
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell. Neurosci. , 21 January 2022
Sec. Cellular Neuropathology
Volume 15 - 2021 | https://doi.org/10.3389/fncel.2021.819182
This article is part of the Research Topic Brain Injury and Repair Following Cerebrovascular Diseases: From Bench to Bedside View all 14 articles
Sepsis susceptibility is significantly increased in patients with intracerebral hemorrhage (ICH), owing to immunosuppression and intestinal microbiota dysbiosis. To date, ICH with sepsis occurrence is still difficult for clinicians to deal with, and the mortality, as well as long-term cognitive disability, is still increasing. Actually, intracerebral hemorrhage and sepsis are mutually exacerbated via similar pathophysiological mechanisms, mainly consisting of systemic inflammation and circulatory dysfunction. The main consequence of these two processes is neural dysfunction and multiple organ damages, notably, via oxidative stress and neurotoxic mediation under the mediation of central nervous system activation and blood-brain barrier disruption. Besides, the comorbidity-induced multiple organ damages will produce numerous damage-associated molecular patterns and consequently exacerbate the severity of the disease. At present, the prospective views are about operating artificial restriction for the peripheral immune system and achieving cross-tolerance among organs via altering immune cell composition to reduce inflammatory damage.
Intracerebral hemorrhage (ICH) is frequently accompanied by infection ranging from 11 to 31% and long-term functional impairment (Ali et al., 2009; Lord et al., 2014). The majority of infectious patients will rapidly deteriorate and finally develop sepsis, due to systemic metabolic disorders and stress caused by excessive release of inflammatory factors and immunosuppression after ICH (Berger et al., 2014; Cheng et al., 2018). Clinically, sepsis complicated by the ICH is common but tricky in the neurosurgical intensive care unit and kills as many as a half (Goncalves et al., 2019). Our retrospective cohort study has shown that approximately 28% of patients with ICH would accompany sepsis, and sepsis is the leading cause of poor outcomes. Furthermore, approximately 80% of survivors will face severe sequelae of various organ damages, especially in the brain (Adam et al., 2013). Actually, there are many synergies in the pathophysiological mechanism of both ICH and sepsis. For example, systemic inflammation after either ICH or sepsis emerges as a crucial trigger and mediator in the progression of secondary insult to the brain (Adam et al., 2013; Fu et al., 2015). In addition, subsequent circulatory dysfunction can be observed in both situations, leading to worse damage progress (Taccone et al., 2010; Kopeikina et al., 2020).
To date, ICH with sepsis is still difficult for clinicians to deal with, and the mortality and long-term cognitive disability are still increasing. Thus, understanding the relevant pathophysiology seems to be imminent and will be beneficial for the exploration of specific therapies. In this review, we focus on the crosstalk between ICH and sepsis and attempt to identify the mechanism of cerebral dysfunction, aiming to provide a unique and systematic insight into the interaction of the two diseases and guide indications for clinical treatment.
Intracerebral hemorrhage and sepsis are mutually exacerbated via several pathophysiological mechanisms mainly consisting of systemic inflammation and circulatory dysfunction (see Figure 1).
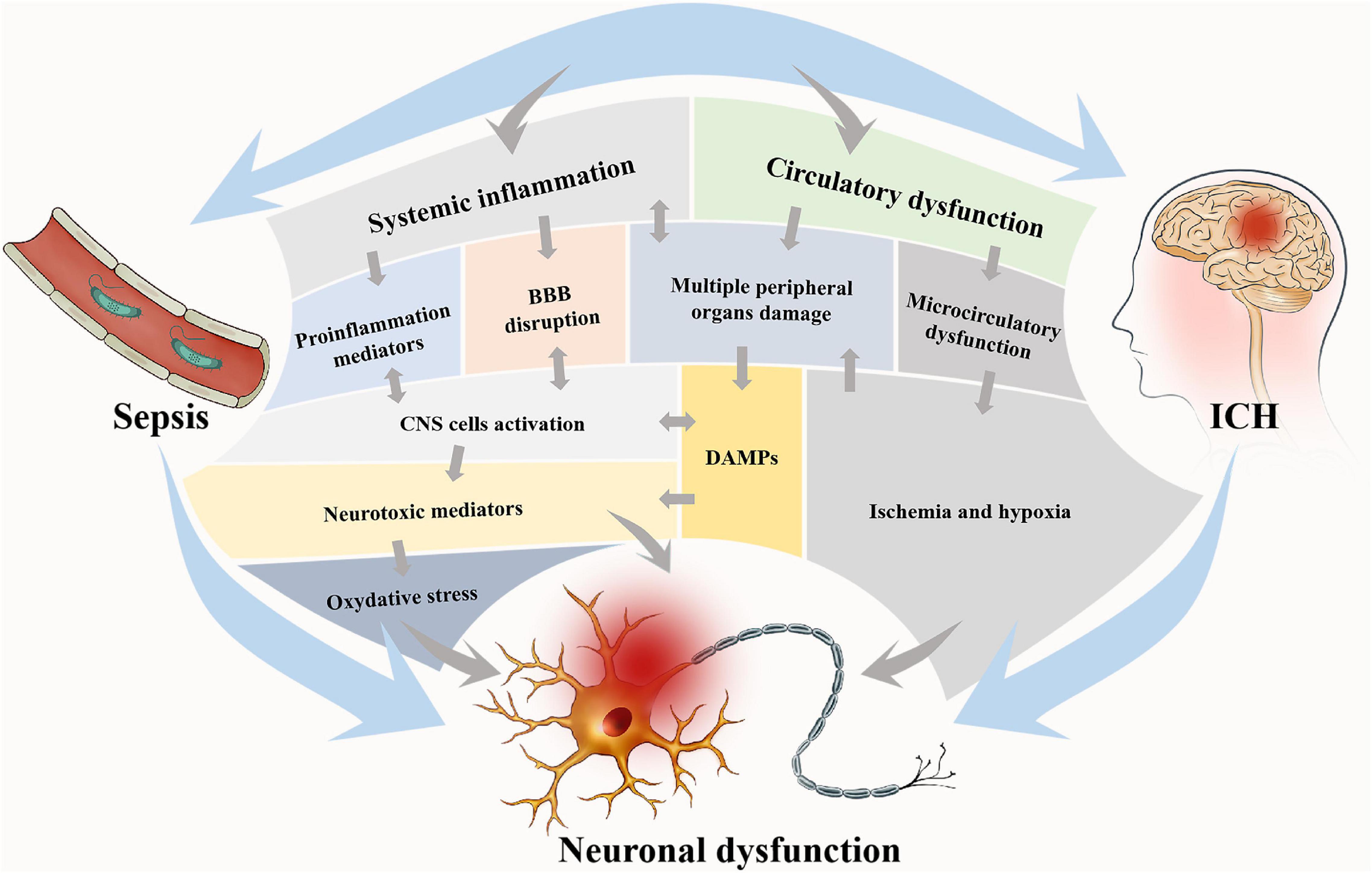
Figure 1. Two main pathophysiological processes are involved in brain dysfunction in ICH with sepsis. These two processes are interdependent, on one hand, and mutually independent, on the other hand. Circulatory dysfunction will be followed by microcirculatory dysfunction and finally result in ischemia and hypoxia of tissues and multiple peripheral organ damages. In systemic inflammation, proinflammatory mediators will be released, and the DAMPs from the periphery will be allowed into the brain due to BBB disruption, which consequently activates CNS cells. The main consequence of these two processes is neural dysfunction, notably via oxidative stress and neurotoxic mediation. Neural dysfunction widely exists in the brain and accounts for the brain dysfunction via the alteration of neurotransmission. DAMPs, damage-associated molecular patterns; BBB, blood-brain barrier; CNS, central nervous system.
On the onset of ICH, primary damage caused by disruption of normal anatomy occurred pathologically in a limited area and time window (Sun et al., 2016). Subsequently, the release of blood components [red blood cells (RBCs), thrombin (Babu et al., 2012), hemoglobin, and hemin (Robinson et al., 2009; Babu et al., 2012)], coagulation factors, complement components, and immunoglobulins activate multiple cerebral cells such as endothelial cells, microglia, and astrocyte, which is followed by proinflammatory cytokines release (Wagner et al., 2002; Nakamura et al., 2005; Aronowski and Zhao, 2011). As a result, the expression of Toll-like receptors (TLRs) and adhesion-related molecules (ARMs) is upregulated (Kodali et al., 2021). Furthermore, TLRs, as a group of class I transmembrane proteins, are critical to identifying the pathogen-associated molecular patterns (PAMPs) from bacteria (Zhu and Mohan, 2010; Fitzgerald and Kagan, 2020) and damage-associated molecular patterns (DAMPs) from systemic inflammatory injury (Kong and Le, 2011). Owing to the above contributors, the cerebral cells will transform into a “hyper-alert state” and become highly sensitive to exogenous substances and active signals. Thus, it is sepsis insult in patients with ICH, which is similar to adding fuel to the fire. The peripheral immune cells are selected and activated by invasive bacteria or toxins in circulation, secreting a series of inflammatory cytokines to induce the systemic inflammatory responses, which further amplify cerebral cell signal cascades (Singer et al., 2018) and bring catastrophic damage to the central nervous system (CNS).
In parallel, circulatory dysfunction can be observed after ICH and sepsis owing to pathological hypoperfusion and coagulation system disorders (Zheng and Wong, 2017; Font et al., 2020). During this process, inflammatory mediators first trigger the endothelial cells to express typical ARMs such as vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule-1 (ICAM-1), endothelin-1, and platelet/endothelial cell adhesion molecule (Machado-Pereira et al., 2017). Especially, endothelin-1 is associated with continuous cerebral vasospasm resulting in local brain ischemia and hypoxia (Zheng and Wong, 2017). Activation of coagulative factors and formation of white/red blood cell plugs are also participating in the ischemic process. Excessive thrombin activation and platelet consumption are implicated with disseminated intravascular coagulation in the late stage (Goyette et al., 2004). Virtually, in the setting of systemic inflammation, the cerebral blood vessels are initially affected by CNS and that mediates further cytokine-dependent signals (Wong et al., 1996; Laflamme and Rivest, 1999). It has been confirmed that macro- and microcirculatory failure occurred rapidly and that is attributed to neurovascular coupling disorder (Rosengarten et al., 2009). Subsequently, extensive cells, especially brain cells, are damaged by compromised supplements of oxygen, nutrients, and metabolites (Sharshar et al., 2004). In turn, these damage signals can feedback to the central and peripheral cells and further augment systemic inflammation, which predisposes to a vicious circle of CNS dysfunction.
The immune system will undergo a profound attenuation process in the setting of severe CNS injury (including traumatic brain injury, stroke, and spinal cord injury) (Fu et al., 2015). A meta-analysis consisting of 137,817 patients has identified the correlation between the high rates of systemic infections and stroke, and reported that approximately 30% of patients with stroke were along with infection including pneumonia or urinary tract infection (Westendorp et al., 2011). Temporary lymphopenia and splenic shrunk can be observed in both humans and animals at the early stage of stroke, via activation of the sympathetic, parasympathetic (cholinergic anti-inflammatory), and hypothalamus-pituitary-adrenal (HPA) axis pathways (Ajmo et al., 2009; Sahota et al., 2013). Thereby, the levels of noradrenaline, acetylcholine, and glucocorticoids in circulation are abruptly elevated by the promotion of these active neuroendocrine pathways, which are responsible for apoptosis and atrophy of lymphoid organs (Wong et al., 2011; Mracsko et al., 2014). This downregulation of immune cell generation and function that originates from the injured brain is aiming to avoid autoimmunity against brain antigens from the death or impaired cells (Fu et al., 2015), whereas it causes systemic immunosuppression and makes the body vulnerable to infections simultaneously (Hug et al., 2009).
The gut vascular barrier (GVB) mainly comprises three defense lines including the biological barrier set up by gut microbiota (Assimakopoulos et al., 2007) and keeps intestinal homeostasis. Intestinal flora displays important metabolic, immunologic, and gut protective functions modulated by the so-called “gut-brain axis” (Haak et al., 2018). Numerous models have shown that microbiota have the potentials to augment the proinflammatory effect of immune cells and even conduct the influx of immune effector cells into distant organs, probably mediated by microbe-associated molecular patterns including lipopolysaccharide (LPS), peptidoglycan, flagellin, and microbiota-derived metabolites (Magnotti et al., 1998; De-Souza and Greene, 2005). Moreover, the microbiota can induce the secretion of antibacterial factors from the gut-epithelial cells and, consequently, augment humoral responses against invading pathogens (Kim et al., 2017). ICH occurrence disturbs GVB integrity and intestinal hemostasis and, ultimately, alters the microbiota composition (Patterson et al., 2019; Rice et al., 2019). A recent study also has confirmed the prominent reduced species diversity and microbiota overgrowth in the dysbiosis induced by ICH, which may reduce intestinal motility and increase gut permeability. While recolonizing, normal health microbiota therapy ameliorated neural deficits and inflammation after ICH (Yu et al., 2021). Thus, it provides an opportunity for the potential translocation of aerobic opportunistic pathogens whereby impaired GVB and, finally, results in the onset of gut-origin sepsis (Donskey, 2004; Haak et al., 2017; Huber-Lang et al., 2018).
There are three pathways to capture sepsis signals by CNS (Ali et al., 2009). Nervous pathways, mainly initiated by PAMPs and inflammatory cytokines via the primary afferent (vagal and trigeminal) and sensorial (olfactory) nerves (Sonneville et al., 2013). At this time, visceral inflammation due to sepsis can be detected by the vagal nerve depending upon its terminal cytokine receptors (Tracey, 2009). In addition, the vagal nucleus transmits signals to the central autonomic system, the neuroendocrine centers, and the amygdala, leading to the alteration of behaviors and emotions (Adam et al., 2013; Lord et al., 2014). Humoral pathways, conducted by circulating inflammatory mediators through choroid plexus (CP) and circumventricular organs (CVOs) (Sonneville et al., 2013). CVOs are defined as the fenestrated regions lacking the intact blood-brain barrier (BBB) around the brain, and hence, molecules and peripheral cells can directly access the cerebral parenchyma through these regions (D’Mello and Swain, 2014). Similar to CVOs structure lacking BBB, CP is constituted of cuboidal epithelium cells and is responsible for secreting cerebrospinal fluid (Ghersi-Egea et al., 2018). Both of these structures express receptors of innate and adaptive immune systems, allowing them to detect central and peripheral inflammatory signals (Adam et al., 2013; Ghersi-Egea et al., 2018). Once relevant signals were captured, they will be amplified and transmitted to deeper areas implicated with controlling behavioral, neuroendocrine, and neurovegetative responses via above two structures (Adam et al., 2013; Berger et al., 2014). BBB alteration, enabling monocytes infiltration, and inflammatory molecules invading in the systemic inflammation (Meneses et al., 2019). Activated endotheliocytes express ARMs and release several inflammatory mediators such as cytokines, prostaglandins, and nitric oxide (Johnston and Webster, 2009). It is involved in the regulation of neurotransmission and neurosecretion (Johnston and Webster, 2009). Studies have reported that both pro-inflammatory cytokines [i.e., tumor necrosis factor (TNF)-α and interleukin (IL)-6 and IL-1β] and anti-inflammatory cytokines (i.e., IL-1Ra and IL-10) collectively participated in these systemic responses and formed inflammation homeostasis (Wong et al., 1997; Ilyin et al., 1998; Pintado et al., 2011). However, the abrupt presence of ICH or sepsis breaks the balance and causes an inflammatory signal cascade.
Endothelial cell activation is a crucial step in the CNS responses to sepsis, which affects microcirculatory function and BBB integrity. Evidence confirmed that LPS could induce various ARM expressions on the endothelial cells such as CD40, E-selectin, VCAM-1, and ICAM-1 (Hess et al., 1996; Wong et al., 1997; Omari and Dorovini-Zis, 2003; Hofer et al., 2008). In addition, several receptors for IL-1, TNF-α, and TLR4 were also upregulated (Wong et al., 1997; Zhou et al., 2009). And these receptors contribute to the secretion of TNF-α, IL-6, and IL-1β, followed by the generation of endothelial/inducible nitric oxide synthase (Freyer et al., 1999; Handa et al., 2008) and type-2 cyclooxygenase (Matsumura et al., 1998). In virtue of these inflammatory mediators, microglia are mobilized to secrete several cytotoxic molecules (Meneses et al., 2019), and astrocytes are also triggered to produce chemokines (the C-C chemokine ligand 2, IL-6, chemokine C-X-C ligand10) via NF-κB pathways (Mayo et al., 2014). Furthermore, the expression of ARMs and TLR4 as well as the conduction of chemokines collectively choose infiltration of peripheral monocytes and their participation in neuroinflammation (Adam et al., 2013). Microcirculatory dysfunction has been widely observed in multiple sepsis models owing to the aggregation of circulating white cells and monocytes in the CNS capillaries, compromising supplements of oxygen, nutrients, and metabolites (Bohatschek et al., 2001; Hofer et al., 2008; Zhou et al., 2009; Taccone et al., 2010). The BBB alteration has been clearly confirmed whereby versatile methods, including blue Evans, fluorescent-labeled dextran clearance, labeled granulocytes, electron microscopy, and magnetic resonance imaging in sepsis models and also in patients (Papadopoulos et al., 1999; Esen et al., 2005, 2012; Sharshar et al., 2007; Handa et al., 2008; Bozza et al., 2010). Furthermore, a recent study has confirmed that BBB displayed a short-term closure at the early stage of inflammation and gradually opened up as the disease progressed (Carloni et al., 2021). Nonetheless, it eventually allows for the entry of neurotoxic molecules, particularly inflammatory mediators, consequently giving rise to brain cell death in systemic inflammation. Likewise, extensive studies have found that the neuroinflammation in the ICH was complicated with gliacyte activation and BBB alteration (Anrather and Iadecola, 2016; Wofford et al., 2019). Therefore, all above mentioned mechanisms indicate that the occurrence of ICH greatly increases the risk of sepsis.
Intracerebral hemorrhage and sepsis can cause similar patterns of neural death and are discussed in the following text (Table 1; Castagna et al., 2016; Oberst, 2016; Lewerenz et al., 2018; Li et al., 2018; Takashima et al., 2019; Nagase et al., 2020). Reactive oxygen species (ROS), a kind of physiological defense molecule, can be maintained at a steady level via mitochondrial oxidative phosphorylation and antioxidant mechanisms (Zhou et al., 2020). However, the onset of ICH or sepsis overgenerates ROS and then causes mitochondrial damage, leading to the deterioration of iron metabolism (Zhou et al., 2020; Liu et al., 2021). Subsequently, hemin released from RBC lysis owing to the elevation of cytokines or bacterial toxins also accounts for excessive free iron in the extracellular matrix (Soares and Weiss, 2015; Ganz, 2016). Subsequently, extracellular iron bounding to the transferrin receptor is internalized by the cells under the drive of inflammatory cytokines (Ludwiczek et al., 2003). Excess iron in the cytoplasm significantly dampens enzyme activity and typically causes potent oxidization, resulting in various cell ferroptosis including neurocytes (Liu et al., 2021). Numerous reports have indicated that ferroptosis is invariably followed by necroptosis (Zhou et al., 2020), and NADPH might be the connectional mediator between the two patterns of cell death (Hou et al., 2019). In systemic inflammation, necroptosis can be initiated by several cytokines including, but not limited to, TNF (Oberst, 2016). Once ferroptosis or necroptosis happened, adjacent cells are likely predisposing to another kind of death pattern especially oxytosis (Zhou et al., 2020). Highly similar to the mechanism of ferroptosis, oxytosis occurrence is also related with the extensive ROS failed to be metabolized because of glutathione depletion (Landshamer et al., 2008; Grohm et al., 2010). Many studies even regarded oxytosis as a component of ferroptosis (Soares and Weiss, 2015; Zhou et al., 2020), and this needs further research to clarify. Apoptosis has been well studied by numerous researchers and mainly conducted by two pathways, namely extrinsic and intrinsic pathways. Among them, the extrinsic pathway is activated by cell surface receptors including TNF receptors (Hasegawa et al., 2011; Fricker et al., 2018; Zhao et al., 2018). Under the condition of systemic inflammation in ICH or sepsis, cerebral proinflammatory factors (e.g., TNF) are released in large quantities, and consequently, the Fas-associated death domain protein can be chosen to activate caspase-8 causing neural apoptosis (Micheau and Tschopp, 2003). Pyroptosis is one of the characteristic manners of cell death upon inflammation. In experimental models, it has been demonstrated to be induced by proinflammatory cytokines (i.e., IL-1β and IL-18) via the combination on the cell membrane between the lipid-selective N-terminal domain and phosphatidylinositol of the lipid plasma membrane (Shi et al., 2015; Ding et al., 2016; Feng et al., 2018). To sum up, inflammatory cytokines and metabolites are prioritized to induce neural death in the ICH with sepsis. Therefore, inhibition of neuroinflammation might be important for curbing brain function deterioration and warrant to be further explored.
Acute brain injury including ICH occurrence will initiate neuroinflammation and then spread inflammatory signals to the periphery, and monocyte infiltration might be a crucial mediator in this process. In an LPS-induced neuroinflammatory mouse model, the infiltrated neutrophils exhibited reverse trans-endothelial migration back to the bloodstream after interacting with microglia (Kim et al., 2020). Subsequently, these reverse-moving neutrophil-transported signals to several organs have been reported in numerous studies. For example, the upregulation of inflammatory cytokines (e.g., IL-8 and IL-10) were observed in the kidney used for organ donation, resulting in the reduction of allograft survival via increasing the number of trafficking inflammatory cells (i.e., FoxP3+ regulatory T cells) (Morariu et al., 2008; Kim et al., 2020). In addition, in the brain injury models, infiltrated monocytes and macrophages were demonstrated to produce several chemoattractants (i.e., leukotriene-B4) and other cytokines (e.g., IL-1β, IL-6, and TNF-α), causing the amplification of pulmonary inflammation (Kalsotra et al., 2007; Mrozek et al., 2015). In addition, other organ damages such as the spleen (Li et al., 2011), gastrointestinal tract (Fung et al., 2017), and liver (D’Mello and Swain, 2014) owing to neuroinflammation have been reported in previous studies. Meanwhile, in sepsis, the reactions of the host to invasive bacteria or toxins typically induce phagocytosis in macrophages and secrete a series of proinflammatory cytokines (Takeuchi and Akira, 2010). Besides, this so-called “cytokines storm” subsequently activates the innate immune system (D’Elia et al., 2013). Apparently, the activation of the innate immune system, which is modulated by pattern-recognition receptors, upregulates the expression of associated inflammatory genes via detecting PAMPs or DAMPs (Raymond et al., 2017). Thus, the simultaneous occurrence of ICH and sepsis can elicit the superposition of inflammatory effects and cause extensive organ damages. Obviously, the inflammatory imbalance represents the most important basis for brain dysfunction pathogenesis in the ICH with sepsis and occurs throughout the whole process of the ICH with sepsis.
Ischemia processes, consisting of microcirculatory dysfunction and macrocirculatory dysfunction, can be observed in the ICH and sepsis development. Furthermore, microcirculatory dysfunction arising from endothelial cell activation has been well-demonstrated in various sepsis models (Taccone et al., 2010). Meanwhile, endothelial cell dysfunction also activates the coagulation system to participate in the ischemia process (Adam et al., 2013). Similar processes have been reported in acute cerebral injury patients with vasospasm. It is presented as persistent narrowing of cerebral arteries and is believed to be contributed by spasmogenic or neuroinflammatory factors (Kassell et al., 1985; Taccone et al., 2010), which indicates the role of inflammation in the ischemia process. In addition, severe inflammatory responses caused by sepsis will disturb neurovascular coupling, followed by a disorder of heart rate and blood pressure and deterioration of macrocirculation (Godin and Buchman, 1996; Jafari and Damani, 2020). It has been reported the autonomic controlling system of the heart and vessel is compromised in polymicrobial sepsis because of the degraded autonomic nervous system (Pancoto et al., 2008). Besides, the parasympathetic nervous system (e.g., vagal nerve) is regarded as one of the critical pathways connecting the center and periphery, suggesting that the autonomic nervous system functions and inflammation may be interdependent (Huston and Tracey, 2011). The damage to the susceptible regions of the CNS, whether chemical or mechanical nature, can augment the sympathetic nerve or HPA axis, further causing the dysregulation of catecholamine and dopamine secretion (Lattanzi et al., 2018). The amount of catecholamine released into the bloodstream will activate α-receptors on the cell surface to produce vasoconstriction for abdominal viscera, leading to consequent hypoperfusion and ischemic injury (Lattanzi et al., 2018). In conclusion, the ischemia process in the ICH accompanied with sepsis is under the co-modulation of inflammation and neuroendocrine changes.
Damage-associated molecular patterns are non-microbial molecules in the host nucleus or cytoplasm and consist of high mobility group box 1 (HMGB1), histones, and adenosine triphosphate (Sunden-Cullberg et al., 2005; Ekaney et al., 2014; Zhou et al., 2015). Once released to the extracellular matrix from injury cells, they will act as the effective activators of the immune system and perpetuate non-infectious inflammatory responses to cause systemic inflammation and cellular injury, even death (Matzinger, 1994; Seong and Matzinger, 2004; Rubartelli and Lotze, 2007). In addition, the exact mechanisms may be implicated with the proinflammatory cytokines and chemokines secreted from active macrophages/microglia, which facilitates excessive neutrophil activation and infiltration into the tissues (Denning et al., 2019). Activated neutrophils can generate several kinds of toxic mediators including ROS and inducible nitric oxide synthase (iNOS) to cause oxidative stress and cellular injury (Gentile and Moldawer, 2013; Schaefer, 2014; Brinkmann, 2018). TLRs are considered as the pivotal signal receptors for DAMPs. Rodriguez-Yanez et al. (2012) have reported in a recent clinical study that the upregulation of TLR2 and TLR4 in the peripheral monocytes is closely related to the undesired prognosis in patients with ICH. In addition, the improved neurological function after ICH onset was demonstrated in the TLR4-knockout rodent (Sansing et al., 2011; Lin et al., 2012). HMGB1, as one of the DAMPs extensively discussed, can enable microglia to increase the NF-κB activity and the transcription of cyclooxygenase-2, TNF-α, and IL-1β (Yang et al., 2011). In turn, the TNF-α can feedback on microglia to facilitate the release of HMGB1 (Wang et al., 2015). Evidence indicated that HMGB1 may be contributive to the poor outcomes after CNS injury and the serum levels, which was associated with the disease severity (Nakahara et al., 2009; Zhou et al., 2010).
In summary, under the effect of inflammatory imbalance and ischemia, injury and/or death cells in multiple organs release DAMPs into circulation and amplify systemic inflammation, accompanying oxidative stress and cytotoxic mediator production, further causing more damage and brain dysfunction worsening.
Increasing evidence has indicated that there is an inextricable inflammatory association between the center and periphery. CNS injury will spread the danger signals to the periphery, and vice versa. Under the mediation of systemic inflammation and circulatory dysfunction, the pathological changes can be observed in multiple organs of the patients with ICH or sepsis. Recent advances in the multi-omics analysis could provide vivid evidence regarding the cascade of biofluids from the injured brain, namely, cerebrospinal fluid and circulation blood, and urine and saliva. In contrast, clinical therapies for patients with ICH with sepsis frequently focus on a single organ or system lacking holistic ideas, causing dissatisfied outcomes. For the ICH process, sepsis presence displays an aggravation to peripheral inflammation imbalance that should not be ignored. Although there are many methods attempting to modulate the peripheral immune system, such as antibiotic prophylaxis and probiotic therapy, and have shown limited achievement (Kim and Cho, 2021), the prospective views considered that we should operate artificial restriction for the peripheral immune system and achieve the cross-tolerance among organs via altering immune cell composition. Based on that, stem cell therapy was extensively used in clinical trials for diverse diseases including hemorrhagic stroke, and exhibited many advantages. Therefore, further study for crosstalk between center and periphery might be beneficial for us to explore potential methods for improving brain dysfunction and prognosis in patients with ICH or sepsis or combination.
JL, BT, and YL drafted the manuscript, and prepared the figure and table. HF and YC proofread and revised the manuscript, then gave the final approval for this submission. All authors contributed to the article and approved the submitted version.
This study was supported by the State Key Laboratory of Trauma, Burn and Combined Injury (SKLYQ202002 to YC), National Natural Science Foundation of China (82030036 to HF), Chongqing Talent Program (4139Z2391 to HF), and Southwest Hospital (SWH2018BJKJ-05 to YC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adam, N., Kandelman, S., Mantz, J., Chretien, F., and Sharshar, T. (2013). Sepsis-induced brain dysfunction. Expert. Rev. Anti. Infect. Ther. 11, 211–221.
Ajmo, C. T. Jr., Collier, L. A., Leonardo, C. C., Hall, A. A., Green, S. M., Womble, T. A., et al. (2009). Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp. Neurol. 218, 47–55. doi: 10.1016/j.expneurol.2009.03.044
Ali, M., Lyden, P., Sacco, R. L., Shuaib, A., Lees, K. R., and VISTA Investigators (2009). Natural history of complications after intracerebral haemorrhage. Eur. J. Neurol. 16, 624–630.
Anrather, J., and Iadecola, C. (2016). Inflammation and stroke: an overview. Neurotherapeutics 13, 661–670. doi: 10.1007/s13311-016-0483-x
Aronowski, J., and Zhao, X. (2011). Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42, 1781–1786. doi: 10.1161/STROKEAHA.110.596718
Assimakopoulos, S. F., Scopa, C. D., and Vagianos, C. E. (2007). Pathophysiology of increased intestinal permeability in obstructive jaundice. World J. Gastroenterol. 13, 6458–6464. doi: 10.3748/wjg.v13.i48.6458
Babu, R., Bagley, J. H., Di, C., Friedman, A. H., and Adamson, C. (2012). Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg. Focus 32:E8. doi: 10.3171/2012.1.FOCUS11366
Berger, B., Gumbinger, C., Steiner, T., and Sykora, M. (2014). Epidemiologic features, risk factors, and outcome of sepsis in stroke patients treated on a neurologic intensive care unit. J. Crit. Care 29, 241–248. doi: 10.1016/j.jcrc.2013.11.001
Bohatschek, M., Werner, A., and Raivich, G. (2001). Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp. Neurol. 172, 137–152. doi: 10.1006/exnr.2001.7764
Bozza, F. A., Garteiser, P., Oliveira, M. F., Doblas, S., Cranford, R., Saunders, D., et al. (2010). Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J. Cereb. Blood Flow Metab. 30, 440–448. doi: 10.1038/jcbfm.2009.215
Brinkmann, V. (2018). Neutrophil extracellular traps in the second decade. J. Innate Immun. 10, 414–421. doi: 10.1159/000489829
Carloni, S., Bertocchi, A., Mancinelli, S., Bellini, M., Erreni, M., Borreca, A., et al. (2021). Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 374, 439–448. doi: 10.1126/science.abc6108
Castagna, C., Merighi, A., and Lossi, L. (2016). Cell death and neurodegeneration in the postnatal development of cerebellar vermis in normal and Reeler mice. Ann. Anat. 207, 76–90. doi: 10.1016/j.aanat.2016.01.010
Cheng, Y., Zan, J., Song, Y., Yang, G., Shang, H., and Zhao, W. (2018). Evaluation of intestinal injury, inflammatory response and oxidative stress following intracerebral hemorrhage in mice. Intern. J. Mol. Med. 42, 2120–2128. doi: 10.3892/ijmm.2018.3755
D’Elia, R. V., Harrison, K., Oyston, P. C., Lukaszewski, R. A., and Clark, G. C. (2013). Targeting the “cytokine storm” for therapeutic benefit. Clin. Vac. Immunol. 20, 319–327.
Denning, N. L., Aziz, M., Gurien, S. D., and Wang, P. (2019). DAMPs and NETs in sepsis. Front. Immunol. 10:2536. doi: 10.3389/fimmu.2019.02536
De-Souza, D. A., and Greene, L. J. (2005). Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit. Care Med. 33, 1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97
Ding, J., Wang, K., Liu, W., She, Y., Sun, Q., Shi, J., et al. (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. doi: 10.1038/nature18590
D’Mello, C., and Swain, M. G. (2014). Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav. Immun. 35, 9–20. doi: 10.1016/j.bbi.2013.10.009
Donskey, C. J. (2004). The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39, 219–226. doi: 10.1086/422002
Ekaney, M. L., Otto, G. P., Sossdorf, M., Sponholz, C., Boehringer, M., Loesche, W., et al. (2014). Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 18:543. doi: 10.1186/s13054-014-0543-8
Esen, F., Erdem, T., Aktan, D., Orhan, M., Kaya, M., Eraksoy, H., et al. (2005). Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit. Care 9, R18–R23. doi: 10.1186/cc3004
Esen, F., Senturk, E., Ozcan, P. E., Ahishali, B., Arican, N., Orhan, N., et al. (2012). Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit. Care Med. 40, 1214–1220. doi: 10.1097/CCM.0b013e31823779ca
Feng, S., Fox, D., and Man, S. M. (2018). Mechanisms of gasdermin family members in inflammasome signaling and cell death. J. Mol. Biol. 430(18 Pt B), 3068–3080. doi: 10.1016/j.jmb.2018.07.002
Fitzgerald, K. A., and Kagan, J. C. (2020). Toll-like receptors and the control of immunity. Cell 180, 1044–1066.
Font, M. D., Thyagarajan, B., and Khanna, A. K. (2020). Sepsis and septic shock - basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. North Am. 104, 573–585. doi: 10.1016/j.mcna.2020.02.011
Freyer, D., Manz, R., Ziegenhorn, A., Weih, M., Angstwurm, K., Docke, W. D., et al. (1999). Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 163, 4308–4314.
Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M., and Brown, G. C. (2018). Neuronal cell death. Physiol. Rev. 98, 813–880.
Fu, Y., Liu, Q., Anrather, J., and Shi, F. D. (2015). Immune interventions in stroke. Nat. Rev. Neurol. 11, 524–535. doi: 10.1038/nrneurol.2015.144
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Ganz, T. (2016). Macrophages and iron metabolism. Microbiol. Spectr. 4:5. doi: 10.1128/microbiolspec.MCHD-0037-2016
Gentile, L. F., and Moldawer, L. L. (2013). DAMPs, PAMPs, and the origins of SIRS in bacterial sepsis. Shock 39, 113–114. doi: 10.1097/SHK.0b013e318277109c
Ghersi-Egea, J. F., Strazielle, N., Catala, M., Silva-Vargas, V., Doetsch, F., and Engelhardt, B. (2018). Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 135, 337–361. doi: 10.1007/s00401-018-1807-1
Godin, P. J., and Buchman, T. G. (1996). Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 24, 1107–1116. doi: 10.1097/00003246-199607000-00008
Goncalves, B., Kurtz, P., Turon, R., Santos, T., Prazeres, M., Righy, C., et al. (2019). Incidence and impact of sepsis on long-term outcomes after subarachnoid hemorrhage: a prospective observational study. Ann. Intens. Care 9:94. doi: 10.1186/s13613-019-0562-3
Goyette, R. E., Key, N. S., and Ely, E. W. (2004). Hematologic changes in sepsis and their therapeutic implications. Semin. Respir. Crit. Care Med. 25, 645–659. doi: 10.1055/s-2004-860979
Grohm, J., Plesnila, N., and Culmsee, C. (2010). Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav. Immun. 24, 831–838. doi: 10.1016/j.bbi.2009.11.015
Haak, B. W., Levi, M., and Wiersinga, W. J. (2017). Microbiota-targeted therapies on the intensive care unit. Curr. Opin. Crit. Care 23, 167–174. doi: 10.1097/MCC.0000000000000389
Haak, B. W., Prescott, H. C., and Wiersinga, W. J. (2018). Therapeutic potential of the gut microbiota in the prevention and treatment of sepsis. Front. Immunol. 9:2042. doi: 10.3389/fimmu.2018.02042
Handa, O., Stephen, J., and Cepinskas, G. (2008). Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am. J. Physiol. Heart Circ. Physiol. 295, H1712–H1719. doi: 10.1152/ajpheart.00476.2008
Hasegawa, Y., Suzuki, H., Sozen, T., Altay, O., and Zhang, J. H. (2011). Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir. Suppl. 110(Pt 1), 43–48. doi: 10.1007/978-3-7091-0353-1_8
Hess, D. C., Thompson, Y., Sprinkle, A., Carroll, J., and Smith, J. (1996). E-selectin expression on human brain microvascular endothelial cells. Neurosci. Lett. 213, 37–40. doi: 10.1016/0304-3940(96)12837-8
Hofer, S., Bopp, C., Hoerner, C., Plaschke, K., Faden, R. M., Martin, E., et al. (2008). Injury of the blood brain barrier and up-regulation of icam-1 in polymicrobial sepsis. J. Surg. Res. 146, 276–281. doi: 10.1016/j.jss.2007.07.021
Hou, L., Huang, R., Sun, F., Zhang, L., and Wang, Q. (2019). NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology 417, 64–73. doi: 10.1016/j.tox.2019.02.011
Huber-Lang, M., Lambris, J. D., and Ward, P. A. (2018). Innate immune responses to trauma. Nat. Immunol. 19, 327–341.
Hug, A., Dalpke, A., Wieczorek, N., Giese, T., Lorenz, A., Auffarth, G., et al. (2009). Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke 40, 3226–3232. doi: 10.1161/STROKEAHA.109.557967
Huston, J. M., and Tracey, K. J. (2011). The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med. 269, 45–53. doi: 10.1111/j.1365-2796.2010.02321.x
Ilyin, S. E., Gayle, D., Flynn, M. C., and Plata-Salaman, C. R. (1998). Interleukin-1beta system (ligand, receptor type I, receptor accessory protein and receptor antagonist), TNF-alpha, TGF-beta1 and neuropeptide Y mRNAs in specific brain regions during bacterial LPS-induced anorexia. Brain Res. Bull. 45, 507–515. doi: 10.1016/s0361-9230(97)00437-1
Jafari, M., and Damani, R. (2020). Blood pressure variability and outcome after acute intracerebral hemorrhage. J. Neurol. Sci. 413:116766.
Johnston, G. R., and Webster, N. R. (2009). Cytokines and the immunomodulatory function of the vagus nerve. Br. J. Anaesth. 102, 453–462. doi: 10.1093/bja/aep037
Kalsotra, A., Zhao, J., Anakk, S., Dash, P. K., and Strobel, H. W. (2007). Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J. Cereb. Blood Flow Metab. 27, 963–974. doi: 10.1038/sj.jcbfm.9600396
Kassell, N. F., Sasaki, T., Colohan, A. R., and Nazar, G. (1985). Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 16, 562–572.
Kim, E., and Cho, S. (2021). CNS and peripheral immunity in cerebral ischemia: partition and interaction. Exp. Neurol. 335:113508. doi: 10.1016/j.expneurol.2020.113508
Kim, S., Covington, A., and Pamer, E. G. (2017). The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 279, 90–105. doi: 10.1111/imr.12563
Kim, Y. R., Kim, Y. M., Lee, J., Park, J., Lee, J. E., and Hyun, Y. M. (2020). Neutrophils return to bloodstream through the brain blood vessel after crosstalk with microglia during LPS-induced neuroinflammation. Front. Cell Dev. Biol. 8:613733. doi: 10.3389/fcell.2020.613733
Kodali, M. C., Chen, H., and Liao, F. F. (2021). Temporal unsnarling of brain’s acute neuroinflammatory transcriptional profiles reveals panendothelitis as the earliest event preceding microgliosis. Mol. Psychiatry 26, 3905–3919. doi: 10.1038/s41380-020-00955-5
Kong, Y., and Le, Y. (2011). Toll-like receptors in inflammation of the central nervous system. Int. Immunopharmacol. 11, 1407–1414. doi: 10.1016/j.intimp.2011.04.025
Kopeikina, E., Dukhinova, M., Yung, A. W. Y., Veremeyko, T., Kuznetsova, I. S., Lau, T. Y. B., et al. (2020). Platelets promote epileptic seizures by modulating brain serotonin level, enhancing neuronal electric activity, and contributing to neuroinflammation and oxidative stress. Prog. Neurobiol. 188:101783. doi: 10.1016/j.pneurobio.2020.101783
Laflamme, N., and Rivest, S. (1999). Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor kappaB alpha within specific cellular populations of the rat brain. J. Neurochem. 73, 309–321. doi: 10.1046/j.1471-4159.1999.0730309.x
Landshamer, S., Hoehn, M., Barth, N., Duvezin-Caubet, S., Schwake, G., Tobaben, S., et al. (2008). Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ. 15, 1553–1563. doi: 10.1038/cdd.2008.78
Lattanzi, S., Cagnetti, C., Rinaldi, C., Angelocola, S., Provinciali, L., and Silvestrini, M. (2018). Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J. Neurol. Sci. 387, 98–102. doi: 10.1016/j.jns.2018.01.038
Lewerenz, J., Ates, G., Methner, A., Conrad, M., and Maher, P. (2018). Oxytosis/Ferroptosis-(Re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front. Neurosci. 12:214. doi: 10.3389/fnins.2018.00214
Li, M., Li, F., Luo, C., Shan, Y., Zhang, L., Qian, Z., et al. (2011). Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J. Trauma 71, 141–147. doi: 10.1097/TA.0b013e3181f30fc9
Li, Q., Weiland, A., Chen, X., Lan, X., Han, X., Durham, F., et al. (2018). Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front. Neurol. 9:581. doi: 10.3389/fneur.2018.00581
Lin, S., Yin, Q., Zhong, Q., Lv, F. L., Zhou, Y., Li, J. Q., et al. (2012). Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 9:46. doi: 10.1186/1742-2094-9-46
Liu, Q., Wu, J., Zhang, X., Wu, X., Zhao, Y., and Ren, J. (2021). Iron homeostasis and disorders revisited in the sepsis. Free Radic. Biol. Med. 165, 1–13. doi: 10.1016/j.freeradbiomed.2021.01.025
Lord, A. S., Langefeld, C. D., Sekar, P., Moomaw, C. J., Badjatia, N., Vashkevich, A., et al. (2014). Infection after intracerebral hemorrhage: risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke 45, 3535–3542. doi: 10.1161/STROKEAHA.114.006435
Ludwiczek, S., Aigner, E., Theurl, I., and Weiss, G. (2003). Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101, 4148–4154. doi: 10.1182/blood-2002-08-2459
Machado-Pereira, M., Santos, T., Bernardino, L., and Ferreira, R. (2017). Vascular inter-regulation of inflammation: molecular and cellular targets for CNS therapy. J. Neurochem. 140, 692–702. doi: 10.1111/jnc.13914
Magnotti, L. J., Upperman, J. S., Xu, D. Z., Lu, Q., and Deitch, E. A. (1998). Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann. Surg. 228, 518–527. doi: 10.1097/00000658-199810000-00008
Matsumura, K., Cao, C., Ozaki, M., Morii, H., Nakadate, K., and Watanabe, Y. (1998). Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J. Neurosci. 18, 6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998
Matzinger, P. (1994). Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045. doi: 10.1146/annurev.iy.12.040194.005015
Mayo, L., Trauger, S. A., Blain, M., Nadeau, M., Patel, B., Alvarez, J. I., et al. (2014). Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156. doi: 10.1038/nm.3681
Meneses, G., Cardenas, G., Espinosa, A., Rassy, D., Perez-Osorio, I. N., Barcena, B., et al. (2019). Sepsis: developing new alternatives to reduce neuroinflammation and attenuate brain injury. Ann. N.Y. Acad. Sci. 1437, 43–56. doi: 10.1111/nyas.13985
Micheau, O., and Tschopp, J. (2003). Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190. doi: 10.1016/s0092-8674(03)00521-x
Morariu, A. M., Schuurs, T. A., Leuvenink, H. G., van Oeveren, W., Rakhorst, G., and Ploeg, R. J. (2008). Early events in kidney donation: progression of endothelial activation, oxidative stress and tubular injury after brain death. Am. J. Transpl. 8, 933–941. doi: 10.1111/j.1600-6143.2008.02166.x
Mracsko, E., Liesz, A., Karcher, S., Zorn, M., Bari, F., and Veltkamp, R. (2014). Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav. Immun. 41, 200–209. doi: 10.1016/j.bbi.2014.05.015
Mrozek, S., Constantin, J. M., and Geeraerts, T. (2015). Brain-lung crosstalk: implications for neurocritical care patients. World J. Crit. Care Med. 4, 163–178. doi: 10.5492/wjccm.v4.i3.163
Nagase, H., Katagiri, Y., Oh-Hashi, K., Geller, H. M., and Hirata, Y. (2020). Reduced sulfation enhanced oxytosis and ferroptosis in mouse hippocampal HT22 cells. Biomolecules 10:92. doi: 10.3390/biom10010092
Nakahara, T., Tsuruta, R., Kaneko, T., Yamashita, S., Fujita, M., Kasaoka, S., et al. (2009). High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocrit. Care 11, 362–368. doi: 10.1007/s12028-009-9276-y
Nakamura, T., Keep, R. F., Hua, Y., Hoff, J. T., and Xi, G. (2005). Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 1039, 30–36. doi: 10.1016/j.brainres.2005.01.036
Oberst, A. (2016). Death in the fast lane: what’s next for necroptosis? FEBS J. 283, 2616–2625. doi: 10.1111/febs.13520
Omari, K. M., and Dorovini-Zis, K. (2003). CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J. Neuroimmunol. 134, 166–178. doi: 10.1016/s0165-5728(02)00423-x
Pancoto, J. A., Correa, P. B., Oliveira-Pelegrin, G. R., and Rocha, M. J. (2008). Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton. Neurosci. 138, 57–63. doi: 10.1016/j.autneu.2007.10.006
Papadopoulos, M. C., Lamb, F. J., Moss, R. F., Davies, D. C., Tighe, D., and Bennett, E. D. (1999). Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin. Sci. 96, 461–466.
Patterson, T. T., Nicholson, S., Wallace, D., Hawryluk, G. W. J., and Grandhi, R. (2019). Complex feed-forward and feedback mechanisms underlie the relationship between traumatic brain injury and the gut-microbiota-brain axis. Shock 52, 318–325. doi: 10.1097/SHK.0000000000001278
Pintado, C., Revilla, E., Vizuete, M. L., Jimenez, S., Garcia-Cuervo, L., Vitorica, J., et al. (2011). Regional difference in inflammatory response to LPS-injection in the brain: role of microglia cell density. J. Neuroimmunol. 238, 44–51. doi: 10.1016/j.jneuroim.2011.06.017
Raymond, S. L., Holden, D. C., Mira, J. C., Stortz, J. A., Loftus, T. J., Mohr, A. M., et al. (2017). Microbial recognition and danger signals in sepsis and trauma. Biochim. Biophys. Acta Mol. Basis Dis. 1863(10 Pt B), 2564–2573. doi: 10.1016/j.bbadis.2017.01.013
Rice, M. W., Pandya, J. D., and Shear, D. A. (2019). Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front. Neurol. 10:875. doi: 10.3389/fneur.2019.00875
Robinson, S. R., Dang, T. N., Dringen, R., and Bishop, G. M. (2009). Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 14, 228–235. doi: 10.1179/135100009X12525712409931
Rodriguez-Yanez, M., Brea, D., Arias, S., Blanco, M., Pumar, J. M., Castillo, J., et al. (2012). Increased expression of Toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J. Neuroimmunol. 247, 75–80. doi: 10.1016/j.jneuroim.2012.03.019
Rosengarten, B., Wolff, S., Klatt, S., and Schermuly, R. T. (2009). Effects of inducible nitric oxide synthase inhibition or norepinephrine on the neurovascular coupling in an endotoxic rat shock model. Crit. Care 13:R139. doi: 10.1186/cc8020
Rubartelli, A., and Lotze, M. T. (2007). Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436. doi: 10.1016/j.it.2007.08.004
Sahota, P., Vahidy, F., Nguyen, C., Bui, T. T., Yang, B., Parsha, K., et al. (2013). Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int. J. Stroke 8, 60–67. doi: 10.1111/ijs.12022
Sansing, L. H., Harris, T. H., Welsh, F. A., Kasner, S. E., Hunter, C. A., and Kariko, K. (2011). Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann. Neurol. 70, 646–656. doi: 10.1002/ana.22528
Schaefer, L. (2014). Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 289, 35237–35245. doi: 10.1074/jbc.R114.619304
Seong, S. Y., and Matzinger, P. (2004). Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478. doi: 10.1038/nri1372
Sharshar, T., Annane, D., de la Grandmaison, G. L., Brouland, J. P., Hopkinson, N. S., and Francoise, G. (2004). The neuropathology of septic shock. Brain Pathol. 14, 21–33. doi: 10.1111/j.1750-3639.2004.tb00494.x
Sharshar, T., Carlier, R., Bernard, F., Guidoux, C., Brouland, J. P., Nardi, O., et al. (2007). Brain lesions in septic shock: a magnetic resonance imaging study. Intens. Care Med. 33, 798–806. doi: 10.1007/s00134-007-0598-y
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. doi: 10.1038/nature15514
Singer, B. H., Dickson, R. P., Denstaedt, S. J., Newstead, M. W., Kim, K., Falkowski, N. R., et al. (2018). Bacterial dissemination to the brain in sepsis. Am. J. Respir. Crit. Care Med. 197, 747–756. doi: 10.1164/rccm.201708-1559OC
Soares, M. P., and Weiss, G. (2015). The Iron age of host-microbe interactions. EMBO Rep. 16, 1482–1500. doi: 10.15252/embr.201540558
Sonneville, R., Verdonk, F., Rauturier, C., Klein, I. F., Wolff, M., Annane, D., et al. (2013). Understanding brain dysfunction in sepsis. Ann. Intens. Care 3:15. doi: 10.1186/2110-5820-3-15
Sun, X., Jones, Z. B., Chen, X. M., Zhou, L., So, K. F., and Ren, Y. (2016). Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship. J. Neuroinflamm. 13:260. doi: 10.1186/s12974-016-0736-y
Sunden-Cullberg, J., Norrby-Teglund, A., Rouhiainen, A., Rauvala, H., Herman, G., Tracey, K. J., et al. (2005). Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 33, 564–573. doi: 10.1097/01.ccm.0000155991.88802.4d
Taccone, F. S., Su, F., Pierrakos, C., He, X., James, S., Dewitte, O., et al. (2010). Cerebral microcirculation is impaired during sepsis: an experimental study. Crit. Care 14:R140. doi: 10.1186/cc9205
Takashima, M., Ichihara, K., and Hirata, Y. (2019). Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food Chem. Toxicol. 132:110669. doi: 10.1016/j.fct.2019.110669
Takeuchi, O., and Akira, S. (2010). Pattern recognition receptors and inflammation. Cell 140, 805–820. doi: 10.1016/j.cell.2010.01.022
Tracey, K. J. (2009). Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428. doi: 10.1038/nri2566
Wagner, K. R., Packard, B. A., Hall, C. L., Smulian, A. G., Linke, M. J., De Courten-Myers, G. M., et al. (2002). Protein oxidation and heme oxygenase-1 induction in porcine white matter following intracerebral infusions of whole blood or plasma. Dev. Neurosci. 24, 154–160. doi: 10.1159/000065703
Wang, R., Zhang, Q., Yang, S., and Guo, Q. (2015). TNF-alpha induces the release of high mobility group protein B1 through p38 mitogen-activated protein kinase pathway in microglia. Zhong Nan Da Xue Xue Bao Yi Xue Ban 40, 967–972. doi: 10.11817/j.issn.1672-7347.2015.09.004
Westendorp, W. F., Nederkoorn, P. J., Vermeij, J. D., Dijkgraaf, M. G., and van de Beek, D. (2011). Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 11:110. doi: 10.1186/1471-2377-11-110
Wofford, K. L., Loane, D. J., and Cullen, D. K. (2019). Acute drivers of neuroinflammation in traumatic brain injury. Neural Regen. Res. 14, 1481–1489. doi: 10.4103/1673-5374.255958
Wong, C. H., Jenne, C. N., Lee, W. Y., Leger, C., and Kubes, P. (2011). Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105. doi: 10.1126/science.1210301
Wong, M. L., Bongiorno, P. B., al-Shekhlee, A., Esposito, A., Khatri, P., and Licinio, J. (1996). IL-1 beta, IL-1 receptor type I and iNOS gene expression in rat brain vasculature and perivascular areas. Neuroreport 7, 2445–2448. doi: 10.1097/00001756-199611040-00008
Wong, M. L., Bongiorno, P. B., Rettori, V., McCann, S. M., and Licinio, J. (1997). Interleukin (IL) 1beta, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proc. Natl. Acad. Sci. U.S.A. 94, 227–232. doi: 10.1073/pnas.94.1.227
Yang, Q. W., Lu, F. L., Zhou, Y., Wang, L., Zhong, Q., Lin, S., et al. (2011). HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J. Cereb. Blood Flow Metab. 31, 593–605. doi: 10.1038/jcbfm.2010.129
Yu, X., Zhou, G., Shao, B., Zhou, H., Xu, C., Yan, F., et al. (2021). Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice. Front. Microbiol. 12:647304. doi: 10.3389/fmicb.2021.647304
Zhao, H., Chen, Y., and Feng, H. (2018). P2X7 receptor-associated programmed cell death in the pathophysiology of hemorrhagic stroke. Curr. Neuropharmacol. 16, 1282–1295. doi: 10.2174/1570159X16666180516094500
Zheng, V. Z., and Wong, G. K. C. (2017). Neuroinflammation responses after subarachnoid hemorrhage: a review. J. Clin. Neurosci. 42, 7–11. doi: 10.1016/j.jocn.2017.02.001
Zhou, H., Andonegui, G., Wong, C. H., and Kubes, P. (2009). Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 183, 5244–5250. doi: 10.4049/jimmunol.0901309
Zhou, S. Y., Cui, G. Z., Yan, X. L., Wang, X., Qu, Y., Guo, Z. N., et al. (2020). Mechanism of ferroptosis and its relationships with other types of programmed cell death: insights for potential interventions after intracerebral hemorrhage. Front. Neurosci. 14:589042. doi: 10.3389/fnins.2020.589042
Zhou, Y., Dong, H., Zhong, Y., Huang, J., Lv, J., and Li, J. (2015). The cold-inducible RNA-binding protein (CIRP) level in peripheral blood predicts sepsis outcome. PLoS One 10:e0137721. doi: 10.1371/journal.pone.0137721
Zhou, Y., Xiong, K. L., Lin, S., Zhong, Q., Lu, F. L., Liang, H., et al. (2010). Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Med. Inflamm. 2010:142458. doi: 10.1155/2010/142458
Keywords: intracerebral hemorrhage, sepsis, inflammatory, neuronal death, brain dysfunction
Citation: Lin J, Tan B, Li Y, Feng H and Chen Y (2022) Sepsis-Exacerbated Brain Dysfunction After Intracerebral Hemorrhage. Front. Cell. Neurosci. 15:819182. doi: 10.3389/fncel.2021.819182
Received: 21 November 2021; Accepted: 27 December 2021;
Published: 21 January 2022.
Edited by:
Yue He, Tongji Hospital, Huazhong University of Science and Technology, ChinaCopyright © 2022 Lin, Tan, Li, Feng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujie Chen, eXVqaWVjaGVuNjg4NkBmb3htYWlsLmNvbQ==; Y2hlbnlqQHRtbXUuZWR1LmNu; Hua Feng, ZmVuZ2h1YTg4ODhAdmlwLjE2My5jb20=; ZmVuZ2h1YUB0bW11LmVkdS5jbg==
†ORCID: Yujie Chen, orcid.org/0000-0002-9905-9138; Hua Feng, orcid.org/0000-0003-4489-9217
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.