- 1Department of Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Anesthesia and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Contrast-induced encephalopathy (CIE) is an uncommon complication associated with contrast exposure during angiographic procedures that is usually transient but occasionally leads to permanent complications or death. Due to the low incidence of CIE, there are still insufficient reports. This study was used to summarize the clinical features of CIE through a case report and systematic review. We summarized and reviewed 127 patients with CIE, and we found that the total incidence of CIE between men and women had no difference (49.61 and 50.39%, respectively), but the average age in female patients with CIE was older than that in male patients (62.19 and 58.77 years, respectively). Interestingly, the incidence of female patients with CIE in the poor prognosis group was significantly higher than that in the good prognosis group (62.50 and 36.51%, respectively), and the average age of these female patients in the poor prognosis group was younger than that in the good prognosis group (61.39 and 62.82 years, respectively). The contrast medium types were mainly nonionic (79.69 and 73.02%, respectively) and low-osmolar (54.69 and 71.43%, respectively) in both groups. Importantly, the total contrast media administrated in patients with poor prognoses was greater than that administrated in patients with good prognoses (198.07 and 188.60 ml, respectively). In addition, comorbidities in both groups included hypertension (55.91%), diabetes mellitus (20.47%), previous contrast history (15.75%), renal impairment (11.81%), and hyperlipidemia (3.15%). The percentage of patients with cerebral angiography was significantly higher in the poor prognosis group than that in the good prognosis group (37.50 and 9.52%, respectively), whereas the percentage of patients with coronary angiography in both groups had the opposite results (35.94 and 77.78%, respectively). In conclusion, CIE may not always have a benign outcome and can cause permanent deficits. Female gender, younger age, the higher dose of contrast medium, and the procedure of cerebral angiography may be related to the patient’s poor prognosis.
Introduction
Contrast-induced encephalopathy (CIE) is an uncommon complication associated with intravenous or intra-arterial exposure to iodinated contrast media during angiographic procedures. The incidence of CIE ranges between 0.3 and 4.0% (de Bono, 1993; Potsi et al., 2012; Liu et al., 2020). Since the first description in 1970, the clinical features of CIE have included headache, memory loss, confusion, visual and speech impairment, seizures, hemiparesis, and even coma. The underlying mechanisms and causes of iodine-based CIE remain unclear. Studies suggest that this may be related to transient blood–brain barrier (BBB) breakdown and increased permeability, which may subsequently contribute to extravasation of contrast medium into the central nervous system, resulting in cerebral edema and altered neuronal excitability (Dangas et al., 2001; Babalova et al., 2021). In addition, some high concentrations of contrast media may cause the clumping of red blood cells and, consequently, occlusion of arterial branches, which may play a role in permanent neurological deficits (Cristaldi et al., 2021). Most patients with CIE have a good prognosis and resolve quickly within 1–2 days (Spina et al., 2017). A minority (approximately 15% of CIE) may develop permanent neurological deficits or fatal cerebral edema (Hamra et al., 2017; Donepudi and Trottier, 2018; Zhao et al., 2019). However, the development of an evidence-based consensus on CIE has been hindered by the low incidence of CIE. Although the current literature on CIE is extensive, only case reports in the literature describe CIE and further analyses on the risk factors of CIE prognosis have been rarely performed. Here, we provided a case report as a reference and summarized existing reports about CIE, aiming to explore pathogenesis, risk factors, diagnosis, treatment strategy, and future exploration direction of the disease.
Case report
A 51-year-old woman was admitted to our hospital with a suspected intracranial aneurysm. The patient had a history of hypertension. On admission, a physical examination showed no signs of neurological deficits. Cerebral angiography was urgently performed through the right femoral approach. The procedure lasted for 60 min. A total of 50 ml iodixanol (Jiangsu Hengrui Pharmaceutical Co., Ltd., China), an iso-osmolar non-ionic dimeric hydrophilic contrast medium, was injected. Notably, 1% lignocaine was administered for local anesthesia prior to cerebral angiography. This was the patient’s first exposure to a contrast medium, and no obvious aneurysm or vascular malformation was found.
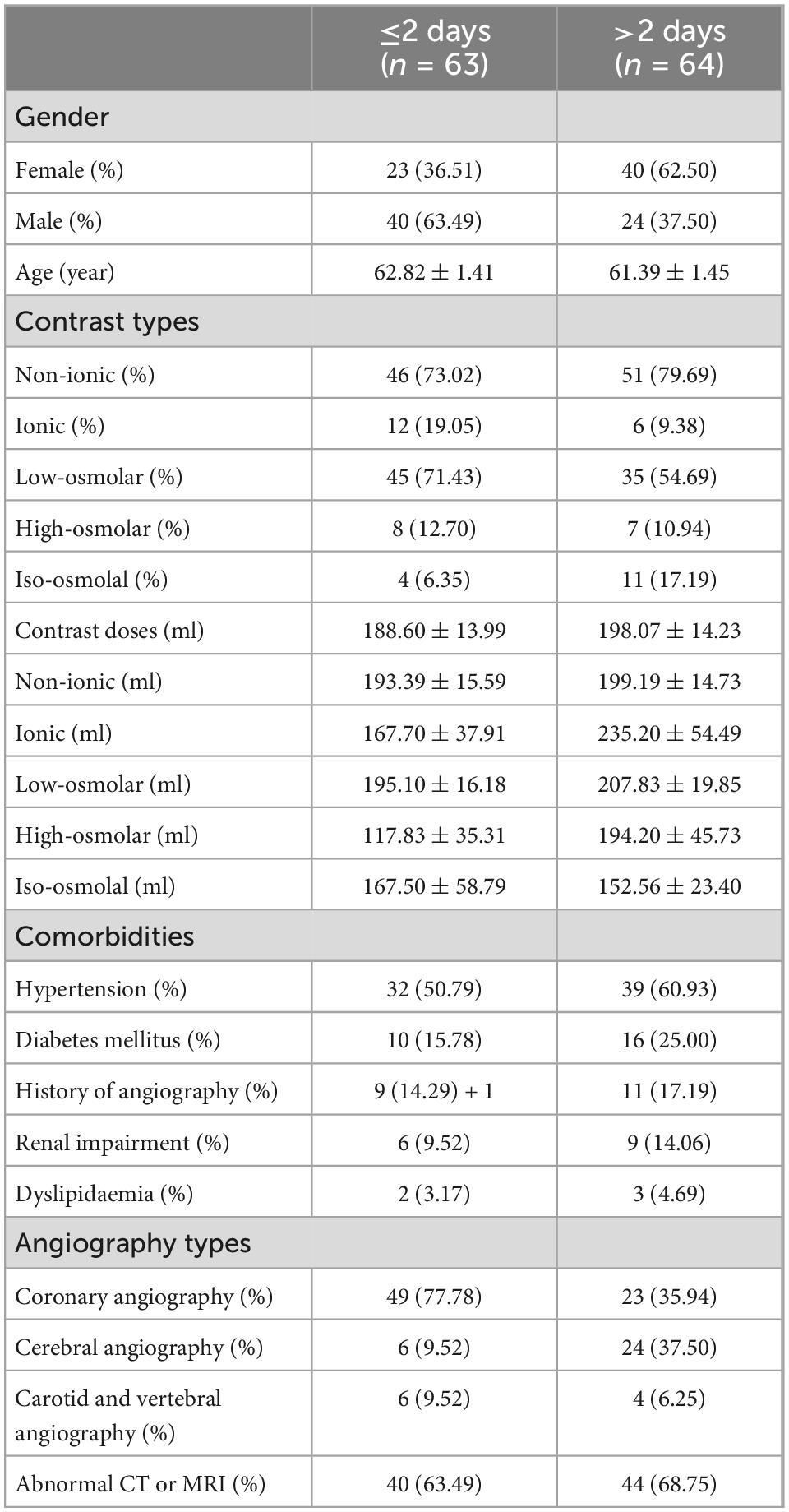
Approximately 5 h after surgical completion, the patient developed a decrease in the upper and lower extremity motor strength, and the pupils were symmetric and reactive. The left muscle strength was grade 2, and the right muscle strength was grade 3. An emergency brain computed tomography (CT) scan was requested and revealed the diffuse contrast enhancement in brain sulci, fissures, cisterns, third ventricle, fourth ventricle, and subarachnoid space with mild global brain edema, and softening foci in the left basal ganglia-insular area (Figures 1A,B). On the second day, the patient’s clinical symptoms further deteriorated and the upper and lower muscle strength was grade 0 with positive pathological signs. Brain CT was reviewed and showed diffuse enhancement disappeared, but the brain parenchyma was diffusely swollen and the lateral ventricles were slightly more compressed than that in the previous scan (Figures 1C,D). A diagnosis of CIE was suspected given the worsening of the clinical manifestations and symptoms compatible with higher functional impairment following the administration of the contrast medium.
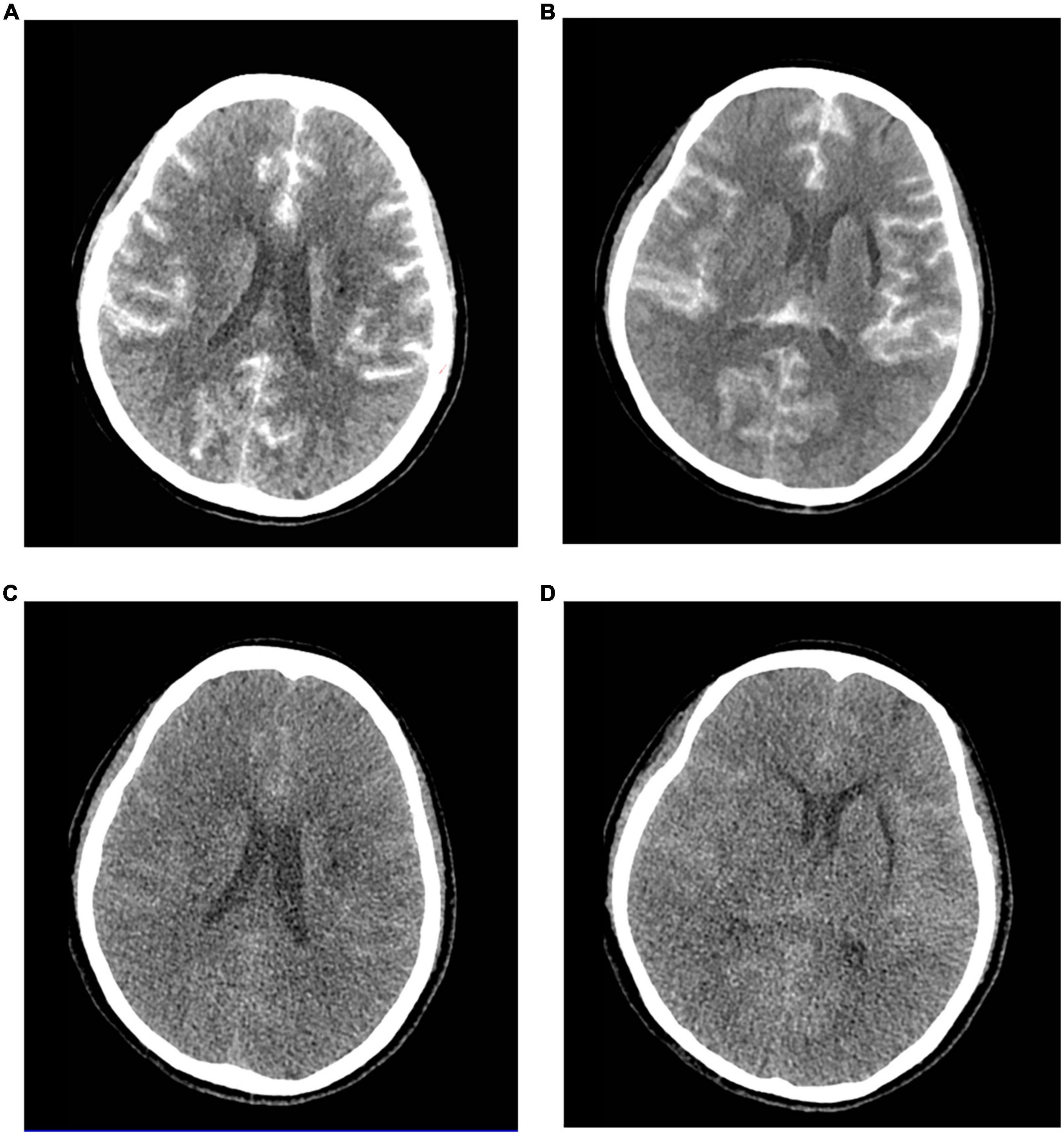
Figure 1. Contrast-induced encephalopathy in brain computed tomography (CT) scans. (A,B) Emergency brain CT 5 h after the procedure showed diffuse contrast enhancement in the brain parenchyma and subarachnoid space with mild global brain edema and the softening foci in the left basal ganglia-insular area. (C,D) Brain CT 2 days after the procedure indicated diffuse enhancement disappeared, and the brain parenchyma was diffusely swollen and the lateral ventricle was slightly more compressed.
The patient routinely received fluids to accelerate the excretion of contrast medium, 1,000 mg of intravenous methylprednisolone once daily for 2 days to mitigate inflammation, 250 ml of mannitol every 8 h to dehydrate and reduce intracranial pressure, 10 mg of nimodipine once daily to prevent vasospasm, 120 mg of sodium valproate once daily to prevent epilepsy, as well as strengthen nutrition to improve clinical symptoms. Furthermore, lumbar cistern drainage was performed to reduce intracranial pressure, and cerebrospinal fluid (CSF) was clear with increased white blood cell count and glucose level and decreased chloride level. In the following hours, the patient experienced further deterioration in mental status and fell into a coma with respiratory insufficiency. Therefore, the patient was transferred to the intensive care unit (ICU) where she underwent tracheal intubation with ventilator-assisted breathing, dehydration, anti-epileptic therapy, body temperature and blood pressure control, and close neurological observation.
On the second day after being admitted to the ICU, the patient regained consciousness, but her motor deficit was unchanged. A neurological examination showed muscle weakness in the upper and lower limbs and sensory loss below the T2 sensory level, which may be related to spinal cord edema. Considering that the patient was temporarily unable to remove the tracheal tube, a tracheotomy was performed 4 days later. A magnetic resonance imaging (MRI) performed at 2 weeks revealed a diffuse hyperintense signal on FLAIR sequences in the cervical cord, which may be consistent with the patient’s motor deficits and sensory disturbances, as well as a softening foci formation in the left basal ganglia-insular area (Figure 2). Dramatically, the patient suffered from a lung infection during hospitalization and was eventually discharged from the neurosurgery ward to another hospital for hyperbaric oxygen therapy after 20 days. A telephone follow-up after 2 months revealed that the patient’s persistent neurological deficits had not improved.

Figure 2. The brain and cervical spine magnetic resonance imaging (MRI) performed at 2 weeks. (A,B) Brain MRI showed the left basal ganglia-insular softening foci in T1 (A) and T2 (B) weighted image. (C–E) Cervical spine MRI showed diffuse hyperintense signal in the cervical cord in T2-weighted image (D) and fluid-attenuated inversion recovery (FLAIR) images (E), and normal findings were observed in T1-weighted image (C).
Literature review
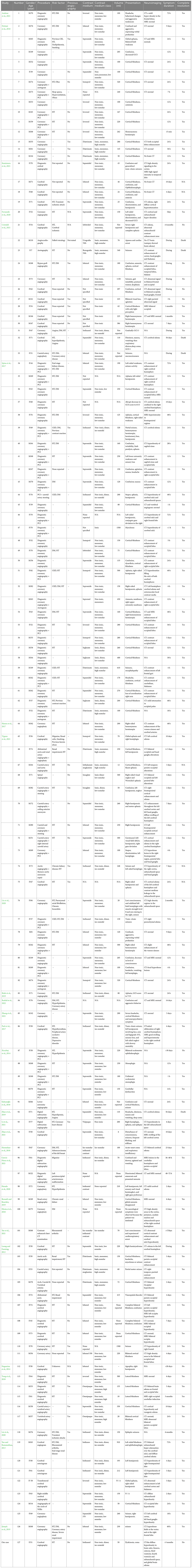
In searching for the keywords “Contrast-induced encephalopathy” and “Angiography” in PubMed, 95 relevant articles were found. A total of 54 papers were selected after screening abstracts and titles. After reading through the whole paper, the reviews, editorial, and duplicate cases were excluded, and 40 papers were left. However, four of them were excluded because it was defined as allergic reactions, vasospasm, and posterior reversible encephalopathy syndrome, and complete data were not available in the other six papers. Finally, we accurately summarized 30 papers (Leong and Fanning, 2012; Yan and Ramanathan, 2013; Kocabay et al., 2014; Nagamine et al., 2014; Hamra et al., 2017; Park et al., 2017; Spina et al., 2017; Dattani et al., 2018; Heemelaar et al., 2018; Hirata et al., 2018; Kahyaoğlu et al., 2018; Tong et al., 2018; Renault and Rouchet, 2019; Riahi et al., 2019; Şimşek et al., 2019; Zhao et al., 2019, 2021; Fernando et al., 2020; Harada et al., 2020; Lei et al., 2020; Liu et al., 2020; Andone et al., 2021; Cristaldi et al., 2021; García-Pérez et al., 2021; Kamimura et al., 2021; Li et al., 2021; Vigano et al., 2021; Yao et al., 2021; Zhang et al., 2021; Rashid et al., 2022). A total of 127 patients were enrolled. Figure 3 shows the screening process. Table 1 shows the basic information of 31 studies (including our case).
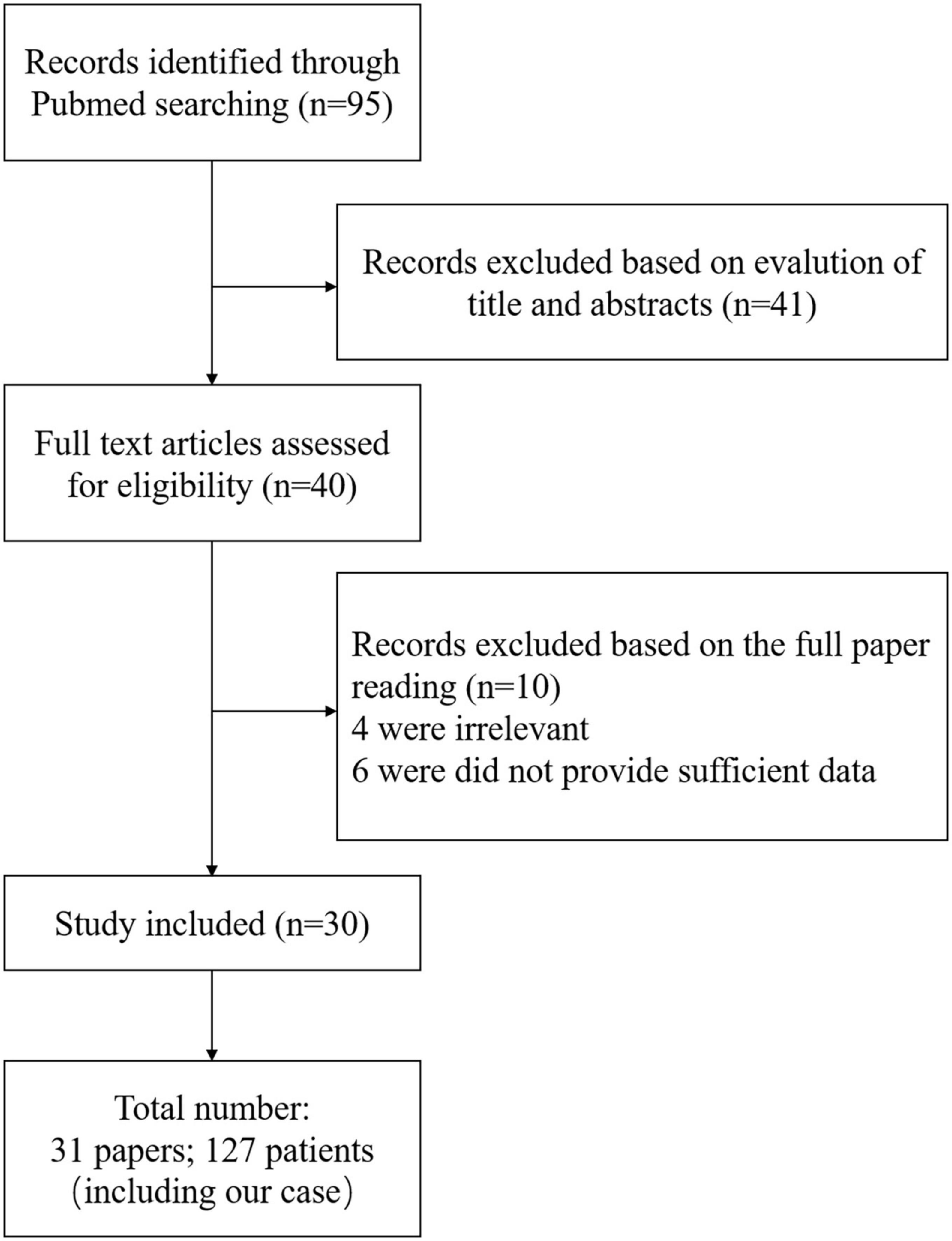
From the search results, we found that the total incidence of CIE between women and men has no difference. A total of 63/127 (49.61%) patients were women and 64/127 (50.39%) patients were men, and the average age in women was older than that in men (62.19 and 58.77 years, respectively). More importantly, we classified the statistical results according to prognosis, and patients who recovered less than or equal to 48 h were included in the good prognosis group and the remaining patients were included in the poor prognosis group. Eventually, 63 patients were included in the good prognosis group and 64 patients were included in the poor prognosis group, and the results are shown in Table 2. We found that the incidence of female patients with CIE in the poor prognosis group was significantly higher than that in the good prognosis group (62.50 and 36.51%, respectively), and the average age of these female patients in the poor prognosis group was younger than that in the good prognosis group (61.39 and 62.82 years, respectively). Furthermore, the poor prognosis group had a wider age range, ranging from 6 to 84 years.
In interventional procedures, the contrast types included non-ionic and ionic, or low-osmolar, high-osmolar, and iso-osmolar in our present study, and we found that both groups were mainly non-ionic (79.69 and 73.02%, respectively) and low-osmolar (54.69 and 71.43%, respectively). Importantly, the total contrast media administrated and the non-ionic, ionic, low-osmolar, or high-osmolar contrast media administrated in patients with poor prognosis were greater than that administrated in patients with good prognosis (198.07 and 188.60 ml, 199.19 and 193.39 ml, 235.20 and 167.70 ml, 207.83 and 195.10 ml, and 194.20 and 117.83 ml, respectively), whereas the iso-osmolar contrast media administrated was lower in patients with poor prognosis compared to patients with good prognosis (152.56 and 167.50, respectively).
The comorbidities in the present study mainly included hypertension (55.91%), diabetes mellitus (20.47%), previous contrast history (15.75%), renal impairment (11.81%), and hyperlipidemia (3.15%). Although there was no significant difference in comorbidities between the two groups, the percentages of hypertension, diabetes mellitus, previous contrast history, renal impairment, and hyperlipidemia in the poor prognosis group were higher than those in the good prognosis group (60.93 and 50.79%, 25.00 and 15.78%, 17.19 and 4.29%, 14.06 and 9.52%, and 4.69 and 3.17%, respectively). The angiography types, mainly coronary angiography (56.69%), cerebral angiography (23.62%), and carotid and vertebral angiography (7.87%), in both groups, were also analyzed. We found that the percentage of patients with cerebral angiography in the poor prognosis group was significantly higher than that in the good prognosis group (37.50 and 9.52%, respectively), whereas the percentage of patients with coronary angiography in both groups had the opposite results (35.94 and 77.78%, respectively). Moreover, brain CT or MRI abnormalities were found in most patients in both groups (68.83 and 62.00%, respectively).
Discussion
Contrast-induced encephalopathy is a rare and reversible complication that can cause neurotoxicity with a favorable prognosis and resolves within 24–48 h in most cases. Based on previous studies (Yan and Ramanathan, 2013; Spina et al., 2017; Cristaldi et al., 2021), the renal elimination of contrast medium, the regression of cerebral edema, and the recovery of BBB function were assumed to play an important role in the pathophysiology of neurological recovery. Here, we described a case of permanent neurological deficit after cerebral angiography and provided a summary and analysis of a series of CIE cases to explore the probable reasons for permanent neurological deficit. Given that most patients resolved completely within 48 h, we performed a prognostic analysis using 48 h as the node. We found that the total incidence of CIE between female patients and male patients had no difference, but female patients were more likely to have a poor prognosis. In addition, the average age of patients with poor prognoses was younger than that of patients with good prognoses. Surprisingly, no reports are currently available on risk factors associated with prognosis in patients with CIE. Only two reports were found to analyze the relationship between the incidence of CIE and gender or age, and the conclusions of the two reports were inconsistent. One report found that the adverse drug reaction incidence of iodinated contrast medium (e.g., CIE) seemed to be associated with gender, with a significantly higher incidence in female patients than in male patients, and it was also associated with age, with a lower occurrence in older (>44 years) patients compared to younger patients (Jiang et al., 2021). The other report summarized 9 CIE cases in 2013 and proposed that male gender and advanced age are the greatest risk factors for developing CIE. These two reports just provide a reference for us, and further research and a more in-depth analysis are necessary.
Studies showed a correlation between contrast medium dose and CIE (Yu and Dangas, 2011; Vigano et al., 2021), and whether the more contrast medium used is related to the poor prognosis of patients has not been directly reported. Although our study showed that the patients with poor prognosis used more contrast medium among different types of contrast media, including the non-ionic, ionic, low-osmolar, and high-osmolar contrast media, as well as the total contrast media used, the results are not absolute. Because in our reported case and 4 other summarized cases, the patient presented with permanent neurological deficits (more than 10 days) after administrating only a low quantity of contrast medium (no more than 50 ml) for angiography. Among these cases, the contrast medium types included non-ionic, low-osmolar, high- osmolar, and iso-osmolar, suggesting that severe neurotoxic symptoms may occur in response to low doses and different types of contrast agents. A previous study has shown that a 49-year-old man developed CIE and completely resolved within 4 h after receiving 610 ml diatrizoate (an ionic high-osmolar contrast medium) for diagnostic coronary angiography (Muruve and Steinman, 1996), indicating that high-dose contrast media do not cause permanent neurological dysfunction. Therefore, we speculated that, in addition to volume, the poor prognosis is generally related to the route and number of administrated, type of contrast medium, and individual patient characteristics.
Previous research has shown that demographic risk factors for CIE are chronic hypertension, diabetes mellitus, renal insufficiency, and previous reactions to contrast media (Yu and Dangas, 2011; Zhao et al., 2019; Cristaldi et al., 2021). Our study showed that the majority of patients (55.91%) had hypertension, 20.47% had diabetes mellitus, 15.75% had a contrast history, and 11.81% had renal insufficiency. Although there is no statistical difference between the poor prognosis group and the good prognosis group, these risk factors have a higher proportion in patients with poor prognosis, suggesting they may be related to worse prognosis, and further research is needed by increasing the sample size.
For the types of angiographic procedures, the present study showed that the proportion of patients with cerebral angiography was significantly higher in the poor-prognosis group than in the good-prognosis group, whereas patients with coronary angiography had the opposite results. Whether the cerebral angiography procedure itself is more likely to aggravate the prognosis than coronary angiography is unclear. This study demonstrated that 170 ml is recommended as the maximum threshold level of toxicity for coronary angiography procedure, and a smaller volume of contrast media may damage the BBB during selective intracranial injection (Kocabay et al., 2014), suggesting that cerebral angiography may be more likely to damage the BBB than coronary angiography. Furthermore, it is unclear whether procedure-related factors and patient-related factors are involved.
The diagnosis of CIE often requires the exclusion of cerebrovascular accidents such as cerebral hemorrhage and cerebral infarction. Neuroimaging plays an important role in distinguishing CIE from other neurological pathologies such as thromboembolism and hemorrhage following angiography. Our research showed that the most common abnormalities on brain CT included cortical or subcortical contrast enhancement, cerebral edema, focal hyperdense lesions, and hyper-density in the cerebral sulci. MRI abnormalities included hyperintensity on T2, FLAIR, and DWI. This study has suggested that CSF examination is also useful to rule out subarachnoid hemorrhage through the absence of xanthochromia or red blood cells (Shahan et al., 2021). The simultaneous detection of high concentrations of iodinated contrast medium in CSF and serum supports contrast medium extravasation rather than hemorrhage. In addition, the exclusion of contrast allergy or allergic-like reactions is also essential for the diagnosis of CIE. A recent study showed that allergic-like or allergic reactions caused by contrast media are rare, which can be severe or even life-threatening (Fusco et al., 2022). It is important to obtain a history of immediate or delayed reactions to a specific contrast medium, which may contribute to predicting the risk for future reactions. Clinical manifestations such as throat tightness, facial edema, and bronchospasm are helpful in distinguishing.
For the treatment of CIE, most patients with CIE have a good prognosis and a rapid recovery. Therefore, supportive care and observation are generally considered sufficient. Based on the literature summarized in the present study, it is recommended that appropriate hydration, steroids, and mannitol can be given immediately after surgery, and benzodiazepines can be used for epileptic seizures.
Conclusion
A contrast-induced encephalopathy is a form of neurotoxicity caused by contrast media that is usually transient but occasionally leads to permanent complications or death. We summarized a series of cases and found that the female gender, younger age, higher contrast medium dose, and cerebral angiography procedure were associated with poor prognosis in patients with CIE. However, the contrast medium types were not associated with the prognosis. In addition, there was no statistical difference between the poor prognosis group and the good prognosis group; hypertension, diabetes mellitus, renal insufficiency, and previous reactions to contrast media were also important risk factors for CIE. Our case and literature review highlight that CIE may not always have a benign outcome and has the potential to cause permanent neurological dysfunction, even with low-dose contrast media. We should not be overlooked, especially following procedures that use contrast medium.
Author contributions
YZ wrote the manuscript. JZ analyzed the data. HS and SY critically revised and edited the manuscript. All authors discussed the content and read and approved the final version.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2501800) and the National Key Research and Development Program of China (2021YFC2501804).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andone, S., Balasa, R., Barcutean, L., Bajko, Z., Ion, V., Motataianu, A., et al. (2021). Contrast medium-induced encephalopathy after coronary angiography- case report. J. Crit. Care Med. 7, 145–149.
Babalova, L., Ruzinak, R., Ballova, J., Sivak, S., Kantorova, E., Kurca, E., et al. (2021). Contrast-induced encephalopathy. Bratisl. Lek. Listy 122, 618–620.
Cristaldi, P. M. F., Polistena, A., Patassini, M., de Laurentis, C., Giussani, C., and Remida, P. (2021). Contrast-induced encephalopathy and permanent neurological deficit: A case report and literature review. Surg. Neurol. Int. 12:273.
Dangas, G., Monsein, L. H., Laureno, R., Peterson, M. A., Laird, J. R. Jr., Satler, L. F., et al. (2001). Transient contrast encephalopathy after carotid artery stenting. J. Endovasc. Ther. 8, 111–113.
Dattani, A., Au, L., Tay, K. H., and Davey, P. (2018). Contrast-induced encephalopathy following coronary angiography with no radiological features: A case report and literature review. Cardiology 139, 197–201.
de Bono, D. (1993). Complications of diagnostic cardiac catheterisation: Results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The joint audit committee of the british cardiac society and royal college of physicians of london. Br. Heart J. 70, 297–300.
Donepudi, B., and Trottier, S. (2018). A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep. Med. 2018:9278526.
Fernando, T. G., Nandasiri, S., Mendis, S., Senanayake, S., Gooneratne, I. K., Navinan, R., et al. (2020). Contrast-induced encephalopathy: A complication of coronary angiography. Pract. Neurol. 20, 482–485.
Fusco, A., Pucci, L., Pierre, K., Wolberg, A., Small, C., Cerillo, J., et al. (2022). Contrast allergies for neurological imaging: When to proceed. AIMS Allergy Immunol. 6, 216–227.
García-Pérez, D., Parra-Serrano, J., Panero, I., Moreno, L. M., Campollo, J., and Alén, J. F. (2021). Transient cortical blindness secondary to contrast-induced encephalopathy following diagnostic cerebral angiography: Report of 2 cases. Acta Neurol. Belg. 121, 585–589.
Hamra, M., Bakhit, Y., Khan, M., and Moore, R. (2017). Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 13, 331–335.
Harada, Y., Kairamkonda, S. R., Ilyas, U., Pothineni, N. V. K., Samant, R. S., Shah, V. A., et al. (2020). Pearls & Oy-sters: Contrast-induced encephalopathy following coronary angiography: A rare stroke mimic. Neurology 94, e2491–e2494.
Heemelaar, J. C., van der Hoeven, N. W., Muller, F. F., and Appelman, Y. (2018). Acute-onset coma after iso-osmolar iodinated contrast injection: A case report of contrast-induced encephalopathy after elective coronary angiography. Eur. Heart J. Case Rep. 2:yty132.
Hirata, S., Koga, M., and Iseki, H. (2018). Contrast-induced encephalopathy after coronary angioplasty in a patient with ST-elevation myocardial infarction. Heart Asia 10:e010987.
Jiang, C., Li, J., Huang, Y., Huang, D., Lin, J., and Jiang, X. (2021). Clinical safety evaluation of contrast agents based on real-world evidence. J. Clin. Pharm. Ther. 46, 1600–1605.
Kahyaoğlu, M., Ağca, M., Çakmak, E., Geçmen, Ç., and Izgi, I. A. (2018). Contrast-induced encephalopathy after percutaneous peripheral intervention. Turk Kardiyol. Dern. Ars. 46, 140–142.
Kamimura, T., Nakamori, M., Imamura, E., Hayashi, Y., Matsushima, H., Mizoue, T., et al. (2021). Low-dose contrast-induced encephalopathy during diagnostic cerebral angiography. Intern. Med. 60, 629–633.
Kocabay, G., Karabay, C. Y., Kalayci, A., Akgun, T., Guler, A., Oduncu, V., et al. (2014). Contrast-induced neurotoxicity after coronary angiography. Herz 39, 522–527.
Lei, P., He, W., Shi, Q., Sun, M., and Sun, Z. (2020). Recurrent epileptic seizures following cardiac catheterization with iodixanol: A case report. BMC Cardiovasc. Disord. 20:79. doi: 10.1186/s12872-020-01341-3
Leong, S., and Fanning, N. F. (2012). Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv. Neuroradiol. 18, 33–41.
Li, J., Qi, G., Zhang, H., Chen, G., Wang, S., Yan, M., et al. (2021). Contrast-induced encephalopathy mimicking stroke after a second cerebral DSA: An unusual case report. BMC Neurol. 21:430. doi: 10.1186/s12883-021-02457-5
Liu, M. R., Jiang, H., Li, X. L., and Yang, P. (2020). Case report and literature review on low-osmolar, non-ionic iodine-based contrast-induced encephalopathy. Clin. Interv. Aging 15, 2277–2289.
Muruve, D. A., and Steinman, T. I. (1996). Contrast-induced encephalopathy and seizures in a patient with chronic renal insufficiency. Clin. Nephrol. 45, 406–409.
Nagamine, Y., Hayashi, T., Kakehi, Y., Yamane, F., Ishihara, S., Uchino, A., et al. (2014). Contrast-induced encephalopathy after coil embolization of an unruptured internal carotid artery aneurysm. Intern. Med. 53, 2133–2138.
Park, J. C., Ahn, J. H., Chang, I. B., Oh, J. K., Kim, J. H., and Song, J. H. (2017). A case of unusual presentation of contrast-induced encephalopathy after cerebral angiography using iodixanol. J. Cerebrovasc. Endovasc. Neurosurg. 19, 184–188.
Potsi, S., Chourmouzi, D., Moumtzouoglou, A., Nikiforaki, A., Gkouvas, K., and Drevelegas, A. (2012). Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol. Sci. 33, 445–448.
Rashid, H., Brown, J., Nix, E., and Fisher Covin, A. (2022). Contrast-Induced encephalopathy following diagnostic coronary angiography. Clin. Case Rep. 10:e05624.
Renault, P., and Rouchet, S. (2019). Transient global amnesia and transient cortical blindness secondary to contrast induced encephalopathy after renal artery angiography. Rev. Neurol. 175, 335–336.
Riahi, L., Mediouni, M., Messelmani, M., and Fehri, W. (2019). A singular manifestation of contrast-induced encephalopathy following coronary angiography. Neurol. India 67, 1525–1527.
Shahan, B., Choi, E. Y., and Nieves, G. (2021). Cerebrospinal fluid analysis. Am. Fam. Phys. 103, 422–428.
Şimşek, E., Ertürk, E., Uçar, R., Yilmaz, A. O., Ekmekçi, C., Mutlu, I., et al. (2019). Transient contrast neurotoxicity after percutaneous coronary intervention mimicking subarachnoid hemorrhage in a patient with chronic kidney disease. Clin. Med. Insights Case Rep. 12:1179547619867671.
Spina, R., Simon, N., Markus, R., Muller, D. W., and Kathir, K. (2017). Contrast-induced encephalopathy following cardiac catheterization. Catheter. Cardiovasc. Interv. 90, 257–268.
Tong, X., Hu, P., Hong, T., Li, M., Zhang, P., Li, G., et al. (2018). Transient Cortical Blindness Associated with Endovascular Procedures for Intracranial Aneurysms. World Neurosurg. 119, 123–131.
Vigano, M., Mantero, V., Basilico, P., Cordano, C., Sangalli, D., Reganati, P., et al. (2021). Contrast-induced encephalopathy mimicking total anterior circulation stroke: A case report and review of the literature. Neurol. Sci. 42, 1145–1150.
Yan, J., and Ramanathan, V. (2013). Severe encephalopathy following cerebral arteriogram in a patient with end-stage renal disease. Semin. Dial. 26, 203–207.
Yao, L. D., Zhu, X. L., Yang, R. L., and Zhang, M. M. (2021). Cardiorespiratory arrest after iso-osmolar iodinated contrast injection: A case report of contrast-induced encephalopathy following contrast-enhanced computed-tomography. Medicine 100:e24035.
Yu, J., and Dangas, G. (2011). Commentary: New insights into the risk factors of contrast-induced encephalopathy. J. Endovasc. Ther. 18, 545–546.
Zhang, W., Huang, H., Jiang, B., Liu, Z. Y., and He, Y. (2021). Iopromide-induced encephalopathy: A case report and literature review. Sichuan Da Xue Xue Bao Yi Xue Ban 52, 528–530.
Zhao, W., Zhang, J., Song, Y., Sun, L., Zheng, M., Yin, H., et al. (2019). Irreversible fatal contrast-induced encephalopathy: A case report. BMC Neurol. 19:46. doi: 10.1186/s12883-019-1279-5
Keywords: cerebral angiography, contrast-induced encephalopathy, neurological deficit, prognosis, risk factor
Citation: Zhang Y, Zhang J, Yuan S and Shu H (2023) Contrast-induced encephalopathy and permanent neurological deficit following cerebral angiography: A case report and review of the literature. Front. Cell. Neurosci. 16:1070357. doi: 10.3389/fncel.2022.1070357
Received: 14 October 2022; Accepted: 21 November 2022;
Published: 04 January 2023.
Edited by:
Shong Lau, Salk Institute for Biological Studies, United StatesReviewed by:
Luis Rafael Moscote-Salazar, Latinamerican Council of Neurocritical Care (CLaNi), ColombiaBrandon Peter Lucke-Wold, University of Florida, United States
Copyright © 2023 Zhang, Zhang, Yuan and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaqing Shu, ✉ aHVhcWluZ19zaHVAMTYzLmNvbQ==; Shiying Yuan, ✉ eXVhbl9zaGl5aW5nQDE2My5jb20=
†These authors have contributed equally to this work
 Yujing Zhang
Yujing Zhang Jiancheng Zhang1,2†
Jiancheng Zhang1,2† Huaqing Shu
Huaqing Shu