2006; Zhang et al., 2014; Kumar et al., 2016; Zhu et al., 2018), and motoneuron and sensory neuron regrowth in vitro (Wu et al., 2020; Cong et al., 2021). Moreover, they can adjust surrounding immunological properties to accelerate myelin debris clearance by recruiting many more macrophages and enhancing inflammatory factor expression (Stratton et al., 2016). The myelination of these cells is higher than that of naïve SKPs in the early stage (McKenzie et al., 2006), and their ability to support sensory functional recovery is equal to or better than that of treatments with isogenic Schwann cells (Shakhbazau et al., 2014). In addition to the great effect of the cells by themselves, acellular matrix and extracellular vesicles from SKP-derived cells are also responsible for neuronal regrowth in vitro (Wu et al., 2020; Cong et al., 2021), but dead cells or the medium was less effective on nerve repair in vivo (Khuong et al., 2014). Although great improvements have been obtained with rat/mouse SKP-derived Schwann cell-like cells, the clinical application of human SKPs still needs many more studies to test the utility of cells from different anatomical regions (Dai et al., 2018). In addition, human pluripotent stem cells can be differentiated into Schwann cell-like cells via self-renewing Schwann cell precursor cells through sequential treatment with conditioned medium in vitro, and the combination with Matrigel successfully improves axonal regeneration and myelin repair (Kim et al., 2017). Another interesting source of Schwann cell-like cells is human fibroblasts, which can be converted with a cellular reprogramming strategy. In vitro and in vivo experiments with gelatin hydrogel showed a potential role of converted Schwann cells in significantly enhancing axonal regrowth, myelin repair and motor functional recovery, which is comparable to treatment with Schwann cells from peripheral nerves (Sowa et al., 2017).
In addition to these Schwann cell-like cells, olfactory ensheathing cells from olfactory bulb and mucosa share many properties with Schwann cells which include the support of axonal regeneration and myelination (Doucette, 1990). They also exhibit great potentials for nerve repair in peripheral nerve injury-induced neuropathy (Radtke et al., 2009b,2011; Radtke and Kocsis, 2012, 2014). Olfactory bulb ensheathing cells from mouse (Goulart et al., 2016) and rat mediate improvements in axonal regeneration, myelination and sciatic nerve functional recovery in mouse sciatic nerve transection with a 3 mm gap, and in rat sciatic nerve transection with a 15 or 12 mm gap (Verdú et al., 1999; You et al., 2011; Zhang et al., 2019), and 20 mm gap (Guérout et al., 2011; Boecker et al., 2018), and with microsurgical nerve repair (Radtke et al., 2009a) and in rat sciatic nerve crush lesion (Dombrowski et al., 2006), as well as in rat facial nerve transection with a 5 mm gap (Guntinas-Lichius et al., 2001). As well, transplantation of olfactory mucosa significantly increases the accuracy of target reinnervation and accelerates the vibrissae movements (Guntinas-Lichius et al., 2002). Notably, olfactory ensheathing cell and Schwann cell, or and epidermal neural crest stem cell co-transplantation effectively enhance anatomical and functional repair after sciatic nerve injury in rats (You et al., 2011; Zhang et al., 2019) through enhancing the level of brain derived factor and nerve growth factor, which indicates that cells co-transplantation may serve as a new method for PNI in future therapies.
Diabetic neuropathy is one of the most common complications of diabetic patients. With the incidences of diabetes increasing annually, especially in type 2 diabetes, studies are focused on understanding the pathogenic mechanisms, most of which are associated with neurons and vessels (Kim et al., 2012). However, accumulating evidence indicates the effect on morphological alterations and dysfunction in Schwann cells following diabetic neuropathy (Mizisin, 2014; Naruse, 2019). Studies on the sural nerves of rodents, cats and patients with diabetic neuropathy indicate that an apparently normal axon is wrapped by an abnormal myelin sheath resulting from segmental demyelination and remyelination (Thomas and Lascelles, 1965; Sima et al., 1988; Malik et al., 2005; Lennertz et al., 2011). In addition, the ultrastructure of abnormal Schwann cells showed mitochondrial enlargement with numerous vacuoles, cytoplasmic expansion, glycogen inclusion, and hyperplasia of the basement membrane (Yagihashi and Matsunaga, 1979; Chowdhury et al., 2013; Mizisin, 2014). Metabolic and molecular perturbations of Schwann cells in diabetic neuropathy include high activity of aldose reductase-mediated polyol pathway flux, oxidative stress and inflammation, as well as damage associated with microvascular changes in Schwann cells, all of which result in decreased neurotrophic factors release and the accumulation of neurotoxic intermediates leading to the dysfunction of interactions between Schwann cells and axons and diabetic neuropathy (Gonçalves et al., 2017, 2018; Naruse, 2019). Therefore, treating Schwann cells offers a potential strategy for diabetic neuropathy. Here, we primarily review the role of Schwann cells in cell therapy for diabetic neuropathy.
As shown in Table 3, different sources of stem cells exhibit potential of treating diabetic neuropathy and have an effect on the function of Schwann cells. BM-derived cells, endothelial progenitor cells (EPCs), and mononuclear cells (MNCs) can effectively reverse the symptoms of diabetic neuropathy through neuroprotective effects and neovascularization (Naruse et al., 2005; Hasegawa et al., 2006; Jeong et al., 2009; Kim et al., 2009). During this process, these neurotrophic and angiogenic factors suppress Schwann cell apoptosis and enhance Schwann cell proliferation and myelination (Jeong et al., 2009; Kim et al., 2012). In addition, treatment with BMSCs in hindlimb muscles, which can be differentiated into Schwann cell-like cells (Caddick et al., 2006), can ameliorate diabetic neuropathy symptoms, such as dysfunction of sensory and motor nerves, as well as demyelination in streptozotocin (STZ)-induced diabetic rats (Han et al., 2016). In addition to BMSCs, ASCs were transplanted by intramuscular injection and had a positive effect on the repair of STZ-induced diabetic neuropathy through the regulation of Schwann cell-related neurotrophic factor expression and remyelination (Yigitturk et al., 2021). Compared with stem cell-based treatment for diabetic neuropathy, additional studies have been performed with dental pulp stem cells (DPSCs). After human DPSCs were injected into the hindlimb skeletal muscle of diabetic mice, increases in vascular endothelial growth factor and nerve growth factor were detected at the injection site, while antibody neutralization reversed the effect of human DPSCs (Hata et al., 2020). Moreover, in STZ-induced diabetic rats, rat DPSCs ameliorated long-term (52 weeks) diabetic neuropathy (Omi et al., 2017). Although GFP-labeled rat DPSCs did not differentiate into Schwann cells after being injected into skeletal muscles (Hata et al., 2015), they had a beneficial effect on Schwann cells, including increasing Schwann cell viability and myelin formation (Omi et al., 2017). Of note, there was no difference in the therapeutic effect on diabetic neuropathy between the injection of rat DPSC-secreted factors and DPSCs (Kanada et al., 2020), and DPSC-secreted factors promoted Schwann cell proliferation and myelin formation (Omi et al., 2017). Conditioned medium from ASCs was also beneficial in preventing foot ulcer formation, ameliorating diabetic neuropathy in diabetic BKS db/db mice, and blocking diabetes-induced Schwann cell apoptosis (De Gregorio et al., 2020). Human DPSCs were used to treat a rat model of diabetic neuropathy through intramuscular or intravenous administration of one or two rounds of transplantation were helpful in contributing to functional recovery, but repeated doses via the intramuscular route was the most effective (Datta et al., 2017), which indicates that different routes and doses produce different effects. Neural crest can differentiate into multiple types of cells, including Schwann cells and peripheral neurons (Bronner and LeDouarin, 2012). However, there has only been one study using neural crest-like cells derived from induced pluripotent stem cells to treat STZ-induced diabetic mice, and the transplanted cells differentiated into Schwann cell-like cells or vascular smooth muscle cells to effectively improve the impaired vascular and neuronal functions (Okawa et al., 2013). Although the effect of Schwann cells as a cell therapy needs further study, Schwann cells are a key player in the treatment of diabetic neuropathy through cell transplantation.
Chemotherapy-induced peripheral neuropathy (CIPN) is the most common secondary effect in cancer patients who receive chemotherapy treatment. Signs of damage to peripheral nerves in CIPN are associated with sensory abnormalities, including allodynia (loss of touch sensation, numbness) or hyperalgesia (pin sensation and tingling), and often manifest as glove-stocking distributions (Bobylev et al., 2015). Some patients also exhibit motor nerve damage and altered musculoskeletal adverse effects (Ibrahim and Ehrlich, 2020). With the increasing numbers of cancer survivors and no ways to predict who will develop symptoms or when, there are no effective approved drugs to prevent or reduce CIPN (Carozzi et al., 2015; Jordan et al., 2019). Therefore, the management of CIPN is still a major challenge for clinical treatment. Despite the lack of direct evidence to illustrate the role of Schwann cell transplantation in CIPN, more attention has been given to the impairment of Schwann cells by chemotherapeutic agents and stem cell therapy for CIPN (Table 4; Al-Massri et al., 2020; Ibrahim and Ehrlich, 2020).
Bortezomib, a proteasome inhibitor, is widely used in the treatment of multiple myeloma and induces axonal-dependent sensory damage and pathological responses in Schwann cells. Bortezomib-treated Schwann cells were analyzed by gene expression microarray, and the results indicated endoplasmic reticulum damage to Schwann cells accompanied by the downregulation of myelin-related genes, which was verified in a patient with high-dose bortezomib-induced peripheral neuropathy (Filosto et al., 2007; Shin et al., 2010). In contrast, compared with that in dorsal rooting ganglion neurons, a lower dose of oxaliplatin, cisplatin or paclitaxel is required in cultured Schwann cells because of cytotoxicity, and these drugs have a negative effect on myelin formation in cocultures but do not affect neurons, which indicates that Schwann cells are more susceptible to CIPN than other cells. Surprisingly, mitochondrial dysfunction occurs in cisplatin- and oxaliplatin-treated Schwann cells but not in paclitaxel-treated Schwann cells, while only paclitaxel induces Schwann cell dedifferentiation (Imai et al., 2017). Consistently, Schwann cell dedifferentiation occurs in epirubicin-docetaxel-induced CIPN, and this effect is suppressed by concomitant duloxetine-allopregnanolone treatment (Matta et al., 2020).
Mesenchymal stem/stromal cell therapy, which is a potential strategy for CIPN treatment, has a beneficial effect on improving symptoms (Al-Massri et al., 2020). MSC treatment could protect both sensory and motor neurons and enhance the efficacy of pregabalin in paclitaxel-induced peripheral neuropathy (Al-Massri et al., 2019). Notably, nasal administration of MSC-based therapy reverses cisplatin- or paclitaxel-induced peripheral neuropathy by Boukelmoune et al. (2021). In addition, ASCs also have a positive role in alleviating oxaliplatin-induced peripheral neuropathy (Di Cesare Mannelli et al., 2018). Induced pluripotent stem cells (iPSCs) can serve as a new method to estimate the neurotoxicity associated with chemotherapy treatment (Wheeler et al., 2015; Wing et al., 2017). The mechanisms of MSC-based therapies, including whether MSCs can differentiate into Schwann-like cells, need further study. MSC-based cell therapy may be a promising strategy for patients suffering from the adverse effects of cancer treatment.
Emerging evidence has demonstrated the important role of Schwann cell/Schwann cell-like cell therapy in alleviating peripheral neuropathy, but a variety of challenges still need to be investigated. The source of both autological and allogenic Schwann cells are primarily nerve biopsies and traumatized nerve stumps, all of which will result in the innervation of anatomical regions for the donor and undesired morbidities. Moreover, nerve-derived Schwann cells need a long expansion time in vitro to produce a large number of cells. The time between injury and transplantation with Schwann cells should be minimized to protect patients from a series of secondary injuries, including muscle degeneration and functional loss. Given these limitations, Schwann cell-like cells from stem cells have become a relatively robust alternative cell for the repair of peripheral neuropathy. The therapeutic application of pluripotent stem cells is associated with safety and technical and ethical constraints compared with other stem cell types, such as BM-MSCs, dM-MSCs and SKPs. However, these cells have a long differentiation time after isolation and can delay treatment, resulting in further damage to the patient. Highly efficient methods for in vitro differentiation and characterization of Schwann cell-like cells may support future clinical applications. On the other hand, direct transplantation with these stem cells, followed by in vivo differentiation associated with the pathological stage of peripheral neuropathy, may become a promising and attractive therapeutic strategy. In addition, in patients transplanted with allogenic Schwann cells or Schwann cell-like cells, drugs still need to be used to avoid immune rejection and potential side effects.
Although there is a large amount of evidence on the role of Schwann cell-like cells in peripheral neuropathy in rodent animal models, including peripheral nerve injury, diabetes and chemotherapy, until now, no direct clinical trials have been developed with these cells. However, for spinal cord injury, several studies reported the therapeutic benefits of treatment with these cells. Most studies have been focused on evaluating safety and adverse events after transplantation (Yazdani et al., 2013; Mendonca et al., 2014; Anderson et al., 2017; Gant et al., 2021). Notably, single MSC administration is safe but less effective than combination treatment with autologous Schwann cells (Oh et al., 2016), which improves sensory and motor functional recovery to some extent, as well as bladder compliance (Oraee-Yazdani et al., 2016, 2021). In addition, the administration of these cells by intravenous infusion, intrathecal administration or direct injection into spinal lesions, and the injury level and size may lead to different outcomes. Due to the effect of advanced age (Tong et al., 2015; Liu et al., 2018) and sexual dimorphism (Magnaghi et al., 2006; Stenberg and Dahlin, 2014) on the characterization of Schwann cells, these factors need to be taken into consideration when choosing a therapeutic strategy. Therefore, many more preclinical studies with cell therapies are needed prior to clinical application.
Schwann cells release a variety of signaling molecules under both physiological and pathological conditions to promote neuronal development and postinjury regeneration (Monje, 2020). Therefore, whether appropriate signaling molecules or drugs are administered in combination with transplanted Schwann cells still needs to be carefully assessed (Balakrishnan et al., 2020). In addition to the variability in the repair response between rodent and human models, relatively long-distance transection or injury occurs in humans compared with rodent models, and a slow rate of nerve regeneration requires a longer time for transplanted Schwann cells or Schwann cell-like cells to support and remyelinate the regenerated axons (Balakrishnan et al., 2020).
In summary, although a great number of challenges remain to be addressed, a growing body of evidence demonstrates the beneficial therapeutic roles of Schwann cells and Schwann cell-like cells in peripheral neuropathy. With deeper insights into the pathology of peripheral neuropathy-related disorders, including peripheral nerve injury, diabetes and chemotherapy, as well as the development of bioengineering systems, Schwann cell-based therapy will soon be a more attractive and effective strategy for treating peripheral neuropathy.
Z-YW and GC conceptualized the topics. Z-YW, QW, Z-ML, F-YC, W-FS, and Y-YZ wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (31900718 and 32070998), Basic Research Program of the Education Department of Jiangsu Province (19KJB180024), Postdoctoral Science Foundation of China (2019M651925); The Key Research and Development of Jiangsu Province (BE2020667); The Foundation of Jiangsu Province “333 Project High-level Talents” (BRA2020076), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Massri, K. F., Ahmed, L. A., and El-Abhar, H. S. (2019). Mesenchymal stem cells therapy enhances the efficacy of pregabalin and prevents its motor impairment in paclitaxel-induced neuropathy in rats: role of Notch1 receptor and JAK/STAT signaling pathway. Behav. Brain Res. 360, 303–311. doi: 10.1016/j.bbr.2018.12.013
Al-Massri, K. F., Ahmed, L. A., and El-Abhar, H. S. (2020). Mesenchymal stem cells in chemotherapy-induced peripheral neuropathy: a new challenging approach that requires further investigations. J. Tissue Eng. Regen. Med. 14, 108–122. doi: 10.1002/term.2972
Anderson, K. D., Guest, J. D., Dietrich, W. D., Bartlett Bunge, M., Curiel, R., Dididze, M., et al. (2017). Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma 34, 2950–2963. doi: 10.1089/neu.2016.4895
Ao, Q., Fung, C. K., Tsui, A. Y., Cai, S., Zuo, H. C., Chan, Y. S., et al. (2011). The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials 32, 787–796. doi: 10.1016/j.biomaterials.2010.09.046
Aszmann, O. C., Korak, K. J., Luegmair, M., and Frey, M. (2008). Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J. Reconstr. Microsurg. 24, 151–158. doi: 10.1055/s-2008-1076091
Balakrishnan, A., Belfiore, L., Chu, T. H., Fleming, T., Midha, R., Biernaskie, J., et al. (2020). Insights into the role and potential of schwann cells for peripheral nerve repair from studies of development and injury. Front. Mol. Neurosci. 13:608442. doi: 10.3389/fnmol.2020.608442
Baradaran, A., El-Hawary, H., Efanov, J. I., and Xu, L. (2021). Peripheral nerve healing: so near and yet so far. Semin. Plast. Surg. 35, 204–210. doi: 10.1055/s-0041-1731630
Barrell, K., and Smith, A. G. (2019). Peripheral Neuropathy. Med. Clin. North Am. 103, 383–397. doi: 10.1016/j.mcna.2018.10.006
Bobylev, I., Elter, T., Schneider, C., Wunderlich, G., Zimmer, P., Streckmann, F., et al. (2015). [Chemotherapy-induced Peripheral Neuropathy]. Fortschr. Neurol. Psychiatr. 83, 427–436. doi: 10.1055/s-0035-1553475
Boecker, A. H., Bozkurt, A., Kim, B. S., Altinova, H., Tank, J., Deumens, R., et al. (2018). Cell-enrichment with olfactory ensheathing cells has limited local extra beneficial effects on nerve regeneration supported by the nerve guide Perimaix. J. Tissue Eng. Regen. Med. 12, 2125–2137. doi: 10.1002/term.2731
Boukelmoune, N., Laumet, G., Tang, Y., Ma, J., Mahant, I., Singh, S. K., et al. (2021). Nasal administration of mesenchymal stem cells reverses chemotherapy-induced peripheral neuropathy in mice. Brain Beha. Immun. 93, 43–54. doi: 10.1016/j.bbi.2020.12.011
Brewer, J. R., Morrison, G., Dolan, M. E., and Fleming, G. F. (2016). Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol. Oncol. 140, 176–183. doi: 10.1016/j.ygyno.2015.11.011
Bronner, M. E., and LeDouarin, N. M. (2012). Development and evolution of the neural crest: an overview. Dev. Biol. 366, 2–9. doi: 10.1016/j.ydbio.2011.12.042
Bryan, D. J., Holway, A. H., Wang, K. K., Silva, A. E., Trantolo, D. J., Wise, D., et al. (2000). Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 6, 129–138. doi: 10.1089/107632700320757
Caddick, J., Kingham, P. J., Gardiner, N. J., Wiberg, M., and Terenghi, G. (2006). Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 54, 840–849. doi: 10.1002/glia.20421
Cai, S., Tsui, Y. P., Tam, K. W., Shea, G. K., Chang, R. S., Ao, Q., et al. (2017). Directed differentiation of human bone marrow stromal cells to fate-committed schwann cells. Stem Cell Rep. 9, 1097–1108. doi: 10.1016/j.stemcr.2017.08.004
Carozzi, V. A., Canta, A., and Chiorazzi, A. (2015). Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 596, 90–107. doi: 10.1016/j.neulet.2014.10.014
Cernea, S., and Raz, I. (2021). Management of diabetic neuropathy. Metabolism 123:154867. doi: 10.1016/j.metabol.2021.154867
Chiu, D. T., Janecka, I., Krizek, T. J., Wolff, M., and Lovelace, R. E. (1982). Autogenous vein graft as a conduit for nerve regeneration. Surgery 91, 226–233.
Chowdhury, S. K., Smith, D. R., and Fernyhough, P. (2013). The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol. Dis. 51, 56–65. doi: 10.1016/j.nbd.2012.03.016
Cong, M., Shen, M., Wu, X., Li, Y., Wang, L., He, Q., et al. (2021). Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res. Ther. 12:80. doi: 10.1186/s13287-020-02125-4
Dai, R., Hua, W., Xie, H., Chen, W., Xiong, L., and Li, L. (2018). The human skin-derived precursors for regenerative medicine: current state, challenges, and perspectives. Stem Cells Int. 2018, 1–11. doi: 10.1155/2018/8637812
Datta, I., Bhadri, N., Shahani, P., Majumdar, D., Sowmithra, S., Razdan, R., et al. (2017). Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 19, 1208–1224. doi: 10.1016/j.jcyt.2017.07.009
De Gregorio, C., Contador, D., Diaz, D., Carcamo, C., Santapau, D., Lobos-Gonzalez, L., et al. (2020). Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 11:168. doi: 10.1186/s13287-020-01680-0
Di Cesare Mannelli, L., Tenci, B., Micheli, L., Vona, A., Corti, F., Zanardelli, M., et al. (2018). Adipose-derived stem cells decrease pain in a rat model of oxaliplatin-induced neuropathy: role of VEGF-A modulation. Neuropharmacology 131, 166–175. doi: 10.1016/j.neuropharm.2017.12.020
di Summa, P. G., Kalbermatten, D. F., Pralong, E., Raffoul, W., Kingham, P. J., and Terenghi, G. (2011). Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181, 278–291. doi: 10.1016/j.neuroscience.2011.02.052
di Summa, P. G., Kingham, P. J., Raffoul, W., Wiberg, M., Terenghi, G., and Kalbermatten, D. F. (2010). Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 63, 1544–1552. doi: 10.1016/j.bjps.2009.09.012
Dombrowski, M. A., Sasaki, M., Lankford, K. L., Kocsis, J. D., and Radtke, C. (2006). Myelination and nodal formation of regenerated peripheral nerve fibers following transplantation of acutely prepared olfactory ensheathing cells. Brain Res. 1125, 1–8. doi: 10.1016/j.brainres.2006.09.089
Doucette, R. (1990). Glial influences on axonal growth in the primary olfactory system. Glia 3, 433–449. doi: 10.1002/glia.440030602
Filosto, M., Rossi, G., Pelizzari, A. M., Buzio, S., Tentorio, M., Broglio, L., et al. (2007). A high-dose bortezomib neuropathy with sensory ataxia and myelin involvement. J. Neurol. Sci. 263, 40–43. doi: 10.1016/j.jns.2007.05.023
Gant, K. L., Guest, J. D., Palermo, A. E., Vedantam, A., Jimsheleishvili, G., Bunge, M. B., et al. (2021). Phase 1 safety trial of autologous human schwann cell transplantation in chronic spinal cord injury. J. Neurotrauma 39, 285–299. doi: 10.1089/neu.2020.7590
Georgiou, M., Golding, J. P., Loughlin, A. J., Kingham, P. J., and Phillips, J. B. (2015). Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 37, 242–251. doi: 10.1016/j.biomaterials.2014.10.009
Gersey, Z. C., Burks, S. S., Anderson, K. D., Dididze, M., Khan, A., Dietrich, W. D., et al. (2017). First human experience with autologous Schwann cells to supplement sciatic nerve repair: report of 2 cases with long-term follow-up. Neurosurg. Focus. 42:E2. doi: 10.3171/2016.12.FOCUS16474
Gonçalves, N. P., Vægter, C. B., Andersen, H., Østergaard, L., Calcutt, N. A., and Jensen, T. S. (2017). Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 13, 135–147. doi: 10.1038/nrneurol.2016.201
Gonçalves, N. P., Vægter, C. B., and Pallesen, L. T. (2018). Peripheral glial cells in the development of diabetic neuropathy. Front. Neurol. 9:268. doi: 10.3389/fneur.2018.00268
Goulart, C. O., Ângelo Durço, D. F., de Carvalho, L. A., Oliveira, J. T., Alves, L., Cavalcante, L. A., et al. (2016). Olfactory ensheathing glia cell therapy and tubular conduit enhance nerve regeneration after mouse sciatic nerve transection. Brain Res. 1650, 243–251. doi: 10.1016/j.brainres.2016.09.021
Griffin, J. W., and Thompson, W. J. (2008). Biology and pathology of nonmyelinating Schwann cells. Glia 56, 1518–1531. doi: 10.1002/glia.20778
Guenard, V., Kleitman, N., Morrissey, T. K., Bunge, R. P., and Aebischer, P. (1992). Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J. Neurosci. 12, 3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992
Guérout, N., Duclos, C., Drouot, L., Abramovici, O., Bon-Mardion, N., Lacoume, Y., et al. (2011). Transplantation of olfactory ensheathing cells promotes axonal regeneration and functional recovery of peripheral nerve lesion in rats. Muscle Nerve 43, 543–551. doi: 10.1002/mus.21907
Guntinas-Lichius, O., Angelov, D. N., Tomov, T. L., Dramiga, J., Neiss, W. F., and Wewetzer, K. (2001). Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp. Neurol. 172, 70–80. doi: 10.1006/exnr.2001.7774
Guntinas-Lichius, O., Wewetzer, K., Tomov, T. L., Azzolin, N., Kazemi, S., Streppel, M., et al. (2002). Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J. Neurosci. 22, 7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002
Hammi, C., and Yeung, B. (2021). Neuropathy. StatPearls. Treasure Island, FL: StatPearls Publishing.
Han, J. W., Choi, D., Lee, M. Y., Huh, Y. H., and Yoon, Y. S. (2016). Bone marrow-derived mesenchymal stem cells improve diabetic neuropathy by direct modulation of both angiogenesis and myelination in peripheral nerves. Cell Transplant. 25, 313–326. doi: 10.3727/096368915X688209
Hanewinckel, R., Ikram, M. A., and Van Doorn, P. A. (2016). Peripheral neuropathies. Handb. Clin. Neurol. 138, 263–282. doi: 10.1016/B978-0-12-802973-2.00015-X
Hasegawa, T., Kosaki, A., Shimizu, K., Matsubara, H., Mori, Y., Masaki, H., et al. (2006). Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp. Neurol. 199, 274–280. doi: 10.1016/j.expneurol.2005.11.001
Hata, M., Omi, M., Kobayashi, Y., Nakamura, N., Miyabe, M., Ito, M., et al. (2020). Transplantation of human dental pulp stem cells ameliorates diabetic polyneuropathy in streptozotocin-induced diabetic nude mice: the role of angiogenic and neurotrophic factors. Stem Cell Res. Ther. 11:236. doi: 10.1186/s13287-020-01758-9
Hata, M., Omi, M., Kobayashi, Y., Nakamura, N., Tosaki, T., Miyabe, M., et al. (2015). Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res Ther. 6:162. doi: 10.1186/s13287-015-0156-4
Hess, J. R., Brenner, M. J., Fox, I. K., Nichols, C. M., Myckatyn, T. M., Hunter, D. A., et al. (2007). Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast. Reconstr. Surg. 119, 246–259. doi: 10.1097/01.prs.0000245341.71666.97
Hoben, G., Yan, Y., Iyer, N., Newton, P., Hunter, D. A., Moore, A. M., et al. (2015). Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand 10, 396–402. doi: 10.1007/s11552-014-9720-0
Holmes, C. J., and Hastings, M. K. (2021). The application of exercise training for diabetic peripheral neuropathy. J. Clin. Med. 10:5042. doi: 10.3390/jcm10215042
Hopf, A., Schaefer, D. J., Kalbermatten, D. F., Guzman, R., and Madduri, S. (2020). Schwann cell-like cells: origin and usability for repair and regeneration of the peripheral and central nervous system. Cells 9:1990. doi: 10.3390/cells9091990
Hudson, T. W., Zawko, S., Deister, C., Lundy, S., Hu, C. Y., Lee, K., et al. (2004). Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 10, 1641–1651. doi: 10.1089/ten.2004.10.1641
Ibrahim, E. Y., and Ehrlich, B. E. (2020). Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit. Rev. Oncol. Hematol. 145:102831. doi: 10.1016/j.critrevonc.2019.102831
Imai, S., Koyanagi, M., Azimi, Z., Nakazato, Y., Matsumoto, M., Ogihara, T., et al. (2017). Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci. Rep. 7:5947. doi: 10.1038/s41598-017-05784-1
Jeong, J. O., Kim, M. O., Kim, H., Lee, M. Y., Kim, S. W., Ii, M., et al. (2009). Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation 119, 699–708. doi: 10.1161/CIRCULATIONAHA.108.789297
Jessen, K. R., and Mirsky, R. (2016). The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531. doi: 10.1113/JP270874
Jordan, B., Jahn, F., Sauer, S., and Jordan, K. (2019). Prevention and management of chemotherapy-induced polyneuropathy. Breast Care 14, 79–84. doi: 10.1159/000499599
Kanada, S., Makino, E., Nakamura, N., Miyabe, M., Ito, M., Hata, M., et al. (2020). Direct comparison of therapeutic effects on diabetic polyneuropathy between transplantation of dental pulp stem cells and administration of dental pulp stem cell-secreted factors. Int. J. Mol. Sci. 21:6064. doi: 10.3390/ijms21176064
Khuong, H. T., Kumar, R., Senjaya, F., Grochmal, J., Ivanovic, A., Shakhbazau, A., et al. (2014). Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 254, 168–179. doi: 10.1016/j.expneurol.2014.01.002
Kidd, G. J., Ohno, N., and Trapp, B. D. (2013). Biology of Schwann cells. Handb. Clin. Neurol. 115, 55–79. doi: 10.1016/B978-0-444-52902-2.00005-9
Kim, H., Kim, J. J., and Yoon, Y. S. (2012). Emerging therapy for diabetic neuropathy: cell therapy targeting vessels and nerves. Endocr. Metab. Immune. Disord. Drug Targets 12, 168–178. doi: 10.2174/187153012800493486
Kim, H., Park, J. S., Choi, Y. J., Kim, M. O., Huh, Y. H., Kim, S. W., et al. (2009). Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells 27, 1686–1696. doi: 10.1002/stem.87
Kim, H. S., Kim, J. Y., Song, C. L., Jeong, J. E., and Cho, Y. S. (2020). Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res. Ther. 11:257. doi: 10.1186/s13287-020-01772-x
Kim, H. S., Lee, J., Lee, D. Y., Kim, Y. D., Kim, J. Y., Lim, H. J., et al. (2017). Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Rep. 8, 1714–1726. doi: 10.1016/j.stemcr.2017.04.011
Kingham, P. J., Kolar, M. K., Novikova, L. N., Novikov, L. N., and Wiberg, M. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 23, 741–754. doi: 10.1089/scd.2013.0396
Kornfeld, T., Vogt, P. M., and Radtke, C. (2019). Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med. Wochenschr. 169, 240–251. doi: 10.1007/s10354-018-0675-6
Kubiak, C. A., Grochmal, J., Kung, T. A., Cederna, P. S., Midha, R., and Kemp, S. W. P. (2020). Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 61, 449–459. doi: 10.1002/mus.26760
Kumar, R., Sinha, S., Hagner, A., Stykel, M., Raharjo, E., Singh, K. K., et al. (2016). Adult skin-derived precursor Schwann cells exhibit superior myelination and regeneration supportive properties compared to chronically denervated nerve-derived Schwann cells. Exp. Neurol. 278, 127–142. doi: 10.1016/j.expneurol.2016.02.006
Lehmann, H. C., and Hoke, A. (2016). Use of engineered Schwann cells in peripheral neuropathy: hopes and hazards. Brain Res. 1638(Pt A), 97–104. doi: 10.1016/j.brainres.2015.10.040
Lennertz, R. C., Medler, K. A., Bain, J. L., Wright, D. E., and Stucky, C. L. (2011). Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J. Neurophysiol. 106, 905–914. doi: 10.1152/jn.01123.2010
Levi, A. D., Burks, S. S., Anderson, K. D., Dididze, M., Khan, A., and Dietrich, W. D. (2016). The use of autologous schwann cells to supplement sciatic nerve repair with a large gap: first in human experience. Cell Transplant. 25, 1395–1403. doi: 10.3727/096368915X690198
Levi, A. D., Guenard, V., Aebischer, P., and Bunge, R. P. (1994). The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J. Neurosci. 14(3 Pt 1), 1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994
Lin, H., Liu, F., Zhang, C., Zhang, Z., Kong, Z., Zhang, X., et al. (2011). Characterization of nerve conduits seeded with neurons and Schwann cells derived from hair follicle neural crest stem cells. Tissue Eng. Part A 17, 1691–1698. doi: 10.1089/ten.tea.2010.0514
Liu, J. H., Tang, Q., Liu, X. X., Qi, J., Zeng, R. X., Zhu, Z. W., et al. (2018). Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages. Neural Regen. Res. 13, 2182–2190. doi: 10.4103/1673-5374.241469
Ma, M. S., Boddeke, E., and Copray, S. (2015). Pluripotent stem cells for Schwann cell engineering. Stem Cell Rev. Rep. 11, 205–218. doi: 10.1007/s12015-014-9577-1
Magnaghi, V., Veiga, S., Ballabio, M., Gonzalez, L. C., Garcia-Segura, L. M., and Melcangi, R. C. (2006). Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J. Peripher. Nerv. Syst. 11, 111–118. doi: 10.1111/j.1085-9489.2006.00075.x
Malik, R. A., Tesfaye, S., Newrick, P. G., Walker, D., Rajbhandari, S. M., Siddique, I., et al. (2005). Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 48, 578–585. doi: 10.1007/s00125-004-1663-5
Matsuse, D., Kitada, M., Kohama, M., Nishikawa, K., Makinoshima, H., Wakao, S., et al. (2010). Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 69, 973–985. doi: 10.1097/NEN.0b013e3181eff6dc
Matta, C., Meyer, L., Mensah-Nyagan, A. G., and Taleb, O. (2020). Behavioral, electrophysiological, and histological characterization of a new rat model for neoadjuvant chemotherapy-induced neuropathic pain: therapeutic potential of duloxetine and allopregnanolone concomitant treatment. Neurotox. Res. 38, 145–162. doi: 10.1007/s12640-020-00176-2
McKenzie, I. A., Biernaskie, J., Toma, J. G., Midha, R., and Miller, F. D. (2006). Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 26, 6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006
Mendonca, M. V., Larocca, T. F., de Freitas Souza, B. S., Villarreal, C. F., Silva, L. F., Matos, A. C., et al. (2014). Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 5:126. doi: 10.1186/scrt516
Menorca, R. M., Fussell, T. S., and Elfar, J. C. (2013). Nerve physiology: mechanisms of injury and recovery. Hand Clin. 29, 317–330. doi: 10.1016/j.hcl.2013.04.002
Mimura, T., Dezawa, M., Kanno, H., Sawada, H., and Yamamoto, I. (2004). Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J. Neurosurg. 101, 806–812. doi: 10.3171/jns.2004.101.5.0806
Mizisin, A. P. (2014). Mechanisms of diabetic neuropathy: schwann cells. Handb. Clin. Neurol. 126, 401–428. doi: 10.1016/B978-0-444-53480-4.00029-1
Monje, P. V. (2020). Schwann cell cultures: biology, technology and therapeutics. Cells 9:1848. doi: 10.3390/cells9081848
Mosahebi, A., Fuller, P., Wiberg, M., and Terenghi, G. (2002). Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp. Neurol. 173, 213–223. doi: 10.1006/exnr.2001.7846
Naruse, K. (2019). Schwann cells as crucial players in diabetic neuropathy. Adv. Exp. Med. Biol. 1190, 345–356. doi: 10.1007/978-981-32-9636-7_22
Naruse, K., Hamada, Y., Nakashima, E., Kato, K., Mizubayashi, R., Kamiya, H., et al. (2005). Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 54, 1823–1828. doi: 10.2337/diabetes.54.6.1823
Nazareth, L., St John, J., Murtaza, M., and Ekberg, J. (2021). Phagocytosis by peripheral glia: importance for nervous system functions and implications in injury and disease. Front. Cell Dev. Biol. 9:660259. doi: 10.3389/fcell.2021.660259
Oh, S. K., Choi, K. H., Yoo, J. Y., Kim, D. Y., Kim, S. J., and Jeon, S. R. (2016). A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 78, 436–447; discussion 447. doi: 10.1227/NEU.0000000000001056
Okawa, T., Kamiya, H., Himeno, T., Kato, J., Seino, Y., Fujiya, A., et al. (2013). Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 22, 1767–1783. doi: 10.3727/096368912X657710
Omi, M., Hata, M., Nakamura, N., Miyabe, M., Ozawa, S., Nukada, H., et al. (2017). Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. Ther. 8:279. doi: 10.1186/s13287-017-0729-5
Oraee-Yazdani, S., Akhlaghpasand, M., Golmohammadi, M., Hafizi, M., Zomorrod, M. S., Kabir, N. M., et al. (2021). Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes. Stem Cell Res. Ther. 12:445. doi: 10.1186/s13287-021-02515-2
Oraee-Yazdani, S., Hafizi, M., Atashi, A., Ashrafi, F., Seddighi, A. S., Hashemi, S. M., et al. (2016). Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord 54, 102–109. doi: 10.1038/sc.2015.142
Orbay, H., Uysal, A. C., Hyakusoku, H., and Mizuno, H. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthet. Surg. 65, 657–664. doi: 10.1016/j.bjps.2011.11.035
Park, H. W., Lim, M. J., Jung, H., Lee, S. P., Paik, K. S., and Chang, M. S. (2010). Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia 58, 1118–1132. doi: 10.1002/glia.20992
Radtke, C., Aizer, A. A., Agulian, S. K., Lankford, K. L., Vogt, P. M., and Kocsis, J. D. (2009a). Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Res. 1254, 10–17. doi: 10.1016/j.brainres.2008.11.036
Radtke, C., Kocsis, J. D., and Vogt, P. M. (2009b). Chapter 22: transplantation of olfactory ensheathing cells for peripheral nerve regeneration. Int. Rev. Neurobiol. 87, 405–415. doi: 10.1016/S0074-7742(09)87022-0
Radtke, C., and Kocsis, J. D. (2012). Peripheral nerve injuries and transplantation of olfactory ensheathing cells for axonal regeneration and remyelination: fact or fiction? Int. J. Mol. Sci. 13, 12911–12924. doi: 10.3390/ijms131012911
Radtke, C., and Kocsis, J. D. (2014). Olfactory-ensheathing cell transplantation for peripheral nerve repair: update on recent developments. Cells Tissues Organs 200, 48–58. doi: 10.1159/000369006
Radtke, C., Wewetzer, K., Reimers, K., and Vogt, P. M. (2011). Transplantation of olfactory ensheathing cells as adjunct cell therapy for peripheral nerve injury. Cell Transplant. 20, 145–152. doi: 10.3727/096368910X522081
Rodriguez, F. J., Verdu, E., Ceballos, D., and Navarro, X. (2000). Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp. Neurol. 161, 571–584. doi: 10.1006/exnr.1999.7315
Santosa, K. B., Jesuraj, N. J., Viader, A., MacEwan, M., Newton, P., Hunter, D. A., et al. (2013). Nerve allografts supplemented with schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve 47, 213–223. doi: 10.1002/mus.23490
Sayad Fathi, S., and Zaminy, A. (2017). Stem cell therapy for nerve injury. World J. Stem Cells 9, 144–151. doi: 10.4252/wjsc.v9.i9.144
Shakhbazau, A., Mohanty, C., Kumar, R., and Midha, R. (2014). Sensory recovery after cell therapy in peripheral nerve repair: effects of naive and skin precursor-derived Schwann cells. J. Neurosurg. 121, 423–431. doi: 10.3171/2014.5.JNS132132
Shimizu, S., Kitada, M., Ishikawa, H., Itokazu, Y., Wakao, S., and Dezawa, M. (2007). Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem. Biophys. Res. Commun. 359, 915–920. doi: 10.1016/j.bbrc.2007.05.212
Shin, Y. K., Jang, S. Y., Lee, H. K., Jung, J., Suh, D. J., Seo, S. Y., et al. (2010). Pathological adaptive responses of Schwann cells to endoplasmic reticulum stress in bortezomib-induced peripheral neuropathy. Glia 58, 1961–1976. doi: 10.1002/glia.21065
Sima, A. A., Bril, V., Nathaniel, V., McEwen, T. A., Brown, M. B., Lattimer, S. A., et al. (1988). Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. N. Engl. J. Med. 319, 548–555. doi: 10.1056/NEJM198809013190905
Sowa, Y., Kishida, T., Tomita, K., Yamamoto, K., Numajiri, T., and Mazda, O. (2017). Direct conversion of human fibroblasts into schwann cells that facilitate regeneration of injured peripheral nerve in vivo. Stem Cells Translat. Med. 6, 1207–1216. doi: 10.1002/sctm.16-0122
Stassart, R. M., and Woodhoo, A. (2021). Axo-glial interaction in the injured PNS. Dev. Neurobiol. 81, 490–506. doi: 10.1002/dneu.22771
Stenberg, L., and Dahlin, L. B. (2014). Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neurosci. 15:107. doi: 10.1186/1471-2202-15-107
Stratton, J. A., Kumar, R., Sinha, S., Shah, P., Stykel, M., Shapira, Y., et al. (2017). Purification and characterization of schwann cells from adult human skin and nerve. Eneuro 4:ENEURO.0307-16.2017. doi: 10.1523/ENEURO.0307-16.2017
Stratton, J. A., Shah, P. T., Kumar, R., Stykel, M. G., Shapira, Y., Grochmal, J., et al. (2016). The immunomodulatory properties of adult skin-derived precursor Schwann cells: implications for peripheral nerve injury therapy. Eur. J. Neurosci. 43, 365–375. doi: 10.1111/ejn.13006
Strauch, B., Ferder, M., Lovelle-Allen, S., Moore, K., Kim, D. J., and Llena, J. (1996). Determining the maximal length of a vein conduit used as an interposition graft for nerve regeneration. J. Reconstr. Microsurg. 12, 521–527. doi: 10.1055/s-2007-1006624
Strauch, B., Rodriguez, D. M., Diaz, J., Yu, H. L., Kaplan, G., and Weinstein, D. E. (2001). Autologous Schwann cells drive regeneration through a 6-cm autogenous venous nerve conduit. J Reconstr Microsurg 17, 589–595; discussion 96–97. doi: 10.1055/s-2001-18812
Sullivan, R., Dailey, T., Duncan, K., Abel, N., and Borlongan, C. V. (2016). Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int. J. Mol. Sci. 17:2101. doi: 10.3390/ijms17122101
Sun, X. H., Che, Y. Q., Tong, X. J., Zhang, L. X., Feng, Y., Xu, A. H., et al. (2009). Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell. Mol. Neurobiol. 29, 347–353. doi: 10.1007/s10571-008-9326-6
Thomas, P. K., and Lascelles, R. G. (1965). Schwann-cell abnormalities in diabetic neuropathy. Lancet 1, 1355–1357. doi: 10.1016/S0140-6736(65)92154-9
Tohill, M. P., Mann, D. J., Mantovani, C. M., Wiberg, M., and Terenghi, G. (2004). Green fluorescent protein is a stable morphological marker for schwann cell transplants in bioengineered nerve conduits. Tissue Eng. 10, 1359–1367. doi: 10.1089/ten.2004.10.1359
Tomita, K., Madura, T., Sakai, Y., Yano, K., Terenghi, G., and Hosokawa, K. (2013). Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience 236, 55–65. doi: 10.1016/j.neuroscience.2012.12.066
Tong, L. L., Ding, Y. Q., Jing, H. B., Li, X. Y., and Qi, J. G. (2015). Differential motor and sensory functional recovery in male but not female adult rats is associated with remyelination rather than axon regeneration after sciatic nerve crush. Neuroreport 26, 429–437. doi: 10.1097/WNR.0000000000000366
Verdú, E., Navarro, X., Gudiño-Cabrera, G., Rodríguez, F. J., Ceballos, D., Valero, A., et al. (1999). Olfactory bulb ensheathing cells enhance peripheral nerve regeneration. Neuroreport 10, 1097–1101. doi: 10.1097/00001756-199904060-00035
Wang, X., Luo, E., Li, Y., and Hu, J. (2011). Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 1383, 71–80. doi: 10.1016/j.brainres.2011.01.098
Weiss, M. L., and Troyer, D. L. (2006). Stem cells in the umbilical cord. Stem Cell Rev. 2, 155–162. doi: 10.1007/s12015-006-0022-y
Wheeler, H. E., Wing, C., Delaney, S. M., Komatsu, M., and Dolan, M. E. (2015). Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One 10:e0118020. doi: 10.1371/journal.pone.0118020
Wing, C., Komatsu, M., Delaney, S. M., Krause, M., Wheeler, H. E., and Dolan, M. E. (2017). Application of stem cell derived neuronal cells to evaluate neurotoxic chemotherapy. Stem Cell Res. 22, 79–88. doi: 10.1016/j.scr.2017.06.006
Wu, X., Wang, L., Cong, M., Shen, M., He, Q., Ding, F., et al. (2020). Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann. Transl. Med. 8:1640. doi: 10.21037/atm-20-5965
Yagihashi, S., and Matsunaga, M. (1979). Ultrastructural pathology of peripheral nerves in patients with diabetic neuropathy. Tohoku J. Exp. Med. 129, 357–366. doi: 10.1620/tjem.129.357
Yazdani, S. O., Hafizi, M., Zali, A. R., Atashi, A., Ashrafi, F., Seddighi, A. S., et al. (2013). Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy 15, 782–791. doi: 10.1016/j.jcyt.2013.03.012
Yigitturk, G., Erbas, O., Karabay Yavasoglu, N. U., Acikgoz, E., Buhur, A., Gokhan, A., et al. (2021). The neuro-restorative effect of adipose-derived mesenchymal stem cell transplantation on a mouse model of diabetic neuropathy. Neurol. Res. doi: 10.1080/01616412.2021.1967679 [Online ahead of print].
You, H., Wei, L., Liu, Y., Oudega, M., Jiao, S. S., Feng, S. N., et al. (2011). Olfactory ensheathing cells enhance Schwann cell-mediated anatomical and functional repair after sciatic nerve injury in adult rats. Exp. Neurol. 229, 158–167. doi: 10.1016/j.expneurol.2010.08.034
Zhang, L., Li, B., Liu, B., and Dong, Z. (2019). Co-transplantation of epidermal neural crest stem cells and olfactory ensheathing cells repairs sciatic nerve defects in rats. Front. Cell. Neurosci. 13:253. doi: 10.3389/fncel.2019.00253
Zhang, P., Lu, X., Chen, J., and Chen, Z. (2014). Schwann cells originating from skin-derived precursors promote peripheral nerve regeneration in rats. Neural Regen. Res. 9, 1696–1702. doi: 10.4103/1673-5374.141805
Keywords: Schwann cells, Schwann cell-like cells, myelination, regeneration, peripheral neuropathy
Citation: Wang Q, Chen F-Y, Ling Z-M, Su W-F, Zhao Y-Y, Chen G and Wei Z-Y (2022) The Effect of Schwann Cells/Schwann Cell-Like Cells on Cell Therapy for Peripheral Neuropathy. Front. Cell. Neurosci. 16:836931. doi: 10.3389/fncel.2022.836931
Received: 16 December 2021; Accepted: 02 February 2022;
Published: 08 March 2022.
Edited by:
Zhaowei Zhu, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Nicolas Guérout, Université de Rouen, FranceCopyright © 2022 Wang, Chen, Ling, Su, Zhao, Chen and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, Y2hlbmdhbmc2NjI2QG50dS5lZHUuY24=; Zhong-Ya Wei, d2Vpemhvbmd5YTA4eWFuQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.