
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 01 December 2020
Sec. Cellular Neurophysiology
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.600777
This article is part of the Research Topic Sensory Processing in Vision and Olfaction – Common Features of Key Players View all 32 articles
 Arlene A. Hirano1,2*
Arlene A. Hirano1,2* Helen E. Vuong1†
Helen E. Vuong1† Helen L. Kornmann1†
Helen L. Kornmann1† Cataldo Schietroma1†
Cataldo Schietroma1† Salvatore L. Stella Jr.1†
Salvatore L. Stella Jr.1† Steven Barnes1,3,4
Steven Barnes1,3,4 Nicholas C. Brecha1,2,3,5,6
Nicholas C. Brecha1,2,3,5,6Feedback inhibition by horizontal cells regulates rod and cone photoreceptor calcium channels that control their release of the neurotransmitter glutamate. This inhibition contributes to synaptic gain control and the formation of the center-surround antagonistic receptive fields passed on to all downstream neurons, which is important for contrast sensitivity and color opponency in vision. In contrast to the plasmalemmal GABA transporter found in non-mammalian horizontal cells, there is evidence that the mechanism by which mammalian horizontal cells inhibit photoreceptors involves the vesicular release of the inhibitory neurotransmitter GABA. Historically, inconsistent findings of GABA and its biosynthetic enzyme, L-glutamate decarboxylase (GAD) in horizontal cells, and the apparent lack of surround response block by GABAergic agents diminished support for GABA's role in feedback inhibition. However, the immunolocalization of the vesicular GABA transporter (VGAT) in the dendritic and axonal endings of horizontal cells that innervate photoreceptor terminals suggested GABA was released via vesicular exocytosis. To test the idea that GABA is released from vesicles, we localized GABA and GAD, multiple SNARE complex proteins, synaptic vesicle proteins, and Cav channels that mediate exocytosis to horizontal cell dendritic tips and axonal terminals. To address the perceived relative paucity of synaptic vesicles in horizontal cell endings, we used conical electron tomography on mouse and guinea pig retinas that revealed small, clear-core vesicles, along with a few clathrin-coated vesicles and endosomes in horizontal cell processes within photoreceptor terminals. Some small-diameter vesicles were adjacent to the plasma membrane and plasma membrane specializations. To assess vesicular release, a functional assay involving incubation of retinal slices in luminal VGAT-C antibodies demonstrated vesicles fused with the membrane in a depolarization- and calcium-dependent manner, and these labeled vesicles can fuse multiple times. Finally, targeted elimination of VGAT in horizontal cells resulted in a loss of tonic, autaptic GABA currents, and of inhibitory feedback modulation of the cone photoreceptor Cai, consistent with the elimination of GABA release from horizontal cell endings. These results in mammalian retina identify the central role of vesicular release of GABA from horizontal cells in the feedback inhibition of photoreceptors.
Horizontal cells receive synaptic input from thousands of photoreceptors and feedback this broad spatial information back to photoreceptors as well as feeding it forward to bipolar cells to generate receptive field surrounds (Thoreson and Mangel, 2012). In 1970, Baylor et al. demonstrated that turtle retinal horizontal cells contribute a negative feedback signal to the cone photoreceptor light response. When our studies began over 30 years later, the proposed cellular mechanisms of horizontal cell neurotransmission were multiple, controversial, and unconventional: voltage- and sodium-dependent, calcium-independent plasmalemmal γ-aminobutyric acid (GABA) transporter (GAT) activity, as characterized in non-mammalian vertebrates (Schwartz, 2002), ephaptic coupling between photoreceptor calcium channel gating and current flow in horizontal cell glutamate receptors and hemichannels shown in fish retina (Byzov and Shura-Bura, 1986; Kamermans et al., 2001), and photoreceptor calcium current regulation by synaptic cleft pH (Hirasawa and Kaneko, 2003; Vessey et al., 2005; Cadetti and Thoreson, 2006; Kreitzer et al., 2012; Wang et al., 2014; Kramer and Davenport, 2015; Tchernookova et al., 2018; Grove et al., 2019). The apparent lack of the cone surround response block by GABAergic pharmacological agents in turtle, goldfish, mouse, and primate retinas (Thoreson and Burkhardt, 1990; Verweij et al., 1996, 2003; Endeman et al., 2012; Kemmler et al., 2014) was used to argue against a direct role for GABA in feedback inhibition. In contrast, early studies reported GABA in horizontal cells (Lam et al., 1978; Mosinger et al., 1986) suggesting that it may be a neurotransmitter used by horizontal cells. However, GABA immunoreactivity in horizontal cells was not consistently observed in adult mammalian retinas (Lam et al., 1978; Schnitzer and Rusoff, 1984; Mosinger et al., 1986; Chun and Wässle, 1989; Wässle and Chun, 1989) raising doubts about its role as a feedback transmitter. Further, unlike other GABAergic neurons, horizontal cells in adult mammalian retina did not take up 3H-GABA or 3H-muscimol (Blanks and Roffler-Tarlov, 1982; Wässle and Chun, 1989) and GATs were not expressed in these cells (Honda et al., 1995; Johnson et al., 1996; Casini et al., 2006; Guo et al., 2009, 2010). In contrast, horizontal cells in cat and monkey retinas showed GABA immunoreactivity (Agardh and Ehinger, 1982; Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Grünert and Wässle, 1990). The GABA synthetic enzymes, glutamic acid decarboxylase65, and 67 (GAD65 and GAD67), were localized to mammalian horizontal cells (Vardi et al., 1994; Johnson and Vardi, 1998). Although like GABA, the GABA synthetic enzymes, GAD65 and GAD67 were observed in horizontal cells during development, and they were not consistently detected in adult horizontal cells (Brandon et al., 1979; Schnitzer and Rusoff, 1984; Mosinger and Yazulla, 1985). In contrast to the lack of GATs, the vesicular GABA transporter (VGAT/VIAAT, vesicular inhibitory amino acid transporter), which loads inhibitory amino acid transmitters into synaptic vesicles (McIntire et al., 1997; Sagné et al., 1997), was observed in amacrine and horizontal cells in multiple mammalian species (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2010; Lee and Brecha, 2010; Hirano et al., 2011). The presence of VGAT in horizontal cell synaptic endings suggested that these unconventional neurons may release the neurotransmitter GABA via vesicular release. When VGAT was deleted from horizontal cells, these cells failed to feedback to photoreceptors (Hirano et al., 2016a) and the same mouse line revealed a lack of autaptic GABA reception by horizontal cells and no influence on cone calcium channels (Grove et al., 2019; Barnes et al., 2020), ending debate, at least in mammalian retinas, about whether horizontal cells utilize vesicular GABA release to send feedback to photoreceptors. Here we marshal evidence for the hypothesis that mammalian horizontal cells possess the cellular structures and proteins that mediate vesicular transmitter release. These include the presence and synthesis of GABA as a neurotransmitter, the essential molecular machinery for vesicular release, the structural basis of vesicular release, namely synaptic vesicles, and the regulated fusion and recycling of synaptic vesicles in mammalian horizontal cells. These findings show that the cellular mechanism underlying feedback inhibition in mammals involves vesicular GABA release by horizontal cells, and this stands to support a new GABA-pH hybrid model wherein autaptic reception of GABA by horizontal cells regulates pH in the synaptic cleft via depolarization and the bicarbonate permeability of the GABA receptors, resulting in the modulation of presynaptic calcium channels in photoreceptors (Grove et al., 2019; Barnes et al., 2020).
Several convergent findings show that GABA is the mammalian horizontal cell transmitter. Mammalian retinas contain typically two morphological types of horizontal cells, an axonless A-type whose dendrites contact only cones and an axon-bearing B-type whose dendrites contact cones and the axonal terminal system, the rods. Some rodents, including mouse and rat, possess only the B-type (Peichl and González-Soriano, 1994). The lack of immunoreactivity for GABA and its synthetic enzymes GAD65 and GAD67 in adult horizonal cells in some studies was used to argue against a role for GABA in horizontal cell neurotransmission. However, many studies have shown evidence for GABA in horizontal cells of cat, rabbit, rat, mouse, guinea pig, and primate retina (Nishimura et al., 1985; Mosinger et al., 1986; Osborne et al., 1986; Agardh et al., 1987; Mosinger and Yazulla, 1987; Wässle and Chun, 1988, 1989; Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Grünert and Wässle, 1990; Pow et al., 1994; Vardi and Auerbach, 1995; Kalloniatis et al., 1996; Johnson and Vardi, 1998; Koulen et al., 1998b; Guo et al., 2010; Deniz et al., 2011; Herrmann et al., 2011), albeit at lower levels than in amacrine cells (Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Vardi et al., 1994; Johnson and Vardi, 1998; Marc et al., 1998). In cat and monkey, horizontal cells in peripheral retina lacked GABA immunoreactivity, whereas they were immunoreactive in central retina (Wässle and Chun, 1989; Grünert and Wässle, 1990). Unlike non-mammalian horizontal cells in which not all subtypes contained GABA (Marc, 1992; Schwartz, 2002; Yang, 2004) both mammalian subtypes appeared to show GABA immunoreactivity (Wässle and Chun, 1989; Grünert and Wässle, 1990; Johnson and Vardi, 1998; Guo et al., 2010). In mouse and rabbit, horizontal cells exhibited high levels of GABA during early retinal development, which then dropped with maturation (Schnitzer and Rusoff, 1984; Osborne et al., 1986; Messersmith and Redburn, 1993; Pow et al., 1994). An example of the GABA immunolabeling is shown in horizontal cells of the adult guinea pig retina, which contains both A- and B-types (Figure 1, Guo et al., 2010) similar to cat and macaque retinas (Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Grünert and Wässle, 1990). GABA immunoreactivity, like the punctate staining of neurotransmitter receptors in retina (Wässle and Chun, 1989; Greferath et al., 1995) was highly sensitive to fixation conditions, favoring weak fixation (e.g., shorter fixation times, lower aldehyde concentrations) for visualization (Guo et al., 2010; Deniz et al., 2011). This lability as well as antibody specificity differences may account for reports of little to no immunostaining observed in well-fixed tissue (Agardh et al., 1986; Osborne et al., 1986; Versaux-Botteri et al., 1989; Messersmith and Redburn, 1992; Yamasaki et al., 1999; Loeliger and Rees, 2005).
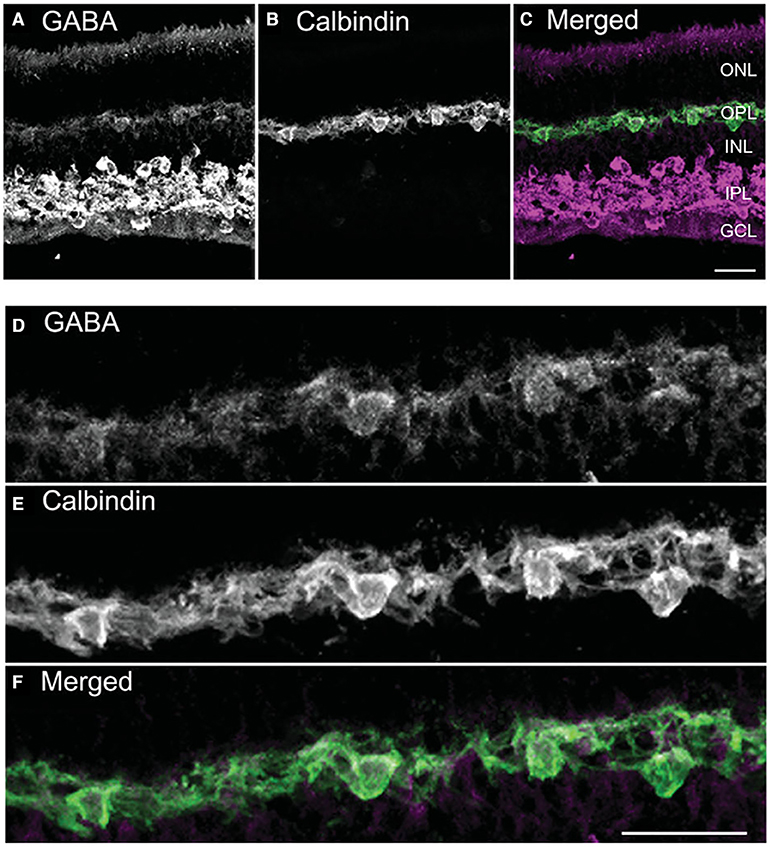
Figure 1. GABA immunoreactivity is localized to horizontal cell bodies and their processes. (A–C) A vertical section through the guinea pig retina was double labeled with antibodies to GABA (A) and calbindin-28K (calbindin, B). Weak, yet distinct, GABA immunolabeling occurs in the outer retina, in contrast to the strong GABA immunolabeling distributed to amacrine cell and displaced amacrine cell bodies and processes in the inner plexiform layer (IPL). (C) Merged image shows the co-localization in horizontal cell bodies and processes. (D–F) Higher magnification views of the outer plexiform layer (OPL) show the GABA immunoreactivity (D) in the calbindin-identified horizontal cells (E) in the merged image (F). Images are maximum intensity projections of 6 optical sections, z = 5 μm. Scale bar, 20 μm in C (applies to A–C), (F) (applies to D–F). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. [Modified from (Guo et al., 2009)].
The GABA-synthesizing enzyme L-glutamate decarboxylase (GAD) exists as two principal isoforms, GAD65 and GAD67 (Erlander et al., 1991; Kaufman et al., 1991). One or both of the GAD isoforms are found in mammalian horizontal cells at both the mRNA (Sarthy and Fu, 1989; Guo et al., 2010; Deniz et al., 2011) and protein levels (Schnitzer and Rusoff, 1984; Vardi et al., 1994; Vardi and Auerbach, 1995; Johnson and Vardi, 1998; Yamasaki et al., 1999; Dkhissi et al., 2001; Guo et al., 2010; Deniz et al., 2011). In rabbit retina, GAD65 and GAD67 immunoreactivities were detected in horizontal cells (Johnson and Vardi, 1998). Several studies report GAD67 immunostaining is present at high levels in horizontal cells of the developing and juvenile mouse, rat, and rabbit retina (Schnitzer and Rusoff, 1984; Osborne et al., 1986; Versaux-Botteri et al., 1989; Pow et al., 1994; Schubert et al., 2010), but at low or non-detectable levels in adult horizontal cells (Brandon et al., 1979; Schnitzer and Rusoff, 1984; Brandon, 1985; Osborne et al., 1986; Wässle and Chun, 1989; Brecha et al., 1991; Yazulla et al., 1997; Koulen et al., 1998b), including mouse (Haverkamp and Wässle, 2000; Schubert et al., 2010; Herrmann et al., 2011). GAD65 immunostaining (Figure 2) and mRNA were detected in adult guinea pig horizontal cells (Guo et al., 2010). Note the concentration of GAD65 immunoreactivity in the horizontal cell endings (Figure 2, arrows) and the scleral portion of the cell body. In rabbit horizontal cells, there are different subcellular localizations of GAD65 and GAD67 protein (Johnson and Vardi, 1998): GAD67 immunolabeling occurred in the dendritic terminals of A type and the dendritic and axonal terminals of the B type horizontal cells; whereas, GAD65 immunolabeling was found in A type somata and primary dendrites within the visual streak. In mouse, horizontal cells appear to express both GAD65 and GAD67 mRNA and protein (Deniz et al., 2011), but whether there is subcellular distribution difference between the two GAD isoform remains an open question.
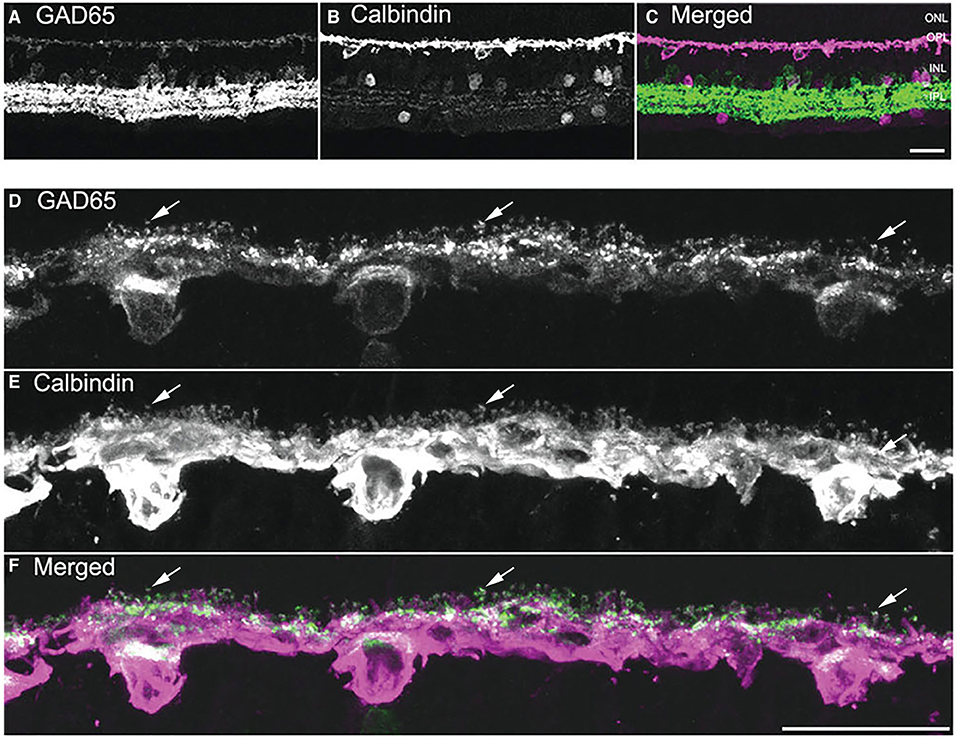
Figure 2. GAD65 immunoreactivity is localized to horizontal cells. (A–C) A vertical section through the guinea pig retina was double labeled with antibodies to GAD65 (A) and calbindin (B). In the outer retina, weak GAD65 immunostaining is present in the cell bodies and processes in the OPL; whereas, strong GAD65 immunoreactivity is in amacrine cell and displaced amacrine cell bodies and processes in the IPL. (B) Horizontal cell somata and processes are labeled with calbindin antibodies. (C) Merged image demonstrates GAD65 immunostaining co-localized with calbindin immunostaining in the outer retina. (D–F) Higher magnification views of the OPL showing the localization of GAD65 immunoreactivity in horizontal cell somata, processes, and endings. (D) GAD65 immunolabeling in the OPL. (E) Calbindin immunolabeling of horizontal cells. (F) Merged image shows the co-expression of GAD65 and calbindin immunoreactivities in the OPL, indicating that GAD65 immunoreactivity is localized to horizontal cell bodies, processes, and tips. Arrows in (D–F) point to GAD65 in horizontal cell endings. Images are maximum intensity projections of 6 optical sections, z = 5 μm. Scale bar, 20 μm in (C) (applies to A–C), (F) (applies to D–F). [Modified from (Guo et al., 2009)].
The Gad1 gene, encoding GAD67, is highly transcriptionally regulated by DNA methylation of the promoter, and exhibits alternative promoter usage and alternative splicing (Martin and Rimvall, 1993; Tao et al., 2018; Lee et al., 2019), that may account for some of the detection variability. Alternative splicing of Gad1 produces proteins of differing molecular weights: the GAD67, GAD44, and GAD25 isoforms (Behar et al., 1993; Trifonov et al., 2014). Whereas GAD67 is thought to be constitutively active, GAD65 activity can be induced by neuronal activity (Lee et al., 2019). In the CNS, GAD65 is enriched in axonal terminals of GABAergic neurons (Esclapez et al., 1994). It is possible that the state of light adaptation and visual experience before collection of the tissue may influence the levels of protein detected (Connaughton et al., 2001). A transiently expressed GAD25/ES isoform was reported in retina (Connaughton et al., 2001; Dkhissi et al., 2001) and may account for the observed loss of GAD67 immunolabeling with retinal maturation. In addition to GAD67, there are at least 10 alternatively spliced isoforms of the full-length Gad1 gene comprised of 19 exons, producing a GAD44 isoform that has enzymatic activity and several GAD25s that do not (Chessler and Lernmark, 2000; Liu et al., 2010; Trifonov et al., 2014; Tao et al., 2018). The Gad2 gene encoding GAD65 appears to produce two splice variants, including a full-length mRNA and a truncated version of undefined function (Davis et al., 2016).
There is also post-transcriptional regulation of GAD, including palmitoylation, phosphorylation, and protein cleavage (Baekkeskov and Kanaani, 2009; Lee et al., 2019) that alters GAD protein activity and conformation, intracellular protein localization, and possibly antibody-targeted epitopes. GAD65 and GAD67 can form heterodimers, during targeting of GAD65 and GAD67 to synaptic vesicles in presynaptic terminals (Dirkx et al., 1995; Kanaani et al., 2010). GAD65 can form a complex with the synaptic vesicle proteins, VGAT, cysteine string protein, and heat shock protein 70 (Wei and Wu, 2008), and thus influence GABA loading into synaptic vesicles (Wei and Wu, 2008; Lee et al., 2019).
The detection of GAD or GABA in the adult retina may be influenced by numerous factors, including the differential expression of GAD isoforms, regulations of levels of Gad transcripts and GAD proteins, and GABA synthesis in horizontal cells, as well as technical issues related to fixation composition, fixation protocols (perfusion or immersion) and antibody specificity (Wässle and Chun, 1989; Pow and Crook, 1994; Vardi et al., 1994; Vardi and Auerbach, 1995; Kalloniatis et al., 1996; Johnson and Vardi, 1998; Deniz et al., 2011). Schubert et al. (2010) confirmed expression of GAD67 during neonatal development in mouse, but never detected GAD65 in horizontal cells. Some investigators observed the volatility of GABA immunoreactivity (Kalloniatis et al., 1996; Deniz et al., 2011) and suggested that it may be due to technical issues with harvesting the retina (Pow and Crook, 1994). GABA immunolabeling in mice was maintained by cardiac perfusion, but not post-dissection, fixation, and under physiological conditions that promoted GAD activity with L-glutamate/glutamine incubation with co-factor pyridoxal phosphate) or intracardiac perfusion with CNQX and cadmium to inhibit transmitter release from horizontal cells prior to fixation (Deniz et al., 2011).
There is evidence that GAD activity and/or level of expression may be regulated by light (Herrmann et al., 2011) and light adaptation (Connaughton et al., 2001) and this may contribute to inconsistencies in detection of GABA in horizontal cells. The GABA immunostaining in horizontal cells increased as mice were subjected to increasing intensity of background light (Herrmann et al., 2011), indicating light increased GABA immunoreactivity. In addition to changes in GAD activity, light stimulation of the retina would result in membrane hyperpolarization of horizontal cells and presumably less release of transmitter. In fish, the levels of the full-length GAD67 mRNA and protein (Connaughton et al., 2001) and GABA were increased in light-adapted retina (Lam, 1972; Starr, 1973; Connaughton et al., 2001). Finally, GAD65 and GAD67 mRNA expression in mouse horizontal cells is consistent with the GFP expression in GAD65-eGFP and GAD67-GFP adult reporter mice (Deniz et al., 2011). These findings suggest expression of both GAD65 and GAD67 in adult mouse horizontal cells occurs (Deniz et al., 2011), but see (Schubert et al., 2010).
The localization of GABA receptors in the outer retina to photoreceptors, bipolar cells, and horizontal cells (Brecha, 1992; Yang, 2004) is congruent with both feedback and feed-forward roles for GABA released from horizontal cells. In non-mammalian retina, such as turtle, fish and salamander, photoreceptors clearly possess functional GABAA receptors, as GABA application generated a chloride conductance (Wu and Dowling, 1980; Tachibana and Kaneko, 1984; Kaneko and Tachibana, 1986; Yazulla et al., 1989; Wu, 1992). Reports of clear-cut expression of GABAA receptors in mammalian photoreceptors are scant, although there are reports of GABAA receptor subunit mRNAs by photoreceptors by in situ hybridization (ISH), single-cell RT-PCR, and GABAA receptor subunit immunohistochemistry (Greferath et al., 1993; Grigorenko and Yeh, 1994; Vardi et al., 1998). In rat retina, GABAA receptor subunit α2 is reported to be expressed at cone photoreceptor terminals and the β1, δ, γ2 mRNAs are expressed in the outer nuclear layer (ONL) (Greferath et al., 1995). However, the α1 subunit mRNA was not detected in the ONL of rat retina (Brecha et al., 1991), consistent with the lack of α1 and ρ1 immunoreactivities in mouse cone pedicles by immunoelectron microscopy (Kemmler et al., 2014). In neonatal rabbit retina, cone photoreceptors transiently express GABAA receptor subunits α1 and β2/3 (Mitchell and Redburn, 1996; Mitchell et al., 1999), when GABA and GAD67 levels are high in horizontal cells (Schnitzer and Rusoff, 1984). Cone terminals of pig and rat were reported to show GABAAρ subunit (ρ subunit) immunoreactivity suggesting the presence of a GABAAρ receptor (Picaud et al., 1998b; Pattnaik et al., 2000). However, Deniz et al. (2019) reported bicuculine-sensitive, but not TPMPA-sensitive, GABA evoked currents in mouse cone photoreceptors in retinal slices, suggesting the presence of ionotropic GABAA receptors, but not those comprising ρ-subunits. Rod photoreceptors from cultured pig retina and in mouse retinal slices were reported to exhibit no response to GABA (Picaud et al., 1998b; Deniz et al., 2019).
Evidence for a horizontal cell feed-forward role includes the expression of GABAA receptor immunoreactivity on bipolar cell dendrites (Wässle and Chun, 1989; Vardi et al., 1992; Greferath et al., 1993; Brecha and Weigmann, 1994; Vardi and Sterling, 1994; Enz et al., 1996; Wässle et al., 1998; Haverkamp and Wässle, 2000; Haverkamp et al., 2000; Hoon et al., 2015). GABAA receptor subunit immunoreactivity is localized to bipolar cell membranes adjacent to horizontal cell endings in cone pedicles and underneath the photoreceptor terminals (Greferath et al., 1994; Vardi and Sterling, 1994; Koulen et al., 1998a; Haverkamp et al., 2000; Puller et al., 2014). The extrasynaptic GABAA receptor α6 subunit is expressed on rod bipolar cell dendrites (Figures 3A–C) (Hirano et al., 2016b), which suggests a role for tonic GABAA receptor currents in feedforward signaling.
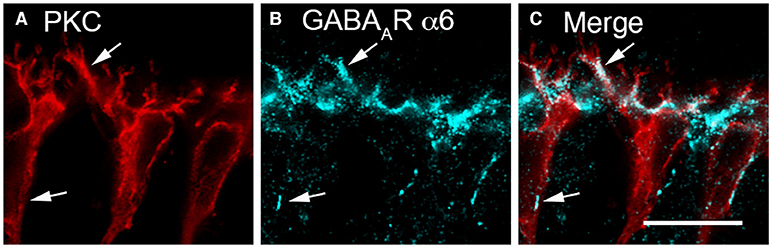
Figure 3. GABAAR α6 subunit immunolabeling occurred in bipolar cell dendrites. (A–C) Rod bipolar cells bear α6 immunoreactivity on their dendrites. (A) PKCα antibodies identify rod bipolar cells (red). (B) α6 immunolabeling (blue) occurs in patches along the bipolar cell dendrites (arrows) in the OPL and in the cell body membrane (arrows). (C) Merged image demonstrates co-localization of α6 and PKCα immunoreactivities in the dendrites of rod bipolar cells (arrows) and to a lesser degree on their somata (arrows). Single optical section (A–C). Scale bar, 10 μm. (Modified from Hirano et al., 2016b).
As functional evidence of a feedforward input, full-field light stimulation, applied in the presence of L-AP4 to block direct photoreceptor input, reduced a gabazine-sensitive current in ON-cone bipolar cells (Yang and Wu, 1991; Chaffiol et al., 2017). This feedforward input results from GABAA receptor activation at ON cone bipolar cell dendrites, which is reduced by horizontal cell hyperpolarization. GABA may evoke responses of opposite polarities in ON and OFF bipolar cells as a result of differing internal chloride concentrations in their dendrites (Duebel et al., 2006). GABA elicited depolarizing inward currents when applied to dendrites of mouse rod bipolar cells and hyperpolarizing currents when applied to OFF-bipolar cells, congruent with feedforward input from horizontal cells (Satoh et al., 2001; Duebel et al., 2006). The basis of the differential intracellular chloride is the expression of Na+-K+-Cl− co-transporter (NKCC), which transports chloride into the cellular compartment, which is prominent in ON bipolar cell dendrites and horizontal cells (Vardi et al., 2000; Dmitriev et al., 2007; Puller et al., 2014). NKCC promotes accumulation of intracellular chloride and generates a chloride equilibrium potential above the resting membrane potential and thus a depolarization when ionotropic GABA receptor chloride channels are opened. In contrast, K+-Cl− co-transporter (KCC2), a chloride extruder, is expressed in OFF bipolar cell dendrites and axonal terminals of ON and OFF bipolar cells (Vardi et al., 2000), where a GABA-activated chloride conductance would elicit a hyperpolarization.
Finally, GABA released by horizontal cells appears to act back on the horizontal cells themselves (Kamermans and Werblin, 1992; Blanco et al., 1996; Feigenspan and Weiler, 2004; Varela et al., 2005; Thoreson and Mangel, 2012). In non-mammalian horizontal cells, GABA elicited currents by activating ionotropic GABAA receptors, including GABAAρ receptors, or electrogenic transporters [fish: (Wu and Dowling, 1980; Schwartz, 1982; Gilbertson et al., 1991; Kamermans and Werblin, 1992; Cammack and Schwartz, 1993; Qian and Dowling, 1993; Takahashi et al., 1994, 1995; Jung et al., 1999) salamander: (Yang and Wu, 1993; Dong and Werblin, 1994; Yang et al., 1999; Wang et al., 2000)]. GABA elicited ionotropic GABAA receptor-mediated currents in mammalian (rabbit, mouse, rat, human) horizontal cells, but not a transporter-mediated current (Blanco et al., 1996; Picaud et al., 1998a; Feigenspan and Weiler, 2004; Varela et al., 2005; Liu et al., 2013). GABA and/or muscimol application activated ionotropic GABAA receptors and elicited chloride currents, blocked by bicuculline and picrotoxin, in whole-cell recordings of isolated rabbit, mouse, and rat horizontal cells (Blanco and de la Villa, 1999; Feigenspan and Weiler, 2004; Liu et al., 2013). In mouse horizontal cells, we showed distinct immunolabeling for GABAAρ ρ2 subunit localized predominantly to their endings at its axon terminals within rod spherules and at its dendrites at cone pedicles (Grove et al., 2019; Barnes et al., 2020), indicating the presence of GABAAρ receptors. Notable characteristics of GABAAρ receptors include high affinity for GABA and non-desensitizing currents, capable of producing tonic currents at ambient levels of interstitial GABA, similar to extrasynaptic GABA receptors in other areas of the CNS (Bormann and Feigenspan, 1995; Bormann, 2000; Farrant and Nusser, 2005). Horizontal cells, recorded in rodent (mouse, rat, guinea pig) retinal slices, maintained a tonic GABA current in the cone terminal synaptic cleft that was sensitive to TPMPA, a GABA Aρ receptor blocker, and this tonic current proved critical for feedback inhibition of cone calcium current (Grove et al., 2019). Recordings in a horizontal cell conditional knockout of VGAT showed this tonic GABA current was abolished in these horizontal cells (Grove et al., 2019), suggesting that horizontal cells were the source of the GABA. In addition to ionotropic GABA receptors, metabotropic GABAB receptors have been reported on rat horizontal cell processes (Koulen et al., 1998b). Taken together, these studies indicate multiple targets for GABA exist in the OPL, which could mediate the action of horizontal cells in the outer retina.
Earlier models of GABA release from non-mammalian horizontal cells posited a central role for a Ca-independent, Na-dependent GABA transporter, GAT-1 (Schwartz, 1987; 2002). GABA uptake or release from the cytoplasm (Schwartz, 2002) is unlikely in mammalian horizontal cells based on several findings. First, uptake studies using radiolabeled GABA or GABA analogs have not reported high affinity uptake of these molecules by adult horizontal cells, although high affinity uptake was readily observed in amacrine cells (Ehinger, 1977; Agardh and Ehinger, 1982; Blanks and Roffler-Tarlov, 1982; Mosinger et al., 1986; Pow et al., 1996). In addition, GABA transporter currents have not been detected in isolated mouse and rabbit horizontal cells (Feigenspan and Weiler, 2004; Varela et al., 2005). These findings are consistent with the failure to detect GAT mRNAs and immunostaining in horizontal cells of mouse, rat, and guinea pig retinas (Brecha and Weigmann, 1994; Honda et al., 1995; Johnson et al., 1996; Guo et al., 2009). In mammalian retinas, GAT-1 and GAT-3 instead are expressed by Müller cells (Johnson et al., 1996; Guo et al., 2009) that take up [3H]-GABA (Marshall and Voaden, 1975; Blanks and Roffler-Tarlov, 1982).
In mammals, a preponderance of evidence shows that GABA meets the criteria for being a neurotransmitter of horizontal cells. There is the synthetic machinery for GABA in horizontal cells, detectable GABA immunoreactivity, and a plethora of GABA receptors in the OPL that would mediate the action of the released GABA (Wässle et al., 1998; Haverkamp et al., 2000). While mammalian horizontal cells do not express GATs, GABA uptake occurs in Müller cell processes that surround photoreceptor terminals, producing a honeycomb pattern in the outer plexiform layer (OPL) (Burris et al., 2002; Guo et al., 2009).
VGAT is a transporter that accumulates inhibitory amino acid transmitters into synaptic vesicles in GABA- and glycine-containing neurons (McIntire et al., 1997; Sagné et al., 1997; Chaudhry et al., 1998; Gasnier, 2000). Whereas mammalian horizontal cells lack plasmalemmal GATs (Johnson et al., 1996; Guo et al., 2009), our laboratory and others showed the presence of the vesicular inhibitory amino acid/GABA transporter (VIAAT/VGAT) in mammalian horizontal cells in mouse (Figure 4A), rat, rabbit and primate retina, where VGAT immunostaining is concentrated in the endings that insert into the rod and cone photoreceptor terminals (Figure 4A, arrows, Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Johnson et al., 2003; Hirano et al., 2005, 2007, 2011; Guo et al., 2010; Lee and Brecha, 2010). Note also the labeled interplexiform process of a tyrosine hydroxylase (TH) amacrine cell (Figure 4A, arrowhead, Witkovsky et al., 2008) and strongly immunolabeled interplexiform layer (IPL) and amacrine cell somata (*). Ultrastructural analysis showed that VGAT immunolabeling was found in the horizontal processes that form the lateral elements at mouse and rat photoreceptor synapses (Figures 4B,C, Cueva et al., 2002). This VGAT localization to synaptic endings suggested that mammalian horizontal cells released GABA via vesicular exocytosis for signaling.
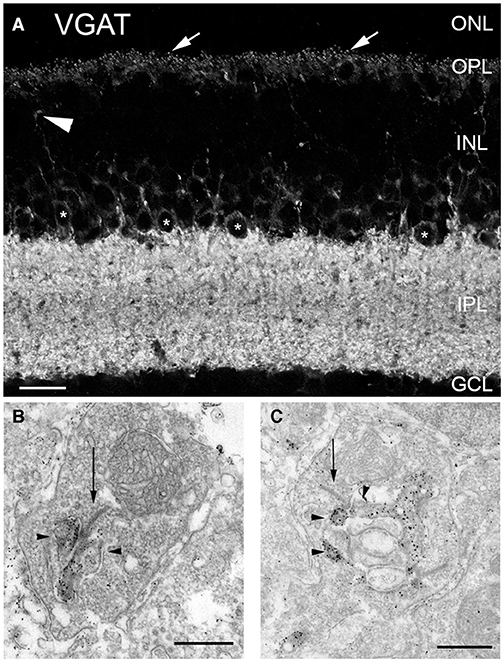
Figure 4. Vesicular γ-aminobutyric acid transporter (VGAT) was present in horizontal cell endings. (A) VGAT antibody staining of a vertical section of mouse retina showed labeled puncta (arrows), weak immunolabeling in the OPL and strong immunolabeling in the IPL, and around cell bodies (*) of the proximal inner nuclear layer (INL). Arrowhead points to a VGAT-containing interplexiform process. ONL, outer nuclear layer; GCL, ganglion cell layer. Scale bar, 20 μm. (B,C) VGAT immunoreactivity is localized in horizontal cell synaptic endings at photoreceptor synapses. Electron micrographs illustrate the dark and granular DAB reaction product of the VGAT immunoreactivity in terminals of horizontal cells of mouse (B) and rat (C) retina. Arrows indicate photoreceptor synaptic ribbons. Arrowheads indicate horizontal cells. Scale bars, 0.5 μm in (B,C). (Modified from (Cueva et al., 2002).
The core complex for fusion of synaptic vesicles with the plasma membrane consist of three soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) proteins: two are plasma membrane proteins, syntaxin-1 and SNAP-25, and the third is the vesicle-associated membrane protein (VAMP-2)/synaptobrevin-2 (Jahn and Scheller, 2006; Südhof, 2013; Yoon and Munson, 2018). Horizontal cell endings contain the SNARE protein isoforms: SNAP-25 (Hirano et al., 2011), syntaxin-1a (Hirano et al., 2005), and VAMP-1, that likely interact to form the minimal machinery for membrane fusion. Figure 5 depicts double labeling for VGAT and SNAP-25, which shows co-localization in horizontal cells in the OPL of mouse (Figures 5A-C,A′-C′), rat (Figures 5D-F), and rabbit retina (Figures 5G-I).
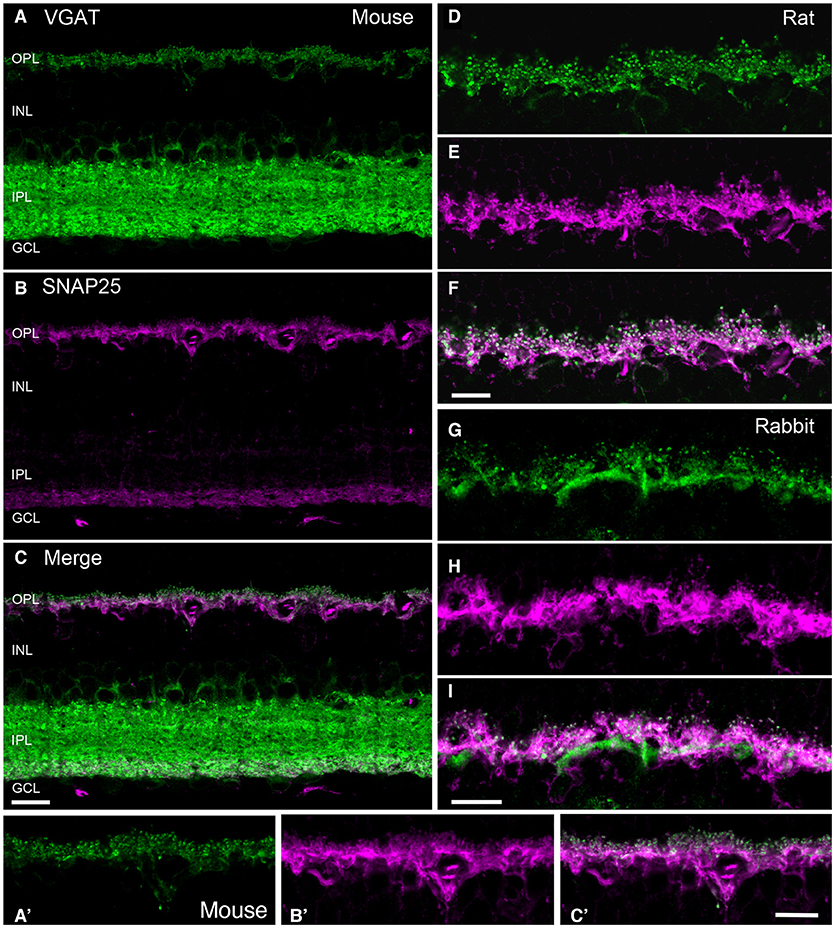
Figure 5. SNARE protein SNAP-25 co-localized with VGAT in horizontal cell processes in mammalian retina. VGAT antibody staining (green) of a vertical section of mouse (A,A'), rat (D), and rabbit (G) retinas showed immunolabeling in the OPL and the IPL, and around cell bodies of the proximal inner nuclear layer (INL). SNAP-25 antibody labeling (magenta) of the same section produced immunolabeling in the OPL and the proximal IPL of mouse (B), and OPL of mouse (A'), rat (E), and rabbit (H) retinae. Merged images of the VGAT and SNAP-25 immunolabeling (white) indicated co-localization of SNAP-25 with VGAT in the tips of horizontal cells in mouse (C,C'), rat (F), and rabbit (I). GCL, ganglion cell layer. Maximum intensity projections. Scale bar, 10 μm in (C) (applies to A–C), (F) (applies to D–F), and (I) (applies to G–I). (Modified from Hirano et al., 2011).
While there were consistent reports of SNAP-25 immunoreactivity in the IPL of mammalian retinas (Catsicas et al., 1992; Ullrich and Südhof, 1994; Brandstätter et al., 1996b; Grabs et al., 1996; Von Kriegstein et al., 1999; Greenlee et al., 2001), there were contradictory reports of its cellular distribution in the outer retina. SNAP-25 immunostaining was reported in horizontal cells of several mammalian species (mouse, rat, monkey, cow) (Catsicas et al., 1992; Grabs et al., 1996; Von Kriegstein et al., 1999; Greenlee et al., 2001). In contrast, other studies reported SNAP-25 immunoreactivity in rat photoreceptor terminals, but not horizontal cells (Ullrich and Südhof, 1994; Brandstätter et al., 1996b; Morgans et al., 1996). Our studies (Lee and Brecha, 2010; Hirano et al., 2011) showed consistent SNAP-25 immunostaining in mouse (Figure 5B), rat (E) and rabbit (H) horizontal cells, identified by calbindin immunoreactivity (Röhrenbeck et al., 1987), with multiple SNAP-25 antibodies (Hirano et al., 2011). SNAP-25 co-localized with VGAT in all three species (Figure 5) and SNAP-25 immunolabeling was found ultrastructurally in horizontal cell processes at photoreceptor terminals (Figure 6A, Hirano et al., 2011). Not surprisingly, as SNAP-25 participates in vesicle trafficking in multiple cellular pathways, one of the SNAP-25 antibody (SMI-31) labeled all retinal cell types (Hirano et al., 2011). Differences in retinal SNAP-25 labeling patterns may be due to the expression of two isoforms of SNAP-25a and b, one of which confers a palmitoylation site for plasma membrane anchoring (Hirano et al., 2011).
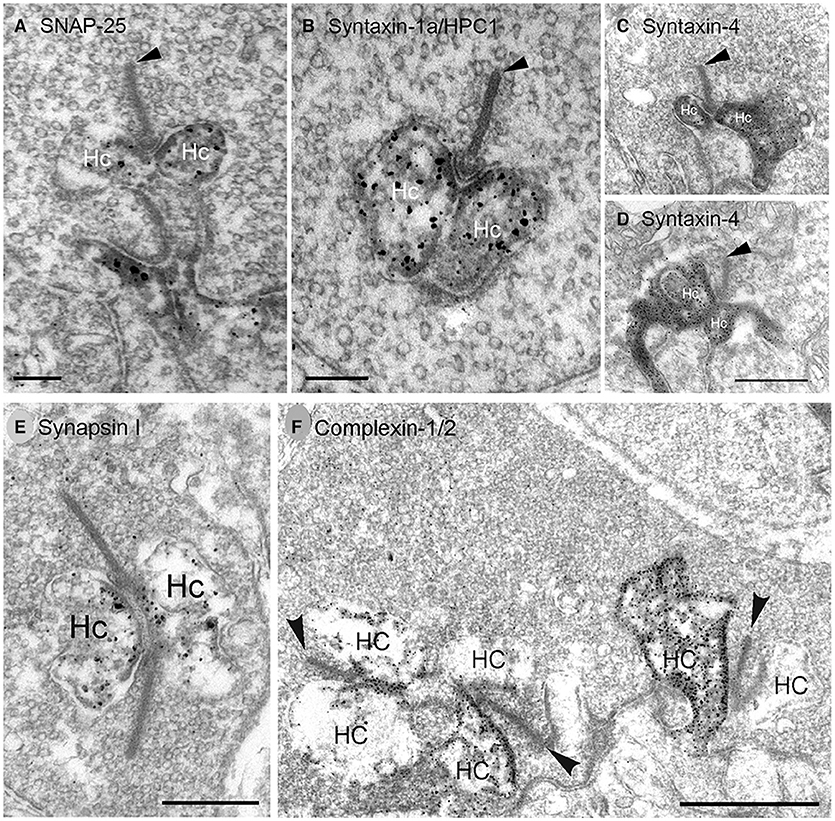
Figure 6. SNARE complex and synaptic proteins localize to horizontal cell synaptic endings. Pre-embedding immunoelectron microscopy with antibodies to (A) SNAP-25, (B) syntaxin-1a/HPC1, (C,D) syntaxin-4, (E) synapsin I, (F) complexin-1/2 produced dark, granular DAB immunolabeling for each SNARE (A–D, SNAP-25, syntaxin-1a, syntaxin-4) or synaptic protein (E,F, synapsin I, complexin-1/2) in horizontal cell (Hc) endings at (A–E) rod photoreceptor synapses. (F) Complexin-1/2 labeling of lateral elements at cone photoreceptor synapses. Arrowheads point to synaptic ribbons. (A,B,E,F), rabbit retina; (C,D), mouse retina. Scale bars, 0.2 μm in (A), 0.3 μm in (B), 0.5 μm in (C,D), 0.4 μm in (E), 1 μm in (F). (Modified from (A) Hirano et al., 2011; (B,E,F) Hirano et al., 2005; (C,D) Hirano et al., 2007).
Syntaxin-1 to−4 direct vesicle targeting to the plasma membrane, with syntaxin-1 typically specialized for presynaptic membranes (Teng et al., 2001; Südhof, 2004; Sherry et al., 2006; Rizo and Xu, 2015). Syntaxin-1a is highly expressed in amacrine cells (Barnstable et al., 1985), but Syntaxin-1a/HPC1 is also present in horizontal cells, albeit at lower levels (Figures 7A–F, Brandstätter et al., 1996b; Morgans et al., 1996; Greenlee et al., 2001; Hirano et al., 2005; Lee and Brecha, 2010), as well as in interplexiform cell processes in the OPL (Brandstätter et al., 1996b; Morgans et al., 1996). Our pre-embedding immunoelectron microscopy findings show syntaxin-1a immunoreactivity in mammalian horizontal cell endings (Figure 6B). In situ proximity ligation assay (PLA, Biolink Bioscience) employs specific antibodies against potentially interacting proteins and visualizes these close (within 40 nm) interactions with oligonucleotide-conjugated secondary antibodies to identify individual sites of protein-protein interactions at the cellular level in tissue sections (Söderberg et al., 2006, 2008). Using PLA, we have shown that SNAP25 and syntaxin-1a are located within 40 nm of each other (red puncta) in the OPL, indicating that they are likely binding partners in horizontal cell processes (Figures 7G,H, Brecha et al., 2010). This interaction likely occurs in the horizontal cells in the OPL, as the SNAP-25 and Syntaxin-1a/HPC1 antibodies only immunolabeled guinea pig horizontal cells in the outer retina (Lee and Brecha, 2010). Furthermore, photoreceptors express a different syntaxin isoform, syntaxin-3b, in their synaptic terminals (Morgans et al., 1996; Curtis et al., 2008; Hays et al., 2020).

Figure 7. Cellular localization of syntaxin-1a to horizontal cells and their processes and endings in photoreceptor synapses. (A) Syntaxin-1a (red) immunolabeling co-localized with that of (B) calbindin (blue) in horizontal cell bodies and processes in the OPL (C), as well as some amacrine cell bodies (arrows) in the INL in rabbit retina. In addition to horizontal cells, calbindin immunoreactivity was present in a subtype of bipolar cell and amacrine cells in rabbit retina. OPL, outer plexiform layer; IPL, inner plexiform layer. (D–F) Higher magnification views of immunolabeling for syntaxin-1a (D), calbindin (E), and merged image (F) in the OPL of rabbit retina. Arrows point to horizontal cell endings. (A–C) Single optical section; (D–F) maximum intensity projection, z = 2.88 μm. (Modified from Hirano et al., 2005). (G,H) In situ proximity ligation assay (PLA) revealed protein interactions between the plasma membrane SNARE proteins SNAP-25 and syntaxin-1a in both plexiform layers. (G) In situ PLA marks close (within 40 nm) protein interactions and identifies these interactions as distinct puncta that are localized to the OPL and more densely in the IPL of guinea pig retina. (H) Negative controls in which one of the antibodies was omitted resulted in no puncta. (G,H) Confocal images were scanned at 0.5 μm intervals, and maximum intensity projection of 9 optical images, z = 4.0 μm. Scale bar, 20 μm (A–C in C, D–F in F, G,H). (Modified from Brecha et al., 2010).
We found syntaxin-4, another isoform that targets vesicles to the plasma membrane (Teng et al., 2001) is highly expressed in horizontal cells at axonal terminals and dendrites (Figure 8), where it is concentrated beneath cone pedicles (Figure 8, arrows), and in the lateral elements at photoreceptor terminals (Hirano et al., 2007). Figures 8A–C shows syntaxin-4 immunolabeling in the OPL of mouse (A), rat (B), and rabbit (C) retina, which co-localizes with the horizontal cell marker, calbindin (Hirano et al., 2007). Syntaxin-4 co-localizes with SNAP-25 in the endings of horizontal cells (Figures 8E–G, Hirano et al., 2007). Immunoelectron microscopy places syntaxin-4 immunoreactivity in the lateral elements at photoreceptor synapses (Figures 6C,D). In other neuronal systems, syntaxin-4 is found in postsynaptic membranes and marks a domain for ionotropic glutamate receptor exocytosis in dendritic spines in hippocampus (Kennedy et al., 2010; Bin et al., 2019) and NGF release from Schwann cells (Lin et al., 2017). At the Drosophila neuromuscular junction, syntaxin-4 is postsynaptic and is involved in retrograde signaling to motoneurons (Harris et al., 2016) to regulate neurotransmitter release and the number of presynaptic active zones and Ca channels (Harris et al., 2018).
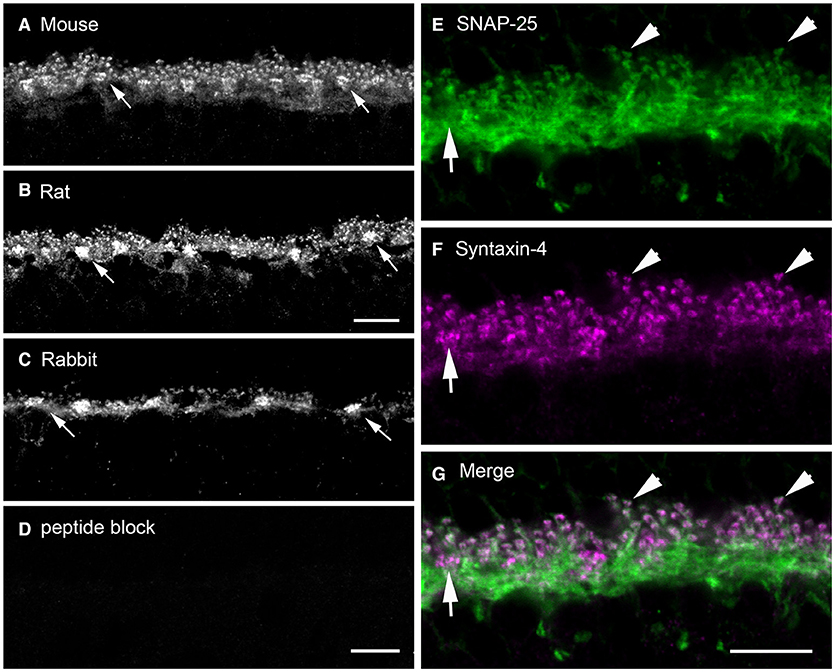
Figure 8. Syntaxin-4 immunolabeling is present in the outer plexiform layer of mouse, rat, and rabbit retinas and colocalizes with that of SNAP-25. (A–C) Localization of syntaxin-4 immunoreactivity in vertical sections of (A) mouse, (B) rat, and (C) rabbit outer retina. Note the prominent immunoreactivity in the OPL of all three species. Arrows point toward thickenings or sandwiches of syntaxin-4 immunolabeling. (D) Pre-adsorption of the antibody with the antigenic peptide abolishes specific labeling in rabbit retina. (E–G) Syntaxin-4 (F) immunolabeling co-localized with that of SNAP-25 in horizontal cell processes and endings (E) as seen in the (G) merged image in mouse retina. Arrows point to horizontal cell dendritic contacts with cone pedicles. Arrowheads point to immunolabeling in horizontal cell axonal endings. (A,C,D) Maximum intensity projection of 3 images, z = 0.6 μm. (B) Maximum intensity projection of 5 images, z = 0.46 μm. (E–G) Maximum intensity projection of 3 images, z = 0.6 μm. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. Scale bars, 10 μm (A,B in B, C,D in D, E–G in G). (Modified from Hirano et al., 2007).
We observed VAMP-1, rather than VAMP-2, in horizontal cell endings by double label immunohistochemistry (Figures 9A–C, (Bitzer and Brecha, 2006; Lee and Brecha, 2009). VAMP-2 is the more common VAMP/synaptobrevin isoform in SNARE complexes at conventional synapses, with VAMP-1 occurring to a lesser degree (Elferink et al., 1989; Brunger et al., 2019). In well-fixed mouse retina, the VAMP-1 labeling was reported to be weaker than that of VAMP-2, in the plexiform layers (Sherry et al., 2003). The strong fixation may have resulted in difficulties in interpretation of VAMP-1 immunostaining, as VAMP-1 immunoreactivity did not appear to label synaptic structures (Sherry et al., 2003).
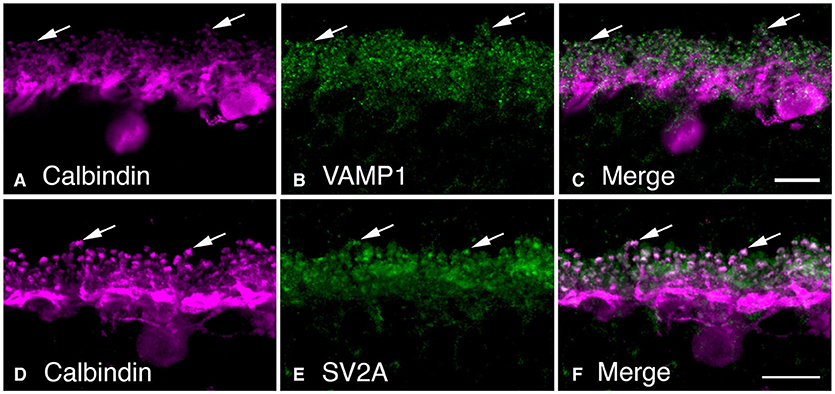
Figure 9. Synaptic vesicle proteins, VAMP-1 and SV2A, are found in horizontal cell synaptic endings. (A–C) VAMP-1 immunolabeling (B, green) was observed in the OPL of mouse retina. (B) Horizontal cells labeled with calbindin antibodies (A, magenta). (C) Merged image demonstrated the co-localization of VAMP-1 and calbindin immunoreactivities in horizontal cell processes and particularly in the synaptic endings (arrows). (D–F) Synaptic vesicle protein SV2A immunolabeling (E) was present in the OPL as large puncta. (D) Horizontal cells identified by calbindin immunoreactivity (magenta). (F) Merged image shows co-localization of SVA and calbindin immunoreactivities in horizontal cell endings. There is also SV2A immunolabeling surrounding the horizontal cell endings, in likely photoreceptor terminals. Maximum intensity projections, A–C, z = 0.6 μm; D–F, z = 6.42 μm. (Modified from Bitzer and Brecha, 2006; Brecha et al., 2010).
Given the prevalent view at the time that there were few or no synaptic vesicles in the horizontal cell endings [(Schwartz, 2002), but see (Dowling and Boycott, 1966; Dowling, 1970; Raviola and Gilula, 1975; Spiwoks-Becker et al., 2001; Zampighi et al., 2011)] we checked whether there were other key synaptic vesicle proteins in addition to VGAT. There are at least 40 different families of vesicle and synaptic proteins, including the synaptotagmins, synapsins, GTP-binding Rab proteins and complexins, that have critical roles in Ca2+-dependent transmitter release, including Ca2+ sensing, vesicle trafficking, and vesicle fusion (Jahn and Scheller, 2006; Takamori et al., 2006). Most of these proteins have multiple isoforms that are differentially expressed in the nervous system (Linial, 1997; Hong, 2005). From this screen, we localized several synaptic vesicle proteins to horizontal cell endings (Hirano et al., 2005, 2007, 2011; Lee and Brecha, 2010), supporting the hypothesis that horizontal cells contain synaptic vesicles, and transmitter is released by a vesicular mechanism.
SV2A is a ubiquitous synaptic vesicle transporter protein in the brain (Buckley and Kelly, 1985; Bajjalieh et al., 1992; Feany et al., 1992; Janz and Südhof, 1999) and is involved in sensing presynaptic calcium levels to prime synaptic vesicles for calcium-dependent exocytosis (Janz et al., 1999; Chang and Südhof, 2009; Wan et al., 2010). Knockout of SV2A resulted in a reduction in hippocampal GABAergic neurotransmission (Crowder et al., 1999). In outer retina, SV2A co-localized with VGAT in horizontal cell endings in likely synaptic vesicles (Lee and Brecha, 2010). Figures 9D–F shows SV2A co-localized with calbindin in horizontal cell endings, as well as in photoreceptor terminals (Brecha et al., 2010). SV2A was reported earlier to be transiently expressed in horizontal cells and cone photoreceptors during mouse retina development, but not in adult retina (Wang et al., 2003). The lack of double labeling for calbindin to clearly identify horizontal cell processes in the OPL in the relatively low-power magnification images makes it difficult to rule out horizontal cell labeling. In well-fixed adult mouse retina, SV2A was reported to be in cone ribbon synapses and a subset of conventional synapses; whereas, SV2B was in photoreceptor and bipolar cell ribbon synapses and SV2C, to sparse conventional synapses in the outer retina and starburst amacrine cells (Wang et al., 2003).
Synaptotagmins form a complex with SV2 proteins in a calcium-dependent manner, in part to regulate presynaptic calcium levels (Marqueze et al., 2000; Südhof, 2002; Wan et al., 2010) and accelerate synaptic vesicle priming and initiate fast, calcium-triggered release (Südhof, 2013). Synaptotagmin-1 and−2 are synaptic vesicle proteins with two calcium binding motifs (C2A and C2B) involved in calcium sensing in calcium-triggered transmitter release (Littleton et al., 1993; Südhof and Rizo, 1996; Südhof, 2002; Grassmeyer et al., 2019). Synaptotagmin-2, but not synaptotagmin-1, is enriched in the horizontal cell endings at both rod and cone photoreceptor terminals in mouse, rat, and guinea pig retina (Fox and Sanes, 2007; Lee and Brecha, 2010). Figures 10D–F show the co-localization of synaptotagmin-2 with Cav2.2, the principal, pore-forming subunit of N-type Ca channels (Hirano and Brecha, 2010). In the cerebellum, synaptotagmin-2 is the fast Ca sensor at the basket cell-Purkinje cell synapse (Chen et al., 2017).
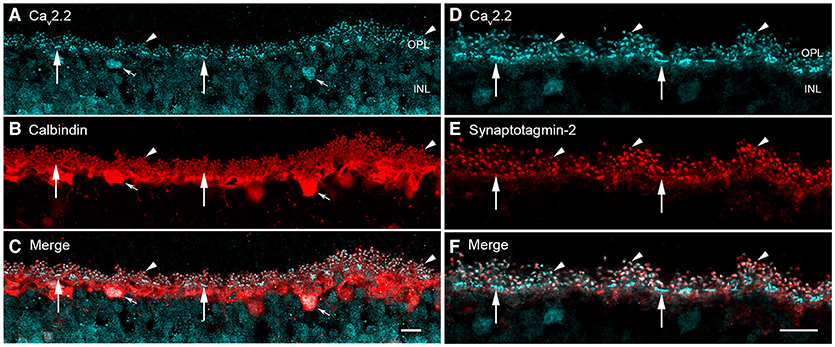
Figure 10. Voltage-gated calcium channels and calcium sensor synaptotagmin-2 are expressed in mouse horizontal cell endings. (A–C) Cav2.2 (α1B, N-type) Ca channels immunolabeling (A, blue) occurred as discrete puncta in the OPL in mouse retina (arrowheads). (B) Calbindin immunolabeling (red) identifies horizontal cells in outer retina (arrowheads). (C) Merged image showed co-localization of Cav2.2 and calbindin immunoreactivities (arrowheads), suggesting Cav2.2 is at horizontal cell synaptic endings. Small arrows point to Cav2.2 immunoreactivity in horizontal cell bodies, suggesting that Cav2.2 is expressed by horizontal cells and not photoreceptors. (D–F) Cav2.2 co-localizes with the calcium sensor synaptotagmin-2 in horizontal cells. (D) Cav2.2 (blue) immunolabeling occurred as puncta (arrowheads) and bars (arrows) in the OPL. (E) Calcium sensor synaptotagmin-2 (red) immunolabeling occurred in horizontal cell processes and is concentrated in the tips. (F) Merged image showed that Cav2.2 and synaptotagmin-2 are present in the same subcellular compartment of the horizontal cell axonal endings. The synaptotagmin-2 immunostaining in horizontal cell dendrites at cones (arrows) appeared to be less intense than at the axonal endings, perhaps simply reflecting the volume of the compartment. Maximum intensity projections, A–F, z = 1.20 μm. Scale bars, 10 μm (A–C in C, D–F in F). (Modified Hirano and Brecha, 2010).
Complexins interact with synaptotagmins and the SNARE complex in a calcium-dependent manner to regulate synchronous transmitter release (Rizo and Xu, 2015; Mortensen et al., 2016). In retina, complexin isoforms are differentially expressed (Hirano et al., 2005; Reim et al., 2005; Lee and Brecha, 2010) with complexin-1/2 found at conventional synapses and complexin-3 and−4 at ribbon synapses (Reim et al., 2005; Vaithianathan et al., 2013; Babai et al., 2016; Mortensen et al., 2016; Bhoi et al., 2020). Complexin-3 is also found at glycinergic synapses in the lobular appendages of AII amacrine cells (Landgraf et al., 2012). Complexin-1/2 localized to rabbit (Figure 6F), mouse and guinea pig horizontal cell endings (Hirano et al., 2005; Reim et al., 2005; Lee and Brecha, 2010) and GABAergic amacrine cells (Hirano et al., 2005; Reim et al., 2005). In addition to interacting with synaptotagmins, complexins bind to SNARE proteins to regulate SNARE complex assembly (Chen et al., 2002; Kummel et al., 2011; Li et al., 2011) to promote synchronous release from a readily releasable pool and to inhibit asynchronous release (Trimbuch and Rosenmund, 2016; Zhou et al., 2017).
Synapsins are a family of 4 abundant synaptic vesicle-associated phosphoproteins that regulate synaptic vesicle availability (Hilfiker et al., 1999) and are markers of conventional synapses in retina, but not of ribbon synapses (Mandell et al., 1990). Synapsin I was expressed at low levels in rabbit horizontal cells (Hirano et al., 2005), consistent with the likely horizontal cell labeling in ferret retina (Karne et al., 1997) and guinea pig horizontal cells, which show strong immunolabeling (Lee and Brecha, 2010). Ultrastructurally, synapsin I immunolabeling was found in the horizontal cell axonal endings at rod photoreceptor synapses (Figure 6E, Hirano et al., 2005). Consistent with the immunolabeling, synapsin mRNA localized to presumed horizonal cells in developing rat retina (Haas et al., 1990).
The localization of numerous synaptic vesicle proteins to horizontal cell processes and endings, including VGAT (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2010; Lee and Brecha, 2010; Hirano et al., 2011), complexin-1/2, synapsin I (Hirano et al., 2005), SV2A (Brecha et al., 2010), and synaptotagmin-2 (Fox and Sanes, 2007; Lee and Brecha, 2010), and SNARE proteins (SNAP-25a/b, syntaxin-1a,−4, VAMP1) (Hirano et al., 2005, 2007, 2011; Bitzer and Brecha, 2006) is consistent with the idea that synaptic vesicles are present in horizontal cells and participate in calcium-triggered exocytosis (Figure 11).
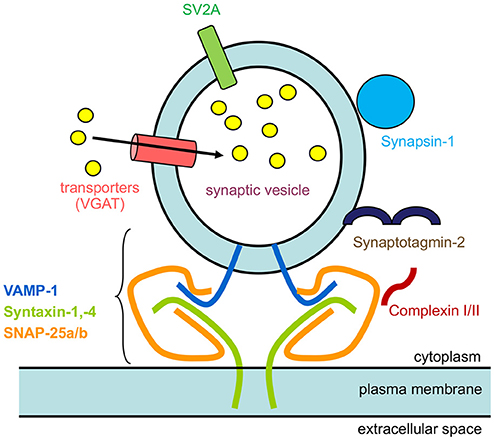
Figure 11. Schematic of synaptic proteins found in mammalian horizontal cells. The diagram depicts a synaptic vesicle studded with synaptic vesicle proteins, VGAT, a neurotransmitter transporter, SV2A, Synaptotagmin-2, a calcium sensor, Synapsin I, and SNARE protein, VAMP-1. The other 2 SNARE proteins that form the minimal complex are Syntaxin-1,−4, and SNAP-25, that brings the synaptic vesicle close to the plasma membrane for fusion. Finally, complexin-1/2 is a SNARE-associated protein. The yellow circles represent GABA that is accumulated inside synaptic vesicles by VGAT.
The localization of synaptotagmin-2 to horizontal cells indicated that a calcium sensor for neurotransmitter release is present in these terminals (Figures 10D–F, Fox and Sanes, 2007; Lee and Brecha, 2010). Rabbit, cat and mouse horizontal cells express L-type voltage-dependent Ca2+ channels (Ueda et al., 1992; Löhrke and Hofmann, 1994; Schubert et al., 2006; Liu et al., 2016), which are known to regulate sustained transmitter release in photoreceptor and bipolar cells and to modulate transmitter release smoothly and continuously with changes in membrane potential that accompany changing levels of illumination (Corey et al., 1984; Wilkinson and Barnes, 1996; de la Villa et al., 1998; Barnes and Kelly, 2002; Morgans et al., 2005; Mercer and Thoreson, 2011; Van Hook et al., 2019). The minimal voltage-dependent inactivation, characteristic of L-type Ca2+ channels, is well-suited for maintaining constant output at these tonic synapses (Juusola et al., 1996). Figures 10A–C shows immunolabeling for Cav2.2 and horizontal cell marker calbindin (Hirano and Brecha, 2010), and the co-localization of Cav2.2 to the horizontal cell axonal terminals and at cone pedicle dendritic contacts suggest N-type Ca channels may play a role in transmitter release. In rat, Cav1.2 (L-type, α1C), Cav2.1 (P/Q-type, α1A), and Cay2.2 (N-type, α1B) were localized by immunohistochemistry to horizontal cell endings (Liu et al., 2013). These findings are consistent with the physiological data supporting three types of voltage-gated Ca channels in mouse horizontal cells based on pharmacological discrimination using nifedipine/verapamil, ω-agatoxin IVA and ω-conotoxin GVIA, respectively (Schubert et al., 2006; Liu et al., 2013).
Initial electron microscopic studies of horizontal cell endings of cat, rabbit, and primate retina (Dowling and Boycott, 1966; Dowling et al., 1966; Raviola and Gilula, 1975) reported infrequent small, clear-core vesicles using different fixation protocols, with the most detailed report in the rat retina (Gray and Pease, 1971). Clear-core vesicles represent a type typically containing small molecule transmitters, such as GABA, glutamate, or acetylcholine, and not catecholamines or peptides. These vesicles are similar in appearance to the small, clear-core vesicles in adjacent photoreceptor terminals (Figure 12A).
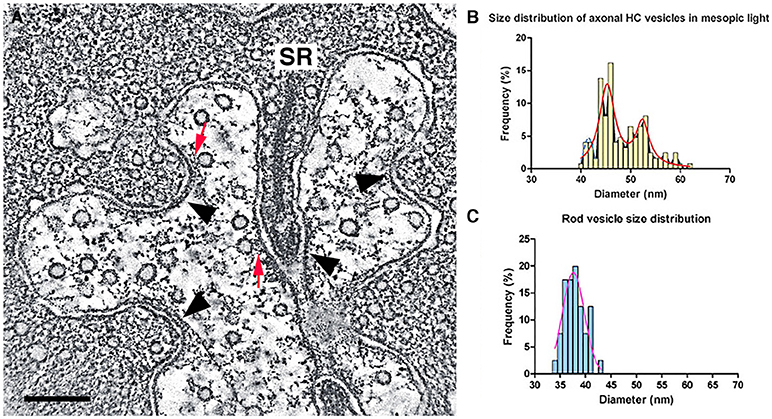
Figure 12. Small, clear-core vesicles are present in horizontal cell endings in rod spherules and cone pedicles. (A) A mouse rod photoreceptor spherule with a horizontal cell axonal terminal near the photoreceptor synaptic ribbon (SR) containing numerous small, clear-core vesicles. Clear-core vesicles represent small neurotransmitter-containing synaptic vesicles. The vesicles show fine fibrils extending from their membrane and in some examples, the vesicles are tethered to the plasma membrane or to plasma membrane specializations (red arrows). Plasma membrane specializations (arrowheads) are seen at infoldings of the horizontal cell endings and near the base of the synaptic ribbon. (B,C) Distribution of vesicle diameters in horizontal cell axonal endings (B) and in rod terminals (C). Horizontal cell axonal vesicle diameters have a bimodal distribution, and overall horizontal cell axonal vesicle diameters are larger than rod photoreceptor vesicle diameters. (A) z-section (orthoslice) of a tomogram. Scale bar, 200 nm. (Modified from Brecha et al., 2010; Zampighi et al., 2011).
We have used conical tomography electron microscopy (Zampighi et al., 2008, 2011) to evaluate horizontal cell dendritic and axonal endings in mouse and guinea pig photoreceptor invaginations. Conical electron microscopy is a high resolution, electron microscopic technique with ~3 nm isotropic resolution in the x-, y-, and z-planes. Essentially, this resolution eliminates the projection artifact common in thicker conventional and scanning block-face electron microscopic images that obscures fine cytoplasmic and membrane detail (Zampighi et al., 2008).
We have identified numerous small, clear-core vesicles, clathrin-coated vesicles, and patches of plasma membrane thickenings with prominent cytoplasmic specializations in the mouse horizontal cell terminals (Figures 12A, 13A, Zampighi et al., 2011). The small, clear-core vesicles have several fine fibrils that are readily seen in the conical tomograms, although they are not seen in conventional electron micrographs. These vesicles are similar in appearance to descriptions of synaptic vesicles in neurons (Peters et al., 1991). A preliminary comparison in mouse horizontal cells indicates a greater number of vesicles in axonal endings compared to dendritic endings. Vesicle diameters in these endings range between 37 and 62 nm with 2 major peaks at 46 and 53 nm, and a smaller peak at 40–41 nm (Brecha et al., 2010). Overall, horizontal cell vesicle size is larger than the rod vesicle size (Figures 12B,C; N = 120; 6 endings). Interestingly, inspection of vesicle sizes in a primate cone terminal and adjacent horizontal cell dendrite (Raviola and Gilula, 1975) also shows that the vesicles in the cone cytoplasm are smaller overall than the vesicles in the horizontal cell dendritic ending and similarly in horizontal cell axon terminals (Moser et al., 2020). In addition, to numerous small vesicles, the horizontal cell terminal occasionally contained endocytotic (Figure 13A, red arrow) and clathrin-coated vesicles (Figure 13B2, Zampighi et al., 2011). Some larger and irregular shaped vesicles were also seen in horizontal cell terminals of rat or guinea pig retina (Gray and Pease, 1971). The presence of both endosomes (Figure 13A, red arrow) and clathrin-coated vesicles is indicative of active processes occurring in these terminals.
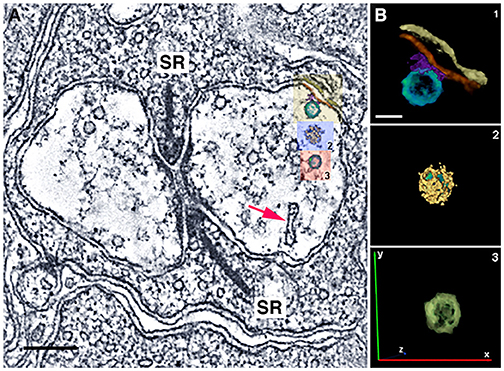
Figure 13. Horizontal cell endings contain different vesicle types. (A) A rod photoreceptor spherule with a horizontal cell axonal terminal with serial reconstructions of selected vesicles in mouse. Small vesicles with a conventional appearance are distributed throughout the terminal. A red arrow points to an endosome. In the right-side horizontal cell terminal, there is a small vesicle tethered to the plasma membrane (yellow box, B1). (B) Reconstructions of vesicles: (B1), A small vesicle (yellow box in A, turquoise vesicle) that is likely attached to the horizontal cell plasma membrane (red) via synaptic proteins (purple). Other structures include the horizontal cell plasma membrane (red) and the rod plasma membrane (yellow-brown). (B2): A clathrin-coated vesicle (blue box in A) in the cytoplasm. Clathrin cage (yellow); vesicle (green). (B3): A small vesicle (red box in A, green) in the cytoplasm. (A) z-section (orthoslice) of a tomogram; (B) Reconstructions of 3 vesicles. Scale bar, 200 nm in A, 40 nm in B. (Modified from Zampighi et al., 2011).
Horizontal cell membranes that are opposite and flanking the arciform density of the mouse photoreceptor terminal are characterized by membrane specialization in conventional electron microscopic preparations (Dowling and Boycott, 1966; Gray and Pease, 1971; Raviola and Gilula, 1975; Linberg and Fisher, 1988). Plasma membrane specializations (arrowheads) also occur along different infoldings of the horizontal cell plasma membrane within the invagination (Figure 12A, Zampighi et al., 2011). There are examples of small vesicles connected by thin tethers to the plasma membrane or are closely associated with these plasma membrane specializations (Figure 12A arrowheads, Figure 13A). In addition, small vesicles are near and adjacent to the plasma membrane in different parts of the horizontal cell terminal (Figures 12A, 13A). Together, these observations suggest the possibility that vesicle fusion and transmitter release sites are located at multiple sites within the horizontal cell terminals.
Vesicle clustering at membrane thickenings typical of many neuronal central synapses was not observed in early reports on primate, cat, rabbit, and rat horizontal cells (Dowling and Boycott, 1966; Raviola and Gilula, 1975; Kolb, 1977; Schaeffer et al., 1982; Peters et al., 1991). These findings may reflect a sampling issue of synapses that are sparsely distributed, as other ultrastructural studies on cat, rabbit, mouse, primate, mudpuppy, salamander, catfish, and turtle retinas demonstrated small clusters of synaptic vesicles in horizontal cell processes adjacent to membrane thickenings in bipolar cell dendrites, suggestive of horizontal cells feedforward synapses (Dowling et al., 1966; Olney, 1968; Dowling and Werblin, 1969; Dowling, 1970; Lasansky, 1973; Fisher and Boycott, 1974; Raviola and Gilula, 1975; Kolb and Jones, 1984; Sakai and Naka, 1986; Linberg and Fisher, 1988; Greferath et al., 1994). In human retina, horizontal cells were shown to make synaptic contacts with rod bipolar cell dendrites and the rod spherule within the invagination (Linberg and Fisher, 1988). Infrequent horizontal cell synapses with interplexiform processes were found in cat and rabbit also (Kolb, 1974; Kolb and West, 1977; Greferath et al., 1994).
The relative dearth and scattered distribution of synaptic vesicles in horizontal cell endings are similar to the observations of dopaminergic neurons that signal by extrasynaptic somatodendritic release, where it has been difficult to unequivocally identify the organelles (small clear-core vesicles, tuberovesicles, and large dense-core vesicles) that mediate dopamine release (Puopolo et al., 2001; Fortin et al., 2006; Hirasawa et al., 2012, 2015; Ludwig et al., 2016). Moreover, the dopaminergic amacrine cell perikaryon does not contain active zones; although, active zones were observed at their dendritic synapses with AII amacrine cells (Puopolo et al., 2001).
Ca2+-regulated transmitter release is a well-established mechanism in the CNS (Südhof, 2013; Kaeser and Regehr, 2014; Rizo, 2018). In the mammalian retina, evidence supports the idea that horizontal cell transmitter release is regulated by Ca2+. The support includes demonstration of voltage-gated Ca2+ currents (ICa) in horizontal cells (Schubert et al., 2006; Liu et al., 2016) and the localization of L-, N-, and P/Q-type Ca2+ channels (Liu et al., 2013) and the Ca2+ sensor, synaptotagmin-2 (Figures 10D–F, Hirano and Brecha, 2010; Lee and Brecha, 2010) to horizontal cell terminals. N-type Ca2+ channels are of particular interest, since they mediate vesicle release at central synapses (Catterall, 2011). Somatodendritic secretion of dopamine and peptides relies on L-type Ca channels primarily (Ludwig et al., 2016). In striatum, dopamine release can involve N-, Q-, T-, and L-type voltage-gated Ca channels, depending on neuronal activity, diverse calcium dependence, and calcium buffering in different cellular domains (Brimblecombe et al., 2015).
Using a luminal VGAT-C antibody in a retinal slice assay, we show that the voltage-gated Ca channels participate in Ca2+-mediated vesicular release from horizontal cells Figure 14A. We developed a retinal slice assay (Lee, 2010; Vuong et al., 2011) to monitor VGAT-expressing vesicles, based on topological studies that showed the C-terminus of VGAT is located within the vesicle lumen and using a fluorophore-conjugated, C-terminal directed VGAT (VGAT-C) antibody (Martens et al., 2008). Depolarization resulted in an Oyster550-VGAT-C terminus antibody labeling of the internal face of exocytosed synaptic vesicles, now exposed to the extracellular milieu containing the Oyster550-VGAT-C antibod (Figure 14A). In retinal slices, depolarization with high [K+] or 50 μM kainate (Figure 14D) in the presence of the VGAT-C antibody resulted in punctate VGAT-C labeling of horizontal cell endings in the OPL (Figures 14B,C), indicative of synaptic vesicles fusion with the plasma membrane. Vesicle fusion is only detected with the VGAT-C antibody and not with a N-terminal, cytoplasmically directed VGAT antibody (Figure 14D), indicating the labeling was not non-specific uptake. Labeling is absent or below detection in control experiments [e.g., basal 3 mM [K+] (Figures 14C',D), Oyster550-VGAT-N antibodies (Figure 14D)]. We showed the VGAT-C antibody uptake in horizontal cell processes occurred in basal 2 and 10 mM [Ca2+]o; whereas, no labeling occurred in nominally 0 mM [Ca2+]o (Figure 14E, Supplementary Figure 1) or in the presence of general (Cd2+, Co2+) and voltage-gated Ca channel subtype-specific blockers (ω-agatoxin, ω-conotoxin, nifedipine) (Supplementary Figure 2). These data indicate that the vesicle fusion in horizontal cell endings was depolarization- and calcium-dependent. Further, multiple rounds of labeling with depolarization, depicted in the schematic in Figure 14A, could be visualized using Alexa488-conjugated secondary antibodies to the VGAT-C primary antibodies (Figures 14B,B',B”,F), suggesting that the initially labeled vesicles are capable of recycling (Lee, 2010; Vuong et al., 2011).
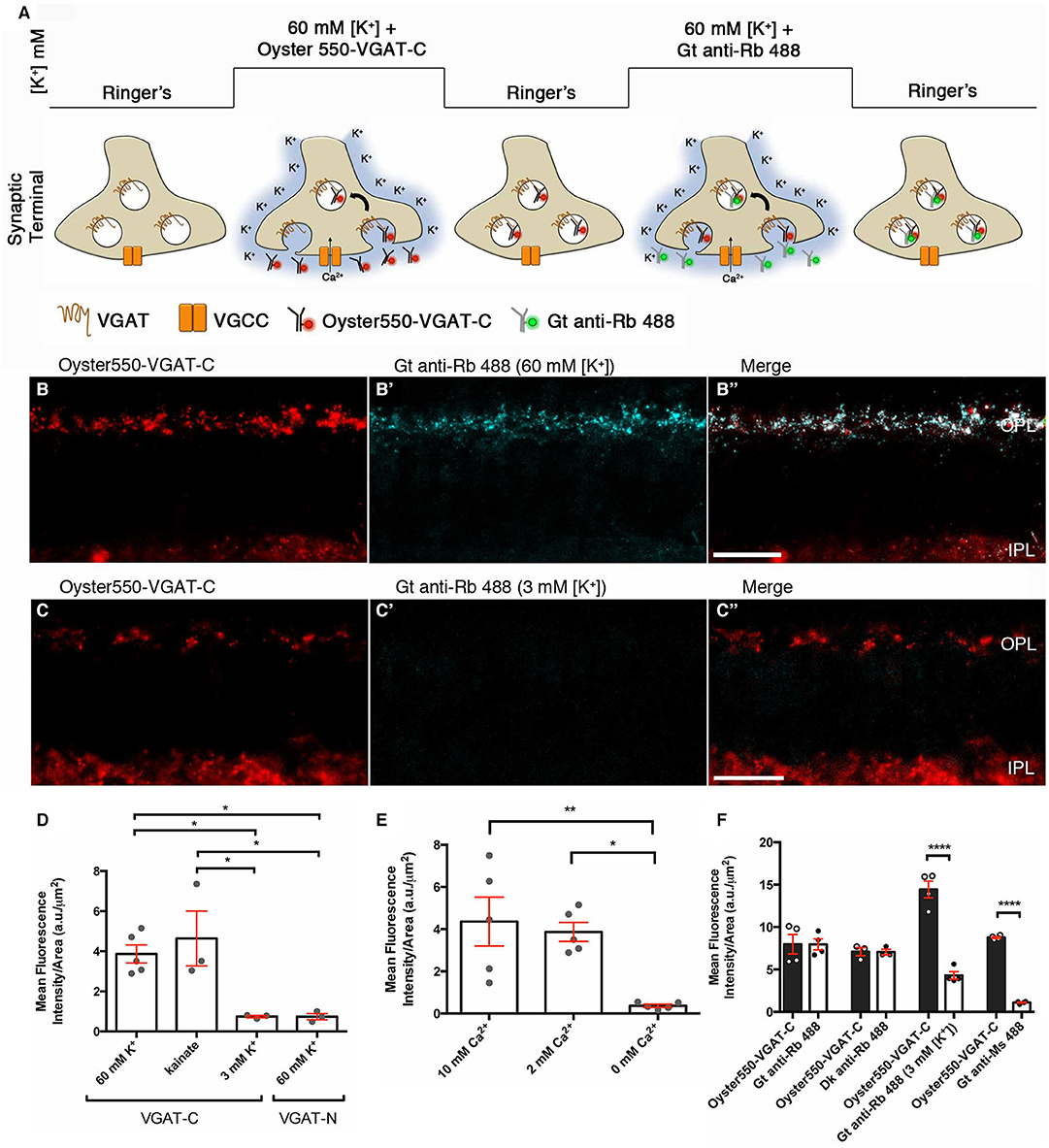
Figure 14. A VGAT-C synaptic vesicle fusion assay demonstrated that vesicle fusion occurs in a depolarization- and calcium-dependent manner in horizontal cell endings. (A) Schematic depicts the luminal VGAT-C vesicle fusion assay protocol. Retinal slices are infused with extracellular medium containing VGAT-C antibodies, then depolarized with high [K+]o that activates voltage-gated calcium channels and the subsequent Ca2+ influx triggers synaptic vesicle fusion with the plasma membrane and, thus, exposure to the VGAT-C antibodies in the extracellular space. (B,C) panels showed the Oyster550-VGAT-C immunolabeling (red) of the fused vesicles. (B') panels showed that a second round of depolarization and vesicle fusion labeled by Alexa488-conjugated secondary antibodies (blue) in the extracellular medium that recognizes the VGAT-C rabbit polyclonal antibodies from the first round of labeling, indicating that the synaptic vesicles recycle, as seen in the merged images (B”, white). (C'-C”,D) Control experiments showed that there was little to no vesicle fusion when slices were maintained in the basal 3 mM [K+]o. (D) Quantification of VGAT-C immunofluorescence was significantly increased when depolarized with high (60 mM) [K+]o or 50 μM kainate, but not with basal 3 mM [K+]o. When VGAT-N antibodies that recognize a cytosolic epitope not exposed to extracellular milieu were used, no specific labeling of horizontal cell endings was observed. This finding indicated that the VGAT-C immunolabeling was not a result of non-specific uptake of antibody *p < 0.02. (E) Quantification of VGAT-C immunofluorescence under different extracellular Ca concentrations. Significant increases in VGAT-C immunolabeling were observed in basal (2 mM) and high (10 mM) [Ca2+]o conditions. In contrast, little to no labeling occurred in nominally calcium-free media (0 mM) *p < 0.01, **p < 0.005. (F) Quantification of VGAT-C immunofluorescence during multiple rounds of depolarization, marked by different fluorophores (Oyster550 vs. Alexa488). Goat or donkey anti-rabbit IgG-Alexa488 recognized the VGAT-C primary antibody from the initial round of immunolabeling. In contrast when goat anti-rabbit-Alexa488 IgG was present during the subsequent incubation period in basal [K+]o, significantly less immunolabeling was observed. Similarly, when a goat anti-mouse IgG-Alexa488 was used, little to no immunolabeling was observed ****p < 0.001. (B,B',B”,C,C',C,”) maximum intensity projections, z = 5.0 μm. Scale bars, 20 μm. (Modified from Lee, 2010; Vuong et al., 2011).
Finally, we showed that feedback inhibition to photoreceptors occurs in a GABA-dependent manner to modulate the photoreceptor calcium current (Figure 15). To assay feedback, photoreceptors in slices (Figure 15E) were loaded with the calcium indicator Fluo-4 (green) in a Cx57-tdTomato retina, where the horizontal cells express the red fluorescent reporter tdTomato (converted to magenta), to show the relationship between horizontal cell processes and the photoreceptor cell bodies that were imaged. The increase in photoreceptor intracellular Ca2+ in response to pulses of 30 mM K+ was evaluated using drugs that depolarized or hyperpolarized horizontal cells (Vessey et al., 2005; Liu et al., 2013; Hirano et al., 2016a). A pulse of 30 mM K+ drove Ca influx through the voltage-gated calcium channels in the photoreceptors in control conditions, and then, when a second pulse was applied in the presence of kainate to depolarize the horizontal cells, the second pulse produced a smaller peak in intracellular Ca2+, showing that horizontal cell depolarization produced an inhibitory signal on the photoreceptor calcium channels (Figure 15A). Conversely, when the horizontal cells were hyperpolarized with 2,3-Dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f ]quinoxaline-7-sulfonamide (NBQX), via blockade of ionotropic glutamate receptors during the second pulse, the calcium signal in photoreceptors is increased, indicating decreased feedback inhibition from horizontal cells to the photoreceptors (Figure 15C). These findings are consistent with reports in mouse retina (Babai and Thoreson, 2009). Kainate did not produce a change in photoreceptor calcium signal upon superfusion prior to the high-K+ pulse, consistent with a lack of ionotropic glutamate receptors on photoreceptors (Babai and Thoreson, 2009). To evaluate the role of vesicular GABA release in this feedback, we conditionally knocked out VGAT by crossing the horizontal cell-specific Cx57-iCre mouse (Hirano et al., 2016a) with a floxed VGAT mouse line (Tong et al., 2008). With the VGAT gene deleted, the neurotransmitter, most likely GABA, cannot be packaged into synaptic vesicles and released. Immunostaining for VGAT confirmed that the VGAT was selectively knocked out in horizontal cells (Hirano et al., 2016a). Whole-cell recordings of VGAT−/− horizontal cells showed that the voltage-gated K+ and Ca2+ membrane currents were normal. In the horizontal cell VGAT knockout, kainate did not produce increased feedback inhibition and NBQX did not result in decreased feedback inhibition (Figures 15B,D,F, Hirano et al., 2016a). These data show that the loss of horizontal cell VGAT eliminated feedback inhibition onto photoreceptors.
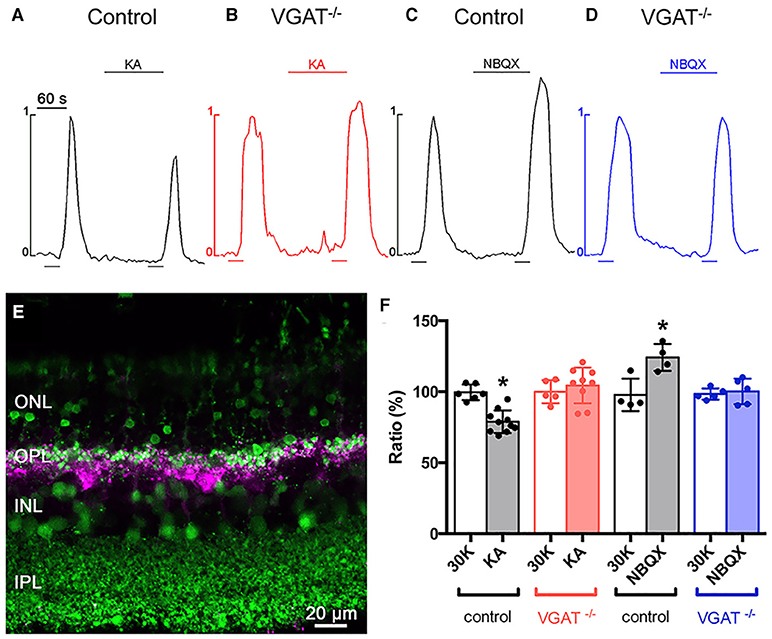
Figure 15. Deletion of VGAT in horizontal cells results in loss of inhibitory feedback modulation of photoreceptor [Ca2+]i. (A–D) Changes to the strength of feedback inhibition, which were increased by kainate and decreased by NBQX, were eliminated in the VGAT KO mice. Twin 30-s pulses (bars below traces) of high [K+]o were applied to mouse retinal slices and, during the second pulse, either 50 μM kainate (bar above trace) was used to pharmacologically depolarize, or 50 μM NBQX to hyperpolarize, the horizontal cell membrane potential. In wild-type (VGAT+/+) retina, (A) kainate decreased the rise in photoreceptor [Ca2+]i (black traces), suggesting an increase in feedback inhibition while, in Cx57-VGAT−/− knockout retinal slices (B) kainate (red traces) produced no change. In Cx57-VGAT+/+ retina, (C) NBQX increased photoreceptor [Ca2+]i (black traces), representing a reduction in feedback inhibition, whereas (D) NBQX (blue traces) did not change the photoreceptor [Ca2+]i in Cx57-VGAT−/− retina, suggesting elimination of feedback inhibition to photoreceptors. These results show that GABA release from horizontal cells is essential for feedback inhibition. (E) Confocal image of a Cx57-tdTomato retinal slice loaded with fluo-4 (green). tdTomato (magenta) identifies the horizontal cells. (F) Summary of photoreceptor Ca signal amplitudes in retinal slices from VGAT+/+ and VGAT−/− mice treated with kainate and NBQX *p < 0.05. ONL, Outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. (Modified from Hirano et al., 2016a).
In mammals, a preponderance of experimental findings indicates that retinal horizontal cells utilize a vesicular mechanism of transmitter release. The evidence for GABA as the horizontal cell neurotransmitter is the presence of GABA immunoreactivity and of GABA synthesizing enzymes (GAD65 and/or GAD67), and postsynaptic targets bearing GABA receptors (photoreceptors, bipolar cells) as well as autoreceptors on horizontal cells. Neonatal rabbit horizontal cells show 3H-GABA uptake that is downregulated after P5 (Redburn and Madtes, 1986); however, adult mammalian horizontal cells are atypical GABAergic neurons, in that they do not express plasmalemmal GABA transporters. The GABA transporters are expressed by Müller cells, whose processes ensheath photoreceptor synapses (Guo et al., 2009). The horizontal cells express SNARE proteins required for membrane fusion of synaptic vesicles (SNAP-25, VAMP-1, and syntaxin-1a & -4). The fusion of VGAT-bearing synaptic vesicles with the plasma membrane is depolarization- and calcium-dependent and show the capacity for multiple rounds of vesicle fusion in retinal slice preparations. The selective knockout of VGAT from horizontal cells that resulted in the loss of the tonic GABA current (Grove et al., 2019) and disrupted feedback inhibition to photoreceptors, showing that GABA release plays an integral role in these cells' neurotransmission (Hirano et al., 2016a).
The low numbers and the scattered appearance of synaptic vesicles in horizontal cell terminals as well as the absence of clearcut active zones (Figures 12, 13) are morphological features found in dendrites that are known to release transmitter; for example, somatodendritic dopamine and GABA release from dopaminergic neurons in the CNS (Hirasawa et al., 2015; Ludwig et al., 2016). In striatum, only a third of the dopaminergic boutons expressed a minimal active zone-like cluster of RIM, bassoon and ELKS (Liu et al., 2018). Ultrastructural analysis of dopaminergic amacrine cell somata revealed no active zones and few synaptic vesicles and tubulovesicular organelles (Puopolo et al., 2001). Nevertheless, somatodendritic release exhibits properties of regulated exocytosis, such as calcium dependence (Chen et al., 2006), the involvement of SNARE protein isoforms SNAP-25, VAMP2, and syntaxin 3b (Fortin et al., 2006; Witkovsky et al., 2009; Rice and Patel, 2015; Ludwig et al., 2016), voltage-gated Ca channels (Puopolo et al., 2001; Brimblecombe et al., 2015; Rice and Patel, 2015), and quantal release (Jaffe et al., 1998; Puopolo et al., 2001). The subtypes of voltage-gated Ca channels involved can differ in dopaminergic neurons and may reflect different modes of release (e.g., somatodendritic vs. axonal, firing patterns) (Ludwig et al., 2016). In retina, dopamine acts at synapses as well as by volume transmission (Witkovsky, 2004). The abundance of GABAA receptors in the OPL along with the relatively few synapses found in horizontal cells to bipolar cell dendrites suggests that horizontal cell GABA may be acting by volume transmission. From the robust presence of syntaxin 4 and SNAP-25 throughout horizontal cell processes, it would be interesting to know if GABA release occurred extrasynaptically as well as synaptically from horizontal cells. Also, these SNARE proteins may function in the regulation of GABA or ionotropic glutamate receptor exocytosis, as syntaxin-4 is reported to be important for postsynaptic dendritic exocytosis in hippocampal neurons (Kennedy et al., 2010; Ovsepian and Dolly, 2011; Gu and Huganir, 2016; Bin et al., 2019).
Transmitter release by a regulated vesicular mechanism would be highly advantageous for fine control of feedback and feed-forward action in the outer retina, as there are multiple molecular control points to modulate secretion from horizontal cells that utilizes the bicarbonate permeability of GABAA receptors to regulate cleft pH (Grove et al., 2019; Barnes et al., 2020). For instance, VGAT's dependence on a proton gradient for GABA uptake would influence vesicular GABA concentrations (Reimer et al., 1998) and, by extension, postsynaptic responses. The possible complex of VGAT and GAD65 (Wei and Wu, 2008) on synaptic vesicles in horizontal cell synaptic endings and its regulation on demand (Buddhala et al., 2009) would also stimulate GABA loading of synaptic vesicles. The highly regulated cascade of SNARE protein interactions in exocytosis would allow for a precise control of the rate and level of transmitter secretion. Local modulation of membrane potential at different endings could also differentially influence presynaptic Ca2+ channel dynamics and influence local GABA release.
The demonstration of GABA and its synthetic enzyme GAD65 and/or GAD67 in adult mammalian horizontal cells supports the notion that GABA is acting as a transmitter, despite not bearing GATs, notably like cerebellar Purkinje cells (Ribak et al., 1996; Guo et al., 2009). Müller cell processes that envelop photoreceptor terminals in the OPL are well placed to take up GABA (Guo et al., 2009), similar to Bergmann glia around the Purkinje cells (Ribak et al., 1996). The GABA receptors on horizontal cells and bipolar cell dendrites (Vardi and Sterling, 1994; Wässle et al., 1998; Haverkamp et al., 2000) indicate the receptor targets of the GABA released by horizontal cell are present.
The presence of GABAA receptor ρ2 (Grove et al., 2019) immunolabeling on horizontal cell terminals implies a significant role for tonic GABA modulation of horizontal cell membrane potential and conductance, signaling which is mediated by graded regulation rather than phasic synaptic transmission. Horizontal cells appear to be the primary source of GABA in the outer retina, as VGAT knockout resulted in a loss of the TPMPA-sensitive GABA-induced current in horizontal cells and feedback regulation of photoreceptor Ca channels (Grove et al., 2019). In primate retina, Haverkamp and colleagues (Haverkamp et al., 2000; Puller et al., 2014) described layers of horizontal cell processes under primate cone pedicles and GABAA receptor-bearing bipolar cell dendrites sandwiched between the two layers, and postulated that a GABA tone may be present. There is an enrichment of syntaxin-4 in horizontal cell processes at S-cones in primate retina and combined with expression of GABAA receptor α1 and ρ subunits and the chloride-accumulating transporter NKCC vitreal to S-cone pedicles is suggestive of HII horizontal cell to blue cone bipolar cells feedforward signal transmission (Puller et al., 2014). There are also interplexiform processes from GABAergic tyrosine hydroxylase (TH) amacrine cells that form synapses in the OPL onto bipolar cell processes (Dowling and Ehinger, 1975; Kolb and West, 1977; Linberg and Fisher, 1986; Chun and Wässle, 1989; Greferath et al., 1994), as well as a non-dopamine containing GABAergic interplexiform cell in mouse (Dedek et al., 2009), that might contribute to GABA levels in the OPL (Chun and Wässle, 1989; Witkovsky et al., 2008). Grove et al. (2019) showed that the high-affinity, non-desensitizing GABAAρ receptors on horizontal cell endings generate a tonic GABA current in the outer retina, most notably within the photoreceptor terminal synapse. Changes in tonic inhibition can alter neuronal and network properties, due to a persistent increase in input conductance that will regulate membrane excitability and alter the gain of a neuron's input-output relationship, and thus a neuron's responsiveness (Semyanov et al., 2004; Walker and Semyanov, 2008; Lee and Maguire, 2014).
In addition to feedback to photoreceptors, GABAA receptors on bipolar cell dendrites relay the horizontal cell feedforward signal (Vardi et al., 1992; Vardi and Sterling, 1994; Enz et al., 1996; Wässle et al., 1998; Haverkamp et al., 2000; Hoon et al., 2015; Chaffiol et al., 2017), although which bipolar cell type and the GABAA receptor subtypes used are not yet well-defined. The GABAA receptors are ρ-containing or extrasynaptic GABAA receptors containing δ subunits, such as those that contain GABAA receptor α6 subunits, are high affinity and non-desensitizing, and mediate tonic inhibition in other CNS areas (Farrant and Nusser, 2005; Glykys and Mody, 2007; Belelli et al., 2009; Brickley and Mody, 2012). Consistent with higher levels of GABA in the interstitial space of the OPL, the background labeling for GABA was observed to be higher in the OPL (Chun and Wässle, 1989).
Conical electron tomography of mouse rod spherules and cone pedicles clearly demonstrate the presence of small, clear-core vesicles in horizontal cell axonal endings and dendritic endings, that are slightly larger compared to synaptic vesicles in rod and cone photoreceptors. Furthermore, the conical tomograms reveal putative active zones and membrane densities in the horizontal cell endings (Zampighi et al., 2011), endocytosis of clathrin-coated vesicles and vesicle tethers, indicative of vesicle specializations and vesicular activity that were not observed in thicker ultrathin sections due to projection artifacts. The presence of synaptic vesicles in horizontal cells is supported by conventional transmission electron micrographs from mouse (Spiwoks-Becker et al., 2001), cat and primates (Dowling and Boycott, 1966; Dowling, 1970; Raviola and Gilula, 1975; Linberg and Fisher, 1988), that depict small, clear-core vesicles in horizontal cell processes at both rod and cone photoreceptor synapses. The presence of synaptic vesicle proteins, such as VGAT and SV2A, in horizontal terminals further supports the conclusion of the presence of synaptic vesicles in these terminals.
These synaptic vesicles are the cellular substrate for the many synaptic vesicle proteins localized to horizontal cell endings, including VGAT, SV2A, synapsin I, complexin-1/2, synaptoporin (Brandstätter et al., 1996a; Hirano et al., 2005, 2007; Lee and Brecha, 2010). The SNARE complex proteins of syntaxin-1a and syntaxin-4, VAMP-1, and SNAP-25 (Hirano et al., 2005, 2007, 2011) along with the SNARE complex associated proteins (complexin-1/2 and synaptotagmin-2; Hirano et al., 2005) mediate and modulate vesicle fusion with the membrane. The use of less common isoforms, e.g. VAMP1, synaptotagmin-2, complexin-1/2, for synaptic vesicle release may reflect different kinetics and/or regulation at this tonic, graded potential GABAergic synapse (Reim et al., 2005; Hua et al., 2011; Chung and Raingo, 2013), similar to the usage of alternative synaptic protein isoforms at ribbon synapses (Moser et al., 2020). The subcellular localization of VAMP1 to horizontal cell terminals suggest it participates in synaptic transmission and that other VAMP isoforms may mediate vesicle trafficking between other cellular compartments within the cell. Although the precise role of syntaxin-4 remains to be determined, its high level of expression in horizontal cells, in addition to syntaxin-1a, likely reflect distinct pools of vesicles trafficked to the membrane. The calcium sensor, synaptotagmin-2, is preferentially expressed at cerebellar GABAergic synapses, where it is the fast Ca sensor and responsible for faster replenishment of the readily releasable pool, necessary for fast feedforward inhibition (Chen et al., 2017).
The functional VGAT-C antibody uptake studies indicated that the VGAT-containing synaptic vesicles fuse with the plasma membrane in a depolarization- and calcium-dependent manner, characteristic of vesicular exocytosis of transmitter (Südhof, 2013). Moreover, the synaptic vesicles recycle, as observed from the multiple rounds of labeling. Finally, the knockout of horizontal cell VGAT resulted in loss of GABA release and eliminated feedback inhibition of photoreceptor calcium channels (Hirano et al., 2016a; Grove et al., 2019). Together these findings show that the vesicular release of GABA from mammalian horizontal cells plays an essential role in horizontal cell synaptic transmission.
Grove et al. (2019) demonstrated that cone photoreceptor calcium currents are modulated by picrotoxin and TPMPA (see Grove et al., Figures 1–2), and that they act at GABAAR-ρ receptors on the horizontal cell endings (see Grove et al., Figures 2I–K), not photoreceptors. This GABAergic modulation is absent in the presence of HEPES, indicating pH sensitivity. Grove et al. (2019) extended the findings in Hirano et al. (2016a) by showing that GABA release by horizontal cells acts back onto its own GABA receptors, and that the GABA release (and cone Ca channel modulation) is abolished in Cx57-VGAT−/− horizontal cells, concluding that the bicarbonate flux through these tonic GABA receptors regulates the synaptic cleft pH. The full hybrid GABA-pH model is much more detailed (see Figure 8, Grove et al., 2019; Figure 12, Barnes et al., 2020), including roles for sodium-proton exchangers (NHEs), the bicarbonate equilibrium potential and horizontal cell membrane potential excursions induced by GABA and glutamate (Grove et al., 2019; Barnes et al., 2020).
AH, SS, SB, and NB designed the experiments. HV and HK performed the VGAT-C functional labeling experiments. AH and HK performed the immunohistochemistry. HK performed PLA. CS performed the conical tomography. SB managed the calcium imaging experiments. AH, SB, and NB wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the NIH EY04067 (NB), NIH EY15573 (NB), NIH EY29869 (NB), VA Merit Award (NB), VA Senior Career Scientist Award (NB), Plum Foundation (SB), Unrestricted Grant from Research to Prevent Blindness, Inc. (UCLA Dept. of Ophthalmology); Jules Stein Eye Institute EyeSTAR Program (HK); NIH T32 EY007026 (HV).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully thank our numerous collaborators and technical associates over the last two decades.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2020.600777/full#supplementary-material
Agardh, E., Bruun, A., Ehinger, B., and Storm-Mathisen, J. (1986). GABA immunoreactivity in the retina. Invest. Ophthalmol. Vis. Sci. 27, 674–678.
Agardh, E., and Ehinger, B. (1982). (3H)-muscimol, (3H)-nipecotic acid and (3H)-isoguvacine as autoradiographic markers for GABA neurotransmission. J. Neural. Transm. 54, 1–18. doi: 10.1007/BF01249274
Agardh, E., Ehinger, B., and Wu, J. Y. (1987). GABA and GAD-like immunoreactivity in the primate retina. Histochemistry 86, 485–490. doi: 10.1007/BF00500621
Babai, N., Sendelbeck, A., Regus-Leidig, H., Fuchs, M., Mertins, J., Reim, K., et al. (2016). Functional roles of complexin3 and complexin4 at mouse photoreceptor ribbon Synapses. J. Neurosci. 36, 6651–6667. doi: 10.1523/JNEUROSCI.4335-15.2016
Babai, N., and Thoreson, W. B. (2009). Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J. Physiol. 587, 2353–2364. doi: 10.1113/jphysiol.2009.169656
Baekkeskov, S., and Kanaani, J. (2009). Palmitoylation cycles and regulation of protein function (Review). Mol. Membr. Biol. 26, 42–54. doi: 10.1080/09687680802680108
Bajjalieh, S. M., Peterson, K., Shinghal, R., and Scheller, R. H. (1992). SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257, 1271–1273. doi: 10.1126/science.1519064
Barnes, S., Grove, J. C. R., McHugh, C. F., Hirano, A. A., and Brecha, N. C. (2020). Horizontal cell feedback to cone photoreceptors in mammalian retina: Novel insights from the GABA-pH hybrid model. Front. Cell. Neurosci. 14:595064. doi: 10.3389/fncel.2020.595064
Barnes, S., and Kelly, M. E. (2002). Calcium channels at the photoreceptor synapse. Adv. Exp. Med. Biol. 514, 465–476. doi: 10.1007/978-1-4615-0121-3_28
Barnstable, C. J., Hofstein, R., and Akagawa, K. (1985). A marker of early amacrine cell development in rat retina. Brain Res. 352, 286–290. doi: 10.1016/0165-3806(85)90116-6
Behar, T., Schaffner, A., Laing, P., Hudson, L., Komoly, S., and Barker, J. (1993). Many spinal cord cells transiently express low molecular weight forms of glutamic acid decarboxylase during embryonic development. Brain Res. Dev. Brain Res. 72, 203–218. doi: 10.1016/0165-3806(93)90185-D
Belelli, D., Harrison, N. L., Maguire, J., Macdonald, R. L., Walker, M. C., and Cope, D. W. (2009). Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 29, 12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009
Bhoi, J. D., Zhang, Z., Janz, R., You, Y., Wei, H., Wu, J., et al. (2020). The SNARE regulator Complexin3 is a target of the cone circadian clock. J. Comp. Neurol. doi: 10.1002/cne.25004
Bin, N. R., Huang, M., and Sugita, S. (2019). Investigating the role of SNARE proteins in trafficking of postsynaptic receptors using conditional knockouts. Neuroscience 420, 22–31. doi: 10.1016/j.neuroscience.2018.11.027
Bitzer, M., and Brecha, N. C. (2006). Expression of different VAMP isoforms in the mouse outer retina. Invest. Ophthalmol. Vision Sci. 47:3720.
Blanco, R., and de la Villa, P. (1999). Ionotropic glutamate receptors in isolated horizontal cells of the rabbit retina. Eur. J. Neurosci. 11, 867–873. doi: 10.1046/j.1460-9568.1999.t01-1-00499.x
Blanco, R., Vaquero, C. F., and de la Villa, P. (1996). The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Res. 36, 3987–3995. doi: 10.1016/S0042-6989(96)00145-9
Blanks, J. C., and Roffler-Tarlov, S. (1982). Differential localization of radioactive gamma-aminobutyric acid and muscimol in isolated and in vivo mouse retina. Exp. Eye Res. 35, 573–584. doi: 10.1016/S0014-4835(82)80071-7
Bormann, J. (2000). The 'ABC' of GABA receptors. Trends Pharmacol. Sci. 21, 16–19. doi: 10.1016/S0165-6147(99)01413-3
Bormann, J., and Feigenspan, A. (1995). GABAC receptors. Trends Neurosci. 18, 515–519. doi: 10.1016/0166-2236(95)98370-E
Brandon, C. (1985). Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res. 344, 286–295. doi: 10.1016/0006-8993(85)90806-6
Brandon, C., Lam, D. M., and Wu, J. Y. (1979). The gamma-aminobutyric acid system in rabbit retina: localization by immunocytochemistry and autoradiography. Proc. Natl. Acad. Sci. U.S.A. 76, 3557–3561. doi: 10.1073/pnas.76.7.3557
Brandstätter, J. H., Löhrke, S., Morgans, C. W., and Wässle, H. (1996a). Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J. Comp. Neurol. 370, 1–10. doi: 10.1002/(SICI)1096-9861(19960617)370:1<1::AID-CNE1>3.0.CO;2-7
Brandstätter, J. H., Wässle, H., Betz, H., and Morgans, C. W. (1996b). The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. Eur. J. Neurosci. 8, 823–828. doi: 10.1111/j.1460-9568.1996.tb01268.x
Brecha, N. C. (1992). Expression of GABAA receptors in the vertebrate retina. Prog. Brain Res. 90, 3–28. doi: 10.1016/S0079-6123(08)63606-7
Brecha, N. C., Sternini, C., and Humphrey, M. F. (1991). Cellular distribution of L-glutamate decarboxylase (GAD) and gamma-aminobutyric acidA (GABAA) receptor mRNAs in the retina. Cell. Mol. Neurobiol. 11, 497–509. doi: 10.1007/BF00734812
Brecha, N. C., and Weigmann, C. (1994). Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J. Comp. Neurol. 345, 602–611. doi: 10.1002/cne.903450410
Brecha, N. C., Hirano, A. A., Lee, H., Schietroma, C., Zampighi, G., Guo, C., et al. (2010). Molecular and cellular machinery underlying vesicular transmitter relaease from horizontal cells. In: International Society of Eye Research Biennial Meeting, Abstract #O441 (Montreal, CA).
Brickley, S. G., and Mody, I. (2012). Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73, 23–34. doi: 10.1016/j.neuron.2011.12.012
Brimblecombe, K. R., Gracie, C. J., Platt, N. J., and Cragg, S. J. (2015). Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol. 593, 929–946. doi: 10.1113/jphysiol.2014.285890
Brunger, A. T., Choi, U. B., Lai, Y., Leitz, J., White, K. I., and Zhou, Q. (2019). The pre-synaptic fusion machinery. Curr. Opin. Struct. Biol. 54, 179–188. doi: 10.1016/j.sbi.2019.03.007
Buckley, K., and Kelly, R. B. (1985). Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 100, 1284–1294. doi: 10.1083/jcb.100.4.1284
Buddhala, C., Hsu, C. C., and Wu, J. Y. (2009). A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem. Int. 55, 9–12. doi: 10.1016/j.neuint.2009.01.020
Burris, C., Klug, K., Ngo, I. T., Sterling, P., and Schein, S. (2002). How Muller glial cells in macaque fovea coat and isolate the synaptic terminals of cone photoreceptors. J. Comp. Neurol. 453, 100–111. doi: 10.1002/cne.10397
Byzov, A. L., and Shura-Bura, T. M. (1986). Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 26, 33–44. doi: 10.1016/0042-6989(86)90069-6
Cadetti, L., and Thoreson, W. B. (2006). Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J. Neurophysiol. 95, 1992–1995. doi: 10.1152/jn.01042.2005
Cammack, J. N., and Schwartz, E. A. (1993). Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J. Physiol. 472, 81–102. doi: 10.1113/jphysiol.1993.sp019938
Casini, G., Rickman, D. W., and Brecha, N. C. (2006). Expression of the gamma-aminobutyric acid (GABA) plasma membrane transporter-1 in monkey and human retina. Invest. Ophthalmol. Vis. Sci. 47, 1682–1690. doi: 10.1167/iovs.05-1117
Catsicas, S., Catsicas, M., Keyser, K. T., Karten, H. J., Wilson, M. C., and Milner, R. J. (1992). Differential expression of the presynaptic protein SNAP-25 in mammalian retina. J. Neurosci. Res. 33, 1–9. doi: 10.1002/jnr.490330102
Catterall, W. A. (2011). Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3:a003947. doi: 10.1101/cshperspect.a003947
Chaffiol, A., Ishii, M., Cao, Y., and Mangel, S. C. (2017). Dopamine regulation of GABAA receptors contributes to light/dark modulation of the ON-cone bipolar cell receptive feld surround in the retina. Curr. Biol. 27, 2600-2609.e2604. doi: 10.1016/j.cub.2017.07.063
Chang, W. P., and Südhof, T. C. (2009). SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J. Neurosci. 29, 883–897. doi: 10.1523/JNEUROSCI.4521-08.2009
Chaudhry, F. A., Reimer, R. J., Bellocchio, E. E., Danbolt, N. C., Osen, K. K., Edwards, R. H., et al. (1998). The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 18, 9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998
Chen, B. T., Moran, K. A., Avshalumov, M. V., and Rice, M. E. (2006). Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca channels contrasted with strong regulation of axonal dopamine release. J. Neurochem. 96, 645–655. doi: 10.1111/j.1471-4159.2005.03519.x
Chen, C., Arai, I., Satterfield, R., Young, S. M. Jr., and Jonas, P. (2017). Synaptotagmin 2 is the fast Ca2+ sensor at a central inhibitory synapse. Cell Rep. 18, 723–736. doi: 10.1016/j.celrep.2016.12.067
Chen, X., Tomchick, D. R., Kovrigin, E., Arac, D., Machius, M., Südhof, T. C., et al. (2002). Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409. doi: 10.1016/S0896-6273(02)00583-4
Chessler, S. D., and Lernmark, A. (2000). Alternative splicing of GAD67 results in the synthesis of a third form of glutamic-acid decarboxylase in human islets and other non-neural tissues. J. Biol. Chem. 275, 5188–5192. doi: 10.1074/jbc.275.7.5188
Chun, M. H., and Wässle, H. (1989). GABA-like immunoreactivity in the cat retina: electron microscopy. J. Comp. Neurol. 279, 55–67. doi: 10.1002/cne.902790106
Chung, C., and Raingo, J. (2013). Vesicle dynamics: how synaptic proteins regulate different modes of neurotransmission. J. Neurochem. 126, 146–154. doi: 10.1111/jnc.12245
Connaughton, V. P., Dyer, K. D., Nadi, N. S., and Behar, T. N. (2001). The expression of GAD67 isoforms in zebrafish retinal tissue changes over the light/dark cycle. J. Neurocytol. 30, 303–312. doi: 10.1023/A:1014404328905
Corey, D. P., Dubinsky, J. M., and Schwartz, E. A. (1984). The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J. Physiol. 354, 557–575. doi: 10.1113/jphysiol.1984.sp015393
Crowder, K. M., Gunther, J. M., Jones, T. A., Hale, B. D., Zhang, H. Z., Peterson, M. R., et al. (1999). Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc. Natl. Acad. Sci. U.S.A. 96, 15268–15273. doi: 10.1073/pnas.96.26.15268
Cueva, J. G., Haverkamp, S., Reimer, R. J., Edwards, R., Wässle, H., and Brecha, N. C. (2002). Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J. Comp. Neurol. 445, 227–237. doi: 10.1002/cne.10166
Curtis, L. B., Doneske, B., Liu, X., Thaller, C., McNew, J. A., and Janz, R. (2008). Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J. Comp. Neurol. 510, 550–559. doi: 10.1002/cne.21806
Davis, K. N., Tao, R., Li, C., Gao, Y., Gondre-Lewis, M. C., Lipska, B. K., et al. (2016). GAD2 Alternative transcripts in the human prefrontal cortex, and in schizophrenia and affective disorders. PLoS ONE 11:e0148558. doi: 10.1371/journal.pone.0148558
de la Villa, P., Vaquero, C. F., and Kaneko, A. (1998). Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur. J. Neurosci. 10, 317–323. doi: 10.1046/j.1460-9568.1998.00051.x
Dedek, K., Breuninger, T., de Sevilla Muller, L. P., Maxeiner, S., Schultz, K., Janssen-Bienhold, U., et al. (2009). A novel type of interplexiform amacrine cell in the mouse retina. Eur. J. Neurosci. 30, 217–228. doi: 10.1111/j.1460-9568.2009.06808.x
Deniz, S., Wersinger, E., Picaud, S., and Roux, M. J. (2019). Evidence for functional GABAA but not GABAC receptors in mouse cone photoreceptors. Vis. Neurosci. 36:E005. doi: 10.1017/S0952523819000038
Deniz, S., Wersinger, E., Schwab, Y., Mura, C., Erdelyi, F., Szabo, G., et al. (2011). Mammalian retinal horizontal cells are unconventional GABAergic neurons. J. Neurochem. 116, 350–362. doi: 10.1111/j.1471-4159.2010.07114.x
Dirkx, R. Jr., Thomas, A., Li, L., Lernmark, A., Sherwin, R. S., De Camilli, P., et al. (1995). Targeting of the 67-kDa isoform of glutamic acid decarboxylase to intracellular organelles is mediated by its interaction with the NH2-terminal region of the 65-kDa isoform of glutamic acid decarboxylase. J. Biol. Chem. 270, 2241–2246. doi: 10.1074/jbc.270.5.2241
Dkhissi, O., Julien, J. F., Wasowicz, M., Dalil-Thiney, N., Nguyen-Legros, J., and Versaux-Botteri, C. (2001). Differential expression of GAD(65) and GAD(67) during the development of the rat retina. Brain Res. 919, 242–249. doi: 10.1016/S0006-8993(01)03022-0
Dmitriev, A. V., Dmitrieva, N. A., Keyser, K. T., and Mangel, S. C. (2007). Multiple functions of cation-chloride cotransporters in the fish retina. Vis. Neurosci. 24, 635–645. doi: 10.1017/S0952523807070629
Dong, C. J., and Werblin, F. S. (1994). Dopamine modulation of GABAC receptor function in an isolated retinal neuron. J. Neurophysiol. 71, 1258–1260. doi: 10.1152/jn.1994.71.3.1258
Dowling, J. E., and Boycott, B. B. (1966). Organization of the primate retina: electron microscopy. Proc. R. Soc. Lond. B. Biol. Sci. 166, 80–111. doi: 10.1098/rspb.1966.0086
Dowling, J. E., Brown, J. E., and Major, D. (1966). Synapses of horizontal cells in rabbit and cat retinas. Science 153, 1639–1641. doi: 10.1126/science.153.3744.1639
Dowling, J. E., and Ehinger, B. (1975). Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science 188, 270–273. doi: 10.1126/science.804181
Dowling, J. E., and Werblin, F. S. (1969). Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J. Neurophysiol. 32, 315–338. doi: 10.1152/jn.1969.32.3.315
Duebel, J., Haverkamp, S., Schleich, W., Feng, G., Augustine, G. J., Kuner, T., et al. (2006). Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron 49, 81–94. doi: 10.1016/j.neuron.2005.10.035
Ehinger, B. (1977). Glial and neuronal uptake of GABA, glutamic acid, glutamine and glutathione in the rabbit retina. Exp. Eye Res. 25, 221–234. doi: 10.1016/0014-4835(77)90089-6
Elferink, L. A., Trimble, W. S., and Scheller, R. H. (1989). Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J. Biol. Chem. 264, 11061–11064.
Endeman, D., Fahrenfort, I., Sjoerdsma, T., Steijaert, M., Ten Eikelder, H., and Kamermans, M. (2012). Chloride currents in cones modify feedback from horizontal cells to cones in goldfish retina. J. Physiol. 590, 5581–5595. doi: 10.1113/jphysiol.2012.240325
Enz, R., Brandstätter, J. H., Wässle, H., and Bormann, J. (1996). Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J. Neurosci. 16, 4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996
Erlander, M. G., Tillakaratne, N. J., Feldblum, S., Patel, N., and Tobin, A. J. (1991). Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100. doi: 10.1016/0896-6273(91)90077-D
Esclapez, M., Tillakaratne, N. J., Kaufman, D. L., Tobin, A. J., and Houser, C. R. (1994). Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J. Neurosci. 14, 1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994
Farrant, M., and Nusser, Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229. doi: 10.1038/nrn1625
Feany, M. B., Lee, S., Edwards, R. H., and Buckley, K. M. (1992). The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 70, 861–867. doi: 10.1016/0092-8674(92)90319-8
Feigenspan, A., and Weiler, R. (2004). Electrophysiological properties of mouse horizontal cell GABAA receptors. J. Neurophysiol. 92, 2789–2801. doi: 10.1152/jn.00284.2004
Fisher, S. K., and Boycott, B. B. (1974). Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc. R. Soc. Lond. B. Biol. Sci. 186, 317–331. doi: 10.1098/rspb.1974.0052
Fortin, G. D., Desrosiers, C. C., Yamaguchi, N., and Trudeau, L. E. (2006). Basal somatodendritic dopamine release requires snare proteins. J. Neurochem. 96, 1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x
Fox, M. A., and Sanes, J. R. (2007). Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503, 280–296. doi: 10.1002/cne.21381
Gasnier, B. (2000). The loading of neurotransmitters into synaptic vesicles. Biochimie 82, 327–337. doi: 10.1016/S0300-9084(00)00221-2
Gilbertson, T. A., Borges, S., and Wilson, M. (1991). The effects of glycine and GABA on isolated horizontal cells from the salamander retina. J. Neurophysiol. 66, 2002–2013. doi: 10.1152/jn.1991.66.6.2002
Glykys, J., and Mody, I. (2007). Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56, 763–770. doi: 10.1016/j.neuron.2007.11.002
Grabs, D., Bergmann, M., Urban, M., Post, A., and Gratzl, M. (1996). Rab3 proteins and SNAP-25, essential components of the exocytosis machinery in conventional synapses, are absent from ribbon synapses of the mouse retina. Eur. J. Neurosci. 8, 162–168. doi: 10.1111/j.1460-9568.1996.tb01177.x
Grassmeyer, J. J., Cahill, A. L., Hays, C. L., Barta, C., Quadros, R. M., Gurumurthy, C. B., et al. (2019). Ca2+ sensor synaptotagmin-1 mediates exocytosis in mammalian photoreceptors. Elife 8:e45946. doi: 10.7554/eLife.45946.018
Gray, E. G., and Pease, H. L. (1971). On understanding the organisation of the retinal receptor synapses. Brain Res. 35, 1–15. doi: 10.1016/0006-8993(71)90591-9
Greenlee, M. H., Roosevelt, C. B., and Sakaguchi, D. S. (2001). Differential localization of SNARE complex proteins SNAP-25, syntaxin, and VAMP during development of the mammalian retina. J. Comp. Neurol. 430, 306–320. doi: 10.1002/1096-986120010212430:3<306::AID-CNE1032>3.0.CO
Greferath, U., Grünert, U., Fritschy, J. M., Stephenson, A., Mohler, H., and Wässle, H. (1995). GABAA receptor subunits have differential distributions in the rat retina: in situ hybridization and immunohistochemistry. J. Comp. Neurol. 353, 553–571. doi: 10.1002/cne.903530407
Greferath, U., Grünert, U., Müller, F., and Wässle, H. (1994). Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 276, 295–307. doi: 10.1007/BF00306115
Greferath, U., Müller, F., Wässle, H., Shivers, B., and Seeburg, P. (1993). Localization of GABAA receptors in the rat retina. Vis. Neurosci. 10, 551–561. doi: 10.1017/S0952523800004764
Grigorenko, E. V., and Yeh, H. H. (1994). Expression profiling of GABAA receptor beta-subunits in the rat retina. Vis. Neurosci. 11, 379–387. doi: 10.1017/S0952523800001723
Grove, J. C. R., Hirano, A. A., de Los Santos, J., McHugh, C. F., Purohit, S., Field, G. D., et al. (2019). Novel hybrid action of GABA mediates inhibitory feedback in the mammalian retina. PLoS Biol. 17:e3000200. doi: 10.1371/journal.pbio.3000200
Grünert, U., and Wässle, H. (1990). GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J. Comp. Neurol. 297, 509–524. doi: 10.1002/cne.902970405
Gu, Y., and Huganir, R. L. (2016). Identification of the SNARE complex mediating the exocytosis of NMDA receptors. Proc. Natl. Acad. Sci. U.S.A. 113, 12280–12285. doi: 10.1073/pnas.1614042113
Guo, C., Hirano, A. A., Stella, S. L. Jr., Bitzer, M., and Brecha, N. C. (2010). Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J. Comp. Neurol. 518, 1647–1669. doi: 10.1002/cne.22294
Guo, C., Stella, S. L. Jr, Hirano, A. A., and Brecha, N. C. (2009). Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J. Comp. Neurol. 512, 6–26. doi: 10.1002/cne.21846
Haas, C. A., DeGennaro, L. J., Muller, M., and Hollander, H. (1990). Synapsin I expression in the rat retina during postnatal development. Exp. Brain Res. 82, 25–32. doi: 10.1007/BF00230834
Harris, K. P., Littleton, J. T., and Stewart, B. A. (2018). Postsynaptic Syntaxin 4 negatively regulates the efficiency of neurotransmitter release. J. Neurogenet. 32, 221–229. doi: 10.1080/01677063.2018.1501372
Harris, K. P., Zhang, Y. V., Piccioli, Z. D., Perrimon, N., and Littleton, J. T. (2016). The postsynaptic t-SNARE Syntaxin 4 controls traffic of Neuroligin 1 and Synaptotagmin 4 to regulate retrograde signaling. Elife 5:e13881. doi: 10.7554/eLife.13881.027
Haverkamp, S., Grünert, U., and Wässle, H. (2000). The cone pedicle, a complex synapse in the retina. Neuron 27, 85–95. doi: 10.1016/S0896-6273(00)00011-8
Haverkamp, S., and Wässle, H. (2000). Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 424, 1–23. doi: 10.1002/1096-9861(20000814)424:1<1::AID-CNE1>3.0.CO;2-V
Hays, C. L., Grassmeyer, J. J., Wen, X., Janz, R., Heidelberger, R., and Thoreson, W. B. (2020). Simultaneous release of multiple vesicles from rods involves synaptic ribbons and syntaxin 3B. Biophys. J. 118, 967–979. doi: 10.1016/j.bpj.2019.10.006
Herrmann, R., Heflin, S. J., Hammond, T., Lee, B., Wang, J., Gainetdinov, R. R., et al. (2011). Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron 72, 101–110. doi: 10.1016/j.neuron.2011.07.030
Hilfiker, S., Pieribone, V. A., Czernik, A. J., Kao, H. T., Augustine, G. J., and Greengard, P. (1999). Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 269–279. doi: 10.1098/rstb.1999.0378
Hirano, A. A., Brandstätter, J. H., and Brecha, N. C. (2005). Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J. Comp. Neurol. 488, 70–81. doi: 10.1002/cne.20577
Hirano, A. A., Brandstätter, J. H., Morgans, C. W., and Brecha, N. C. (2011). SNAP25 expression in mammalian retinal horizontal cells. J. Comp. Neurol. 519, 972–988. doi: 10.1002/cne.22562
Hirano, A. A., Brandstätter, J. H., Vila, A., and Brecha, N. C. (2007). Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis. Neurosci. 24, 489–502. doi: 10.1017/S0952523807070198
Hirano, A. A., and Brecha, N. C. (2010). N-Type calcium channels localize to mammalian horizontal cell endings. Invest. Ophthalmol. Vis. Sci. 51:3283.
Hirano, A. A., Liu, X., Boulter, J., Grove, J., Pérez de Sevilla Müller, L., Barnes, S., et al. (2016a). Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium channels. eNeuro 3:ENEURO.0148-0115.2016. doi: 10.1523/ENEURO.0148-15.2016
Hirano, A. A., Solomon, A., Barnes, S. A., and Brecha, N. C. (2016b). “Extrasynaptic GABAA receptor Gabra6 and Gabra4 are expressed in mouse outer retina,” in Neuroscience Meeting Planner (Washington, DC: Society for Neuroscience, 2016) Online Program No. 239.212
Hirasawa, H., Betensky, R. A., and Raviola, E. (2012). Corelease of dopamine and GABA by a retinal dopaminergic neuron. J. Neurosci. 32, 13281–13291. doi: 10.1523/JNEUROSCI.2213-12.2012
Hirasawa, H., Contini, M., and Raviola, E. (2015). Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370:140186. doi: 10.1098/rstb.2014.0186
Hirasawa, H., and Kaneko, A. (2003). pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J. Gen. Physiol. 122, 657–671. doi: 10.1085/jgp.200308863
Honda, S., Yamamoto, M., and Saito, N. (1995). Immunocytochemical localization of three subtypes of GABA transporter in rat retina. Brain Res. Mol. Brain Res. 33, 319–325. doi: 10.1016/0169-328X(95)00150-Q
Hong, W. (2005). SNAREs and traffic. Biochim. Biophys. Acta 1744, 493–517. doi: 10.1016/j.bbamcr.2005.03.014
Hoon, M., Sinha, R., Okawa, H., Suzuki, S. C., Hirano, A. A., Brecha, N., et al. (2015). Neurotransmission plays contrasting roles in the maturation of inhibitory synapses on axons and dendrites of retinal bipolar cells. Proc. Natl. Acad. Sci. U.S.A. 112, 12840–12845. doi: 10.1073/pnas.1510483112
Hua, Z., Leal-Ortiz, S., Foss, S. M., Waites, C. L., Garner, C. C., Voglmaier, S. M., et al. (2011). v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71, 474–487. doi: 10.1016/j.neuron.2011.06.010
Jaffe, E. H., Marty, A., Schulte, A., and Chow, R. H. (1998). Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J. Neurosci. 18, 3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998
Jahn, R., and Scheller, R. H. (2006). SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell. Biol. 7, 631–643. doi: 10.1038/nrm2002
Janz, R., Goda, Y., Geppert, M., Missler, M., and Sudhof, T. C. (1999). SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24, 1003–1016. doi: 10.1016/S0896-6273(00)81046-6
Janz, R., and Südhof, T. C. (1999). SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94, 1279–1290. doi: 10.1016/S0306-4522(99)00370-X
Jellali, A., Stussi-Garaud, C., Gasnier, B., Rendon, A., Sahel, J. A., Dreyfus, H., et al. (2002). Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J. Comp. Neurol. 449, 76–87. doi: 10.1002/cne.10272
Johnson, J., Chen, T. K., Rickman, D. W., Evans, C., and Brecha, N. C. (1996). Multiple gamma-Aminobutyric acid plasma membrane transporters (GAT-1, GAT-2, GAT-3) in the rat retina. J Comp Neurol. 375, 212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5
Johnson, J., Tian, N., Caywood, M. S., Reimer, R. J., Edwards, R. H., and Copenhagen, D. R. (2003). Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J. Neurosci. 23, 518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003
Johnson, M. A., and Vardi, N. (1998). Regional differences in GABA and GAD immunoreactivity in rabbit horizontal cells. Vis. Neurosci. 15, 743–753. doi: 10.1017/S0952523898154135
Jung, C. S., Lee, S. J., Paik, S. S., and Bai, S. H. (1999). The GABA(C) receptor is present in cone-horizontal cell axon terminals isolated from catfish retina. Neurosci. Lett. 260, 185–188. doi: 10.1016/S0304-3940(98)00964-1
Juusola, M., French, A. S., Uusitalo, R. O., and Weckstrom, M. (1996). Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 19, 292–297. doi: 10.1016/S0166-2236(96)10028-X
Kaeser, P. S., and Regehr, W. G. (2014). Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 76, 333–363. doi: 10.1146/annurev-physiol-021113-170338
Kalloniatis, M., Marc, R. E., and Murry, R. F. (1996). Amino acid signatures in the primate retina. J. Neurosci. 16, 6807–6829. doi: 10.1523/JNEUROSCI.16-21-06807.1996
Kamermans, M., Fahrenfort, I., Schultz, K., Janssen-Bienhold, U., Sjoerdsma, T., and Weiler, R. (2001). Hemichannel-mediated inhibition in the outer retina. Science 292, 1178–1180. doi: 10.1126/science.1060101
Kamermans, M., and Werblin, F. (1992). GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J. Neurosci. 12, 2451–2463. doi: 10.1523/JNEUROSCI.12-07-02451.1992
Kanaani, J., Kolibachuk, J., Martinez, H., and Baekkeskov, S. (2010). Two distinct mechanisms target GAD67 to vesicular pathways and presynaptic clusters. J. Cell Biol. 190, 911–925. doi: 10.1083/jcb.200912101
Kaneko, A., and Tachibana, M. (1986). Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J. Physiol. 373, 443–461. doi: 10.1113/jphysiol.1986.sp016057
Karne, A., Oakley, D. M., Wong, G. K., and Wong, R. O. (1997). Immunocytochemical localization of GABA, GABAA receptors, and synapse-associated proteins in the developing and adult ferret retina. Vis. Neurosci. 14, 1097–1108. doi: 10.1017/S0952523800011809
Kaufman, D. L., Houser, C. R., and Tobin, A. J. (1991). Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 56, 720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x
Kemmler, R., Schultz, K., Dedek, K., Euler, T., and Schubert, T. (2014). Differential regulation of cone calcium signals by different horizontal cell feedback mechanisms in the mouse retina. J. Neurosci. 34, 11826–11843. doi: 10.1523/JNEUROSCI.0272-14.2014
Kennedy, M. J., Davison, I. G., Robinson, C. G., and Ehlers, M. D. (2010). Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 141, 524–535. doi: 10.1016/j.cell.2010.02.042
Kolb, H. (1974). The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J. Comp. Neurol. 155, 1–14. doi: 10.1002/cne.901550102
Kolb, H. (1977). The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J. Neurocytol. 6, 131–153. doi: 10.1007/BF01261502
Kolb, H., and Jones, J. (1984). Synaptic organization of the outer plexiform layer of the turtle retina: an electron microscope study of serial sections. J. Neurocytol. 13, 567–591. doi: 10.1007/BF01148080
Kolb, H., and West, R. W. (1977). Synaptic connections of the interplexiform cell in the retina of the cat. J. Neurocytol. 6, 155–170. doi: 10.1007/BF01261503
Koulen, P., Brandstätter, J. H., Enz, R., Bormann, J., and Wässle, H. (1998a). Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur. J. Neurosci. 10, 115–127. doi: 10.1046/j.1460-9568.1998.00005.x
Koulen, P., Malitschek, B., Kuhn, R., Bettler, B., Wässle, H., and Brandstätter, J. H. (1998b). Presynaptic and postsynaptic localization of GABA(B) receptors in neurons of the rat retina. Eur. J. Neurosci. 10, 1446–1456. doi: 10.1046/j.1460-9568.1998.00156.x
Kramer, R. H., and Davenport, C. M. (2015). Lateral Inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoS Biol. 13:e1002322. doi: 10.1371/journal.pbio.1002322
Kreitzer, M. A., Jacoby, J., Naylor, E., Baker, A., Grable, T., Tran, E., et al. (2012). Distinctive patterns of alterations in proton efflux from goldfish retinal horizontal cells monitored with self-referencing H(+)-selective electrodes. Eur. J. Neurosci. 36, 3040–3050. doi: 10.1111/j.1460-9568.2012.08226.x
Kummel, D., Krishnakumar, S. S., Radoff, D. T., Li, F., Giraudo, C. G., Pincet, F., et al. (2011). Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. 18, 927–933. doi: 10.1038/nsmb.2101
Lam, D. M. (1972). The biosynthesis and content of gamma-aminobutyric acid in the goldifsh retina. J. Cell Biol. 54, 225–231. doi: 10.1083/jcb.54.2.225
Lam, D. M., Lasater, E. M., and Naka, K. I. (1978). gamma-Aminobutyric acid: a neurotransmitter candidate for cone horizontal cells of the catfish retina. Proc. Natl. Acad. Sci. U.S.A. 75, 6310–6313. doi: 10.1073/pnas.75.12.6310
Landgraf, I., Muhlhans, J., Dedek, K., Reim, K., Brandstätter, J. H., and Ammermüller, J. (2012). The absence of complexin 3 and complexin 4 differentially impacts the ON and OFF pathways in mouse retina. Eur. J. Neurosci. 36, 2470–2481. doi: 10.1111/j.1460-9568.2012.08149.x
Lasansky, A. (1973). Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 265, 471–489. doi: 10.1098/rstb.1973.0033
Lee, H. (2010). Vesicular synaptic transmitter release in mammalian horizontal cells. (Ph.D. dissertation) University of California, United States, Los Angeles, CA.
Lee, H., and Brecha, N. C. (2010). Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur. J. Neurosci. 31, 1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x
Lee, H., and Brecha, N. C. (2009). Evidence of vesicular exocytosis in Guinea Pig retinal horizontal cells. Invest. Ophthalmol. Vis. Sci. 50:1037.
Lee, S. E., Lee, Y., and Lee, G. H. (2019). The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res. 42, 1031–1039. doi: 10.1007/s12272-019-01196-z
Lee, V., and Maguire, J. (2014). The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circ. 8:3. doi: 10.3389/fncir.2014.00003
Li, F., Pincet, F., Perez, E., Giraudo, C. G., Tareste, D., and Rothman, J. E. (2011). Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat. Struct. Mol. Biol. 18, 941–946. doi: 10.1038/nsmb.2102
Lin, M., Jiang, M., Ding, F., and Cao, Z. (2017). Syntaxin-4 and SNAP23 act as exocytic SNAREs to release NGF from cultured Schwann cells. Neurosci. Lett. 653, 97–104. doi: 10.1016/j.neulet.2017.01.031
Linberg, K. A., and Fisher, S. K. (1986). An ultrastructural study of interplexiform cell synapses in the human retina. J. Comp. Neurol. 243, 561–576. doi: 10.1002/cne.902430410
Linberg, K. A., and Fisher, S. K. (1988). Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J. Comp. Neurol. 268, 281–297. doi: 10.1002/cne.902680211
Linial, M. (1997). SNARE proteins–why so many, why so few? J. Neurochem. 69, 1781–1792. doi: 10.1046/j.1471-4159.1997.69051781.x
Littleton, J. T., Bellen, H. J., and Perin, M. S. (1993). Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118, 1077–1088.
Liu, C., Kershberg, L., Wang, J., Schneeberger, S., and Kaeser, P. S. (2018). Dopamine secretion is mediated by sparse active zone-like release sites. Cell 172, 706–718.e715. doi: 10.1016/j.cell.2018.01.008
Liu, H., Zhang, Y., Li, S., Yan, Y., and Li, Y. (2010). Dynamic regulation of glutamate decarboxylase 67 gene expression by alternative promoters and splicing during rat testis maturation. Mol. Biol. Rep. 37, 3111–3119. doi: 10.1007/s11033-009-9889-4
Liu, X., Grove, J. C., Hirano, A. A., Brecha, N. C., and Barnes, S. (2016). Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J. Neurophysiol. 116, 686–697. doi: 10.1152/jn.00990.2015
Liu, X., Hirano, A. A., Sun, X., Brecha, N. C., and Barnes, S. (2013). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J. Physiol. 591, 3309–3324. doi: 10.1113/jphysiol.2012.248179
Loeliger, M., and Rees, S. (2005). Immunocytochemical development of the guinea pig retina. Exp. Eye Res. 80, 9–21. doi: 10.1016/j.exer.2004.08.003
Löhrke, S., and Hofmann, H. D. (1994). Voltage-gated currents of rabbit A- and B-type horizontal cells in retinal monolayer cultures. Vis. Neurosci. 11, 369–378. doi: 10.1017/S0952523800001711
Ludwig, M., Apps, D., Menzies, J., Patel, J. C., and Rice, M. E. (2016). Dendritic Release of Neurotransmitters. Compr. Physiol. 7, 235–252. doi: 10.1002/cphy.c160007
Mandell, J. W., Townes-Anderson, E., Czernik, A. J., Cameron, R., Greengard, P., and De Camilli, P. (1990). Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron 5, 19–33. doi: 10.1016/0896-6273(90)90030-J
Marc, R. E. (1992). Structural organization of GABAergic circuitry in ectotherm retinas. Prog. Brain Res. 90, 61–92. doi: 10.1016/S0079-6123(08)63609-2
Marc, R. E., Murry, R. F., Fisher, S. K., Linberg, K. A., and Lewis, G. P. (1998). Amino acid signatures in the detached cat retina. Invest. Ophthalmol. Vis. Sci. 39, 1694–1702
Marqueze, B., Berton, F., and Seagar, M. (2000). Synaptotagmins in membrane traffic: which vesicles do the tagmins tag? Biochimie 82, 409–420. doi: 10.1016/S0300-9084(00)00220-0
Marshall, J., and Voaden, M. (1975). Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 15, 459–461. doi: 10.1016/0042-6989(75)90102-9
Martens, H., Weston, M. C., Boulland, J. L., Gronborg, M., Grosche, J., Kacza, J., et al. (2008). Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J. Neurosci. 28, 13125–13131. doi: 10.1523/JNEUROSCI.3887-08.2008
Martin, D. L., and Rimvall, K. (1993). Regulation of gamma-aminobutyric acid synthesis in the brain. J. Neurochem. 60, 395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x
McIntire, S. L., Reimer, R. J., Schuske, K., Edwards, R. H., and Jorgensen, E. M. (1997). Identification and characterization of the vesicular GABA transporter. Nature 389, 870–876. doi: 10.1038/39908
Mercer, A. J., and Thoreson, W. B. (2011). The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis. Neurosci. 28, 453–471. doi: 10.1017/S0952523811000356
Messersmith, E. K., and Redburn, D. A. (1992). gamma-Aminobutyric acid immunoreactivity in multiple cell types of the developing rabbit retina. Vis. Neurosci. 8, 201–211. doi: 10.1017/S0952523800002856
Messersmith, E. K., and Redburn, D. A. (1993). The role of GABA during development of the outer retina in the rabbit. Neurochem. Res. 18, 463–470. doi: 10.1007/BF00967250
Mitchell, C. K., Huang, B., and Redburn-Johnson, D. A. (1999). GABA(A) receptor immunoreactivity is transiently expressed in the developing outer retina. Vis. Neurosci. 16, 1083–1088. doi: 10.1017/S0952523899166082
Mitchell, C. K., and Redburn, D. A. (1996). GABA and GABA-A receptors are maximally expressed in association with cone synaptogenesis in neonatal rabbit retina. Brain Res. Dev. Brain Res. 95, 63–71. doi: 10.1016/0165-3806(96)00064-8
Morgans, C. W., Bayley, P. R., Oesch, N. W., Ren, G., Akileswaran, L., and Taylor, W. R. (2005). Photoreceptor calcium channels: insight from night blindness. Vis. Neurosci. 22, 561–568. doi: 10.1017/S0952523805225038
Morgans, C. W., Brandstätter, J. H., Kellerman, J., Betz, H., and Wässle, H. (1996). A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J. Neurosci. 16, 6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996
Mortensen, L. S., Park, S. J. H., Ke, J. B., Cooper, B. H., Zhang, L., Imig, C., et al. (2016). Complexin 3 increases the fidelity of signaling in a retinal circuit by regulating exocytosis at ribbon synapses. Cell Rep. 15, 2239–2250. doi: 10.1016/j.celrep.2016.05.012
Moser, T., Grabner, C. P., and Schmitz, F. (2020). sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 100, 103–144. doi: 10.1152/physrev.00026.2018
Mosinger, J., and Yazulla, S. (1987). Double-label analysis of GAD- and GABA-like immunoreactivity in the rabbit retina. Vision Res. 27, 23–30. doi: 10.1016/0042-6989(87)90139-8
Mosinger, J. L., and Yazulla, S. (1985). Colocalization of GAD-like immunoreactivity and 3H-GABA uptake in amacrine cells of rabbit retina. J. Comp. Neurol. 240, 396–406. doi: 10.1002/cne.902400407
Mosinger, J. L., Yazulla, S., and Studholme, K. M. (1986). GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp. Eye Res. 42, 631–644. doi: 10.1016/0014-4835(86)90052-7
Nishimura, Y., Schwartz, M. L., and Rakic, P. (1985). Localization of gamma-aminobutyric acid and glutamic acid decarboxylase in rhesus monkey retina. Brain Res. 359, 351–355. doi: 10.1016/0006-8993(85)91449-0
Olney, J. W. (1968). An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest. Ophthalmol. 7, 250–268
Osborne, N. N., Patel, S., Beaton, D. W., and Neuhoff, V. (1986). GABA neurones in retinas of different species and their postnatal development in situ and in culture in the rabbit retina. Cell Tissue Res. 243, 117–123. doi: 10.1007/BF00221859
Ovsepian, S. V., and Dolly, J. O. (2011). Dendritic SNAREs add a new twist to the old neuron theory. Proc. Natl. Acad. Sci. U.S.A. 108, 19113–19120. doi: 10.1073/pnas.1017235108
Pattnaik, B., Jellali, A., Sahel, J., Dreyfus, H., and Picaud, S. (2000). GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J. Neurosci. 20, 6789–6796. doi: 10.1523/JNEUROSCI.20-18-06789.2000
Peichl, L., and González-Soriano, J. (1994). Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 11, 501–517. doi: 10.1017/S095252380000242X
Peters, A., Webster, H.,d., Palay, S. L. (1991). The Fine Structure of the Nervous System : Neurons and Their Supporting Cells. New York, NY: Oxford University Press
Picaud, S., Hicks, D., Forster, V., Sahel, J., and Dreyfus, H. (1998a). Adult human retinal neurons in culture: physiology of horizontal cells. Invest. Ophthalmol. Vis. Sci. 39, 2637–2648.
Picaud, S., Pattnaik, B., Hicks, D., Forster, V., Fontaine, V., Sahel, J., et al. (1998b). GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J. Physiol. 513, 33–42. doi: 10.1111/j.1469-7793.1998.033by.x
Pourcho, R. G., and Owczarzak, M. T. (1989). Distribution of GABA immunoreactivity in the cat retina: a light- and electron-microscopic study. Vis. Neurosci. 2, 425–435. doi: 10.1017/S0952523800012323
Pow, D. V., Baldridge, W., and Crook, D. K. (1996). Activity-dependent transport of GABA analogues into specific cell types demonstrated at high resolution using a novel immunocytochemical strategy. Neuroscience 73, 1129–1143. doi: 10.1016/0306-4522(96)00097-8
Pow, D. V., and Crook, D. K. (1994). Rapid postmortem changes in the cellular localisation of amino acid transmitters in the retina as assessed by immunocytochemistry. Brain Res. 653, 199–209. doi: 10.1016/0006-8993(94)90390-5
Pow, D. V., Crook, D. K., and Wong, R. O. (1994). Early appearance and transient expression of putative amino acid neurotransmitters and related molecules in the developing rabbit retina: an immunocytochemical study. Vis. Neurosci. 11, 1115–1134. doi: 10.1017/S0952523800006933
Puller, C., Haverkamp, S., Neitz, M., and Neitz, J. (2014). Synaptic elements for GABAergic feed-forward signaling between HII horizontal cells and blue cone bipolar cells are enriched beneath primate S-cones. PLoS ONE 9:e88963. doi: 10.1371/journal.pone.0088963
Puopolo, M., Hochstetler, S. E., Gustincich, S., Wightman, R. M., and Raviola, E. (2001). Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 30, 211–225. doi: 10.1016/S0896-6273(01)00274-4
Qian, H., and Dowling, J. E. (1993). GABA responses on retinal bipolar cells. Biol. Bull. 185:312. doi: 10.1086/BBLv185n2p312a
Raviola, E., and Gilula, N. B. (1975). Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J. Cell Biol. 65, 192–222. doi: 10.1083/jcb.65.1.192
Redburn, D. A., and Madtes, P. Jr. (1986). Postnatal development of 3H-GABA-accumulating cells in rabbit retina. J. Comp. Neurol. 243, 41–57. doi: 10.1002/cne.902430105
Reim, K., Wegmeyer, H., Brandstätter, J. H., Xue, M., Rosenmund, C., Dresbach, T., et al. (2005). Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 169, 669–680. doi: 10.1083/jcb.200502115
Reimer, R. J., Fon, E. A., and Edwards, R. H. (1998). Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr. Opin. Neurobiol. 8, 405–412. doi: 10.1016/S0959-4388(98)80068-8
Ribak, C. E., Tong, W. M., and Brecha, N. C. (1996). Astrocytic processes compensate for the apparent lack of GABA transporters in the axon terminals of cerebellar Purkinje cells. Anat. Embryol. 194, 379–390. doi: 10.1007/BF00198540
Rice, M. E., and Patel, J. C. (2015). Somatodendritic dopamine release: recent mechanistic insights. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370:1672. doi: 10.1098/rstb.2014.0185
Rizo, J. (2018). Mechanism of neurotransmitter release coming into focus. Protein Sci. 27, 1364–1391. doi: 10.1002/pro.3445
Rizo, J., and Xu, J. (2015). The synaptic vesicle release machinery. Annu. Rev. Biophys. 44, 339–367. doi: 10.1146/annurev-biophys-060414-034057
Röhrenbeck, J., Wässle, H., and Heizmann, C. W. (1987). Immunocytochemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci. Lett. 77, 255–260. doi: 10.1016/0304-3940(87)90508-8
Sagné, C., El Mestikawy, S., Isambert, M. F., Hamon, M., Henry, J. P., Giros, B., et al. (1997). Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 417, 177–183. doi: 10.1016/S0014-5793(97)01279-9
Sakai, H. M., and Naka, K. (1986). Synaptic organization of the cone horizontal cells in the catfish retina. J. Comp. Neurol. 245, 107–115. doi: 10.1002/cne.902450108
Sarthy, P. V., and Fu, M. (1989). Localization of L-glutamic acid decarboxylase mRNA in cat retinal horizontal cells by in situ hybridization. J. Comp. Neurol. 288, 593–600. doi: 10.1002/cne.902880406
Satoh, H., Kaneda, M., and Kaneko, A. (2001). Intracellular chloride concentration is higher in rod bipolar cells than in cone bipolar cells of the mouse retina. Neurosci. Lett. 310, 161–164. doi: 10.1016/S0304-3940(01)02120-6
Schaeffer, S. F., Raviola, E., and Heuser, J. E. (1982). Membrane specializations in the outer plexiform layer of the turtle retina. J. Comp. Neurol. 204, 253–267. doi: 10.1002/cne.902040305
Schnitzer, J., and Rusoff, A. C. (1984). Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J. Neurosci. 4, 2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984
Schubert, T., Huckfeldt, R. M., Parker, E., Campbell, J. E., and Wong, R. O. (2010). Assembly of the outer retina in the absence of GABA synthesis in horizontal cells. Neural Dev. 5:15. doi: 10.1186/1749-8104-5-15
Schubert, T., Weiler, R., and Feigenspan, A. (2006). Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J. Neurophysiol. 96, 1278–1292. doi: 10.1152/jn.00191.2006
Schwartz, E. A. (1982). Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J. Physiol. 323, 211–227. doi: 10.1113/jphysiol.1982.sp014069
Schwartz, E. A. (1987). Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science 238, 350–355. doi: 10.1126/science.2443977
Schwartz, E. A. (2002). Transport-mediated synapses in the retina. Physiol. Rev. 82, 875–891. doi: 10.1152/physrev.00010.2002
Semyanov, A., Walker, M. C., Kullmann, D. M., and Silver, R. A. (2004). Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 27, 262–269. doi: 10.1016/j.tins.2004.03.005
Sherry, D. M., Mitchell, R., Standifer, K. M., and du Plessis, B. (2006). Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 7:54. doi: 10.1186/1471-2202-7-54
Sherry, D. M., Wang, M. M., and Frishman, L. J. (2003). Differential distribution of vesicle associated membrane protein isoforms in the mouse retina. Mol. Vis. 9, 673–688
Söderberg, O., Gullberg, M., Jarvius, M., Ridderstrale, K., Leuchowius, K. J., Jarvius, J., et al. (2006). Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000. doi: 10.1038/nmeth947
Söderberg, O., Leuchowius, K. J., Gullberg, M., Jarvius, M., Weibrecht, I., Larsson, L. G., et al. (2008). Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227–232. doi: 10.1016/j.ymeth.2008.06.014
Spiwoks-Becker, I., Vollrath, L., Seeliger, M. W., Jaissle, G., Eshkind, L. G., and Leube, R. E. (2001). Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience 107, 127–142. doi: 10.1016/S0306-4522(01)00345-1
Starr, M. S. (1973). Effect of dark adaptation on the GABA system in retina. Brain Res. 59, 331–338. doi: 10.1016/0006-8993(73)90271-0
Südhof, T. C. (2002). Synaptotagmins: why so many? J. Biol. Chem. 277, 7629–7632. doi: 10.1074/jbc.R100052200
Südhof, T. C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. doi: 10.1146/annurev.neuro.26.041002.131412
Südhof, T. C. (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80, 675–690. doi: 10.1016/j.neuron.2013.10.022
Südhof, T. C., and Rizo, J. (1996). Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron 17, 379–388. doi: 10.1016/S0896-6273(00)80171-3
Tachibana, M., and Kaneko, A. (1984). gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc. Natl. Acad. Sci. U.S.A. 81, 7961–7964. doi: 10.1073/pnas.81.24.7961
Takahashi, K., Miyoshi, S., and Kaneko, A. (1994). Two components of GABA-induced currents in catfish retinal horizontal cells. Jpn. J. Physiol. 44(Suppl 2):S141–144.
Takahashi, K., Miyoshi, S., and Kaneko, A. (1995). GABA-induced chloride current in catfish horizontal cells mediated by non-GABAA receptor channels. Jpn. J. Physiol. 45, 437–456. doi: 10.2170/jjphysiol.45.437
Takamori, S., Holt, M., Stenius, K., Lemke, E. A., Gronborg, M., Riedel, D., et al. (2006). Molecular anatomy of a trafficking organelle. Cell 127, 831–846. doi: 10.1016/j.cell.2006.10.030
Tao, R., Davis, K. N., Li, C., Shin, J. H., Gao, Y., Jaffe, A. E., et al. (2018). GAD1 alternative transcripts and DNA methylation in human prefrontal cortex and hippocampus in brain development, schizophrenia. Mol. Psychiatry 23, 1496–1505. doi: 10.1038/mp.2017.105
Tchernookova, B. K., Heer, C., Young, M., Swygart, D., Kaufman, R., Gongwer, M., et al. (2018). Activation of retinal glial (Muller) cells by extracellular ATP induces pronounced increases in extracellular H+ flux. PLoS ONE 13:e0190893. doi: 10.1371/journal.pone.0190893
Teng, F. Y., Wang, Y., and Tang, B. L. (2001). The syntaxins. Genome Biol. 2:REVIEWS3012. doi: 10.1186/gb-2001-2-11-reviews3012
Thoreson, W. B., and Burkhardt, D. A. (1990). Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis. Neurosci. 5, 571–583. doi: 10.1017/S0952523800000730
Thoreson, W. B., and Mangel, S. C. (2012). Lateral interactions in the outer retina. Prog. Retin. Eye Res. 31, 407–441. doi: 10.1016/j.preteyeres.2012.04.003
Tong, Q., Ye, C. P., Jones, J. E., Elmquist, J. K., and Lowell, B. B. (2008). Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000. doi: 10.1038/nn.2167
Trifonov, S., Yamashita, Y., Kase, M., Maruyama, M., and Sugimoto, T. (2014). Glutamic acid decarboxylase 1 alternative splicing isoforms: characterization, expression and quantification in the mouse brain. BMC Neurosci. 15:114. doi: 10.1186/1471-2202-15-114
Trimbuch, T., and Rosenmund, C. (2016). Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 17, 118–125. doi: 10.1038/nrn.2015.16
Ueda, Y., Kaneko, A., and Kaneda, M. (1992). Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J. Neurophysiol. 68, 1143–1150. doi: 10.1152/jn.1992.68.4.1143
Ullrich, B., and Südhof, T. C. (1994). Distribution of synaptic markers in the retina: implications for synaptic vesicle traffic in ribbon synapses. J. Physiol. Paris. 88, 249–257. doi: 10.1016/0928-4257(94)90088-4
Vaithianathan, T., Akmentin, W., Henry, D., and Matthews, G. (2013). The ribbon-associated protein C-terminal-binding protein 1 is not essential for the structure and function of retinal ribbon synapses. Mol. Vis. 19, 917–926.
Van Hook, M. J., Nawy, S., and Thoreson, W. B. (2019). Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res 72:100760. doi: 10.1016/j.preteyeres.2019.05.001
Vardi, N., and Auerbach, P. (1995). Specific cell types in cat retina express different forms of glutamic acid decarboxylase. J. Comp. Neurol. 351, 374–384. doi: 10.1002/cne.903510305
Vardi, N., Kaufman, D. L., and Sterling, P. (1994). Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Vis. Neurosci. 11, 135–142. doi: 10.1017/S0952523800011172
Vardi, N., Masarachia, P., and Sterling, P. (1992). Immunoreactivity to GABAA receptor in the outer plexiform layer of the cat retina. J. Comp. Neurol. 320, 394–397. doi: 10.1002/cne.903200310
Vardi, N., Morigiwa, K., Wang, T. L., Shi, Y. J., and Sterling, P. (1998). Neurochemistry of the mammalian cone 'synaptic complex'. Vision Res. 38, 1359–1369. doi: 10.1016/S0042-6989(98)00007-8
Vardi, N., and Sterling, P. (1994). Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 34, 1235–1246. doi: 10.1016/0042-6989(94)90198-8
Vardi, N., Zhang, L. L., Payne, J. A., and Sterling, P. (2000). Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J. Neurosci. 20, 7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000
Varela, C., Rivera, L., Blanco, R., and De la Villa, P. (2005). Depolarizing effect of GABA in horizontal cells of the rabbit retina. Neurosci. Res. 53, 257–264. doi: 10.1016/j.neures.2005.07.001
Versaux-Botteri, C., Pochet, R., and Nguyen-Legros, J. (1989). Immunohistochemical localization of GABA-containing neurons during postnatal development of the rat retina. Invest. Ophthalmol. Vis. Sci. 30, 652–659.
Verweij, J., Hornstein, E. P., and Schnapf, J. L. (2003). Surround antagonism in macaque cone photoreceptors. J. Neurosci. 23, 10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003
Verweij, J., Kamermans, M., and Spekreijse, H. (1996). Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 36, 3943–3953. doi: 10.1016/S0042-6989(96)00142-3
Vessey, J. P., Stratis, A. K., Daniels, B. A., Da Silva, N., Jonz, M. G., Lalonde, M. R., et al. (2005). Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J. Neurosci. 25, 4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005
Von Kriegstein, K., Schmitz, F., Link, E., and Südhof, T. C. (1999). Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur. J. Neurosci. 11, 1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x
Vuong, H. E., Kornmann, H. L., Stella, J. S. L., and Brecha, N. (2011). Gabaergic synaptic vesicles in guinea pig horizontal cells participate in Ca2+ dependent recycling. Invest. Ophthalmol. Vis. Sci. 52:4110.
Walker, M. C., and Semyanov, A. (2008). Regulation of excitability by extrasynaptic GABA(A) receptors. Results Probl. Cell Differ. 44, 29–48. doi: 10.1007/400_2007_030
Wan, Q. F., Zhou, Z. Y., Thakur, P., Vila, A., Sherry, D. M., Janz, R., et al. (2010). SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 66, 884–895. doi: 10.1016/j.neuron.2010.05.010
Wang, H., Standifer, K. M., and Sherry, D. M. (2000). GABA(A) receptor binding and localization in the tiger salamander retina. Vis. Neurosci. 17, 11–21. doi: 10.1017/S0952523800171020
Wang, M. M., Janz, R., Belizaire, R., Frishman, L. J., and Sherry, D. M. (2003). Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. J. Comp. Neurol. 460, 106–122. doi: 10.1002/cne.10636
Wang, T. M., Holzhausen, L. C., and Kramer, R. H. (2014). Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat. Neurosci. 17, 262–268. doi: 10.1038/nn.3627
Wässle, H., and Chun, M. H. (1988). Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. J. Neurosci. 8, 3383–3394. doi: 10.1523/JNEUROSCI.08-09-03383.1988
Wässle, H., and Chun, M. H. (1989). GABA-like immunoreactivity in the cat retina: light microscopy. J. Comp. Neurol. 279, 43–54. doi: 10.1002/cne.902790105
Wässle, H., Koulen, P., Brandstätter, J. H., Fletcher, E. L., and Becker, C. M. (1998). Glycine and GABA receptors in the mammalian retina. Vision Res. 38, 1411–1430. doi: 10.1016/S0042-6989(97)00300-3
Wei, J., and Wu, J. Y. (2008). Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem. Res. 33, 1459–1465. doi: 10.1007/s11064-008-9600-5
Wilkinson, M. F., and Barnes, S. (1996). The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J. Gen. Physiol. 107, 621–630. doi: 10.1085/jgp.107.5.621
Witkovsky, P. (2004). Dopamine and retinal function. Doc. Ophthalmol. 108, 17–40. doi: 10.1023/B:DOOP.0000019487.88486.0a
Witkovsky, P., Gabriel, R., and Krizaj, D. (2008). Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. J. Comp. Neurol. 510, 158–174. doi: 10.1002/cne.21784
Witkovsky, P., Patel, J. C., Lee, C. R., and Rice, M. E. (2009). Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience 164, 488–496. doi: 10.1016/j.neuroscience.2009.08.017
Wu, S. M. (1992). Feedback connections and operation of the outer plexiform layer of the retina. Curr. Opin. Neurobiol. 2, 462–468. doi: 10.1016/0959-4388(92)90181-J
Wu, S. M., and Dowling, J. E. (1980). Effects of GABA and glycine on the distal cells of the cyprinid retina. Brain Res. 199, 401–414. doi: 10.1016/0006-8993(80)90697-6
Yamasaki, E. N., Barbosa, V. D., De Mello, F. G., and Hokoc, J. N. (1999). GABAergic system in the developing mammalian retina: dual sources of GABA at early stages of postnatal development. Int. J. Dev. Neurosci. 17, 201–213. doi: 10.1016/S0736-5748(99)00002-7
Yang, X. L. (2004). Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 73, 127–150. doi: 10.1016/j.pneurobio.2004.04.002
Yang, X. L., Gao, F., and Wu, S. M. (1999). Modulation of horizontal cell function by GABA(A) and GABA(C) receptors in dark- and light-adapted tiger salamander retina. Vis. Neurosci. 16, 967–979. doi: 10.1017/S0952523899165167
Yang, X. L., and Wu, S. M. (1991). Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc. Natl. Acad. Sci. U.S.A. 88, 3310–3313. doi: 10.1073/pnas.88.8.3310
Yang, X. L., and Wu, S. M. (1993). Effects of GABA on horizontal cells in the tiger salamander retina. Vision Res. 33, 1339–1344. doi: 10.1016/0042-6989(93)90041-T
Yazulla, S., Studholme, K. M., and Pinto, L. H. (1997). Differences in the retinal GABA system among control, spastic mutant and retinal degeneration mutant mice. Vision Res. 37, 3471–3482. doi: 10.1016/S0042-6989(96)00223-4
Yazulla, S., Studholme, K. M., Vitorica, J., and de Blas, A. L. (1989). Immunocytochemical localization of GABAA receptors in goldfish and chicken retinas. J. Comp. Neurol. 280, 15–26. doi: 10.1002/cne.902800103
Yoon, T. Y., and Munson, M. (2018). SNARE complex assembly and disassembly. Curr. Biol. 28, R397–R401. doi: 10.1016/j.cub.2018.01.005
Zampighi, G. A., Fain, N., Zampighi, L. M., Cantele, F., Lanzavecchia, S., and Wright, E. M. (2008). Conical electron tomography of a chemical synapse: polyhedral cages dock vesicles to the active zone. J. Neurosci. 28, 4151–4160. doi: 10.1523/JNEUROSCI.4639-07.2008
Zampighi, G. A., Schietroma, C., Zampighi, L. M., Woodruff, M., Wright, E. M., and Brecha, N. C. (2011). Conical tomography of a ribbon synapse: structural evidence for vesicle fusion. PLoS ONE 6:e16944. doi: 10.1371/journal.pone.0016944
Keywords: retina, horizontal cell, GABA, exocytosis, tonic current
Citation: Hirano AA, Vuong HE, Kornmann HL, Schietroma C, Stella SL Jr, Barnes S and Brecha NC (2020) Vesicular Release of GABA by Mammalian Horizontal Cells Mediates Inhibitory Output to Photoreceptors. Front. Cell. Neurosci. 14:600777. doi: 10.3389/fncel.2020.600777
Received: 31 August 2020; Accepted: 04 November 2020;
Published: 01 December 2020.
Edited by:
Wallace B. Thoreson, University of Nebraska Medical Center, United StatesReviewed by:
Andreas Feigenspan, University of Erlangen Nuremberg, GermanyCopyright © 2020 Hirano, Vuong, Kornmann, Schietroma, Stella, Barnes and Brecha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arlene A. Hirano, YWhpcmFub0BtZWRuZXQudWNsYS5lZHU=
†Present address: Helen E. Vuong, Department of Integrative Biology and Physiology, University of California, Los Angeles, Los Angeles, CA, United States
Helen L. Kornmann, Glaucoma Associates of Texas, Dallas, TX, United States
Cataldo Schietroma, AbbeLight Biotech, Cachan, France
Salvatore L. Stella Jr., Department of Neural and Behavioral Sciences, Penn State University College of Medicine, Hershey, PA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.